SUMMARY
Nitric oxide (NO) plays a key role in the pathogenesis of inflammation and has been implicated in endotoxin-induced tissue injury. Chicken feather meal is a rich source of amino acids that may serve as a peptide hydrolysate to inhibit NO activity. Anti-inflammatory hydrolysates of chicken feather meal were prepared and fractionated into five samples based on molecular mass. The smallest fraction (<0.65 kDa) exhibited the highest NO inhibitory activity without cytotoxicity towards macrophage RAW 264.7 cells. Further subfractions were sufficient to obtain amino acid sequences by Q-TOF LC-MS/MS ESI analysis. Of these, the SNPSVAGVR (885.97 Da) peptide and its corresponding pure synthetic peptide have inhibitory activity against NO production by RAW 264.7 cells (IC50=(55.2±0.2) mM) without cytotoxicity. Reverse transcriptase polymerase chain reaction (RT-PCR) and quantitative real-time RT-PCR results revealed that the peptide of the obtained fraction reduced transcript expression levels of the pro-inflammatory cytokines iNOS, TNF-α, COX-2 and IL-6 in lipopolysaccharide-stimulated RAW 264.7 cells. These results suggest that the peptides derived from the chicken feather meal protein could potentially be used as a promising ingredient in functional foods or nutraceuticals against inflammatory diseases.
Key words: nitric oxide, anti-inflammatory activity, chicken feather meal, protein hydrolysate, polypeptide, macrophage RAW 264.7
INTRODUCTION
Inflammation is a host defence mechanism that involves physiological and pathological processes within an organism, and is induced by the invasion of pathogens or tissue injury caused by biological, chemical or physical damage (1). The activation of several immune cells (monocytes and macrophages) produces inflammation mediators, such as nitric oxide (NO), cyclooxygenase-2 (COX-2), prostaglandins E2 (PGE2) and other pro-inflammatory cytokines (iNOS), including tumour necrosis factor alpha (TNF-α), interleukin-6 (IL-6) and interleukin-1β (2). Macrophages play an important role in inflammation and this cellular response is initiated by bacterial lipopolysaccharide (LPS), which is part of the outer membrane of Gram-negative bacteria, interferon-γ (IFN-γ) and pro-inflammatory cytokines (3). Oxidative stress is involved in the pathogenesis of several diseases. Physical and biochemical processes in the human body generate free radicals as byproducts that trigger a wide range of diseases (4). The interaction of reactive nitrogen species and reactive oxygen species (ROS), e.g. NO, with toxic agents is strongly linked with inflammation, while the uncontrolled production of these species is linked to several diseases, including cancer, coronary heart disease, rheumatoid arthritis, asthma and Alzheimer’s (5). During inflammatory processes, inflammatory mediators, such as NO and PGE2, are produced via the oxidation of l-arginine by inducible nitric oxide synthase (iNOS) and the conversion of arachidonic acid by COX-2. Moreover, NO is a key signalling biological molecule involved in vasodilation, regulation of blood pressure, neurotransmission and the host immune defence system. Inflammation can be regulated by suppression of the pro-inflammatory cytokines and NO production (6).
Currently, there is an increasing interest in food proteins and their constituent peptides as potential candidates for use as antioxidant and anti-inflammatory agents (7). The production and development of functional food or dietary supplements has increased significantly and these products have been used to aid human health (8). Peptides are highly selective, efficient and completely safe for humans (9). These peptides are often functionally inactive within native proteins and must be released by hydrolysis using enzymes such as Alcalase, Flavourzyme and Neutrase to achieve their specific bioactive roles (10). Recently, significant pharmaceutical research has been undertaken in an effort to use bioactive peptides from plants or animals as potential medicines and to underpin research into drug development (11). Currently, bioactive peptides that display anticancer (12), antimicrobial (13), hypocholesterolemic (14), antihypertensive (15), and anti-inflammatory properties (16) have been identified.
Poultry processing plants and related industries are expanding in many countries, especially in Thailand. Although chicken feather meal represents around 5–7% of the body mass of chicken, it is a major waste byproduct of poultry processing, with large amounts produced annually, and its substantial accumulation causes potential environmental problems and pollution. Instead, chicken feather meal can be used as an alternative value-added product as animal feed or feed supplement and organic fertilizer due to its high (80–90%) protein content, as well as being rich in hydrophobic amino acids (17). The objective of this study is to prepare peptides from chicken feather meal by enzymatic hydrolysis using microbial proteases and then to determine the in vitro anti-inflammatory effect of the isolated peptide samples on macrophage RAW 264.7 cells. The results indicate that chicken feather meal is a suitable source of anti-inflammatory peptides, which can be further developed in the pharmaceutical industry or as an ingredient in cosmetic products on the global market.
MATERIALS AND METHODS
Biological materials
The chicken feather meal used in this study was obtained from Betagro Science Center Co., Ltd. (Pathumthani, Thailand) and was ground to small particles and dried at 60 °C overnight. Then it was filtered through a 150-μm sieve to give a more homogenous particle size distribution for better accuracy and consistency of the results.
Chemicals
Alcalase and Flavourzyme were purchased from Brentag (Mülheim, Germany). Neutrase was purchased from Novozymes (Bagsværd, Denmark). Acetic acid, ethanol and phosphoric acid were purchased from Merck (Gibbstown, NJ, USA). Acetonitrile (ACN), l-α-amino-n-butyric acid, bovine serum albumin (BSA), budesonide, curcumin from Curcuma longa (turmeric), Coomassie brilliant blue G-250, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), disodium hydrogen phosphate, Dulbecco’s modified Eagle medium (DMEM), foetal bovine serum (FBS), phosphoric acid, formic acid, hydrochloric acid, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), methanol, monosodium dihydrogen orthophosphate, mouse interferon gamma (IFN-γ), lipopolysaccharides (LPS) from Escherichia coli, potassium persulfate, sodium nitrite, (1-naphthyl)ethylenediamine (NED), sodium nitroprusside (SNP), sodium pyruvate, streptomycin sulphate, sulphanilamide, and trifluoroacetic acid (TFA) were purchased from Sigma-Aldrich, Merck (St. Louis, MO, USA).
Determination of the chicken feather meal amino acid content
The amino acid content of the chicken feather meal was determined based on the standard AOAC method 994.12 (18). One gram of chicken feather meal was dissolved in 5 mL of 6 M HCl in a test tube and then placed into a heating block at 110 °C for 24 h to liberate the amino acids. The internal standard (10 mL 2.5 mM l-α-amino-n-butyric acid in 0.1 M HCl) was added to this sample, diluted with deionized water to 250 mL and placed in a heating block at 55 °C for 10 min. Amino acids were analysed by reversed-phase high performance liquid chromatography (RP-HPLC; Waters Corporation, Milford, MA, USA) using a Hypersil GOLD column C18 (50 mm×0.5 mm, 5 µm; Thermo Fisher Scientific, San Jose, CA, USA) shielded by a guard column on a Hypersil GOLD (30 mm×0.5 mm, 5 µm C18; Thermo Fisher Scientific, San Jose, CA, USA, elution buffer of 20 mM sodium acetate (pH=4.90) and 60% acetonitrile (ACN) at a flow rate of 0.3 mL/min.
Preparation of enzymatic hydrolysate from chicken feather meal
The sieved chicken feather meal (5 µg) was mixed with 100 mL of phosphate-buffered saline (PBS; 20 mM phosphate buffer, 0.15 M NaCl, pH=7.2) and stirred at 4 °C overnight. The suspension was then hydrolysed with one of three types of microbial proteases (Alcalase, Flavourzyme (both from Bren-tag) and Neutrase (Novozymes)) at 0, 1, 2.5 or 5% (m/V) for 4 h at 50 °C and pH=7 (except for Alcalase treatment which was at pH=8) with shaking (180 rpm; model Innova 4330 refrigerated floor incubator shaker; New Brunswick Scientific (UK) Ltd., Hatfield, Herts, UK). After hydrolysis, the mixtures were heated to 90 °C for 10 min to inactivate the enzymes and the samples were clarified by centrifugation (model Kubota 6500; Shimadzu, Kyoto, Japan) at 6440×g for 15 min. The supernatant (i.e. hydrolysate) was collected and stored at –20 °C until use.
Protein content determination
The concentration of the chicken feather meal protein hydrolysate was determined according to the Bradford procedure (19), using BSA as the standard protein to construct the calibration curve. For each sample three aliquots (20 μL) were separately mixed with 200 μL of the Bradford working solution (50 mg of Coomassie brilliant blue G-250 in 50 mL of methanol and 100 mL 85% (m/V) phosphoric acid) in a 96-well plate (flat-bottom 96-well; Bio-Rad Laboratories, Inc., Hercules, MA, USA), incubated for 20 min and then the absorbance was measured at A540 nm with a microplate reader (Multiskan GO; Thermo Fisher Scientific, Waltham, MA, USA). The protein content in each sample was determined from the standard curve derived from the BSA measurements.
NO radical scavenging assay
The NO radical scavenging assay was slightly modified from that previously reported by Chantaranothai et al. (20). The peptide hydrolysates (5 μL) were mixed with 10 mM of SNP in PBS and incubated at room temperature for 150 min before the addition of 100 μL of Griess reagent (0.33% sulfanilamide in 5% phosphoric acid) and incubation for 5 min. Next, 100 μL NED were added and the samples were incubated for 30 min at room temperature before measurement of the absorbance at 540 nm using a microplate reader (model Multiskan GO, Thermo Fisher Scientific Inc., San Jose, CA, USA). The percentage of NO inhibition (%) and concentration of hydrolysate that inhibited 50% of the NO radical production (IC50) were calculated, the latter using the GraphPad Prism v. 6.01 for Windows (GraphPad Software Inc., San Diego, CA, USA) (21). Curcumin was used as the positive control. The NO scavenging activity (%) was calculated from:
| NO inhibition = ((Acontrol−Ablank)−(Asample−Abackground/(Acontrol−Ablank)) ·100 /1/ |
where Acontrol is the absorbance of the control (no sample), Asample is the absorbance of the sample, Abackground is the absorbance of the background (colour of the sample), and Ablank is the absorbance of blank (deionized water).
Fractionation and enrichment of the chicken feather meal peptide hydrolysate
Molecular mass cut-off by ultrafiltration
The chicken feather meal peptide hydrolysate was fractionated through ultrafiltration membranes with molecular mass cut- -off (MMCO; Pellicon XL filter; Merck Millipore, Billerica, MA, USA) values of 10, 5, 3 and 0.65 kDa to give five different size fractions of >10, 5–10, 3–5, 0.65–3 and <0.65 kDa. The obtained protein hydrolysate fractions were stored at –20 °C until further use.
Gel filtration chromategraphy
The fraction with the highest NO radical scavenging activity from the ultrafiltration step (i.e.<0.65 kDa fraction) was loaded onto a preparative Sephacryl S-100 gel filtration column (1.6 cm×60 cm, AKTA™ prime with Hitrap™; Amersham Biosciences, Uppsala, Sweden). The samples were eluted in distilled water at a flow rate of 0.5 mL/min and collected in 5-mL fractions (Fraction Collector Frac-950; Amersham Biosciences), and the absorbance was monitored at A280 nm to determine protein concentration. Fractions in each protein peak were pooled and the resulting separate fractions (F1 to F4) were assayed for NO radical scavenging activity.
RP-HPLC
The protein fraction obtained by gel filtration chromatography with the highest NO radical scavenging activity (F2) was further fractionated by RP-HPLC using a C18 column (250 mm×4.6 mm, Luna 5U; Phenomenex, Torrance, CA, USA). The peptides were eluted at room temperature using a gradient (0–100% mobile phase B for 20 min) of mobile phase A (0.1%, V/V, trifluoroacetic acid; TFA) and B (70%, V/V, ACN in 0.05%, V/V, TFA) at a flow rate of 0.7 mL/min. Chromatographic analyses were performed using the ChromQuest software (Thermo Fisher Scientific Inc., Waltham, MA, USA) (22). Peptides were detected by measuring the absorbance at A280 nm, the principal subfractions (F2-1 to F2-5) were isolated and collected, and the NO radical scavenging activity of three aliquots was determined while the rest was lyophilized.
Identification of anti-inflammatory peptides
The principal subfractions (enriched peptides) obtained from the RP-HPLC fractionation that showed a marked NO radical scavenging activity and a sufficient yield (F2-1 to F2-3 and F2-5) were identified by amino acid sequencing using quadrupole time-of-flight (Q-TOF) liquid chromatography-tandem mass spectrometry (Q-TOF LC-MS/MS) coupled with electrospray ionization (ESI; model Amazon SL, Bruker, Bremen, Germany). The MS/MS data were searched against the Swiss-Prot database with the MASCOT package (www.matrixscience.com) (23).
Comparison between the NO radical scavenging activity of the pure synthetic peptide and enriched enzymatic subfraction
The peptide sequences obtained from the Q-TOF LC-MS/MS ESI analysis were synthesized using a 433A Synergy solid phase peptide synthesizer (model ABI 433A; Applied Biosystems, Foster City, CA, USA). The purity of the peptides was verified by analytical mass spectrometry (model Finnigan™ LXQ™; Thermo Fisher Scientific Inc., Waltham) coupled to a Surveyor HPLC. Ionization was performed in the positive mode. The separation was performed at a flow rate of 100 m/min under a linear gradient of 5–80% B for 50 min, where A was 0.1% (V/V) formic acid in water and B was 100% ACN. Mass spectral data from 300–1500 m/z were collected in positive ionization mode and HyStar v. 3.2 software (Bruker Daltonics Inc., Billerica, MA, USA) (24) was used to interface the HPLC and MS systems. All data obtained from LC-MS/MS were analysed using de novo sequencing. The NO radical scavenging activity of the pure synthesized peptides was determined in comparison with those obtained from the RP-HPLC fractionation.
Cell culture
The RAW 264.7 cell line was maintained in complete medium (CM: DMEM supplemented with 10%, V/V, FBS, 100 U/mL penicillin G, 0.4 mg/mL streptomycin sulfate, 1%, m/V, sodium pyruvate and 1%, m/V, HEPES) at 37 °C in a humidified atmosphere with 5% (V/V) CO2. For routine maintenance in the culture (passage) (22), the cells were seeded in non-tissue culture treated dishes at approx. 10% confluency and grown to approx. 80% confluency, which typically took 2 days. The used medium was aspirated, cells were gently rinsed with PBS, then dislodged by gently scraping with a rubber spatula and harvested by centrifugation (Hettich, Tuttlingen, Germany) at 15 000×g and 4 °C for 5 min.
Pretreatment of macrophage RAW 264.7 cells
The macrophage RAW 264.7 cells were seeded in 96-well plates at N=104 cells/well in 100 μL CM and incubated overnight at 37 °C in a humidified atmosphere with 5% CO2. The medium was replaced with CM alone (negative control) or with different concentrations of the chicken feather meal protein hydrolysate (or fractions thereafter), or with budesonide (2.5 μg/mL) as a positive control, and incubated for 1 h. NO production was stimulated by the addition of 100 ng/mL of LPS and incubated for 12 h.
Measurement of cell viability/proliferation by the MTT assay
The cytotoxicity activity of the F2 and the peptide from F2-1 (SNPSVAGVR) were determined according to Saisavoey et al. (22) with slight modifications. The macrophage RAW 264.7 cells were plated at N=104 cells/well in a 96-well plate and then incubated at 37 °C with 5% (V/V) CO2 for 24 h. Cells were then treated with different concentrations of the test sample (peptide) and LPS. Subsequently, 100 μL of a 5 mg/mL MTT solution (in PBS) were added to each well. Cells were incubated at 37 °C in a humidified atmosphere with 5% CO2 for 4 h before the medium was removed and DMSO added at 100 μL/well to solubilise the formed formazan crystals, and subsequently the A540 nm was measured (model Multiskan GO; Thermo Fisher Scientific Inc.).
Determination of NO production by macrophage RAW 264.7 cells
The level of NO production was determined by measuring nitrite production in the CM according to the Griess reaction. Macrophage RAW 264.7 cells were incubated with the test sample at various concentrations (control 0 μg/mL). Then 50 μL of sulfanilamide were added to 50 μL of the culture supernatant in a 96-well plate and incubated at room temperature for 10 min before 50 μL of the NED solution were added and incubated for 10 min. Finally, the A540 nm was measured using a microplate reader (model Multiskan GO; Thermo Fisher Scientific Inc.).
Detection of iNOS, TNF-α, COX-1, COX-2 and IL-6 mRNA by two-stage reverse transcriptase (RT)-PCR or quantification by two-stage quantitative real time (qrt)-RT-PCR analyses
Macrophage RAW 264.7 cells were pretreated for 1 h with a solvent (negative control), and synthesized peptide at various concentrations or 2.5 μg/mL of budesonide (positive control). The pretreated cells were then stimulated by the addition of 100 ng/mL of LPS and incubated for 12 h before their total RNA was harvested using the MasterPure™ Complete DNA and RNA Purification Kit (Epicentre; Lucigen, a part of LGC, Biosearch Technologies, Middleton, WI, USA) according to the manufacturer’s instruction. The RNA concentration was measured using a Nanodrop 2000 UV-Vis spectrophotometer (Thermo Fisher Scientific, Inc.) at 260 nm. Reverse transcription of the total RNA (1 μg) was performed with oligo-dT primers using a Precision nanoScript II Reverse Transcription Kit (PrimerDesign, Camberley, UK) according to the manufacturer’s protocol. The PCR was performed using selective primers for the β-actin (5’- ACCAACTGGGACGACATGGAGAA-3’ and 5’-GTGGTGGTGAAGCTGTAGCC-3’), iNOS (5’- CCATCATGGACCACCACACA-3’ and 5’-CCATGCAGACAACCTTGGTG-3’), IL-6 (5’- CATGTTCTCTGGGAAATCGTGG-3’ and 5’-AACGCACTAGGTTTGCCGAGTA-3’), TNF-α (5’-CCTGTAGCCCACGTCGTAGC-3’ and 5’-TGACCTCAGCGCTGAGTTG-3’), COX-1 (5’- AGTGCGGTCCAACCTTATCC-3’ and 5’- GGTAAAGCCAGGACCCATCTTTC-3’), and COX-2 (5’- GGAGAGACTATCAAGATAGT-3’ and 5’- ATGGTCAGTAGACTTTTACA-3’).
For the (qrt)-RT-PCR analysis, the PCR mixture comprised 1 μL cDNA, 1 μL of each primer (10 mM), 7 μL ultrapure water and 10 μL 2× qPCRBIO SyGreen Mix (PCR Biosystems Ltd, London, UK) to give a final volume of 20 μL. The (qrt)-RT-PCR reactions (performed separately per gene) were amplified using a MyGo Pro® Real time PCR apparatus (IT-IS International Ltd, Stokesley, UK) and thermal cycles at 95 °C for 2 min, followed by 40 cycles at 95 °C for 10 s, 68 °C (or 60 °C for TNF-α and IL-6) for 20 s and 72 °C for 30 s with melting at 55–95 °C for 1 min. The RT-PCR was performed using selective primers for the β-actin (5’-GATCAAGATCATTGCTCCTCCTG-3’ and 5’-CGCAGCTCAGTAACAGTCCG-3’), iNOS (5’-CGGCAAACATGACTTCAGGC-3’ and 5’-TAGGTCGATGCACAACTGGG-3’), IL-6 (5’-CTCTCTGCAAGAGACTTCCATCC-3’ and 5’-ACAGGTCTGTTGGGAGTGGTATC-3’), TNF-α (5’-GGGCAGGTCTACTTTGGAGTCA-3’ and 5’-ACAGACTGGGGGCTCTGAGG-3’), COX-1 (5’-AGCTGCTGCTGAGAAGGGAGTT-3’ and 5’-GGTAAAGCCAGGACCCATCTTTC-3’), and COX-2 (5’-CTGACCCCCAAGGCTCAAAT-3’ and 5’-AAGTCCACTCCATGGCCCAG -3’). β-Actin was used as the internal reference gene and the three q-PCR reactions were analysed by relative quantitation. The relative gene expression level was determined using the Ct (threshold cycle) value by calculating as follows:
| Relative gene expression=2–∆∆Ct /2/ |
where ∆∆Ct correlates with the increase in the threshold cycle of the gene (25).
Statistical analysis
Numerical data are shown as the mean value±standard deviation, derived from three independent repeats. The data were subjected to analysis of variance followed by Duncan’s multiple range post hoc tests, accepting significance at the p<0.05 level. The analysis was performed using the SPSS statistical software (26).
RESULTS AND DISCUSSION
Amino acid composition of chicken feather meal
The chicken feather meal contains essential and non-essential amino acids (Table 1). Glutamic acid, proline, serine, glycine and leucine are present in higher mass fractions than other amino acids. Active anti-inflammatory peptides from Mytilus coruscus were reported to consist of glycine, valine, serine, leucine, glutamine and phenylalanine (5, 27). Moreover, it was reported that peptides consisting of glycine, cysteine, alanine, valine and serine could inhibit NO production, while peptides comprising glycine, cysteine and histidine exhibited anti-inflammatory activity by inhibiting the NF-κB signalling pathway (28). Certain amino acids, such as glycine, histidine, cysteine, glutamine and tryptophan, have been reported to possess anti-inflammatory properties (29).
Table 1. Amino acid profile of chicken feather meal.
| Amino acid | w/% |
|---|---|
| Alanine (Ala) | 3.80 |
| Arginine (Arg) | 6.30 |
| Glycine (Gly) | 6.82 |
| Aspatic acid (Asp) | 5.70 |
| Cysteine (Cys) | 2.90 |
| Glutamic acid (Glu) | 10.6 |
| Leucine (Leu) | 6.46 |
| Isoleucine (Ile) | 3.94 |
| Histidine (His) | 0.59 |
| Threonine (Thr) | 3.96 |
| Proline (Pro) | 8.37 |
| Lysine (Lys) | 1.45 |
| Methionine (Met) | 0.67 |
| Serine (Ser) | 7.84 |
| Phenylalanine (Phe) | 4.03 |
| Tyrosine (Tyr) | 1.10 |
| Tryptophan (Trp) | 0.20 |
| Valine (Val) | 5.85 |
| Total | 80.58 |
In vitro NO radical scavenging assay
The anti-inflammatory activity of the crude protein hydrolysates of chicken feather meal from the three protease digests was tested using a NO radical scavenging assay. NO radical scavenging activity of hydrolysates obtained with Alcalase and Neutrase increased with the increase of enzyme concentration up to 25 mg/mL, and then at 50 mg/mL the activity decreased. However, a dramatically increased NO radical scavenging activity was observed after treatment with 10 mg/mL Flavourzyme (Table 2). Overall, the hydrolysate obtained with 10 mg/mL Flavourzyme exhibited the highest NO radical scavenging activity, with an IC50=(5.5±1.0) μg/mL. Several studies have suggested that the antioxidant activity of peptides in hydrolysates varies depending on their amino acid composition, sequence and length (30). This suggests that enzymatic hydrolysis with different proteolytic enzymes and conditions would lead to the formation of different peptide sequences. In the same way, the anti-inflammatory activity of the Flavourzyme hydrolysate from M. coruscus showed a strong inhibition of NO production in macrophage RAW 264.7 cells (27). Since the hydrolysate obtained from chicken feather meal with 10 mg/mL Flavourzyme had the highest NO radical scavenging activity, it was selected for further study.
Table 2. Effect of enzyme concentration on NO radical scavenging activity of chicken feather meal hydrolysates expressed as concentration of hydrolysate that inhibited 50% of NO production (IC50).
| Enzyme | γ(enzyme)/(µg/mL) | |||
|---|---|---|---|---|
| 0 | 10 | 25 | 50 | |
| IC50/(µg/mL) | ||||
| Alcalase | (134.0±4.7)e | (80.6±5.4)d | (30.0±1.8)a | (40.2±0.8)b |
| Flavourzyme | (158.4±1.5)E | (5.5±1.0)A | (82.1±7.0)D | (68.7±3.6)C |
| Neutrase | (175.7±4.0)ee | (133.0±5.4)dd | (37.0±0.4)aa | (78.7±3.9)cc |
Data are shown as mean value±standard deviation of triplicates. Different superscripts indicate significant difference (p<0.05). Curcumin was used as positive control (IC50=(60.5±3.2) µg/mL)
Enrichment of NO inhibitory peptides from the chicken feather meal hydrolysate obtained with 10 µg/mL Flavourzyme
The 50% inhibitory concentrations of the obtained >10, 5–10, 3–5, 0.65–3 and <0.65 kDa fractions were (21.9±0.7), (10.4±0.7), (7.2±0.5), (5.8±0.6) and (3.6±0.3) μg/mL respectively (Table S1). Thus, it can be concluded that the inhibitory activity increased with the decrease of peptide fraction size. The molecular mass is an important parameter that correlates with the bioactivity of protein hydrolysates. Previous research has shown that the effective hydrolysates depend on their molecular mass distribution, e.g. Li et al. (31) reported that the peptide fraction with a molecular mass of 200–3000 Da was probably associated with a higher antioxidant activity, Nalinanon et al. (32) reported that low molecular mass peptides contributed to the antioxidant activity, and Wang et al. (33) reported that the biological activity of hydrolysates depended on the molecular mass of each peptide.
Arrays of NO inhibitory peptides with low molecular mass fractionated by ultrafiltration were previously shown to be effective in interacting with internal barriers to promote biological activities including antioxidant and anti-inflammatory activities (34). In addition, Lee et al. (35) also identified low molecular mass (<1.3 kDa) anti-inflammatory egg white peptides that were able to attenuate the symptoms of inflammatory bowel disease. Anti-inflammatory Leu-Asp-Ala-Val-Asn-Arg (683 Da) and Met-Met-Leu-Asp-Phe (655 Da) from Spirulina maxima (11), Gln-Cys-Gln-Gln-Ala-Val-Gln-Ser-Ala-Val (1061 Da) from Ruditapes philippinarum (5) and Gln-Cys-Gln-Cys-Ala-Val-Glu-Gly-Gly-Leu (1007 Da) from Crassostrea gigas were also isolated from marine organisms (36), while the tripeptide Val-Pro-Tyr (377 Da) from soybean exhibited anti-inflammatory effects (37). Therefore, the <0.65 kDa fraction from chicken feather meal was selected for further study.
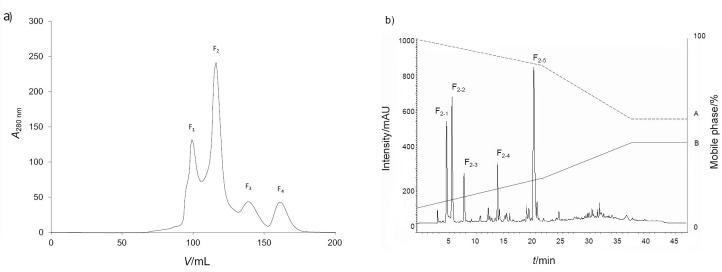
Since peptide length is closely related to biological activities (antioxidant, antihypertensive, antimicrobial and immunomodulatory), gel filtration chromatography is suitable for fractionation, enrichment and concentration of proteins and peptides (Fig. 1). It has previously been reported to be useful for improving the anticancer activities of protein hydrolysates (38). Thus, further separation of lyophilized active <0.65 kDa fraction yielded four (F1–4) protein fractions based upon segregation of the A280 nm profile (Fig. 1a). Measurement of NO inhibitory activity of each of the four fractions revealed that F1 and F2 had NO inhibitory activity with IC50 values of (73.5±2.8) and (34.6±3.4) μg/mL (Table S2), respectively, whereas the yields of fractions F3 and F4 were too low to determine their IC50 values.
Fig. 1.
Enrichment of bioactive peptides from the <0.65 kDa fraction of chicken feather meal hydrolysate obtained with 10 mg/mL Flavourzyme: a) the absorbance values of four fractions obtained after gel filtration chromatography of bioactive peptide, and b) RP-HPLC chromatograph of the F2 fractionation into five principal subfractions (F2-1 to F2-5)
Given the higher inhibitory activity (lower IC50 value) of fraction F2, it was selected for further fractionation. The F2 fraction was divided into five principal subfractions (F2-1, F2-2, F2-3, F2-4 and F2-5) (Fig. 1b) all with NO radical scavenging activity. The yield of subfraction F2-4 was too low to determine its IC50 value, while the IC50 values of the remaining four fractions were: (16.8±1.5), (15.3±0.7), (17.4±0.4) and (34.0±1.9) μg/mL (Table S3), respectively, and they were subjected to amino acid sequencing by mass spectrometry.
Identification of peptides by Q-TOF mass spectrometry and comparison of the NO radical scavenging activity between synthetic and enzymatic peptides
The amino acid sequences and molecular masses of F2-1, F2-2, F2-3 and F2-5 are shown in Table 3. The de novo sequencing yielded a homology identification of 100% based on the Phasianidae family. Each sequence contained 9–13 amino acid residues. Fraction F2-1 was identified as Ser-Asn-Pro-Ser-Val-Ala-Gly-Val-Arg (SNPSVAGVR; 886 Da), F2-2 as Ser-Leu-Phe-Leu-His-Thr-His-Ser-Ile-Val-Ala-Asp-Lys (SLFLHTHSIVADK; 1468 Da), F2-3 as Ala-Val-Leu-Lys-Lys-Lys-Val-Thr-Ser-Thr-Phe-Gly-Arg (AVLKKKVTSTFGR; 1435 Da) and F2-5 as Leu-Ser-Pro-Trp-Pro-Val-Lys-Gly-Val (LSPWPVKGV; 982 Da).
Table 3. Amino acid sequences of RP-HPLC subfractions obtained from the chicken feather meal hydrolysate, identified by Q-TOF LC/MS/MS.
| Fraction | Amino acid sequence | M/Da | Protein name | Organism | Accession number |
|---|---|---|---|---|---|
| F2-1 | SNPSVAGVR | 886 | Putative E3 ubiquitin-protein ligase SH3RF2 | Gallus gallus | XP_015157384.1 |
| Putative E3 ubiquitin-protein ligase SH3RF2 ioform X2 | Gallus gallus | XP_015157384.1 | |||
| Putative E3 ubiquitin-protein ligase SH3RF2 isoform X1 | Gallus gallus | XP_414662.3 | |||
| PRELI domain containing protein 3B | Gallus gallus | NP_001026037.1 | |||
| Hypothetical protein RCJMB04_17b4, partial | Gallus gallus | CAG32065.1 | |||
| F2-2 | SLFLHTHSIVADK | 1468 | Lactate dehydrogenase | Phodilus badius | GI629677270 |
| F2-3 | AVLKKKVTSTFGR | 1435 | Cystine/glutamate transporter | Gallus gallus | XP_426289.3 |
| Dynein heavy chain 1, axonemal | Gallus gallus | XP_015148334.1 | |||
| Unconventional myosin-XVI isoform X2 | Gallus gallus | XP_004938593.1 | |||
| Unconventional myosin-XVI isoform X1 | Gallus gallus | XP_416950.3 | |||
| Transcription initiation factor TFIID subunit 9B | Gallus gallus | NP_001264725.1 | |||
| F2-5 | LSPWPVKGV | 982 | Zinc finger matrin-type protein 1-like isoform X1 | Gallus gallus | XP_004936323.1 |
| Neogenin isoform X6 | Gallus gallus | XP_015134538.1 | |||
| Neogenin isoform X5 | Gallus gallus | XP_015134537.1 | |||
| Neogenin isoform X4 | Gallus gallus | XP_015134536.1 | |||
| Neogenin isoform X3 | Gallus gallus | XP_015134535.1 |
Since F2-1, F2-2, F2-3 and F2-5 exhibited NO radical scavenging activity, these obtained peptide sequences were synthesized and evaluated for their NO radical scavenging activity. However, the SNPSVAGVR peptide showed a high NO radical scavenging activity with an IC50 value of (55.2±0.2) mM (Table S4). Regarding the relationship between the properties and anti-inflammatory activity of the amino acids, all of the peptide sequences contained mainly hydrophobic (leucine, histidine, phenylalanine, proline, tryptophan and valine) and positively charged (arginine and lysine) amino acids. These have been reported previously to have a high antioxidant activity (39). Hasegawa et al. (28) reported the anti-inflammatory activity of glycine and histidine, suggesting that glycine, histidine and cysteine act as anti-inflammatory agents by reducing NF-κB activation and inhibiting the expression of IL-6 in human coronary arterial endothelial cells. The relationships between the structure and the activity of the peptides and their mechanism of anti-inflammatory activities are not yet fully understood. However, the inhibition of NO production (%) by these peptides might be related to their amino acid composition and sequences since hydrophobic amino acid sequences in peptides have been suggested to play important roles in anti-inflammatory activities (40).
Anti-inflammatory effect of the F2 fraction in LPS-induced macrophage RAW 264.7 cells
The F2-1 fraction showed no cytotoxicity to LPS-induced RAW 264.7 cells up to the highest tested concentration (120 µg/mL), where a cell viability of (92.6±0.6) % was still observed (Fig. 2a). Thus, the F2-1 fraction can be used at concentrations up to 120 μg/mL. Likewise, the bioactive peptides of Lupinus angustifolius were previously shown not to be cytotoxic against RAW 264.7 cells (41). Therefore, the nontoxic F2-1 peptide was evaluated further by testing its NO inhibitory activity.
Fig. 2.
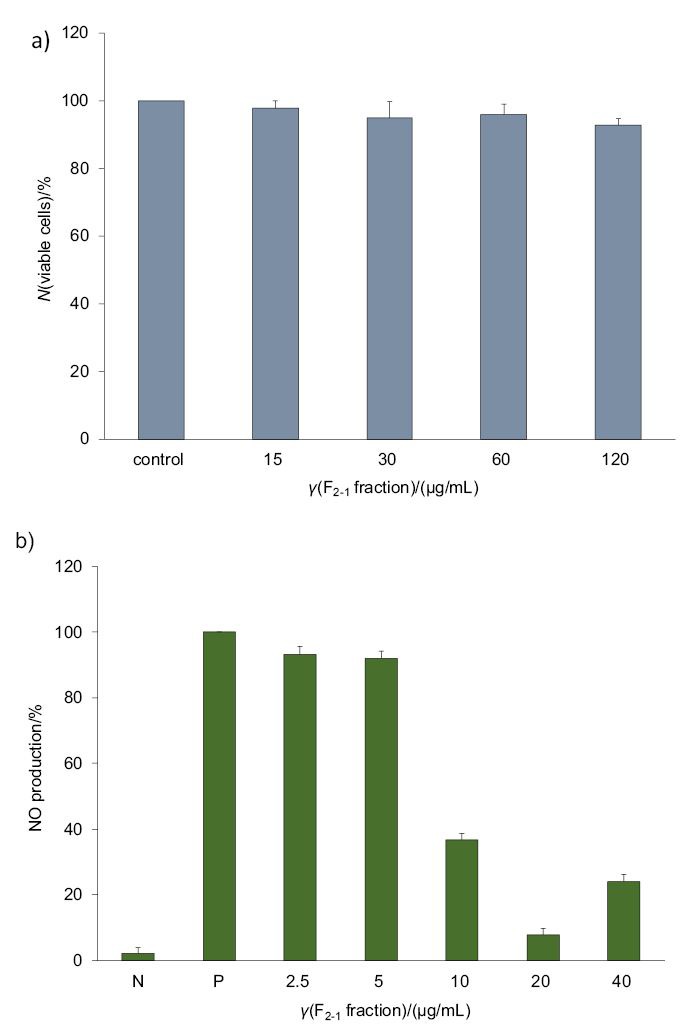
Effect of different concentrations of F2-1 fraction on: a) viability of and b) NO production by lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophage cells, where N and P represent the negative (cells alone) and positive (cells+LPS) controls respectively
In this study, an inflammation-type response was created in RAW 264.7 cells by treating them with 100 ng/mL LPS. The NO released from the LPS-stimulated RAW 264.7 cells was found to decrease with increasing concentrations of F2-1 (2.5-40 μg/mL), where LPS treatment alone was considered as the control (P in Fig. 2b). The enriched F2-1 peptide significantly inhibited NO production: at the concentration of 5 μg/mL by <15% and at 20 μg/mL by >85% (Fig. 2b). Peptides derived from marine sources were previously shown to possess NO inhibitory activities, such as the lupine protein hydrolysate from L. angustifolius in LPS-stimulated THP-1 cells (41) and R. philippinarum (5) and M. coruscus (27) in LPS-stimulated RAW264.7 cells. Both NO and proinflammatory cytokines play a critical role in the physiology and pathology of diverse tissues, including the immune system of the body.
Fig. 3 showed that in unstimulated macrophage RAW 264.7 cells the transcript levels of iNOS, COX-2, TNF-α and IL-6 were undetectable, but they were all expressed after treatment with LPS, and the F2-1 fraction significantly inhibited these expression levels without affecting the constitutive expression of COX-1 and β-actin transcripts. Cotreatment of the RAW 264.7 cells with LPS and F2-1 fraction still revealed the upregulation of COX-2 and TNF-α, whereas the expression of IL-6 and iNOS transcripts was downregulated. On the other hand, cotreatment with F2-1 fraction negated the LPS-induced expression of COX-2 and TNF-α as well as IL-6 and iNOS. It is known that iNOS responds to various proinflammatory cytokines, including INF-γ, TNF-α and IL-6, and mediates several inflammatory responses (42). Moreover, it has been reported that the inducible isoforms of NOS and COX-2 are involved in the production of large amounts of NO and PGE2, respectively (43). The activation of NF-κB is responsible for the induced expression of iNOS and COX-2 (44).
Fig. 3.
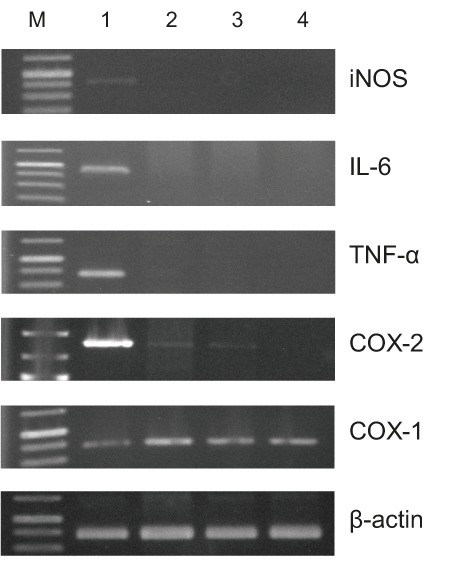
Effect of F2-1 fraction on transcript expression levels of iNOS, IL-6, TNF-α, COX-1, COX-2 and β-actin in lipopolysaccharide (LPS)-induced RAW 264.7 macrophage cells. Lane 1=LPS, lane 2=LPS+γ(F2-1 fraction)=40 µg/mL, lane 3=LPS+budesonide; 2.5 μg/mL (positive control), lane 4=no addition of LPS or F2-1, and lane M=1000-bp size markers
Oxidative stress has been reported to promote inflammation-related diseases through activation of the NF-κB pathway (45). Therefore, an antioxidative effect is a possible mechanism of limiting the damage induced by inflammatory reactions, although a detailed mechanism remains unclear. The NO radical scavenging assays in both in vitro and cellular models indicated that all the chicken feather meal hydrolysate-based fractions, especially fraction F2–1, can potentially impair oxidative stress. The effects of peptide length or peptide mixture were not observed in the cellular anti-inflammatory assays, and this could be due to complex interactions of the constituents in each hydrolysate with the cell matrices, and the likelihood of further proteolytic processing of the peptides within the cell cultures.
Several studies have reported anti-inflammatory activities of low-molecular-mass peptides derived from food proteins, such as soybean (46), milk (47), soybean flour (48), egg (49) and whey (50). However, a high-molecular-mass peptide lunasin derived from soybean was reported to down-regulate the production of IL-6, IL-1β, NF-κB, iNOS and NO, indicating an anti-inflammatory function (46). Moreover, other factors, such as the peptide sequence and amino acid composition (not evaluated for all the hydrolysates in this study), can also determine the bioactivity of protein hydrolysates. This activity could be due to electron donation by the sulfhydryl group of cysteine leading to dimerization, although this mechanism would be more relevant in free radical quenching than ROS scavenging. Therefore, the structure-function relationships of antioxidative and anti-inflammatory peptides and protein hydrolysates appear complex, especially within physiological matrices.
Anti-inflammatory activity of the synthesized SNPSVAGVR peptide
The SNPSVAGVR peptide displayed no cytotoxic effect against the RAW 264.7 cells at various concentrations (15–120 mM), as shown in Fig. 4a. Stimulation of RAW264.7 cells with LPS (100 ng/mL) resulted in a significant increase in NO production compared to the unstimulated group, and SNPSVAGVR inhibited this NO production in a dose-dependent manner (Fig. 4b). The potential mechanism underlying the anti-inflammatory activity of SNPSVAGVR in LPS-induced RAW 264.7 macrophages was evaluated in terms of the transcript expression level of proinflammatory mediators. As shown in Fig. 5, LPS treatment markedly stimulated the expression of iNOS, IL-6, TNF-α and COX-2 (but not COX-1) in RAW 264.7 cells. When added to the cells, 60 mM of SNPSVAGVR blocked the stimulatory effects of LPS on these genes (at p<0.05 and p<0.001). It is known that IL-6 and COX-2 have important effects on inflammation, and so it is relevant that SNPSVAGVR at 60 mM clearly decreased the IL-6 and COX-2 transcript expression levels by 96 and 83% respectively. All of these results support the conclusion that the synthetic peptide had in vitro anti-inflammatory effect.
Fig. 4.
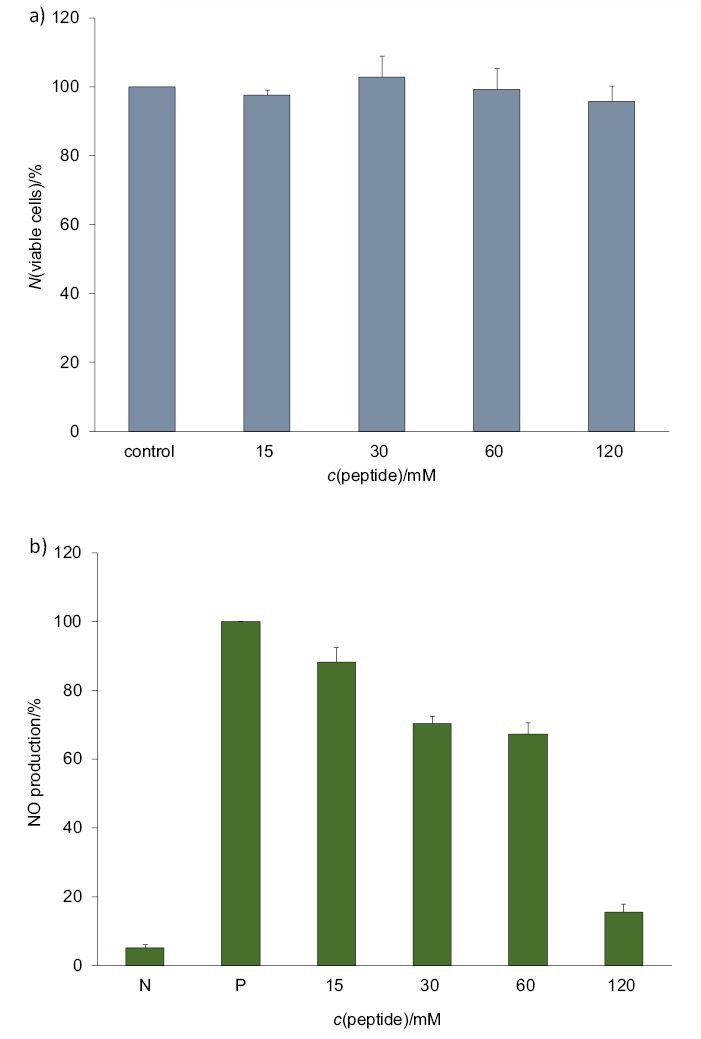
Effect of different concentrations of synthesized SNPSVAGVR peptide on: a) viability of and b) NO production by lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophage cells, where N and P represent the negative (cells alone) and positive (cells+LPS) controls respectively
Fig. 5.
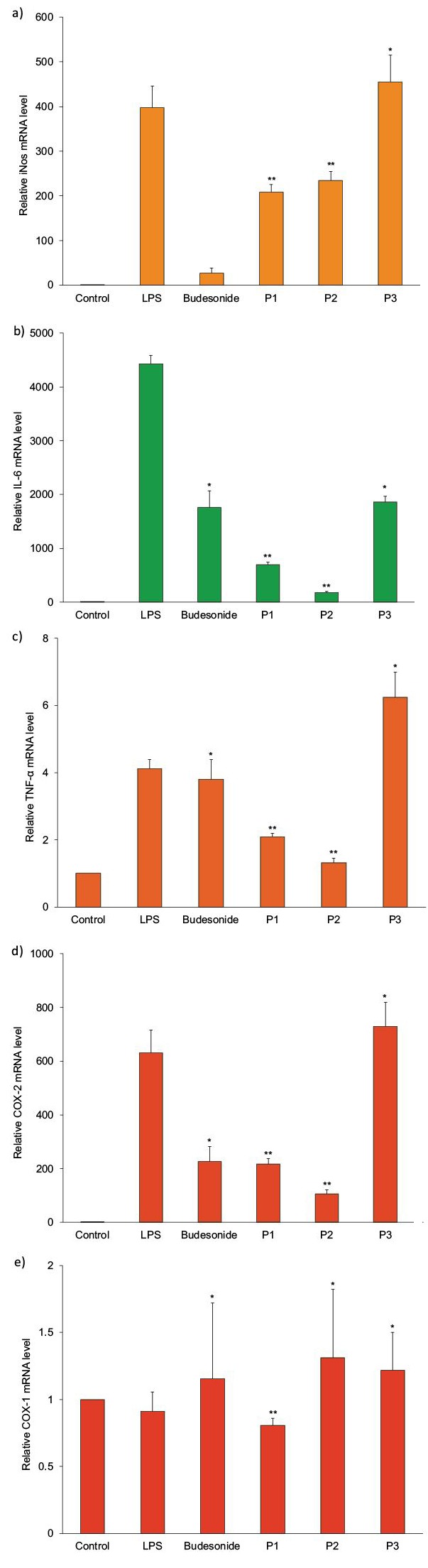
Effects of synthesized SNPSVAGVR peptide on transcript expression levels of: a) inducible nitric oxide synthase (iNOS), b) interleukin 6 (IL-6), c) tumour necrosis factor (TNF-α), d) cyclooxygenase-2 (COX-2) and e) cyclooxygenase-1 (COX-1) in lipopolysaccharide (LPS)-stimulated RAW264.7 cells measured by qrtRT-PCR. Cells were incubated for 12 h with γ(LPS)=100 ng/mL in the presence of c(peptide)=30 (P1), 60 (P2) and 120 (P3) mM, or with γ(budesonide)=2.5 µg/mL as positive control. Statistical significance is expressed as *p<0.05 and **p<0.001 compared to LPS
Conclusions
Our results show that chicken feather meal peptides obtained by microbial enzyme hydrolysis exhibit anti-inflammatory activity, as evaluated by the NO radical scavenging assay. The chicken feather meal hydrolysate obtained with 10 mg/mL Flavourzyme had the highest NO radical scavenging activity. Peptides with the lowest molecular mass (<0.65 kDa) have the highest NO radical scavenging activity. Moreover, the F2-1 fraction and pure SNPSVAGVR peptide are potent inhibitors of the LPS-induced expression of pro-inflammatory cytokines, including iNOS, TNF-α, COX-2 and IL-6. These results suggest that chicken feather meal hydrolysate can potentially be used as a natural anti-inflammatory agent in functional foods or pharmaceutical products.
SUPPLEMENTARY MATERIAL
All supplementary material is available at www.ftb.com.hr.
AcknowledgEments
The authors would like to thank the Institute of Biotechnology and Genetic Engineering, Chulalongkorn University, for their support and providing access to their facilities. The authors thank the Edanz Group (www.edanzediting.com/ac) for editing the draft of this manuscript.
Footnotes
FUNDING: We acknowledge the financial support from the Research and Researcher for Industry: MAG under grant number MSD57I0073, the Grant for Research: Government Budget, Chulalongkorn University under grant number GRB_BSS_ 99_59_61_06, and The Center of Excellence on Medical Biotechnology (CEMB), S&T Postgraduate Education and Research Development Office (PERDO), Office of Higher Education Commission (OHEC), Thailand (SN-60-003-909) for providing the financial support for this research.
CONFLICT OF INTEREST: The authors report no conflict of interest. The authors alone are responsible for the content and writing of this article.
REFERENCES
- 1.Zhou HY, Shin EM, Guo LY, Youn UJ, Bae K, Kang SS, et al. Anti-inflammatory activity of 4-methyxyhonokiol is a function of the inhibition of iNOS and COX-2 expression in RAW 264.7 macrophages via NF-κB, JNK and p38 MAPK inactivation. Eur J Pharmacol. 2008;586(1-3):340–9. 10.1016/j.ejphar.2008.02.044 [DOI] [PubMed] [Google Scholar]
- 2.Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. 10.1155/2014/561459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13(2):85–94. 10.1016/S0898-6568(00)00149-2 [DOI] [PubMed] [Google Scholar]
- 4.Dia VP, Wang W, Oh VL, de Lumen BO, de Mejia EG. Isolation, purification and characterisation of lunasin from defatted soybean flour and in vitro evaluation of its anti-inflammatory activity. Food Chem. 2009;114(1):108–15. 10.1016/j.foodchem.2008.09.023 [DOI] [Google Scholar]
- 5.Lee SJ, Kim EK, Kim YS, Hwang JW, Lee KH, Choi DK, et al. Purification and characterization of a nitric oxide inhibitory peptide from Ruditapes philippinarum. Food Chem Toxicol. 2012;50(5):1660–6. 10.1016/j.fct.2012.02.021 [DOI] [PubMed] [Google Scholar]
- 6.Lee SJ, Cheong SH, Kim YS, Hwang JW, Kwon HJ, Kang SH, et al. Antioxidant activity of a novel synthetic hexa-peptide derived from an enzymatic hydrolysate of duck skin by-products. Food Chem Toxicol. 2013;62:276–80. 10.1016/j.fct.2013.08.054 [DOI] [PubMed] [Google Scholar]
- 7.Chakrabarti S, Jahandideh F, Wu J. Food-derived bioactive peptides on inflammation and oxidative stress. BioMed Res Int. 2014;2014:608979. 10.1155/2014/608979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cian RE, López-Posadas R, Drago SR, de Medina FS, Martínez-Augustin O. A Porphyra columbina hydrolysate upregulates IL-10 production in rat macrophages and lymphocytes through an NF-κB, and p38 and JNK dependent mechanism. Food Chem. 2012;134(4):1982–90. 10.1016/j.foodchem.2012.03.134 [DOI] [PubMed] [Google Scholar]
- 9.Fosgerau K, Hoffmann T. Peptide therapeutics: Current status and future directions. Drug Discov Today. 2015;20(1):122–8. 10.1016/j.drudis.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 10.Hartmann R, Meisel H. Food-derived peptides with biological activity: From research to food applications. Curr Opin Biotechnol. 2007;18(2):163–9. 10.1016/j.copbio.2007.01.013 [DOI] [PubMed] [Google Scholar]
- 11.Vo TS, Ryu B, Kim SK. Purification of novel anti-inflammatory peptides from enzymatic hydrolysate of the edible microalgal Spirulina maxima. J Funct Foods. 2013;5(3):1336–46. 10.1016/j.jff.2013.05.001 [DOI] [Google Scholar]
- 12.Chi CF, Hu FY, Wang B, Li T, Ding GF. Antioxidant and anticancer peptides from the protein hydrolysate of blood clam (Tegillarca granosa) muscle. J Funct Foods. 2015;15:301–13. 10.1016/j.jff.2015.03.045 [DOI] [Google Scholar]
- 13.Mine Y, Ma F, Lauriau S. Antimicrobial peptides released by enzymatic hydrolysis of hen egg white lysozyme. J Agric Food Chem. 2004;52(5):1088–94. 10.1021/jf0345752 [DOI] [PubMed] [Google Scholar]
- 14.Nagaoka S, Shimizu K, Kaneko H, Shibayama F, Morikawa K, Kanamaru Y, et al. A novel protein C-phycocyanin plays a crucial role in the hypocholesterolemic action of Spirulina platensis concentrate in rats. J Nutr. 2005;135(10):2425–30. 10.1093/jn/135.10.2425 [DOI] [PubMed] [Google Scholar]
- 15.Hong F, Ming L, Yi S, Zhanxia L, Yongquan W, Chi L. The antihypertensive effect of peptides: A novel alternative to drugs? Peptides. 2008;29(6):1062–71. 10.1016/j.peptides.2008.02.005 [DOI] [PubMed] [Google Scholar]
- 16.Mukhopadhya A, Noronha N, Bahar B, Ryan MT, Murray BA, Kelly PM, et al. Anti-inflammatory effects of a casein hydrolysate and its peptide-enriched fractions on TNFα-challenged Caco-2 cells and LPS-challenged porcine colonic explants. Food Sci Nutr. 2014;2(6):712–23. 10.1002/fsn3.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tiwary E, Gupta R. Rapid conversion of chicken feather to feather meal using dimeric keratinase from Bacillus licheniformis ER-15. J Bioprocess Biotech. 2012;2(4):1000123 10.4172/2155-9821.1000123 [DOI] [Google Scholar]
- 18.Official Method AOAC. 994.12. Amino acids in feeds. Rockville, MD, USA: AOAC International; 2011. [Google Scholar]
- 19.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248–54. 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- 20.Chantaranothai C, Palaga T, Karnchanatat A, Sangvanich P. Inhibition of nitric oxide production in the macrophage-like RAW 264.7 cell line by protein from the rhizomes of Zingiberaceae plants. Prep Biochem Biotechnol. 2013;43(1):60–78. 10.1080/10826068.2012.697958 [DOI] [PubMed] [Google Scholar]
- 21.Ren J, Zhao M, Shi J, Wang J, Jiang Y, Cui C, et al. Purification and identification of antioxidant peptides from grass carp muscle hydrolysates by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chem. 2008;108(2):727–36. 10.1016/j.foodchem.2007.11.010 [DOI] [PubMed] [Google Scholar]
- 22.Saisavoey T, Sangtanoo P, Reamtong O, Karnchanatat A. Antioxidant and anti-inflammatory effects of defatted rice bran (Oryza sativa L.) protein hydrolysates on RAW 264.7 macrophage cells. J Food Biochem. 2016;40(6):731–40. 10.1111/jfbc.12266 [DOI] [Google Scholar]
- 23.Cooper B. Doubling down on phosphorylation as a variable peptide modification. Proteomics. 2016;16(18):2444–7. 10.1002/pmic.201500440 [DOI] [PubMed] [Google Scholar]
- 24.Drummond e Silva FG, Hernández-Ledesma B, Amigo L, Netto FM, Miralles B. Identification of peptides released from flaxseed (Linum usitatissimum) protein by Alcalase® hydrolysis: Antioxidant activity. LWT - Food Sci Technol. 2017;76(Part A):140–6. https://doi.org/ 10.1016/j.lwt.2016.10.049 [DOI]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–8. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 26.Wu Y, Shu J, He C, Li M, Wang Y, Ou W, et al. ROCK inhibitor Y27632 promotes proliferation and diminishes apoptosis of marmoset induced pluripotent stem cells by suppressing expression and activity of caspase 3. Theriogenology. 2016;85(2):302–14. 10.1016/j.theriogenology.2015.09.020 [DOI] [PubMed] [Google Scholar]
- 27.Kim EK, Kim YS, Hwang JW, Kang SH, Choi DK, Lee KH, et al. Purification of a novel nitric oxide inhibitory peptide derived from enzymatic hydrolysates of Mytilus coruscus. Fish Shellfish Immunol. 2013;34(6):1416–20. 10.1016/j.fsi.2013.02.023 [DOI] [PubMed] [Google Scholar]
- 28.Hasegawa S, Ichiyama T, Sonaka I, Ohsaki A, Okada S, Wakiguchi H, et al. Cysteine, histidine and glycine exhibit anti-inflammatory effects in human coronary arterial endothelial cells. Clin Exp Immunol. 2012;167(2):269–74. 10.1111/j.1365-2249.2011.04519.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsune I, Ikejima K, Hirose M, Yoshikawa M, Enomoto N, Takei Y, et al. Dietary glycine prevents chemical-induced experimental colitis in the rat. Gastroenterology. 2003;125(3):775–85. 10.1016/S0016-5085(03)01067-9 [DOI] [PubMed] [Google Scholar]
- 30.Kim SK, Kim YT, Byun HG, Nam KS, Joo DS, Shahidi F. Isolation and characterization of antioxidative peptides from gelatin hydrolysate of Alaska pollack skin. J Agric Food Chem. 2001;49(4):1984–9. 10.1021/jf000494j [DOI] [PubMed] [Google Scholar]
- 31.Li XX, Han LJ, Chen LJ. In vitro antioxidant activity of protein hydrolysates prepared from corn gluten meal. J Sci Food Agric. 2008;88(9):1660–6. 10.1002/jsfa.3264 [DOI] [Google Scholar]
- 32.Nalinanon S, Benjakul S, Visessanguan W, Kishimura H. Improvement of gelatin extraction from bigeye snapper skin using pepsin-aided process in combination with protease inhibitor. Food Hydrocoll. 2008;2(4)2:615–22. https://doi.org/ 10.1016/j.foodhyd.2007.01.012 [DOI]
- 33.Wang AY, Zhou MY, Lin WC. Antioxidative and anti-inflammatory properties of Citrus sulcata extracts. Food Chem. 2011;124(3):958–63. 10.1016/j.foodchem.2010.07.035 [DOI] [Google Scholar]
- 34.Ahn CB, Je JY, Cho YS. 2012. Antioxidant and anti-inflammatory peptide fraction from salmon byproduct protein hydrolysates by peptic hydrolysis. Food Res Int. 2011;49(1):92–8. 10.1016/j.foodres.2012.08.002 [DOI] [Google Scholar]
- 35.Lee M, Kovacs-Nolan J, Archbold T, Fan MZ, Juneja LR, Okubo T, et al. Therapeutic potential of hen egg white peptides for the treatment of intestinal inflammation. J Funct Foods. 2009;1(2):161–9. 10.1016/j.jff.2009.01.005 [DOI] [Google Scholar]
- 36.Hwang JW, Lee SJ, Kim YS, Kim EK, Ahn CB, Jeon YJ, et al. Purification and characterization of a novel peptide with inhibitory effects on colitis induced mice by dextran sulfate sodium from enzymatic hydrolysates of Crassostrea gigas. Fish Shellfish Immunol. 2012;33(4):993–9. 10.1016/j.fsi.2012.08.017 [DOI] [PubMed] [Google Scholar]
- 37.Kovacs-Nolan J, Zhang H, Ibuki M, Nakamori T, Yoshiura K, Turner PV, et al. The PepT1-transportable soy tripeptide VPY reduces intestinal inflammation. Biochim Biophys Acta. 2012;1820(11):1753–63. 10.1016/j.bbagen.2012.07.007 [DOI] [PubMed] [Google Scholar]
- 38.Hsu KC, Li-Chan ECY, Jao CL. Antiproliferative activity of peptides prepared from enzymatic hydrolysates of tuna dark muscle on human breast cancer cell line MCF-7. Food Chem. 2011;126(2):617–22. 10.1016/j.foodchem.2010.11.066 [DOI] [Google Scholar]
- 39.Bougatef A, Nedjar-Arroume N, Manni L, Ravallec R, Barkia A, Guillochon D, et al. Purification and identification of novel antioxidant peptides from enzymatic hydrolysates of sardinelle (Sardinella aurita) by-products proteins. Food Chem. 2010;118(3):559–65. 10.1016/j.foodchem.2009.05.021 [DOI] [Google Scholar]
- 40.Jang A, Jo C, Kang KS, Lee M. Antimicrobial and human cancer cell cytotoxic effect of synthetic angiotensin-converting enzyme (ACE) inhibitory peptides. Food Chem. 2008;107(1):327–36. 10.1016/j.foodchem.2007.08.036 [DOI] [Google Scholar]
- 41.Del Carmen Millán-Linares M, Bermúdez B, del Mar Yust M, Millán F, Pedroche J. Anti-inflammatory activity of lupine (Lupinus angustifolius L.) protein hydrolysates in THP- -1-derived macrophages. J Funct Foods. 2014;8:224–33. 10.1016/j.jff.2014.03.020 [DOI] [Google Scholar]
- 42.Wang SY, Lan XY, Xiao JH, Yang JC, Kao YT, Chang ST. Antiinflammatory activity of Lindera erythrocarpa fruits. Phytother Res. 2008;22(2):213–6. 10.1002/ptr.2289 [DOI] [PubMed] [Google Scholar]
- 43.Brown CM, Dela Cruz CD, Yang E, Wise PM. Inducible nitric oxide synthase and estradiol exhibit complementary neuroprotective roles after ischemic brain injury. Exp Neurol. 2008;210(2):782–7. 10.1016/j.expneurol.2007.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park EM, Cho S, Frys KA, Glickstein SB, Zhou P, Anrather J, et al. Inducible nitric oxide synthase contributes to gender differences in ischemic brain injury. J Cereb Blood Flow Metab. 2006;26(3):392–401. 10.1038/sj.jcbfm.9600194 [DOI] [PubMed] [Google Scholar]
- 45.Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J. 2006;28:219–42. 10.1183/09031936.06.00053805 [DOI] [PubMed] [Google Scholar]
- 46.Hwang JS, Yoo HJ, Song HJ, Kim KK, Chun YJ, Matsui T, et al. Inflammation-related signaling pathways implicating TGFβ are revealed in the expression profiling of MCF7 cell treated with fermented soybean, chungkookjang. Nutr Cancer. 2011;63(4):645–52. 10.1080/01635581.2011.551987 [DOI] [PubMed] [Google Scholar]
- 47.Nielsen DSG, Theil PK, Larsen LB, Purup S. Effect of milk hydrolysates on inflammation markers and drug-induced transcriptional alterations in cell-based models. J Anim Sci. 2012;90 Suppl 4:403–5. 10.2527/jas.53953 [DOI] [PubMed] [Google Scholar]
- 48.Vernaza MG, Dia VP, de Mejia Gonzalez E, Chang YK. Antioxidant and antiinflammatory properties of germinated and hydrolysed Brazilian soybean flours. Food Chem. 2012;134(4):2217–25. 10.1016/j.foodchem.2012.04.037 [DOI] [PubMed] [Google Scholar]
- 49.Majumder K, Chakrabarti S, Davidge ST, Wu J. Structure and activity study of egg protein ovotransferrin derived peptides (IRW and IQW) on endothelial inflammatory response and oxidative stress. J Agric Food Chem. 2013;61(9):2120–9. 10.1021/jf3046076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iskandar MM, Dauletbaev N, Kubow S, Mawji N, Lands LC. Whey protein hydrolysates decrease IL-8 secretion in lipopolysaccharide (LPS)-stimulated respiratory epithelial cells by affecting LPS binding to Toll-like receptor 4. Br J Nutr. 2013;110(1):58–68. 10.1017/S0007114512004655 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All supplementary material is available at www.ftb.com.hr.