Abstract
The hedgehog (HH) signaling pathway is central to the regulation of bone development and homeostasis. HH signaling is not only involved in osteoblast differentiation from bone marrow mesenchymal stem cells (BM-MSCs), but also acts upstream within osteoblasts via the OPG/RANK/RANKL axis to control the expression of RANKL. HH signaling has been found to up-regulate parathyroid hormone related protein (PTHrP) expression in osteoblasts, which in turn activates its downstream targets nuclear factor of activated T cells (NFAT) and cAMP responsive element binding protein (CREB), and as a result CREB and NFAT cooperatively increase RANKL expression and osteoclastogenesis. Osteoblasts must remain in balance with osteoclasts in order to avoid excessive bone formation or resorption, thereby maintaining bone homeostasis. This review systemically summarizes the mechanisms whereby HH signaling induces osteoblast development and controls RANKL expression through PTHrP in osteoblasts. Proper targeting of HH signaling may offer a therapeutic option for treating bone homeostasis disorders.
Keywords: hedgehog, RANKL, osteoblast, PTHrP, CREB, NFAT
1. Introduction
The hedgehog (HH) protein family, first identified in Drosophila, is a highly conserved secretory glycoprotein family that is now known to play key roles in regulating embryonic development, cell differentiation and proliferation, and tissue homeostasis in all vertebrates [1,2,3,4,5]. The hedgehog gene was so named because the hedgehog mutation in Drosophila resulted in the larvae that resembled frightened hedgehogs [6]. When abnormally activated, HH signaling is closely linked to tumor development and metastasis [7,8,9], and tumor cell-derived HH signaling can induce receptor activator of nuclear factor-κB ligand (RANKL) production in osteoblasts, stimulating osteoclastogenesis and increasing bone resorption [10]. The maintenance of bone homeostasis, through the balancing of osteoblast-mediated bone formation and osteoclast-mediated bone resorption, is essential, and the osteoprotegerin (OPG)/RANK/RANKL axis acts as a key regulatory mechanism controlling the differentiation and activity of both osteoblasts and osteoclasts. Both OPG and RANKL can be produced by osteoblasts, with RANKL signaling through RANK expressed on pre-osteoclasts to drive their maturation, thus bone resorption, whereas OPG can also bind RANK, competing with RANKL to block osteoclast induction and inhibit bone resorption [11,12,13,14,15,16]. HH signaling in mature osteoblasts has also been shown to induce parathyroid hormone related protein (PTHrP) expression, which further up-regulates RANKL expression, increasing osteoclast maturation and bone resorption [17,18,19]. HH signaling is therefore thought to regulate many key signals upstream of the OPG/RANK/RANKL axis. HH signaling has also recently been found to stimulate bone marrow mesenchymal stem cells (BM-MSC) differentiation into osteoblasts via influencing Runt-related transcription factor 2 (RUNX2) and Osterix (OSX) expression. Both of these transcription factors are key regulators of osteoblast development and consequent bone formation [3,20,21,22,23]. HH signaling thus serves a key dual role in bone homeostasis by regulating bone formation and resorption. With ongoing research on HH signaling, an increasing number of signaling intermediaries and downstream signaling molecules have been found. In this review, we summarize the molecular mechanisms by which HH signaling induces osteoblast differentiation and regulates RANKL expression through PTHrP in osteoblasts.
2. HH Signaling in Mammals
In mammals, three homologous proteins encoded by the hedgehog gene are sonic hedgehog (SHH), desert hedgehog (DHH), and Indian hedgehog (IHH) [24,25,26]. SHH expression is widespread in embryonic tissues and plays critical roles in nervous system, limb, and somite patterning [27,28,29], and it also controls the development of the skin, hair follicles, bones, and gastrointestinal tract [30,31,32]. IHH primarily regulates chondrocyte development and endochondral bone formation, and DHH is expressed predominantly in the male reproductive tract and is essential for the maintenance of the male germ line and spermatogenesis [32,33]. The above three HH proteins serve as secreted signaling molecules and signal through the same highly conserved HH signaling pathway. On target cells, HH signal transduction is controlled by smoothened (SMO) and patched (PTCH, with two mammalian isoforms–PTCH1 and PTCH2), which are 7- and 12-pass transmembrane proteins. The PTCH proteins, encoded by the tumor suppressor gene Patched, serve as the primarily HH receptors, binding all three HH proteins to negatively regulate HH signaling [34,35,36]. SMO, encoded by the proto-oncogene Smoothened, is a G protein-coupled receptor (GPCR) essential for signal transduction [37,38]. In addition, PTCH is enriched on and around primary cilium of the target cell surface, whereas inactive SMO is located on the membrane of intracellular endosomes and maintained away from the cilium [6,39,40]. The primary cilium is an organelle composed of microtubules and protruding from the surface of most mammalian cells, where it serves as a key mediator of HH signaling [41,42,43].
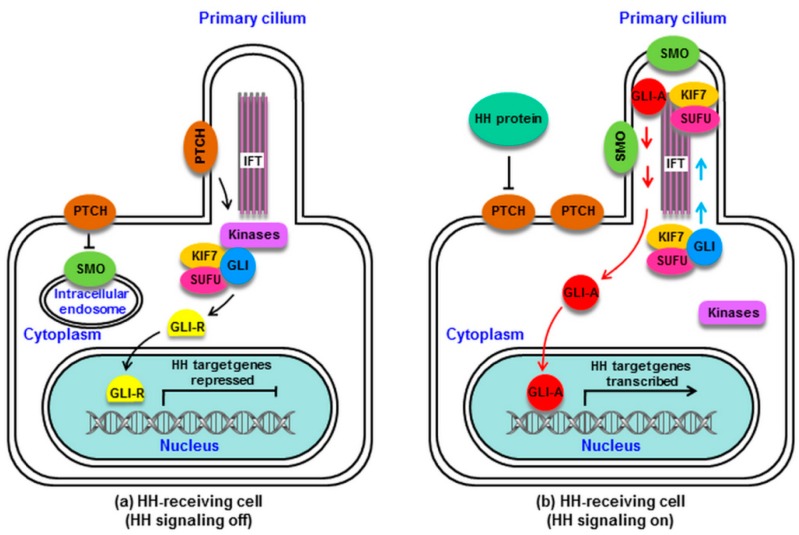
In the absence of HH proteins, PTCH remains near the cilium and represses the activity of SMO. As the final downstream effectors of SMO, GLI proteins, which are zinc finger transcription factors (GLI1-3), are complexed with the suppressor of fused (SUFU) and the kinesin family protein 7 (KIF7). SUFU serves as a major GLI inhibitor, with KIF7 serving a more minor inhibitory role [44,45]. The GLI/SUFU/KIF7 complex can recruit casein kinase I (CKI), protein kinase A (PKA), and glycogen synthase kinase 3 (GSK3). PTCH mediates the activation of these kinases at the cilium base, leading GLI to become phosphorylated. This can in turn promote full-length GLI (GLI-F) cleavage into GLI-R, which is a repressive form that undergoes nuclear translocation to suppress HH target genes important for cellular differentiation, migration, and proliferation (Figure 1a) [46,47].
Figure 1.
Mammalian hedgehog (HH) signaling. (a): Without HH proteins, patched (PTCH) is located at the cilium of the HH-receiving cell, and smoothened (SMO) remains distant from the cilium. PTCH inhibits SMO via preventing its cilial entry. Several kinases (PKA, CKI, GSK3) are recruited by the GLI/suppressor of fused (SUFU)/kinesin family protein 7 (KIF7) complex and activated by PTCH at the base of the cilium. GLI is phosphorylated by these kinases, which promotes its processing into the repressor GLI-R, which undergoes nuclear translocation to block HH target gene transcription. (b): HH protein binding to PTCH relieves SMO inhibition. PTCH is displaced from the cilium, and SMO accumulates in the cilium and converts from an inactive to active state. The GLI/SUFU/KIF7 complex is recruited by active SMO, traveling via intraflagellar transport (IFT) to the cilial top. SMO activation results in the detachment of GLI from SUFU and KIF7. GLI remains as an active GLI-A, which is translocated into the nucleus and goes on to induce HH target gene transcription. HH signaling is activated. CKI, casein kinase I; GLI, GLI protein (a zinc finger transcription factor); GLI-A, GLI activator; GLI-R, GLI repressor; GSK3, glycogen synthase kinase 3; IFT, intraflagellar transport protein; KIF7, kinesin family protein 7 (a minor inhibitor of GLI); PKA, protein kinase A; PTCH, Patched; SMO, Smoothened; SUFU, suppressor of fused (a major inhibitor of GLI).
When HH proteins are present, HH binding to PTCH removes PTCH from the cilium, relieving its repression of SMO. SMO then migrates from the intracellular endosome to the ciliary membrane [48], where HH induces SMO phosphorylation via CKI and GPCR kinase 2 (GRK2), leading to ciliary SMO accumulation [49]. SMO then undergoes a conformational change from an inactive to active state [50]. Activated SMO can recruit the GLI/SUFU/KIF7 complex, which undergoes translocation via intraflagellar transport (IFT) proteins to the cilial tip [51,52], where it can mediate the dissociation of GLI from SUFU and KIF7 [53]. The released GLI-F remains in its activator form (GLI-A), traveling through IFT into the cytoplasm and undergoing nuclear translocation to drive HH target gene activation (Figure 1b) [54,55].
3. Regulation of BM-MSC Osteogenic Differentiation by HH Signaling in Mammals
Mesenchymal stem cells (MSCs) serve as pluripotent progenitor cells which arise from bone marrow that can be induced to differentiate into osteoblasts, chondrocytes, and adipocytes. Osteoblasts and chondrocytes are key cells composing the mammalian skeletal system [56,57]. Bone formation relies upon two essential processes: Intramembranous ossification in certain bones (such as craniofacial bones), and endochondral ossification in the majority of bones (such as long bones). During the former process, BM-MSCs condense and differentiate into osteoblasts and form bone directly, whereas the latter process requires a cartilage intermediate. Endochondral ossification consists of several phases, beginning with the development of a cartilage model, followed by primary and secondary ossification center formation and appositional bone growth. Condensed BM-MSC are able to differentiate into osteoblasts and chondrocytes, with the former differentiating and maturing into bone collars in the cartilage periphery, and the latter forming the initial cartilage model [34,58]. In addition to osteoblasts and chondrocytes, the skeleton also contains hematopoietic-derived osteoclasts, which play vital roles in both cartilage and bone resorption and remodeling [59]. The interaction between osteoblasts and osteoclasts activities results in the necessary bone homeostasis between the formation and resorption of bone.
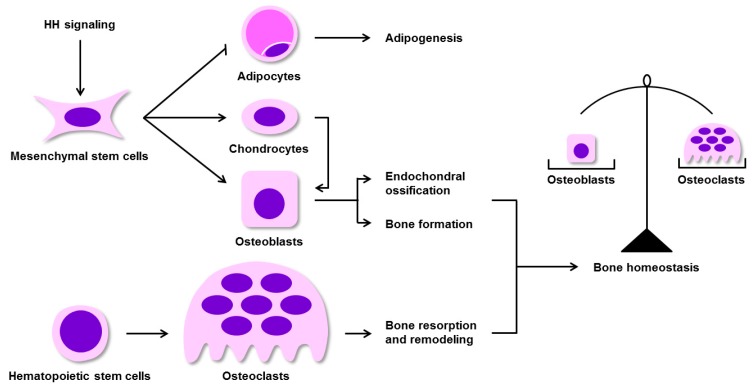
Numerous studies have found that osteoblast differentiation is regulated by a number of signaling proteins and transcription factors. HH signaling, and primarily SHH and IHH, are key regulators of embryonic bone development and homeostasis (Figure 2) [18,60,61]. Chiang et al. [27] demonstrated that lack of Shh in mice can inhibit endochondral ossification, causing vertebrae and limbs to fail to form. Likewise, Yang et al. [6] found that SHH provides key signals necessary for proper limb bud patterning during early embryonic limb development. Work with mutant Ihh mice has revealed that these animals exhibit abnormal endochondral bone formation accompanied by a significant reduction in chondrocyte proliferation, with most chondrocytes being of a mature phenotype and a lack of normal osteoblast development, clearly indicating that IHH plays a central role in the bone formation process [62]. In addition, a recent study established Ihh limb-deficient mice, revealing IHH to play a key role in mesenchymal cell differentiation in the limbs, as long bones in these animals showed evidence of severe bone dysplasia, with a loss of normal bone structures due a lack of normal osteoblast activity [63]. HH signaling thus plays a key role in governing normal BM-MSC differentiation into osteoblasts in the context of endochondral ossification.
Figure 2.
HH signaling is an essential for maintaining bone homeostasis. HH signaling promotes BM-MSC differentiation into chondrocytes and osteoblasts (two cell types involved in endochondral ossification), and inhibits BM-MSC differentiation into adipocytes (associated with adipogenesis). Osteoblasts mediate bone formation, whereas the hematopoietic system-derived osteoclasts drive bone resorption and remodeling. The balance of osteoblast and osteoclast activities is essential for the maintenance of bone homeostasis. BM-MSCs, bone marrow mesenchymal stem cells.
RUNX2 and OSX are two essential transcription factors which mediate ossification and osteoblast differentiation processes [64,65,66]. RUNX2 was the first described osteoblast-specific transcription factor, and is also known as core binding factor α 1 (CBFA1). OSX is a downstream target of RUNX2, and is an osteoblast-specific zinc finger transcription factor [66,67]. Luo et al. [3] reported that HH signaling promotes osteoblast differentiation of BM-MSCs via up-regulating the expression of RUNX2 and OSX, inhibiting BM-MSC differentiation into adipocytes. Several studies have further demonstrated that in osteoblastic cells treated with SHH, both Runx2 and Osx gene expression were increased, with Osx being up-regulated at an earlier time than of Runx2, indicating that factors other than RUNX2 also likely regulate OSX expression, with SHH potentially directly targeting and driving OSX transcription in the context of osteoblastogenesis [68,69]. Moreover, Shimoyama et al. [70] have found that IHH up-regulates the expression and osteogenic action of RUNX2 through GLI2 and consequently stimulates the osteoblastic differentiation of BM-MSCs. Accordingly, GLI2 deficiency in BM-MSCs can significantly inhibit IHH-induced osteoblast differentiation. In addition, IHH treatment or GLI2 overexpression also up-regulates the expression and function of RUNX2. In this study, co-immunoprecipitation further demonstrated that GLI2 and RUNX2 physically interact with one another, suggesting that GLI2 plays a critical role in IHH-induced osteoblastogenesis via regulating RUNX2. Notably, Tu et al. [71] confirmed by histological and molecular analyses that overexpression of RUNX2 in the endochondral skeleton promotes ossification in Runx2-null embryos, whereas the same is not true in Ihh-null embryos. Therefore, it is possible that osteoblastogenesis in response to IHH necessitates additional factors beyond RUNX2. Other studies have shown that HH family members up-regulate expression of bone morphogenetic proteins (BMPs) in several tissues and cells, and BMPs are pleiotropic cytokines can control osteoblast differentiation and bone formation [72,73,74]. It is thus also likely that IHH achieves its osteogenic action through up-regulation of BMPs expression. In summary, the mechanisms by which HH induces BM-MSC osteoblast differentiation remain poorly understood and necessitate further clarification.
4. Activation of HH Signaling Is Able to Increase Osteoblast Activity
HH signaling is induced during mammalian development and is involved in skeletal formation. During skeletogenesis, HH proteins are produced by differentiating mesenchymal cells, where SHH and IHH drive or maintain cell differentiation programs, regulating cartilage and bone formation, and they are particularly essential for osteoblast generation [2,75]. In addition, HH signaling is involved in homeostatic osteoblast activity and in the regulation of bone remodeling [17]. Indeed, several studies have found that activation of HH signaling can increase osteoblastogenesis and osteoblast activity in cell lines from rodent systems [70,72]. Using a murine bone fracture model researchers demonstrated SHH is activated in osteoblasts at the remodeling site of the fracture and modulates the proliferation and differentiation of osteoblasts [76]. Matsumoto et al. [77] also found that SHH is detected in osteoblasts at the callus remodeling stage in young fractured mice, and it stimulates significantly more alkaline phosphatase (ALP)-positive osteoblast formation. Moreover, Baht et al. [78] found that HH pathway activation in osteoblasts leads to increased matrix deposition at the site of the fracture in mice, and then in vitro osteogenic culture systems further confirmed this pathway activation enhances osteoblast activity. Thus, this suggests that the effect of HH pathway regarding fracture repair is mainly on osteoblasts.
5. Activation of HH Signaling in Mature Osteoblasts Enhances Bone Resorption via Up-Regulation of RANKL
A recent study has shown increased HH signaling activity in mature murine osteoblasts in vivo can indirectly enhance the proliferation and differentiation of osteoclasts [17]. This study further noted that in bones of postnatal mice, HH signaling gradually decreased as osteoblast differentiation and maturation progressed. Up-regulating HH signaling specifically within osteoblasts enhanced the ossification process, however, the resultant bone was highly fragile with a high degree of porosity, and there was clear evidence of bone resorption that eventually led to severe osteopenia in mutant mice. Thus, these lines of evidence suggested that HH signaling in mature osteoblasts drove both bone formation and resorption, with resorption being the dominant process. This may be because enhancement of HH signaling can simultaneously drive osteoblast proliferation as well as osteoclast generation via promotion of RANKL up-regulation, given its central role as an inducer of osteoclastogenesis and osteoclastic function [79,80], thereby accelerating bone resorption.
5.1. HH Signaling Promotes the Expression of RANKL through PTHrP
Accumulating evidence indicates that HH signaling regulates RANKL expression in osteoblasts in a manner inextricably linked to PTHrP activity [6,10,81]. PTHrP is a paracrine regulator secreted by periarticular chondrocytes and osteoblasts [82,83,84], while its receptor is mainly expressed on osteogenic cells [85,86,87]. PTHrP binding to its cognate receptor drives endochondral bone development and the maintenance of the adult skeleton [85,88,89]. In line with previous findings [90], Mak and colleagues [17] reported that PTHrP controls HH-mediated regulation of RANKL expression, promoting RANKL expression via activating multiple downstream cascades. In addition, they further found that activation of SHH promoted enhanced expression of both RANKL and PTHrP in skull osteoblasts from normal mice, but not in those from PTHrP-knockout mice. PTHrP thus serves as critical downstream target of HH signaling in osteoblasts to up-regulate RANKL expression, as has been confirmed by other studies [91,92].
RANKL belongs to the tumor necrosis factor (TNF) superfamily, and is secreted by osteoblasts and functions as a major regulator of osteoclast differentiation [93,94]. RANKL binds to the receptor RANK on the surface of pre-osteoclasts and mature osteoclasts and facilitates their activation and differentiation and increases bone resorption [95,96]. In a previous study, Mak and colleagues [17] determined that when HH signaling was specifically enhanced in murine osteoblasts, this led to elevated RANKL and PTHrP expression. Interestingly, the expression of these factors rose more quickly than did osteoblast marker up-regulation, indicating that their expression is independent of such osteoblast differentiation in response to HH pathway activation. In summary, in murine osteoblasts HH signaling can drive osteoclastogenesis and bone resorption via PTHrP-mediated modulation of the RANKL to OPG expression ratio. As Vega et al. [97] and Tat et al. [98] have previously described, this RANKL/OPG ratio is an important signal that reflects the bone remodeling environment. A low ratio suggests an environment that favors bone formation, while a high ratio favors bone resorption.
5.2. The Molecular Mechanisms by which PTHrP Regulates RANKL Expression
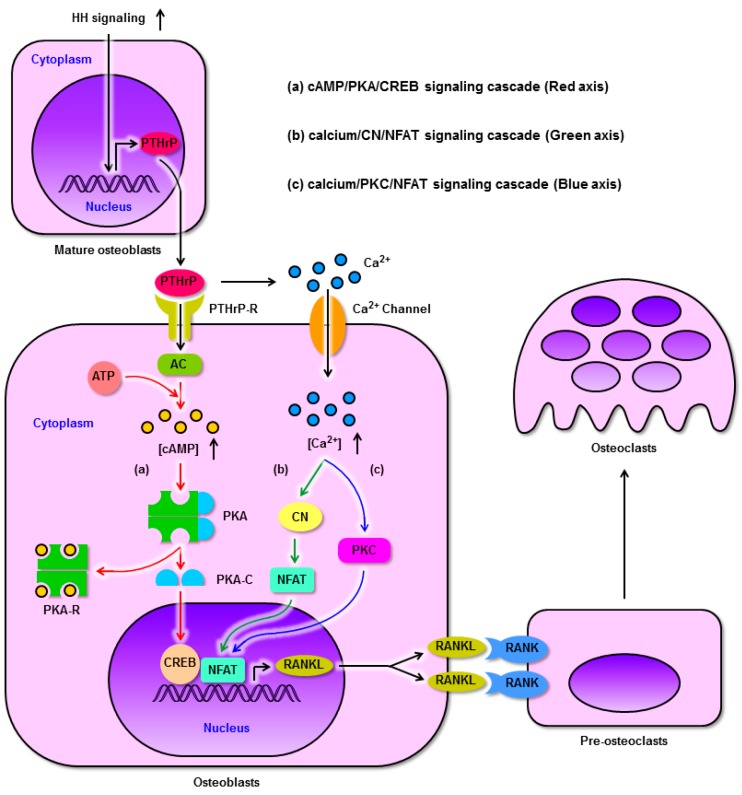
Research using Ptch-knockout mouse models has revealed that enhanced HH signaling in mature osteoblasts ultimately results in elevated osteoclast activity, decreased bone mass, and osteoporosis. The authors of these studies speculated abnormal skeletal phenotype was closely related to PKA and cyclic 3′, 5′-adenosine monophosphate (cAMP) responsive element binding protein (CREB)-mediated RANKL up-regulation [17]. Similarly, Park et al. [99] also proposed that the intracellular cAMP/PKA/CREB cascade is a key signaling pathway important for PTHrP-induced RANKL expression in murine osteoblasts, while CREB downstream of this pathway may be a central regulator of RANKL expression. PKA is made up of 2 regulatory (R) and 2 catalytic (C) subunits, stimulating bone formation owing to its role in osteoblastic cells, enhancing their proliferation and differentiation [100,101,102]. PTHrP binds to its receptor on osteoblastic cells, and the activation of the PTHrP receptor in turn causes the activation of adenylate cyclase (AC) on the surface of osteoblasts [86,99]. Activated AC begins to convert ATP to cAMP. The resultant cAMP accumulates within cells and is able to bind the R subunits of PKA, leading the C subunits to be released. Once activated, these C subunits are able to enter the nucleus and phosphorylate CREB [103,104]. Phosphorylated CREB then binds distal RANKL enhancer regions in order to promote elevated expression of RANKL (Figure 3a) [105,106]. The cAMP/PKA/CREB signaling cascade is thus initiated by PTHrP, whereas PTHrP itself is regulated by HH signaling. These signaling molecules thus constitute a signaling axis that regulates RANKL expression in osteoblasts.
Figure 3.
HH signaling regulates RANKL expression through PTHrP in osteoblasts and promotes osteoclast differentiation. (a): Enhanced HH signaling up-regulates the expression of PTHrP in mature osteoblasts, after which PTHrP binds its receptor on the osteoblast surface and activates AC. Activated AC converts ATP to cAMP. cAMP accumulates within cells and binds PKA-R, freeing the PKA-C subunits which undergo nuclear translocation and phosphorylate CREB. Subsequently, phosphorylated CREB binds to the RANKL promoter. (b) and (c): Meanwhile, PTHrP elevates intracellular calcium concentrations to activate both CN and PKC, in turn promoting the binding of NFAT to the RANKL promoter. As a result, CREB and NFAT cooperatively induce RANKL expression. Finally, RANKL binds RANK on the pre-osteoclast surface, driving osteoclastogenesis. AC, adenylate cyclase; cAMP, cyclic AMP; CN, calcineurin; CREB, cAMP responsive element binding protein; NFAT, nuclear factor of activated T cells; PKA, protein kinase A; PKA-C, PKA catalytic (C) subunit; PKA-R, PKA regulatory (R) subunit; PKC, protein kinase C; PTHrP, parathyroid hormone related protein; PTHrP-R, PTHrP receptor; RANK, receptor activator of nuclear factor-κB; RANKL, RANK ligand.
Recent work suggests that in murine osteoblasts, PTHrP-induced RANKL up-regulation is dependent upon CREB and nuclear factor of activated T cells (NFAT) cooperating to bind the promoter region of RANKL promoter, such that a reduction in NFAT expression impairs this up-regulation. However, NFAT knockdown did not impair the PTHrP-induced up-regulation of CREB, suggesting it is only essential for up-regulating RANKL in response to PTHrP [99,107]. The NFAT transcription factor family is composed of 5 members (NFATC1-C4 and NFAT 5), each of which undergo specific phosphorylation events that dictate their activity. In the absence of stimulation, NFAT remains in the cytosol wherein it is heavily phosphorylated, whereas upon stimulation high levels of intracellular calcium release mediated activation of the Ca2+-dependent phosphatase calcineurin (CN), which dephosphorylates NFAT, leading it to translocate to the nucleus wherein it can regulate gene expression [108,109]. Therein NFAT can cooperate with activated CREB to mediate the binding and transactivation of the RANKL promoter in response to PTHrP (Figure 3b). Consistent with this, Takami and his coworkers [110] determined that the calcium ionophore ionomycin can induce RANKL and OPG upon stimulation of osteoblasts, with the former being up-regulated twice as readily as the latter, suggesting that intracellular calcium is capable of increasing the RANKL/OPG ratio, thus driving osteoclastogenesis. We therefore speculate that NFAT overexpression may be a key mediator of intracellular calcium-mediated RANKL expression in osteoblasts, thus driving osteoclast development.
PTHrP is a key factor responsible for raising circulating calcium levels in the blood, leading to the development of hypercalcemia in certain contexts in addition to playing normal roles in many physiological processes [111,112,113,114]. Increasing intracellular calcium levels in osteoblasts arise due to the influx of extracellular calcium through membrane channels, and/or through the activity of calcium-sensing receptors (CaSR) [115,116,117]. Once these levels of calcium rise to a particular threshold, this is sufficient to drive CN-mediated NFAT activation and consequent changes in gene expression. Interestingly, of the 5 known NFAT transcription factors, Lee et al. [107] observed only NFATC1 and NFATC3 expression in osteoblasts, and they were able to demonstrate that NFATC3 serves as a target gene of NFATC1 in the RANKL regulatory pathway, such that NFATC1 is necessary to mediate NFATC3 up-regulation and activation, whereas overexpressing both NFATC1 and NFATC3 can induce increased expression of RANKL. Park et al. [99], in contrast, proposed that NFATC1 and NFATC3 both bound to the promoter of RANKL in murine osteoblasts, with NFATC1 binding being dependent on its interactions with CREB.
When murine bone marrow cells and primary osteoblasts are cultured together, then treating osteoblasts using ionomycin can drive elevations in intracellular calcium levels, inducing RANKL expression and consequent osteoclastogenesis in the bone marrow cells in a dose-dependent fashion. There was also an initial and unexpected dose-dependent increase in OPG in primary osteoblasts following ionomycin addition, although this expression then decreased over time [110]. This suggests that cytosolic calcium plays a key role in regulating the expression of RANKL, and the ability of RANKL to drive osteoclastogenesis is evidently dominant over the ability of OPG to inhibit this process in a co-culture system. This may in part be due to OPG being a secreted molecule whereas RANKL is a cell-surface molecule [118,119,120,121], potentially leading the former to be more readily diluted than the latter and thus allowing RANKL signaling to dominate and drive osteoclast differentiation. Further, Takami et al. [110] determined that direct protein kinase C (PKC) resulted in comparable outcomes as did increasing intracellular calcium levels, with increases in both RANKL and OPG expression and osteoclastogenesis in a co-culture setting, while PKC inhibitors produced the opposite phenotype. These findings thus clearly reveal a role for PKC downstream of calcium signaling as a mediator of osteoblast RANKL expression. PKC is well-known to act as a kinase central to many fundamental processes within cells [122]. Shin et al. [123] found PKCβ to both induce and activate NFATC1, thereby offering a mechanism whereby it can drive RANKL expression and osteoclastogenesis. These studies thus provide clear evidence that PTHrP, together with PKC signaling, serve as key regulators of osteoblast RANKL expression through their connection to intracellular calcium levels. The calcium/PKC/NFAT signaling axis is regulated by PTHrP as a means of controlling RANKL levels in osteoblasts (Figure 3c).
6. Pharmacologic Management of the OPG/RANK/RANKL Axis
Normal bone is always undergoing remodeling in which osteoclast-mediated bone resorption is balanced by osteoblast-mediated bone formation. In addition, bone remodeling also helps to heal injured bones. When this balance is disrupted, bone homeostasis disorders occur, including the most common such disease—osteoporosis. With ongoing research on bone cell biology, specific targets for regulating bone homeostasis have been revealed, which are based on approaches aimed at promoting bone formation or inhibiting bone resorption. To date, the drugs used to improve bone mass mainly include bone-forming agents and bone resorption inhibitors. The former, including parathyroid hormone peptides and strontium ranelate, increase osteogenesis by enhancing osteoblastic activity, proliferation, survival and differentiation, while the latter, including bisphosphonates, selective estrogen receptor modulators, and denosumab, inhibit osteoclastogenesis by reducing osteoclastic activity, survival, and differentiation [124]. As previously mentioned, the OPG/RANK/RANKL axis acts as a key regulatory mechanism maintaining bone homeostasis to prevent bone loss and ensure a normal bone turnover. Thus, as an important target of drug development, manipulation of the RANKL signaling system has attracted increasing attention and research. Currently, denosumab is the only RANKL-targeted therapeutic drug, providing a new treatment option for osteoporosis. This fully human monoclonal antibody is able to bind RANKL with high specificity and affinity and block RANKL-RANK interactions. As a result, osteoclast maturation, function and survival are inhibited, bone resorption slows, and bone mass increases [125]. Numerous studies have confirmed that denosumab markedly increases bone mineral density, with an associated reduction in the risk of fractures [125,126], and that it also decreases the expression of specific markers of bone resorption in postmenopausal women [127]. Therefore, the good application value of denosumab and its unique mechanism of action differ substantially from other bone resorption inhibitors, thus providing meaningful indications for the screening and development of other signaling pathway-targeted drugs.
7. The Effects of HH Signaling on Skeletal Homeostasis Remodeling and Repair
As shown by these previous studies, HH signaling plays a central role in the regulation of bone formation, repair, and homeostasis. Kashiwagi et al. [128] previously found that by locally administering a Smoothened agonist in a murine bone fracture model, they were able to enhance the rate of callus formation in a manner associated with enhanced chondrocyte proliferation in cartilaginous regions and enhanced osteoblastogenesis in bony regions, thus providing direct evidence that enhancing HH signaling can enhance bone repair. In another report, Lee et al. [129] determined that combining a Smoothened agonist with the osteoinductive Nel-like protein-1, they were able to enhance bone healing in a murine calvarial bone defect model in a manner associated with increased osteoblastogenesis, decreased adipogenesis, and an associated enhancement of bone formation. HH signaling is activated in chondrocytes during the early phases of fracture healing and is known to modulate chondrogenesis. Research using mouse models of fracture repair have further indicated that HH pathway activation in osteoblasts positively influences osteogenesis during the later phases of fracture healing, when osteoblasts are maximally activated so as to enhance matrix production at the site of the fracture to form new bone [78]. This suggests that activation of the HH pathway in osteoblasts is more important for normal fracture healing. As described in previous studies, oxygenated derivatives of cholesterol (oxysterols), which are naturally present in the vascular system and tissues of higher mammals, have important functions in many biological processes including cholesterol homeostasis, apoptosis, platelet aggregation, and sphingolipid metabolism [130], and which also have osteogenic potential [131]. Li et al. [132] demonstrated that a novel semi-synthetic oxysterol analogue, Oxy133, strongly induces osteogenic differentiation in cultured rabbit bone mesenchymal stem cells (rBMSCs) in a manner that increases ALP activity, osteogenic gene marker expression, and mineralization, and that mediates successful healing in a rabbit model of intramembranous bone healing in a manner that induces the osteogenic differentiation of undifferentiated mesenchymal stem cells which accumulate at the site of injury. They further confirmed that the osteogenic potency of Oxy133 both in vitro and in vivo is inhibited by the HH signaling inhibitor cyclopamine, suggesting that Oxy133 activates the HH signaling to promote osteogenesis in the context of intramembrenous bone formation. These findings further support the idea that bone injury will heal as long as undifferentiated mesenchymal cells can differentiate into osteoblasts.
Given that such HH signaling was able to simultaneously promote osteogenesis and suppress adipogenesis, this suggests that controlling HH signaling offers an opportunity to better regulate bone density and treat osteoporosis. Osteoporosis is a very common age-related disease in which bone mass and density are reduced and risk of fracture is increased [133]. Nakamura et al. [69] found that the small molecule HH agonist Hh-Ag 1.7 identified through a high-throughput screening effort strongly activates HH signaling in cultured murine mesenchymal stem cells via activating Gli1 gene expression and stimulates the differentiation of mesenchymal stem cells into osteoblasts via up-regulating Osx gene expression, thereby inducing osteogenesis. As such, Hh-Ag 1.7 may have a great potential for the treatment of bone fractures, especially in patients with osteoporosis or the elderly. As previously reported, speckle-type POZ protein (Spop), an E3-ubiquitin ligase adaptor protein, is responsible for the ubiquitination and degradation of mammalian GLI proteins [134,135]. Cai and Liu [136] found that the loss of Spop causes osteoblast differentiation defects in mutant Spop mice, thereby leading to lower bone density and osteopenia. Of note, the loss of Spop leads to increased GLI3 repressor (GLI3-R) levels and decreased IHH signaling. In addition, they further revealed that Spop directly targets GLI3-R for degradation. As GLI3-R is known to antagonize IHH signaling during endochondral ossification [137], they concluded that Spop positively modulates IHH signaling to promote bone development and homeostasis remodeling via specifically down-regulating GLI3-R, and that the Spop protein may thus be a potential target of intervention for osteoporosis.
Osteoarthritis (OA) is a common degenerative disorder of articular cartilage, especially in the aging population and is associated with obesity. Notably during OA, articular cartilage chondrocytes undergo changes in gene expression and phenotype leading to a phenotype similar to that of chondrocyte hypertrophy in the growth plate during skeletogenesis, indicating the central role for chondrocytes in the progression of OA [138]. As important signaling proteins for the growth and differentiation of chondrocytes, HH signaling has also been shown to play a key role during OA development. Using human osteoarthritic samples and OA mice, Lin et al. [139] found that HH downstream targets, including GLI1, PTCH and hedgehog-interacting protein (HHIP), are highly expressed in articular cartilage accompanied by up-regulation of OA markers, including a disintegrin and metalloproteinase with thrombospondin type 1 motif-5 (ADAMTS5), collagen type X α1 (COL10A1), RUNX2 and matrix metallopeptidase-13 (MMP13), confirming that activation of HH signaling is associated with OA. They used transgenic mice in which HH signaling is activated and further suggested that the level of HH signaling activation correlates positively with the severity of OA. In contrast, when this signaling is inhibited through genetic or pharmacological means, the severity of OA in mice is reduced, and which process potentially is mediated by RUNX2 up-regulating ADAMTS5, which is known to be a key mediator of damage in the development of OA. Thus, inhibiting HH pathway may offer an approach to treating or preventing OA development. Ali et al. [140] also proposed that activation of HH pathway leads to changes in OA progression, with the higher levels of GLI-mediated transcriptional activation in chondrocytes linked to the more severity of OA. Using genetically modified mice, they further demonstrated that the level of GLI-mediated transcription correlates positively with the level of chondrocyte-specific cholesterol accumulation and the severity of OA, suggesting that HH pathway positively regulates intracellular cholesterol biosynthesis, while this cholesterol accumulation in turn partially mediates the effect of HH pathway in chondrocytes in OA. Furthermore, cholesterol blockade, such as statin treatment attenuates the severity of OA in the mouse model with HH activation. Thus, these findings provide new insight into the therapy of OA. Together, there is thus clear clinical value in developing a more in-depth understanding of the mechanisms whereby HH signaling governs skeletal homeostasis remodeling and repair.
8. Conclusions and Future Prospects
As highlighted in this review, HH signaling pathway activation progressively decreased over the course of osteoblast differentiation under normal physiological conditions. Pre-osteoblasts can be driven to undergo more rapid proliferation and differentiation in response to HH signaling, ultimately resulting in increases in bone mass. However, when mature osteoblasts are instead exposed to increased HH signaling then this can result in increased PTHrP expression, activating the cAMP/PKA/CREB pathway and leading CREB to bind the promoter region of the RANKL gene, while increased intracellular calcium levels simultaneously activate CN and PKC to promote NFAT activation and binding to the RANKL promoter. Together, NFAT and CREB are then able to cooperate to promote the expression of RANKL, thereby driving pre-osteoclasts to mature and thus enhancing rates of bone resorption (Figure 3).
HH signaling, though often regarded as a linear pathway, is in reality quite complex, with extensive crosstalk between different signaling pathways together serving to regulate physiological processes. While the specific molecules that serve to regulate and execute HH signaling responses are incompletely understood and in some cases remain controversial, there is nonetheless clear evidence that HH signaling is a key regulator of BM-MSC differentiation into osteoblasts, in addition to governing embryonic bone development [141,142]. Indeed, several studies have found that pharmacological modulation of HH signaling can control the rate of fracture healing in model systems. Early during fracture repair, Ihh and Ptch1 are both induced, with osteoblasts exhibiting an increase in SHH signaling at the site of the fracture, thereby regulating osteoblast and osteoclast proliferation and differentiation, in addition to controlling local vascularization [76,143,144]. When HH agonists are administered systemically, they have also been shown to enhance bone and vascular healing in murine fracture model systems [145]. This is consistent with a central role for HH early during osteoblast development and in promoting the proliferation of these cells. As such, there is a clear need to better understand the HH signaling pathway so as to leverage it to enhance reparative osteogenesis in therapeutic contexts of fracture healing of skeletal weakness.
Major strides in the understanding of HH signaling have been made in recent years, allowing for a more mechanistic understanding of how this pathway induces osteoblast RANKL expression. When activated in mature osteoblasts, HH signaling can simultaneously promote bone formation and resorption, with the latter being more dominant. This occurs due to HH-induced PTHrP expression, which in turn drives RANKL expression and osteoclastogenesis. In these mature osteoblasts, HH signaling thus controls the delicate balance between the development and removal of bone tissue through PTHrP and RANKL regulation. The exact outcomes of this homeostatic process rely on context-dependent cues such as cell type, signal strength, and signal timing. For example, Tomimori Y et al. [146] recently found that soluble RANKL administration was able to rapidly drive bone loss in mice through activation of bone resorption. The authors were then able to use this soluble RANKL to induce a bone loss model in which they could assess the efficacy of inhibitors of bone resorption and other compounds through modulation of the balance between bone loss and bone formation. Work conducted to date thus provides clear evidence that control of the HH signaling pathway may represent an optimal means of tipping the balance of bone homeostasis in a clinically advantageous manner in a wide range of human diseases.
Acknowledgments
The authors acknowledge the helpful advice of Aorigele.
Funding
This work was financially supported by the National Natural Science Foundation of China (Nos. 31760720).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Nusslein-Volhard C., Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 2.Dashti M., Peppelenbosch M.P., Rezaee F. Hedgehog signalling as an antagonist of ageing and its associated diseases. Bioessays News Rev. Mol. Cell. Dev. Biol. 2012;34:849–856. doi: 10.1002/bies.201200049. [DOI] [PubMed] [Google Scholar]
- 3.Luo M., Huang H.X., Huang H., Li Z.T., Lai Y.Y. Hedgehog signaling pathway and osteoporosis. Zhongguo Gu Shang China J. Orthop. Traumatol. 2014;27:169–172. doi: 10.3969/j.issn.1003-0034.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 4.Petrova R., Joyner A.L. Roles for Hedgehog signaling in adult organ homeostasis and repair. Development. 2014;141:3445–3457. doi: 10.1242/dev.083691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gradilla A.-C., Sanchez-Hernandez D., Brunt L., Scholpp S. From top to bottom: Cell polarity in Hedgehog and Wnt trafficking. BMC Biol. 2018;16:37. doi: 10.1186/s12915-018-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang J., Andre P., Ye L., Yang Y.Z. The Hedgehog signalling pathway in bone formation. Int. J. Oral Sci. 2015;7:73–79. doi: 10.1038/ijos.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scales S.J., de Sauvage F.J. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharm. Sci. 2009;30:303–312. doi: 10.1016/j.tips.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson S.E., Furic L., Buchanan G., Larsson O., Pedersen J., Frydenberg M., Risbridger G.P., Taylor R.A. Hedgehog signaling is active in human prostate cancer stroma and regulates proliferation and differentiation of adjacent epithelium. Prostate. 2013;73:1810–1823. doi: 10.1002/pros.22720. [DOI] [PubMed] [Google Scholar]
- 9.He Y., Guo Q., Cheng Y., Qu Y., Sun L., Kong C., Lei L., Zhang G. Abnormal activation of the sonic hedgehog signaling pathway in endometriosis and its diagnostic potency. Fertil. Steril. 2018;110:128–136.e2. doi: 10.1016/j.fertnstert.2018.02.138. [DOI] [PubMed] [Google Scholar]
- 10.Cannonier S.A., Sterling J.A. The Role of Hedgehog Signaling in Tumor Induced Bone Disease. Cancers (Basel) 2015;7:1658–1683. doi: 10.3390/cancers7030856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee B., Iwaniec U.T., Turner R.T., Lin Y.W., Clarke B.L., Gingery A., Wei L.N. RIP140 in monocytes/macrophages regulates osteoclast differentiation and bone homeostasis. JCI Insight. 2017;2:e90517. doi: 10.1172/jci.insight.90517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao B. TNF and Bone Remodeling. Curr. Osteoporos. Rep. 2017;15:126–134. doi: 10.1007/s11914-017-0358-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roshandel D., Holliday K.L., Pye S.R., Boonen S., Borghs H., Vanderschueren D., Huhtaniemi I.T., Adams J.E., Ward K.A., Bartfai G., et al. Genetic variation in the RANKL/RANK/OPG signaling pathway is associated with bone turnover and bone mineral density in men. J. Bone Min. Res. 2010;25:1830–1838. doi: 10.1002/jbmr.78. [DOI] [PubMed] [Google Scholar]
- 14.Ledesma-Colunga M.G., Adán N., Ortiz G., Solís-Gutiérrez M., López-Barrera F., Martínez de la Escalera G., Clapp C. Prolactin blocks the expression of receptor activator of nuclear factor κB ligand and reduces osteoclastogenesis and bone loss in murine inflammatory arthritis. Arthritis Res. Ther. 2017;19:93. doi: 10.1186/s13075-017-1290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L., Liu S., Zhao Y., Liu D., Liu Y., Chen C., Karray S., Shi S., Jin Y. Osteoblast-induced osteoclast apoptosis by fas ligand/FAS pathway is required for maintenance of bone mass. Cell Death Differ. 2015;22:1654–1664. doi: 10.1038/cdd.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sobacchi C., Menale C., Villa A. The RANKL-RANK Axis: A Bone to Thymus Round Trip. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mak K.K., Bi Y., Wan C., Chuang P.T., Clemens T., Young M., Yang Y. Hedgehog signaling in mature osteoblasts regulates bone formation and resorption by controlling PTHrP and RANKL expression. Dev. Cell. 2008;14:674–688. doi: 10.1016/j.devcel.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Li L., Dong Q., Wang Y., Feng Q., Zhou P., Ou X., Meng Q., He T., Luo J. Hedgehog signaling is involved in the BMP9-induced osteogenic differentiation of mesenchymal stem cells. Int. J. Mol. Med. 2015;35:1641–1650. doi: 10.3892/ijmm.2015.2172. [DOI] [PubMed] [Google Scholar]
- 19.Ye L., Lou F., Yu F., Zhang D., Wang C., Wu F., Li X., Ping Y., Yang X., Yang J., et al. NUMB maintains bone mass by promoting degradation of PTEN and GLI1 via ubiquitination in osteoblasts. Bone Res. 2018;6:32. doi: 10.1038/s41413-018-0030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliveira F.S., Bellesini L.S., Defino H.L., da Silva Herrero C.F., Beloti M.M., Rosa A.L. Hedgehog signaling and osteoblast gene expression are regulated by purmorphamine in human mesenchymal stem cells. J. Cell Biochem. 2012;113:204–208. doi: 10.1002/jcb.23345. [DOI] [PubMed] [Google Scholar]
- 21.Zhang C. Molecular mechanisms of osteoblast-specific transcription factor Osterix effect on bone formation. Beijing Da Xue Xue Bao Yi Xue Ban. 2012;44:659–665. doi: 10.3969/j.issn.1671-167X.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Ohata Y., Ozono K. Bone and Stem Cells. The mechanism of osteogenic differentiation from mesenchymal stem cell. Clin. Calcium. 2014;24:501–508. [PubMed] [Google Scholar]
- 23.Komori T. Roles of Runx2 in Skeletal Development. Adv. Exp. Med. Biol. 2017;962:83–93. doi: 10.1007/978-981-10-3233-2_6. [DOI] [PubMed] [Google Scholar]
- 24.Yang L., Xie G., Fan Q., Xie J. Activation of the hedgehog-signaling pathway in human cancer and the clinical implications. Oncogene. 2010;29:469–481. doi: 10.1038/onc.2009.392. [DOI] [PubMed] [Google Scholar]
- 25.Fernandes-Silva H., Correia-Pinto J., Moura R.S. Canonical Sonic Hedgehog Signaling in Early Lung Development. J. Dev. Biol. 2017;5:3. doi: 10.3390/jdb5010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dimou A., Bamias A., Gogas H., Syrigos K. Inhibition of the Hedgehog pathway in lung cancer. Lung Cancer. 2019;133:56–61. doi: 10.1016/j.lungcan.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Chiang C., Litingtung Y., Lee E., Young K.E., Corden J.L., Westphal H., Beachy P.A. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- 28.Borycki A.G., Mendham L., Emerson C.P., Jr. Control of somite patterning by Sonic hedgehog and its downstream signal response genes. Development. 1998;125:777–790. doi: 10.1242/dev.125.4.777. [DOI] [PubMed] [Google Scholar]
- 29.Ho K.S., Scott M.P. Sonic hedgehog in the nervous system: Functions, modifications and mechanisms. Curr. Opin. Neurobiol. 2002;12:57–63. doi: 10.1016/S0959-4388(02)00290-8. [DOI] [PubMed] [Google Scholar]
- 30.van den Brink G.R., Hardwick J.C., Nielsen C., Xu C., ten Kate F.J., Glickman J., van Deventer S.J., Roberts D.J., Peppelenbosch M.P. Sonic hedgehog expression correlates with fundic gland differentiation in the adult gastrointestinal tract. Gut. 2002;51:628–633. doi: 10.1136/gut.51.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ellis T., Smyth I., Riley E., Bowles J., Adolphe C., Rothnagel J.A., Wicking C., Wainwright B.J. Overexpression of Sonic Hedgehog suppresses embryonic hair follicle morphogenesis. Dev. Biol. 2003;263:203–215. doi: 10.1016/S0012-1606(03)00394-4. [DOI] [PubMed] [Google Scholar]
- 32.James A.W. Review of Signaling Pathways Governing MSC Osteogenic and Adipogenic Differentiation. Scientifica. 2013;2013:684736. doi: 10.1155/2013/684736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Hara W.A., Azar W.J., Behringer R.R., Renfree M.B., Pask A.J. Desert hedgehog is a mammal-specific gene expressed during testicular and ovarian development in a marsupial. BMC Dev. Biol. 2011;11:72. doi: 10.1186/1471-213X-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maeda Y., Nakamura E., Nguyen M.T., Suva L.J., Swain F.L., Razzaque M.S., Mackem S., Lanske B. Indian Hedgehog produced by postnatal chondrocytes is essential for maintaining a growth plate and trabecular bone. Proc. Natl. Acad. Sci. USA. 2007;104:6382–6387. doi: 10.1073/pnas.0608449104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bai Y., Bai Y., Dong J., Li Q., Jin Y., Chen B., Zhou M. Hedgehog Signaling in Pancreatic Fibrosis and Cancer. Medicine. 2016;95:e2996. doi: 10.1097/MD.0000000000002996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao L., Wang L., Chi C., Lan W., Su Y. The emerging roles of phosphatases in Hedgehog pathway. Cell Commun. Signal. 2017;15:35. doi: 10.1186/s12964-017-0191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murone M., Rosenthal A., de Sauvage F.J. Sonic hedgehog signaling by the patched-smoothened receptor complex. Curr. Biol. 1999;9:76–84. doi: 10.1016/S0960-9822(99)80018-9. [DOI] [PubMed] [Google Scholar]
- 38.Dey J., Ditzler S., Knoblaugh S.E., Hatton B.A., Schelter J.M., Cleary M.A., Mecham B., Rorke-Adams L.B., Olson J.M. A distinct Smoothened mutation causes severe cerebellar developmental defects and medulloblastoma in a novel transgenic mouse model. Mol. Cell Biol. 2012;32:4104–4115. doi: 10.1128/MCB.00862-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rohatgi R., Milenkovic L., Scott M.P. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 40.Briscoe J., Therond P.P. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 2013;14:416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 41.Goetz S.C., Anderson K.V. The primary cilium: A signalling centre during vertebrate development. Nat. Rev. Genet. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson C.W., Chuang P.T. Mechanism and evolution of cytosolic Hedgehog signal transduction. Development. 2010;137:2079–2094. doi: 10.1242/dev.045021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han Y., Xiong Y., Shi X., Wu J., Zhao Y., Jiang J. Regulation of Gli ciliary localization and Hedgehog signaling by the PY-NLS/karyopherin-beta2 nuclear import system. PLoS Biol. 2017;15:e2002063. doi: 10.1371/journal.pbio.2002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kasper M., Regl G., Frischauf A.M., Aberger F. GLI transcription factors: Mediators of oncogenic Hedgehog signalling. Eur. J. Cancer. 2006;42:437–445. doi: 10.1016/j.ejca.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 45.Cox B., Briscoe J., Ulloa F. SUMOylation by Pias1 regulates the activity of the Hedgehog dependent Gli transcription factors. PLoS ONE. 2010;5:e11996. doi: 10.1371/journal.pone.0011996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan Y., Bai C.B., Joyner A.L., Wang B. Sonic hedgehog signaling regulates Gli2 transcriptional activity by suppressing its processing and degradation. Mol. Cell Biol. 2006;26:3365–3377. doi: 10.1128/MCB.26.9.3365-3377.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koury J., Zhong L., Hao J. Targeting Signaling Pathways in Cancer Stem Cells for Cancer Treatment. Stem Cells Int. 2017;2017:2925869. doi: 10.1155/2017/2925869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caro I., Low J.A. The role of the hedgehog signaling pathway in the development of basal cell carcinoma and opportunities for treatment. Clin. Cancer Res. 2010;16:3335–3339. doi: 10.1158/1078-0432.CCR-09-2570. [DOI] [PubMed] [Google Scholar]
- 49.Chen Y., Sasai N., Ma G., Yue T., Jia J., Briscoe J., Jiang J. Sonic Hedgehog dependent phosphorylation by CK1alpha and GRK2 is required for ciliary accumulation and activation of smoothened. PLoS Biol. 2011;9:e1001083. doi: 10.1371/journal.pbio.1001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao Y., Tong C., Jiang J. Hedgehog regulates smoothened activity by inducing a conformational switch. Nature. 2007;450:252–258. doi: 10.1038/nature06225. [DOI] [PubMed] [Google Scholar]
- 51.Kim J., Kato M., Beachy P.A. Gli2 trafficking links Hedgehog-dependent activation of Smoothened in the primary cilium to transcriptional activation in the nucleus. Proc. Natl. Acad. Sci. USA. 2009;106:21666–21671. doi: 10.1073/pnas.0912180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Humke E.W., Dorn K.V., Milenkovic L., Scott M.P., Rohatgi R. The output of Hedgehog signaling is controlled by the dynamic association between Suppressor of Fused and the Gli proteins. Genes Dev. 2010;24:670–682. doi: 10.1101/gad.1902910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tukachinsky H., Lopez L.V., Salic A. A mechanism for vertebrate Hedgehog signaling: Recruitment to cilia and dissociation of SuFu-Gli protein complexes. J. Cell Biol. 2010;191:415–428. doi: 10.1083/jcb.201004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Varjosalo M., Taipale J. Hedgehog: Functions and mechanisms. Genes Dev. 2008;22:2454–2472. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- 55.Teglund S., Toftgard R. Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim. Biophys. Acta. 2010;1805:181–208. doi: 10.1016/j.bbcan.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 56.Wang L., Jie Q., Yang L. Chondrocytes-osteoblast transition in endochondral ossification. Ann. Jt. 2017;2 doi: 10.21037/aoj.2017.01.06. [DOI] [Google Scholar]
- 57.Lin H., Sohn J., Shen H., Langhans M.T., Tuan R.S. Bone marrow mesenchymal stem cells: Aging and tissue engineering applications to enhance bone healing. Biomaterials. 2019;203:96–110. doi: 10.1016/j.biomaterials.2018.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Day T.F., Guo X., Garrett-Beal L., Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev. Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 59.Miclea R.L., Karperien M., Bosch C.A., van der Horst G., van der Valk M.A., Kobayashi T., Kronenberg H.M., Rawadi G., Akcakaya P., Lowik C.W., et al. Adenomatous polyposis coli-mediated control of beta-catenin is essential for both chondrogenic and osteogenic differentiation of skeletal precursors. BMC Dev. Biol. 2009;9:26. doi: 10.1186/1471-213X-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang C. Transcriptional regulation of bone formation by the osteoblast-specific transcription factor Osx. J. Orthop. Surg. Res. 2010;5:37. doi: 10.1186/1749-799X-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shi Y., Chen J., Karner C.M., Long F. Hedgehog signaling activates a positive feedback mechanism involving insulin-like growth factors to induce osteoblast differentiation. Proc. Natl. Acad. Sci. USA. 2015;112:4678–4683. doi: 10.1073/pnas.1502301112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.St-Jacques B., Hammerschmidt M., McMahon A.P. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amano K., Densmore M.J., Lanske B. Conditional Deletion of Indian Hedgehog in Limb Mesenchyme Results in Complete Loss of Growth Plate Formation but Allows Mature Osteoblast Differentiation. J. Bone Min. Res. 2015;30:2262–2272. doi: 10.1002/jbmr.2582. [DOI] [PubMed] [Google Scholar]
- 64.Komori T. Regulation of osteoblast differentiation by transcription factors. J. Cell Biochem. 2006;99:1233–1239. doi: 10.1002/jcb.20958. [DOI] [PubMed] [Google Scholar]
- 65.Baek W.Y., Lee M.A., Jung J.W., Kim S.Y., Akiyama H., de Crombrugghe B., Kim J.E. Positive regulation of adult bone formation by osteoblast-specific transcription factor osterix. J. Bone Min. Res. 2009;24:1055–1065. doi: 10.1359/jbmr.081248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Z., Xu Z., Duan C., Liu W., Sun J., Han B. Role of TCF/LEF Transcription Factors in Bone Development and Osteogenesis. Int. J. Med. Sci. 2018;15:1415–1422. doi: 10.7150/ijms.26741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pang X.G., Cong Y., Bao N.R., Li Y.G., Zhao J.N. Quercetin Stimulates Bone Marrow Mesenchymal Stem Cell Differentiation through an Estrogen Receptor-Mediated Pathway. BioMed Res. Int. 2018;2018:4178021. doi: 10.1155/2018/4178021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tian Y., Xu Y., Fu Q., Dong Y. Osterix is required for Sonic hedgehog-induced osteoblastic MC3T3-E1 cell differentiation. Cell Biochem. Biophys. 2012;64:169–176. doi: 10.1007/s12013-012-9369-7. [DOI] [PubMed] [Google Scholar]
- 69.Nakamura T., Naruse M., Chiba Y., Komori T., Sasaki K., Iwamoto M., Fukumoto S. Novel hedgehog agonists promote osteoblast differentiation in mesenchymal stem cells. J. Cell Physiol. 2015;230:922–929. doi: 10.1002/jcp.24823. [DOI] [PubMed] [Google Scholar]
- 70.Shimoyama A., Wada M., Ikeda F., Hata K., Matsubara T., Nifuji A., Noda M., Amano K., Yamaguchi A., Nishimura R., et al. Ihh/Gli2 signaling promotes osteoblast differentiation by regulating Runx2 expression and function. Mol. Biol. Cell. 2007;18:2411–2418. doi: 10.1091/mbc.e06-08-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tu X., Joeng K.S., Long F. Indian hedgehog requires additional effectors besides Runx2 to induce osteoblast differentiation. Dev. Biol. 2012;362:76–82. doi: 10.1016/j.ydbio.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakamura T., Aikawa T., Iwamoto-Enomoto M., Iwamoto M., Higuchi Y., Pacifici M., Kinto N., Yamaguchi A., Noji S., Kurisu K., et al. Induction of osteogenic differentiation by hedgehog proteins. Biochem. Biophys. Res. Commun. 1997;237:465–469. doi: 10.1006/bbrc.1997.7156. [DOI] [PubMed] [Google Scholar]
- 73.Yamaguchi A., Komori T., Suda T. Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocr. Rev. 2000;21:393–411. doi: 10.1210/edrv.21.4.0403. [DOI] [PubMed] [Google Scholar]
- 74.Hojo H., Ohba S., Taniguchi K., Shirai M., Yano F., Saito T., Ikeda T., Nakajima K., Komiyama Y., Nakagata N., et al. Hedgehog-Gli activators direct osteo-chondrogenic function of bone morphogenetic protein toward osteogenesis in the perichondrium. J. Biol. Chem. 2013;288:9924–9932. doi: 10.1074/jbc.M112.409342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Long F., Zhang X.M., Karp S., Yang Y., McMahon A.P. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development. 2001;128:5099–5108. doi: 10.1242/dev.128.24.5099. [DOI] [PubMed] [Google Scholar]
- 76.Horikiri Y., Shimo T., Kurio N., Okui T., Matsumoto K., Iwamoto M., Sasaki A. Sonic hedgehog regulates osteoblast function by focal adhesion kinase signaling in the process of fracture healing. PLoS ONE. 2013;8:e76785. doi: 10.1371/journal.pone.0076785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matsumoto K., Shimo T., Kurio N., Okui T., Obata K., Masui M., Pang P., Horikiri Y., Sasaki A. Expression and Role of Sonic Hedgehog in the Process of Fracture Healing with Aging. In Vivo. 2016;30:99–105. [PubMed] [Google Scholar]
- 78.Baht G.S., Silkstone D., Nadesan P., Whetstone H., Alman B.A. Activation of hedgehog signaling during fracture repair enhances osteoblastic-dependent matrix formation. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2014;32:581–586. doi: 10.1002/jor.22562. [DOI] [PubMed] [Google Scholar]
- 79.Lacey D.L., Timms E., Tan H.L., Kelley M.J., Dunstan C.R., Burgess T., Elliott R., Colombero A., Elliott G., Scully S., et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/S0092-8674(00)81569-X. [DOI] [PubMed] [Google Scholar]
- 80.Bi H., Chen X., Gao S., Yu X., Xiao J., Zhang B., Liu X., Dai M. Key Triggers of Osteoclast-Related Diseases and Available Strategies for Targeted Therapies: A Review. Front. Med. 2017;4:234. doi: 10.3389/fmed.2017.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Otsuka A., Dreier J., Cheng P.F., Nageli M., Lehmann H., Felderer L., Frew I.J., Matsushita S., Levesque M.P., Dummer R. Hedgehog pathway inhibitors promote adaptive immune responses in basal cell carcinoma. Clin. Cancer Res. 2015;21:1289–1297. doi: 10.1158/1078-0432.CCR-14-2110. [DOI] [PubMed] [Google Scholar]
- 82.Martin T.J., Moseley J.M., Williams E.D. Parathyroid hormone-related protein: Hormone and cytokine. J. Endocrinol. 1997;154:S23–S37. [PubMed] [Google Scholar]
- 83.Zhang W., Chen J., Zhang S., Ouyang H.W. Inhibitory function of parathyroid hormone-related protein on chondrocyte hypertrophy: The implication for articular cartilage repair. Arthritis Res. 2012;14:221. doi: 10.1186/ar4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martin T.J. Parathyroid Hormone-Related Protein, Its Regulation of Cartilage and Bone Development, and Role in Treating Bone Diseases. Physiol. Rev. 2016;96:831–871. doi: 10.1152/physrev.00031.2015. [DOI] [PubMed] [Google Scholar]
- 85.Karaplis A.C. PTHrP: Novel roles in skeletal biology. Curr. Pharm. Des. 2001;7:655–670. doi: 10.2174/1381612013397753. [DOI] [PubMed] [Google Scholar]
- 86.Datta N.S., Abou-Samra A.B. PTH and PTHrP signaling in osteoblasts. Cell Signal. 2009;21:1245–1254. doi: 10.1016/j.cellsig.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leder B.Z. Parathyroid Hormone and Parathyroid Hormone-Related Protein Analogs in Osteoporosis Therapy. Curr. Osteoporos. Rep. 2017;15:110–119. doi: 10.1007/s11914-017-0353-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kronenberg H.M. PTHrP and skeletal development. Ann. N. Y. Acad Sci. 2006;1068:1–13. doi: 10.1196/annals.1346.002. [DOI] [PubMed] [Google Scholar]
- 89.Ono W., Sakagami N., Nishimori S., Ono N., Kronenberg H.M. Parathyroid hormone receptor signalling in osterix-expressing mesenchymal progenitors is essential for tooth root formation. Nat. Commun. 2016;7:11277. doi: 10.1038/ncomms11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thomas R.J., Guise T.A., Yin J.J., Elliott J., Horwood N.J., Martin T.J., Gillespie M.T. Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology. 1999;140:4451–4458. doi: 10.1210/endo.140.10.7037. [DOI] [PubMed] [Google Scholar]
- 91.Das S., Samant R.S., Shevde L.A. Hedgehog signaling induced by breast cancer cells promotes osteoclastogenesis and osteolysis. J. Biol. Chem. 2011;286:9612–9622. doi: 10.1074/jbc.M110.174920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang X., Cheng Q., Wang Y., Leung P.S., Mak K.K. Hedgehog signaling in bone regulates whole-body energy metabolism through a bone-adipose endocrine relay mediated by PTHrP and adiponectin. Cell Death Differ. 2017;24:225–237. doi: 10.1038/cdd.2016.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yamashita T., Takahashi N., Udagawa N. New roles of osteoblasts involved in osteoclast differentiation. World J. Orthop. 2012;3:175–181. doi: 10.5312/wjo.v3.i11.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu F., Dong Y., Huang X., Chen P., Guo F., Chen A., Huang S. Pioglitazone affects the OPG/RANKL/RANK system and increase osteoclastogenesis. Mol. Med. Rep. 2016;14:2289–2296. doi: 10.3892/mmr.2016.5515. [DOI] [PubMed] [Google Scholar]
- 95.Boyle W.J., Simonet W.S., Lacey D.L. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 96.Silva I., Branco J.C. Rank/Rankl/opg: Literature review. Acta Reum. Port. 2011;36:209–218. [PubMed] [Google Scholar]
- 97.Vega D., Maalouf N.M., Sakhaee K. CLINICAL Review #: The role of receptor activator of nuclear factor-kappaB (RANK)/RANK ligand/osteoprotegerin: Clinical implications. J. Clin. Endocrinol. Metab. 2007;92:4514–4521. doi: 10.1210/jc.2007-0646. [DOI] [PubMed] [Google Scholar]
- 98.Tat S.K., Pelletier J.P., Velasco C.R., Padrines M., Martel-Pelletier J. New perspective in osteoarthritis: The OPG and RANKL system as a potential therapeutic target? Keio J. Med. 2009;58:29–40. doi: 10.2302/kjm.58.29. [DOI] [PubMed] [Google Scholar]
- 99.Park H.J., Baek K., Baek J.H., Kim H.R. The cooperation of CREB and NFAT is required for PTHrP-induced RANKL expression in mouse osteoblastic cells. J. Cell Physiol. 2015;230:667–679. doi: 10.1002/jcp.24790. [DOI] [PubMed] [Google Scholar]
- 100.Kvissel A.K., Orstavik S., Oistad P., Rootwelt T., Jahnsen T., Skalhegg B.S. Induction of Cbeta splice variants and formation of novel forms of protein kinase A type II holoenzymes during retinoic acid-induced differentiation of human NT2 cells. Cell Signal. 2004;16:577–587. doi: 10.1016/j.cellsig.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 101.Jilka R.L. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434–1446. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Greenfield E.M. Anabolic effects of intermittent PTH on osteoblasts. Curr. Mol. Pharm. 2012;5:127–134. doi: 10.2174/1874467211205020127. [DOI] [PubMed] [Google Scholar]
- 103.Sands W.A., Palmer T.M. Regulating gene transcription in response to cyclic AMP elevation. Cell Signal. 2008;20:460–466. doi: 10.1016/j.cellsig.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 104.Tascau L., Gardner T., Anan H., Yongpravat C., Cardozo C.P., Bauman W.A., Lee F.Y., Oh D.S., Tawfeek H.A. Activation of Protein Kinase A in Mature Osteoblasts Promotes a Major Bone Anabolic Response. Endocrinology. 2016;157:112–126. doi: 10.1210/en.2015-1614. [DOI] [PubMed] [Google Scholar]
- 105.Fu Q., Manolagas S.C., O’Brien C.A. Parathyroid hormone controls receptor activator of NF-kappaB ligand gene expression via a distant transcriptional enhancer. Mol. Cell Biol. 2006;26:6453–6468. doi: 10.1128/MCB.00356-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim S., Yamazaki M., Shevde N.K., Pike J.W. Transcriptional control of receptor activator of nuclear factor-kappaB ligand by the protein kinase A activator forskolin and the transmembrane glycoprotein 130-activating cytokine, oncostatin M, is exerted through multiple distal enhancers. Mol. Endocrinol. 2007;21:197–214. doi: 10.1210/me.2006-0315. [DOI] [PubMed] [Google Scholar]
- 107.Lee H.L., Bae O.Y., Baek K.H., Kwon A., Hwang H.R., Qadir A.S., Park H.J., Woo K.M., Ryoo H.M., Baek J.H. High extracellular calcium-induced NFATc3 regulates the expression of receptor activator of NF-kappaB ligand in osteoblasts. Bone. 2011;49:242–249. doi: 10.1016/j.bone.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 108.Rao A. Signaling to gene expression: Calcium, calcineurin and NFAT. Nat. Immunol. 2009;10:3–5. doi: 10.1038/ni0109-3. [DOI] [PubMed] [Google Scholar]
- 109.Musson R.E., Cobbaert C.M., Smit N.P. Molecular diagnostics of calcineurin-related pathologies. Clin. Chem. 2012;58:511–522. doi: 10.1373/clinchem.2011.167296. [DOI] [PubMed] [Google Scholar]
- 110.Takami M., Takahashi N., Udagawa N., Miyaura C., Suda K., Woo J.T., Martin T.J., Nagai K., Suda T. Intracellular calcium and protein kinase C mediate expression of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin in osteoblasts. Endocrinology. 2000;141:4711–4719. doi: 10.1210/endo.141.12.7852. [DOI] [PubMed] [Google Scholar]
- 111.Philbrick W.M., Wysolmerski J.J., Galbraith S., Holt E., Orloff J.J., Yang K.H., Vasavada R.C., Weir E.C., Broadus A.E., Stewart A.F. Defining the roles of parathyroid hormone-related protein in normal physiology. Physiol. Rev. 1996;76:127–173. doi: 10.1152/physrev.1996.76.1.127. [DOI] [PubMed] [Google Scholar]
- 112.Kitazawa R., Kitazawa S., Maeda S., Kobayashi A. Expression of parathyroid hormone-related protein (PTHrP) in parathyroid tissue under normal and pathological conditions. Histol. Histopathol. 2002;17:179–184. doi: 10.14670/hh-17.179. [DOI] [PubMed] [Google Scholar]
- 113.Li J., Karaplis A.C., Huang D.C., Siegel P.M., Camirand A., Yang X.F., Muller W.J., Kremer R. PTHrP drives breast tumor initiation, progression, and metastasis in mice and is a potential therapy target. J. Clin. Investig. 2011;121:4655–4669. doi: 10.1172/JCI46134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Camirand A., Goltzman D., Gupta A., Kaouass M., Panda D., Karaplis A. The Role of Parathyroid Hormone-Related Protein (PTHrP) in Osteoblast Response to Microgravity: Mechanistic Implications for Osteoporosis Development. PLoS ONE. 2016;11:e0160034. doi: 10.1371/journal.pone.0160034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Barry E.L. Expression of mRNAs for the alpha 1 subunit of voltage-gated calcium channels in human osteoblast-like cell lines and in normal human osteoblasts. Calcif Tissue Int. 2000;66:145–150. doi: 10.1007/s002230010029. [DOI] [PubMed] [Google Scholar]
- 116.Francis M.J., Lees R.L., Trujillo E., Martin-Vasallo P., Heersche J.N., Mobasheri A. ATPase pumps in osteoclasts and osteoblasts. Int. J. Biochem. Cell Biol. 2002;34:459–476. doi: 10.1016/S1357-2725(01)00142-X. [DOI] [PubMed] [Google Scholar]
- 117.Zayzafoon M. Calcium/calmodulin signaling controls osteoblast growth and differentiation. J. Cell Biochem. 2006;97:56–70. doi: 10.1002/jcb.20675. [DOI] [PubMed] [Google Scholar]
- 118.Koch F.P., Merkel C., Ziebart T., Smeets R., Walter C., Al-Nawas B. Influence of bisphosphonates on the osteoblast RANKL and OPG gene expression in vitro. Clin. Oral Investig. 2012;16:79–86. doi: 10.1007/s00784-010-0477-8. [DOI] [PubMed] [Google Scholar]
- 119.Soysa N.S., Alles N., Aoki K., Ohya K. Osteoclast formation and differentiation: An overview. J. Med. Dent. Sci. 2012;59:65–74. doi: 10.11480/jmds.590301. [DOI] [PubMed] [Google Scholar]
- 120.Peng X., Guo W., Ren T., Lou Z., Lu X., Zhang S., Lu Q., Sun Y. Differential expression of the RANKL/RANK/OPG system is associated with bone metastasis in human non-small cell lung cancer. PLoS ONE. 2013;8:e58361. doi: 10.1371/journal.pone.0058361. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 121.Streicher C., Heyny A., Andrukhova O., Haigl B., Slavic S., Schuler C., Kollmann K., Kantner I., Sexl V., Kleiter M., et al. Estrogen Regulates Bone Turnover by Targeting RANKL Expression in Bone Lining Cells. Sci. Rep. 2017;7:6460. doi: 10.1038/s41598-017-06614-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Saito N., Kikkawa U., Nishizuka Y. The family of protein kinase C and membrane lipid mediators. J. Diabetes Complicat. 2002;16:4–8. doi: 10.1016/S1056-8727(01)00200-8. [DOI] [PubMed] [Google Scholar]
- 123.Shin J., Jang H., Lin J., Lee S.Y. PKCbeta positively regulates RANKL-induced osteoclastogenesis by inactivating GSK-3beta. Mol. Cells. 2014;37:747–752. doi: 10.14348/molcells.2014.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nardone V., D’Asta F., Brandi M.L. Pharmacological management of osteogenesis. Clinics. 2014;69:438–446. doi: 10.6061/clinics/2014(06)12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hanley D.A., Adachi J.D., Bell A., Brown V. Denosumab: Mechanism of action and clinical outcomes. Int. J. Clin. Pr. 2012;66:1139–1146. doi: 10.1111/ijcp.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cummings S.R., San Martin J., McClung M.R., Siris E.S., Eastell R., Reid I.R., Delmas P., Zoog H.B., Austin M., Wang A., et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 2009;361:756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 127.McClung M.R., Lewiecki E.M., Cohen S.B., Bolognese M.A., Woodson G.C., Moffett A.H., Peacock M., Miller P.D., Lederman S.N., Chesnut C.H., et al. Denosumab in postmenopausal women with low bone mineral density. N. Engl. J. Med. 2006;354:821–831. doi: 10.1056/NEJMoa044459. [DOI] [PubMed] [Google Scholar]
- 128.Kashiwagi M., Hojo H., Kitaura Y., Maeda Y., Aini H., Takato T., Chung U.I., Ohba S. Local administration of a hedgehog agonist accelerates fracture healing in a mouse model. Biochem. Biophys. Res. Commun. 2016;479:772–778. doi: 10.1016/j.bbrc.2016.09.134. [DOI] [PubMed] [Google Scholar]
- 129.Lee S., Wang C., Pan H.C., Shrestha S., Meyers C., Ding C., Shen J., Chen E., Lee M., Soo C., et al. Combining Smoothened Agonist and NEL-Like Protein-1 Enhances Bone Healing. Plast Reconstr. Surg. 2017;139:1385–1396. doi: 10.1097/PRS.0000000000003367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kha H.T., Basseri B., Shouhed D., Richardson J., Tetradis S., Hahn T.J., Parhami F. Oxysterols regulate differentiation of mesenchymal stem cells: Pro-bone and anti-fat. J. Bone Min. Res. 2004;19:830–840. doi: 10.1359/jbmr.040115. [DOI] [PubMed] [Google Scholar]
- 131.Hokugo A., Sorice S., Parhami F., Yalom A., Li A., Zuk P., Jarrahy R. A novel oxysterol promotes bone regeneration in rabbit cranial bone defects. J. Tissue Eng. Regen. Med. 2016;10:591–599. doi: 10.1002/term.1799. [DOI] [PubMed] [Google Scholar]
- 132.Li A., Hokugo A., Segovia L.A., Yalom A., Rezzadeh K., Zhou S., Zhang Z., Parhami F., Stappenbeck F., Jarrahy R. Oxy133, a novel osteogenic agent, promotes bone regeneration in an intramembranous bone-healing model. J. Tissue Eng. Regen. Med. 2017;11:1490–1499. doi: 10.1002/term.2047. [DOI] [PubMed] [Google Scholar]
- 133.Castrogiovanni P., Trovato F.M., Szychlinska M.A., Nsir H., Imbesi R., Musumeci G. The importance of physical activity in osteoporosis. From the molecular pathways to the clinical evidence. Histol. Histopathol. 2016;31:1183–1194. doi: 10.14670/hh-11-793. [DOI] [PubMed] [Google Scholar]
- 134.Wang C., Pan Y., Wang B. Suppressor of fused and Spop regulate the stability, processing and function of Gli2 and Gli3 full-length activators but not their repressors. Development. 2010;137:2001–2009. doi: 10.1242/dev.052126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wen X., Lai C.K., Evangelista M., Hongo J.A., de Sauvage F.J., Scales S.J. Kinetics of hedgehog-dependent full-length Gli3 accumulation in primary cilia and subsequent degradation. Mol. Cell Biol. 2010;30:1910–1922. doi: 10.1128/MCB.01089-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cai H., Liu A. Spop promotes skeletal development and homeostasis by positively regulating Ihh signaling. Proc. Natl. Acad. Sci. USA. 2016;113:14751–14756. doi: 10.1073/pnas.1612520114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hilton M.J., Tu X., Cook J., Hu H., Long F. Ihh controls cartilage development by antagonizing Gli3, but requires additional effectors to regulate osteoblast and vascular development. Development. 2005;132:4339–4351. doi: 10.1242/dev.02025. [DOI] [PubMed] [Google Scholar]
- 138.Alman B.A. The role of hedgehog signalling in skeletal health and disease. Nat. Rev. Rheumatol. 2015;11:552–560. doi: 10.1038/nrrheum.2015.84. [DOI] [PubMed] [Google Scholar]
- 139.Lin A.C., Seeto B.L., Bartoszko J.M., Khoury M.A., Whetstone H., Ho L., Hsu C., Ali S.A., Alman B.A. Modulating hedgehog signaling can attenuate the severity of osteoarthritis. Nat. Med. 2009;15:1421–1425. doi: 10.1038/nm.2055. [DOI] [PubMed] [Google Scholar]
- 140.Ali S.A., Al-Jazrawe M., Ma H., Whetstone H., Poon R., Farr S., Naples M., Adeli K., Alman B.A. Regulation of Cholesterol Homeostasis by Hedgehog Signaling in Osteoarthritic Cartilage. Arthritis Rheumatol. 2016;68:127–137. doi: 10.1002/art.39337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tanabe S. Signaling involved in stem cell reprogramming and differentiation. World J. Stem Cells. 2015;7:992–998. doi: 10.4252/wjsc.v7.i7.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sari I.N., Phi L.T.H., Jun N., Wijaya Y.T., Lee S., Kwon H.Y. Hedgehog Signaling in Cancer: A Prospective Therapeutic Target for Eradicating Cancer Stem Cells. Cells. 2018;7:208. doi: 10.3390/cells7110208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kazmers N.H., McKenzie J.A., Shen T.S., Long F., Silva M.J. Hedgehog signaling mediates woven bone formation and vascularization during stress fracture healing. Bone. 2015;81:524–532. doi: 10.1016/j.bone.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dohle E., Fuchs S., Kolbe M., Hofmann A., Schmidt H., Kirkpatrick C.J. Sonic hedgehog promotes angiogenesis and osteogenesis in a coculture system consisting of primary osteoblasts and outgrowth endothelial cells. Tissue Eng. Part A. 2010;16:1235–1237. doi: 10.1089/ten.tea.2009.0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McKenzie J.A., Maschhoff C., Liu X., Migotsky N., Silva M.J., Gardner M.J. Activation of hedgehog signaling by systemic agonist improves fracture healing in aged mice. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2019;37:51–59. doi: 10.1002/jor.24017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tomimori Y., Mori K., Koide M., Nakamichi Y., Ninomiya T., Udagawa N., Yasuda H. Evaluation of pharmaceuticals with a novel 50-h animal model of bone loss. J. Bone Min. Res. 2009;24:1194–1205. doi: 10.1359/jbmr.090217. [DOI] [PubMed] [Google Scholar]