Abstract
With increasing drug resistance and the poor state of current antifungals, the need for new antifungals is urgent and growing. Therefore, we tested a variety of essential oils for antifungal activity. We report the minimum inhibitory concentrations (MIC) values for a common set of 82 essential oils against Aspergillus niger, Candida albicans, and Cryptococcus neoformans. Generally, narrow-spectrum activity was found. However, C. neoformans was much more susceptible to inhibition by essential oils with over one-third of those tested having MIC values below 160 ppm. GC-MS analysis showed the essential oils to be chemically diverse, yet, the potentially active major constituents typically fell into a few general categories (i.e., terpenes, terpenoids, terpenols). While essential oils remain a rich source of potential antifungals, focus should shift to prioritizing activity from novel compounds outside the commonalities reported here, instead of simply identifying antifungal activity. Further, capitalizing on bigger data approaches can provide significant returns in expediting the identification of active components.
Keywords: Aspergillus niger, Candida albicans, Cryptococcus neoformans, essential oil, antifungal, minimum inhibitory concentration, natural product, exclusionary principle, phytochemicals, bioactivity, drug discovery
1. Introduction
Worldwide, invasive fungal infections are responsible for greater than 1.5 million deaths annually [1,2,3]. Aspergillus, Candida, and Cryptococcus are responsible for a majority of these infections. Invasive aspergillosis is estimated to infect over 200,000 people per year with a mortality rate between 30% and 95%, invasive candidiasis is estimated to infect over 1 million people per year with a mortality rate between 10% and 75%, and invasive cryptococcosis is estimated to infect over 400,000 people per year with a mortality rate between 20% and 70% [2,3,4,5,6,7,8]. Even more dire, fungal infection and mortality rates are increasing despite current treatment options [9,10,11,12]. The increase is largely attributed to the emergence of drug-resistant fungal strains and an increasing immunocompromised population [13,14,15]. Exacerbating the situation, current antifungals generally require long-term adherence and have significant negative side effects. Thus, the need for new, safe, and effective antifungals is urgent and growing.
Phytochemicals have long been a source of medicinal compounds. This is particularly true for plant essential oils, largely due to their abundance in nature, unique composition, and molecular complexity [13,16,17]. Additionally, the location, time of year, environmental exposure, contact with fertilizer or pesticides, and a variety of other factors may significantly impact the chemical composition of plant essential oils [18,19,20,21]. Since plants can gain important benefits, like mitigating development of resistance, from multiple compounds with multiple targets and multiple modes of action (cocktail approach), there is the potential for multiple antimicrobial compounds in an essential oil. Regardless, one of the major bottlenecks facing natural product drug discovery is the identification of active components. Traditionally, this involves bioactive fractionation which requires a significant amount of natural product sample and is resource and time intensive—especially, when a large number of essential oils are involved.
To determine if bigger data methods could increase throughput and reduce the current bottleneck, we expanded a typical screen to include a large number of essential oils while still coupling quantification of antifungal efficacy with molecular characterization. Overall, we tested 82 plant essential oils for antifungal activity against A. niger, C. albicans, and C. neoformans. Minimum inhibitory concentrations (MICs) were determined and GC-MS spectra were acquired for each essential oil. It was found that all of the pathogenic fungi were inhibited to varying degrees. Notably, the susceptibility of C. neoformans was extremely high, with 32 of the essential oils tested having an MIC value of 160 ppm or lower. Conversely, seven essential oils showed activity at or below 160 ppm for A. niger, while C. albicans only had two essential oils showing the same level of inhibitory activity. Comparing the major constituents of the essential oils that showed antifungal activity, the constituents were largely accounted for by similar classes of molecules (i.e., terpenes, terpenoids, terpenols). While more data would be needed to apply more rigorous mathematical approaches (like singular value decomposition or principle component analysis) to deconvolute trends in inhibitory properties, simple exclusionary principles can be implemented to reduce the number of potential active components. Overall, from a bigger data perspective, the priority for essential oil antifungal discovery should focus on compounds outside of the commonalities reported here. Also, bigger data approaches can contribute to more efficient identification of active components if data are available, potentially reducing one of the major bottlenecks faced by traditional natural product drug discovery.
2. Results
Essential oils were randomly chosen for inclusion in the study. Oils from a variety of plant families (citrus, spices, evergreens, deciduous tree, shrubs, ornamental) and geographical locations were included. For antifungal activity, we focused on the three pathogens that are responsible for a majority of human fungal infections, namely, those fungi are the filamentous, mold-like ascomycete Aspergillus niger, the biofilm forming, yeast-like ascomycete Candida albicans, and the encapsulated basidiomycete Cryptococcus neoformans.
2.1. Fungal Susceptibility
For each of the three pathogenic fungal species studied, MIC values for all 82 essential oils are reported (see Appendix A). Given the unique natures of the fungal pathogens, it is not surprising that significant differences in susceptibility are observed. Most notable is the high degree to which C. neoformans is susceptible to inhibition by essential oils, with 37 of 82, or 45%, having MIC values less than or equal to 160 ppm (see Figure 1). In contrast, for A. niger, only seven essential oils and for C. albicans, only two essential oils show similar levels of inhibition (see Table 1). Using the previously suggested MIC of 100 µg/mL (equivalent to 100 ppm for essential oils) as a cutoff for significant antimicrobial potential to continue the study of crude natural products [22], 15 of the essential oils active against C. neoformans are of significant interest. In contrast, by this criteria, A. niger was the least susceptible and not significantly inhibited whereas for C. albicans, only one essential oil meets the significance criteria.
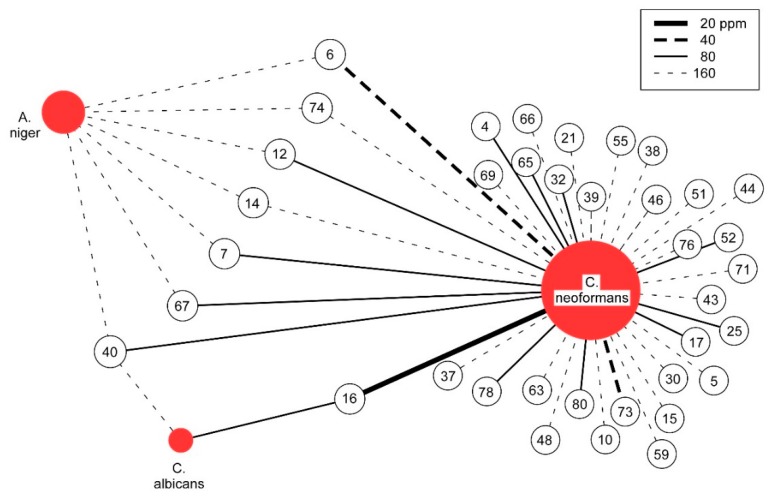
Figure 1.
Graphical Analysis of Antifungal Activity. The antifungal activity of essential oils with MIC values at or below 160 ppm are depicted. Unshaded circles represent essential oils, numbered as noted in Appendix A. The strength of MIC is indicated by the line (see legend).
Table 1.
Fungal Susceptibility to Essential Oils. The number of essential oils having the indicated MIC value are shown for each fungal pathogen. Those meeting the MIC < 100 ppm significance criteria are shaded.
| Fungal Pathogen | MIC = 160 ppm | 80 ppm | 40 ppm | 20 ppm |
|---|---|---|---|---|
| A. niger | 7 | 0 | 0 | 0 |
| C. albicans | 1 | 1 | 0 | 0 |
| C. neoformans | 22 | 12 | 2 | 1 |
When considering the range of inhibition, an initial inspection would suggest that narrow-spectrum inhibition is mostly observed. For the 15 essential oils that significantly inhibited C. neoformans, only one shows significant activity against C. albicans and none against A. niger. However, the most potent essential oil that inhibits C. neoformans is also the most potent inhibitor of C. albicans. Relaxing the significance criteria reveals more commonalities, blurring the narrow spectrum distinction (see Table 2). This is readily apparent from five of the seven essential oils having 160 ppm MICs against A. niger, just outside the 100 ppm significance cutoff, inhibiting C. neoformans with an MIC at or below 80 ppm. All seven inhibit C. neoformans at or below the same 160 ppm level that they inhibit A. niger. Therefore, the initial antifungal specificity of essential oils is not as pronounced as it first appears.
Table 2.
Essential Oil Efficacy against Pathogenic Fungi. The more stringent significance cutoff of 100 ppm applies to C. neoformans; the asterisk (*) indicates a more relaxed susceptibility cutoff of 160 ppm for C. albicans and A. niger. N/A indicates no activity beyond the vehicle carrier control.
| Essential Oil Name | C. neoformans | C. albicans * | A. niger * |
|---|---|---|---|
| Cedrus atlantica (2) | 20 | 80 | |
| Amyris balsamifera | 40 | N/A | 160 |
| Santalum spicatum | 40 | N/A | |
| Ferula galbaniflua | 80 | 160 | 160 |
| Ajowan Trachyspermum ammi | 80 | ||
| Callitris intratropica | 80 | N/A | 160 |
| Pogostemon cablin | 80 | N/A | 160 |
| Citrus aurantium ssp. Amara | 80 | N/A | N/A |
| Chamaecyparis obtuse | 80 | N/A | N/A |
| Aquilaria sinensis | 80 | N/A | 160 |
| Kunzea ericoides | 80 | N/A | |
| Vitex agnuscastus | 80 | N/A | N/A |
| Citrus clementina | 80 | N/A | |
| Coriandrum sativum | 80 | N/A | N/A |
| Turnera diffusa | 80 | N/A | N/A |
2.2. Essential Oil Composition
The composition of the essential oils was characterized by GC-MS and the data are shown in Supplementary Figure S1. From the 82 essential oils, it was found that approximately 750 different compounds could be confidently identified, accounting for greater than 99% of the observed constituents. Focusing on the major constituents, defined as a component being present at 5% or greater of the total composition, we found 157 different major constituents from the 82 essential oils. Of the 157 major constituents, 114 were unique, found in only one of the essential oils (see Figure 2). Therefore, each essential oil, on average, introduced 1.35 new major constituents into the analysis. Of the 43 non-unique major constituents, six were very common, appearing as major constituents in 10 or more of the essential oils (right half of Figure 2). All are terpenes/terpenoids, representatives from one of the major chemical classes common in essential oils [23]. Overall, while there is a considerable degree of commonality in essential oils, there is still a significant degree of difference in terms of major constituents. Thus, foregoing the antifungal activity of common, well known major constituents, essential oils represent a rich source of small molecules for antimicrobial drug discovery.
Figure 2.
Major Constituent Frequency Histogram. Shown are the frequencies of occurrence for the major constituents of essential oils. A total of 114 major constituents are unique to one essential oil (far left, in gray), while one major constituent is found in 19 essential oils (far right). Red numbers indicate the population of a given column.
2.3. Major Constituents of Antifungal Essential Oils
Using bigger data approaches to analyze antifungal activity of essential oils was constrained by the uniqueness of the major constituents which generated a large, but sparse data set. Approaches using singular-value decomposition or principle component analysis to correlate major constituents to MIC efficacy require more than one entry for each constituent for meaningful convergence. This can be somewhat overcome using a hierarchical clustering analysis [18] where major constituents are grouped based on chemical similarities and then the groups correlated to antifungal efficacy. While this approach is useful, unique identification of active constituents is still not possible. Given this limitation, we used an exclusionary principle to readily eliminate major constituents that could not be responsible for the antifungal activity. Simply by removing major constituents that have higher concentrations but less potent MIC values than in other essential oils, the number of possible antifungal constituents could be significantly reduced. For the 15 essential oils that significantly inhibited C. neoformans, there were a combined total of 48 major constituents. Using the simple exclusion principle, 13 of these major components could be eliminated from consideration, a 27% reduction. For the two essential oils with significant activity against C. albicans, there were six major constituents in total. The simple exclusion removed two. Therefore, by considering larger data (i.e., having more data sets) and consistent (at least relatively) MIC and GC-MS measurements, the identification of active components can be expedited. With more data, the convergence of singular-value decomposition or principal component analysis approaches would improve, potentially providing unique identification of antifungal constituents. However, the number of data sets needed for unique identification could not be realistically estimated beyond a rudimentary approximation of several thousand.
2.4. Chemical Similarity of Major Constituents
While individual active compounds could not be discerned with complete confidence, structural analysis of the potentially active constituents does provide additional insight. Structures of major constituents found in essential oils demonstrating antifungal activity are shown in Supplementary Figure S2. Using the clustering capability of ChemMineR [24], the structures of major constituents for essential oils with significant antifungal activity were grouped based on similarity. It should be noted that using the cutoff of 100 ppm meant that the significant oils found for C. neoformans accounted for all antifungal activity (since none were found for A. niger and the one essential oil for C. albicans was also found for C. neoformans). Thus, the analysis could be considered general, but really most relevant for and centered on C. neoformans.
The chemical similarity clustering revealed different categories, the members of which are henceforth referred to as Bins with a numbering distinction. Four Bins have multiple compounds. Bin 1 consists of sesquiterpenoids, sesquiterpenes, and sesquiterpenols. Bin 3 contains three different decane derivatives. Bin 25 has monoterpenes and monoterpenoids. Bin 20 contains two santalol derivatives. The compounds in their own individual Bins were Bin 13, an aromatic ketone, Bin 15, a monoterpene, Bin 18, a terpenoid, and Bin 27, a monoterpene. Most of these compounds have already been reported in the literature to be major components of multiple essential oils [25,26,27]. Also, numerous reports of antimicrobial activity have been reported for essential oils containing many of these individual constituents. However, no MIC quantification is available for individual constituents against A. niger, C. albicans, nor C. neoformans using the same methods reported here, further emphasizing the need for methodology to more efficiently and effectively identify the active constituents, not just general antifungal activity.
2.5. Cedrol: A Test Case for Individual Component Screening
The essential oil from Cedrus atlantica (2), #16 in our study, most potently inhibited both C. albicans (80 ppm) and C. neoformans (20 ppm). The major constituent cedrol has been reported in other essential oils to have antifungal properties [28,29] but has not been studied in isolation. Therefore, the individual compound was screened against both C. albicans and C. neoformans. Cedrol was obtained commercially, and samples were prepared in DMSO in both 1% concentrations, exactly as the full essential oils were, and in 0.23% concentrations, which corresponds to the amount of cedrol in the active essential oil. The screening results revealed that commercial cedrol inhibited C. neoformans, but was inactive against C. albicans. The 0.23% solution did not account for the full activity of the Cedrus atlantica (2) essential oil, having an MIC of 160 ppm (compared to the 20 ppm for the essential oil). Thus, it appears that other constituents contribute to the antifungal activity of the entire essential oil. As before, this cocktail approach can provide the advantage of multiple modes of action which limits the development of resistance. Having multiple active components needs to be accounted for in further algorithm development. This example also illustrates the need for antimicrobial susceptibility data to advance essential oil contribution to antimicrobial drug discovery.
3. Discussion
The trend towards larger data sets and bigger data methodology is growing in all fields of science. Therefore, application of these methods to essential oils antifungal discovery is natural. Before further discussion of the results, a discussion of the limitations is warranted. To maximize the beneficial outcome, the same laboratory equipment and personnel conducted the GC-MS characterization and antifungal studies under as reproducible conditions as possible. Thus, the 82 essential oil data set used is internally consistent and approaches an ideal case. Difficulties arise when comparing GC-MS and fungal susceptibility data from different studies due to many factors. These include different extraction methods, varying climates that can affect essential oil composition, and different methods to obtain susceptibility results (such as different media, assay techniques, and cell concentrations) [30,31,32,33]. Therefore, caution must be taken when comparing essential oil bioactivity results across various studies, especially when considering interlaboratory differences [34]. Another limitation is that inhibition of only one strain of each pathogenic fungi is reported, a particularly important point for Cryptococcus neoformans where only one serotype is represented. Other studies show variations in essential oil antifungal activity exist across different strains of the same fungal species [35,36,37], again requiring further study for meaningful generalization. Even with these caveats, the benefits of essential oils in antifungal drug discovery and use of larger data approaches is readily apparent.
The finding that C. neoformans is highly susceptible to essential oil inhibition was encouraging, albeit unrelated to the methodology. While this may have some relation to the capsule and/or fungal membrane, further discussion is beyond the scope of this study. With respect to essential oil composition, comparison to previously published data underscores the need for consistent reporting. For Cedrus atlantica (2), the three individual components found with the highest percentage were alpha-cedrene (31.4%), cedrol (23.52%), and cis-thujopsene (20.45%). From other studies, one reported that the three individual components found with the highest percentage were alpha-pinene (14.85%), himachalane (10.14%), and beta-himachalene (9.89%) [38], a different study reported E-gamma-atlantone (19.73%), E-alpha-atlantone (16.86%), and 5-isocedranol (11.68%) [39], while another reported limonene (29.18%), myrcene (16.9%), and ocimene + alpha-pinene (18.6%) [40]. For all studies, the essential oil was collected in or around Morocco via hydrodistillation. Similarly, previous studies have reported Coriandrum sativum to have activity against C. albicans [41,42], which was not observed herein. However, antifungal activity of C. sativum essential oil was noted against C. neoformans. Therefore, antifungal activity must be associated with the major constituents, not the essential oil.
Our investigation of bigger data approaches was constrained by the diversity of major constituents in the essential oils tested. In many cases, a major constituent of an essential oil with potent antifungal activity was only present in that oil. Thus, while the potential exists, discrimination of unique antifungal activity in terms of individual major constituents is not feasible without more data. While underscoring the utility of essential oils as being a large source of chemically different compounds, we and others [28,43,44] were limited by active compound identification. Even limited to a simple exclusion, using bigger data tools to facilitate analysis of essential oil data had an immediate positive impact.
With regard to identification of active compounds, it is necessary to bring up the possibility that more than one component of the essential oil may be responsible for the antifungal activity. Generally, it is the major component(s) that will be responsible for bioactivity, and not those found in trace amounts [45]; therefore, our focus has been centered on major constituents. Previous studies report that one or two of the major components found in an essential oil will be responsible for the bioactivity seen against a microorganism [21,23]. However, it has been shown in other studies that a synergistic effect between several different essential oil components is responsible for the full bioactivity [46,47]. It has also been shown across several different studies that some essential oils possess a single mechanism of action, while others possess two or more [45], meaning that sometimes interactions amongst different constituents are important for biological activity. As shown for cedrol, it is possible that even after identifying the potentially active major component(s), full activity may not be found and synergy between multiple essential oil components must be considered. Thus, bigger data approaches and algorithms must have the ability to account for such combinatorial outcomes.
4. Materials and Methods
4.1. Essential Oils
Essential oils were obtained commercially (doTerra, Pleasant Grove, UT, USA). All oils were tested as 1% solutions in DMSO made by suspending 10 µL of essential oil in 990 µL of DMSO.
4.2. Fungal Cultures
Pathogenic fungal strains of Aspergillus niger (ATCC #16888), Candida albicans (ATCC #18804), and Cryptococcus neoformans 24,067 (serotype D or var. neoformans) were utilized. A. niger cultures were initially grown on potato dextrose agar (PDA) at room temperature (~22 °C) before the conidia were filtered and resuspended in potato dextrose broth (PDB). Conidia density in the broth was adjusted with PDB to a density of 4000 conidia/mL from previously reported methods [48]. C. albicans and C. neoformans were grown on potato dextrose agar plates for 48–72 h, respectively, before a single colony was picked and grown in PDB to create initial cultures. Cells from these cultures were diluted to a concentration of 4000 cells/mL using RPMI 1640 (Roswell Park Memorial Institute) buffered with 167 mM MOPS (3-(N-morpholino)propanesulfonic acid) at pH 7.0.
4.3. Microdilution
Screening was performed according to CLSI guidelines. For all fungi, the 96-well microdilution method was used with a final well volume of 200 µL. For C. albicans and C. neoformans, 100 µL of RPMI was first added to all wells. Subsequently, 100 µL of the antifungal sample or control was added to the respective well in row A, mixed and then serially diluted in each row of the plate. To this, 100 µL of the initial fungal inoculum (described above) was added, for a final cell concentration of 2000 cells/mL. The microplates for C. albicans and C. neoformans were incubated at 37 °C for 24 and 72 h, respectively, before being analyzed. For A. niger, 100 µL of RPMI was added to all wells followed by serial 2-fold dilution of test samples or controls. Added last was 100 µL of the A. niger conidia solution (described above), for a final conidia concentration of 2000 conidia/mL. The A. niger microplates were incubated at room temperature for 7 days before being analyzed. All MICs were determined from triplicate measurements. RPMI was used as a negative control while 100% DMSO and 25 µg/mL amphotericin B were used as positive controls.
4.4. GC-MS
The essential oils were analyzed by GC-MS using a Shimadzu GCMS-QP2010 Ultra operated in the electron impact (EI) mode (electron energy = 70 eV), scan range = 40–400 atomic mass units, scan rate = 3.0 scans/s, and GC-MS solution software. The GC column was a ZB-5 fused silica capillary column with a (5% phenyl)-polymethylsiloxane stationary phase and a film thickness of 0.25 μm. The carrier gas was helium with a column head pressure of 552 kPa and flow rate of 1.37 mL/min. The injector temperature was 250 °C and the ion source temperature was 200 °C. The GC oven temperature was initially 50 °C followed by the temperature increased at a rate of 2 °C/min to 260 °C. A 5% w/v solution of the sample in CH2Cl2 was prepared and 0.1 μL was injected with a splitting mode (30:1). Identification of the oil components was based on their retention indices determined by reference to a homologous series of n-alkanes, and by comparison of their mass spectral fragmentation patterns with those reported in the literature [49] and stored in our in-house MS library.
4.5. ChemMineR
PubChem IDs for the potential active antifungal major constituents were uploaded to the ChemMineR server. Binning was performed using the default similarity cutoff of 0.4. The reported binning output was converted to a graphical figure manually.
4.6. Cedrol from Commercial Sources
Cedrol (TCI America, Portland, OR, USA) was obtained commercially. For a 1% solution, 10 µL of cedrol was added to 990 µL of DMSO. For the 0.23% solution, 23 µL of the 1% solution was added to 77 µL of DMSO.
5. Conclusions
With the need for new treatment options for fungal infections, essential oils offer a promising avenue for antifungal discovery and development. A growing essential oil knowledge base enables efforts to reduce the bottleneck of active component identification. Our investigation of bigger data approaches underscores this utility, showing that correlating efficacy to composition is possible to facilitate identification of active constituents. Currently a complement to, not substitute for, bioactive fractionation, this methodology holds great potential to expedite the identification of active constituents. As more and larger data sets become available, bigger data approaches will improve. (A curated database with relatively consistent data would greatly improve the performance of any such big/bigger data approaches.) The high susceptibility of C. neoformans to essential oil inhibition was also encouraging, serving to emphasize the priority for antifungal essential oil screening should move towards oils with compounds outside the common, generally active classes reported here. Overall, while essential oils remain a staple source for antimicrobial inhibitors, improving processes that efficiently identify lead candidates is of great benefit, expediting the next generation of drug discovery.
Acknowledgments
The authors thank Erin McClelland of Middle Tennessee State University for useful discussion and providing C. neoformans.
Supplementary Materials
The following are available online, Figure S1: GC-MS data; Figure S2: Structures of Potentially Active Major Constituents.
Appendix A
Table A1.
Antifungal Activity of Essential Oils. MIC data are shown for each essential oil against the three pathogenic fungi tested herein.
| Antifungal Activity of Essential Oils | ||||||
|---|---|---|---|---|---|---|
| Scientific Name | Common Name | Location | MIC (ppm) | |||
| A. nig. | C. alb. | C. neo. | ||||
| 1 | Abelmoschus moschatus | Ambrette Seed/Musk Mallow | India | 625 | 1250 | 625 |
| 2 | Achillea millefolium | Yarrow | Bulgaria | 625 | 625 | 310 |
| 3 | Agonis fragrans | Fragonia | Australia | 625 | 625 | 310 |
| 4 | Ajowan Trachyspermum ammi | Ajwain/Ajowan | Finland | 310 | 310 | 80 |
| 5 | Alpinia zerumbet | Shell Ginger/Getto | Japan | 625 | 625 | 160 |
| 6 | Amyris balsamifera | Amyris | Haiti | 160 | 625 | 40 |
| 7 | Aquilaria sinensis | Agarwood/Aloeswood | Vietnam | 160 | 625 | 80 |
| 8 | Artemisia dracunculus | Tarragon | France | 625 | 1250 | 625 |
| 9 | Artemisia pallens | Davana/Dhavanam | India | 625 | 625 | 310 |
| 10 | Artemisia vulgaris | Mugwort/Titepati | Nepal | 625 | 625 | 160 |
| 11 | Bursera graveolens | Palo Santo “Holy Wood” | Ecuador | 625 | 625 | 310 |
| 12 | Callitris intratropica | Blue Cypress | Australia | 160 | 625 | 80 |
| 13 | Carum carvi | Caraway | Finland | 625 | 625 | 310 |
| 14 | Cedrelopsis grevei Baill | Katrafay | Madagascar | 160 | 625 | 160 |
| 15 | Cedrus atlantica (1) | Atlas Cedarwood | Morocco | 625 | 625 | 160 |
| 16 | Cedrus atlantica (2) | Atlas Cedarwood | Morocco | 310 | 80 | 20 |
| 17 | Chamaecyparis obtusa | Hinoki Cypress | Japan | 625 | 625 | 80 |
| 18 | Chamaemelum nobile | Chamomile/Whig Plant | China | 625 | 1250 | 310 |
| 19 | Chrysopogon zizanioides | Wild Vetiver/Khus | India | 625 | 625 | 625 |
| 20 | Cinnamomum camphora | Camphor Tree/Ravintsara | China | 625 | 625 | 310 |
| 21 | Cinnamomum glaucescens | Sugandha Kokila | Nepal | 625 | 625 | 160 |
| 22 | Citrus aurantium | Neroli | Egypt | 625 | 1250 | 625 |
| 23 | Citrus aurantium ssp. amara | Petitgrain | Paraguay | 625 | 625 | 310 |
| 24 | Citrus bergamia | Bergamot | Italy | 625 | 625 | 625 |
| 25 | Citrus clementina | Clementine | France | 625 | 310 | 80 |
| 26 | Citrus depressa Hayata | Shiikuwasha | Japan | 625 | 625 | 310 |
| 27 | Citrus junos | Yuzu | Japan | 625 | 1250 | 310 |
| 28 | Citrus limon | Lemon | Italy | 625 | 625 | 625 |
| 29 | Citrus reticulata | Mandarin/Tangerine | Brazil | 625 | 625 | 625 |
| 30 | Comiphora myrrh | Myrrh | Ethiopia | 310 | 625 | 160 |
| 31 | Coriandrum sativum (1) | Coriander/Cilantro | Russia | 625 | 625 | 310 |
| 32 | Coriandrum sativum (2) | Coriander/Cilantro | Russia | 625 | 625 | 80 |
| 33 | Cupressus semiperviens | Cypress | Morocco | 625 | 625 | 310 |
| 34 | Curcuma zedoaria | Zedoary/White Turmeric | Nepal | 310 | 625 | 310 |
| 35 | Cymbopogon jwarancusa | Sotigrass | Nepal | 625 | 625 | 310 |
| 36 | Cymbopogon martinii var motia | Palmarosa | Nepal | 310 | 625 | 310 |
| 37 | Cymbopogon winterianus | Citronella/Lemon Grass | Indonesia | 310 | 625 | 160 |
| 38 | Elettaria cardamomum | Cardamom | Guatemala | 625 | 625 | 160 |
| 39 | Eugenia uniflora | Pitanga/Brazilian or Surinam Cherry | Brazil | 310 | 625 | 160 |
| 40 | Ferula galbaniflua | Galbanum Resin | Germany | 160 | 160 | 80 |
| 41 | Foenicul vulgare | Fennel | Bulgaria | 625 | 625 | 625 |
| 42 | Gautheria fragrantissima | Wintergreen | Nepal | 625 | 1250 | 625 |
| 43 | Geranium macrorrhizum | Zdravetz | Bulgaria | 310 | 625 | 160 |
| 44 | Hedychium coronarium (1) | White Garland or Ginger Lily | India | 625 | 625 | 160 |
| 45 | Hedychium coronarium (2) | White Garland or Ginger Lily | India | 625 | 625 | 310 |
| 46 | Helichrysum italicum | Helichrysum | Albania | 310 | 625 | 160 |
| 47 | Homalomena aromatica | Ghandi Root | India | 310 | 625 | 310 |
| 48 | Hyssopus officinalis | Hyssop | France | 625 | 310 | 160 |
| 49 | Illicium verum | Star Anise | Egypt | 625 | 625 | 625 |
| 50 | Juniperus communis | Juniper | Albania | 625 | 625 | 625 |
| 51 | Kunzea ambigua | White Kunzea | Australia | 310 | 625 | 160 |
| 52 | Kunzea ericoides | Kanuka/White Tea Tree | Australia | 310 | 625 | 80 |
| 53 | Laurus nobilis | Laurel Leaf/Bay Laurel | Austria | 625 | 625 | 310 |
| 54 | Lavandula hybrida | Lavandin | France | 625 | 625 | 625 |
| 55 | Litsea cubeba | May Chang | China | 310 | 625 | 160 |
| 56 | Matricaria chamomilla | Blue Chamomile | Nepal | 625 | 625 | 310 |
| 57 | Melaleuca cajuputi | Cajeput | Indonesia | 625 | 625 | 625 |
| 58 | Myrtle communis | Myrtle | Albania | 625 | 625 | 625 |
| 59 | Nardostachys jatamansi | Spikenard/Nard/Muskroot | Nepal | 625 | 625 | 160 |
| 60 | Nymphaea caerulea | Blue Lotus/Blue Water Lily | India | 625 | 1250 | 625 |
| 61 | Ocimum basilicum | Basil | Egypt | 625 | 625 | 310 |
| 62 | Ocimum sanctum | Holy Basil/Tulsi | India | 310 | 625 | 310 |
| 63 | Osmanthus fragrans | Osmanthus | China | 625 | 625 | 160 |
| 64 | Pelargonium graveolens | Geranium | Egypt | 310 | 625 | 310 |
| 65 | Picea mariana | Black Spruce | New Zealand | 310 | 625 | 80 |
| 66 | Piper nigrum | Black Pepper | Madagascar | 310 | 625 | 160 |
| 67 | Pogostemon cablin | Patchouli | Indonesia | 160 | 625 | 80 |
| 68 | Polianthes tuberosa | Tuberose | India | 625 | 625 | 310 |
| 69 | Pseudotsuga menziesii | Douglas Fir | New Zealand | 625 | 625 | 160 |
| 70 | Rhododendron anthopogon | Anthopogon | Nepal | 625 | 625 | 310 |
| 71 | Rhododendron anthopogon | Rhododendron | Nepal | 625 | 625 | 160 |
| 72 | Rosa damascena | Damask Rose/Rose of Castile | Bulgaria | 625 | 625 | 625 |
| 73 | Santalum spicatum | Sandalwood | Australia | 310 | 625 | 40 |
| 74 | Satureja montana | Winter Savory | Turkey | 160 | 310 | 160 |
| 75 | Tagetes minuta | Tagetes | Madagascar | 625 | 1250 | 625 |
| 76 | Tanacetum annuum | Tansy Oil/Blue Tansy | Morocco | 625 | 625 | 160 |
| 77 | Thumus vulgaris | Thyme | Turkey | 310 | 625 | 625 |
| 78 | Turnera diffusa | Damiana | Mexico | 625 | 625 | 80 |
| 79 | Valeriana officinalis | Valerian Root | Nepal | 625 | 625 | 310 |
| 80 | Vitex agnuscastus | Chasteberry/Vitex | Albania | 625 | 625 | 80 |
| 81 | Zingiber cassumunar | Plai | Indonesia | 310 | 625 | 310 |
| 82 | Zingiber officinale | Ginger | Madagascar | 625 | 1250 | 310 |
Note: The minimum inhibitory concentrations (MIC) for the amphotericin B control for A. niger and C. albicans was 0.78 µg/mL and for C. neoformans was 0.39 µg/mL. For the DMSO vehicle control, A. niger and C. neoformans were inhibited at 625 ppm whereas C. albicans was inhibited at 1250 ppm.
Author Contributions
Conceptualization, C.N.P., H.M., and R.L.M.; methodology, C.N.P., P.S., H.M. and J.A.M.; investigation and formal analysis, C.N.P., P.S., and H.M.; resources, P.S. and R.L.M.; data curation, C.N.P., P.S. and R.L.M.; writing—original draft preparation, C.N.P. and R.L.M.; writing—review and editing, J.A.M. and H.M.; project administration, R.L.M.; funding acquisition, R.L.M.
Funding
C.N.P. was supported in part by the National Institute of General Medical Sciences of the National Institutes of Health under award number R15GM119052 to R.L.M. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors until stocks are consumed.
References
- 1.Brown G.D., Denning D.W., Gow N.A., Levitz S.M., Netea M.G., White T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012;4:165rv113. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 2.Campoy S., Adrio J.L. Antifungals. Biochem. Pharmacol. 2017;133:86–96. doi: 10.1016/j.bcp.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Pianalto K.M., Alspaugh J.A. New horizons in antifungal therapy. J. Fungi. 2016;2:26. doi: 10.3390/jof2040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diekema D., Arbefeville S., Boyken L., Kroeger J., Pfaller M. The changing epidemiology of healthcare-associated candidemia over three decades. Diagn. Microbiol. Infect. Dis. 2012;73:45–48. doi: 10.1016/j.diagmicrobio.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Guinea J., Torres-Narbona M., Gijon P., Munoz P., Pozo F., Pelaez T., de Miguel J., Bouza E. Pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: Incidence, risk factors, and outcome. Clin. Microbiol. Infect. 2010;16:870–877. doi: 10.1111/j.1469-0691.2009.03015.x. [DOI] [PubMed] [Google Scholar]
- 6.Marr K.A., Schlamm H.T., Herbrecht R., Rottinghaus S.T., Bow E.J., Cornely O.A., Heinz W.J., Jagannatha S., Koh L.P., Kontoyiannis D.P., et al. Combination antifungal therapy for invasive aspergillosis: A randomized trial. Ann. Intern. Med. 2015;162:81–89. doi: 10.7326/M13-2508. [DOI] [PubMed] [Google Scholar]
- 7.Park B.J., Wannemuehler K.A., Marston B.J., Govender N., Pappas P.G., Chiller T.M. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 8.Pfaller M.A., Diekema D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrillo-Munoz A.J., Finquelievich J., Tur-Tur C., Eraso E., Jauregizar N., Quindos G., Giusiano G. Combination antifungal therapy: A strategy for the management of invasive fungal infections. Revista Espanola de Quimioterapia. 2014;27:141–158. [PubMed] [Google Scholar]
- 10.Dignani M.C. Epidemiology of invasive fungal diseases on the basis of autopsy reports. F1000prime Rep. 2014;6:81. doi: 10.12703/P6-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfaller M.A., Diekema D.J. Epidemiology of invasive mycoses in North America. Crit. Rev. Microbiol. 2010;36:1–53. doi: 10.3109/10408410903241444. [DOI] [PubMed] [Google Scholar]
- 12.Schelenz S., Barnes R.A., Barton R.C., Cleverley J.R., Lucas S.B., Kibbler C.C., Denning D.W. British Society for Medical Mycology best practice recommendations for the diagnosis of serious fungal diseases. Lancet Infect. Dis. 2015;15:461–474. doi: 10.1016/S1473-3099(15)70006-X. [DOI] [PubMed] [Google Scholar]
- 13.Balouiri M., Sadiki M., Ibnsouda S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016;6:71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Favre-Godal Q., Queiroz E.F., Wolfender J.L. Latest developments in assessing antifungal activity using TLC-bioautography: A review. J. AOAC Int. 2013;96:1175–1188. doi: 10.5740/jaoacint.SGEFavre-Godal. [DOI] [PubMed] [Google Scholar]
- 15.Mokoka T.A., McGaw L.J., Eloff J.N. Antifungal efficacy of ten selected South African plant species against Cryptococcus neoformans. Pharm. Biol. 2010;48:397–404. doi: 10.3109/13880200903150393. [DOI] [PubMed] [Google Scholar]
- 16.Swamy M.K., Akhtar M.S., Sinniah U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid. Based Complement. Altern. Med. 2016;2016:3012462. doi: 10.1155/2016/3012462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bansod S., Rai M. Antifungal Activity of essential oils from Indian medicinal plants against human pathogenic Aspergillus fumigatus and A. niger. World J. Med. Sci. 2008;3.2:81–88. [Google Scholar]
- 18.Satyal P., Murray B.L., McFeeters R.L., Setzer W.N. Essential oil characterization of Thymus vulgaris from various geographical locations. Foods. 2016;5:70. doi: 10.3390/foods5040070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turek C., Stintzing F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013;12:40–53. doi: 10.1111/1541-4337.12006. [DOI] [Google Scholar]
- 20.Hussain A.I., Anwar F., Hussain Sherazi S.T., Przybylski R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008;108:986–995. doi: 10.1016/j.foodchem.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Raut J.S., Karuppayil S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014;250:250–264. doi: 10.1016/j.indcrop.2014.05.055. [DOI] [Google Scholar]
- 22.Cos P., Vlietinck A.J., Berghe D.V., Maes L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006;106:290–302. doi: 10.1016/j.jep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 24.Backman T.W., Cao Y., Girke T. ChemMine tools: An online service for analyzing and clustering small molecules. Nucleic Acids Res. 2011;39:486–491. doi: 10.1093/nar/gkr320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heghes S.C., Vostinaru O., Rus L.M., Mogosan C., Iuga C.A., Filip L. Antispasmodic effect of essential oils and their constituents: A review. Molecules. 2019;24:1675. doi: 10.3390/molecules24091675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salehi B., Valussi M., Morais-Braga M.F.B., Carneiro J.N.P., Leal A., Coutinho H.D.M., Vitalini S., Kregiel D., Antolak H., Sharifi-Rad M., et al. Tagetes spp. essential oils and other extracts: Chemical characterization and biological activity. Molecules. 2018;23:2847. doi: 10.3390/molecules23112847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winska K., Maczka W., Lyczko J., Grabarczyk M., Czubaszek A., Szumny A. Essential oils as antimicrobial agents-myth or real alternative? Molecules. 2019;24:2130. doi: 10.3390/molecules24112130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powers C.N., Osier J.L., McFeeters R.L., Brazell C.B., Olsen E.L., Moriarity D.M., Satyal P., Setzer W.N. antifungal and cytotoxic activities of sixty commercially-available essential oils. Molecules. 2018;23:1549. doi: 10.3390/molecules23071549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng S.S., Lin C.Y., Gu H.J., Chang S.T. Antifungal activities and chemical composition of wood and leaf essential oils from Cunninghamia konishii. J. Wood Chem. Technol. 2011;31:204–217. doi: 10.1080/02773813.2010.515049. [DOI] [Google Scholar]
- 30.Hammer K.A., Carson C.F., Riley T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999;86:985–990. doi: 10.1046/j.1365-2672.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 31.Janssen A.M., Scheffer J.J., Baerheim Svendsen A. Antimicrobial activity of essential oils: A 1976–1986 literature review. Aspects of the test methods. Planta Med. 1987;53:395–398. doi: 10.1055/s-2006-962755. [DOI] [PubMed] [Google Scholar]
- 32.Serra E., Hidalgo-Bastida L.A., Verran J., Williams D., Malic S. Antifungal activity of commercial essential oils and biocides against Candida albicans. Pathogens. 2018;7:15. doi: 10.3390/pathogens7010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lahlou M. Methods to study the phytochemistry and bioactivity of essential oils. Phytother. Res. 2004;18:435–448. doi: 10.1002/ptr.1465. [DOI] [PubMed] [Google Scholar]
- 34.Negri M., Salci T.P., Shinobu-Mesquita C.S., Capoci I.R., Svidzinski T.I., Kioshima E.S. Early state research on antifungal natural products. Molecules. 2014;19:2925–2956. doi: 10.3390/molecules19032925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vieira P.R.N., de Morais S.M., Bezerra F.H.Q., Ferreira P.A.T., Oliveira I.R., Silva M.G.V. Chemical composition and antifungal activity of essential oils from Ocimum species. Ind. Crops Prod. 2014;55:267–271. doi: 10.1016/j.indcrop.2014.02.032. [DOI] [Google Scholar]
- 36.Lago J.H., Souza E.D., Mariane B., Pascon R., Vallim M.A., Martins R.C., Baroli A.A., Carvalho B.A., Soares M.G., dos Santos R.T., et al. Chemical and biological evaluation of essential oils from two species of Myrtaceae—Eugenia uniflora L. and Plinia trunciflora (O. Berg) Kausel. Molecules. 2011;16:9827–9837. doi: 10.3390/molecules16129827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinto E., Goncalves M.J., Cavaleiro C., Salgueiro L. Antifungal activity of Thapsia villosa essential oil against Candida, Cryptococcus, Malassezia, Aspergillus and Dermatophyte species. Molecules. 2017;22:1595. doi: 10.3390/molecules22101595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Derwich E., Benziane Z., Boukir A. Chemical composition and in vitro antibacterial activity of the essential oil of Cedrus atlantica. Int. J. Agricult. Biol. 2010;12:381–385. [Google Scholar]
- 39.Fidah A., Salhi N., Rahouti M., Kabouchi B., Ziani M., Aberchane M., Famiri A. Natural durability of Cedrus atlantica wood related to the bioactivity of its essential oil against wood decaying fungi. Maderas Ciencia y Tecnología. 2016;18:49. doi: 10.4067/S0718-221X2016005000049. [DOI] [Google Scholar]
- 40.Lamiri A.S., Lhaloui B.B., Berrada M. Insecticidal Effects of essential oils against hessian fly, mayetiola destructor (say) Field Crops Res. 2001;71:9–15. doi: 10.1016/S0378-4290(01)00139-3. [DOI] [Google Scholar]
- 41.Silva F., Ferreira S., Duarte A., Mendonca D.I., Domingues F.C. Antifungal activity of Coriandrum sativum essential oil, its mode of action against Candida species and potential synergism with amphotericin B. Phytomedicine. 2011;19:42–47. doi: 10.1016/j.phymed.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 42.Begnami A.F., Duarte M.C.T., Furletti V., Rehder V.L.G. Antimicrobial potential of Coriandrum Sativum L. against different Candida species in vitro. Food Chem. 2010;118:74–77. doi: 10.1016/j.foodchem.2009.04.089. [DOI] [Google Scholar]
- 43.Monzote L., Scull R., Cos P., Setzer W.N. Essential oil from Piper aduncum: Chemical analysis, antimicrobial assessment, and literature review. Medicines. 2017;4:49. doi: 10.3390/medicines4030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elaissi A., Rouis Z., Mabrouk S., Salah K.B.H., Aouni M., Khouja M.L., Farhat F., Chemli R., Harzallah-Skhiri F. Correlation between chemical composition and antibacterial activity of essential oils from fifteen Eucalyptus species growing in the Korbous and Jbel Abderrahman Arboreta (North East Tunisia) Molecules. 2012;17:3044–3057. doi: 10.3390/molecules17033044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chouhan S., Sharma K., Guleria S. Antimicrobial activity of some essential oils-present status and future perspectives. Medicines. 2017;4:20. doi: 10.3390/medicines4030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Isman M.B., Wilson J.A., Bradbury R. Insecticidal activities of commercial rosemary oils (Rosmarinus officinalis) against Larvae of Pseudaletia unipuncta and Trichoplusia Ni in relation to their chemical compositions. Pharm. Biol. 2008;46:82–87. doi: 10.1080/13880200701734661. [DOI] [Google Scholar]
- 47.Mundy L., Pendry B., Rahman M. Antimicrobial resistance and synergy in herbal medicine. J. Herb. Med. 2016;6:53–58. doi: 10.1016/j.hermed.2016.03.001. [DOI] [Google Scholar]
- 48.Araujo R., Rodrigues A.G., Pina-Vaz C. A fast, practical and reproducible procedure for the standardization of the cell density of an Aspergillus suspension. J. Med. Microbiol. 2004;53:783–786. doi: 10.1099/jmm.0.05425-0. [DOI] [PubMed] [Google Scholar]
- 49.Satyal P., Jones T.H., Lopez E.M., McFeeters R.L., Ali N.A., Mansi I., Al-Kaf A.G., Setzer W.N. Chemotypic characterization and biological activity of Rosmarinus officinalis. Foods. 2017;6:20. doi: 10.3390/foods6030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.