Abstract
Glucagon exerts pleiotropic actions on energy balance and has emerged as an attractive target for the treatment of diabetes and obesity in the last few years. Glucagon reduces body weight and adiposity by suppression of appetite and by modulation of lipid metabolism. Moreover, this hormone promotes weight loss by activation of energy expenditure and thermogenesis. In this review, we cover these metabolic actions elicited by glucagon beyond its canonical regulation of glucose metabolism. In addition, we discuss recent developments of therapeutic approaches in the treatment of obesity and diabetes by dual- and tri-agonist molecules based on combinations of glucagon with other peptides. New strategies using these unimolecular polyagonists targeting the glucagon receptor (GCGR), have become successful approaches to evaluate the multifaceted nature of glucagon signaling in energy balance and metabolic syndrome.
Keywords: glucagon, energy balance, food intake, body weight, energy expenditure, thermogenesis, lipid metabolism, obesity
1. Introduction
1.1. Background
In the early 1920s, Kimball and Murlin [1] reported that extracts of pancreatic tissue produced a hyperglycemic response, due to a circulating factor that was identified as glucagon. This pancreatic hormone is a 29-amino-acid peptide released from the α-cells of the islet of Langerhans, and has for long been recognized as the principal counter-regulatory hormone to insulin in response to low glucose [2,3]. Glucagon and glucagon-like peptides are transcribed from a common proglucagon gene, a peptide precursor of 160 amino acids, that is expressed in pancreatic islet α-cells, in a specific population of enteroendocrine cells (L-cells) of the intestinal mucosa, and in a set of neurons in the nucleus tractus solitarius (NTS) of the medulla oblongata [4,5].
1.2. Glucagon Peptides
The posttranslational regulation of proglucagon gene generates a number of well-known hormones, such as glucagon and glucagon like peptide 1 (GLP-1), and also other bioactive peptides like glucagon like peptide 2 (GLP-2), glicentin, glicentin-related pancreatic polypeptide (GRPP), and oxyntomodulin (OXM). Most of these proglucagon-derived peptides show very specific effects on glucose and energy balance, although some of them exhibit unclear functional roles [6,7,8,9,10]. The tissues-specific expression of various products from the proglucagon gene depend on posttranslational modifications by specific prohormone convertases (PC) [11]. It is important to note that glucagon in the α-cells is cleaved from the proglucagon gene by the prohormone convertase 2 (PC2), encoded by the Proprotein Convertase Subtilisin/Kexin Type 2 gene [12]. GLP-1, GLP-2, GRPP, and OXM, on the other hand, are cleaved from the proglucagon gene in the brain and intestine through the prohormone convertase 1 (PC1), also known as prohormone convertase 3 and usually abbreviated as PC1/3, encoded by the Proprotein Convertase Subtilisin/Kexin Type 1 gene [13].
1.3. Glucagon Receptor
Glucagon acts through the binding and activation of glucagon receptor (GCGR). The GCGR belongs to class B of G protein-coupled receptors (GPCRs) located on the cell surface [14]. GCGR is mainly present in liver and kidney and to a lesser extent in intestinal smooth muscle, brain, adipose tissue, the adrenal gland, heart, and in both α- and β-pancreatic cells [15,16]. The GCGR has been considered an important drug target in the treatment of type 2 diabetes mellitus (T2DM) due to its effect on pancreatic alpha-cells. However, additional and novel effects for glucagon, such as modulation of satiety, thermogenesis, energy expenditure, and control of lipid metabolism have more recently been garnering scientific attention [3,17].
1.4. Objectives
In this review article, we highlight the specific metabolic actions exerted by glucagon that participates in the control of food intake and energy balance. In addition, we discuss the novel therapeutic approaches in the treatment of obesity and diabetes by dual- and tri-agonist molecules based on glucagon in combination with other peptides.
2. The Effect of Glucagon on Satiety and Appetite Suppression
Glucagon exerts effects on metabolism beyond the regulation of glucose metabolism, including modulation of satiety as demonstrated both in humans [18,19] and in rats [20,21]. The effect of glucagon on satiety was shown to be mainly due to the inhibition of meal size via the liver–brain axis [22]. Concordantly, intraperitoneal injections of antibodies against glucagon caused an increase in meal size in rats [23]. Moreover, it has also been shown that a considerable amount of glucagon is released during meals. These facts support the idea of a physiological role of glucagon in the termination of meals or postprandial satiety [24,25]. The anorexigenic action of glucagon is initiated in the liver, which is able to sense the glucagon levels in the hepatic portal vein and send the information through the vagal afferents to the central nervous system (CNS) (Figure 1).
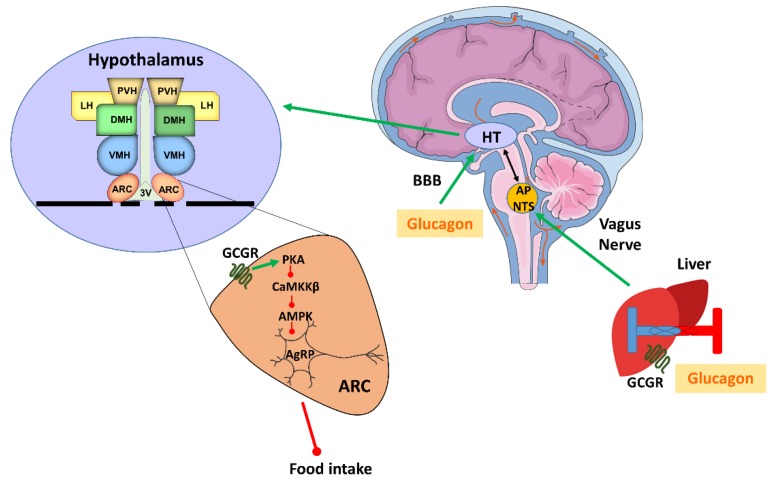
Figure 1.
Glucagon control on food intake. Diagram of the neuronal pathway that regulates the anorexigenic action of glucagon. Green arrows indicate an increase or activation, while red arrows indicate a decrease or inhibition. AgRP: Agouti related peptide; AMPK: Adenosine monophosphate -activated protein kinase; AP: Area Postrema; ARC: Hypothalamic arcuate nucleus; BBB: Blood brain barrier; CaMKKβ: Ca2+/calmodulin-dependent protein kinase kinase β; DMH: Dorsomedial hypothalamic nucleus; GCGR: Glucagon receptor; HT: Hypothalamus; LH: Lateral hypothalamic area; NTS: Nucleus tractus solitarius; PKA: Protein kinase A; PVN: Paraventricular hypothalamic nucleus; VMH: Ventromedial hypothalamic nucleus; 3V: Third ventricle. Figure made with Servier Medical Art resources.
Thus, the hepatic branch of the vagus nerve conveys the satiety signal to the area postrema (AP) and the NTS, and from there the neuronal signal is transported to the hypothalamus. The existence of this neuronal loop is supported by experiments in rats, where glucagon administration into the portal vein was unable to induce satiety after hepatic vagotomy [22]. Moreover, specific lesions of the AP or NTS are also able to block glucagon anorexigenic action [26]. There is also considerable experimental evidence indicating that the hypothalamus plays a main role in the action of glucagon on food intake. For example, the administration of glucagon at low doses into the third ventricle of rats suppressed feeding with a potency greater than 1000 times than that of peripheral administration [27]. Also, glucagon is able to cross the blood–brain barrier [28], and multiple regions in the brain, including the hypothalamus, have demonstrated significant binding to glucagon via GCGR [29]. Indeed, mechanistic studies recently deciphered the neuronal pathway that regulates the anorexigenic actions of glucagon [20]. This process takes place in the hypothalamic arcuate nucleus (ARC) via the GCGR and includes the protein kinase A/ Ca2+−calmodulin-dependent protein kinase kinase β/AMP-activated protein kinase/Agouti related protein (PKA/CaMKKβ/AMPK/AgRP) signaling pathway [20] (Figure 1). An important aspect of this study was that, in diet-induced obese (DIO) rats, the amount of CaMKKβ and pAMPK in the hypothalamus and the food intake did not change after glucagon treatment. Nevertheless, the anorectic action of central glucagon in obese rats could be restored by using virogenetic tools to inhibit CaMKKβ activity within the ARC, suggesting that DIO-induced resistance to the anorexic function of glucagon can be explained by deficient CaMKKβ signaling [20]. These results suggest that the diet-induced resistance to the anorexigenic action of glucagon might contribute to the development of obesity.
It is important to note that not all studies found an effect of glucagon on food intake. For example, glucagon administration for more than six months does not affect feeding in a model with impaired leptin signaling [30]. However, is important to note that in this study glucagon was not able to change other relevant metabolic parameters such as glucose, insulin, ketone bodies, or circulating lipids, suggesting that the differences observed with other studies (see next sections) may be explained by the singularity of the model used and in the different doses and time of administration of glucagon. Apart from this, since obesity is mainly induced by high caloric consumption and that glucagon induces satiety, it is conceivable that this hormone could have an impact on energy metabolism and body weight control.
3. Glucagon on Body Weight, Energy Expenditure and Thermogenesis
3.1. Glucagon on Body Weight
In addition to the modulating role of glucagon on satiety, glucagon also controls body weight by promoting weight loss in physiological and pathological states in humans and rodents [18,31,32]. To evaluate the effect of glucagon signaling on body weight, loss of function models for the study of glucagon receptor were developed. In this regard, GCGR (–/–) mice have normal body weight when fed normal chow [33] but exhibit lower blood glucose, hyperglucagonemia, and pancreatic α-cell hyperplasia [33]. However, GCGR (–/–) mice are resistant to DIO with decreased body weight (~30%), reduced food intake, improved glucose tolerance, and less gastric emptying [34]. According to the lean phenotype, GCGR (–/–) mice on high fat diet (HFD) exhibit significantly lower white adipose tissue (WAT) and brown adipose tissue (BAT) mass than wild type (WT) mice. Consistent with reduced adipose tissue, plasma leptin levels were also significantly lower in those mice [34]. As we commented on before, another study evaluated the effect of glucagon on body weight in Zucker rats, a genetic model of obesity that exhibit decreased release of glucagon from pancreatic islets, as a consequence of leptin receptor mutation [35]. In these rats, glucagon caused a marked reduction of body weight in a food intake independent manner [30]. These data suggest that the glucagon action on body weight is complex and involve food dependent and independent mechanisms.
3.2. Glucagon on Energy Expenditure
In a seminal study in 1957 by Davidson et al., it was reported that glucagon injected subcutaneously increased oxygen consumption in rats [36]. These effects were also found in humans, indicating that hyperglucagonemia during insulin deficiency promotes an increase in energy expenditure (EE), which may contribute to the catabolic state in many conditions [37]. Moreover, this notion is supported by reports which show that rats treated with glucagon gain less weight and less fat compared to pair-fed rats [38]. Accordingly, it was also noted that long-term administration of glucagon reduced body weight (~20%) with no change in feeding, suggesting that the reduced body weight is related to an increase in EE [30]. Recent studies with glucagon agonists provide more mechanistic data on this issue. In this sense, Habegger et al. showed that the subcutaneous administration of the glucagon agonist IUB288 decreased body weight and fat mass in DIO mice by stimulating EE and locomotor activity through the action of fibroblast growth factor 21 (FGF21) [39]. A follow up study determined that the farnesoid X receptor signaling in the liver in concert with FGF21, were responsible for this stimulation of EE and fatty oxidation [40]. Therefore, it seems clear that glucagon impacts body weight through feeding-independent mechanisms, most probably by stimulating EE and fatty oxidation (Figure 2).
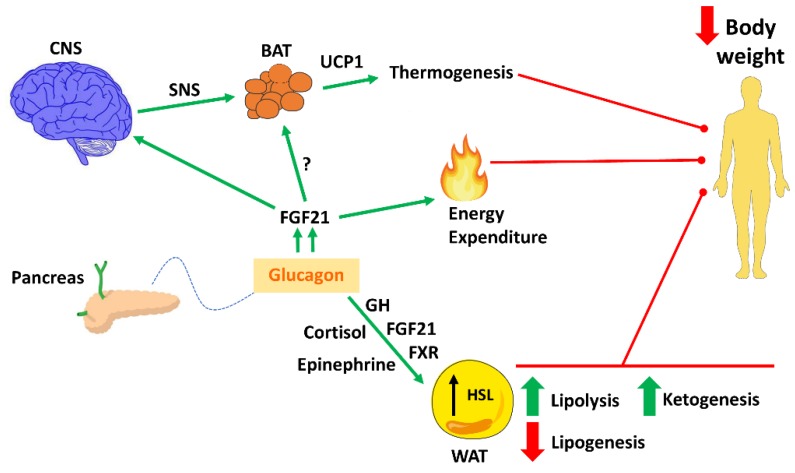
Figure 2.
Glucagon on energy balance regulation. Schematic overview of the effects of glucagon on energy balance that finally leads to loss of body weight. Green arrows indicate an increase or improvement, while red arrows indicate a decrease or inhibition. BAT: Brown adipose tissue; CNS: Central nervous system; GH: Growth hormone; HSL: Hormone sensitive lipase; FGF21: Fibroblast growth factor 21; FXR: Farnesoid X receptor; SNS: Sympathetic nervous system; UCP-1: Uncoupling protein 1; WAT: White adipose tissue. Figure made with Servier Medical Art resources.
3.3. Glucagon on Thermogenesis
In addition to enhance metabolic rate in rats, measured by significant elevation of oxygen consumption, administration of glucagon is also able to increase BAT mass and to stimulate BAT thermogenic capacity in vivo and in vitro [41]. It is important to note that although most of the studies of glucagon on BAT activity were made under supraphysiological doses, another study also recapitulated the thermogenic aspects of glucagon by using physiological doses [42]. Accordingly, after cold exposure the levels of circulating glucagon are significantly elevated in humans and rats [43], suggesting that glucagon participates in the cold-induced thermogenesis. To elucidate this aspect, Kinoshita et al. used mice deficient in proglucagon-derived peptides (GCGKO mice) [44] and showed that GCGKO mice displayed cold intolerance and impaired thermogenesis after cold stimulus that was partially ameliorated by glucagon supplementation [44]. In addition, glucagon administration also restored uncoupling protein 1 (UCP1) expression in BAT and significantly increased circulating FGF21 in these mice [44], suggesting that glucagon modulates BAT function through FGF21 signaling. Accordingly, a recent study also reported that glucagon increased EE in normal mice but this effect was partially blocked in FGF21 (–/–) [45]. However, in the same study the activation of EE by glucagon was also observed in UCP1 (–/–) mice and GCGRBAT conditional knockout mice, suggesting that glucagon increases EE through UCP1 independent pathways [45]. In humans, Salem et al. described the effects of glucagon infusion on EE through a BAT-independent mechanism [46]. Therefore, the importance of BAT in the thermogenic action of glucagon is an issue that remains controversial. Nevertheless, pharmacological studies in rodents provide evidence towards a CNS-sympathetic nervous system-dependent action of glucagon on BAT thermogenesis [47,48,49]. In fact, the inhibition with the β-adrenergic blocker propanol [49] or surgically denervation of BAT [47] blunted the thermogenic effects of exogenous glucagon in rodents. However, more studies are needed to evaluate the contribution of glucagon on BAT thermogenesis in humans, but it seems clear that beyond inducing satiety, glucagon decreases body weight by activating EE and thermogenesis (Figure 2). Due to the fact that glucagon improves body weight by the simultaneous activation of the abovementioned processes and that WAT constitute around 20% of total body composition, it is reasonable to assume that this hormone also modulates WAT lipid metabolism (Figure 2).
4. The Effect of Glucagon on Lipid Metabolism
Studies in the early 1960s showed that glucagon administration had a potent hypolipidemic effect [50,51,52]. Glucagon, significantly decreased cholesterol levels and plasma total lipids after its intravenous administration [53]. Glucagon also decreased triglycerides (TG) production [54] and circulating TG, very low-density lipoprotein, and cholesterol in hyperlipidemic and non-hyperlipidemic rats [55]. Thus, it may contribute to the hypolipidemic effect by an inhibition of amino acid incorporation into hepatic lipoprotein [55]. However, these studies did not find lower levels of lipids in liver or erythrocytes. Accordingly, another study demonstrated that subcutaneously injections of glucagon during 21 days, decreased cholesterol and triacylglycerol levels by 40 and 70% in plasma but not in the liver, suggesting a transformation of cholesterol into bile acids and higher urinary secretion of cholesterol by chronic administration of this peptide [56]. The effect of chronic treatment of glucagon on lipoprotein composition demonstrated it to be a potent hypolipidemic agent affecting mainly the apo-E-rich lipoproteins [57,58]. The effect of glucagon on lipid metabolism is mediated by inhibition of lipogenesis and stimulation of lipolysis. Glucagon promotes lipolysis by enhancing the activity of hormone-sensitive lipase, the key enzyme that mobilizes the stored fats by TG hydrolysis in adipocytes [59]. Another route by which glucagon regulates lipid metabolism involves the up-regulation of other lipolytic hormones such as growth hormone [60], cortisol [61], or epinephrine [62]. It was also reported that glucagon receptor agonism improved body fat and plasma cholesterol via FGF21 in rodents and human [39].
On the other hand, studies in GCGR (–/–) mice generated controversial data on hypolipidemic effect of glucagon. Connarello et al. demonstrated that GCGR (–/–) mice were resistant to HFD and the development of hepatic steatosis, probably due to elevated circulating GLP-1 in these mice [34]. However, Longet et al. reported increased hepatic TG secretion following fasting and enhanced susceptibility to hepatosteatosis following exposure to a HFD in the same strain, suggesting that disruption of GCGR signaling impairs the control of fatty acid oxidation during fasting [63]. These studies showed contradictory results despite identical background in both models and similar diets, which demonstrates that further research is needed to elucidate the contribution of glucagon on hepatic steatosis.
Glucagon also affects lipid metabolism via a direct effect on hepatic ketogenesis [64,65]. Glucagon activate fatty acids oxidation under conditions of limited energy supply, such as fasting or uncontrolled type 1 diabetes, resulting in ketogenesis [66]. The lipolytic effect of glucagon provides a constant supply of non-esterified fatty acids to the liver, which leads to maintained fatty acid oxidation and ketone bodies production [67,68]. The role of glucagon in the regulation of ketogenesis was reported by Gerich et al., who showed that suppression of glucagon, caused by the treatment with somatostatin, prevented the development of ketoacidosis in patients with type 1 diabetes mellitus after insulin withdrawal [69]. However, the normal ketone response to physiological elevations of glucagon in healthy humans involves an increase in circulating insulin and decreased ketone bodies production [70], suggesting that the activity of glucagon on ketogenesis can be modulated by the simultaneous actions of other hormones or substrates like insulin [70]. Accordingly, in perfused liver, exposure to insulin inhibits ketone body production [70,71]. These effects were also demonstrated in human ketoacidosis by administration of insulin that rapidly reduced plasma ketone [72].
In conclusion, glucagon acts directly on the main fat storage tissues to control lipid metabolism. However, is important to note that while glucagon induces a hypolipidemic action in WAT, and this is not a matter of discussion, the actions of glucagon on hepatic steatosis and ketogenesis remain controversial, and in the case of the ketogenesis could be indirectly dependent of other hormones such as insulin. Collectively all of these studies have positioned glucagon as a key hormone in the control of lipid metabolism as a mechanism to reduce body weight.
5. Novel Approaches against Obesity and Diabetes Targeting the Glucagon Receptor
The pleiotropic actions of glucagon on energy balance have, over the last years, made it an attractive molecule for the treatment of diabetes and obesity. The most promising approach to use glucagon as a therapy against obesity comes from its combination with other hormones. Specifically, the design of single-molecule peptides that integrate the complementary actions of multiple endogenous metabolically-related hormones, known as unimolecular polypharmacy, has become successful (Figure 3).
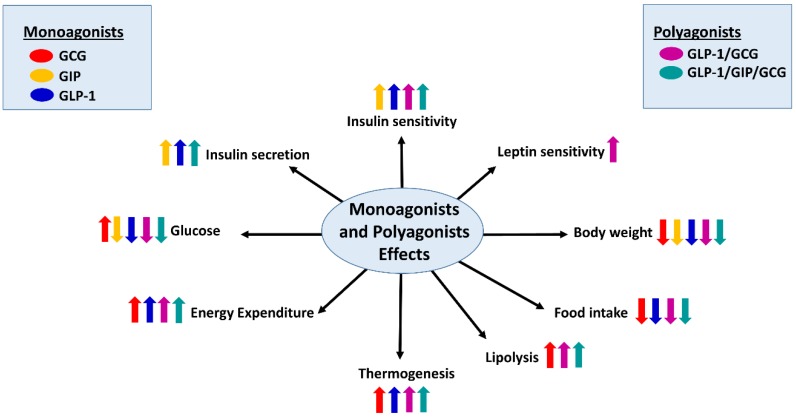
Figure 3.
Unimolecular polypharmacy targeting the glucagon receptor: Schematic overview of the effects of polyagonists of glucagon receptor on energy balance regulation. GCG [Glucagon (red)], GIP [glucose insulinotropic peptide (orange)], GLP-1 [Glucagon like peptide-1 (blue)], GLP-1/GCG (purple), and GLP-1/GIP/GCG (green) (right and left panels). Arrows up indicate increase, while arrows down indicate decrease. Figure made with Servier Medical Art resources.
Remarkably, this strategy has been shown to be more effective than approaches that combine single hormones as separate molecules [73,74]. In this last section, we will focus in the unimolecular polypharmacy of GCGR-based drugs [75]. This strategy presents tremendous efficacy in preclinical animal models and has showed some promising results in preliminary clinical trials. Although this aspect has been extensively reviewed before [75,76,77], we will summarize some of the main studies using these compounds targeting GCGR.
Dual and Triple Agonists Approach Targeting GCGR
A pioneer compound simultaneously activated the GCGR and the glucagon-like peptide-1 receptor (GLP1R), which promoted weight loss and decreased adiposity while stimulating lipolysis and enhance glucose tolerance and leptin sensitivity in DIO mice [78,79].
Another dual agonist that was based on the sequence of the hormone OXM, a gastrointestinal peptide that is able to act through GLP-1R and GCGR with balanced potency, displayed remarkable effects on satiety, body weight and glucose levels [80]. A third class of dual agonist for these receptors was based in the sequence of the native GLP-1 agonist Exendin-4 peptide and a modified glucagon sequence [81]. This chimera showed impressive metabolic actions in DIO mice and indeed doubled the body weight loss induced by the approved drugs for the treatment of obesity [81]. GLP-1/Glucagon co-agonist, in addition to decrease body weight in rodents and cynomolgus monkeys [82], also showed beneficial effects in humans. One such co-agonist, known with the name of MEDI0382, decreased hyperglycemia and body weight in a Phase II clinical trial in diabetic patients [83]. A recent study with another dual agonist of GLP-1/GCGR, known as SAR425899, also reported similar effects in patients with T2DM [84].
Taken together, available evidence obtained with different molecules acting as dual agonists of GLP-1R and GCGR have fulfilled the original expectations of obtaining a much more efficacious treatment than treatment with single agonists. Due to the success with dual agonists in preclinical and clinical studies, this novel strategy has advanced towards the development of single molecules targeting three target receptors at once. Although several early tri-agonists were proved in preclinical studies with suboptimal effects due to an unbalanced agonism [85,86,87], a novel class of a tri-agonist peptides, targeting GLP-1/Glucagon/Glucose Insulinotropic Peptide (GIP) receptors, was developed with promising results [88]. This tri-agonist peptide exhibited a high synergistic activity at each of the three target receptors and greater potency than the native ligands separately [88]. In this study, a low dose of the tri-agonist drastically reduced body weight and adiposity and improved glucose tolerance. Remarkable, this tri-agonist displays higher efficiency compared to other approved drugs, and indeed improves additional parameters such as lipid metabolism and hepatic steatosis in different rodent models of obesity [88,89]. Noticeable, the results obtained showed that this molecule has similar effectiveness on both genders. All these facts implicate that this tri-agonist exhibits high potential translational value.
In summary, this promising strategy showed impressive results on metabolic profile in preclinical animal models targeting GCGR (Figure 3), and due to the superior efficacy compared to currently approved drugs, most of them are now undergoing clinical trials. These studies highlight this strategy as a new attractive path for the managing of metabolic diseases.
6. Conclusions
In sum, we have reviewed studies of glucagon action focusing on the energy balance regulation beyond glucose control. Moreover, we summarized some of therapeutic efforts directed at manipulating GCGR signaling for the treatment of metabolic diseases. Glucagon promotes satiety when it is sensed in the hepatic-portal vein through the vagus nerve and further activation of CNS. However, glucagon also promotes weight loss by activation of EE, thermogenesis and fatty oxidation, suggesting that this hormone impacts on body weight through feeding-dependent and independents mechanisms (Figure 2). In line with these glucagon actions leading to reduced body weight and fat loss, the possible involvement of this hormone in other metabolic disease related to obesity such as hepatic steatosis deserves further investigation.
Despite that the physical–chemical properties of glucagon were for long a significant barrier for chronic studies due to the poor solubility in physiological buffers, new strategies using agonists targeting GCGR have become successful approaches to evaluate the multifaceted nature of glucagon signaling in energy balance and metabolic syndrome. Likewise, although substantial progress has been made in the last decades in the understanding of the molecular pathways and physiological systems that governed energy balance, no successful pharmacological treatments for obesity have been developed yet. Currently the most effective method to improve body weight and glucose levels is bariatric surgery. However, bariatric surgery is restricted to patients with morbid obesity due to its perioperative risks by which a pharmacological option to treat obesity is largely desired. In this scenario, we believe that therapeutic approaches based on unimolecular polypharmacy, such as GCGR-based drugs, may represent a relevant alternative to surgical procedures in the treatment of metabolic diseases.
Acknowledgments
We apologize to the colleagues whose work could not be cited here due to space limitations. This work is supported by grants from Ministerio de Economia y Competitividad (RN: BFU2015-70664R), Xunta de Galicia (RN: 2015-CP080 and 2016-PG057), Centro de Investigación Biomédica en Red (CIBER) de Fisiopatología de la Obesidad y Nutrición (CIBERobn), and European Community Seventh Framework Programme (RN: ERC-2011-StG-OBESITY53-281408). CIBERobn is an initiative of the Instituto de Salud Carlos III (ISCIII) of Spain which is supported by FEDER funds. M.Q. is a recipient of a Postdoctoral contract from Galician Government (Xunta de Galicia ED481B2018/004). Western Norway Regional Health Authority (Helse Vest RHF). The figures were generated by using materials from Servier Medical Art (Servier) under consideration of a Creative Commons Attribution 3.0 Unported License.
Author Contributions
All authors contributed substantially to the writing and editing of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kimball CP M.J. Aqueous extracts of pancreas. III. Some precipitaton reactions to insulin. J. Biol. Chem. 1923;58:337–346. [Google Scholar]
- 2.Quesada I., Tuduri E., Ripoll C., Nadal A. Physiology of the pancreatic alpha-cell and glucagon secretion: Role in glucose homeostasis and diabetes. J. Endocrinol. 2008;199:5–19. doi: 10.1677/JOE-08-0290. [DOI] [PubMed] [Google Scholar]
- 3.Habegger K.M., Heppner K.M., Geary N., Bartness T.J., DiMarchi R., Tschop M.H. The metabolic actions of glucagon revisited. Nat. Rev. Endocrinol. 2010;6:689–697. doi: 10.1038/nrendo.2010.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han V.K., Hynes M.A., Jin C., Towle A.C., Lauder J.M., Lund P.K. Cellular localization of proglucagon/glucagon-like peptide I messenger RNAs in rat brain. J. Neurosci. Res. 1986;16:97–107. doi: 10.1002/jnr.490160110. [DOI] [PubMed] [Google Scholar]
- 5.Drucker D.J., Asa S. Glucagon gene expression in vertebrate brain. J. Biol. Chem. 1988;263:13475–13478. [PubMed] [Google Scholar]
- 6.Ohneda A., Ohneda M. Effect of glicentin-related peptides upon the secretion of insulin and glucagon in the canine pancreas. Tohoku J. Exp. Med. 1988;155:197–204. doi: 10.1620/tjem.155.197. [DOI] [PubMed] [Google Scholar]
- 7.Whiting L., Stewart K.W., Hay D.L., Harris P.W., Choong Y.S., Phillips A.R., Brimble M.A., Cooper G.J. Glicentin-related pancreatic polypeptide inhibits glucose-stimulated insulin secretion from the isolated pancreas of adult male rats. Physiol. Rep. 2015;3 doi: 10.14814/phy2.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi X., Zhou F., Li X., Chang B., Li D., Wang Y., Tong Q., Xu Y., Fukuda M., Zhao J.J., et al. Central GLP-2 enhances hepatic insulin sensitivity via activating PI3K signaling in POMC neurons. Cell Metab. 2013;18:86–98. doi: 10.1016/j.cmet.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt P.T., Naslund E., Gryback P., Jacobsson H., Hartmann B., Holst J.J., Hellstrom P.M. Peripheral administration of GLP-2 to humans has no effect on gastric emptying or satiety. Regul. Pept. 2003;116:21–25. doi: 10.1016/S0167-0115(03)00175-7. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt W.E., Siegel E.G., Creutzfeldt W. Glucagon-like peptide-1 but not glucagon-like peptide-2 stimulates insulin release from isolated rat pancreatic islets. Diabetologia. 1985;28:704–707. doi: 10.1007/BF00291980. [DOI] [PubMed] [Google Scholar]
- 11.Sandoval D.A., D’Alessio D.A. Physiology of proglucagon peptides: Role of glucagon and GLP-1 in health and disease. Physiol. Rev. 2015;95:513–548. doi: 10.1152/physrev.00013.2014. [DOI] [PubMed] [Google Scholar]
- 12.Holst J.J., Bersani M., Johnsen A.H., Kofod H., Hartmann B., Orskov C. Proglucagon processing in porcine and human pancreas. J. Biol. Chem. 1994;269:18827–18833. [PubMed] [Google Scholar]
- 13.Larsen P.J., Tang-Christensen M., Holst J.J., Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997;77:257–270. doi: 10.1016/S0306-4522(96)00434-4. [DOI] [PubMed] [Google Scholar]
- 14.Segre G.V., Goldring S.R. Receptors for secretin, calcitonin, parathyroid hormone (PTH)/PTH-related peptide, vasoactive intestinal peptide, glucagonlike peptide 1, growth hormone-releasing hormone, and glucagon belong to a newly discovered G-protein-linked receptor family. Trends Endocrinol. Metab. 1993;4:309–314. doi: 10.1016/1043-2760(93)90071-L. [DOI] [PubMed] [Google Scholar]
- 15.Svoboda M., Tastenoy M., Vertongen P., Robberecht P. Relative quantitative analysis of glucagon receptor mRNA in rat tissues. Mol. Cell. Endocrinol. 1994;105:131–137. doi: 10.1016/0303-7207(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 16.Kieffer T.J., Heller R.S., Unson C.G., Weir G.C., Habener J.F. Distribution of glucagon receptors on hormone-specific endocrine cells of rat pancreatic islets. Endocrinology. 1996;137:5119–5125. doi: 10.1210/endo.137.11.8895386. [DOI] [PubMed] [Google Scholar]
- 17.Muller T.D., Finan B., Clemmensen C., DiMarchi R.D., Tschop M.H. The New Biology and Pharmacology of Glucagon. Physiol. Rev. 2017;97:721–766. doi: 10.1152/physrev.00025.2016. [DOI] [PubMed] [Google Scholar]
- 18.Schulman J.L., Carleton J.L., Whitney G., Whitehorn J.C. Effect of glucagon on food intake and body weight in man. J. Appl. Physiol. 1957;11:419–421. doi: 10.1152/jappl.1957.11.3.419. [DOI] [PubMed] [Google Scholar]
- 19.Penick S.B., Hinkle L.E., Jr. Depression of food intake induced in healthy subjects by glucagon. N. Engl. J. Med. 1961;264:893–897. doi: 10.1056/NEJM196105042641801. [DOI] [PubMed] [Google Scholar]
- 20.Quinones M., Al-Massadi O., Gallego R., Ferno J., Dieguez C., Lopez M., Nogueiras R. Hypothalamic CaMKKbeta mediates glucagon anorectic effect and its diet-induced resistance. Mol. Metab. 2015;4:961–970. doi: 10.1016/j.molmet.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geary N., Asarian L. Estradiol increases glucagon’s satiating potency in ovariectomized rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R1290–R1294. doi: 10.1152/ajpregu.2001.281.4.R1290. [DOI] [PubMed] [Google Scholar]
- 22.Geary N., Le Sauter J., Noh U. Glucagon acts in the liver to control spontaneous meal size in rats. Am. J. Physiol. 1993;264:R116–R122. doi: 10.1152/ajpregu.1993.264.1.R116. [DOI] [PubMed] [Google Scholar]
- 23.Le Sauter J., Noh U., Geary N. Hepatic portal infusion of glucagon antibodies increases spontaneous meal size in rats. Am. J. Physiol. 1991;261:R162–R165. doi: 10.1152/ajpregu.1991.261.1.R162. [DOI] [PubMed] [Google Scholar]
- 24.Raben A., Andersen H.B., Christensen N.J., Madsen J., Holst J.J., Astrup A. Evidence for an abnormal postprandial response to a high-fat meal in women predisposed to obesity. Am. J. Physiol. 1994;267:E549–E559. doi: 10.1152/ajpendo.1994.267.4.E549. [DOI] [PubMed] [Google Scholar]
- 25.Nilsson G., Uvnas-Wallensten K. Effect of teasing and sham feeding on plasma glucagon concentration in dogs. Acta Physiol. Scand. 1977;100:298–302. doi: 10.1111/j.1748-1716.1977.tb05953.x. [DOI] [PubMed] [Google Scholar]
- 26.Weatherford S.C., Ritter S. Lesion of vagal afferent terminals impairs glucagon-induced suppression of food intake. Physiol. Behav. 1988;43:645–650. doi: 10.1016/0031-9384(88)90220-X. [DOI] [PubMed] [Google Scholar]
- 27.Inokuchi A., Oomura Y., Nishimura H. Effect of intracerebroventricularly infused glucagon on feeding behavior. Physiol. Behav. 1984;33:397–400. doi: 10.1016/0031-9384(84)90160-4. [DOI] [PubMed] [Google Scholar]
- 28.Banks W.A., Kastin A.J. Peptides and the blood-brain barrier: Lipophilicity as a predictor of permeability. Brain Res. Bull. 1985;15:287–292. doi: 10.1016/0361-9230(85)90153-4. [DOI] [PubMed] [Google Scholar]
- 29.Hoosein N.M., Gurd R.S. Identification of glucagon receptors in rat brain. Proc. Natl. Acad. Sci. USA. 1984;81:4368–4372. doi: 10.1073/pnas.81.14.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan E.K., Mackey M.A., Snover D.C., Schneider P.D., Rucker R.D., Jr., Allen C.E., Buchwald H. Suppression of weight gain by glucagon in obese Zucker rats. Exp. Mol. Pathol. 1984;40:320–327. doi: 10.1016/0014-4800(84)90049-2. [DOI] [PubMed] [Google Scholar]
- 31.Bloom S.R., Polak J.M. Glucagonoma syndrome. Am. J. Med. 1987;82:25–36. doi: 10.1016/0002-9343(87)90424-4. [DOI] [PubMed] [Google Scholar]
- 32.De Castro J.M., Paullin S.K., DeLugas G.M. Insulin and glucagon as determinants of body weight set point and microregulation in rats. J. Comp. Physiol. Psychol. 1978;92:571–579. doi: 10.1037/h0077485. [DOI] [PubMed] [Google Scholar]
- 33.Gelling R.W., Du X.Q., Dichmann D.S., Romer J., Huang H., Cui L., Obici S., Tang B., Holst J.J., Fledelius C., et al. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc. Natl. Acad. Sci. USA. 2003;100:1438–1443. doi: 10.1073/pnas.0237106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conarello S.L., Jiang G., Mu J., Li Z., Woods J., Zycband E., Ronan J., Liu F., Roy R.S., Zhu L., et al. Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. Diabetologia. 2007;50:142–150. doi: 10.1007/s00125-006-0481-3. [DOI] [PubMed] [Google Scholar]
- 35.Bryce G.F., Johnson P.R., Sullivan A.C., Stern J.S. Insulin and glucagon: Plasma levels and pancreatic release in the genetically obese Zucker rat. Horm. Metab. Res. 1977;9:366–370. doi: 10.1055/s-0028-1093529. [DOI] [PubMed] [Google Scholar]
- 36.Davidson I.W., Salter J.M., Best C.H. Calorigenic action of glucagon. Nature. 1957;180:1124. doi: 10.1038/1801124a0. [DOI] [PubMed] [Google Scholar]
- 37.Nair K.S. Hyperglucagonemia increases resting metabolic rate in man during insulin deficiency. J. Clin. Endocrinol. Metab. 1987;64:896–901. doi: 10.1210/jcem-64-5-896. [DOI] [PubMed] [Google Scholar]
- 38.JM S. Metabolic Effects of Glucagon in the Wistar Rat. Am. J. Clin. Nutr. 1960;8:535–539. [Google Scholar]
- 39.Habegger K.M., Stemmer K., Cheng C., Müller T.D., Heppner K.M., Ottaway N., Holland J., Hembree J.L., Smiley D., Gelfanov V., et al. Fibroblast growth factor 21 mediates specific glucagon actions. Diabetes. 2013;62:1453–1463. doi: 10.2337/db12-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim T., Nason S., Holleman C., Pepin M., Wilson L., Berryhill T.F., Wende A.R., Steele C., Young M.E., Barnes S., et al. Glucagon Receptor Signaling Regulates Energy Metabolism via Hepatic Farnesoid X Receptor and Fibroblast Growth Factor 21. Diabetes. 2018;67:1773–1782. doi: 10.2337/db17-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doi K., Kuroshima A. Modified metabolic responsiveness to glucagon in cold-acclimated and heat-acclimated rats. Life Sci. 1982;30:785–791. doi: 10.1016/0024-3205(82)90614-2. [DOI] [PubMed] [Google Scholar]
- 42.Billington C.J., Briggs J.E., Link J.G., Levine A.S. Glucagon in physiological concentrations stimulates brown fat thermogenesis in vivo. Am. J. Physiol. 1991;261:R501–R507. doi: 10.1152/ajpregu.1991.261.2.R501. [DOI] [PubMed] [Google Scholar]
- 43.Seitz H.J., Krone W., Wilke H., Tarnowski W. Rapid rise in plasma glucagon induced by acute cold exposure in man and rat. Pflug. Arch. Eur. J. Physiol. 1981;389:115–120. doi: 10.1007/BF00582100. [DOI] [PubMed] [Google Scholar]
- 44.Kinoshita K., Ozaki N., Takagi Y., Murata Y., Oshida Y., Hayashi Y. Glucagon is essential for adaptive thermogenesis in brown adipose tissue. Endocrinology. 2014;155:3484–3492. doi: 10.1210/en.2014-1175. [DOI] [PubMed] [Google Scholar]
- 45.Beaudry J.L., Kaur K.D., Varin E.M., Baggio L.L., Cao X., Mulvihill E.E., Stern J.H., Campbell J.E., Scherer P.E., Drucker D.J., et al. The brown adipose tissue glucagon receptor is functional but not essential for control of energy homeostasis in mice. Mol. Metab. 2019;22:37–48. doi: 10.1016/j.molmet.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salem V., Izzi-Engbeaya C., Coello C., Thomas D.B., Chambers E.S., Comninos A.N., Buckley A., Win Z., Al-Nahhas A., Rabiner E.A., et al. Glucagon increases energy expenditure independently of brown adipose tissue activation in humans. DiabetesObes. Metab. 2016;18:72–81. doi: 10.1111/dom.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atrens D.M., Menendez J.A. Glucagon and the paraventricular hypothalamus: Modulation of energy balance. Brain Res. 1993;630:245–251. doi: 10.1016/0006-8993(93)90663-8. [DOI] [PubMed] [Google Scholar]
- 48.Billington C.J., Bartness T.J., Briggs J., Levine A.S., Morley J.E. Glucagon stimulation of brown adipose tissue growth and thermogenesis. Am. J. Physiol. 1987;252:R160–R165. doi: 10.1152/ajpregu.1987.252.1.R160. [DOI] [PubMed] [Google Scholar]
- 49.Dicker A., Zhao J., Cannon B., Nedergaard J. Apparent thermogenic effect of injected glucagon is not due to a direct effect on brown fat cells. Am. J. Physiol. 1998;275:R1674–R1682. doi: 10.1152/ajpregu.1998.275.5.R1674. [DOI] [PubMed] [Google Scholar]
- 50.Caren R., Corbo L. Glucagon and cholesterol metabolism. Metab. Clin. Exp. 1960;9:938–945. [PubMed] [Google Scholar]
- 51.Paloyan E., Harper P.V., Jr. Glucagon as a regulating factor of plasma lipids. Metab. Clin. Exp. 1961;10:315–323. [PubMed] [Google Scholar]
- 52.Guettet C., Rostaqui N., Navarro N., Lecuyer B., Mathe D. Effect of chronic glucagon administration on the metabolism of triacylglycerol-rich lipoproteins in rats fed a high sucrose diet. J. Nutr. 1991;121:24–30. doi: 10.1093/jn/121.1.24. [DOI] [PubMed] [Google Scholar]
- 53.Caren R., Corbo L. Transfer of plasma lipid to platelets by action of glucagon. Metab. Clin. Exp. 1970;19:598–607. doi: 10.1016/0026-0495(70)90016-8. [DOI] [PubMed] [Google Scholar]
- 54.Penhos J.C., Wu C.H., Daunas J., Reitman M., Levine R. Effect of glucagon on the metabolism of lipids and on urea formation by the perfused rat liver. Diabetes. 1966;15:740–748. doi: 10.2337/diab.15.10.740. [DOI] [PubMed] [Google Scholar]
- 55.Eaton R.P. Hypolipemic action of glucagon in experimental endogenous lipemia in the rat. J. Lipid Res. 1973;14:312–318. [PubMed] [Google Scholar]
- 56.Guettet C., Mathe D., Riottot M., Lutton C. Effects of chronic glucagon administration on cholesterol and bile acid metabolism. Biochim. Et Biophys. Acta. 1988;963:215–223. doi: 10.1016/0005-2760(88)90283-4. [DOI] [PubMed] [Google Scholar]
- 57.Guettet C., Mathe D., Navarro N., Lecuyer B. Effects of chronic glucagon administration on rat lipoprotein composition. Biochim. Et Biophys. Acta. 1989;1005:233–238. doi: 10.1016/0005-2760(89)90042-8. [DOI] [PubMed] [Google Scholar]
- 58.Guettet C., Rostaqui N., Mathe D., Lecuyer B., Navarro N., Jacotot B. Effect of chronic glucagon administration on lipoprotein composition in normally fed, fasted and cholesterol-fed rats. Lipids. 1991;26:451–458. doi: 10.1007/BF02536072. [DOI] [PubMed] [Google Scholar]
- 59.Perea A., Clemente F., Martinell J., Villanueva-Penacarrillo M.L., Valverde I. Physiological effect of glucagon in human isolated adipocytes. Horm. Metab. Res. 1995;27:372–375. doi: 10.1055/s-2007-979981. [DOI] [PubMed] [Google Scholar]
- 60.Mitchell M.L., Byrne M.J., Silver J. Growth-hormone release by glucagon. Lancet. 1969;1:289–290. doi: 10.1016/S0140-6736(69)91041-1. [DOI] [PubMed] [Google Scholar]
- 61.Waldhausl W., Haydl H., Nowotny P. ACTH and cortisol responses to glucagon stimulation. J. Clin. Endocrinol. Metab. 1976;43:675–678. doi: 10.1210/jcem-43-3-675. [DOI] [PubMed] [Google Scholar]
- 62.Sarcione E.J., Back N., Sokal J.E., Mehlman B., Knoblock E. Elevation of plasma epinephrine levels produced by glucagon in vivo. Endocrinology. 1963;72:523–526. doi: 10.1210/endo-72-4-523. [DOI] [PubMed] [Google Scholar]
- 63.Longuet C., Sinclair E.M., Maida A., Baggio L.L., Maziarz M., Charron M.J., Drucker D.J. The glucagon receptor is required for the adaptive metabolic response to fasting. Cell Metab. 2008;8:359–371. doi: 10.1016/j.cmet.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pegorier J.P., Garcia-Garcia M.V., Prip-Buus C., Duee P.H., Kohl C., Girard J. Induction of ketogenesis and fatty acid oxidation by glucagon and cyclic AMP in cultured hepatocytes from rabbit fetuses. Evidence for a decreased sensitivity of carnitine palmitoyltransferase I to malonyl-CoA inhibition after glucagon or cyclic AMP treatment. Biochem. J. 1989;264:93–100. doi: 10.1042/bj2640093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vons C., Pegorier J.P., Girard J., Kohl C., Ivanov M.A., Franco D. Regulation of fatty-acid metabolism by pancreatic hormones in cultured human hepatocytes. Hepatology. 1991;13:1126–1130. doi: 10.1002/hep.1840130620. [DOI] [PubMed] [Google Scholar]
- 66.Dunning B.E., Gerich J.E. The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr. Rev. 2007;28:253–283. doi: 10.1210/er.2006-0026. [DOI] [PubMed] [Google Scholar]
- 67.Ontko J.A., Zilversmit D.B. Correlation between concentrations of circulating free fatty acids and ketone bodies. Proc. Soc. Exp. Biol. Med. 1966;121:319–321. doi: 10.3181/00379727-121-30768. [DOI] [PubMed] [Google Scholar]
- 68.Van Harken D.R., Dixon C.W., Heimberg M. Hepatic lipid metabolism in experimental diabetes. V. The effect of concentration of oleate on metabolism of triglycerides and on ketogenesis. J. Biol. Chem. 1969;244:2278–2285. [PubMed] [Google Scholar]
- 69.Gerich J.E., Lorenzi M., Bier D.M., Schneider V., Tsalikian E., Karam J.H., Forsham P.H. Prevention of human diabetic ketoacidosis by somatostatin. Evidence for an essential role of glucagon. N. Engl. J. Med. 1975;292:985–989. doi: 10.1056/NEJM197505082921901. [DOI] [PubMed] [Google Scholar]
- 70.Schade D.S., Eaton R.P. Modulation of fatty acid metabolism by glucagon in man. IV. Effects of a physiologic hormone infusion in normal man. Diabetes. 1976;25:978–983. doi: 10.2337/diab.25.10.978. [DOI] [PubMed] [Google Scholar]
- 71.Parrilla R., Goodman M.N., Toews C.J. Effect of glucagon: Insulin ratios on hepatic metabolism. Diabetes. 1974;23:725–731. doi: 10.2337/diab.23.9.725. [DOI] [PubMed] [Google Scholar]
- 72.Genuth S.M. Constant intravenous insulin infusion in diabetic ketoacidosis. JAMA. 1973;223:1348–1351. doi: 10.1001/jama.1973.03220120020004. [DOI] [PubMed] [Google Scholar]
- 73.Wellman P.J., Maher T.J. Synergistic interactions between fenfluramine and phentermine. Int. J. Obes. Relat. Metab. Disord. 1999;23:723–732. doi: 10.1038/sj.ijo.0800920. [DOI] [PubMed] [Google Scholar]
- 74.Gault V.A., Kerr B.D., Harriott P., Flatt P.R. Administration of an acylated GLP-1 and GIP preparation provides added beneficial glucose-lowering and insulinotropic actions over single incretins in mice with Type 2 diabetes and obesity. Clin. Sci. (Lond.) 2011;121:107–117. doi: 10.1042/CS20110006. [DOI] [PubMed] [Google Scholar]
- 75.Tschop M.H., Finan B., Clemmensen C., Gelfanov V., Perez-Tilve D., Muller T.D., DiMarchi R.D. Unimolecular Polypharmacy for Treatment of Diabetes and Obesity. Cell Metab. 2016;24:51–62. doi: 10.1016/j.cmet.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 76.Muller T.D., Clemmensen C., Finan B., DiMarchi R.D., Tschop M.H. Anti-Obesity Therapy: From Rainbow Pills to Polyagonists. Pharmacol. Rev. 2018;70:712–746. doi: 10.1124/pr.117.014803. [DOI] [PubMed] [Google Scholar]
- 77.Quinones M., Ferno J., Dieguez C., Nogueiras R., Al-Massadi O. Exciting advances in GPCR-based drugs discovery for treating metabolic disease and future perspectives. Expert Opin. Drug Discov. 2019;14:421–431. doi: 10.1080/17460441.2019.1583642. [DOI] [PubMed] [Google Scholar]
- 78.Day J.W., Ottaway N., Patterson J.T., Gelfanov V., Smiley D., Gidda J., Findeisen H., Bruemmer D., Drucker D.J., Chaudhary N., et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat. Chem. Biol. 2009;5:749–757. doi: 10.1038/nchembio.209. [DOI] [PubMed] [Google Scholar]
- 79.Clemmensen C., Chabenne J., Finan B., Sullivan L., Fischer K., Küchler D., Sehrer L., Ograjsek T., Hofmann S.M., Schriever S.C., et al. GLP-1/glucagon coagonism restores leptin responsiveness in obese mice chronically maintained on an obesogenic diet. Diabetes. 2014;63:1422–1427. doi: 10.2337/db13-1609. [DOI] [PubMed] [Google Scholar]
- 80.Pocai A., Carrington P.E., Adams J.R., Wright M., Eiermann G., Zhu L., Du X., Petrov A., Lassman M.E., Jiang G., et al. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes. 2009;58:2258–2266. doi: 10.2337/db09-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Evers A., Haack T., Lorenz M., Bossart M., Elvert R., Henkel B., Stengelin S., Kurz M., Glien M., Dudda A., et al. Design of Novel Exendin-Based Dual Glucagon-like Peptide 1 (GLP-1)/Glucagon Receptor Agonists. J. Med. Chem. 2017;60:4293–4303. doi: 10.1021/acs.jmedchem.7b00174. [DOI] [PubMed] [Google Scholar]
- 82.Henderson S.J., Konkar A., Hornigold D.C., Trevaskis J.L., Jackson R., Fritsch Fredin M., Jansson-Löfmark R., Naylor J., Rossi A., Bednarek M., et al. Robust anti-obesity and metabolic effects of a dual GLP-1/glucagon receptor peptide agonist in rodents and non-human primates. Diabetes Obes. Metab. 2016;18:1176–1190. doi: 10.1111/dom.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ambery P., Parker V.E., Stumvoll M., Posch M.G., Heise T., Plum-Moerschel L., Tsai L.F., Robertson D., Jain M., Petrone M., et al. MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: A randomised, controlled, double-blind, ascending dose and phase 2a study. Lancet. 2018;391:2607–2618. doi: 10.1016/S0140-6736(18)30726-8. [DOI] [PubMed] [Google Scholar]
- 84.Tillner J., Posch M.G., Wagner F., Teichert L., Hijazi Y., Einig C., Keil S., Haack T., Wagner M., Bossart M., et al. A novel dual glucagon-like peptide and glucagon receptor agonist SAR425899: Results of randomized, placebo-controlled first-in-human and first-in-patient trials. Diabetes Obes. Metab. 2019;21:120–128. doi: 10.1111/dom.13494. [DOI] [PubMed] [Google Scholar]
- 85.Bhat V.K., Kerr B.D., Flatt P.R., Gault V.A. A novel GIP-oxyntomodulin hybrid peptide acting through GIP, glucagon and GLP-1 receptors exhibits weight reducing and anti-diabetic properties. Biochem. Pharmacol. 2013;85:1655–1662. doi: 10.1016/j.bcp.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 86.Gault V.A., Bhat V.K., Irwin N., Flatt P.R. A novel glucagon-like peptide-1 (GLP-1)/glucagon hybrid peptide with triple-acting agonist activity at glucose-dependent insulinotropic polypeptide, GLP-1, and glucagon receptors and therapeutic potential in high fat-fed mice. J. Biol. Chem. 2013;288:35581–35591. doi: 10.1074/jbc.M113.512046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bhat V.K., Kerr B.D., Vasu S., Flatt P.R., Gault V.A. A DPP-IV-resistant triple-acting agonist of GIP, GLP-1 and glucagon receptors with potent glucose-lowering and insulinotropic actions in high-fat-fed mice. Diabetologia. 2013;56:1417–1424. doi: 10.1007/s00125-013-2892-2. [DOI] [PubMed] [Google Scholar]
- 88.Finan B., Yang B., Ottaway N., Smiley D.L., Ma T., Clemmensen C., Chabenne J., Zhang L., Habegger K.M., Fischer K., et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat. Med. 2015;21:27–36. doi: 10.1038/nm.3761. [DOI] [PubMed] [Google Scholar]
- 89.Jall S., Sachs S., Clemmensen C., Finan B., Neff F., DiMarchi R.D., Tschop M.H., Muller T.D., Hofmann S.M. Monomeric GLP-1/GIP/glucagon triagonism corrects obesity, hepatosteatosis, and dyslipidemia in female mice. Mol. Metab. 2017;6:440–446. doi: 10.1016/j.molmet.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]