SUMMARY
Bacteriocins are a large group of antimicrobial compounds that are synthesized by representatives of the genus Bacillus and lactic acid bacteria. They are used extensively in the food industry as biopreservatives. Incorporated in the composition of edible coatings, bacteriocins can reduce microbial growth and decay incidence in perishable fruits, thus improving product shelf-life and commercial appearance. The present study aims to investigate the effect of edible coatings of 0.5% carboxymethyl cellulose (CMC) enriched with a purified bacteriocin from Bacillus methylotrophicus BM47 on the shelf-life extension of fresh strawberries. During storage at 4 °C and 75% relative humidity for 16 days, the measurements of mass loss, decay percentage, total soluble solids (TSS), titratable acidity (TA), pH, organic acids, total phenolic and anthocyanin contents and antioxidant activity were made. The results demonstrate that the application of edible coatings with 0.5% CMC and 0.5% CMC with bacteriocin (CMC+B) led to a significant decrease of mass loss in the treated strawberries compared to the uncoated fruit. After the 8th day of storage, significant reductions in decay percentage along with the absence of fungal growth in CMC+B-coated fruit were observed in comparison with the CMC-coated and control strawberries. During the second half of the storage period, CMC and CMC+B treatments reduced TSS amount in the coated fruit compared to the control, but did not affect the increase of TA and decrease of pH values that are normally associated with postharvest changes. The CMC and CMC+B coatings did not prevent the decrease of ascorbic acid, and total phenolic and anthocyanin contents during cold storage. The application of CMC and CMC+B coatings had a significant inhibitory effect on decreasing the antioxidant activity throughout the storage period and maintained the antioxidant levels in both treatments close to the initial value of 76.8 mmol Trolox equivalents per 100 g of fresh mass.
Key words: bacteriocin, biopreservation, edible coatings, strawberry, Bacillus methylotrophicus
INTRODUCTION
The cultivated or garden strawberry (Fragaria × ananassa Duch.) is among the most widely spread fruits and is commonly used as a dessert in the human diet. Strawberry fruit in its fresh or processed form has a delicious taste and unique flavour that is widely accepted by consumers. Strawberries are also very nutritious and exert a strong antioxidant activity that is related to the high levels of anthocyanins, phenolic compounds, flavonoids, nitrogenous compounds, tocopherols, carotenoids and ascorbic acid. In addition to antioxidants, strawberry fruit contains a variety of volatiles such as esters, aldehydes, alcohols and sulfur compounds. The rich phytochemical composition and high antioxidant capacity of strawberries exhibit a protective effect against chronic and degenerative diseases, cardiovascular disorders, and have been found to possess anticarcinogenic and antimutagenic activities (1, 2).
Strawberries are extremely sensitive and perishable. They have a short shelf-life due to the exposure to postharvest activities that make them susceptible to physical injuries, fungal decay, desiccation and other disorders during storage (3). Despite the use of rapid cooling to avoid postharvest decay and to maintain the quality of the fruit during the storage, the shelf-life of fresh strawberries at low temperatures (0–4 °C) is limited to 5 days (4).
In recent years, more advanced trends in food biopreservation have resulted in the use of non-conventional approaches such as the application of edible coatings that help to extend the shelf-life of strawberries and other perishable fruits. Edible coatings are thin layers of edible materials that are applied to the surface of food products and play an important role in the product conservation. The basic function of edible coatings is to protect the product from mechanical damage, physical, chemical and microbiological activities (5). The edible coatings act as protective barriers against dehydration by reducing water/moisture loss, which is the primary reason for deterioration of fruit quality. In addition to decreasing the loss of product mass and firmness, edible coatings suppress respiration, improve textural quality, help to retain natural colour and volatile flavour compounds, and retard microbial growth, especially fungal growth (4, 6).
The effectiveness of edible coatings depends on the selection of coating materials, which can be used alone or in various combinations. Numerous studies of the composition and application of edible coatings have shown that polysaccharides, proteins, lipids or their derivatives are among the most commonly used structural matrices (7). For example, cellulose is a suitable natural polysaccharide of plant origin used in the production of edible coatings. To overcome the limitations of the native form of this biopolymer (water-insolubility and highly associated crystalline structure), some commercially available derivatives such as carboxymethyl cellulose (CMC), methyl cellulose, hydroxypropyl cellulose and hydroxypropyl methyl cellulose have been developed for incorporation into edible coatings used for perishable fruits (8). Cellulose derivatives are water-soluble, odourless and tasteless, possess good film-forming characteristics and provide a moderate barrier to moisture and oxygen transmission (9). In addition to its edible coating application, CMC is considered to be a promising raw material to replace non-degradable polymers in the packaging of fruits and vegetables (10).
One of the most challenging problems associated with the shelf-life, storage and distribution of perishable fruits, especially strawberries, is fungal spoilage. Recently, the increasing consumer demand for fresh and minimally processed food has resulted in research into the development and application of new methods for biopreservation by safe and non-toxic biologically active compounds as alternatives to traditional chemical treatments and fungicides. In this regard, bacteriocins as antimicrobial peptides produced by some Bacillus spp. and lactic acid bacteria (LAB) represent a promising solution to problems associated with fungal decay. A number of recent studies have reported the potential of LAB bacteriocins (nisin, bovicin CH5, enterocins AS-48 and 416K1, bificin C6165 and pediocin) in the biopreservation of fruits and fruit products (11, 12). Some other LAB bacteriocins such as those produced by Lactobacillus plantarum and Lactobacillus Y153 have been shown to possess a strong inhibitory effect on the most severe etiological agents of postharvest fungal decay in strawberries – Rhizopus stolonifer and Botrytis cinerea (13, 14). However, there are no data available in the scientific literature related to the application of other Bacillus sp. bacteriocins in fruit biopreservation.
Therefore, the purpose of the present study is to investigate the addition of a bacteriocin produced by Bacillus methylotrophicus BM47 to the composition of CMC edible coatings and to evaluate the effect of bacteriocin treatment on the prolongation of shelf-life and the preservation of quality in fresh strawberries.
MATERIALS AND METHODS
Fruit material
Fresh strawberries (Fragaria × ananassa Duch.) were purchased from a local fruit market in Plovdiv, Bulgaria. The strawberries were selected by uniform size, normal shape without defects or physical damage and saturated red colour. Fruits were kept in brown paper bags and transferred immediately to the laboratory to conduct the experimental work.
Bacteriocin
A bacteriocin produced by Bacillus methylotrophicus strain BM47 isolated from a natural thermal spring in the Haskovo region of Bulgaria was used. The purified bacteriocin substance contained an antimicrobial peptide of intermediate molecular size (19 578 Da) as described in our previous work (15).
Preparation of edible coatings
An edible coating solution was prepared according to the method described by Gol et al. (16) with slight modification. Carboxymethyl cellulose (CMC; 0.5%) was prepared by dissolving 2 g of CMC powder (Sigma-Aldrich, Merck, St. Louis, MO, USA) in 400 mL of distilled water and ethyl alcohol mixture (3:1 V/V) at 75 °C by stirring on a magnetic stirrer IKA® RCT classic (IKA®-Werke GmbH & Co. KG, Staufen, Germany) at 800 rpm for 20 min. Next, 0.25% glycerol monostearate Cutina® GMS (Henkel, Düsseldorf, Germany) was added to the mixture, and the solution was stirred under the same conditions for 15 min. Ethyl alcohol (Sigma-Aldrich, Merck) in the solution was used to reduce the drying time and to obtain transparent and shiny coatings, while glycerol monostearate was used as a plasticizer to improve the flexibility and strength of the coatings. The coating solution was divided into two portions of 200 mL each. After cooling, 100 AU/mL (0.15 mg/mL) of purified and lyophilized bacteriocin substance were added to the second portion and stirred without heating at 800 rpm for 15 min.
Application of edible coatings
Strawberries were surface-disinfected by dipping in 1% sodium hypochlorite (Sigma-Aldrich, Merck) for 3 min, then washed three times and dried at 25 °C for 2 h in a drying chamber with forced air (MLW; Labexchange, Burladingen, Germany). The fruits were divided into three experimental groups of 25 strawberries each, with uncoated strawberries labelled as the control (group 1), strawberries with a CMC coating as group 2 and strawberries with a CMC coating and bacteriocin (CMC+B) as group 3. After drying, the strawberries from groups 2 and 3 were dipped into the CMC and CMC+B coating solutions for 2–3 min. The processed fruit was then dried at 25 °C for 2 h in a drying chamber with forced air (MLW; Labexchange). Next, the strawberries from all groups were placed into plastic boxes and stored at 4 °C and 75% relative humidity (RH) for 16 days. The control fruit were examined at the beginning of the experiment (i.e. day 0). During storage, all groups were observed for morphological changes and fungal growth on the 4th, 8th, 12th and 16th day, and the samples for physicochemical analyses were collected (16).
Mass loss and decay percentages
To estimate these two parameters, three separate groups consisting of ten strawberries with the same treatments (control, CMC and CMC+B) were prepared and stored under the same conditions. To determine the mass loss percentage, each group was weighed at the beginning of the experiment (i.e. day 0) and on the 4th, 8th, 12th and 16th day of storage. The difference between the initial mass of each experimental group and the mass of the same group determined on each sampling day was defined as the mass loss, and the results were calculated as a percentage of loss of the initial mass using the following equation:
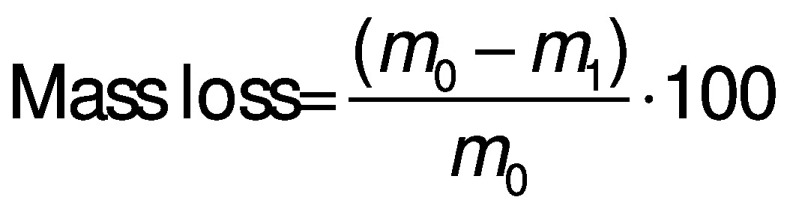 |
where m0 is the initial mass of each experimental group (day 0), and m1 is the measured mass of the same group on each sampling day (17).
The decay percentage was determined as follows: the number of strawberries with visible decay or morphological changes was expressed as the percentage of the initial number of all strawberries in the relevant experimental group (16).
Total soluble solids, titratable acidity and pH
The total soluble solids (TSS) of the strawberries were measured using a portable Abbe refractrometer (Officine Galileo, Campi Bisenzio, Italy). Strawberries from each experimental group were homogenized with a homogenizer Polytron (Kinematica AG, Luzern, Switzerland). A few drops of the juice were placed on the prism glass, and the TSS value was read directly and recorded. The titratable acidity (TA) was determined by titration of 2 mL of strawberry juice with 0.1 M NaOH (Sigma-Aldrich, Merck) using phenolphthalein (Sigma-Aldrich, Merck) as an indicator until the appearance of a pale pink colour persisted over 1 min. The results were calculated as the mean value of three consecutive experiments and expressed as the percentage of citric acid. The pH values of each group were measured by a pH-meter InoLab® pH 7110 (WTW, Weilheim, Germany) at room temperature (23 °C) (16).
Total phenolic content
Total phenolic content was measured by the method of Stintzing et al. (18). In brief, 1 mL of Folin-Ciocalteu reagent (Sigma-Aldrich, Merck) diluted five times was mixed with a 0.2-mL sample and 0.8 mL 7.5% Na2CO3 (Sigma-Aldrich, Merck). The reaction was performed in darkness at room temperature for 20 min. Next, the absorbance was measured by UV/Vis spectrophotometer Camspec M107 (Spectronic-Camspec Ltd., Leeds, UK) at λ=765 nm against a blank. The results were expressed in mg of gallic acid equivalents (GAE) per 100 g of fresh mass (fm), according to a calibration curve (19).
Total anthocyanin content
Total anthocyanin content was determined according to the pH differential method, described by Lee et al. (20). Samples of the strawberry juice (0.2 mL) were mixed with buffers (Sigma-Aldrich, Merck) at pH=1.0 and pH=4.5 (1.8 mL), and the absorbance was measured against a blank at λ=510 and 700 nm using UV/Vis spectrophotometer Camspec M107 (Spectronic-Camspec Ltd.). The results were expressed in mg cyanidin-3-glycoside equivalents (CGE) per 100 g of fm for a minimum of three replicates.
Antioxidant activity
The antioxidant activity was determined by ferric reducing antioxidant power (FRAP) assay according to the Benzie and Strain (21) method with slight modification. The FRAP reagent was freshly prepared by mixing 10 parts 0.3 M acetate buffer (pH=3.6), 1 part 10 mM 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ) in 40 mM HCl and 1 part 20 mM FeCl3·6H2O (all from Sigma-Aldrich, Merck) in distilled water. The reaction was started by mixing 3.0 mL FRAP reagent with 0.1 mL strawberry juice. The reaction time was 10 min at 37 °С in darkness, and the absorbance was measured at λ=593 nm using UV/Vis spectrophotometer Camspec M107 (Spectronic-Camspec Ltd.) against a blank prepared with methanol. Antioxidant activity was expressed in mmol Trolox equivalents (TE) per 100 g of fm (19).
High-pressure liquid chromatography analysis of organic acids
The organic acid analysis was performed on an Elite LaChrom HPLC-DAD system (VWR™ Hitachi, Tokyo, Japan) as previously described by Ivanov et al. (22). The separation was conducted on a Discovery® HS C18 column (25 cm×4.6 mm, 5 µm; Sigma-Aldrich, Merck) at 30 °С by isocratic elution with the mobile phase consisting of 25 mM KН2РО4 (pH=2.4 with 85% H3PO4) at a flow rate of 0.5 mL/min. The detection of l-(+)-ascorbic acid (vitamin C) was monitored at λ=244 nm and for citric and l-malic acids at λ=210 nm. The results were expressed in mg per 100 g of fm.
Statistical analysis
The experiments were performed in triplicates, data were presented as mean value, and the standard deviation (S.D.) was calculated.
RESULTS AND DISCUSSION
Mass loss and decay percentages of treated strawberries
The results of the determination of the mass loss percentage are presented in Table 1. During the first four days of storage at 4 °C and 75% RH, a slight increase in mass loss was observed, which was more pronounced in the control fruit than in the strawberries with carboxymethyl cellulose (CMC) and CMC with bacteriocin (CMC+B) coatings. The same trend was recorded on the 8th and 12th day of observation – the mass loss of uncoated strawberries was higher than of the coated fruit; this parameter was 1.22 to 1.88% lower in CMC coatings, and 1.28 to 1.84% lower in CMC+B coatings. The mass loss continued to increase, and by the end of storage (16th day) the difference between the control fruit and treated groups (CMC and CMC+B) reached 2.91 and 2.96% respectively, which demonstrated that these coatings prevented moisture/water loss in the treated fruit.
Table 1. Effects of carboxymethyl cellulose (CMC, 0.5%) and CMC with bacteriocin from Bacillus methylotrophicus BM47 (CMC+B) edible coatings on the physicochemical parameters of fresh strawberries during storage at 4 °C and 75% relative humidity.
| t/day | Coating | Parameter | ||||
|---|---|---|---|---|---|---|
| Mass loss/% | Decay/% | w(TSS)/% | TA/% | pH | ||
| 0 | Control | n.d. | n.d. | 8.4 | 0.90±0.03 | 3.63±0.02 |
| CMC | n.d. | n.d. | n.d. | n.d. | n.d. | |
| CMC+B | n.d. | n.d. | n.d. | n.d. | n.d. | |
| 4 | Control | 4.8 | 0 | 8.4 | 0.91±0.06 | 3.60±0.03 |
| CMC | 3.7 | 0 | 8.4 | 0.92±0.05 | 3.61±0.04 | |
| CMC+B | 3.9 | 0 | 8.4 | 0.94±0.06 | 3.58±0.02 | |
| 8 | Control | 9.11 | 10 | 8.7 | 0.96±0.07 | 3.54±0.01 |
| CMC | 7.89 | 10 | 8.4 | 0.97±0.04 | 3.51±0.05 | |
| CMC+B | 7.83 | 0 | 8.4 | 0.99±0.05 | 3.41±0.03 | |
| 12 | Control | 12.34 | 20 | 9.1 | 0.99±0.06 | 3.41±0.06 |
| CMC | 10.46 | 10 | 8.6 | 1.01±0.08 | 3.40±0.05 | |
| CMC+B | 10.5 | 0 | 8.6 | 1.09±0.07 | 3.34±0.04 | |
| 16 | Control | 17.37 | 60 | 9.4 | 1.10±0.04 | 3.32±0.07 |
| CMC | 14.46 | 40 | 8.9 | 1.12±0.06 | 3.30±0.02 | |
| CMC+B | 14.41 | 20 | 8.9 | 1.13±0.08 | 3.24±0.01 | |
The results are expressed as mean value±standard deviation. TSS=total soluble solids, TA=titratable acidity, n.d.=not determined
Our data also demonstrated that no morphological changes were observed in any of the experimental groups until the 4th day of storage (Table 1). The first visible signs of fungal decay in the control and CMC-coated fruit appeared on the 8th day of storage, while the strawberries with CMC+B coatings remained unaffected. Until the end of the storage, the percentage of decay and fungal growth in CMC+B-coated fruit were reduced significantly. This result would suggest that the preventive effect of the bacteriocin of B. methylotrophicus BM47 and the concomitant decrease in the incidence of mould decay in the CMC+B-covered strawberries were related to the antifungal activity of the bacteriocin as described in our earlier studies (23).
Our results are in agreement with those reported by Gol et al. (16), who described the effectiveness of edible coatings with 1% CMC on the quality of strawberries, showing a significant reduction in mass loss during storage and high reduction in decay percentage from 11.5% (control) to 5.89% (1% CMC) on the 4th day, and from 95.59% (control) to 28.57% (1% CMC) on the 12th day of storage. Dong and Wang (24) also reported a significant delay in the mass loss of fresh strawberries covered with CMC-based coatings. They found a lower mass loss of 16.23% and a decay percentage of 71.8% of the treated fruit than the control fruit levels of 24.73 and 93.2%, respectively, on the 6th day of storage.
Hussain et al. (25) reported that the application of CMC coatings (0.5 and 0.75%) alone had no effect on delaying decay or inhibiting fungal growth in strawberry fruit. However, compared with other treatments, the combination of 1% CMC coatings with gamma irradiation (2.0 kGy) had a significant effect on delaying the decay and appearance of fungal growth on strawberries kept in refrigerated storage up to 18 days.
Changes in total soluble solids, titratable acidity and pH
In the control group only, a slight increase in total soluble solids (TSS) was detected on the 8th day of storage. This change was related to the migration of water in the environment and the higher moisture loss of the uncoated fruit. The TSS levels in CMC- and CMC+B-coated strawberries were equal to the levels noted at the beginning of the experiment (8.4%). The TSS continued to increase gradually, and the value of the uncoated group by the end of the storage was 0.5% higher than of the CMC- and CMC+B-coated fruit (Table 1).
A slight increase in titratable acidity (TA) and a decrease in pH values were detected in all experimental groups. These findings are usually associated with postharvest physiological activities such as the decomposition of sugars and decay changes. The results summarized in Table 1 show that the application of CMC and CMC+B coatings did not consistently influence these two parameters, which remained similar to those of the control samples until the end of the storage.
The changes in TSS were in agreement with the results published by Gol et al. (16), who also found that TSS levels increased during storage in both CMC-coated and uncoated strawberries. In contrast to our results, the authors reported a decrease in TA levels and an increase in pH values during the entire storage period.
According to Hussain et al. (25) the combination of 1% CMC edible coatings and gamma irradiation (2.0 kGy) was helpful in maintaining higher levels of TSS in strawberries than after the treatments with 0.5 and 0.75% CMC and controls. The authors also noted decreasing TA levels in all treatments up to the 15th day, followed by an increase in TA levels after the 18th day of storage.
Changes in organic acid content
The results obtained from the HPLC analysis of organic acids demonstrated that the content of ascorbic acid (vitamin C) in the strawberries gradually decreased in all experimental groups with the prolongation of storage (Table 2). During the first 4 days, vitamin C levels in the three experimental groups decreased but were close to the initial level of 30.5 mg/100 g fm (i.e. day 0). Vitamin C mass fractions continued to decline, and by the end of the monitoring period (16th day), they reached levels, on fresh mass basis, of (in mg/100 g): 17 (control), 16 (CMC coatings) and 15.7 (CMC+B coatings). One explanation for the reduction of ascorbic acid content in strawberry fruit is the high thermal instability of ascorbic acid and the activity of ascorbate oxidase which converts ascorbic acid into dehydroascorbic acid with prolonged storage (2).
Table 2. Effects of carboxymethyl cellulose (CMC, 0.5%) and CMC with bacteriocin from Bacillus methylotrophicus BM47 (CMC+B) edible coatings on organic acid content of fresh strawberries during 16 days of storage at 4 °C and 75% relative humidity.
| t/day | Coating | w(organic acid)/(mg/100 g) | ||
|---|---|---|---|---|
| Ascorbic | Malic | Citric | ||
| 0 | Control | 30.5±0.06 | 22.0±0.09 | 54.5±0.04 |
| CMC | n.d. | n.d. | n.d. | |
| CMC+B | n.d. | n.d. | n.d. | |
| 4 | Control | 30.0±0.08 | 21.5±0.07 | 56.0±0.05 |
| CMC | 28.5±0.09 | 21.3±0.03 | 57.2±0.02 | |
| CMC+B | 28.2±0.01 | 20.6±0.04 | 58.5±0.09 | |
| 8 | Control | 29.0±0.05 | 21.4±0.07 | 59.5±0.06 |
| CMC | 27.9±0.08 | 21.0±0.02 | 60.4±0.04 | |
| CMC+B | 27.4±0.09 | 20.5±0.04 | 61.3±0.01 | |
| 12 | Control | 25.7±0.01 | 19.6±0.05 | 61.5±0.08 |
| CMC | 24.9±0.07 | 19.0±0.03 | 63.6±0.02 | |
| CMC+B | 24.5±0.04 | 18.8±0.09 | 65.1±0.06 | |
| 16 | Control | 17.0±0.03 | 18.7±0.08 | 69.8±0.05 |
| CMC | 16.0±0.06 | 18.2±0.01 | 71.7±0.04 | |
| CMC+B | 15.7±0.08 | 18.0±0.07 | 71.8±0.02 | |
The results are expressed as mean value±standard deviation, n.d.=not determined
Treatment with other biologically active substances could have a protective effect on the ascorbic acid retention. Jia et al. (13) reported a postharvest decrease in vitamin C in fresh strawberries. However, the authors observed a significantly higher content of vitamin C in fruit treated with bacteriocin from Lactobacillus plantarum LF-1 alone than in the control and nisin-treated fruit. After six days of storage, the vitamin C content in the fruit treated with the bacteriocin from L. plantarum LF-1 was 63 mg/100 g, and in the fruit treated with nisin 50 mg/100 g. Strawberries treated with bacteriocin from L. plantarum LF-1 had a 33 mg/100 g higher vitamin C level than the untreated fruit. Zhou et al. (14) reported that the application of a bacteriocin from Lactobacillus Y153 had an inhibitory effect on the progressive decrease of vitamin C in fresh strawberries, thus preserving the nutritional qualities of the fruit. Similar results were obtained by Gol et al. (16), who concluded that edible coatings made up of CMC (1%) with or without the addition of chitosan (1%) exerted a significant effect on the level of ascorbic acid and delayed its decrease in the treated strawberries.
The edible coatings of CMC and CMC+B did not affect the malic acid content of the strawberries during storage (Table 2). The malic acid content remained relatively constant during the first half of the storage compared to the initial level of 22 mg/100 g fm. However, by the end of the storage (16th day), malic acid content, on fresh mass basis, had decreased to the values of (in mg/100 g): 18.7 (control), 18.2 (CMC-coated strawberries) and 18.0 (CMC+B-coated strawberries).
The mass fraction of citric acid, one of the primary acids in strawberries, gradually increased in both the control and coated fruit during storage, reaching levels, on fresh mass basis, of (in mg/100 g): 69.8 (control), 71.7 (CMC) and 71.8 (CMC+B) on the 16th day of storage, compared to 54.5 on day 0 (Table 2). The CMC and CMC+B edible coatings had no inhibitory effect on the increasing mass fraction of citric acid that is normally associated with postharvest changes in the fruit. These results correspond to the increasing titratable acidity and decreasing pH values (Table 1). Our results are in agreement with those reported by Nunes et al. (26), who observed increasing levels of citric acid in strawberries stored for 8 days under refrigerated conditions (1 °C and 90–95% RH).
Changes in total anthocyanin content
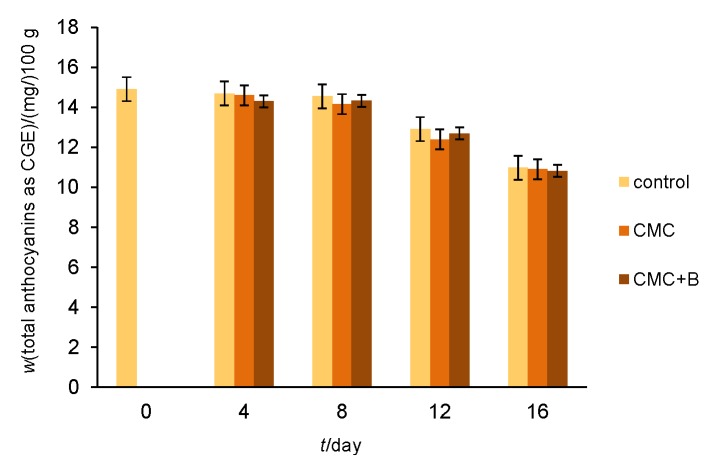
Anthocyanins, a type of flavonoid, are the pigments that give strawberries their characteristic red colour. The anthocyanins showed relatively constant levels in both uncoated and coated fruit during the first half of the storage. The total anthocyanins retained mass fractions, on fresh mass basis, of (in mg/100 g): 14.55 (control), 14.16 (CMC) and 14.33 (CMC+B), which were close to the initial level of 14.91 (Fig. 1). Thereafter, the total anthocyanins began to decrease slightly. This trend was observed until the end of monitoring period when total anthocyanins reached levels of (in mg/100 g): 10.98, 10.9 and 10.82 respectively (Fig. 1), demonstrating that CMC and CMC+B coatings did not delay the reduction of anthocyanin content in strawberries during storage.
Fig. 1.
Effects of carboxymethyl cellulose (CMC) and CMC with bacteriocin (CMC+B) edible coatings on total anthocyanin content of fresh strawberries during 16 days of storage at 4 °C and 75% relative humidity (error bars indicate standard deviation)
One of the explanations for decreasing anthocyanin and vitamin C levels in strawberries is the negative effect of low temperatures on their accumulation levels during cold storage, as reported by Cordenunsi et al. (27). However, the authors observed a positive effect on soluble sugars, while flavonols, ellagic acid, total phenolic content and antioxidant activity maintained constant levels or even decreased at all storage temperatures (6, 16 and 25 °C). Kalt et al. (28) also proved the negative effect of low temperatures on anthocyanin biosynthesis during the cold storage of small fruits (i.e. strawberries). Likewise, Hussain et al. (25) observed a declining trend in the anthocyanin content, and reported that strawberries treated with a combination of CMC edible coatings (0.5–1.0%) and gamma irradiation (2.0 kGy) exhibited an initial increase in anthocyanin levels, followed by a sustainable decrease in both the control and treated fruit after prolonged storage.
According to some research, the addition of essential oils in the composition of CMC-based edible coatings could prevent the decrease in the anthocyanin content. Dong and Wang (24) ascertained that the anthocyanin content in the strawberries coated with CMC (1%) decreased during storage. However, the authors found that the addition of garlic essential oil (2%) into the CMC coatings was an effective way to delay fruit senescence and the decrease in anthocyanins. They concluded that such coatings acted as a gas barrier and modified the internal atmosphere of the fruit, thus retarding the biochemical reactions leading to anthocyanin synthesis.
Changes in total polyphenolic content
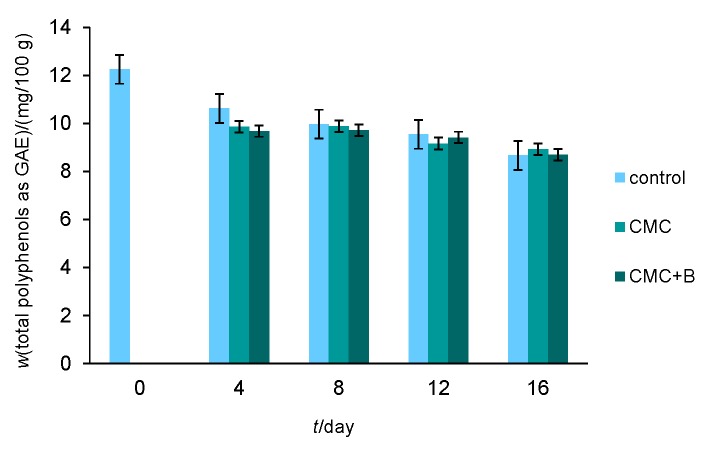
The total polyphenols maintained relatively high and almost equal mass fractions in all experimental groups during the entire storage, corresponding to the results obtained for total anthocyanins. Until the 8th day, the total polyphenolic content remained stable, and a slight decrease in their mass fraction was observable on the 12th day. On the 16th day, the total polyphenolic level (expressed as GAE) decreased to (in mg/100 g): 8.66 (uncoated fruit), 8.92 (CMC) and 8.69 (CMC+B) compared to the 12.25 at day 0 (Fig. 2).
Fig. 2.
Effects of carboxymethyl cellulose (CMC) and CMC with bacteriocin (CMC+B) edible coatings on total phenolic content of fresh strawberries during 16 days of storage at 4 °C and 75% relative humidity (error bars indicate standard deviation)
Our results are in agreement with those reported by Gol et al. (16), who also observed a progressive decrease in total phenols in both control and strawberries coated with 1% CMC. However, the incorporation of chitosan (1%) in these coatings helped to maintain higher phenolic content in the processed fruit. The noted decrease in phenolic compounds, which might be associated with the breakdown of cell structures caused by natural fruit senescence during storage, was also stated by Dong and Wang (24), who determined that the decrease in phenolic content was greater in the control than in the fruit treated with CMC coating with added garlic essential oil.
The use of non-conventional methods in combination with edible coatings may also have a beneficial effect on the phenolic compounds in strawberries. The combined application of CMC edible coatings (1.0%) and gamma irradiation (2.0 kGy) led to a significant increase in the total phenols in treated strawberries during 21 days of storage compared with the phenolic levels in fruit coated with 0.5, 0.75 and 1% CMC alone as reported by Hussain et al. (25).
Changes in antioxidant activity
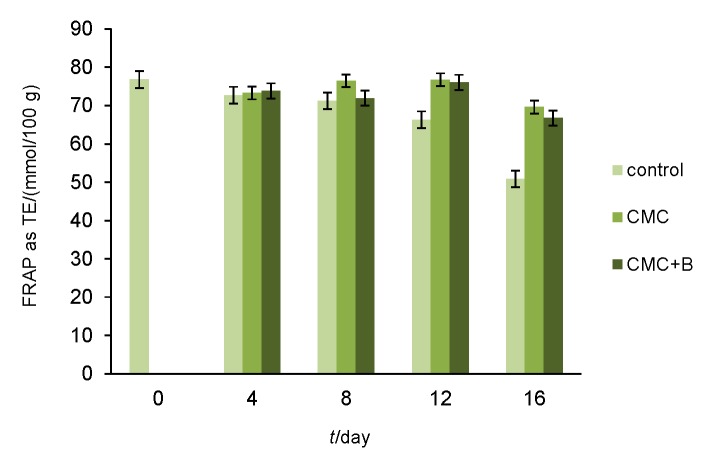
The results obtained by FRAP assay (Fig. 3) revealed that the antioxidant activity (expressed as TE) of uncoated strawberries gradually decreased throughout the storage, reaching its lowest value of 50.86 mmol/100 g in the control fruit on the 16th day. This result correlated with decreasing levels of compounds with antioxidant activity – ascorbic acid, anthocyanins and polyphenols. The application of CMC and CMC+B edible coatings inhibited this decrease and maintained the antioxidant activity of the coated fruit at levels higher than those of the uncoated ones and kept the antioxidant values close to the initial one of 76.8 mmol/100 g (day 0). The protective effect of the edible coatings on the antioxidant activity could be associated with a reduction in the respiration rate of the coated fruit and a probable positive effect on other biologically active compounds possessing antioxidant activity (phenolic acids, hydrolysable tannins, vitamin E, carotenoids, minerals, enzymes, etc.), which may be explored in a further study.
Fig. 3.
Effects of carboxymethyl cellulose (CMC) and CMC with bacteriocin (CMC+B) edible coatings on antioxidant activity of fresh strawberries during 16 days of storage at 4 °C and 75% relative humidity (error bars indicate standard deviation)
CONCLUSIONS
Our results showed that the application of carboxymethyl cellulose (CMC) and CMC with bacteriocin (CMC+B) coatings had a positive impact on some physicochemical parameters of strawberries by reducing the total soluble solid levels and mass loss percentage. The addition of bacteriocin from Bacillus methylotrophicus BM47 to the composition of CMC-based coatings (CMC+B) led to a significant decrease in decay incidence and effectively inhibited the fungal growth on the coated fruit. CMC and CMC+B edible coatings positively influenced one of the primary health beneficial properties of fresh strawberries – their antioxidant activity. Thus, we can conclude that CMC-based edible coatings in combination with the bacteriocin produced by B. methylotrophicus BM47 could be effective means for improving the shelf-life, quality, commercial appearance and safety of processed strawberries. These findings are important not only from a commercial point of view, but also represent a sustainable vision of the fruits postharvest, which is every day more requested. Nowadays, agricultural waste management is one of the major challenges, and both edible coatings address this issue, prolonging the shelf-life of strawberries and at the same time reducing their waste. Moreover, this preservation technique is simple, ecofriendly and can be applied without the need for expensive equipment, and should therefore be considered for application in industrial fruit biopreservation.
Footnotes
CONFLICT OF INTEREST: The authors declare that no conflict of interest exists.
REFERENCES
- 1.Meyers KJ, Watkins CB, Pritts MP, Liu RH. Antioxidant and antiproliferative activities of strawberries. J Agric Food Chem. 2003;51(23):6887–92. 10.1021/jf034506n [DOI] [PubMed] [Google Scholar]
- 2.Mishra R, Kar A. Effect of storage on the physicochemical and flavour attributes of two cultivars of strawberry cultivated in Northern India. ScientificWorldJournal. 2014;2014:794926. 10.1155/2014/794926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vu KD, Hollingsworth RG, Leroux E, Salmieri S, Lacroix M. Development of edible bioactive coating based on modified chitosan for increasing the shelf life of strawberries. Food Res Int. 2011;44(1):198–203. 10.1016/j.foodres.2010.10.037 [DOI] [Google Scholar]
- 4.Han C, Zhao Y, Leonard SW, Traber MG. Edible coatings to improve storability and enhance nutritional value of fresh and frozen strawberries (Fragaria × ananassa) and raspberries (Rubus ideaus). Postharvest Biol Technol. 2004;33(1):67–78. 10.1016/j.postharvbio.2004.01.008 [DOI] [Google Scholar]
- 5.Falguera V, Quintero JP, Jimenez A, Munoz JA, Ibarz A. Edible films and coatings: Structures, active functions and trends in their use. Trends Food Sci Technol. 2011;22(6):292–303. 10.1016/j.tifs.2011.02.004 [DOI] [Google Scholar]
- 6.Corbo MR, Campaniello D, Speranza B, Bevilacqua A, Sinigaglia M. Non-conventional tools to preserve and prolong the quality of minimally-processed fruits and vegetables. Coatings. 2015;5(4):931–61. 10.3390/coatings5040931 [DOI] [Google Scholar]
- 7.Costa Garcia L, Mendes Pereira L, de Luca Sarantópoulos CIG, Dupas Hubinger M. Effect of antimicrobial starch edible coating on shelf-life of fresh strawberries. Packag Technol Sci. 2012;25(7):413–25. 10.1002/pts.987 [DOI] [Google Scholar]
- 8.Chander Mahajan BV, Tandon R, Kapoor S, Kaur Sidhu M. Natural coatings for shelf-life enhancement and quality maintenance of fresh fruits and vegetables – A review. J Postharvest Technol. 2018;6(1):12–26. [Google Scholar]
- 9.Bourtoom T. Edible films and coatings: Characteristics and properties. Int Food Res J. 2008;15(3):237–48. [Google Scholar]
- 10.Shahbazi M, Ahmadi SJ, Seif A, Rajabzadeh G. Carboxymethyl cellulose film modification through surface photo-crosslinking and chemical crosslinking for food packaging applications. Food Hydrocoll. 2016;61:378–89. 10.1016/j.foodhyd.2016.04.021 [DOI] [Google Scholar]
- 11.Barbosa AA, Mantovani HC, Jain S. Bacteriocins from lactic acid bacteria and their potential in the preservation of fruit products. Crit Rev Biotechnol. 2017;37(7):852–64. 10.1080/07388551.2016.1262323 [DOI] [PubMed] [Google Scholar]
- 12.Tumbarski Y, Lante A, Krastanov A. Immobilization of bacteriocins from lactic acid bacteria and possibilities for application in food biopreservation. Open Biotechnol J. 2018;12(1):25–32. 10.2174/1874070701812010025 [DOI] [Google Scholar]
- 13.Jia N, Xie YH, Zhang HX, Liu H, Feng JJ, Zhu LD, et al. Effect of bacteriocin treatment on storage and quality of postharvest strawberry fruit. Adv Mat Res. 2012;554–556:1547–52. 10.4028/www.scientific.net/AMR.554-556.1547 [DOI] [Google Scholar]
- 14.Zhou HM, Jia N, Zhang HX, Xie YH, Liu H, Kong BH, et al. Study on antifungal properties of bacteriocin produced by Lactobacillus and application in fruit preservation. Adv Mat Res. 2013;781–784:1315–21. 10.4028/www.scientific.net/AMR.781-784.1315 [DOI] [Google Scholar]
- 15.Tumbarski Y, Deseva I, Mihaylova D, Stoyanova M, Krastev L, Nikolova R, et al. Isolation, characterization and amino acid composition of a bacteriocin produced by Bacillus methylotrophicus strain BM47. Food Technol Biotechnol. 2018;56(4):546–52. 10.17113/ftb.56.04.18.5905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gol NB, Patel PR, Ramana Rao TV. Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharvest Biol Technol. 2013;85:185–95. 10.1016/j.postharvbio.2013.06.008 [DOI] [Google Scholar]
- 17.Zhang C, Li W, Zhu B, Chen H, Chi H, Li L, et al. The quality evaluation of postharvest strawberries stored in nano-Ag packages at refrigeration temperature. Polymers (Basel). 2018;10(8):894. 10.3390/polym10080894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stintzing FC, Herbach KM, Mosshammer MR, Carle R, Yi W, Sellappan S, et al. Color, betalain pattern, and antioxidant properties of cactus pear (Opuntia spp.) clones. J Agric Food Chem. 2005;53(2):442–51. 10.1021/jf048751y [DOI] [PubMed] [Google Scholar]
- 19.Ivanov IG, Vrancheva RZ, Marchev AS, Petkova NT, Aneva IY, Denev PP, et al. Antioxidant activities and phenolic compounds in Bulgarian Fumaria species. Int J Curr Microbiol Appl Sci. 2014;3(2):296–306. [Google Scholar]
- 20.Lee J, Durst RW, Wrolstad RE. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J AOAC Int. 2005;88(5):1269–78. [PubMed] [Google Scholar]
- 21.Benzie IFF, Strain JJ. Ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996;239(1):70–6. 10.1006/abio.1996.0292 [DOI] [PubMed] [Google Scholar]
- 22.Ivanov I, Vrancheva R, Mihaylova D, Popova A. Adaptation of HPLC method for quantity evaluation of organic acids in food products. In: Proceedings of the 7th National Scientific Conference for students, PhD students and young scientists, 15 years of Federation “Science and Higher Education”; 2017 May 27, Plovdiv, Bulgaria: Imeon, Plovdiv, Bulgaria; 2017. pp. 41–6 (in Bulgarian). [Google Scholar]
- 23.Tumbarski Y, Yanakieva V, Nikolova R, Mineva G, Deseva I, Mihaylova D, et al. Antifungal effect of a bacteriocin of Bacillus methylotrophicus BM47 and its potential application as a biopreservative in traditional Bulgarian yogurt. J Microbiol Biotechnol Food Sci. 2018;8(1):659–62. 10.15414/jmbfs.2018.8.1.659-662 [DOI] [Google Scholar]
- 24.Dong F, Wang X. Effects of carboxymethyl cellulose incorporated with garlic essential oil composite coatings for improving quality of strawberries. Int J Biol Macromol. 2017; 104(Pt A):821–6. https://doi.org/ 10.1016/j.ijbiomac.2017.06.091 [DOI] [PubMed]
- 25.Hussain PR, Dar MA, Wani AM. Effect of edible coating and gamma irradiation on inhibition of mould growth and quality retention of strawberry during refrigerated storage. Int J Food Sci Technol. 2012;47(11):2318–24. 10.1111/j.1365-2621.2012.03105.x [DOI] [Google Scholar]
- 26.Nunes MCN, Brecht JK, Morais AMMB, Sargent SA. Physicochemical changes during strawberry development in the field compared with those that occur in harvested fruit during storage. J Sci Food Agric. 2005;86(2):180–90. 10.1002/jsfa.2314 [DOI] [Google Scholar]
- 27.Cordenunsi BR, Genovese MI, Oliveira do Nascimento JR, Hassimotto NM, dos Santos RJ, Lajolo FM. Effects of temperature on the chemical composition and antioxidant activity of three strawberry cultivars. Food Chem. 2005;91(1):113–21. 10.1016/j.foodchem.2004.05.054 [DOI] [Google Scholar]
- 28.Kalt W, Forney CF, Martin A, Prior RL. Antioxidant capacity, vitamin C, phenolics, and anthocyanins after fresh storage of small fruits. J Agric Food Chem. 1999;47(11):4638–44. 10.1021/jf990266t [DOI] [PubMed] [Google Scholar]