Abstract
A multi-residue method for the determination of 107 pesticide residues in wolfberry has been developed and validated. Similar pretreatment approaches were compared, and the linearity, matrix effect, analysis limits, precision, stability and accuracy were validated, which verifies the satisfactory performance of this new method. The LODs and LOQs were in the range of 0.14–1.91 µg/kg and 0.46–6.37 µg/kg, respectively. The recovery of analytes at three fortification levels (10 µg/kg, 50 µg/kg, 100 µg/kg) ranged from 63.3–123.0%, 72.0–118.6% and 67.0–118.3%, respectively, with relative standard deviations (RSDs) below 15.0%. The proposed method was applied to the analysis of fifty wolfberry samples collected from supermarkets, pharmacies and farmers’ markets in different cities of Shandong Province. One hundred percent of the samples analyzed included at least one pesticide, and a total of 26 pesticide residues was detected in fifty samples, which mainly were insecticides and bactericide. Several pesticides with higher detection rates were 96% for acetamiprid, 82% for imidacloprid, 54% for thiophanate-methyl, 50% for blasticidin-S, 42% for carbendazim, 42% for tebuconazole and 36% for difenoconazole in wolfberry samples. This study proved the adaptability of the developed method to the detection of multiple pesticide residues in wolfberry and provided basis for the research on the risks to wolfberry health.
Keywords: multi-residue, pesticide, wolfberry, Sin-QuEChERS Nano, UPLC/MS/MS
1. Introduction
Lycium barbarum, belonging to the Solanaceous family, also called Gouqizi in Chinese or wolfberry in English, has been widely cultivated in the northwest of China and the Mediterranean region [1,2]. Wolfberry is a kind of traditional Chinese medicine (TCM) and food homologous plant with health-protection functions. It has been widely used for the prevention and treatment of various diseases for more than thousands of years [3,4]. In recent years, wolfberry, a functional dietary supplement with nutritive value, has become increasingly prevalent in the Western countries and regions [5]. Wolfberry is rich in minerals, proteins, polysaccharides, amino acids, carotenoids, flavonoids and so on [6]. This characteristic makes it vulnerable to diseases and insect pests during its growth. Rust mite, gall mite, psylla, mealybug, aphid, anthracnose and powdery mildew are often encountered at the same time or alternately. As chemical agents for diseases and insect pests control as well as plant growth regulation, pesticides play an important role in the growth process and standardized management of wolfberry. Traditional Chinese medicine, as a gem of the Chinese nation, has a unique concept of treating diseases, which has been by degrees accepted both at home and abroad [7]. With the rapid growth of import and export trade of Chinese herbal medicines, China has developed into the largest supplier of wolfberry products [8,9,10]. Artificial planting has become an inevitable trend due to the large demand and the lack of wild wolfberry resources. Consequently, the issue of pesticide residues which are inevitably caused by the extensive use of pesticides in plant cultivation [11], has attracted wide attention of the international community [12,13,14]. Organophosphorus and pyrethroid pesticides are frequently applied to improve the quality and yield during the course of the growth of wolfberry. At present, there are more than 2000 kinds of pesticides registered in the world, of which about 500 are commonly used, while new pesticides are also being developed and applied. Biodegradation, photolysis, chemical oxidation and plant metabolism will decompose most pesticides, but there will still be a minor part of pesticide residues in plants and soil [15]. This small fraction of pesticide not only has serious adverse effects on the quality of wolfberry, but also gives rise to tremendous potential hazard to human health [16]. Simultaneously, there are increasing requirements and new challenges for pesticide residue detection technology [16,17].
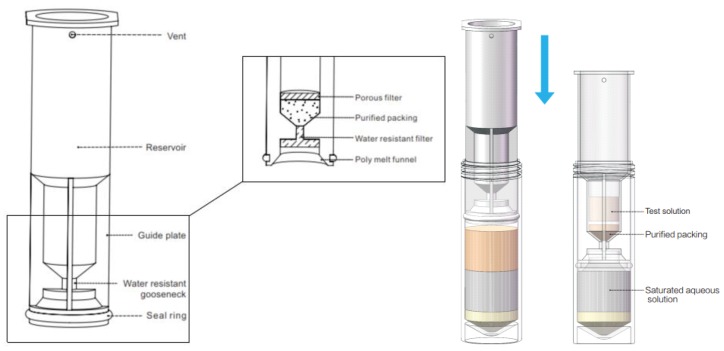
Pesticide residue analysis is a complex trace analysis technology in which the pretreatment method plays a vital role in the detection process. The traditional extraction and purification technology cannot meet the requirements of modern pesticide residue analysis, especially under the condition that not only is the concentration of food pollutants getting lower [18,19], but also the matrices are becoming more and more complex [20]. Appropriate pretreatment technology can improve the sensitivity, detection range, precision and accuracy of the detection [21,22,23,24]. In recent years, mass spectrometry (MS) technology has been developing continuously, among which LC coupled with tandem MS (LC-MS/MS) is an effective and sensitive method for the detection of pesticide residues [25,26]. Common sample pretreatment techniques including liquid-liquid extraction (LLE) [27], solid phase extraction (SPE) [28,29], microwave-assisted extraction (MAE) [29,30], supercritical fluid extraction (SFE) [31], accelerated solvent extraction (ASE) [32,33,34], magnetic solid phase extraction (MSPE) [35,36], gel permeation chromatography (GPC) [37,38,39], as well as the QuEChERS method (quick, easy, cheap, effective, rugged and safe) which was firstly published by Anastassiades et al. in 2003 [40]. Nowadays, the QuEChERS protocol has been widely used for the determination of pesticide residues due to its significant advantages [41]. After the continuous improvement, two buffer versions, namely AOAC Official Method 2007.01 (acetate buffering) [42] and European Committee for Standardization (CEN) Standard Method EN 15,662 (citrate buffering) [43], were gradually formed on the basis of QuEChERS technology and widely utilized in sample pretreatment process prior to chromatographic analysis. Multi-pesticide residue analysis mostly used modified QuEChERS method [44,45,46]. Multi-walled carbon nanotubes is a new type of pretreatment adsorption material, which can effectively remove pigments and hydrophobic substances by combining with target molecules as non-covalent interaction. In recent years, it has attracted great attention in the purification process with the advantages of good chemical stability, high surface area, strong adsorption capacity, wide application range of pH value and low cost [47,48,49]. Multi-walled carbon nanotubes (MWCNTs) [50,51,52] and magnetic amino (m-MWCNTs-NH2) [53] or amino (MWCNTs-NH2) [20] modified multi-walled carbon nanotubes are extensively used as sorbent materials for dispersed solid phase extraction in the detection of multi-pesticide residues. Sin-QuEChERS Nano purification column is a new sample preparation product based on the QuEChERS method. The clean-up column with sorbents as multi-walled carbon nanotubes and PSA packed in has good purification effects of plant pigments, lipids, some sugars, sterols, phenol, wax, alkaline interferers, organic acids, etc. with the function of dehydration. With the extraction and purification combined into one-step, the loss of target components via solvent transfer can be avoided, resulting in fewer interferences during determination and longer instrument maintenance periods, meanwhile the sample preparation time is greatly saved. After the organic extract enters the liquid storage tank during the purification, the liquid level rises, and the air in the liquid storage tank is discharged through the vent hole. The Sin-QuEChERS Nano purification column is reversely guided in the downward process of the centrifuge tube, and the purified extract is stored in the liquid storage tank, then the built-in water blocking filter is bonded to the strong hydrophobic water blocking group to ensure that the aqueous solution does not contact with the purification packing and reservoir. The seal is used for sealing between the cylinder and the centrifuge tube.
In this study, in order to monitor the quality of wolfberry in China, a method with acetate-buffered salt extraction and Sin-QuEChERS nanocolumn purification coupled with ultra performance liquid chromatography tandem mass spectrometry was developed for the determination of pesticide residues in wolfberry samples. Different versions of buffer salts for sample extraction and several SPE cartridges for clean-up were compared. The linearity, matrix effect, analysis limits, precision, stability and accuracy were validated in detail. Finally, the method was applied to determinate 50 wolfberry samples to validate the feasibility.
2. Results
2.1. Optimization and Comparison of the Extraction Procedure
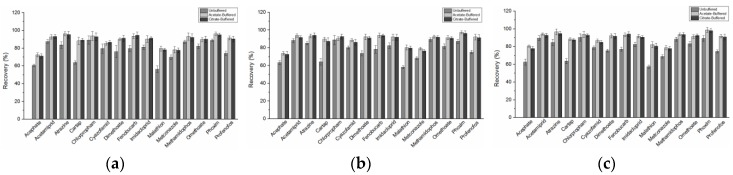
In this study, three versions of buffer salts were compared, and the extraction efficiency was evaluated using three levels (10, 25, 50 µg/kg) spiked recovery with five parallel samples. The results, were in accordance with previous studies [54], where most pesticides except for pH-sensitive ones gave excellent results when extracted with three different versions of buffer salts. As shown in Figure 1, the QuEChERS version using acetate buffering or citrate buffering more often gave higher recoveries compared with the unbuffered method for pH-dependent pesticides. For acephate, dimethoate, fenobucarb, methamidophos, omethoate and profenofos that are unstable in alkaline medium, it is easier to obtain higher recovery in buffer salt system with the pH of the matrix was maintained between 5.0 and 5.5 throughout the experiment. In addition to, as Figure 1 results demonstrate, the recovery rate of pesticides in acetate buffer version (AOAC) is slightly higher than that in citrate buffer version (CEN), but the difference is not obvious. Further, referring to previous research results [42,54], the ionization efficiency of acetonitrile and the ability of the matrix itself to interfere with acidity and alkalinity is enhanced in the acetate-buffered version, so subsequent experiments used acetate buffer version.
Figure 1.
Average recoveries of 15 pesticides using the three different versions of QuEChERS (n = 5), (a) 10 µg/kg; (b) 25 µg/kg; (c) 50 µg/kg.
2.2. Optimization and Comparison of the Clean-up Procedure
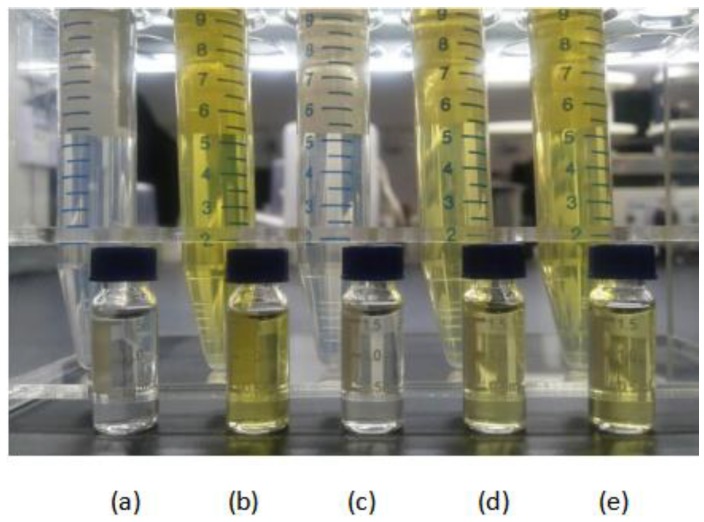
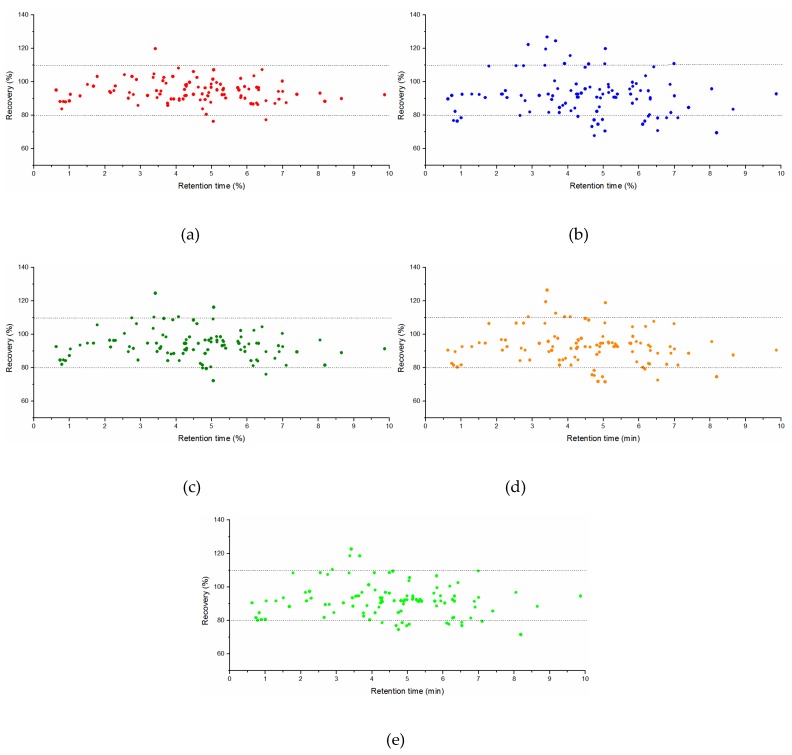
Five kinds of SPE cartridges were selected for optimization and comparison of the purification procedure, and were activated by 4 mL methanol before use. Extraction solution obtained as described in Section 3.3 was passed through the SPE cartridges and 4 mL of eluent was collected for the next process which involved treatment the same as the filtrate in Section 3.3. As the Figure 2 results demonstrate vividly, compared with the other three SPE cartridges, the solution purified by the Sin-QuEChERS Nano and HyperSep NH2 catridges showed a colorless and transparent state. PSA was used for adsorbing pigments, organic acids, some carbohydrates and fatty acids while C18 and MWCNTs absorbed the pigments. The Sin-QuEChERS Nano cartridge is packed with a certain amount of MWCNTs in addition to PSA, which can better remove carotene, lutein and other colored interferents in wolfberry with less co-extracted compounds. Recoveries with the different purification processes are displayed in Figure 3, where most pesticides displayed higher recoveries for the Sin-QuEChERS Nano cartridge in comparison with other SPE columns, while all pesticides also gave satisfactory recoveries when purified by HyperSep NH2 columns.
Figure 2.
Colour comparison of extracts purified by different SPE catridges. (a) Sin-QuEChERS Nano; (b) SampliQ Florisil; (c) HyperSep NH2; (d) HyperSep C18; (e) ProElut GLASS PSA.
Figure 3.
Comparison for recoveries of samples with different purification process. (a) Sin-QuEChERS Nano; (b) SampliQ Florisil; (c) HyperSep NH2; (d) HyperSep C18; (e) ProElut GLASS PSA.
Some pesticides gave unsatisfactory recoveries (>120% or <70%) with the other three SPE cartridges owing to matrix enhancement or inhibition effects caused by matrix co-extracted compounds. Therefore, considering the recoveries, the purification effect and the simplicity of operation, Sin-QuEChERS Nano cartridges were considered the optimal choice for purification of wolfberry.
2.3. Method Validation
2.3.1. Linearity, LOD and LOQ
The linearity was assessed by calibration curves at eight concentration levels, prepared by diluting the mixed stock solution. As shown in Table 1, the linear range of most pesticides was 5–1000 µg/kg with eight points, except for acetochlor, bendiocarb, clothianidin, cyproconazole, flutriafol, metalaxyl, propham were 2–1000 µg/kg, butralin, diethofencarb, fenbuconazole, fosthiazate, isocarbophos, phorate-sulfoxide, propiconazole were 2–500 µg/kg, and cartap, iprodione, phenthoate, phorate, thifluzamide, triflumizole were 10–1000 µg/kg. The results indicated that all targets obtained satisfactory linearity with the correlation coefficient (R2) of the regression curve was better than 0.9900.
Table 1.
Liquid chromatography tandem mass spectrometry analysis parameters, LODs, LOQs, Linear range and Calibration Curve Coefficients (R2) of 107 pesticides.
| No. | Compound | Elemental composition |
Precursor ion | Retention Time (min) | Precursor ions (m/z) |
Products (m/z) |
Cone voltage (V) | Collision energy (qv) |
LOD (µg/kg) |
LOQ (µg/kg) |
Linear range (µg/kg) |
R2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Abamectin | C48H72O14 | [M + NH4]+ | 8.65 | 890.7 | 305.1 | 18 | 22 | 1.48 | 3.94 | 5–1000 | 0.9998 |
| 890.7 | 567.2 | 18 | 10 | |||||||||
| 2 | Acephate | C4H10NO3PS | [M + H]+ | 0.79 | 184.0 | 49.2 | 10 | 16 | 1.17 | 3.91 | 5–1000 | 0.9981 |
| 3 | Acetamiprid | C10H11ClN4 | [M + H]+ | 2.29 | 223.0 | 56.1 | 22 | 10 | 0.46 | 1.54 | 5–1000 | 0.9999 |
| 223.0 | 126.0 | 22 | 12 | |||||||||
| 4 | Acetochlor | C14H20ClNO2 | [M + H]+ | 5.14 | 270.0 | 148.1 | 15 | 20 | 0.27 | 0.90 | 2–1000 | 0.9995 |
| 270.0 | 224.0 | 15 | 10 | |||||||||
| 5 | Aldicarb | C7H14N2O2S | [M + Na]+ | 2.80 | 213.1 | 89.1 | 25 | 16 | 0.45 | 1.50 | 5–1000 | 0.9697 |
| 213.1 | 116.1 | 25 | 11 | |||||||||
| 6 | Aldicarb-Sulfone | C7H14N2O4S | [M + H]+ | 1.30 | 223.0 | 86.0 | 25 | 10 | 0.72 | 2.39 | 5–1000 | 0.9990 |
| 223.0 | 148.0 | 25 | 8 | |||||||||
| 7 | Aldicarb-Sulfoxide | C7H14N2O3S | [M + H]+ | 0.89 | 207.1 | 89.0 | 10 | 14 | 0.68 | 2.27 | 5–1000 | 0.9911 |
| 207.1 | 132.0 | 10 | 5 | |||||||||
| 8 | Ametryn | C9H17N5S | [M + H]+ | 2.89 | 228.1 | 68.1 | 25 | 25 | 0.50 | 1.66 | 5–1000 | 0.9998 |
| 228.1 | 186.0 | 25 | 10 | |||||||||
| 9 | Atrazine | C8H14ClN5 | [M + H]+ | 3.66 | 216.1 | 96.1 | 22 | 18 | 0.90 | 3.00 | 5–1000 | 0.9996 |
| 216.1 | 174.1 | 22 | 14 | |||||||||
| 10 | Azoxystrobin | C22H17N3O5 | [M + H]+ | 4.63 | 404.0 | 329.0 | 25 | 25 | 0.33 | 1.08 | 5–1000 | 0.9995 |
| 404.0 | 372.0 | 25 | 12 | |||||||||
| 11 | Bendiocarb | C11H13NO4 | [M + H]+ | 3.38 | 224.1 | 109.0 | 17 | 18 | 0.24 | 0.79 | 2–1000 | 0.9987 |
| 224.1 | 167.0 | 17 | 8 | |||||||||
| 12 | Blasticidin-S | C17H26N8O5 | [M + H]+ | 2.76 | 423.5 | 189.3 | 18 | 12 | 0.98 | 3.27 | 5–1000 | 0.9994 |
| 423.5 | 210.6 | 18 | 12 | |||||||||
| 13 | Boscalid | C18H12Cl2N2O | [M − H]− | 4.75 | 342.9 | 139.9 | 32 | 20 | 0.60 | 2.01 | 5–1000 | 0.9994 |
| 342.9 | 307.0 | 32 | 20 | |||||||||
| 14 | Bupirimate | C13H24N4O3S | [M + H]+ | 5.06 | 317.0 | 108.0 | 31 | 28 | 0.48 | 1.61 | 5–1000 | 0.9995 |
| 317.0 | 166.0 | 31 | 28 | |||||||||
| 15 | Buprofezin | C16H23N3OS | [M + H]+ | 5.36 | 306.1 | 116.0 | 20 | 10 | 0.82 | 2.74 | 5–1000 | 0.9997 |
| 306.1 | 201.0 | 20 | 10 | |||||||||
| 16 | Butralin | C14H21N3O4 | [M + H]+ | 8.19 | 296.3 | 222.2 | 20 | 20 | 0.21 | 0.70 | 2–500 | 0.9997 |
| 296.3 | 240.3 | 20 | 17 | |||||||||
| 17 | Carbaryl | C12H11NO2 | [M + H]+ | 3.58 | 202.0 | 117.0 | 19 | 28 | 0.68 | 2.26 | 5–1000 | 0.9998 |
| 202.0 | 145.0 | 19 | 22 | |||||||||
| 18 | Carbendazim | C9H9N3O2 | [M + H]+ | 6.32 | 192.0 | 132.0 | 17 | 22 | 1.11 | 3.70 | 5–1000 | 0.9996 |
| 192.0 | 160.0 | 17 | 22 | |||||||||
| 19 | Carbofuran | C12H15NO3 | [M + H]+ | 3.42 | 222.1 | 123.0 | 22 | 12 | 0.71 | 2.38 | 5–1000 | 0.9991 |
| 222.1 | 165.1 | 22 | 12 | |||||||||
| 20 | Carbosulfan | C20H32N2O3S | [M + H]+ | 4.27 | 381.1 | 117.9 | 24 | 18 | 0.96 | 3.20 | 5–1000 | 0.9987 |
| 381.1 | 159.9 | 24 | 18 | |||||||||
| 21 | Cartap | C7H15N3O2S2 | [M + H]+ | 0.63 | 238.0 | 61.1 | 25 | 22 | 1.57 | 5.22 | 10–1000 | 0.9999 |
| 238.0 | 105.0 | 25 | 16 | |||||||||
| 22 | Chlorantraniliprole | C18H14BRCl2N5O2 | [M + H]+ | 4.20 | 484.1 | 286.1 | 25 | 20 | 0.42 | 1.39 | 5–1000 | 0.9998 |
| 484.1 | 453.0 | 25 | 18 | |||||||||
| 23 | Chlorfenvinphos | C12H14Cl3O4P | [M − H]− | 5.30 | 358.9 | 99.0 | 20 | 30 | 0.96 | 3.22 | 5–1000 | 0.9989 |
| 358.9 | 155.0 | 20 | 12 | |||||||||
| 24 | Chlorpropham | C10H12ClNO2 | [M + H]+ | 4.85 | 214.6 | 126.0 | 20 | 15 | 0.72 | 2.41 | 5–1000 | 0.9983 |
| 214.6 | 154.0 | 20 | 10 | |||||||||
| 25 | Clodinafop-propargyl | C17H13ClFNO4 | [M + H]+ | 6.78 | 350.0 | 266.0 | 22 | 20 | 0.86 | 2.87 | 5–1000 | 0.9997 |
| 350.0 | 91.0 | 22 | 18 | |||||||||
| 26 | Clothianidin | C6H8ClN5O2S | [M + H]+ | 2.16 | 249.9 | 132.0 | 22 | 15 | 0.22 | 0.74 | 2–1000 | 0.9995 |
| 249.9 | 169.0 | 22 | 10 | |||||||||
| 27 | Cyantraniliprole | C19H14BrClN6O2 | [M + H]+ | 3.77 | 475.0 | 286.0 | 25 | 15 | 0.31 | 1.03 | 5–1000 | 0.9995 |
| 475.0 | 444.0 | 25 | 15 | |||||||||
| 28 | Cyazofamid | C13H13ClN4O2S | [M + H]+ | 5.34 | 325.0 | 107.9 | 20 | 20 | 0.36 | 1.19 | 5–1000 | 0.9991 |
| 325.0 | 261.0 | 20 | 10 | |||||||||
| 29 | Cymoxanil | C7H10N4O3 | [M + H]+ | 2.55 | 199.0 | 111.0 | 15 | 18 | 0.86 | 2.87 | 5–1000 | 0.9989 |
| 199.0 | 128.0 | 15 | 8 | |||||||||
| 30 | Cyproconazole | C15H18ClN3O | [M + H]+ | 5.32 | 292.2 | 70.2 | 30 | 18 | 0.22 | 0.73 | 2–1000 | 0.9996 |
| 292.2 | 125.1 | 30 | 24 | |||||||||
| 31 | Cyprodinil | C14H15N3 | [M + H]+ | 4.09 | 226.0 | 93.0 | 30 | 26 | 0.84 | 2.81 | 5–1000 | 0.9992 |
| 226.0 | 108.0 | 30 | 20 | |||||||||
| 32 | Cyromazine | C6H10N6 | [M + H]+ | 2.66 | 167.2 | 60.0 | 28 | 20 | 1.03 | 3.43 | 5–1000 | 0.9992 |
| 167.2 | 108.0 | 28 | 20 | |||||||||
| 33 | Diazinon | C12H21N2O3PS | [M + H]+ | 6.99 | 305.1 | 96.9 | 30 | 35 | 0.63 | 2.10 | 5–1000 | 0.9990 |
| 305.1 | 169.0 | 30 | 22 | |||||||||
| 34 | Diethofencarb | C14H21NO4 | [M + H]+ | 4.49 | 268.3 | 124.0 | 19 | 40 | 0.26 | 0.86 | 2–500 | 0.9989 |
| 268.3 | 226.0 | 19 | 10 | |||||||||
| 35 | Difenoconazole | C19H17Cl2N3O3 | [M + H]+ | 5.40 | 406.0 | 111.1 | 20 | 60 | 0.60 | 1.99 | 5–1000 | 0.9994 |
| 406.0 | 251.1 | 20 | 25 | |||||||||
| 36 | Dimethoate | C5H12NO3PS2 | [M + H]+ | 2.25 | 230.1 | 125.0 | 22 | 10 | 0.40 | 1.34 | 5–1000 | 0.9991 |
| 230.1 | 199.0 | 22 | 6 | |||||||||
| 37 | Dimethomorph | C21H22ClNO4 | [M + H]+ | 4.25 | 388.1 | 165.0 | 35 | 30 | 0.20 | 0.65 | 5–1000 | 0.9994 |
| 388.1 | 300.9 | 35 | 20 | |||||||||
| 38 | Diniconazole | C15H17Cl2N3O | [M + H]+ | 6.30 | 326.1 | 70.2 | 37 | 25 | 0.71 | 2.38 | 5–1000 | 0.9994 |
| 326.1 | 159.0 | 37 | 34 | |||||||||
| 39 | Dinotefuran | C7H14N4O3 | [M + H]+ | 1.00 | 203.0 | 129.0 | 13 | 12 | 0.86 | 2.88 | 5–1000 | 0.9983 |
| 203.0 | 156.9 | 13 | 6 | |||||||||
| 40 | Diuron | C9H10Cl2N2O | [M + H]+ | 3.76 | 233.0 | 46.3 | 28 | 14 | 0.27 | 0.91 | 5–1000 | 0.9974 |
| 233.0 | 72.1 | 28 | 18 | |||||||||
| 41 | Epoxiconazole | C17H13ClFN3O | [M + H]+ | 4.68 | 330.0 | 101.0 | 25 | 50 | 0.55 | 1.84 | 5–1000 | 0.9997 |
| 330.0 | 121.0 | 25 | 22 | |||||||||
| 42 | Ethion | C9H22O4P2S4 | [M + H]+ | 8.05 | 384.9 | 97.0 | 30 | 46 | 0.80 | 2.66 | 5–1000 | 0.9994 |
| 384.9 | 199.1 | 30 | 10 | |||||||||
| 43 | Fenbuconazole | C19H17ClN4 | [M + H]+ | 4.89 | 337.0 | 70.1 | 32 | 20 | 0.24 | 0.82 | 2–500 | 0.9990 |
| 337.0 | 125.0 | 32 | 36 | |||||||||
| 44 | Fenhexamid | C14H17Cl2NO2 | [M + H]+ | 4.74 | 302.1 | 55.3 | 32 | 38 | 0.34 | 1.15 | 5–1000 | 0.9998 |
| 302.1 | 97.2 | 32 | 22 | |||||||||
| 45 | Fenobucarb | C12H17NO2 | [M + H]+ | 4.38 | 208.0 | 94.9 | 16 | 14 | 0.87 | 2.89 | 5–1000 | 0.9966 |
| 208.0 | 152.0 | 16 | 8 | |||||||||
| 46 | Flonicamid | C9H6F3N3O | [M + H]+ | 1.78 | 230.2 | 148.1 | 35 | 25 | 1.36 | 4.52 | 5–1000 | 0.9992 |
| 230.2 | 203.1 | 35 | 15 | |||||||||
| 47 | Fluazifop-butyl | C19H20F3NO4 | [M + H]+ | 6.32 | 384.1 | 282.1 | 28 | 20 | 0.45 | 1.49 | 5–1000 | 0.9985 |
| 384.1 | 328.1 | 28 | 14 | |||||||||
| 48 | Fluazinam | C13H4Cl2F6N4O4 | [M + H]+ | 6.17 | 465.0 | 338.1 | 23 | 47 | 0.27 | 0.91 | 5–1000 | 0.9997 |
| 465.0 | 373.0 | 23 | 26 | |||||||||
| 49 | Fluopicolide | C14H8Cl3F3N2O | [M + H]+ | 4.82 | 386.2 | 173.0 | 35 | 25 | 0.43 | 1.45 | 5–1000 | 0.9995 |
| 386.2 | 175.0 | 35 | 25 | |||||||||
| 50 | Flusilazole | C16H15F2N3Si | [M + H]+ | 5.93 | 316.0 | 165.0 | 30 | 28 | 0.42 | 1.39 | 5–1000 | 0.9993 |
| 316.0 | 247.0 | 30 | 18 | |||||||||
| 51 | Flutriafol | C16H13F2N3O | [M + H]+ | 4.24 | 302.1 | 70.2 | 30 | 18 | 0.14 | 0.46 | 2–1000 | 0.9999 |
| 302.1 | 123.1 | 30 | 29 | |||||||||
| 52 | Fosthiazate | C9H18NO3PS2 | [M + H]+ | 3.63 | 284.0 | 104.0 | 20 | 18 | 0.20 | 0.68 | 2–500 | 0.9998 |
| 284.0 | 228.0 | 20 | 6 | |||||||||
| 53 | Haloxyfop-methyl | C16H13ClF3NO4 | [M + H]+ | 5.82 | 376.0 | 91.1 | 25 | 25 | 0.23 | 0.77 | 5–1000 | 0.9998 |
| 376.0 | 316.1 | 25 | 10 | |||||||||
| 54 | Hexaconazole | C14H17Cl2N3O | [M + H]+ | 6.05 | 315.0 | 70.1 | 31 | 22 | 0.46 | 1.52 | 5–1000 | 0.9992 |
| 315.0 | 159.0 | 31 | 28 | |||||||||
| 55 | Hexythiazox | C17H21ClN2O2S | [M + H]+ | 6.53 | 353.0 | 168.1 | 24 | 26 | 0.90 | 2.98 | 5–1000 | 0.9995 |
| 353.0 | 228.1 | 24 | 14 | |||||||||
| 56 | Imazalil | C14H14Cl2N2O | [M + H]+ | 2.93 | 296.9 | 158.9 | 25 | 20 | 0.60 | 2.00 | 5–1000 | 0.9999 |
| 296.9 | 201.0 | 25 | 15 | |||||||||
| 57 | Imidacloprid | C9H10ClN5O2 | [M + H]+ | 2.13 | 256.1 | 175.1 | 22 | 20 | 0.31 | 1.02 | 5–1000 | 0.9999 |
| 256.1 | 209.1 | 22 | 15 | |||||||||
| 58 | Indoxacarb | C22H17ClF3N3O7 | [M + H]+ | 5.83 | 528.0 | 150.0 | 25 | 22 | 0.37 | 1.23 | 5–1000 | 0.9993 |
| 528.0 | 203.0 | 25 | 40 | |||||||||
| 59 | Iprodione | C13H13Cl2N3O3 | [M + H]+ | 4.98 | 330.0 | 244.7 | 15 | 16 | 1.57 | 5.23 | 10–1000 | 0.9995 |
| 330.0 | 288.0 | 15 | 15 | |||||||||
| 60 | Isocarbophos | C11H16NO4PS | [M + H]+ | 4.30 | 291.1 | 121.1 | 12 | 30 | 0.21 | 0.70 | 2–500 | 0.9992 |
| 291.1 | 231.1 | 12 | 13 | |||||||||
| 61 | Isoprocarb | C11H15NO2 | [M + H]+ | 3.91 | 194.1 | 95.1 | 18 | 14 | 0.33 | 1.09 | 5–1000 | 0.9981 |
| 194.1 | 137.1 | 18 | 8 | |||||||||
| 62 | Isoprothiolane | C12H18O4S2 | [M + H]+ | 5.04 | 291.1 | 188.8 | 15 | 18 | 0.22 | 0.72 | 5–1000 | 0.9991 |
| 291.1 | 230.9 | 15 | 10 | |||||||||
| 63 | Isoproturon | C12H18N2O | [M + H]+ | 3.72 | 207.0 | 72.0 | 28 | 22 | 0.30 | 0.99 | 5–1000 | 0.9982 |
| 207.0 | 165.1 | 28 | 15 | |||||||||
| 64 | Malathion | C10H19O6PS2 | [M + H]+ | 4.99 | 331.0 | 99.0 | 14 | 24 | 0.21 | 0.71 | 5–1000 | 0.9998 |
| 331.0 | 127.0 | 14 | 12 | |||||||||
| 65 | Metalaxyl | C15H21NO4 | [M + H]+ | 4.49 | 280.1 | 192.1 | 30 | 17 | 0.21 | 0.70 | 2–1000 | 0.9997 |
| 280.1 | 220.1 | 30 | 13 | |||||||||
| 66 | Metconazole | C17H22ClN3O | [M + H]+ | 5.05 | 320.1 | 70.0 | 25 | 22 | 0.52 | 1.73 | 5–1000 | 0.9993 |
| 320.1 | 125.0 | 25 | 36 | |||||||||
| 67 | Methamidophos | C2H8NO2PS | [M + H]+ | 0.74 | 142.0 | 94.0 | 22 | 10 | 0.44 | 1.48 | 5–1000 | 0.9999 |
| 142.0 | 124.9 | 22 | 10 | |||||||||
| 68 | Methomyl | C5H10N2O2S | [M + H]+ | 1.51 | 163.0 | 88.0 | 20 | 10 | 0.32 | 1.06 | 5–1000 | 0.9992 |
| 163.0 | 106.0 | 20 | 10 | |||||||||
| 69 | Methoxyfenozide | C22H28N2O3 | [M + H]+ | 4.90 | 369.1 | 149.1 | 25 | 18 | 0.53 | 1.78 | 5–1000 | 0.9994 |
| 369.1 | 313.2 | 25 | 8 | |||||||||
| 70 | Metolachlor | C15H22ClNO2 | [M + H]+ | 6.27 | 284.1 | 176.1 | 30 | 25 | 0.40 | 1.35 | 5–1000 | 0.9997 |
| 284.1 | 252.1 | 30 | 15 | |||||||||
| 71 | Metribuzin | C8H14N4OS | [M + H]+ | 3.86 | 215.0 | 131.0 | 10 | 18 | 0.62 | 2.07 | 5–1000 | 0.9987 |
| 215.0 | 89.0 | 20 | 16 | |||||||||
| 72 | Myclobutanil | C15H17ClN4 | [M + H]+ | 4.59 | 289.1 | 70.2 | 28 | 18 | 0.35 | 1.18 | 5–1000 | 0.9992 |
| 289.1 | 125.1 | 28 | 32 | |||||||||
| 73 | Omethoate | C5H12NO4PS | [M + H]+ | 0.83 | 214.0 | 125.0 | 20 | 22 | 0.63 | 2.09 | 5–1000 | 0.9983 |
| 214.0 | 183.0 | 20 | 11 | |||||||||
| 74 | Paclobutrazol | C15H20ClN3O | [M + H]+ | 5.15 | 294.1 | 70.2 | 30 | 20 | 0.40 | 1.34 | 5–1000 | 0.9995 |
| 294.1 | 125.1 | 30 | 38 | |||||||||
| 75 | Penconazole | C13H15Cl2N3 | [M + H]+ | 4.99 | 284.0 | 70.1 | 28 | 16 | 0.38 | 1.26 | 5–1000 | 0.9999 |
| 284.0 | 159.0 | 28 | 34 | |||||||||
| 76 | Phenthoate | C12H17O4PS2 | [M + H]+ | 6.88 | 321.0 | 135.0 | 30 | 20 | 1.91 | 6.37 | 10–1000 | 0.9999 |
| 321.0 | 163.0 | 30 | 12 | |||||||||
| 77 | Phorate | C7H17O2PS3 | [M + H]+ | 5.82 | 261.0 | 75.0 | 10 | 12 | 1.62 | 5.40 | 10–1000 | 0.9949 |
| 78 | Phorate-sulfone | C7H17O4PS3 | [M + NH4]+ | 4.29 | 293.0 | 115.0 | 16 | 24 | 0.51 | 1.71 | 5–1000 | 0.9979 |
| 293.0 | 171.0 | 16 | 6 | |||||||||
| 79 | Phorate-sulfoxide | C7H17O3PS3 | [M + H]+ | 3.55 | 277.0 | 96.9 | 18 | 32 | 0.15 | 0.50 | 2–500 | 0.9989 |
| 277.0 | 143.0 | 18 | 20 | |||||||||
| 80 | Phoxim | C12H15N2O3PS | [M + H]+ | 5.77 | 299.0 | 129.0 | 12 | 13 | 0.45 | 1.49 | 5–1000 | 0.9977 |
| 299.0 | 153.0 | 12 | 7 | |||||||||
| 81 | Piperonyl-butoxide | C19H30O5 | [M + NH4]+ | 6.27 | 356.3 | 119.0 | 20 | 30 | 0.57 | 1.91 | 5–1000 | 0.9999 |
| 356.3 | 176.9 | 20 | 10 | |||||||||
| 82 | Pirimiphos-methyl | C11H20N3O3PS | [M + H]+ | 6.90 | 306.1 | 108.1 | 30 | 32 | 0.55 | 1.84 | 5–1000 | 0.9991 |
| 306.1 | 164.1 | 30 | 22 | |||||||||
| 83 | Prochloraz | C15H16Cl3N3O2 | [M + H]+ | 9.87 | 376.1 | 266.0 | 22 | 10 | 0.89 | 2.97 | 5–1000 | 0.9978 |
| 376.1 | 308.0 | 22 | 10 | |||||||||
| 84 | Profenofos | C11H15BrClO3PS | [M − H]− | 7.40 | 372.9 | 127.9 | 30 | 40 | 1.14 | 3.80 | 5–1000 | 0.9999 |
| 372.9 | 302.6 | 30 | 20 | |||||||||
| 85 | Prometryn | C10H19N5S | [M + H]+ | 3.45 | 242.0 | 158.0 | 20 | 16 | 0.69 | 2.31 | 5–1000 | 0.9995 |
| 242.0 | 200.1 | 20 | 12 | |||||||||
| 86 | Propham | C10H13NO2 | [M + H]+ | 4.07 | 180.0 | 77.0 | 18 | 20 | 0.26 | 0.88 | 2–1000 | 0.9991 |
| 180.0 | 120.0 | 18 | 10 | |||||||||
| 87 | Propiconazole | C15H17Cl2N3O2 | [M + H]+ | 5.15 | 342.0 | 69.0 | 25 | 20 | 0.16 | 0.54 | 2–500 | 0.9996 |
| 342.0 | 159.0 | 25 | 30 | |||||||||
| 88 | Propoxur | C11H15NO3 | [M + H]+ | 3.36 | 210.0 | 111.0 | 12 | 16 | 0.44 | 1.45 | 5–1000 | 0.9968 |
| 89 | Pyridaben | C19H25ClN2OS | [M + H]+ | 7.00 | 365.1 | 147.0 | 22 | 20 | 0.24 | 0.80 | 5–1000 | 0.9985 |
| 365.1 | 309.1 | 22 | 8 | |||||||||
| 90 | Semiamitraz | C10H14N2 | [M + H]+ | 3.94 | 163.0 | 96.3 | 18 | 10 | 1.32 | 4.40 | 5–1000 | 0.9921 |
| 163.0 | 118.4 | 18 | 10 | |||||||||
| 91 | Sethoxydim | C17H29NO3S | [M + H]+ | 6.42 | 328.3 | 254.3 | 23 | 15 | 1.50 | 4.99 | 5–1000 | 0.9976 |
| 328.3 | 282.0 | 23 | 10 | |||||||||
| 92 | Simazine | C7H12ClN5 | [M + H]+ | 3.47 | 202.0 | 96.0 | 25 | 20 | 0.30 | 0.99 | 5–1000 | 0.9999 |
| 202.0 | 124.0 | 25 | 14 | |||||||||
| 93 | Spirodiclofen | C21H24Cl2O4 | [M + H]+ | 7.10 | 411.1 | 71.2 | 25 | 13 | 0.78 | 2.61 | 5–1000 | 0.9997 |
| 411.1 | 313.0 | 25 | 13 | |||||||||
| 94 | Tebuconazole | C16H22ClN3O | [M + H]+ | 4.82 | 308.0 | 70.1 | 34 | 22 | 0.24 | 0.79 | 5–1000 | 0.9996 |
| 308.0 | 125.0 | 34 | 40 | |||||||||
| 95 | Tebufenozide | C22H28N2O2 | [M + H]+ | 5.25 | 353.0 | 105.0 | 12 | 22 | 0.28 | 0.95 | 5–1000 | 0.9996 |
| 353.0 | 133.0 | 12 | 18 | |||||||||
| 96 | Thiabendazole | C10H7N3S | [M + H]+ | 1.03 | 202.0 | 131.0 | 25 | 25 | 0.30 | 0.99 | 5–1000 | 0.9998 |
| 202.0 | 175.0 | 25 | 20 | |||||||||
| 97 | Thiacloprid | C10H9ClN4S | [M + H]+ | 2.69 | 253.0 | 90.1 | 25 | 30 | 0.20 | 0.67 | 5–1000 | 0.9997 |
| 253.0 | 126.0 | 25 | 10 | |||||||||
| 98 | Thiamethoxam | C8H10ClN5O3S | [M + H]+ | 1.68 | 292.0 | 132.0 | 22 | 22 | 0.46 | 1.53 | 5–1000 | 0.9999 |
| 292.0 | 211.2 | 22 | 12 | |||||||||
| 99 | Thifluzamide | C13H6Br2F6N2O2S | [M + H]+ | 6.53 | 526.8 | 148.0 | 20 | 25 | 1.69 | 5.63 | 10–1000 | 0.9993 |
| 526.8 | 168.0 | 20 | 25 | |||||||||
| 100 | Thiophanate-methyl | C12H14N4O4S2 | [M + H]+ | 3.20 | 343.1 | 93.0 | 20 | 46 | 0.72 | 2.39 | 5–1000 | 0.9996 |
| 343.1 | 151.0 | 20 | 22 | |||||||||
| 101 | Tolfenpyrad | C21H22ClN3O2 | [M + H]+ | 6.11 | 384.2 | 171.0 | 30 | 20 | 1.13 | 3.78 | 5–1000 | 0.9997 |
| 384.2 | 197.0 | 30 | 18 | |||||||||
| 102 | Triadimefon | C14H16ClN3O2 | [M + H]+ | 5.74 | 294.1 | 69.3 | 30 | 20 | 0.20 | 0.68 | 5–1000 | 0.9988 |
| 294.1 | 197.2 | 30 | 15 | |||||||||
| 103 | Triazophos | C12H16N3O3PS | [M + H]+ | 6.19 | 314.1 | 118.9 | 30 | 35 | 0.49 | 1.63 | 5–1000 | 0.9996 |
| 314.1 | 161.9 | 30 | 18 | |||||||||
| 104 | Tribenuron-methyl | C15H17N5O6S | [M + H]+ | 4.09 | 396.1 | 154.9 | 18 | 14 | 0.30 | 1.00 | 5–1000 | 0.9885 |
| 396.1 | 180.9 | 18 | 22 | |||||||||
| 105 | Tridemorph | C19H39NO | [M + H]+ | 4.29 | 298.1 | 57.0 | 40 | 28 | 1.24 | 4.14 | 5–1000 | 0.9997 |
| 298.1 | 98.0 | 40 | 34 | |||||||||
| 106 | Trifloxystrobin | C20H19F3N2O4 | [M + H]+ | 5.94 | 409.0 | 145.0 | 25 | 25 | 0.48 | 1.58 | 5–1000 | 0.9994 |
| 409.0 | 186.0 | 25 | 8 | |||||||||
| 107 | Triflumizole | C15H15ClF3N3O | [M + H]+ | 4.92 | 346.0 | 277.9 | 13 | 10 | 1.75 | 5.82 | 10–1000 | 0.9998 |
| 359.0 | 139.1 | 20 | 35 |
Under the optimal experimental conditions, the lowest concentration or the lowest content of the target component can be detected is considered as the detection limit (LOD). The limit of quantitation (LOQ) is the lowest concentration or the lowest quantity of the components to be measured in the sample by analytical method. The LOD and LOQ for each analytes were determined at the lowest concentration at a signal-to-noise ratio (S/N) of 3 and 10, respectively. The results were shown in Table 1 that the LOD and LOQ were 0.14–1.91 µg/kg and 0.46–6.37 µg/kg, respectively.
2.3.2. Precision and Stability
In this study, the stability and precision of the established method were tested. The precision was evaluated by measuring the intra-day and inter-day variations in the relative standard deviation (RSD%) of the peak area of each analyte. In order to estimate intra-day precision, the blank spiked samples at concentration levels of 10 and 100 µg/kg were pretreated and analyzed in one day, with each spike level with five parallel samples. Inter-day precision was achieved by measuring the spiked samples at concentration levels of 10 and 100 µg/kg once a day within five consecutive days. As shown in Table 2, the relative standard deviation values (RSD) of intra-day were 0.6–9.4% (10 µg/kg) and 0.8–9.1% (100 µg/kg), inter-day were 0.8–12.6% (10 µg/kg) and 1.5–11.7% (100 µg/kg). The stability was evaluated by injecting the same volume of wolfberry spiked samples (100 µg/kg) into the UPLC/MS/MS system at 0, 2, 4, 8, 12, 18 and 24 h under the same condition in one day. The RSD values were less than 11.7 for all analytes (Table 2), which indicating that the sample solutions were stable and unchanged.
Table 2.
Recovery (RE) values, Precision, Stability and Matrix effect (ME) of 107 Pesticides.
| No. | Compound | 10 µg/kg | 50 µg/kg | 100 µg/kg | Intra-day Precision (RSD%, n = 5) |
Inter-day Precision (RSD%, n = 5) |
Stability (RSD, %) |
ME (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rec. (%) |
RSD (%) |
Rec. (%) |
RSD (%) |
Rec. (%) |
RSD (%) |
10 µg/kg | 100 µg/kg | 10 µg/kg | 100 µg/kg | 100 µg/kg | 10 µg/kg | ||
| 1 | Abamectin | 83.3 | 8.1 | 86.0 | 13.1 | 87.0 | 8.2 | 3.2 | 2.7 | 5.4 | 1.9 | 6.2 | 90.0 |
| 2 | Acephate | 71.7 | 1.0 | 80.0 | 2.0 | 67.0 | 0.9 | 0.7 | 3.1 | 3.6 | 6.3 | 5.4 | 83.8 |
| 3 | Acetamiprid | 85.0 | 8.8 | 96.0 | 9.0 | 93.5 | 6.8 | 1.6 | 1.6 | 2.1 | 2.8 | 3.9 | 97.5 |
| 4 | Acetochlor | 86.7 | 7.9 | 104.3 | 5.9 | 102.3 | 3.3 | 2.1 | 5.4 | 6.3 | 4.6 | 4.8 | 99.6 |
| 5 | Aldicarb | 96.7 | 5.6 | 92.3 | 3.7 | 86.8 | 0.6 | 6.2 | 1.0 | 4.8 | 11.7 | 1.9 | 92.4 |
| 6 | Aldicarb-Sulfone | 90.0 | 13.5 | 93.3 | 10.4 | 94.7 | 6.6 | 0.6 | 2.2 | 7.2 | 2.7 | 7.2 | 91.6 |
| 7 | Aldicarb-Sulfoxide | 85.0 | 5.5 | 78.0 | 4.1 | 102.0 | 4.7 | 0.8 | 3.6 | 1.9 | 10.8 | 10.8 | 87.9 |
| 8 | Ametryn | 90.0 | 2.7 | 92.3 | 5.7 | 102.0 | 3.6 | 2.3 | 7.8 | 4.5 | 11.6 | 3.5 | 101.4 |
| 9 | Atrazine | 103.3 | 9.1 | 92.0 | 4.1 | 96.5 | 5.3 | 5.7 | 1.2 | 2.6 | 3.8 | 7.2 | 102.6 |
| 10 | Azoxystrobin | 63.3 | 9.1 | 77.3 | 2.6 | 104.7 | 2.7 | 1.0 | 4.3 | 5.6 | 4.6 | 5.6 | 96.8 |
| 11 | Bendiocarb | 103.3 | 2.8 | 104.6 | 4.3 | 92.8 | 0.6 | 9.3 | 3.2 | 10.2 | 7.2 | 6.3 | 104.7 |
| 12 | Blasticidin-S | 96.5 | 3.1 | 101.2 | 1.6 | 99.8 | 0.7 | 5.6 | 7.6 | 2.8 | 6.3 | 8.2 | 103.2 |
| 13 | Boscalid | 80.0 | 10.8 | 79.0 | 7.4 | 77.0 | 3.3 | 7.2 | 4.5 | 4.6 | 2.1 | 6.3 | 79.8 |
| 14 | Bupirimate | 116.0 | 8.9 | 93.7 | 5.2 | 93.2 | 4.3 | 1.4 | 6.2 | 7.9 | 1.6 | 4.9 | 107.2 |
| 15 | Buprofezin | 103.3 | 5.6 | 98.0 | 2.0 | 85.8 | 0.9 | 5.3 | 1.9 | 5.3 | 1.9 | 5.1 | 92.4 |
| 16 | Butralin | 91.7 | 8.3 | 92.6 | 0.6 | 85.0 | 3.8 | 5.9 | 8.2 | 4.1 | 2.0 | 7.2 | 88.3 |
| 17 | Carbaryl | 103.3 | 12.9 | 79.0 | 5.0 | 92.0 | 2.4 | 2.7 | 4.1 | 2.2 | 3.6 | 4.1 | 94.5 |
| 18 | Carbendazim | 92.4 | 6.3 | 96.3 | 3.2 | 102.6 | 1.8 | 0.5 | 2.0 | 1.0 | 5.4 | 2.8 | 95.2 |
| 19 | Carbofuran | 113.3 | 2.5 | 118.6 | 6.2 | 118.3 | 3.3 | 9.4 | 3.3 | 3.2 | 8.2 | 2.2 | 121.6 |
| 20 | Carbosulfan | 106.3 | 2.1 | 98.2 | 1.9 | 104.2 | 4.1 | 1.6 | 6.1 | 5.1 | 7.5 | 1.6 | 98.2 |
| 21 | Cartap | 108.3 | 2.6 | 85.6 | 3.5 | 89.0 | 6.1 | 4.1 | 2.9 | 11.3 | 1.9 | 4.3 | 95.1 |
| 22 | Chlorantraniliprole | 100.0 | 13.2 | 82.5 | 4.5 | 79.0 | 6.7 | 6.8 | 2.5 | 0.9 | 3.6 | 5.3 | 89.7 |
| 23 | Chlorfenvinphos | 95.0 | 3.6 | 88.7 | 5.5 | 89.7 | 3.9 | 2.4 | 1.8 | 2.8 | 9.2 | 5.9 | 92.3 |
| 24 | Chlorpropham | 100.0 | 5.0 | 106.7 | 10.1 | 75.3 | 13.6 | 2.2 | 5.5 | 5.6 | 1.6 | 11.2 | 80.6 |
| 25 | Clodinafop-propargyl | 83.3 | 3.4 | 86.0 | 11.0 | 85.8 | 7.3 | 1.0 | 4.9 | 8.4 | 5.5 | 9.7 | 87.1 |
| 26 | Clothianidin | 88.3 | 8.6 | 90.3 | 3.5 | 104.5 | 6.3 | 1.3 | 5.6 | 7.6 | 8.0 | 3.8 | 93.5 |
| 27 | Cyantraniliprole | 75.0 | 13.3 | 77.5 | 4.8 | 83.8 | 5.6 | 3.0 | 8.2 | 3.5 | 7.9 | 4.9 | 85.9 |
| 28 | Cyazofamid | 100.0 | 3.1 | 86.7 | 2.4 | 94.5 | 1.9 | 1.8 | 7.1 | 6.1 | 3.6 | 7.4 | 96.1 |
| 29 | Cymoxanil | 115.0 | 11.5 | 99.0 | 6.0 | 96.3 | 3.5 | 2.6 | 2.3 | 4.8 | 2.1 | 6.6 | 104.2 |
| 30 | Cyproconazole | 96.7 | 2.9 | 87.7 | 1.7 | 103.2 | 6.1 | 2.8 | 1.1 | 6.6 | 9.7 | 5.9 | 95.3 |
| 31 | Cyprodinil | 80.0 | 12.7 | 78.3 | 10.3 | 88.9 | 11.8 | 4.2 | 1.0 | 7.4 | 4.8 | 8.7 | 88.9 |
| 32 | Cyromazine | 90.7 | 6.8 | 95.4 | 3.2 | 94.6 | 5.7 | 3.1 | 2.0 | 2.5 | 5.1 | 9.2 | 90.2 |
| 33 | Diazinon | 96.7 | 2.9 | 97.3 | 0.5 | 96.7 | 1.6 | 6.5 | 2.4 | 4.6 | 3.2 | 7.4 | 100.4 |
| 34 | Diethofencarb | 100.0 | 5.0 | 97.3 | 4.2 | 114.0 | 7.0 | 5.1 | 3.5 | 1.9 | 1.9 | 5.4 | 106.2 |
| 35 | Difenoconazole | 75.0 | 6.7 | 75.7 | 2.5 | 87.8 | 4.4 | 3.1 | 6.1 | 8.4 | 5.6 | 3.2 | 90.3 |
| 36 | Dimethoate | 95.0 | 2.6 | 97.0 | 2.0 | 92.2 | 0.8 | 5.0 | 2.2 | 7.6 | 4.9 | 2.6 | 94.8 |
| 37 | Dimethomorph | 86.7 | 3.3 | 92.0 | 1.0 | 90.3 | 4.9 | 6.2 | 0.8 | 3.8 | 8.5 | 2.1 | 91.7 |
| 38 | Diniconazole | 76.7 | 3.7 | 79.0 | 4.8 | 76.3 | 7.3 | 1.8 | 1.2 | 5.4 | 4.0 | 4.4 | 86.3 |
| 39 | Dinotefuran | 88.3 | 8.6 | 92.0 | 3.9 | 103.0 | 6.5 | 0.9 | 1.9 | 2.9 | 3.9 | 7.0 | 88.6 |
| 40 | Diuron | 75.0 | 6.6 | 76.0 | 4.8 | 78.3 | 4.4 | 2.2 | 3.7 | 0.8 | 2.1 | 6.2 | 87.2 |
| 41 | Epoxiconazole | 72.0 | 4.0 | 82.5 | 9.4 | 81.5 | 7.1 | 2.3 | 2.6 | 11.6 | 6.3 | 5.9 | 89.1 |
| 42 | Ethion | 78.3 | 13.2 | 96.3 | 4.3 | 89.2 | 7.3 | 1.6 | 5.4 | 12.3 | 9.7 | 7.6 | 93.2 |
| 43 | Fenbuconazole | 80.0 | 2.2 | 83.5 | 5.6 | 81.2 | 10.3 | 1.8 | 5.7 | 2.2 | 8.2 | 3.9 | 91.7 |
| 44 | Fenhexamid | 111.0 | 11.2 | 87.3 | 11.0 | 75.2 | 7.1 | 3.3 | 6.1 | 2.0 | 5.1 | 9.8 | 92.8 |
| 45 | Fenobucarb | 105.0 | 8.2 | 90.7 | 9.1 | 91.7 | 4.7 | 2.9 | 3.3 | 6.5 | 4.2 | 11.7 | 99.7 |
| 46 | Flonicamid | 101.7 | 2.8 | 93.0 | 2.8 | 103.0 | 2.7 | 4.2 | 1.8 | 1.9 | 1.2 | 7.9 | 103.2 |
| 47 | Fluazifop-butyl | 88.3 | 6.5 | 95.7 | 2.1 | 84.5 | 4.3 | 3.6 | 1.1 | 7.1 | 2.9 | 5.9 | 96.5 |
| 48 | Fluazinam | 81.7 | 9.3 | 82.0 | 12.2 | 76.7 | 5.5 | 5.1 | 2.6 | 6.3 | 3.5 | 4.2 | 86.7 |
| 49 | Fluopicolide | 83.3 | 3.4 | 93.3 | 7.5 | 100.8 | 3.6 | 1.8 | 3.7 | 1.8 | 7.4 | 1.8 | 96.3 |
| 50 | Flusilazole | 93.3 | 3.0 | 93.0 | 2.1 | 91.8 | 5.8 | 1.7 | 8.6 | 7.7 | 6.1 | 4.1 | 97.2 |
| 51 | Flutriafol | 88.3 | 3.2 | 91.0 | 4.7 | 106.8 | 5.1 | 2.2 | 9.0 | 6.5 | 9.8 | 3.1 | 95.4 |
| 52 | Fosthiazate | 96.7 | 7.9 | 96.3 | 0.6 | 93.8 | 2.1 | 1.3 | 5.7 | 13.6 | 4.2 | 5.8 | 100.7 |
| 53 | Haloxyfop-methyl | 91.7 | 3.1 | 88.3 | 3.6 | 87.7 | 5.7 | 3.3 | 3.1 | 5.8 | 6.6 | 4.2 | 90.6 |
| 54 | Hexaconazole | 86.7 | 12.0 | 92.7 | 8.3 | 101.7 | 6.4 | 2.9 | 6.8 | 6.2 | 1.5 | 1.9 | 95.7 |
| 55 | Hexythiazox | 75.0 | 6.6 | 89.0 | 2.9 | 91.0 | 10.0 | 8.2 | 7.2 | 4.9 | 4.6 | 7.4 | 77.3 |
| 56 | Imazalil | 68.3 | 11.1 | 80.0 | 8.0 | 70.2 | 3.1 | 7.6 | 1.9 | 7.5 | 5.1 | 4.8 | 85.9 |
| 57 | Imidacloprid | 76.7 | 3.7 | 86.3 | 2.6 | 102.3 | 2.2 | 1.4 | 4.4 | 4.6 | 8.4 | 4.6 | 94.3 |
| 58 | Indoxacarb | 88.3 | 3.2 | 93.7 | 4.0 | 82.3 | 9.9 | 6.2 | 4.7 | 1.8 | 7.4 | 5.0 | 91.6 |
| 59 | Iprodione | 90.0 | 5.5 | 72.0 | 2.7 | 86.8 | 9.2 | 9.4 | 2.8 | 4.2 | 3.3 | 2.7 | 87.8 |
| 60 | Isocarbophos | 80.0 | 12.5 | 96.0 | 8.1 | 96.3 | 6.2 | 8.0 | 3.9 | 1.3 | 2.9 | 6.0 | 98.4 |
| 61 | Isoprocarb | 106.7 | 2.7 | 95.0 | 4.8 | 93.7 | 1.3 | 7.2 | 6.5 | 2.6 | 6.1 | 4.8 | 103.2 |
| 62 | Isoprothiolane | 115.0 | 11.5 | 105.0 | 4.3 | 89.2 | 7.7 | 4.4 | 2.2 | 5.8 | 3.2 | 7.1 | 101.6 |
| 63 | Isoproturon | 101.7 | 2.8 | 94.7 | 0.6 | 106.0 | 3.1 | 4.6 | 1.3 | 7.1 | 1.9 | 4.2 | 99.1 |
| 64 | Malathion | 80.0 | 2.3 | 87.3 | 1.1 | 89.5 | 2.1 | 2.8 | 7.2 | 6.3 | 7.8 | 6.6 | 96.7 |
| 65 | Metalaxyl | 90.0 | 5.5 | 97.3 | 2.1 | 93.5 | 1.4 | 3.9 | 4.5 | 6.9 | 6.4 | 5.9 | 92.5 |
| 66 | Metconazole | 76.7 | 13.5 | 76.0 | 5.9 | 73.2 | 5.5 | 4.1 | 6.1 | 5.5 | 1.4 | 8.2 | 76.3 |
| 67 | Methamidophos | 76.7 | 3.7 | 81.0 | 2.4 | 96.7 | 3.0 | 3.2 | 2.8 | 1.8 | 0.8 | 8.0 | 88.2 |
| 68 | Methomyl | 88.3 | 11.7 | 95.0 | 4.5 | 115.8 | 3.2 | 6.1 | 7.0 | 8.2 | 1.9 | 10.6 | 98.4 |
| 69 | Methoxyfenozide | 85.0 | 11.7 | 87.7 | 9.2 | 90.3 | 3.3 | 5.8 | 8.2 | 3.6 | 2.8 | 2.1 | 91.5 |
| 70 | Metolachlor | 100.0 | 1.8 | 97.7 | 3.1 | 95.8 | 2.6 | 4.6 | 9.1 | 5.8 | 4.6 | 1.9 | 96.7 |
| 71 | Metribuzin | 91.7 | 6.3 | 94.0 | 6.5 | 93.5 | 3.2 | 0.8 | 2.4 | 6.2 | 7.1 | 1.5 | 89.7 |
| 72 | Myclobutanil | 118.0 | 6.4 | 93.7 | 2.2 | 105.7 | 4.9 | 1.1 | 3.3 | 2.9 | 7.3 | 4.1 | 102.7 |
| 73 | Omethoate | 81.7 | 3.5 | 76.3 | 5.4 | 94.2 | 3.2 | 2.3 | 5.1 | 7.1 | 5.9 | 3.3 | 88.2 |
| 74 | Paclobutrazol | 95.0 | 5.2 | 90.0 | 6.7 | 98.8 | 4.3 | 2.9 | 2.9 | 4.4 | 4.5 | 2.3 | 95.4 |
| 75 | Penconazole | 100.0 | 5.0 | 95.0 | 2.8 | 99.5 | 3.5 | 2.2 | 4.6 | 1.8 | 2.9 | 6.5 | 98.7 |
| 76 | Phenthoate | 85.0 | 5.8 | 99.7 | 1.2 | 93.0 | 1.9 | 5.1 | 4.1 | 2.6 | 8.7 | 4.9 | 94.2 |
| 77 | Phorate | 86.7 | 13.8 | 105.3 | 5.5 | 110.1 | 5.1 | 1.6 | 2.9 | 3.7 | 12.6 | 7.3 | 102.1 |
| 78 | Phorate-sulfone | 80.0 | 6.2 | 100.7 | 4.6 | 103.0 | 3.2 | 1.1 | 4.1 | 5.6 | 5.9 | 5.4 | 97.4 |
| 79 | Phorate-sulfoxide | 90.0 | 5.5 | 95.7 | 1.2 | 98.8 | 1.0 | 7.5 | 6.2 | 4.4 | 4.8 | 6.1 | 92.7 |
| 80 | Phoxim | 88.3 | 6.5 | 98.7 | 1.5 | 100.0 | 1.1 | 8.6 | 5.7 | 4.4 | 8.9 | 9.7 | 95.1 |
| 81 | Piperonyl butoxide | 81.7 | 3.5 | 85.0 | 3.1 | 90.7 | 4.2 | 8.3 | 6.0 | 2.8 | 4.6 | 8.2 | 87.3 |
| 82 | Pirimiphos-methyl | 85.0 | 4.1 | 95.7 | 3.7 | 106.1 | 3.0 | 1.9 | 2.8 | 7.6 | 7.6 | 8.9 | 89.6 |
| 83 | Prochloraz | 89.6 | 1.4 | 91.6 | 0.9 | 96.3 | 2.5 | 6.7 | 4.9 | 3.6 | 1.6 | 4.9 | 92.3 |
| 84 | Profenofos | 91.7 | 3.1 | 90.0 | 5.7 | 85.5 | 5.1 | 3.2 | 6.6 | 5.9 | 5.2 | 4.7 | 92.5 |
| 85 | Prometryn | 90.0 | 3.8 | 94.3 | 0.6 | 109.5 | 1.6 | 4.1 | 7.4 | 5.6 | 1.3 | 5.4 | 94.8 |
| 86 | Propham | 116.6 | 2.4 | 89.3 | 6.5 | 92.2 | 3.6 | 8.8 | 2.9 | 3.8 | 5.6 | 8.2 | 108.2 |
| 87 | Propiconazole | 83.3 | 6.9 | 87.0 | 3.0 | 98.7 | 6.9 | 8.4 | 3.6 | 4.2 | 4.3 | 1.7 | 95.1 |
| 88 | Propoxur | 101.7 | 10.2 | 90.0 | 8.9 | 103.5 | 1.0 | 6.2 | 1.9 | 1.9 | 6.3 | 4.6 | 102.7 |
| 89 | Pyridaben | 80.0 | 1.9 | 95.3 | 2.4 | 103.8 | 11.2 | 8.5 | 1.3 | 4.4 | 2.6 | 6.2 | 94.3 |
| 90 | Semiamitraz | 85.6 | 8.9 | 89.3 | 2.6 | 87.6 | 3.5 | 1.9 | 3.5 | 7.5 | 1.9 | 3.9 | 89.7 |
| 91 | Sethoxydim | 123.0 | 8.4 | 101.7 | 4.0 | 90.3 | 4.0 | 2.7 | 4.9 | 6.3 | 4.6 | 7.4 | 107.3 |
| 92 | Simazine | 91.7 | 3.1 | 89.0 | 6.0 | 89.8 | 10.2 | 3.6 | 8.2 | 6.9 | 2.5 | 1.5 | 90.6 |
| 93 | Spirodiclofen | 85.0 | 12.9 | 97.3 | 2.4 | 82.5 | 7.3 | 1.8 | 7.1 | 5.5 | 1.0 | 8.4 | 87.5 |
| 94 | Tebuconazol | 95.0 | 5.2 | 80.0 | 7.6 | 81.0 | 3.2 | 2.2 | 7.5 | 8.0 | 6.8 | 5.6 | 89.4 |
| 95 | Tebufenozide | 111.7 | 8.5 | 99.3 | 7.4 | 84.7 | 2.3 | 2.2 | 2.9 | 10.6 | 7.2 | 9.2 | 98.6 |
| 96 | Tetraconazole | 85.0 | 5.8 | 91.7 | 1.6 | 100.8 | 4.2 | 6.3 | 6.2 | 2.8 | 2.2 | 9.1 | 92.5 |
| 97 | Thiacloprid | 88.3 | 3.2 | 93.7 | 0.6 | 92.7 | 2.1 | 4.1 | 5.9 | 4.5 | 2.6 | 4.8 | 93.6 |
| 98 | Thiamethoxam | 88.3 | 3.2 | 90.0 | 2.9 | 92.7 | 1.1 | 3.9 | 3.8 | 11.3 | 5.1 | 4.3 | 97.4 |
| 99 | Thifluzamide | 71.7 | 4.0 | 103.7 | 8.2 | 89.5 | 8.7 | 5.7 | 1.6 | 1.7 | 4.3 | 2.2 | 88.7 |
| 100 | Thiophanate-methyl | 88.3 | 3.2 | 92.0 | 3.7 | 92.2 | 12.4 | 6.2 | 4.6 | 12.8 | 6.2 | 2.6 | 91.8 |
| 101 | Tolfenpyrad | 83.3 | 9.1 | 80.0 | 3.3 | 84.2 | 2.0 | 2.4 | 1.5 | 3.3 | 1.8 | 9.0 | 86.9 |
| 102 | Triadimefon | 91.7 | 3.1 | 91.7 | 4.9 | 92.0 | 3.8 | 3.3 | 2.3 | 5.7 | 4.1 | 8.1 | 96.5 |
| 103 | Triazophos | 100.0 | 5.0 | 90.7 | 9.2 | 84.7 | 12.7 | 0.8 | 6.2 | 4.9 | 2.9 | 6.1 | 103.6 |
| 104 | Tribenuron-methyl | 100.0 | 3.3 | 73.3 | 13.2 | 82.8 | 2.1 | 1.9 | 5.5 | 8.1 | 3.6 | 7.2 | 89.7 |
| 105 | Tridemorph | 105.0 | 4.7 | 81.7 | 11.4 | 79.5 | 7.7 | 5.1 | 1.9 | 6.2 | 1.1 | 3.6 | 91.8 |
| 106 | Trifloxystrobin | 91.7 | 3.1 | 95.3 | 0.6 | 83.3 | 2.2 | 3.3 | 2.1 | 5.0 | 3.2 | 4.9 | 90.2 |
| 107 | Triflumizole | 90.0 | 5.5 | 86.3 | 8.8 | 95.2 | 4.9 | 2.9 | 4.6 | 4.1 | 5.1 | 6.2 | 93.6 |
2.3.3. Accuracy
The accuracy of this method was tested by the blank spike recovery experiment of 107 pesticides. And the spiking levels were 10, 50, and 100 µg/kg, respectively, meanwhile the determination had five parallel samples for each spiking level. Average recoveries of 107 kinds of pesticides at three fortification levels using the analytical procedure, as presented in Table 2, were 63.3–123.0%, 72.0–118.6%, and 67.0–118.3%, respectively, which were within the range of the acceptable values. Under the premise of ensuring the accuracy and reproducibility of the results, a better recovery rate can be obtained, which is fully in line with the AOAC 2007.01 and EN 15,662 standards. All samples were analyzed on the same day and the RSD values below 15.0% at the three fortification levels indicated the accuracy was acceptable.
2.4. Matrix Effects
Matrix effects are caused by the co-elution of matrix constituents that play an important role in multi-residue analysis of pesticides, which can affect the ionization efficiency of target pesticides [55,56] and then influence the quantitative results by matrix enhancement and attenuation effects caused by quality of chromatographic separation, the ionization type, the amount and the type of the sample matrix, and sample preparation procedure [57,58]. For complex matrix like wolfberry is wealthy in mineral substance, proteins, polysaccharose, amino acids, carotinoid, flavonoids and so on [6], it is critical to eliminate or attenuate matrix effects by minimizing matrix co-extractives through the sample preparation procedure. In this research, the matrix effect (ME) was calculated by the following equation:
| (1) |
where AMatrix is the peak area of matrix standard sample and As is the peak area of pure solvent standard sample. The matrix effect of the individual pesticide was studied at the level of 10 µg/kg with five parallel samples, which was deemed to be ignored if the ME value is between 90% and 110%, while it was regarded to be matrix suppression or enhancement effect when the value was less than 90% or greater than 110%, respectively [55,58,59]. The results showed that 71.96% of the pesticides presented a negligible ME, whereas 27.10% of the analytes presented matrix suppression effect, and only one pesticide (carbofuran) showed a matrix enhancement effect. Table 2 shows the specific ME values of each pesticide. Most of the compounds showed negligible matrix effect or mild matrix inhibition effect, suggesting that this method was suitable for the determination of 107 pesticide residues in wolfberry. However, there are still several pesticides with strong matrix effect: ME < 80% (boscalid, hexythiazox, metconazole) or ME > 120% (carbofuran). Therefore, to overcome and compensate for these matrix effects, matrix-matched standard curves were used in the quantitative analysis.
2.5. Real Samples Analysis
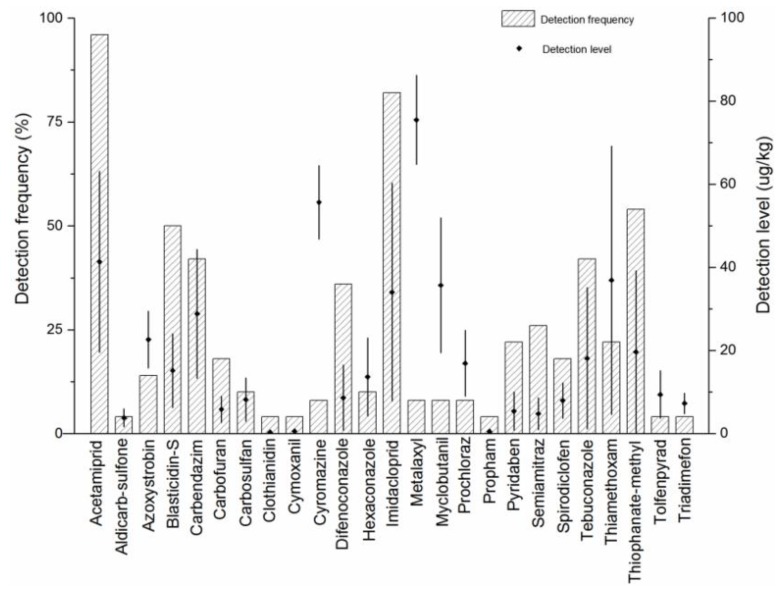
The applicability of the developed method was evaluated by analyzing a total of fifty wolfberry samples collected from supermarkets, pharmacies and farmers’ markets in different cities of Shandong Province and three replicates of each sample were analysed. One hundred percent of the samples analyzed included at least one pesticide, and a total of 26 pesticide residues was detected in fifty samples, which mainly were insecticides and bactericides. Ninety-six percent of the samples was found to be acetamiprid with concentration ranging from 19.54 to 63.15 µg/kg, and imidacloprid was detected in eighty-two percent of the samples with concentration ranging from 7.80 to 60.26 µg/kg. In addition, several pesticides with higher detection frequency in wolfberry were thiophanate-methyl (54%), blasticidin-S (50%), carbendazim (42%), tebuconazole (42%) and difenoconazole (36%). The detection frequency of cyromazine and metalaxyl in wolfberry is only 8%, but the detection concentration is as high as 46.78–64.56 µg/kg and 64.75–86.22 µg/kg, respectively. Moreover, carbendazim (44.36 µg/kg), myclobutanil (51.95 µg/kg) and thiamethoxam (69.21 µg/kg) were found higher concentration in some samples occasionally. All pesticide residues detected in wolfberry were below the MRLs specified by the EU and China, except for pesticides without MRLs. The residue level of imidacloprid with a detection frequency of up to 82% was lower than the MRL (1 mg/kg) set by China (GB 2763-2016). Figure 4 reveals the types, frequency and concentration range of 26 pesticides detected in 50 wolfberry samples. From this test, the adaptability of the developed method to the detection of multiple pesticide residues in wolfberry was determined.
Figure 4.
Detection results of pesticide residues in real samples with the developed method.
3. Materials and Methods
3.1. Chemicals and Reagents
Pesticide reference standards of all analytes were of purity > 98%, purchased from Aldrich-Sigma (Shanghai, China) and Dr. Ehrenstorfer GmbH (Augsburg, Germany). Each kind of pesticide standard substances was accurately labeled as 10 mg in a 100 mL volumetric flask, then dissolved with methanol and fixed to the scale line. A composite sample working standard solution was prepared by combining aliquots of each stock solution and diluting in methanol to obtain a final concentration of 10 mg/L, a series of matrix-matched standard solutions was obtained by gradually diluting the stock solution with blank matrix solution. All solutions were stored at 4 °C before use.
Acetonitrile (MeCN) and methanol (MeOH) were of HPLC grade, purchased from Fisher Scientific (Pittsburgh, PA, USA). High purity water was obtained using a Milli-Q POD water purification system (Millipore, Schwalbach, Germany). LUMTECHTM Sin-QuEChERS Nano catridges (2 g Na2SO4, 0.6 g MgSO4, 90 mg PSA, 15 mg MWCNTs) and the three kinds of salting out packages for the traditional QuEChERS method (4 g MgSO4, 1 g NaCl), AOAC 2007.01 Method (6 g MgSO4, 1.5 g NaOAc) and EN 15,662 Method (4 g MgSO4, 1 g NaCl, 1 g TSCD, 0.5 g DHS) respectively were provided by Lumiere Technologies (Beijing, China). HyperSep C18 and HyperSep NH2 solid phase extraction catridges were purchased from Thermo Scientific (Waltham, MA, USA), SampliQ Florisil solid phase extraction catridges were obtained from Agilent Technologies (Santa Clara, CA, USA), and ProElut GLASS PSA solid phase extraction catridges were obtained from Dikma Technologies (Beijing, China), respectively.
3.2. Instrument
An ACQUITY Quattro Premier XE system (Waters Corp., Milford, MA, USA) was used for analysis. Chromatographic separation was performed at 35 °C with an ACQUITY UPLC HSS T3 (1.8 μm, 2.1 × 100 mm, Waters Corp., Milford, MA, USA). The mobile phase A was 0.1% formic acid solution and the mobile phase B was 0.1% formic acid acetonitrile. Initial composition of the mobile phase was 80% of solvent A and 20% of solvent B, reaching the 95% of solvent B at 6 min. The concentration of B remained at 95% for 2 min before returning to the initial state in 1 min. Re-equilibration of the column was performed for 1 min before the next injection was conducted. The flow rate of the eluent was 0.4 mL/min and the injection volume was 1 μL. The mass spectrometer was equipped with an electrospray ionization (ESI) interface operating at positive (ESI+) or negative (ESI−) mode, and MRM mode was adopted for date collection. The electrospray voltage, the atomizing gas pressure, the auxiliary airflow speed and the ion source temperature were set as 3000 V, 7.0 Bar, 150 L/h and 400 °C, respectively. The 107 analytical parameters of liquid chromatography tandem mass spectrometry are listed in Table 1.
3.3. Sample Preparation
Wolfberry samples were collected from supermarkets, pharmacies and farmers’markets in different cities of Shandong Province, then the samples were ground into powder, passed through mesh screen (0.42 mm). The prepared samples were stored at 4 ℃ and analysed withinn 24 h following the procedure described below. Wolfberry (pesticide-free) obtained from an organic production base was used as blank matrix for preparing standard curve and the recovery studies.
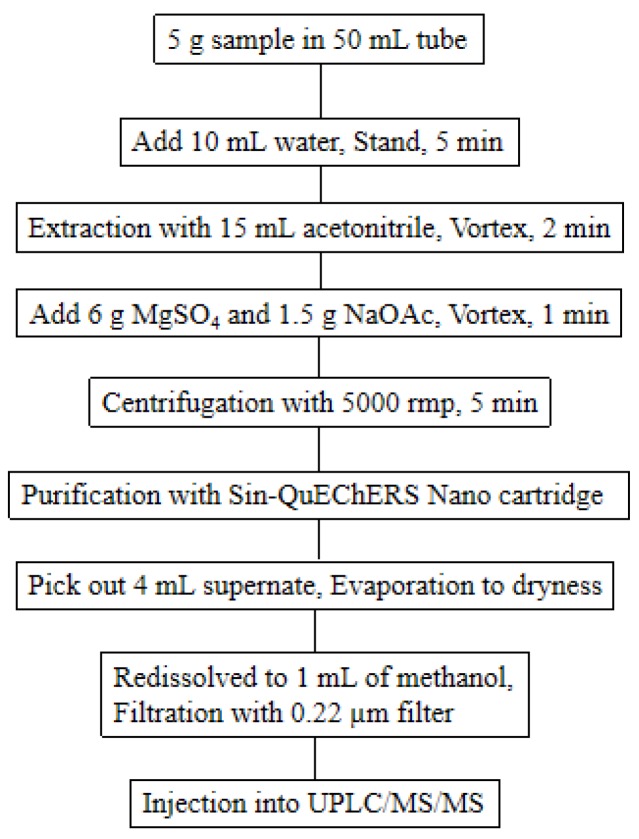
Five grams of ground wolfberry sample were weighed exactly into a 50 mL polypropylene centrifuge tube and soaked with 10 mL water for 5 min. Subsequently, 15 mL acetonitrile was added, next vortexed for 2 min, after following AOAC 2007. 01 extraction salt pack was added, after that vortexed for 1 min, succeeding centrifuged at 5000 rpm for 5 min. The Sin-QuEChERS Nano purification tube was taken into the 50 mL centrifuge tube, which was filled with extraction liquid, eventually the purification tube was slowly pressed down by an automatic apparatus to making up about 4 mL supernatant in the storage tank of the purification pipe. The structure and usage of Sin-QuEChERS Nano catridges were shown in Figure 5. The supernatant of 4 mL was accurately transferred to 10 mL centrifugal tube, dried with nitrogen gas stream and resolved with 1 mL methanol. Before UPLC/MS/MS analysis, the solution was filtered through a 0.22 µm filter (Dikma, Technologies). The flow chart of sample preparation procedure is presented in Figure 6.
Figure 5.
A schematic diagram of the structure and use of Sin-QuEChERS Nano catridges.
Figure 6.
Scheme of pesticide extraction procedures from wolfberry samples.
4. Conclusions
In this work, a simple and rapid method has been developed for the determination of 107 pesticide residues in wolfberry samples using UPLC/MS/MS analysis. The qualitative and quantitative analysis of 107 pesticides can be accomplished in 10 min at a time with one injection. The Sin-QuEChERS Nano purification column can not only effectively remove pigments, organic acids, alkaline interferents, fat and water, but also save sample preparation time and avoid the losses caused by solvent transfers. It greatly simplifies the purification process of the samples, while improving the detection efficiency and accuracy. The linearity, matrix effect, analysis limits, precision, stability and accuracy were validated in detail. The LODs and LOQs of the target pesticides obtained by this method were in the range of 0.14–1.91 µg/kg and 0.46–6.37 µg/kg, respectively. Under the premise of ensuring the accuracy and reproducibility of the results, the average recoveries (80%–120%) and RSD (<15%) of most target pesticides at the three spike levels were acceptable, which met the requirements of conventional pesticide residues screening. Fifty commercial wolfberry samples were tested for pesticide residues by the developed method, all of which were positive, and 26 pesticides were detected. These results demonstrated that the proposed method was sensitive, fast, simple and reliable for the simultaneous determination of 107 pesticide residues in wolfberry, furthermore did not require complex purification process to allow routine analysis of a large quantity of samples. Furthermore, the developed approach can further expand the types of target pesticides and be applied to the detection of pesticide residues in more other traditional Chinese medicine. This will be the focus of our future work.
Acknowledgments
The authors would like to thank the Agricultural College of Shandong Agricultural University for helping to collect the wolfberry samples for analysis.
Author Contributions
J.-N.C. carried out the experiments, data acquisition and analysis, and wrote the manuscript; Y.-J.L. collated experimental data and provided technical assistance; Y.-R.Z. carried out data analysis and revised the manuscript; M.-H.W. carried out date acquisition and analysis; X.-Q.Z. provided technical assistance; J.-H.W. provided the wolfberry samples; Y.-N.W. provided technical assistance and revised the manuscript; M.-L.W. designed the experiments and carried out data analysis.
Funding
This research was funded by National Key Research and Development Plan for the “Thirteenth Five-Year Plan” (Multi-target high-throughput screening technology research on potential hazardous materials for high-value agricultural products), grant number 2017YFF0211304), and Shandong Modern Agriculture Industry Technology System Project, grant number SDAIT-20-04.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Zhang X.F., Chen J., Yang J., Shi Y.P. UPLC-MS/MS analysis for antioxidant components of Lycii Fructus based on spectrum-effect relationship. Talanta. 2018;180:389–395. doi: 10.1016/j.talanta.2017.12.078. [DOI] [PubMed] [Google Scholar]
- 2.Zhao J.H., Li H.X., Xi W.P., An W., Niu L.L., Cao Y.L., Wang H.F., Wang Y.J., Yin Y. Changes in sugars and organic acids in wolfberry (Lycium barbarum L.) fruit during development and maturation. Food Chem. 2015;173:718–724. doi: 10.1016/j.foodchem.2014.10.082. [DOI] [PubMed] [Google Scholar]
- 3.Jin M.L., Huang Q.S., Zhao K.Z., Shang P. Biological activities and potential health benefit effects of polysaccharides isolated from Lycium barbarum L. Int. J. Biol. Macromol. 2013;54:16–23. doi: 10.1016/j.ijbiomac.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 4.Amagase H., Farnsworth N.R. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji) Food Res. Int. 2011;44:1702–1717. doi: 10.1016/j.foodres.2011.03.027. [DOI] [Google Scholar]
- 5.Qian D., Zhao Y., Yang G., Huang L. Systematic review of chemical constituents in the genus Lycium (Solanaceae) Molecules. 2017;22:911. doi: 10.3390/molecules22060911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo G.P., Dang T.T., Deng Y.N., Han J.L., Zou Z.H., Jing S., Zhang Y., Lin Q., Huang L.J., Wang Z.F. Physicochemical properties and biologicai activities of polysaccharides from Lycium barbarum prepared by fractional precipitation. Int. J. Biol. Macromol. 2018;109:611–618. doi: 10.1016/j.ijbiomac.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 7.Wang W.J., Zhang T. Integration of traditional Chinese medicine and Western medicine in the era of precision medicine. J. Integr. Med. 2017;15:1–7. doi: 10.1016/S2095-4964(17)60314-5. [DOI] [PubMed] [Google Scholar]
- 8.Wu D.T., Guo H., Lin S., Lam S.C., Zhao L., Lin D.R., Qin W. Review of the structural characterization, quality evaluation, and industrial application of Lycium barbarum polysaccharides. Trends Food. Sci. Technol. 2018;79:171–183. doi: 10.1016/j.tifs.2018.07.016. [DOI] [Google Scholar]
- 9.Xiao J.J., Xu X., Wang F., Ma J.J., Liao M., Shi Y.H., Fang Q.K., Cao H.Q. Analysis of exposure to pesticide residues from Traditional Chinese Medicine. J. Hazard. Mater. 2019;365:857–867. doi: 10.1016/j.jhazmat.2018.11.075. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y., Rukeya J., Tao W., Sun P., Ye X. Bioactive compounds and antioxidant activity of wolfberry infusion. Sci. Rep. 2017;7:40605. doi: 10.1038/srep40605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan X.L., Dong F.S., Wu X.H., Xu J., Liu X.G., Zheng Y.Q. Progress of the discovery, application, and control technologies of chemical pesticides in China. J. Integr. Agric. 2019;18:840–853. doi: 10.1016/S2095-3119(18)61929-X. [DOI] [Google Scholar]
- 12.Zhan X.P., Ma L., Huang L.Q., Chen J.B., Zhao L. The optimization and establishment of QuEChERS-UPLC-MS/MS method for simultaneously detecting various kinds of pesticides residues in fruits and vegetables. J. Chromatogr. B. 2017;1060:281–290. doi: 10.1016/j.jchromb.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Diop A., Diop Y.M., Thiare D.D., Cazier F., Sarr S.O., Kasprowiak A., Landy D., Delattre F. Monitoring survey of the use patterns and pesticide residues on vegetables in the Niayes zone, Senegal. Chemosphere. 2016;144:1715–1721. doi: 10.1016/j.chemosphere.2015.10.058. [DOI] [PubMed] [Google Scholar]
- 14.Chen X.X., Li X., Pang K.J., Fan X.Q., Ma Y.C., Hu J.Y. Dissipation behavior and residue distribution of fluazaindolizine and its seven metabolites in tomato ecosystem based on SAX SPE procedure using HPLC-QqQ-MS/MS technique. J. Hazard. Mater. 2018;342:698–704. doi: 10.1016/j.jhazmat.2017.08.056. [DOI] [PubMed] [Google Scholar]
- 15.Farajzadeh M.A., Khoshmaram L. Air-assisted liquid-liquid microextraction-gas chromatography-flame ionisation detection: A fast and simple method for the assessment of triazole pesticides residues in surface water, cucumber, tomato and grape juices samples. Food Chem. 2013;141:1881–1887. doi: 10.1016/j.foodchem.2013.05.088. [DOI] [PubMed] [Google Scholar]
- 16.Taha S.M., Gadalla S.A. Development of an efficient method for multi residue analysis of 160 pesticides in herbal plant by ethyl acetate hexane mixture with direct injection to GC-MS/MS. Talanta. 2017;174:767–779. doi: 10.1016/j.talanta.2017.06.080. [DOI] [PubMed] [Google Scholar]
- 17.Ji W.H., Sun R.H., Duan W.J., Wang X., Wang T., Mu Y., Guo L.P. Selective solid phase extraction of chloroacetamide herbicides from environmental water samples by amphiphilic magnetic molecularly imprinted polymers. Talanta. 2017;170:111–118. doi: 10.1016/j.talanta.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Bandforuzi S.R., Hadjmohammadi M.R. Modified magnetic chitosan nanoparticles based on mixed hemimicelle of sodium dodecyl sulfate for enhanced removal and trace determination of three organophosphorus pesticides from natural waters. Anal. Chim. Acta. 2019;1078:90–100. doi: 10.1016/j.aca.2019.06.026. [DOI] [PubMed] [Google Scholar]
- 19.Gil García M.D., Uclés Duque S., Lozano Fernández A.B., Sosa A., Fernández-Alba A.R. Multiresidue method for trace pesticide analysis in honeybee wax comb by GC-QqQ-MS. Talanta. 2017;163:54–64. doi: 10.1016/j.talanta.2016.10.083. [DOI] [PubMed] [Google Scholar]
- 20.Wang S.C., Qi P.P., Di S.S., Wang J., Wu S.G., Wang X.Y., Wang Z.W., Wang Q., Wang X.Q., Zhao C.S., et al. Significant role of supercritical fluid chromatography-mass spectrometry in improving the matrix effect and analytical efficiency during multi-pesticides residue analysis of complex chrysanthemum samples. Anal. Chim. Acta. 2019;1074:108–116. doi: 10.1016/j.aca.2019.04.063. [DOI] [PubMed] [Google Scholar]
- 21.Chen X.Y., Zhao K.X., Ge B.K., Chen Q.Y. Simultaneous determination of 44 pesticides in tobacco by UPLC/MS/MS and a modified QuEChERS procedure. J. AOAC Int. 2013;96:422–431. doi: 10.5740/jaoacint.11-524. [DOI] [PubMed] [Google Scholar]
- 22.Madej K., Kalenik T.K., Piekoszewski W. Sample preparation and determination of pesticides in fat-containing foods. Food Chem. 2018;269:527–541. doi: 10.1016/j.foodchem.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Samsidar A., Siddiquee S., Shaarani S.M. A review of extraction, analytical and advanced methods for determination of pesticides in environment and foodstuffs. Trends. Food Sci. Technol. 2018;71:188–201. doi: 10.1016/j.tifs.2017.11.011. [DOI] [Google Scholar]
- 24.Bresin B., Piol M., Fabbro D., Mancini M.A., Casetta B., Bianco C.D. Analysis of organo-chlorine pesticides residue in raw coffee with a modified “quick easy cheap effective rugged and safe” extraction/clean up procedure for reducing the impact of caffeine on the gas chromatography-mass spectrometry measurement. J. Chromatogr. A. 2015;1376:167–171. doi: 10.1016/j.chroma.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 25.Jian W., Wendy C. UHPLC/ESI-MS/MS Determination of 187 Pesticides in Wine. J. AOAC Int. 2016;99:539–557. doi: 10.5740/jaoacint.15-0265. [DOI] [PubMed] [Google Scholar]
- 26.Guo J.G., Tong M.M., Tang J., Bian H.Z., Wan X.C., He L.L., Hou R.Y. Analysis of multiple pesticide residues in polyphenol-rich agricultural products by UPLC-MS/MS using a modified QuEChERS extraction and dilution method. Food Chem. 2019;274:452–459. doi: 10.1016/j.foodchem.2018.08.134. [DOI] [PubMed] [Google Scholar]
- 27.Tighrine A., Amir Y., Alfaro P., Mamou M., Nerín C. Simultaneous extraction and analysis of preservatives and artificial sweeteners in juices by salting out liquid-liquid extraction method prior to ultra-high performance liquid chromatography. Food Chem. 2019;277:586–594. doi: 10.1016/j.foodchem.2018.10.107. [DOI] [PubMed] [Google Scholar]
- 28.Castro G., Rodríguez I., Ramil M., Cela R. Selective determination of sartan drugs in environmental water samples by mixed-mode solid-phase extraction and liquid chromatography tandem mass spectrometry. Chemosphere. 2019;224:562–571. doi: 10.1016/j.chemosphere.2019.02.137. [DOI] [PubMed] [Google Scholar]
- 29.Bartosiak M., Jankowski K., Giersz J. Determination of cobalt species in nutritional supplements using ICP-OES after microwave-assisted extraction and nutritional supplements using solid-phase extraction. J. Pharm. Biomed. 2018;155:135–140. doi: 10.1016/j.jpba.2018.03.058. [DOI] [PubMed] [Google Scholar]
- 30.Di X., Wang X., Liu Y.P., Guo X.J., Di X. Microwave assisted extraction in combination with solid phase purification and switchable hydrophilicity solvent-based homogeneous liquid-liquid microextraction for the determination of sulfonamides in chicken meat. J. Chromatogr. B. 2019;1118–1119:109–115. doi: 10.1016/j.jchromb.2019.04.036. [DOI] [PubMed] [Google Scholar]
- 31.Yen H.W., Yang S.C., Chen C.H., Chang J.S. Supercritical fluid extraction of valuable compounds from microalgal biomass. Bioresour. Technol. 2015;184:291–296. doi: 10.1016/j.biortech.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 32.Ahmad R., Ahmad N., Shehzad A. Solvent and temperature effects of accelerated solvent extraction (ASE) with Ultra-high pressure liquid chromatography (UHPLC-PDA) technique for determination of Piperine and its ICP-MS analysis. Ind. Crops Prod. 2019;136:37–49. doi: 10.1016/j.indcrop.2019.04.016. [DOI] [Google Scholar]
- 33.Chuang Y.H., Zhang Y.J., Zhang W., Boyd S.A., Li H. Comparison of accelerated solvent extraction and quick, easy, cheap, effective, rugged and safe method for extraction and determination of pharmaceuticals in vegetables. J. Chromatogr. A. 2015;1404:1–9. doi: 10.1016/j.chroma.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 34.Saha S., Walia S., Kundu A., Sharma K., Paul R.K. Optimal extraction and fingerprinting of carotenoids by accelerated solvent extraction and liquid chromatography with tandem mass spectrometry. Food Chem. 2015;177:369–375. doi: 10.1016/j.foodchem.2015.01.039. [DOI] [PubMed] [Google Scholar]
- 35.Liang L., Wang X.H., Sun Y., Ma P.Y., Li X.P., Piao H.L., Jiang Y.X., Song D.Q. Magnetic solid-phase extraction of triazine herbicides from rice using metal-organic framework MIL-101(Cr) functionalized magnetic particles. Talanta. 2018;179:512–519. doi: 10.1016/j.talanta.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 36.Ma J.P., Wu G.G., Li S., Tan W.Q., Wang X.Y., Li J.H., Chen L.X. Magnetic solid-phase extraction of heterocyclic pesticides in environmental water samples using metal-organic frameworks coupled to high performance liquid chromatography determination. J. Chromatogr. A. 2018;1553:57–66. doi: 10.1016/j.chroma.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 37.Lian W.L., Ren F.L., Tang L.Y., Dong D.Z. Analysis of polycyclic aromatic hydrocarbons in cigarette samples using gel permeation chromatography clean-up by gas chromatography-tandem mass spectrometry. Microchem. J. 2016;129:194–199. doi: 10.1016/j.microc.2016.06.021. [DOI] [Google Scholar]
- 38.Qian M.R., Zhang H., Wu L.Q., Jin N., Wang J.M., Jiang K.Z. Simultaneous determination of zearalenone and its derivatives in edible vegetable oil by gel permeation chromatography and gas chromatography-triple quadrupole mass spectrometry. Food Chem. 2015;166:23–28. doi: 10.1016/j.foodchem.2014.05.133. [DOI] [PubMed] [Google Scholar]
- 39.Zheng G.C., Han C., Liu Y., Wang J., Zhu M.W., Wang C.J., Shen Y. Multiresidue analysis of 30 organochlorine pesticides in milk and milk powder by gel permeation chromatography-solid phase extraction-gas chromatography-tandem mass spectrometry. J. Dairy Sci. 2014;97:6016–6026. doi: 10.3168/jds.2014-8192. [DOI] [PubMed] [Google Scholar]
- 40.Anastassiades M., Lehotay S.J., Stajnbaher D., Schenck F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003;86:412–431. [PubMed] [Google Scholar]
- 41.Villaverde J.J., Sevilla-Morán B., López-Goti C., Alonso-Prados J.L., Sandín-España P. Computational-Based Study of QuEChERS Extraction of Cyclohexanedione Herbicide Residues in Soil by Chemometric Modeling. Molecules. 2018;23:2009. doi: 10.3390/molecules23082009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lehotay S.J. Determination of pesticide residues in foods by acetonitrile extraction and partitioning with magnesium sulfate: Collaborative study. J. AOAC Int. 2007;90:485–520. [PubMed] [Google Scholar]
- 43.Foods of Plant Origin—Determination of Pesticide Residues Using GC-MS and/or LC-MS/MS Following Acetonitrile Extraction/Partitioning and Clean-Up by Dispersive SPE-QuEChERS-Method. [(accessed on 20 February 2009)]; Available online: www.cen.eu.
- 44.Chen L.N., Song F.R., Liu Z.Q., Zheng Z., Xing J.P., Liu S.Y. Multi-residue method for fast determination of pesticide residues in plants used in traditional chinese medicine by ultra-high-performance liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A. 2012;1225:132–140. doi: 10.1016/j.chroma.2011.12.071. [DOI] [PubMed] [Google Scholar]
- 45.Liu H.M., Kong W.J., Qi Y., Gong B., Miao Q., Wei J.H., Yang M.H. Streamlined pretreatment and GC-FPD analysis of multi-pesticide residues in perennial Morinda roots: A tropical or subtropical plant. Chemosphere. 2014;95:33–40. doi: 10.1016/j.chemosphere.2013.07.085. [DOI] [PubMed] [Google Scholar]
- 46.Miao Q., Kong W.J., Yang S.H., Yang M.H. Rapid analysis of multi-pesticide residues in lotus seeds by a modified QuEChERS-based extraction and GC-ECD. Chemosphere. 2013;91:955–962. doi: 10.1016/j.chemosphere.2013.01.104. [DOI] [PubMed] [Google Scholar]
- 47.Han Y.G., Zou N., Song L., Li Y.J., Qin Y.H., Liu S.W., Li X.S., Pan C.P. Simultaneous determination of 70 pesticide residues in leek, leaf lettuce and garland chrysanthemum using modified QuEChERS method with multi-walled carbon nanotubes as reversed-dispersive solid-phase extraction materials. J. Chromatogr. B. 2015;1005:56–64. doi: 10.1016/j.jchromb.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Fan S.F., Zhao P.Y., Yu C.S., Pan C.P., Li X.S. Simultaneous determination of 36 pesticide residues in spinach and cauliflower by LC-MS/MS using multi-walled carbon nanotubes-based dispersive solid-phase clean-up. Food Addit. Contam. A. 2014;31:73–82. doi: 10.1080/19440049.2013.853324. [DOI] [PubMed] [Google Scholar]
- 49.Wang S., Zhao P., Min G., Fang G.Z. Multi-residue determination of pesticides in water using multi-walled carbon nanotubes solid-phase extraction and gas chromatography-mass spectrometry. J. Chromatogr. A. 2007;1165:166–171. doi: 10.1016/j.chroma.2007.07.061. [DOI] [PubMed] [Google Scholar]
- 50.Hou X., Lei S.R., Qin S.T., Guo L.A., Yi S.G., Lin W. A multi-residue method for the determination of pesticides in tea using multi-walled carbon nanotubes as a dispersive solid phase extraction absorbent. Food Chem. 2014;153:121–129. doi: 10.1016/j.foodchem.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 51.Zhu B.Q., Xu X.Y., Luo J.W., Jin S.Q., Chen W.Q., Lin Z., Tian C.X. Simultaneous determination of 131 pesticides in tea by on-line GPC-GC-MS/MS using graphitized multi-walled carbon nanotubes as dispersive solid phase extraction sorbent. Food Chem. 2019;276:202–208. doi: 10.1016/j.foodchem.2018.09.152. [DOI] [PubMed] [Google Scholar]
- 52.Han Y.T., Song L., Zou N., Chen R.H., Qin Y.H., Pan C.P. Multi-residue determination of 171 pesticides in cowpea using modified QuEChERS method with multi-walled carbon nanotubes as reversed-dispersive solid-phase extraction materials. J. Chromatogr. B. 2016;1031:99–108. doi: 10.1016/j.jchromb.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 53.Zhao P.F., Wang Z.K., Li K.J., Guo X.J., Zhao L.S. Multi-residue enantiomeric analysis of 18 chiral pesticides in water, soil and river sediment using magnetic solid-phase extraction based on amino modified multiwalled carbon nanotubes and chiral liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. A. 2018;1568:8–21. doi: 10.1016/j.chroma.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 54.Lehotay S.J., Son K.A., Kwon H., Koesukwiwat U., Fu W., Mastovska K., Hoh E., Leepipatpiboon N. Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruits and vegetables. J. Chromatogr. A. 2010;1217:2548–2560. doi: 10.1016/j.chroma.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 55.Zhang H.L., Wang J.H., Li L., Wang Y. Determination of 103 Pesticides and Their Main Metabolites in Animal Origin Food by QuEChERS and Liquid Chromatography-Tandem Mass Spectrometry. Food Anal. Methods. 2017;10:1826–1843. doi: 10.1007/s12161-016-0736-7. [DOI] [Google Scholar]
- 56.Han Y.T., Xu J., Dong F.S., Li W.M., Liu X.G., Li Y.B., Kong Z.Q., Zhu Y.L., Liu N., Zheng Y.Q. The fate of spirotetramat and its metabolite spirotetramat-enol in apple samples during apple cider processing. Food Control. 2013;34:283–290. doi: 10.1016/j.foodcont.2013.05.009. [DOI] [Google Scholar]
- 57.Niessen W., Manini P., Andreoli R. Matrix effects in quantitative pesticide analysis using liquid chromatography-mass spectrometry. Mass Spectrom. Rev. 2006;25:881–899. doi: 10.1002/mas.20097. [DOI] [PubMed] [Google Scholar]
- 58.Pan X.L., Dong F.S., Xu J., Liu X.G., Chen Z.L., Liu N., Chen X.X., Tao Y., Zhang H.J., Zheng Y.Q. Simultaneous determination of chlorantraniliprole and cyantraniliprole in fruits, vegetables and cereals using ultra-high-performance liquid chromatography-tandem mass spectrometry with the isotope-labelled internal standard method. Anal. Biolanal. Chem. 2015;407:4111–4120. doi: 10.1007/s00216-015-8603-8. [DOI] [PubMed] [Google Scholar]
- 59.Matuszewski B.K., Constanzer M.L., Chavez-Eng C.M. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS (Article) Anal. Chem. 2003;75:3019–3030. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]