Abstract
A library of isopulegol-based bi-, tri- and tetrafunctional chiral ligands has been developed from commercially available (−)-isopulegol and applied as chiral catalysts in the addition of diethylzinc to benzaldehyde. Michael addition of primary amines towards α-methylene-γ-butyrolactone, followed by reduction, was accomplished to provide aminodiols in highly stereoselective transformations. Stereoselective epoxidation of (+)-neoisopulegol, derived from natural (−)-isopulegol, and subsequent oxirane ring opening with primary amines afforded aminodiols. The regioselective ring closure of N-substituted aminodiols with formaldehyde was also investigated. Hydroxylation of (+)-neoisopulegol resulted in diol, which was then transformed into aminotriols by aminolysis of its epoxides. Dihydroxylation of (+)-neoisopulegol or derivatives with OsO4/NMO gave neoisopulegol-based di-, tri- and tetraols in highly stereoselective reactions. The antimicrobial activity of aminodiol and aminotriol derivatives as well as di-, tri- and tetraols was also explored. In addition, structure–activity relationships were examined by assessing substituent effects on the aminodiol and aminotriol systems.
Keywords: aminodiols, aminotriols, diols, triols, tetraols, chiral catalysts, antimicrobial activity
1. Introduction
In recent years, the discovery of aminodiols and their applications as building moieties of complex bioactive molecules have attracted significant attention due to their biological activities. The aminodiol moieties possess a cardiovascular, cytostatic, and antiviral effect [1]. For example, aristeromycin, first isolated from Streptomyces citricolor, and its modified derivatives belong to an important group of carbocyclic nucleosides that exhibit a wide range of pharmacological properties such as antiviral, anticancer and antitoxoplasma activities. Aristeromycin analogues, in particular, are widely used as antiviral agents against a range of viruses, including the human immunodeficiency, hepatitis B, herpes simplex, varicella-zoster, influenza and hepatitis C viruses [2,3,4]. (2R,3R,7Z)-2-Aminotetradec-7-ene-1,3-diol, a new sphingosine derivative of the Caribbean sponge Haliclona vansoesti, is a potent antimicrobial metabolite [5]. The Abbott aminodiol, found to be a useful building block for the synthesis of the potent renin inhibitor Zankiren®, and Enalkiren®, was introduced into the therapy of hypertension [6,7]. Aminodiols can also exert antidepressive activity. For example, (S,S)-reboxetine, a selective norepinephrine reuptake inhibitor, was approved in many countries for the treatment of unipolar depression [8], while some aminodiols have been investigated as selective antagonists on receptor P2X1 [9]. Other aminodiols may serve as starting materials for the synthesis of biologically active natural compounds. For example, cytoxazone, a microbial metabolite isolated from Streptomyces species, is a selective modulator of the secretion of TH2 cytokine [10,11]. Some bicyclic aminodiol-based carbocyclic nucleoside analogues exert antiviral activity [12].
Besides their biological interest, aminodiols have also been applied as starting materials in asymmetric syntheses or as chiral auxiliaries and ligands in enantioselective transformations [13]. To develop new, efficient and commercially available chiral catalysts, chiral natural products including (+)- and (−)-α-pinene [14,15], (+)-carene [16,17], (-)-menthone [18], (−)-fenchone [19], (+)-sabinol [20], (−)-nopinone [21] or (−)-pulegone [22] can serve as important starting materials for the synthesis of aminodiols. Monoterpene-based aminodiols have been demonstrated to be excellent chiral auxiliaries in a wide range of stereoselective transformations including intramolecular radical cyclisation [23], intramolecular [2+2] photocycloaddition [24] and Grignard addition [25,26].
Monoterpene-based diols or triols have also proved to be good chiral auxiliaries and catalysts [27,28]. They also possess marked biological properties; e.g., antimicrobial, antifungal or enzyme inhibitor activities [29,30,31].
In the present contribution, we report the preparation of a new library of isopulegol-based chiral bi-, tri- and tetrafunctional synthons, such as aminodiols, aminotriols, di-, tri- and tetraols, starting from commercially available natural (−)-isopulegol. Our study also involved the evaluation of the resulting ligands as catalysts in the asymmetric transformation and antimicrobial activity on multiple bacterial and fungal strains of new isopulegol derivatives.
2. Results
2.1. Synthesis of (−)-α-methylene-γ-butyrolactone 4
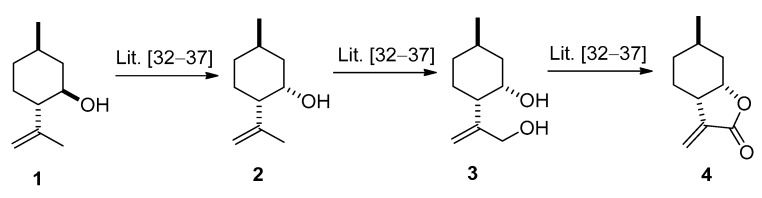
The key intermediate (−)-α-methylene-γ-butyrolactone 4 was prepared from commercially available (−)-isopulegol 1 by oxidizing its hydroxy group, followed by stereoselective reduction of the resulting carbonyl group providing (+)-neoisopulegol 2. Regioselective allylic hydroxylation of 2 gave diol 3, which was transformed to 4 by oxidation and ring closure of the obtained γ-hydroxy-substituted α,β-unsaturated carboxylic acid applying literature methods [32,33,34,35,36,37] (Figure 1).
Figure 1.
Synthesis of (−)-isopulegol-based α-methylene-γ-butyrolactone 4.
2.2. Synthesis of Isopulegol-based Aminodiols
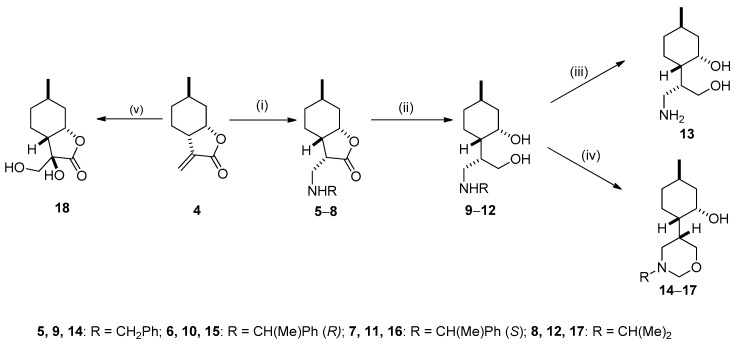
Nucleophilic addition of primary amines to α-methylene-γ-butyrolactone 4 has proved to be an efficient method for the preparation of a highly diversified library of β-aminolactones 5–8 [38,39,40]. Treatment of β-aminolactones with LiAlH4 resulted in secondary aminodiols 9–12 [16]. Secondary aminodiols 9–11 were transformed into primary diol 13 with debenzylation through hydrogenolysis over Pd/C. In order to study the regioselectivity of ring closure of the aminodiol function, we attempted to incorporate the hydroxy groups of aminodiols into products with 1,3-oxazine or 1,3-oxazepine ring [16,22,41]. When aminodiols 9–12 were reacted with HCHO under mild conditions, 1,3-oxazine 14–17 were obtained in highly regioselective ring closure (Scheme 1).
Scheme 1.
(i) RNH2 (1 equivalent), dry EtOH, 25 °C, 20 h, 60–70%; (ii) LiAlH4 (2 equivalent), dry Et2O, 25 °C, 4 h, 74–99%, (iii) 5% Pd/C, H2 (1 atm), 25 °C, 24 h, 50%, (iv) 35% HCHO, Et2O, 25 °C, 1 h, 64–83%; (v) 2% OsO4/t-BuOH, 50% NMO/H2O, acetone, 25 °C, 24 h, 50%.
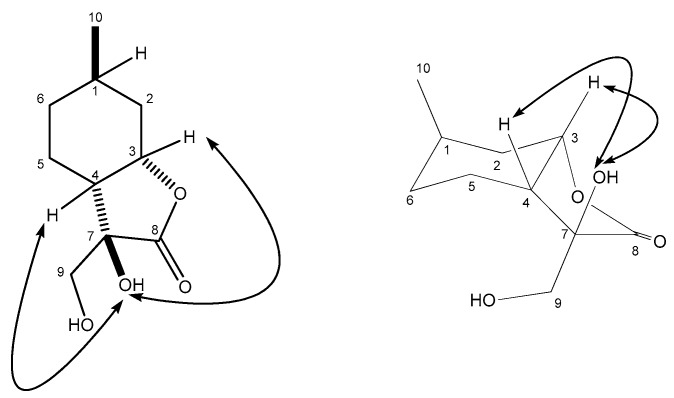
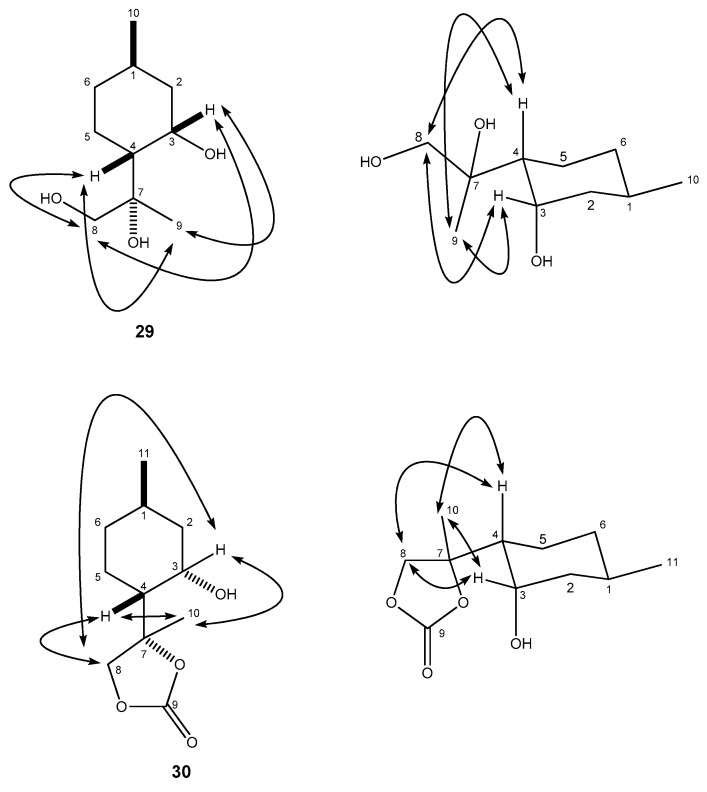
Dihydroxylation of 4 with OsO4 and NMO (4-Methylmorpholine N-oxide) furnished 18 in an acceptable yield [16,22] (Scheme 1). The relative configuration of compound 18 was determined by means of NOESY (Nuclear Overhauser Effect SpecroscopY) experiments: clear NOE (Nuclear Overhauser Effect) signals were observed between the OH-7 and H-3 as well as OH-7 and H-4 protons (Figure 2).
Figure 2.
Determination of the structure of diol 18 by NOESY.
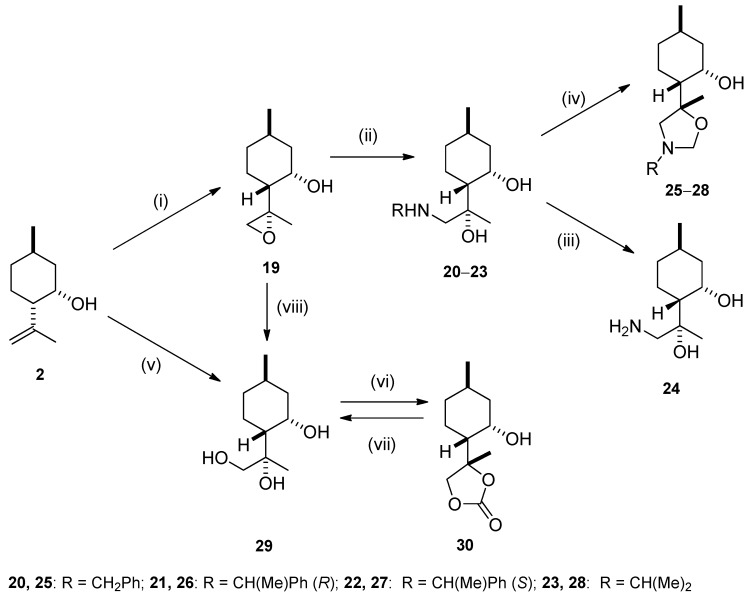
Epoxidation of 2 with t-BuOOH in the presence of vanadyl acetylacetonate (VO(acac)2) as catalyst furnished epoxide 19 in a stereospecific reaction [35,42,43,44]. Since our earlier results clearly demonstrated that substituents at nitrogen of aminodiols exerted definite influence on the efficiency of their catalytic activity, aminodiol library 20–23 was prepared by aminolysis of 19 with different primary amines and LiClO4 as catalyst [17,41,45,46], whereas exposure of 19 to NaOH furnished 29 with retention of stereochemistry [47]. Debenzylation via hydrogenolysis of compounds 20–22 over Pd/C in MeOH resulted in primary aminodiol 24 in moderate yield. When aminodiols 20–23 were treated with HCHO at room temperature, oxazolidines 25–28 were obtained in highly regioselective ring closures, similarly to the regioisomeric oxazine analogues (Scheme 2).
Scheme 2.
(i) VO(acac)2, 70% t-BuOOH (2 equivalent), dry toluene, 25 °C, 12 h, 76%; (ii) RNH2 (2 equivalent), LiClO4 (1 equivalent), MeCN, 70–80 °C, 6 h, 40–88%; (iii) 5% Pd/C, H2 (1 atm), MeOH, 25 °C, 24 h, 72%; (iv) 35% HCHO, Et2O, 25 °C, 1 h, 60–70%; (v) 2% OsO4/t-BuOH, 50% NMO/H2O, acetone, 25 °C, 24 h, 61%; (vi) triphosgene (0.5 equivalent), dry pyridine (4 equivalent), dry CH2Cl2, 25 °C, 2 h, 60%; (vii) LiAlH4 (2 equivalent), dry ether, 25 °C, 4 h, 80%, (viii) 3 M NaOH, DMSO, 80 °C, 2 h, 60%.
The syn-selective dihydroxylation of compound 2 with OsO4 in the presence of a stoichiometric amount of the co-oxidant NMO furnished product 29 as a single diastereomer in moderate yield (Scheme 2). The relative configuration of compound 29 was determined by means of NOESY experiments: clear NOE signals were observed between the H-9 and H-3, H-4 as well as H-8 and H-3, H-4 protons (Figure 3).
Figure 3.
Determination of the structure of diol 29 and carbonate 30 by NOESY.
The structure of compound 29 was confirmed by its five-membered cyclic carbonate 30 synthesized from 29 by the reaction with triphosgene (Scheme 2) [48]. It is well known that this carbonation reaction maintains the stereochemical configuration of 29 [49,50]. The stereochemical structure of carbonate 30 was identified by NOESY analyses: characteristic NOE signals were observed between the protons H-8 and H-3, H-4 together with the protons H-10 and H-3, H-4 (Figure 3).
Reduction of 30 with LAH (Lithium aluminum hydride) proceeded smoothly giving 29 in an excellent yield (Scheme 2). It has been reported that reduction of the cyclic carbonate moiety of 30 with LAH gave the corresponding diol with the same stereochemical configuration at the carbon atoms as of the original 29 moiety [51,52,53].
2.3. Synthesis of Isopulegol-based Aminotriols
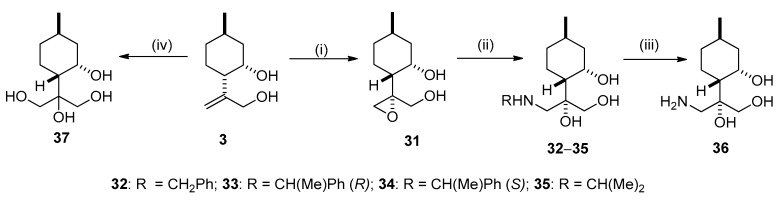
Stereospecific epoxidation of allylic diol 3 with t-BuOOH and VO(acac)2 was successfully applied to prepare epoxy diol 31 [35,43,44] (Scheme 3). The relative configuration of epoxide 31 was determined by means of NOESY experiments. Significant NOE signals were shown between the H-8 and H-3, H-4 as well as the H-3 and H-9 protons (Figure 4).
Scheme 3.
(i) VO(acac)2, 70% t-BuOOH (2 equivalent), dry toluene, 25 °C, 12 h, 80%; (ii) RNH2 (2 equivalent), LiClO4 (1 equivalent), MeCN, 70–80 °C, 6 h, 60–80%; (iii) 5% Pd/C, H2 (1 atm), MeOH, 25 °C, 24 h, 72%; (iv) 2% OsO4/t-BuOH, 50% NMO/H2O, acetone, 25 °C, 24 h, 40%.
Figure 4.
Determination of the structure of epoxide 31 by NOESY.
The oxirane ring of 31 was opened with primary amines and LiClO4 as catalyst to give aminotriol library 32–35 [45,46]. Primary aminotriol 36 was obtained by debenzylation of the corresponding aminotriols 32–34 under standard condition by hydrogenation in the presence of a Pd/C catalyst. The synthesis of tetraol 37 was effectively performed by selective dihydroxylation of compound 3 with the OsO4/NMO system [16,22] (Scheme 3).
2.4. Application of Aminodiol Derivatives and Aminotriols as Chiral Ligands for Catalytic Addition of Diethylzinc to Benzaldehyde
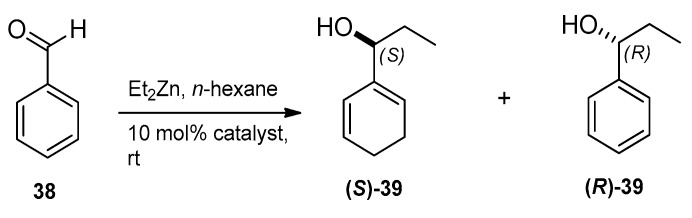
Aminodiol derivatives 9–17 and 20–28 together with aminotriols 32–36 were applied as chiral catalysts in the enantioselective addition of diethylzinc to benzaldehyde 38 to form (S)- and (R)-1-phenyl-1-propanol 39 (Scheme 4).
Scheme 4.
Model reaction for enantioselective catalysis.
The enantiomeric purity of 1-phenyl-1-propanols (S)-39 and (R)-39 was determined by GC on a CHIRASIL-DEX CB column using literature methods [14,54]. Low to moderate enantioselectives were observed. The results obtained (see Table 1) clearly show that all aminodiol derivatives favoured the formation of the (R)-enantiomer, whereas aminotriols led to the corresponding (S)-enantiomer. Aminodiol 10 afforded the best ee value (ee = 60%) with an (R)-selectivity, while aminotriol 34 showed the best ee value (ee = 28%) with an (S)-selectivity. Other compounds were also examined but their selectivities were less than 10% when applied as chiral ligands.
Table 1.
Addition of diethylzinc to benzaldehyde, catalyzed by aminodiol derivatives and aminotriols.
| Entry | Ligand | Yield a (%) | eeb (%) | Configuration c |
|---|---|---|---|---|
| 1 | 9 | 90 | 18 | (R) |
| 2 | 10 | 92 | 60 | (R) |
| 3 | 11 | 95 | 43 | (R) |
| 4 | 12 | 95 | 17 | (R) |
| 5 | 22 | 97 | 18 | (R) |
| 6 | 25 | 93 | 37 | (R) |
| 7 | 32 | 86 | 19 | (S) |
| 8 | 34 | 87 | 28 | (S) |
| 9 | 35 | 80 | 16 | (S) |
2.5. Antimicrobial Effects
Since several aminodiols [5], as well as polyols [29,30,31], exerted antimicrobial activities on various bacterial and fungal strains, antimicrobial activities of the prepared aminodiol analogues and polyols were also tested against two yeasts as well as two Gram-positive and two Gram-negative bacteria (Table 2, only the outstanding results are shown). Compounds 10 and 20 inhibited over 20% against the applied Gram-negative bacteria, while other derivatives showed weak activities. Furthermore, 11 showed an inhibition activity over 40% for P. aeruginosa, while it had no effect against E. coli. All compounds presented low to moderate inhibitions against the Gram-positive bacteria in the range of 5%–55% and 9%–35% for B. subtilis and S. aureus, respectively. In the case of B. subtilis, 24 showed much more potential antimicrobial activity, while for S. aureus, 18 and 29 proved to be the most effective agents. In the yeast assays, only 24 exhibited moderate inhibition against C. albicans, whereas significant effects were observed against C. krusei almost in all cases reaching up to 50% with analogue 16.
Table 2.
Antimicrobial activities of the synthesized compounds.
| Inhibitory Effect (%) ± RSD (%) | |||||||
|---|---|---|---|---|---|---|---|
| Yeast | Gram-negative | Gram-positive | |||||
| Analogue | Conc. (µg/mL) | C. albicans | C. krusei | E. coli | P. aeruginosa | B. subtilis | S. aureus |
| 9 | 10 | − | 38.2 ± 4.2 | − | − | − | − |
| 100 | − | 41.8 ± 1.2 | 24.8 ± 1.3 | − | − | − | |
| 10 | 10 | − | − | 21.1 ± 8.0 | − | − | − |
| 100 | − | − | 25.8 ± 10.1 | 19.5 ± 0.5 | − | − | |
| 11 | 10 | 0.2 ± 0.9 | 0.9 ± 0.5 | − | 22.6 ± 2.1 | 18.4 ± 0.7 | 8.5 ± 0.6 |
| 100 | 2.2 ± 2.3 | 3.3 ± 4.7 | 0.2 ± 1.8 | 41.6 ± 12.2 | 21.0 ± 7.5 | 9.8 ± 4.0 | |
| 12 | 10 | − | − | − | 5.4 ± 0.3 | − | 8.5 ± 6.3 |
| 100 | − | − | − | 14.3 ± 4.5 | − | 9.4 ± 5.4 | |
| 13 | 10 | − | − | − | − | 26.1 ± 8.3 | 24.5 ± 15.6 |
| 100 | − | 40.4 ± 2.4 | − | − | 42.9 ± 20.0 | 25.2 ± 1.1 | |
| 14 | 10 | − | 8.4 ± 4.1 | − | 3.2 ± 7.1 | − | − |
| 100 | − | 6.7 ± 2.0 | 25.6 ± 2.1 | 4.4 ± 5.8 | − | 12.6 ± 4.5 | |
| 15 | 10 | − | 1.9 ± 0.7 | − | 8.6 ± 2.5 | − | 3.6 ± 1.4 |
| 100 | − | 2.8 ± 4.2 | 18.0 ± 1.8 | 20.1 ± 0.2 | − | 10.4 ± 1.5 | |
| 16 | 10 | − | − | − | 4.4 ± 5.8 | − | − |
| 100 | − | 48.9 ± 0.1 | 15.7 ± 1.7 | 8.4 ± 5.1 | 4.6 ± 12.5 | − | |
| 17 | 10 | − | − | − | 15.2 ± 10.4 | − | − |
| 100 | − | 33.1 ± 0.4 | 8.5 ± 2.06 | 16.7 ± 7.2 | − | − | |
| 18 | 10 | − | − | − | − | 16.9 ± 17.7 | 34.4 ± 11.7 |
| 100 | − | − | − | − | 27.1 ± 16.0 | 34.2 ± 2.6 | |
| 20 | 10 | − | − | − | 2.0 ± 0.9 | 10.6 ± 6.4 | − |
| 100 | 4.1 ± 1.6 | − | 35.4 ± 0.8 | 19.9 ± 4.8 | 11.5 ± 1.3 | 19.7 ± 7.2 | |
| 24 | 10 | − | 34.6 ± 3.3 | − | − | 47.6 ± 10.6 | 30.0 ± 2.0 |
| 100 | 23.6 ± 1.2 | 37.8 ± 3.6 | − | − | 55.1 ± 19.9 | 33.9 ± 4.0 | |
| 29 | 10 | − | − | − | − | − | 32.4 ± 4.1 |
| 100 | − | − | − | − | 21.2 ± 5.2 | 34.7 ± 6.6 | |
| 32 | 10 | − | − | − | − | − | − |
| 100 | − | − | − | − | − | 24.6 ± 11.9 | |
| 10 | − | − | − | − | 14.9 ± 13.8 | 31.2 ± 7.9 | |
| 36 | 100 | 39.9 ± 4.1 | − | − | 43.1 ± 2.6 | 33.3 ± 2.1 | |
| 37 | 10 | − | − | − | − | − | 31.6 ± 15.1 |
| 100 | − | 26.8 ± 7.9 | − | − | 40.9 ± 16.6 | 32.8 ± 8.2 | |
Comparing the antimicrobial activities of the two families of aminodiols, our results suggest that both 3-aminomethyl-1,4-diols 9–13 and 4-amino-1,3-diols 20–24 have moderate antifungal activity against C. krusei and Gram-negative and Gram-positive bacteria. An interesting difference was observed between 1,3-oxazines and oxazolidines. Namely, oxazines 14–17 possess moderate antifungal and antibacterial activity, whereas oxazolidines 25–28 proved ineffective.
Polyols 29 and 37 have antibacterial effect only against the examined Gram-positive bacteria. From aminotriol library only primary aminotriol 36 has substantial antifungal and antibacterial effect. In contrast, N-substitution led to the loss of antimicrobial activity.
3. Discussion
Starting from the commercially available (−)-isopugeol, a new family of isopulegol-based chiral aminodiol and aminotriol libraries were prepared through chiral (+)-neoisopulegol as key intermediate via stereoselective transformations. Moreover, isopulegol-based chiral di-, tri- and tetraols, promising chiral substrates for the synthesis of chiral crown ethers were synthesized.
The resulting aminodiols exert moderate antimicrobial action on a panel of bacterial and fungal strains. The in vitro pharmacological studies have clearly shown that these primary aminodiols have significant microbiological effects. In addition, aminodiol and aminotriol derivatives were applied as chiral catalysts in the enantioselective addition of diethylzinc to benzaldehyde with moderate but significantly opposite stereoselectivity.
4. Materials and Methods
4.1. General Methods
Commercially available compounds were used as obtained from suppliers (Molar Chemicals Ltd, Halásztelek, Hungary; Merck Ltd., Budapest, Hungary and VWR International Ltd., Debrecen, Hungary), while applied solvents were dried according to standard procedures. Optical rotations were measured in MeOH at 20 °C with a Perkin-Elmer 341 polarimeter (PerkinElmer Inc., Shelton, CT, USA). Chromatographic separations and monitoring of reactions were carried out on Merck Kieselgel 60 (Merck Ltd., Budapest, Hungary). Elemental analyses for all prepared compounds were performed on a Perkin-Elmer 2400 Elemental Analyzer (PerkinElmer Inc., Waltham, MA, USA). GC measurements for direct separation of commercially available enantiomers of isopulegol to determine the enantiomeric purity of starting material 1 and separation of O-acetyl derivatives of enantiomers were performed on a Chirasil-DEX CB column (2500 × 0.25 mm I.D.) on a Perkin-Elmer Autosystem XL GC consisting of a Flame Ionization Detector (Perkin-Elmer Corporation, Norwalk, CT, USA) and a Turbochrom Workstation data system (Perkin-Elmer Corp., Norwalk, CT, USA). Melting points were determined on a Kofler apparatus (Nagema, Dresden, Germany) and are uncorrected. 1H- and 13C-NMR were recorded on Brucker Avance DRX 500 spectrometer [500 MHz (1H) and 125 MHz (13C), δ = 0 (TMS)]. Chemical shifts are expressed in ppm (δ) relative to TMS as the internal reference. J values are given by Hz. Supplementary material, all 1H/13C NMR spectra, in addition to NOESY, also 2D-HMBC and 2D-HMQC spectra are involved in Supporting Information file.
4.2. Starting Materials
(−)-Isopulegol 1 is available commercially from Merck Co with ee = 95%. (+)-Neoisopulegol 2, diol 3 and (−)-α-methylene-γ-butyrolactone 4 were prepared according to literature procedures, and all spectroscopic data were similar to those described therein [35]. The nucleophilic addition of α-methylene-γ-butyrolactone to amines were carried out according to our literature procedures with all spectroscopic data of compounds 5–7 being consistent with literature values [38].
(3S,3aS,6R,7aS)-3-((Isopropylamino)methyl)-6-methylhexahydrobenzofuran-2(3H)-one (8)
Yield: 70%, white crystals, m.p.: 192–195 °C. = +42.0 (c 0.22, MeOH). 1H NMR (500 MHz, CDCl3): δ = 0.91 (3H, d, J = 6.5 Hz), 0.95–1.01 (2H, m), 1.20–1.26 (1H, m), 1.47 (6H, d, J = 6.5 Hz), 1.53–1.54 (1H, m), 1.67–1.70 (1H, m), 1.82–1.92 (1H, m), 2.22 (1H, d, J = 15.0 Hz), 2.72–2.76 (1H, m), 3.12–3.16 (1H, m), 3.22–3.26 (1H, m), 3.41–3.47 (1H, m), 3.62 (1H, q, J = 6.4 Hz), 4.60 (1H, d, J = 2.0 Hz), 8.69 (1H, brs), 10.04 (1H, brs). 13C NMR (125 MHz, CDCl3): δ = 18.9, 19.4, 21.9, 23.2, 26.1, 31.6, 35.7, 37.5, 40.5, 45.0, 51.5, 79.5, 177.1. Anal. Calcd for C13H23NO2: C, 69.29; H, 10.29; N, 6.22. Found: C, 69.30; H, 10.27; N, 6.25.
4.3. General Procedure for Reduction with LiAlH4
To a stirred suspension of LiAlH4 (8 mmol) in dry ether (16 mL), a solution of compounds 5–8 (4 mmol) in dry ether (20 mL) was added at 0 °C. The reaction mixture was stirred for 4 h at room temperature, while the reaction progress was monitored by TLC. A mixture of H2O (1.0 mL) and THF (10 mL) was then added dropwise with cooling. The inorganic material was filtered off and washed with Et2O (for compounds 9–12) or EtOAc (for compound 29). The filtrate was dried (Na2SO4) and evaporated to dryness. The crude product was purified by column chromatography on silica gel using CHCl3:MeOH = 9:1 (for compounds 9–12) or n-hexane:EtOAc = 1:4 (for compound 29).
4.3.1. (1S,2S,5R)-2-((S)-1-(Benzylamino)-3-hydroxypropan-2-yl)-5-methylcyclohexanol (9)
Yield: 98%, white crystals, m.p.: 101–103 °C. = +8.0 (c 0.29, MeOH). 1H NMR (500 MHz, CDCl3): δ = 0.86 (3H, d, J = 6.4 Hz), 0.88–0.94 (1H, m), 1.05–1.10 (1H, m), 1.32–1.36 (1H, m), 1.40–1.43 (1H, m), 1.53–1.58 (1H, m), 1.66–1.73 (2H, m), 1.78–1.87 (2H, m), 2.78 (1H, dd, J = 3.8, 12.1 Hz), 2.87 (1H, dd, J = 5.7, 12.2 Hz), 3.60 (1H, q, J = 7.7 Hz), 3.72–3.75 (1H, m), 3.77 (2H, s), 3.94 (1H, s), 7.25–7.34 (5H, m). 13C NMR (125 MHz, CDCl3): δ = 22.5, 25.2, 26.2, 35.4, 42.1, 42.3, 43.7, 49.1, 54.2, 64.6, 66.5, 127.5, 128.5, 128.7, 138.8. Anal. Calcd for C17H27NO2: C, 73.61; H, 9.81; N, 5.05. Found: C, 73.65; H, 9.80; N, 5.10.
4.3.2. (1S,2S,5R)-2-((S)-1-Hydroxy-3-(((R)-1-phenylethyl)amino)propan-2-yl)-5-methylcyclohexanol (10)
Yield: 74%, colorless oil. = +33.0 (c 0.26, MeOH). 1H NMR (500 MHz, CDCl3): δ = 0.85 (3H, d, J = 6.5 Hz), 0.82–0.95 (1H, m), 1.05–1.10 (1H, m), 1.25–1.29 (1H, m), 1.39 (3H, d, J = 6.6 Hz), 1.42–1.48 (1H, m), 1.57–1.59 (1H, m), 1.68 (1H, d, J = 12.9 Hz), 1.78–1.87 (2H, m), 2.57 (1H, dd, J = 4.9, 12.1 Hz), 2.76 (1H, dd, J = 5.6, 12.1 Hz), 3.16 (2H, brs), 3.57 (1H, q, J = 7.4 Hz), 3.71–3.75 (2H, m), 3.96 (1H, s), 7.24–7.35 (5H, m). 13C NMR (125 MHz, CDCl3): δ = 22.5, 23.8, 25.1, 26.1, 35.3, 41.9, 42.4, 43.8, 48.3, 59.0, 65.3, 66.8, 126.8, 127.4, 128.7, 144.3. Anal. Calcd for C18H29NO2: C, 74.18; H, 10.03; N, 4.81. Found: C, 74.20; H, 10.05; N, 4.85.
4.3.3. (1S,2S,5R)-2-((S)-1-Hydroxy-3-(((S)-1-phenylethyl)amino)propan-2-yl)-5-methylcyclohexanol (11)
Yield: 74%, white crystals, m.p.: 144–146 °C. = −16.0 (c 0.25, MeOH). 1H NMR (500 MHz, CDCl3): δ = 0.86 (3H, d, J = 6.3 Hz), 0.88–0.94 (1H, m), 1.06 (1H, t, J = 12.5 Hz), 1.30–1.34 (1H, m), 1.39 (3H, d, J = 6.6 Hz), 1.38–1.42 (1H, m), 1.56–1.64 (2H, m), 1.71–1.74 (1H, m), 1.75–1.90 (2H, m), 2.60 (1H, dd, J = 6.0, 12.2 Hz), 2.66 (1H, dd, J = 3.3 Hz, 12.2 Hz), 3.11 (2H, brs), 3.58 (1H, q, J = 3.0 Hz), 3.69–3.72 (2H, m), 3.86 (1H, s), 7.26–7.35 (5H, m). 13C NMR (125 MHz, CDCl3): δ = 22.5, 23.7, 25.4, 26.2, 35.4, 42.0, 42.2, 44.1, 47.0, 58.9, 64.5, 66.2, 126.8, 127.5, 128.7, 144.2. Anal. Calcd for C18H29NO2: C, 74.18; H, 10.03; N, 4.81. Found: C, 74.15; H, 10.00; N, 4.79.
4.3.4. (1S,2S,5R)-2-((S)-1-Hydroxy-3-(isopropylamino)propan-2-yl)-5-methylcyclohexanol (12)
Yield: 99%, white crystals, m.p.: 155–160 °C. = +18.0 (c 0.275, MeOH). 1H NMR (500 MHz, CDCl3): δ = 0.85 (3H, d, J = 6.4 Hz), 0.85–0.95 (1H, m), 1.09 (1H, t, J = 12.8 Hz), 1.43 (6H, dd, J = 5.9, 11.6 Hz), 1.41–1.54 (3H, m), 1.72–1.79 (2H, m), 1.91 (1H, d, J = 13.3 Hz), 2.17 (1H, s), 3.25–3.30 (2H, m), 3.71 (1H, t, J = 9.9 Hz), 3.85–3.87 (1H, m), 4.06 (1H, s), 8.96 (1H, brs), 9.04 (1H, brs). 13C NMR (125 MHz, CDCl3): δ = 19.1, 19.5, 22.3, 24.8, 25.9, 35.0, 41.0, 42.1, 42.2, 43.6, 51.3, 62.1, 65.6. Anal. Calcd for C13H27NO2: C, 68.08; H, 11.87; N, 6.11. Found: C, 68.10; H, 11.90; N, 6.15.
4.4. General Procedure of Epoxidation
To a stirred pale red solution of 9 or 31 (5.87 mmol) and vanadyl acetylacetonate (10 mg) in dry toluene (30 mL) t-BuOOH (70% solution in H2O, 11.8 mmol) dried briefly (Na2CO3) was added dropwise at 25 °C. The red colour of the solution darkened during the addition then faded to brownish yellow. Stirring was continued (12 h), whereupon KOH (9.8 mmol) in brine (25 mL) was added. The mixture was extracted with toluene (3 × 100 mL) and the organic layer was washed with brine before drying (Na2SO4) and evaporation. Compounds 10 and 32 were isolated after flash column chromatography (n-hexane:EtOAc = 4:1 for 10 and n-hexane:EtOAc = 1:1 for 32).
4.4.1. (1S,2R,5R)-5-Methyl-2-((S)-2-methyloxiran-2-yl)cyclohexanol (19)
Yield: 76%, white crystals, m.p.: 38–41 °C. = +69.0 (c 0.25, MeOH). All spectroscopic data of compound 19 was consistent with literature data [35,42].
4.4.2. (1S,2R,5R)-2-((R)-2-(Hydroxymethyl)oxiran-2-yl)-5-methylcyclohexanol (31)
Yield: 80%, colorless oil. = +36.0 (c 0.275, MeOH). 1H NMR (500 MHz, CDCl3): δ= 0.77 (3H, d, J = 6.3 Hz), 0.81–0.86 (1H, m), 1.00 (1H, t, J = 12.9 Hz), 1.26 (1H, d, J = 12.4 Hz), 1.43–1.46 (1H, m), 1.60–1.72 (4H, m), 2.67 (2H, dd, J = 4.5, 19.8 Hz), 3.18 (1H, d, J = 11.7 Hz), 3.81 (1H, d, J = 11.7 Hz), 4.04 (1H, s), 4.14 (1H, s), 4.62 (1H, brs). 13C NMR (125 MHz, CDCl3): δ = 22.0, 22.1, 25.6, 34.4, 41.7, 45.5, 50.4, 61.6, 63.2, 68.0. Anal. Calcd for C10H18O3: C, 64.49; H, 9.74. Found: C, 64.45; H, 9.70.
4.5. General Procedure for Ring-Opening of Epoxide with Primary Amines
To a solution of epoxide 10 or 32 (2.94 mmol) in MeCN (30 mL) was added a solution of the appropriate amine (5.88 mmol) in MeCN (10 mL) and LiClO4 (2.94 mmol). The mixture was kept at reflux temperature for 4 hours. When the reaction completed (indicated by TLC), the mixture was evaporated to dryness, the residue was dissolved in water (15 mL) and extracted with CH2Cl2 (3 × 50 mL). The combined organic phase was dried (Na2SO4), filtered and concentrated. The crude product was purified by column chromatography on silica gel with an appropriate solvent mixture (CHCl3:MeOH = 19:1). Purification by recrystallization from a mixture of n-hexane:Et2O resulted in compounds 20–23 or 32–35, respectively.
4.5.1. (1S,2R,5R)-2-((S)-1-(Benzylamino)-2-hydroxypropan-2-yl)-5-methylcyclohexanol (20)
Yield: 40%, white crystals, m.p.: 146–148 °C. = +15.0 (c 0.27, MeOH). 1H NMR (500 MHz, CDCl3): δ = 0.83 (3H, d, J = 6.4 Hz), 0.83–0.93 (1H, m), 0.95–1.03 (1H, m), 1.07–1.17 (1H, m), 1.31 (3H, s), 1.50 (1H, d, J = 12.4 Hz), 1.64 (1H, d, J = 12.5 Hz), 1.70–1.80 (2H, m), 1.89 (1H, brs), 2.78 (1H, t, J = 11.4 Hz), 2.95 (1H, t, J = 10.6 Hz), 3.29 (1H, brs), 3.74 (1H, s), 3.92–3.96 (1H, m), 4.49–4.53 (2H, m), 6.89 (1H, brs), 7.42 (5H, s), 10.2 (1H, s). 13C NMR (125 MHz, CDCl3): δ = 21.8, 21.9, 25.6, 29.6, 35.0, 42.2, 50.2, 51.2, 51.3, 65.2, 71.6, 129.5, 129.8, 130.2, 130.4. Anal. Calcd for C17H27NO2: C, 73.61; H, 9.81; N, 5.05. Found: C, 73.65; H, 9.80; N, 5.10.
4.5.2. (1S,2R,5R)-2-((S)-2-Hydroxy-1-(((R)-1-phenylethyl)amino)propan-2-yl)-5-methylcyclohexanol (21)
Yield: 43%, white crystals, m.p.: 118–119 °C. = +5.0 (c 0.295, MeOH). 1H NMR (500 MHz, CDCl3): δ = 0.85 (3H, d, J = 6.3 Hz), 0.84–0.89 (2H, m), 1.05–1.15 (2H, m), 1.25–1.33 (1H, m), 1.28 (3H, s), 1.52 (1H, d, J = 9.3 Hz), 1.63–1.65 (1H, m), 1.70–1.88 (3H, m), 1.79 (3H, s), 2.56 (1H, s), 2.97 (1H, s), 4.06 (1H, brs), 4.26 (1H, s), 4.59 (1H, s), 6.99 (1H, s), 7.30–7.50 (5H, m), 10.3 (1H, s). 13C NMR (125 MHz, CDCl3): δ = 20.7, 21.8, 22.0, 25.8, 29.8, 35.1, 42.3, 50.3, 51.1, 59.4, 65.4, 71.6, 127.9, 129.6, 129.9, 135.8. Anal. Calcd for C18H29NO2: C, 74.18; H, 10.03; N, 4.81. Found: C, 74.20; H, 10.05; N, 4.80.
4.5.3. (1S,2R,5R)-2-((S)-2-hydroxy-1-(((S)-1-phenylethyl)amino)propan-2-yl)-5-methylcyclohexanol (22)
Yield: 43%, white crystals, m.p.: 160–161 °C. = −9.0 (c 0.255, MeOH). 1H NMR (500 MHz, CDCl3): δ = 0.87 (3H, d, J = 6.3 Hz), 0.87–0.95 (1H, m), 1.04 (1H, t, J = 14.5 Hz), 1.19 (3H, s), 1.34 (2H, d, J = 10.5 Hz), 1.42 (3H, d, J = 6.87 Hz), 1.59–1.68 (1H, m), 1.75–1.77 (1H, m), 1.90–1.97 (1H, m), 2.36 (1H, d, J = 12.1 Hz), 2.54 (1H, d, J = 12.1 Hz), 3.73 (1H, q, J = 6.6 Hz), 4.29 (1H, s), 7.26–7.36 (5H, m). 13C NMR (125 MHz, CDCl3): δ = 22.1, 22.4, 23.1, 26.0, 29.0, 35.6, 42.3, 52.3, 52.7, 58.5, 64.6, 74.1, 126.6, 127.5, 128.8, 143.9. Anal. Calcd for C18H29NO2: C, 74.18; H, 10.03; N, 4.81. Found: C, 74.15; H, 10.02; N, 4.85.
4.5.4. (1S,2R,5R)-2-((S)-2-Hydroxy-1-(isopropylamino)propan-2-yl)-5-methylcyclohexanol (23)
Yield: 88%, yellow oil. = +20.0 (c 0.245, MeOH). 1H NMR (500 MHz, CDCl3): δ = 0.87 (3H, d, J = 6.1 Hz), 0.95–1.01 (1H, m), 1.16 (1H, t, J = 13.3 Hz), 1.35 (3H, d, J = 6.5 Hz), 1.39 (3H, s), 1.389 (3H, d, J = 6.8 Hz), 1.30–1.53 (3H, m), 1.59 (1H, d, J = 12.0 Hz), 1.78–1.87 (3H, m), 2.91 (1H, t, J = 11.1 Hz), 2.97–3.03 (1H, m), 3.39 (1H, quin, J = 6.0 Hz), 3.85 (1H, brs), 4.54 (1H, s), 6.37 (1H, s), 9.77 (1H, s). 13C NMR (125 MHz, CDCl3): δ =19.1, 19.6, 22.0, 22.2, 25.8, 29.6, 35.1, 42.1, 49.0, 51.0., 51.2, 65.3, 71.6. Anal. Calcd for C13H27NO2: C, 68.08; H, 11.87; N, 6.11. Found: C, 68.10; H, 11.85; N, 6.13.
4.5.5. (S)-3-(Benzylamino)-2-((1R,2S,4R)-2-hydroxy-4-methylcyclohexyl)propane-1,2-diol (32)
Yield: 60%, colorless oil. = +20.0 (c 0.26, MeOH). 1H NMR (500 MHz, DMSO-d6): δ = 0.81 (3H. d, J = 6.3 Hz), 0.80–0.87 (1H, m), 1.01 (1H, t, J = 13.2 Hz), 1.30–1.38 (2H, m), 1.50 (1H, t, J = 7.8 Hz), 1.63 (1H, d, J = 12.5 Hz), 1.67–1.77 (2H, m), 2.85 (1H, dd, J = 12.8, 21.0 Hz), 3.32 (1H, d, J = 11.4 Hz), 3.41 (1H, d, J = 11.3 Hz), 4.11 (1H, q, J = 12.8 Hz), 4.98 (1H, s), 7.41–7.47 (5H, m). 13C NMR (125 MHz, DMSO-d6): δ = 21.0, 22.1, 25.3, 34.6, 41.8, 45.6, 48.4, 50.8, 64.3, 66.0, 73.7, 128.7, 128.8, 129.9, 132.4. Anal. Calcd for C13H27NO3: C, 69.59; H, 9.28; N, 4.77. Found: C, 69.60; H, 9.25; N, 4.82.
4.5.6. (S)-2-((1R,2S,4R)-2-Hydroxy-4-methylcyclohexyl)-3-(((R)-1-phenylethyl)amino)propane-1,2-diol (33)
Yield: 60%, colorless oil. = +10.0 (c 0.24, MeOH). 1H NMR (500 MHz, DMSO-d6): δ = 0.81 (3H, d, J = 6.3 Hz), 0.80–0.85 (1H, m), 1.01 (1H, t, J = 12.9 Hz), 1.10–1.15 (1H, m), 1.19–1.23 (1H, m), 1.49 (1H. d, J = 12.5 Hz), 1.55 (3H, d, J = 6.7 Hz), 1.54–1.58 (1H, m), 1.67–1.75 (2H, m), 2.54 (1H, d, J = 13.0 Hz), 2.83 (1H, d, J = 12.7 Hz), 3.27 (1H, d, J = 11.3 Hz), 4.12 (1H, s), 4.32 (1H, d, J = 6.3 Hz), 5.03 (1H, s), 5.11 (1H, brs), 6.05 (1H, brs), 7.39–7.50 (5H, m), 8.72 (1H, brs), 9.40 (1H, brs). 13C NMR (125 MHz, DMSO-d6): δ = 19.0, 21.2, 22.1, 25.3, 34.6, 41.7, 45.6, 46.7, 57.5, 64.1, 66.2, 73.9, 127.9, 128.9, 129.0, 137.1. Anal. Calcd for C18H27NO3: C, 70.32; H, 9.51; N, 4.56. Found: C, 70.35; H, 9.55; N, 4.52.
4.5.7. (S)-2-((1R,2S,4R)-2-Hydroxy-4-methylcyclohexyl)-3-(((S)-1-phenylethyl)amino)propane-1,2-diol (34)
Yield: 80%, colorless oil. = +3.0 (c 0.235, MeOH). 1H NMR (500 MHz, DMSO-d6): δ = 0.83 (3H, d, J = 6.2 Hz), 0.82–0.92 (1H, m), 1.04 (1H, t, J = 12.9 Hz), 1.31–1.44 (2H, m), 1.49–1.55 (1H, m), 1.54 (3H, d, J = 6.8 Hz), 1.65 (1H, d, J = 12.5 Hz), 1.70–1.79 (2H, m), 2.56 (1H, d, J = 12.7 Hz), 2.73 (1H, d, J = 12.7 Hz), 3.27 (1H, d, J = 11.3 Hz), 3.33 (1H, s), 4.19 (1H, s), 4.33 (1H, q, J = 6.6 Hz), 5.02 (1H, s), 7.39–7.53 (5H, m). 13C NMR (125 MHz, DMSO-d6): δ = 19.4, 21.2, 22.1, 25.4, 34.6, 41.8, 45.6, 47.1, 57.5, 64.3, 66.0, 73.7, 127.7, 128.9, 137.2. Anal. Calcd for C18H27NO3: C, 70.32; H, 9.51; N, 4.56. Found: C, 70.30; H, 9.48; N, 4.60.
4.5.8. (S)-2-((1R,2S,4R)-2-Hydroxy-4-methylcyclohexyl)-3-(isopropylamino)propane-1,2-diol (35)
Yield: 75%, colorless oil. = +15.0 (c 0.29, MeOH). 1H NMR (500 MHz, DMSO-d6): δ = 0.83 (3H, d, J = 6.2 Hz), 0.83–0.90 (1H, m), 1.05 (1H, t, J = 12.8 Hz), 1.20 (6H, d, J = 6.3 Hz), 1.40–1.47 (2H, m), 1.54–1.57 (1H, m), 1.65–1.80 (3H, m), 2.86 (1H, dd, J = 12.8, 25.4 Hz), 3.25 (1H, quin, J = 6.4 Hz), 3.43 (1H, d, J = 11.3 Hz), 4.19 (1H, s), 5.03 (1H, s). 13C NMR (125 MHz, DMSO-d6): δ = 18.2, 18.8, 21.2, 22.1, 25.4, 34.7, 42.0, 45.6, 46.0, 50.0, 64.3, 65.7, 73.7. Anal. Calcd for C13H27NO3: C, 63.64; H, 11.09; N, 5.71. Found: C, 63.63; H, 11.05; N, 4.75.
4.6. General Procedure for Ring Closure with Formaldehyde
To a solution of aminodiols 9–12 or 20–23 (1.8 mmol) in 5 mL Et2O was added 20 mL of 35% aqueous formaldehyde and the mixture was stirred at room temperature. After 1 h, it was made alkaline with 10% aqueous KOH (20 mL) and extracted with Et2O (3 × 50 mL). After drying (Na2SO4) and solvent evaporation crude products 14–17 or 25–28 were purified by column chromatography (CHCl3:MeOH = 19:1).
4.6.1. (1S,2S,5R)-2-((S)-3-Benzyl-1,3-oxazinan-5-yl)-5-methylcyclohexanol (14)
Yield: 64%, colorless oil. = −4.0 (c, 0.285 MeOH). 1H NMR (500 MHz, CDCl3): δ = 0.86 (3H, d, J = 6.4 Hz), 0.86–0.94 (1H, m), 1.07 (1H, t, J = 12.6 Hz), 1.25–1.33 (2H, m), 1.56–1.62 (1H, m), 1.70–1.73 (1H, m), 1.75–1.90 (3H, m), 2.64 (1H, s), 2.85 (1H, s), 3.58–3.67 (3H, m), 3.90 (2H, d, J = 7.4 Hz), 4.13 (1H, s), 4.32 (1H, d, J = 8.2 Hz), 7.25–7.33 (5H, m). 13C NMR (125 MHz, CDCl3): δ = 22.5, 24.5, 26.0, 35.3, 42.3, 44.7, 52.3, 57.4, 65.9, 72.1, 85.0, 127.6, 128.6, 129.3, 136.6. Anal. Calcd for C18H27NO2: C, 74.70; H, 9.40; N, 4.84. Found: C, 74.73; H, 9.37; N, 4.85.
4.6.2. (1S,2S,5R)-5-Methyl-2-((S)-3-((R)-1-phenylethyl)-1,3-oxazinan-5-yl)cyclohexanol (15)
Yield: 65%, colorless oil. = +11.0 (c 0.28, MeOH). 1H NMR (500 MHz, CDCl3): δ = 0.84 (3H, d, J = 6.3 Hz), 0.84–0.89 (1H, m), 1.03 (1H, t, J = 12.8 Hz), 1.15–1.27 (2H, m), 1.41 (3H, d, J = 6.6 Hz), 1.42–1.50 (1H, m), 1.65–1.70 (1H, m), 1.70–1.85 (3H, m), 2.43 (1H, s), 2.65 (1H, s), 3.66 (1H, s), 3.71 (1H, s), 3.85 (1H, d, J = 8.2 Hz), 4.00 (1H, s), 4.11 (1H, brs), 4.54 (1H, d, J = 5.4 Hz), 7.26–7.36 (5H, m). 13C NMR (125 MHz, CDCl3): δ = 20.2, 22.5, 24.3, 26.0, 35.3, 42.2, 44.5, 49.7, 59.8, 65.8, 71.9, 83.5, 127.6, 127.7, 128.6. Anal. Calcd for C19H29NO2: C, 75.21; H, 9.63; N, 4.62. Found: C, 75.25; H, 9.60; N, 4.65.
4.6.3. (1S,2S,5R)-5-Methyl-2-((S)-3-((S)-1-phenylethyl)-1,3-oxazinan-5-yl)cyclohexanol (16)
Yield: 65%, colorless oil. = −22.0 (c 0.27, MeOH). 1H NMR (500 MHz, CDCl3): δ = 0.88 (3H, d, J = 6.4 Hz), 0.91–0.99 (1H, m), 1.11 (1H, td, J = 1.9, 12.9 Hz), 1.25–1.30 (1H, m), 1.35–1.45 (1H, m), 1.43 (3H, d, J = 6.7 Hz), 1.70–1.80 (3H, m), 1.90 (2H, d, J = 12.0 Hz), 2.60 (1H, brs), 3.14 (1H, d, J = 8.7 Hz), 3.54 (1H, s), 3.64 (1H, s), 3.72 (1H, d, J = 10.2 Hz), 3.79 (1H, d, J = 8.6 Hz), 4.22 (1H, s), 4.26 (1H, d, J = 8.2 Hz), 7.23–7.33 (5H, m). 13C NMR (125 MHz, CDCl3): δ = 20.3, 22.5, 24.9, 26.1, 35.5, 42.2, 45.5, 49.5, 60.5, 65.8, 72.1, 83.8, 127.4, 127.5, 128.7, 142.5. Anal. Calcd for C19H29NO2: C, 75.21; H, 9.63; N, 4.62. Found: C, 75.20; H, 9.65; N, 4.65.
4.6.4. (1S,2S,5R)-2-((S)-3-Isopropyl-1,3-oxazinan-5-yl)-5-methylcyclohexanol (17)
Yield: 83%, colorless oil. = −2.0 (c 0.20, MeOH). 1H NMR (500 MHz, CDCl3): δ = 13C NMR (125 MHz, CDCl3): δ = 0.85 (3H, d, J = 6.4 Hz), 0.85–0.90 (1H, m), 0.90–0.97 (1H, m), 1.06–1.11 (1H, m), 1.09 (3H, d, J = 4.7 Hz), 1.10 (3H, d, J = 4.8 Hz), 1.19–1.30 (3H, m), 1.40–1.45 (1H, m), 1.72–1.75 (3H, m), 1.80–1.90 (2H, m), 2.55–2.75 (1H, m), 2.83 (1H, quin, J = 6.4 Hz), 2.90 (1H, d, J = 11.1 Hz), 3.79 (2H, s), 3.98 (1H, s), 4.22 (1H, s), 4.45 (1H, d, J =7.7 Hz). 13C NMR (125 MHz, CDCl3): δ = 18.6, 18.8, 22.5, 25.0, 26.1, 29.8, 35.6, 42.2, 45.8, 47.3, 51.2, 65.7, 72.2, 82.8. Anal. Calcd for C14H27NO2: C, 69.66; H, 11.27; N, 5.80. Found: C, 69.70; H, 9.29; N, 4.78.
4.6.5. (1S,2R,5R)-2-((S)-3-Benzyl-5-methyloxazolidin-5-yl)-5-methylcyclohexanol (25)
Yield: 60%, colorless oil. = +29.0 (c 0.24, MeOH). 1H NMR (500 MHz, CDCl3): δ = 0.86 (3H, d, J = 6.4 Hz), 0.85–0.90 (1H, m), 1.00–1.05 (1H, m), 1.33 (3H, s), 1.33–1.38 (2H, m), 1.52–1.56 (1H, m), 1.73 (1H, d, J = 12.8 Hz), 1.82–1.92 (2H, m), 2.06 (1H, d, J = 9.4 Hz), 3.35 (1H, d, J = 9.3 Hz), 3.53 (1H, d, J = 12.6 Hz), 3.73 (1H, d, J = 12.6 Hz), 3.90 (1H, s), 4.26 (1H, s), 4.57 (1H, s), 5.80 (1H, brs), 7.26–7.34 (5H, m). 13C NMR (125 MHz, CDCl3): δ = 22.4, 23.1, 26.2, 27.2, 35.3, 41.8, 51.1, 56.5, 58.9, 65.4, 84.9, 85.8, 127.8, 128.7, 128.9, 136.9. Anal. Calcd for C18H27NO2: C, 74.70; H, 9.40; N, 4.84. Found: C, 74.68; H, 9.35; N, 4.80.
4.6.6. (1S,2R,5R)-5-Methyl-2-((S)-5-methyl-3-((R)-1-phenylethyl)oxazolidin-5-yl)cyclohexanol (26)
Yield: 65%, colorless oil. = +3.0 (c 0.30, MeOH). 1H NMR (500 MHz, CDCl3): δ = 0.84 (3H, d, J = 6.5 Hz), 0.83–0.89 (1H, m), 1.02 (1H, td, J = 2.0, 12.2 Hz), 1.19–1.30 (2H, m), 1.26 (3H, s), 1.29–1.43 (2H, m), 1.36 (3H, t, J = 6.1 Hz), 1.66 (1H, d, J = 12.8 Hz), 1.80–1.92 (2H, m), 2.92 (1H, d, J = 7.8 Hz), 3.30 (1H, d, J = 4.8 Hz), 4.20 (1H, s), 4.30 (1H, s), 4.91 (1H, s), 5.94 (1H, brs), 7.27–7.37 (5H, m). 13C NMR (125 MHz, CDCl3): δ = 22.4, 22.5, 22.9, 26.1, 27.3, 35.3, 41.8, 51.2, 58.0, 63.4, 65.4, 85.0, 85.4, 127.0, 127.8, 129.0, 143.0. Anal. Calcd for C19H29NO2: C, 75.21; H, 9.63; N, 4.62. Found: C, 75.19; H, 9.65; N, 4.63.
4.6.7. (1S,2R,5R)-5-Methyl-2-((S)-5-methyl-3-((S)-1-phenylethyl)oxazolidin-5-yl)cyclohexanol (27)
Yield: 65%, colorless oil. = +28.0 (c 0.20, MeOH). 1H NMR (500 MHz, CDCl3): δ = 0.88 (3H, d, J = 6.3 Hz), 0.89–1.08 (1H, m), 1.05 (1H, t, J = 12.8 Hz), 1.34 (3H, s), 1.41 (3H, d, J = 7.7 Hz), 1.37–1.42 (2H, m), 1.62–1.70 (1H, m), 1.75–1.82 (1H, m), 1.85–1.95 (2H, m), 2.04 (1H, d, J = 8.4 Hz), 3.27 (1H, s), 3.59 (1H, d, J = 7.3 Hz), 3.77 (1H, s), 4.26 (2H, s), 7.26–7.34 (5H, m). 13C NMR (125 MHz, CDCl3): δ = 22.5, 23.2, 26.3, 27.2, 35.4, 41.8, 51.2, 57.3, 62.6, 65.4, 85.2, 85.5, 126.7, 127.8, 129.0, 143.2. Anal. Calcd for C19H29NO2: C, 75.21; H, 9.63; N, 4.62. Found: C, 75.25; H, 9.60; N, 4.60.
4.6.8. (1S,2R,5R)-2-((S)-3-Isopropyl-5-methyloxazolidin-5-yl)-5-methylcyclohexanol (28)
Yield: 70%, colorless oil. = +14.0 (c 0.25, MeOH). 1H NMR (500 MHz, CDCl3): δ = 0.84 (3H, d, J = 6.4 Hz), 0.85–0.92 (1H, m), 1.00–1.06 (1H, m), 1.05 (3H, d, J = 6.2 Hz), 1.10 (3H, d, J = 6.3 Hz), 1.30 (3H, s), 1.32–1.37 (2H, m), 1.52–1.61 (1H, m), 1.71–1.74 (1H, m), 1.77–1.87 (2H, m), 1.91 (1H, d, J = 9.1 Hz), 2.40 (1H, quin, J = 6.2 Hz), 3.37 (1H, d, J = 9.1 Hz), 3.89 (1H, s), 4.22 (1H, s), 4.73 (1H, s), 6.17 (1H, brs). 13C NMR (125 MHz, CDCl3): δ = 21.7, 21.9, 22.5, 23.1, 26.2, 27.1, 35.4, 41.9, 51.4, 52.2, 56.8, 65.3, 84.8, 85.0. Anal. Calcd for C14H27NO2: C, 69.66; H, 11.27; N, 5.80. Found: C, 69.70; H, 11.25; N, 4.84.
4.7. General Procedure for Debenzylation
To a suspension of palladium-on-carbon (5% Pd, 0.22 g) in MeOH (50 mL) was added aminodiols 9–11, 20–22 or aminotriols 32–34 (14.0 mmol) in MeOH (100 mL), and the mixture was stirred under a H2 atmosphere (1 atm) at room temperature. After completion of the reaction (as monitored by TLC, 24 h), the mixture was filtered through a Celite pad and the solution was evaporated to dryness. The crude products were recrystallized in diethyl ether, resulting in primary aminodiols 13 and 24 or aminotriol 36, respectively.
4.7.1. (1S,2S,5R)-2-((S)-1-Amino-3-hydroxypropan-2-yl)-5-methylcyclohexanol (13)
Yield: 50%, colorless oil. = +21.0 (c 0.25, MeOH). 1H NMR (500 MHz, CDCl3): δ = 0.85 (3H, d, J = 5.8 Hz), 0.90–0.95 (1H, m), 1.10 (1H, t, J = 12.3 Hz), 1.24 (1H, s), 1.35–1.45 (2H, m) 1.58 (1H, t, J = 12.0 Hz), 1.60–1.87 (5H, m), 2.85–3.00 (2H, m), 3.42 (4H, brs), 3.60–3.70 (1H, m), 3.70–3.80 (1H, m), 3.98 (1H, s). 13C NMR (125 MHz, CDCl3): δ = 22.5, 24.7, 26.2, 35.4, 40.9, 42.0, 42.4, 44.7, 62.8, 63.7, 66.7. Anal. Calcd for C10H21NO2: C, 64.13; H, 11.30; N, 7.48. Found: C, 64.15; H, 11.28; N, 7.50.
4.7.2. (1S,2R,5R)-2-((S)-1-Amino-2-hydroxypropan-2-yl)-5-methylcyclohexanol (24)
Yield: 72%, white crystals, m.p.: 139–140 °C. = +12.0 (c 0.295, MeOH). 1H NMR (500 MHz, DMSO-d6): δ = 0.82 (3H, d, J = 6.3 Hz), 0.78–0.87 (1H, m), 1.01 (1H, t, J = 12.6 Hz), 1.17 (3H, s), 1.30–1.33 (1H, m), 1.45–1.48 (2H, m), 1.70–1.75 (3H, m), 2.62 (1H, d, J = 12.8 Hz), 2.92 (1H, d, J = 12.9 Hz), 4.10 (1H, s), 4.95 (1H, s). 13C NMR (125 MHz, DMSO-d6): δ = 20.9, 22.3, 25.1, 25.4, 34.8, 42.6, 45.1, 49.7, 64.4, 71.0. Anal. Calcd for C10H21NO2: C, 64.13; H, 11.30; N, 7.48. Found: C, 64.15; H, 11.28; N, 7.50.
4.7.3. (S)-3-Amino-2-((1R,2S,4R)-2-hydroxy-4-methylcyclohexyl)propane-1,2-diol (36)
Yield: 72%, colorless oil. = +22.0 (c 0.275, MeOH). 1H NMR (500 MHz, DMSO-d6): δ = 0.82 (3H, d, J = 6.3 Hz), 0.82–0.88 (1H, m), 1.02 (1H, t, J = 13.2 Hz), 1.45–1.50 (3H, m), 1.67–1.74 (3H, m), 2.78–2.87 (2H, m), 3.31–3.47 (2H, m), 4.12 (1H, s), 4.89 (1H, brs), 5.14 (1H, brs), 5.37 (1H, brs), 7.65 (3H, s). 13C NMR (125 MHz, DMSO-d6): δ = 20.6, 22.2, 25.4, 34.7, 45.5, 42.2, 45.2, 64.5, 65.8, 73.2. Anal. Calcd for C10H21NO3: C, 59.08; H, 10.41; N, 6.89. Found: C, 59.10; H, 10.45; N, 6.85.
4.8. General Procedure for Dihydroxylation
To a solution of compounds 2–4 (14 mmol) in acetone (60 mL), an aqueous solution of NMO (12 mL, 50% aqueous solution) and a solution of OsO4 in t-BuOH (6 mL, 2% t-BuOH solution) were added in one portion. The reaction mixture was stirred at room temperature for 24 h, and then quenched by the addition of a saturated aqueous solution of Na2SO3 (100 mL) and extracted with EtOAc (3 x 80 mL). The organic layer was dried and evaporated. The crude products were purified by chromatography on silica gel by using n-hexane:EtOAc (1:4 for compounds 18 and 29 and 1:9 for compound 37). The products after purification were recrystallized in diethyl ether resulting in compounds 18, 29 and 37 as white crystals.
4.8.1. (3R,3aR,6R,7aS)-3-Hydroxy-3-(hydroxymethyl)-6-methylhexahydrobenzofuran-2(3H)-one (18)
Yield: 50%, white crystals, m.p.: 73–75 °C. = −63.0 (c 0.26, MeOH). 1H NMR (500 MHz, DMSO-d6): δ = 0.86 (3H, d, J = 6.4 Hz), 0.83–0.95 (2H, m), 1.19–1.25 (1H, m), 1.34–1.39 (1H, m), 1.56 (1H, d, J = 12.4 Hz), 1.73–1.77 (1H, m), 2.05–2.13 (2H, m), 3.46 (1H, dd, J = 6.0, 11.9 Hz), 3.54 (1H, dd, J = 4.8, 12.0 Hz), 4.77 (1H, t, J = 5.7 Hz), 4.81 (1H, q, J = 3.4 Hz), 5.84 (1H, s). 13C NMR (125 MHz, DMSO-d6): δ = 21.1, 21.2, 25.9, 31.6, 35.4, 43.0, 61.0, 77.1, 79.5, 176.0. Anal. Calcd for C10H16O4: C, 63.80; H, 10.71. Found: C, 63.83; H, 10.69.
4.8.2. (R)-2-((1R,2S,4R)-2-Hydroxy-4-methylcyclohexyl)propane-1,2-diol (29)
Yield: 61%, white crystals, m.p.: 137–139 °C. = +12.0 (c 0.275, MeOH). 1H NMR (500 MHz, DMSO-d6): δ = 0.80 (3H, d, J = 6.5 Hz), 0.85–0.79 (1H, m), 0.95 (1H, t, J = 11.9 Hz), 1.05 (3H, s), 1.30–1.32 (1H, m), 1.48–1.57 (2H, m), 1.65–1.74 (3H, m), 3.11 (1H, dd, J = 5.7, 10.5 Hz), 3.43 (1H, dd, J = 5.2, 10.5 Hz), 4.12 (1H, s), 4.40 (1H, s), 4.80 (1H, t, J = 5.4 Hz), 4.83 (1H, d, J = 1.9 Hz). 13C NMR (125 MHz, DMSO-d6): δ = 19.8, 22.4, 23.8, 25.4, 34.9, 42.4, 45.3, 65.8, 67.3, 73.9. Anal. Calcd for C10H20O3: C, 63.80; H, 10.71. Found: C, 63.78; H, 10.75.
4.8.3. 2-((1R,2S,4R)-2-Hydroxy-4-methylcyclohexyl)propane-1,2,3-triol (37)
Yield: 40%, white crystals, m.p.: 93–95 °C. = +17.0 (c 0.235, MeOH). 1H NMR (500 MHz, DMSO-d6): δ = 0.80 (3H, d, J = 6.5 Hz), 0.82–0.85 (1H, m), 0.96 (1H, t, J = 12.2 Hz), 1.46–1.57 (3H, m), 1.65–1.68 (2H, m), 1.73–1.75 (1H, m), 3.38–3.43 (2H, m), 4.09 (1H, s), 4.26 (1H, s), 4.49 (1H, t, J = 5.6 Hz), 4.73 (1H, t, J = 5.4 Hz), 4.95 (1H, d, J = 1.0 Hz). 13C NMR (125 MHz, DMSO-d6): δ = 19.8, 22.4, 25.4, 35.0, 42.3, 43.3, 63.1, 63.2, 65.4, 75.6, 75.6. Anal. Calcd for C10H20O4: C, 58.80; H, 9.87. Found: C, 58.83; H, 9.85.
4.9. (S)-4-((1R,2S,4R)-2-Hydroxy-4-methylcyclohexyl)-4-methyl-1,3-dioxolan-2-one (30)
To the solution of 29 (2.00 mmol) in dry pyridine (8.22 mmol) and dry CH2Cl2 (20 mL), triphosgene (1.00 mmol) in dry CH2Cl2 (5 mL) was added under cooling in an ice bath. After stirring at room temperature for 2 h under Ar atmosphere, the consumption of 29 was confirmed by TLC. Then water (20 mL) was added to the solution and then the organic layer was washed with saturated aqueous NH4Cl solution. The organic layer was collected, dried over anhydrous Na2SO4 and filtered. The crude product after evaporation was purified by column chromatography on silica gel (n-hexane:EtOAc = 4:1) to obtain 30 in a 60% yield.
Yield: 60%, colorless oil. = +35.0 (c 0.28, MeOH). 1H NMR (500 MHz, CDCl3): δ = 0.95 (3H, d, J = 6.2 Hz), 1.16–1.25 (1H, m), 1.57 (3H, s), 1.58–1.70 (2H, m), 1.74–1.80 (1H, m), 1.94–2.08 (2H, m), 2.13–2.20 (1H, m), 4.11 (1H, d, J = 8.2 Hz), 4.27 (1H, d, J = 8.2 Hz), 5.78 (1H, t, J = 1.8 Hz). 13C NMR (125 MHz, CDCl3): δ = 21.6, 24.0, 24.3, 28.1, 30.6, 33.4, 74.2, 85.0, 123.4, 135.6, 154.8. Anal. Calcd for C11H18O4: C, 61.66; H, 8.47. Found: C, 66.63; H, 10.51.
(R)-2-((1R,2S,4R)-2-Hydroxy-4-methylcyclohexyl)propane-1,2-diol (29)
Compound 19 (0.60 mmol) was treated with DMSO (3.0 mL) and 3 M NaOH (3.0 mL). The resulting homogenous solution was stirred at 80 °C for 2 hours. After being cooled to room temperature, EtOAc (20 mL) was added and the aqueous layer was washed with EtOAc (3 x 20 mL). The combined organic layers were dried over Na2SO4, filtered and concentrated in vacuo. The crude material was purified by column chromatography on silica gel (n-hexane:EtOAc = 1:4) then recrystallized in Et2O to provide compound 29 (60%).
4.10. General Procedure for the Reaction of Benzaldehyde with Diethylzinc in the Presence of Chiral Catalysts
To the respective catalyst (0.1 mmol), 1 M Et2Zn in n-hexane solution (3 mL, 3 mmol) was added under an argon atmosphere at room temperature. The solution was stirred for 25 min at room temperature then benzaldehyde (1 mmol) was added. After stirring at room temperature for a further 20 h, the reaction was quenched with saturated NH4Cl solution (15 mL) and the mixture was extracted with EtOAc (2 × 20 mL). The combined organic phase was washed with H2O (10 mL), dried (Na2SO4) and evaporated under vacuum. The crude secondary alcohols obtained were purified by flash column chromatography (n-hexane:EtOAc = 4:1). The ee and absolute configuration of the resulting material were determined by chiral GC on CHIRASIL-DEX CB column after O-acetylation in Ac2O/DMPA/pyridine.
4.11. Antimicrobial Analyses
For the antimicrobial analyses, the pure compounds were first dissolved in MeOH and diluted with H2O to two concentration levels (400 µg/mL and 40 µg/mL)—keeping the final MeOH content at 10%. Then, these solutions were investigated in microdilution assay with two Gram-positive bacteria including Bacillus subtilis SZMC 0209 and Staphylococcus aureus SZMC 14611, two Gram-negative bacteria Escherichia coli SZMC 6271 and Pseudomonas aeruginosa SZMC 23290, as well as two yeast strains Candida albicans SZMC 1533 and C. krusei SZMC 1352 according to the M07-A10 CLSI guideline [55] and our previous work [56]. Suspensions of the test microbes were prepared from overnight cultures cultivated in ferment broth (bacteria: 10 g/L peptone, 5 g/L NaCl, 5 g/L yeast extract; yeast: 20 g/L peptone, 10 g/L yeast extract, 20 g/L glucose) at 37 °C. Then the concentrations of the suspensions were set to 2 x 105 cells/mL with sterile media. For the assay, 96-well plates were prepared by dispensing into each well 100 μL of suspension containing the bacterial or yeast cells and 50 μL of sterile broth as well as 50 μL of the test solutions and incubated for 24 h at 37 °C. The mixture of 150 μL broth and 50 μL of 10% methanol was used as the blank sample for the background correction, while 100 μL of microbial suspension supplemented with 50 μL sterile broth and 50 μL of 10% methanol was applied as negative control. The positive control contained ampicillin (Sigma) or nystatin (Sigma) for bacteria or fungi, respectively at two concentration levels (100 µg/mL and 10 µg/mL). The inhibitory effects of the derivatives were observed spectrophotometrically at 620 nm after the incubation, and inhibition was calculated as the percentage of the positive control after blank correction.
5. Conclusions
Starting from the commercially available (−)-isopugeol, a new family of isopulegol-based chiral aminodiol, aminotriol libraries and di-, tri- and tetraols were prepared through chiral (+)-neoisopulegol as key intermediate via stereoselective transformations.
The resulting aminodiols exert moderate antimicrobial action on a panel of bacterial and fungal strains, while aminodiol and aminotriol derivatives were applied as chiral catalysts in the enantioselective addition of diethylzinc to benzaldehyde with moderate but significantly opposite stereoselectivity.
Abbreviations
| Et2O | Diethyl ether |
| EtOH | Ethanol |
| HCHO | Formaldehyde |
| EtOAc | Ethyl acetate |
| t-BuOOH | tert-Butyl hydroperoxide |
| VO(acac)2 | Vanadyl aceatylacetonate |
| NMO | 4-Methylmorpholine N-oxide |
| MeCN | Acetonitrile |
| THF | Tetrahydrofuran |
| LiAlH4 | Lithium aluminum hydride |
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/20/16/4050/s1.
Author Contributions
Z.S. and A.S. conceived and designed the experiments; T.M.L., T.S. and B.V. performed the experiments, analyzed the data and wrote the experimental part; Z.S., A.S. and F.F. discussed the results and contributed to write the paper.
Funding
We are grateful for financial supports from the Hungarian Research Foundation (NKFI K112442, K115731) and for the EU-funded Hungarian grant GINOP-2.3.2-15-2016-00012.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results. Sources of funding for the study.
References
- 1.Largy E., Liu W., Hasan A., Perrin D.M. A pyrimidopyrimidine Janus-AT nucleoside with improved base-pairing properties to both A and T within a DNA duplex: The stabilizing effect of a second endocyclic ring nitrogen. Chem. Eur. J. 2014;20:1495–1499. doi: 10.1002/chem.201303867. [DOI] [PubMed] [Google Scholar]
- 2.Grajewska A., Rozwadowska M.D. Stereoselective synthesis of cytoxazone and its analogues. Tetrahedron Asymmetry. 2007;18:803–813. doi: 10.1016/j.tetasy.2007.03.027. [DOI] [Google Scholar]
- 3.Mishra R.K., Coates C.M., Revell K.D., Turos E. Synthesis of 2-Oxazolidinones from β-lactams: Stereospecific total synthesis of (−)-cytoxazone and all of its stereoisomers. Org. Lett. 2007;9:575–578. doi: 10.1021/ol062752p. [DOI] [PubMed] [Google Scholar]
- 4.Allepuz A.C., Badorrey R., Díaz-de-Villegas M.D., Gálvez J.A. Diastereoselective reduction of Ketimines derived from (R)-3,4-dihydroxybutan-2-one: An alternative route to key intermediates for the synthesis of anticancer agent ES-285. Tetrahedron Asymmetry. 2010;21:503–506. doi: 10.1016/j.tetasy.2010.02.012. [DOI] [Google Scholar]
- 5.Richelle-Maurer E., Braekman J.C., Kluijver M., Gomez R., de Vyver G., Soest R., Devijver C. Cellular location of (2R,3R,7Z)-2-aminotetradec-7-ene-1, 3-diol, a potent antimicrobial metabolite produced by the Caribbean sponge Haliclona vansoesti. Cell Tissue Res. 2001;306:157–165. doi: 10.1007/s004410100437. [DOI] [PubMed] [Google Scholar]
- 6.Kleinert H.D., Rosenberg S.H., Baker W.R., Stein H.H., Klinghofer V., Barlow J., Spina K., Polakowski J., Kovar P., Cohen J. Discovery of a peptide-based renin inhibitor with oral bioavailability and efficacy. Science. 1992;257:1940–1943. doi: 10.1126/science.1411510. [DOI] [PubMed] [Google Scholar]
- 7.Chandrasekhar S., Mohapatra S., Yadav J.S. Practical synthesis of Abbott amino-diol: A core unit of the potent renin inhibitor Zankiren. Tetrahedron. 1999;55:4763–4768. doi: 10.1016/S0040-4020(99)00148-9. [DOI] [Google Scholar]
- 8.Toribatake K., Miyata S., Naganawa Y., Nishiyama H. Asymmetric synthesis of optically active 3-amino-1,2-diols from N-acyl-protected allylamines via catalytic diboration with Rh[bis(oxazolinyl)phenyl] catalysts. Tetrahedron. 2015;71:3203–3208. doi: 10.1016/j.tet.2015.04.011. [DOI] [Google Scholar]
- 9.Jaime-Figueroa S., Greenhouse R., Padilla F., Dillon M.P., Gever J.R., Ford A.P.D.W. Discovery and synthesis of a novel and selective drug-like P2X1 antagonist. Bioorg. Med. Chem. Lett. 2005;15:3292–3295. doi: 10.1016/j.bmcl.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 10.Paraskar A.S., Sudalai A. Enantioselective synthesis of (−)-cytoxazone and (+)-epi-cytoxazone, novel cytokine modulators via Sharpless asymmetric epoxidation and L-proline catalyzed Mannich reaction. Tetrahedron. 2006;62:5756–5762. doi: 10.1016/j.tet.2006.03.079. [DOI] [Google Scholar]
- 11.Kakeya H., Morishita M., Koshino H., Morita Ti T., Kobayashi K., Osada H. Cytoxazone: A novel cytokine modulator containing a 2-Oxazolidinone ring produced by Streptomyces sp. J. Org. Chem. 1999;64:1052–1053. doi: 10.1021/jo981922b. [DOI] [PubMed] [Google Scholar]
- 12.Zhu W., Burnette A., Dorjsuren D., Roberts P.E., Huleihel M., Shoemaker R.H., Marquez V.E., Agbaria R., Sei S. Potent antiviral activity of north-methanocarbathymidine against Kaposi’s sarcoma-associated herpesvirus. Antimicrob. Agents Chemother. 2005;49:4965–4973. doi: 10.1128/AAC.49.12.4965-4973.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin G.Q., You Q.D., Cheng J.F. Chiral Drugs: Chemistry and Biological Action. Wiley; Hoboken, NJ, USA: 2011. [Google Scholar]
- 14.Tanaka T., Yasuda Y., Hayashi M. New chiral Schiff base as a tridentate ligand for catalytic enantioselective addition of diethylzinc to aldehydes. J. Org. Chem. 2006;71:7091–7093. doi: 10.1021/jo060964u. [DOI] [PubMed] [Google Scholar]
- 15.Koneva E.A., Korchagina D.V., Gatilov Y.V., Genaev A.M., Krysin A.P., Volcho K.P., Tolstikov A.G., Salakhutdinov N.F. New chiral ligands based on (+)-α-pinene. Russ. J. Org. Chem. 2010;46:1109–1115. doi: 10.1134/S1070428010080014. [DOI] [Google Scholar]
- 16.Szakonyi Z., Csőr Á., Csámpai A., Fülöp F. Stereoselective synthesis and modelling-driven optimisation of carane-based aminodiols and 1,3-oxazines as catalysts for the enantioselective addition of diethylzinc to benzaldehyde. Chem. Eur. J. 2016;22:7163–7173. doi: 10.1002/chem.201600749. [DOI] [PubMed] [Google Scholar]
- 17.Szakonyi Z., Csillag K., Fülöp F. Stereoselective synthesis of carane-based aminodiols as chiral ligands for the catalytic addition of diethylzinc to aldehydes. Tetrahedron Asymmetry. 2011;22:1021–1027. doi: 10.1016/j.tetasy.2011.06.013. [DOI] [Google Scholar]
- 18.Panev S., Linden A., Dimitrov V. Chiral aminoalcohols with a menthane skeleton as catalysts for the enantioselective addition of diethylzinc to benzaldehyde. Tetrahedron Asymmetry. 2001;12:1313–1321. doi: 10.1016/S0957-4166(01)00206-3. [DOI] [Google Scholar]
- 19.Goldfuss B., Steigelmann M., Khan S.I., Houk K.N. Rationalization of enantioselectivities in dialkylzinc additions to benzaldehyde catalyzed by fenchone derivatives. J. Org. Chem. 2000;65:77–82. doi: 10.1021/jo991070v. [DOI] [PubMed] [Google Scholar]
- 20.Tashenov Y., Daniels M., Robeyns K., Van Meervelt L., Dehaen W., Suleimen Y., Szakonyi Z. Stereoselective syntheses and application of chiral bi- and tridentate ligands derived from (+)-sabinol. Molecules. 2018;23:771. doi: 10.3390/molecules23040771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szakonyi Z., Gonda T., Ötvös S.B., Fülöp F. Stereoselective syntheses and transformations of chiral 1,3-aminoalcohols and 1,3-diols derived from nopinone. Tetrahedron Asymmetry. 2014;25:1138–1145. doi: 10.1016/j.tetasy.2014.06.017. [DOI] [Google Scholar]
- 22.Gonda T., Szakonyi Z., Csámpai A., Haukka M., Fülöp F. Stereoselective synthesis and application of tridentate aminodiols derived from (+)-pulegone. Tetrahedron Asymmetry. 2016;27:480–486. doi: 10.1016/j.tetasy.2016.04.009. [DOI] [Google Scholar]
- 23.Pedrosa R., Andrés C., Duque-Soladana J.P., Rosón C.D. Regio- and stereoselective 6- exo - trig radical cyclisations onto chiral perhydro-1,3-benzoxazines: Synthesis of enantiopure 3-alkylpiperidines. Tetrahedron Asymmetry. 2000;11:2809–2821. doi: 10.1016/S0957-4166(00)00230-5. [DOI] [Google Scholar]
- 24.Pedrosa R., Andrés C., Nieto J., del Pozo S. Synthesis of enantiopure 3-azabicyclo[3.2.0]heptanes by diastereoselective intramolecular [2+2] photocycloaddition reactions on chiral perhydro-1,3-benzoxazines. J. Org. Chem. 2003;68:4923–4931. doi: 10.1021/jo034251c. [DOI] [PubMed] [Google Scholar]
- 25.Alberola A., Andrés C., Pedrosa R. Diastereoselective ring opening of 2-substituted N-benzyl-4,4, 7α-trimethyl-trans-octahydro-1,3-benzoxazines by Grignard reagents. Highly enantioselective synthesis of primary amines. Synlett. 1990;1990:763–765. doi: 10.1055/s-1990-21244. [DOI] [Google Scholar]
- 26.Andrés C., Nieto J., Pedrosa R., Villamañán N. Synthesis of enantiopure primary amines by stereoselective ring opening of chiral octahydro-1,3-benzoxazines by Grignard and organoaluminum reagents. J. Org. Chem. 1996;61:4130–4135. doi: 10.1021/jo960047w. [DOI] [PubMed] [Google Scholar]
- 27.Cherng Y.J., Fang J.M., Lu T.J. Pinane-type tridentate reagents for enantioselective reactions: Reduction of ketones and addition of diethylzinc to aldehydes. J. Org. Chem. 1999;64:3207–3212. doi: 10.1021/jo982403b. [DOI] [PubMed] [Google Scholar]
- 28.Roy C.D., Brown H.C. A study of transesterification of chiral (−)-pinanediol methylboronic ester with various structurally modified diols. Monatshefte Für Chem. Chem. Mon. 2007;138:747–753. doi: 10.1007/s00706-007-0681-7. [DOI] [Google Scholar]
- 29.Ardashov O.V., Pavlova A.V., Il’ina I.V., Morozova E.A., Korchagina D.V., Karpova E.V., Volcho K.P., Tolstikova T.G., Salakhutdinov N.F. Highly potent activity of (1R,2R,6S)-3-methyl-6-(prop-1-en-2-yl)cyclohex-3-ene-1,2-diol in animal models of Parkinson’s Disease. J. Med. Chem. 2011;54:3866–3874. doi: 10.1021/jm2001579. [DOI] [PubMed] [Google Scholar]
- 30.Radulović N.S., Mladenović M.Z., Randjelovic P.J., Stojanović N.M., Dekić M.S., Blagojević P.D. Toxic essential oils. Part IV: The essential oil of Achillea falcata L. as a source of biologically/pharmacologically active trans-sabinyl esters. Food Chem. Toxicol. 2015;80:114–129. doi: 10.1016/j.fct.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Tuberoso C.I.G., Kowalczyk A., Coroneo V., Russo M.T., Dessì S., Cabras P. Chemical composition and antioxidant, antimicrobial, and antifungal activities of the essential oil of Achillea ligustica All. J. Agric. Food Chem. 2005;53:10148–10153. doi: 10.1021/jf0518913. [DOI] [PubMed] [Google Scholar]
- 32.Brocksom T.J., dos Santos R.B., Varanda N.A., Brocksom U. An Efficient synthesis of monoterpene α-methylene-γ-butyrolactones. Synth. Commun. 1988;18:1403–1410. doi: 10.1080/00397918808078810. [DOI] [Google Scholar]
- 33.Brocksom T.J., Tercio J., Ferreira B. A biomimetic synthesis of α-methylene-γ-butyrolactones. Synth. Commun. 1981;11:105–119. doi: 10.1080/00397918108064290. [DOI] [Google Scholar]
- 34.Carda M., Marco J.A. Total synthesis of the monoterpenes (−)-mintlactone and (+)-isomintlactone. Tetrahedron. 1992;48:9789–9800. doi: 10.1016/S0040-4020(01)81195-9. [DOI] [Google Scholar]
- 35.Friedrich D., Bohlmann F. Total synthesis of various elemanolides. Tetrahedron. 1988;44:1369–1392. doi: 10.1016/S0040-4020(01)85916-0. [DOI] [Google Scholar]
- 36.Schlosser M., Kotthaus M. Isopulegol as a model compound: Metalation and substitution of an allylic position in the presence of an unprotected hydroxy function. Eur. J. Org. Chem. 1999;1999:459–462. doi: 10.1002/(SICI)1099-0690(199902)1999:2<459::AID-EJOC459>3.0.CO;2-F. [DOI] [Google Scholar]
- 37.Serra S., Fuganti C. Enzyme-mediated preparation of enantiomerically pure p-menthan- 3,9-diols and their use for the synthesis of natural p-menthane lactones and ethers. Helv. Chim. Acta. 2002;85:2489–2502. doi: 10.1002/1522-2675(200208)85:8<2489::AID-HLCA2489>3.0.CO;2-9. [DOI] [Google Scholar]
- 38.Le T., Bérdi P., Zupkó I., Fülöp F., Szakonyi Z. Synthesis and transformation of (-)-isopulegol-based chiral β-aminolactones and β-aminoamides. Int. J. Mol. Sci. 2018;19:3522. doi: 10.3390/ijms19113522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kupchan S.M., Fessler D.C., Eakin M.A., Giacobbe T.J. Reactions of alpha methylene lactone tumor inhibitors with model biological nucleophiles. Science. 1970;168:376–378. doi: 10.1126/science.168.3929.376. [DOI] [PubMed] [Google Scholar]
- 40.Lawrence N.J., McGown A.T., Nduka J., Hadfield J.A., Pritchard R.G. Cytotoxic michael-type amine adducts of α-methylene lactones alantolactone and isoalantolactone. Bioorg. Med. Chem. Lett. 2001;11:429–431. doi: 10.1016/S0960-894X(00)00686-7. [DOI] [PubMed] [Google Scholar]
- 41.Szakonyi Z., Hetényi A., Fülöp F. Synthesis and application of monoterpene-based chiral aminodiols. Tetrahedron. 2008;64:1034–1039. doi: 10.1016/j.tet.2007.07.065. [DOI] [Google Scholar]
- 42.Kocienski P.J., Pontiroli A., Qun L. Enantiospecific syntheses of pseudopterosin aglycones. Part 2. Synthesis of pseudopterosin K–L aglycone and pseudopterosin A–F aglycone via a B→BA→BAC annulation strategy. J. Chem. Soc. Perkin Trans. 1. 2001:2356–2366. doi: 10.1039/b102961b. [DOI] [Google Scholar]
- 43.Rodríguez-Berríos R.R., Torres G., Prieto J.A. Stereoselective VO(acac)(2) catalyzed epoxidation of acyclic homoallylic diols. Complementary preparation of C2-syn-3,4-epoxy alcohols. Tetrahedron. 2011;67:830–836. doi: 10.1016/j.tet.2010.11.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carman R.M., Handley P.N. 9,10-Dihydroxy-1,8-cineole (1,3,3-Trimethyl-2-oxabicyclo[2.2.2]octane-10,11-diol) Aust. J. Chem. 2001;54:769. doi: 10.1071/CH01168. [DOI] [Google Scholar]
- 45.Shivani, Pujala B., Chakraborti A.K. Zinc(II) perchlorate hexahydrate catalyzed opening of epoxide ring by amines: Applications to synthesis of (RS)/(R)-propranolols and (RS)/(R)/(S)-naftopidils. J. Org. Chem. 2007;72:3713–3722. doi: 10.1021/jo062674j. [DOI] [PubMed] [Google Scholar]
- 46.Bergmeier S.C. The Synthesis of vicinal amino alcohols. Tetrahedron. 2000;56:2561–2576. doi: 10.1016/S0040-4020(00)00149-6. [DOI] [Google Scholar]
- 47.Chen K., Baran P.S. Total synthesis of eudesmane terpenes by site-selective C–H oxidations. Nature. 2009;459:824–828. doi: 10.1038/nature08043. [DOI] [PubMed] [Google Scholar]
- 48.Morikawa H., Yamaguchi J., Sugimura S., Minamoto M., Gorou Y., Morinaga H., Motokucho S. Systematic synthetic study of four diastereomerically distinct limonene-1,2-diols and their corresponding cyclic carbonates. Beilstein J. Org. Chem. 2019;15:130–136. doi: 10.3762/bjoc.15.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paterson I., Anderson E.A., Dalby S.M., Lim J.H., Maltas P. The stereocontrolled total synthesis of spirastrellolide A methyl ester. Fragment coupling studies and completion of the synthesis. Org. Biomol. Chem. 2012;10:5873. doi: 10.1039/c2ob25101a. [DOI] [PubMed] [Google Scholar]
- 50.Superchi S., Donnoli M.I., Proni G., Spada G.P., Rosini C. Induction of cholesteric mesophases by simple cyclic derivatives of p,p’-disubstituted 1,2-diphenylethane-1,2-diols: Importance of shape and polarizability effects. J. Org. Chem. 1999;64:4762–4767. doi: 10.1021/jo990038y. [DOI] [PubMed] [Google Scholar]
- 51.Morikawa H., Minamoto M., Gorou Y., Yamaguchi J., Morinaga H., Motokucho S. Two diastereomers of d-limonene-derived cyclic carbonates from d-limonene oxide and carbon dioxide with a tetrabutylammonium chloride catalyst. Bull. Chem. Soc. Jpn. 2018;91:92–94. doi: 10.1246/bcsj.20170300. [DOI] [Google Scholar]
- 52.Wender P.A., Buschmann N., Cardin N.B., Jones L.R., Kan C., Kee J.M., Kowalski J.A., Longcore K.E. Gateway synthesis of daphnane congeners and their protein kinase C affinities and cell-growth activities. Nat. Chem. 2011;3:615–619. doi: 10.1038/nchem.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marcos I.S., Castañeda L., Basabe P., Díez D., Urones J.G. Synthesis of sibiricinone A, sibiricinone B and leoheterin. Tetrahedron. 2008;64:10860–10866. doi: 10.1016/j.tet.2008.09.007. [DOI] [Google Scholar]
- 54.Jimeno C., Pastó M., Riera A., Pericàs M.A. Modular amino alcohol ligands containing bulky alkyl groups as chiral controllers for Et2Zn addition to aldehydes: Illustration of a design principle. J. Org. Chem. 2003;68:3130–3138. doi: 10.1021/jo034007l. [DOI] [PubMed] [Google Scholar]
- 55.Weinstein M.P. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. 11th ed. Clinical and Laboratory; Wayne, PA, USA: 2018. [Google Scholar]
- 56.Béni Z., Dékány M., Kovács B., Csupor-Löffler B., Zomborszki Z., Kerekes E., Szekeres A., Urbán E., Hohmann J., Ványolós A. Bioactivity-guided isolation of antimicrobial and antioxidant metabolites from the mushroom. Tapinella Atrotomentosa Mol. 2018;23:1082. doi: 10.3390/molecules23051082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.