Abstract
Low skeletal muscle mass (sarcopenia) is an important prognostic risk factor for the outcome of a variety of cancer types. The current study investigated whether skeletal muscle area (SMA), psoas area (PA) and psoas major volume (PV) are associated with progression-free survival (PFS) and overall survival (OS) in patients with epithelial ovarian cancer (OC). A total of 92 OC patients were enrolled in the present study. Pre-treatment with SMA and PA was assessed using computed tomography (CT) and PV was calculated using a three-dimensional-CT (3D-CT). The clinical factors associated with sarcopenia and prognosis were retrospectively evaluated. For all patients, the median PFS and OS were 19 and 32 months, respectively. Patients exhibiting lower PV (<195.6 cm3) had significantly poorer PFS and OS compared with patients exhibiting higher PV (≥195.6 cm3; P=0.018 and P=0.006), while those with low SMA (<92.92 cm2) had significantly worse OS than patients with higher SMA (≥92.92 cm2; P=0.030). PV was also demonstrated to be superior to SMA and PA in prognosis prediction. PV by 3D-CT can serve as an indicator of poor prognosis in patients with OC.
Keywords: low skeletal muscle mass, psoas major volume, skeletal muscle area, psoas area, prognostic factor, ovarian cancer
Introduction
Ovarian cancer (OC) is the second most common gynecological malignancy in the United States, accounting for approximately 21,500 new cases of cancer and 14,600 deaths in 2009 (1). In Japan, OC is the fourth most common gynecological malignancy with an estimated incidence of nearly 8,000 cases and more than 4,000 deaths every year (2). Poor prognostic factors for OC are thought to include the existence of residual tumor tissue and chemotherapy response (3,4). However, such parameters are insufficient for predicting the prognosis of patients with OC. Therefore, a new approach for pre-treatment assessment of OC is pivotal in improving clinical outcomes for OC.
Low skeletal muscle mass (sarcopenia) is useful because it reflects not only a state of degenerative body weight, but also muscle mass wasting in cancer patients. Therefore, sarcopenia is an important physiological change that occurs during the development of cancer cachexia (5,6). Low skeletal muscle mass can be easily investigated using abdominal computed tomography (CT). Cross-sectional CT measurement of the skeletal muscle area (SMA) and psoas area (PA) at the level of the third lumbar vertebra (L3) has been demonstrated to give reliable muscle mass indices for the whole body (7,8). Loss of skeletal muscle mass, which is symptomatic of poor physical condition, is a risk factor for the outcome of various cancer types (9–13). Volumetric measurements on a three-dimensional (3D) scale are more accurate compared with conventional measurements on a one- or two-dimensional scale (14,15). Multi-detector CT scanning is widely undertaken in routine clinical practice, and 3D constructive CT (3D-CT) can provide volumetric data. This is the first study to investigate whether SMA and PA calculated by CT and psoas major volume (PV) calculated by 3D-CT are associated with clinical parameters and prognosis in patients with OC.
Patients and methods
Study population
A total of 92 patients with epithelial OC who underwent preoperative assessment at the Department of Obstetrics and Gynecology of Okayama University Hospital between January 2002 and December 2017 were enrolled in this study. The study protocol was approved by the Institutional Review Board of Okayama University Hospital (1704-012). Disease staging was performed according to the International Federation of Gynecology and Obstetrics (FIGO) criteria. All enrolled patients were examined by CT or positron emission tomography-CT (PET-CT) to locate tumor deposits before debulking surgery. Our standard surgical treatment for OC of primary debulking surgery (PDS)/interval debulking surgery (IDS) consists of laparotomy for total abdominal hysterectomy, bilateral salpingo-oophorectomy, and infracolic or total omentectomy to debulk peritoneal tumor masses with maximum effects. All cases of primary debulking surgery (PDS) successfully achieved no residual tumor (R0). Patients with more extensive disease and those unable to undergo surgery started neoadjuvant chemotherapy. Surgical resection was classified as curative (R0) or non-curative (R1 or R2, microscopic or gross residual tumor) in the IDS group. Patients who underwent PDS were treated with or without three to eight cycles of standard chemotherapy. This included three to six cycles of neo-adjuvant chemotherapy with IDS followed by two to five cycles of adjuvant chemotherapy. For standard chemotherapy, we used the TC regimen consisting of paclitaxel (175 mg/m2 infused over 3 h) and an area under the curve value of 5 for carboplatin.
CT imaging analysis
For SMA or PA, a single axial image corresponding to the L3 vertebral body was selected and measured for each CT scan. Cross-sectional CT measurement of the SMA and PA within predefined validated boundaries of −29 to +150 Hounsfield units were measured by software (Synapse Vincent; Fujifilm Medical, Tokyo, Japan). SMA consisting of the abdominal muscles, psoas muscles, and para spinal muscles was demarcated. Selected PA only included the psoas muscle area (right and left). All recipients also underwent 3D-CT. Psoas major volume in the lesion by 3D-CT was measured using image recognition software (Synapse Vincent; Fujifilm Medical, Tokyo, Japan).
Laboratory data collection
All subjects had their serum albumin, C-reactive protein (CRP) and CA125 levels measured within 1 week prior to treatment. Levels of serum albumin and CRP were measured using latex nephelometry (LT Auto Wako, Osaka, Japan). Serum CA125 levels were measured by electrochemiluminescence immunoassay on the Roche/Hitachi Modular Analysis E170 (Roche Diagnostics, Tokyo, Japan). Briefly, the high Glasgow prognostic score (GPS) group included patients with GPS 2: CRP levels >1.0 mg/dl and hypoalbuminemia (<3.5 g/dl). The low GPS group included patients with only one of these factors showing abnormal levels (GPS 1) or none of these abnormalities (GPS 0).
Statistical analysis
Statistical analysis was performed using SPSS version 20.0 (SPSS Inc., Chicago, IL, USA). Statistical analyses were performed using the Kruskal-Wallis test for comparisons with controls. Spearman correlation analysis was performed between PA and SMA, PV and PA, PV and SMA metric values, which we used to report the correlation and P-values. PFS and OS of the groups were analyzed using the Kaplan-Meier method. Differences between the recurrence and survival curves were examined using the log-rank test. We performed univariate and multivariate analyses using Cox's proportional hazards model to determine which factors predict PFS and OS after adjusting for the effects of known prognostic factors. P<0.05 was considered statistically significant.
Results
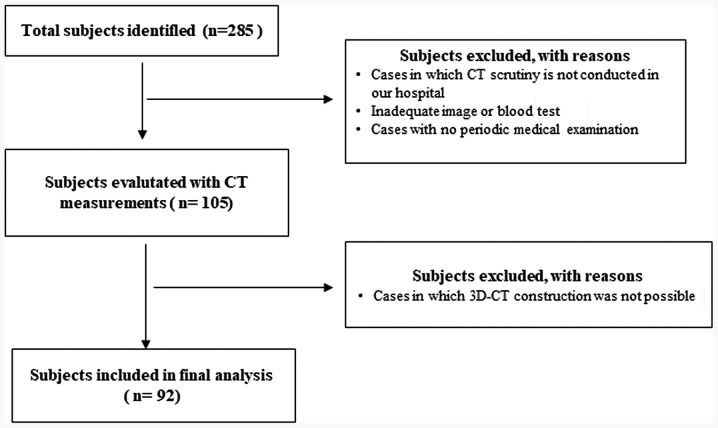
Of 285 patients who attended our institutions for treatment of OC during the study period, 92 were found to be eligible following exclusion of ineligible patients after completion of the study measurements by CT and 3D-CT (Fig. 1). The patients were aged from 15 to 78 years (mean, 55.3 years). FIGO stage, histology, lymph node metastasis, no residual tumor (R0), ascites, neo-adjuvant chemotherapy, GPS, and CA125 are shown in Table I.
Figure 1.
Study cohort selection flow chart. Flow chart indicated the selection of the study cohort. CT, computed tomography.
Table I.
Patient and tumor characteristics
| Baseline characteristics | ||
|---|---|---|
| Mean | Range | |
| Age at diagnosis | 55.3 | 15–78 |
| Numbers | (%) | |
| Stage | ||
| I | 32 | 34.8 |
| II | 6 | 6.5 |
| III | 34 | 37 |
| IV | 20 | 21.7 |
| Histology | ||
| Serous carcinoma | 39 | 42.4 |
| Clear cell carcinoma | 8 | 8.7 |
| Mucinous carcinoma | 12 | 13 |
| Endometrioid carcinoma | 11 | 12 |
| Other carcinoma | 22 | 23.9 |
| Lymph node metastasis | ||
| Absent | 60 | 65.2 |
| Present | 32 | 34.8 |
| Macroscopic tumor free (R0) | ||
| Absent | 7 | 7.6 |
| Present | 85 | 92.4 |
| Ascites | ||
| Absent | 76 | 82.6 |
| Present | 16 | 17.4 |
| Neo-adjuvant chemotherapy | ||
| Absent | 61 | 66.3 |
| Present | 31 | 33.7 |
| GPS | ||
| 0 | 38 | 41.3 |
| 1 | 30 | 32.6 |
| 2 | 24 | 26.1 |
| CA125 | ||
| <35.0 U/ml | 9 | 9.8 |
| ≥35.0 U/ml | 83 | 90.2 |
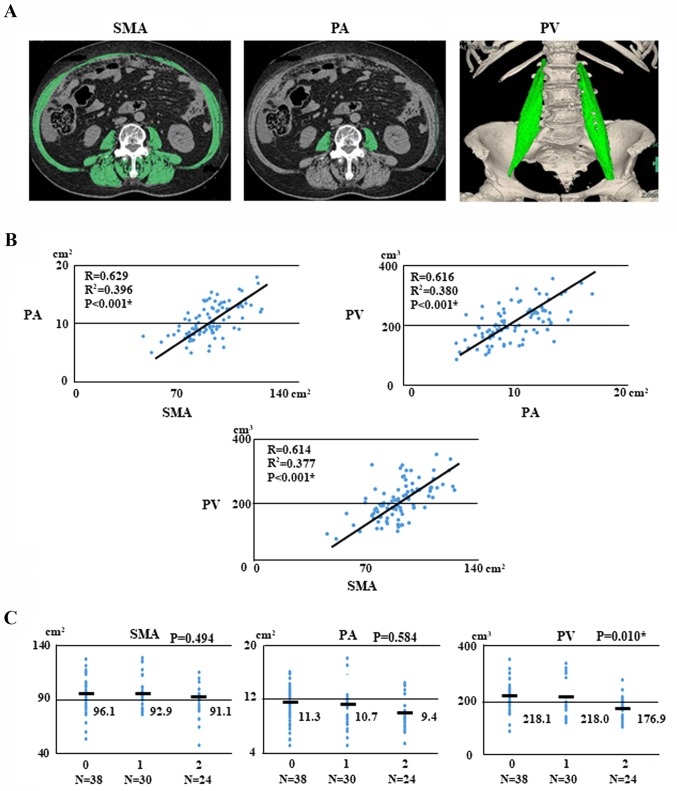
With respect to body composition, the median (range) pre-treatment SMA, PA, and PV were 92.92 (range, 47.41–128.91) cm2, 9.96 (range, 5.07–18.05) cm2, and 195.6 (range, 83.89–351.49) cm3, respectively. Inter-measurement correlations of SMA, PA, and PV were analyzed with data from CT scans. The correlations between SMA and PA, PV and PA, and PV and SMA were 0.629, 0.616, and 0.614, respectively (Fig. 2B).
Figure 2.
Sarcopenia images, regression analysis and associations with ovarian cancer. (A) Patient with sarcopenia. Pre-treatment SMA (102.71 cm2), PA (10.73 cm2) and PV (191.0 cm3) measured according to attenuation thresholds of −29 to +150 Hounsfield units. (B) Regression analysis for PA and SMA, SMA and PV, and PV and PA for 92 patients with ovarian cancer. (C) Associations of GPS and SMA, PA, and PV with ovarian cancer. SMA, skeletal muscle area; PA, psoas area; PV, psoas major volume; GPS, Glasgow prognostic score.
We examined the correlations between pre-treatment GPS and body composition such as SMA, PA, and PV with OC. The pre-treatment GPSs were as follows: GPS 0, 38 patients (41.3%); GPS 1, 30 (32.6%); and GPS 2, 24 (26.1%). Interestingly, PV was significantly associated with GPS (P=0.010, respectively; Fig. 2C).
We next investigated whether SMA, PA, and PV are associated with clinical parameters in patients with OC. We found that PA was significantly associated with stage (P<0.001), histology (P=0.017), lymph node metastasis (P=0.01), ascites (P=0.048), neo-adjuvant chemotherapy (P=0.007), and GPS (P=0.012). Furthermore, PV was significantly associated with age (P=0.019), stage (P<0.001), histology (P=0.001), lymph node metastasis (P=0.005), ascites (P=0.024), neo-adjuvant chemotherapy (P<0.001), and GPS (P=0.002; Table II).
Table II.
Associations of SMA, PA, PV with clinical factors on ovarian cancer.
| Numbers | SMA | P-value | PA | P-value | PV | P-value | |
|---|---|---|---|---|---|---|---|
| Age | 0.31 | 0.06 | 0.019a | ||||
| <70 years | 81 | 94.34±15.82 | 10.34±2.84 | 204.82±57.85 | |||
| ≥70 years | 11 | 89.19±14.90 | 8.61±2.76 | 161.77±38.14 | |||
| BMI | 0.497 | 0.182 | 0.498 | ||||
| <24.9 | 71 | 92.91±16.37 | 9.78±2.71 | 194.9±56.44 | |||
| ≥25.0 | 21 | 95.57±13.17 | 10.73±3.29 | 204.82±66.02 | |||
| Stage | 0.05 | <0.001a | <0.001a | ||||
| Stage I, II | 39 | 97.94±16.93 | 12.32±2.88 | 237.48±54.62 | |||
| Stage III, IV | 53 | 91.56±13.89 | 9.41±2.57 | 180.83±50.90 | |||
| Histology | 0.236 | 0.017a | 0.001a | ||||
| Serous adenocarinoma | 39 | 96.44±17.76 | 11.06±2.92 | 219.91±61.14 | |||
| Non-serous carcinoma | 53 | 92.46±12.61 | 9.63±2.71 | 182.88±50.30 | |||
| Lymph node metastasis | 0.187 | 0.01a | 0.005a | ||||
| Absent | 62 | 95.24±15.74 | 10.98±2.81 | 213.27±59.07 | |||
| Present | 30 | 90.7±15.36 | 9.38±2.72 | 178.74±46.73 | |||
| Macroscopic tumor free (R0) | 1 | 0.085 | 0.325 | ||||
| Absent | 7 | 92.92±11.63 | 11.74±2.79 | 177.52±48.62 | |||
| Present | 85 | 92.92±16.26 | 9.78±2.87 | 197.92±59.51 | |||
| Ascites | 0.983 | 0.048a | 0.024a | ||||
| Absent | 76 | 92.98±17.12 | 10.7±2.84 | 205.66±57.73 | |||
| Present | 16 | 92.92±8.35 | 9.14±2.80 | 169.65±53.99 | |||
| Neo-adjuvant chemotherapy | 0.06 | 0.007a | <0.001a | ||||
| Absent | 61 | 95.57±17.72 | 11.1±2.99 | 220.47±61.37 | |||
| Present | 31 | 89.84±11.03 | 9.41±2.39 | 180.83±40.65 | |||
| GPS | 0.315 | 0.012a | 0.002a | ||||
| 0+1 | 68 | 94.91±15.98 | 11.08±2.95 | 218±58.89 | |||
| 2 | 24 | 91.15±14.83 | 9.38±2.39 | 176.91±43.49 | |||
| CA125 | 0.998 | 0.155 | 0.066 | ||||
| <35.0 U/ml | 9 | 92.91±19.27 | 11.22±2.70 | 233.13±77.05 | |||
| ≥35.0 U/ml | 83 | 92.92±15.64 | 9.78±2.88 | 194.9±56.58 |
P<0.05; SMA, skeletal muscle area; PA, psoas area; PV, psoas major volume.
Patients underwent follow-up examinations approximately every 1–2 months for the first 6 months, every 3 months for the next 2 years, and every 6 months thereafter. The median PFS and OS times for all patients who were alive at the time of last follow-up were 19.0 and 32.0 months, respectively (range for follow-up periods was 1 to 144 months for both PFS and OS). At the last follow-up point, 44 patients were without evidence of disease (47.8%), 32 patients had died of disease (34.8%), and 16 patients had recurrence and were currently alive with the disease (17.4%).
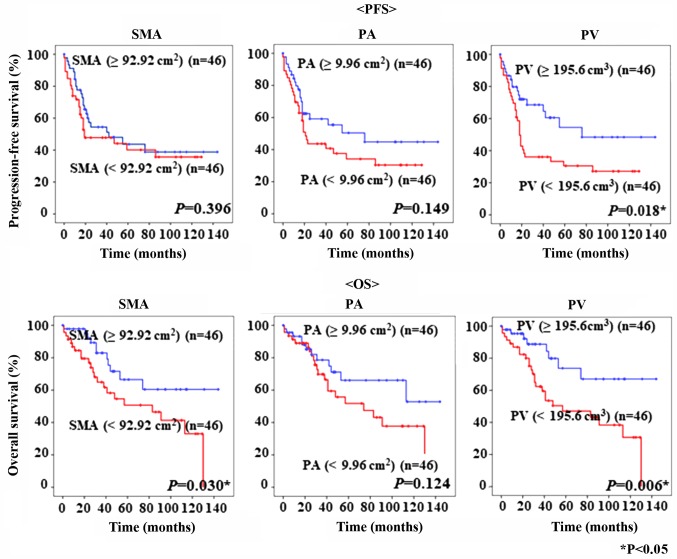
The PFS and OS of the 92 patients with epithelial OC are shown in Fig. 3. The cut-off values for SMA, PA, and PV, based on the median values, were 92.92 cm2, 9.96 cm2, and 195.6 cm3, respectively. Kaplan-Mayer curves showed that patients with lower PV had poorer PFS and OS than patients with significantly higher PV (P=0.018 and P=0.006, respectively). Moreover, patients with low SMA had significantly worse OS than those with higher SMA (P=0.030, respectively). Interestingly, PV was superior to SMA and PA in prognosis prediction (Fig. 3).
Figure 3.
Kaplan-Meier curves. Kaplan-Meier curves for PFS and OS rates of 92 patients with ovarian cancer according to their pre-treatment SMA, PA and PV. PFS, progression-free survival; OS, overall survival; SMA, skeletal muscle area; PA, psoas area; PV, psoas major volume.
The correlations between clinical factors and PFS or OS were assessed in univariate and multivariate analyses (Table III). In a univariate analysis of PFS and OS, GPS (P<0.001), age (P=0.001), PV (P=0.021), neo-adjuvant chemotherapy (P<0.001), stage (P<0.001), histology (P<0.001), lymph node metastasis (P<0.001), no residual tumor (P=0.001), and ascites (P<0.001) were significantly associated with PFS, whereas GPS (P<0.001), age (P=0.005), SMA (P=0.035), PV (P=0.009), neo-adjuvant chemotherapy (P=0.003), stage (P<0.001), lymph node metastasis (P<0.001), no residual tumor (P<0.001), and ascites (P=0.008) were significantly associated with OS. In a multivariate analysis, GPS (P=0.048 and P=0.008) and stage (P<0.001 and P=0.008) were significantly associated with PFS and OS. However, the multivariate analysis showed that PV was not an independent predictor of recurrence and survival in patients with OC.
Table III.
Prognostic factors for progression-free survival and overall survival with ovarian cancer selected by Cox's univariate and multivariate analysis.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value | |
| Progression-free survival | ||||||
| GPS 2 | 5.246 | 2.909–9.460 | <0.001a | 2.034 | 1.006–4.115 | 0.048a |
| CA125 (≥35.0 U/ml) | 3.99 | 0.967–16.461 | 0.056 | – | ||
| Age (≥70 years) | 3.235 | 1.606–6.516 | 0.001a | 1.673 | 0.740–3.780 | 0.216 |
| BMI (≥25.0) | 0.805 | 0.411–1.576 | 0.527 | – | ||
| SMA(<92.92 cm2) | 1.272 | 0.725–2.230 | 0.402 | – | ||
| PA (<9.96 cm2) | 1.51 | 0.854–2.670 | 0.156 | – | ||
| PV (<195.6 cm3) | 1.998 | 1.109–3.601 | 0.021a | 0.818 | 0.404–1.654 | 0.576 |
| Neo-adjuvant chemotherapy | 3.778 | 2.113–6756 | <0.001a | 1.162 | 0.580–2.326 | 0.672 |
| Stage (Stage III–IV) | 11.099 | 4.636–26.572 | <0.001a | 8.065 | 2.620–24.826 | <0.001a |
| Histology (Serous Ca) | 2.824 | 1.587–5.024 | <0.001a | 0.979 | 0.490–1.958 | 0.952 |
| Lymph node metastasis | 3.309 | 1.876–5.834 | <0.001a | 0.85 | 0.423–1.707 | 0.648 |
| No residual tumor (R0) | 4.24 | 1.826–9.845 | 0.001a | 1.92 | 0.761–4.842 | 0.167 |
| Ascites | 3.424 | 1.836–6.386 | <0.001a | 0.979 | 0.476–2.011 | 0.953 |
| Overall survival | ||||||
| GPS 2 | 6.978 | 3.336–14.596 | <0.001a | 3.37 | 1.381–8.223 | 0.008a |
| CA125 (≥35.0 U/ml) | 2.654 | 0.632–11.149 | 0.183 | – | ||
| Age (≥70 years) | 3.076 | 1.410–6.706 | 0.005a | 1.209 | 0.459–3.186 | 0.702 |
| BMI (≥25.0) | 0.848 | 0.382–1.882 | 0.685 | – | ||
| SMA (<92.92 cm2) | 2.186 | 1.057–4.518 | 0.035a | 2.106 | 0.765–5.802 | 0.15 |
| PA (<9.96 cm2) | 1.734 | 0.851–3.532 | 0.13 | – | ||
| PV (<195.6 cm3) | 2.882 | 1.296–6.406 | 0.009a | 0.981 | 0.368–2.617 | 0.969 |
| Neo-adjuvant chemotherapy | 2.937 | 1.455–5.929 | 0.003a | 0.709 | 0.307–1.638 | 0.42 |
| Stage (stage III–IV) | 9.956 | 3.499–28.659 | <0.001a | 5.896 | 1.586–21.923 | 0.008a |
| Histology (Serous Ca) | 1.812 | 0.912–3.600 | 0.09 | – | ||
| Lymph node metastasis | 3.843 | 1.917–7.703 | <0.001a | 0.922 | 0.400–2.125 | 0.849 |
| No residual tumor (R0) | 5.611 | 2.197–14.335 | <0.001a | 2.85 | 0.917–8.851 | 0.07 |
| Ascites | 2.902 | 1.323–6.367 | 0.008a | 0.905 | 0.358–2.286 | 0.833 |
P<0.05; SMA, skeletal muscle area; PA, Psoas area; PV, psoas major volume.
Discussion
Poor prognostic factors for OC are thought to include the existence of residual tumor tissue and chemotherapy response (3,4). Sarcopenia is an important prognostic risk factor for the outcome of various cancer types. This study aimed to evaluate whether pre-treatment SMA and PA measurements by cross-sectional CT and PV by 3D-CT, which predicts poor prognosis for patients with OC, are associated with recurrence or survival.
Sarcopenia, often observed in patients with cancer, is usually regarded as a marker of malnutrition, cachexia, progressive weight loss, fatigue, and anorexia. It is associated with the loss of body composition of patients with cancer because it causes a reduction in SMA. The relationship between poor prognosis and progressive atrophy of SMA has been reported in various cancer types (16–18). Muscle tissue areas can be objectively measured using standard abdominal CT. Areas of SMA and PA can also be accurately estimated using this approach. A low SMA area has been associated with poor outcome in various types of cancer (5).
Inflammatory markers such as malnutrition and cachexia are important prognostic factors for survival in various cancer types. CRP and albumin have prominent roles in tumor inflammation (19–21). Inflammation-based prognostic scores, including the GPS, which is a combination of CRP and albumin parameters, is associated with survival in various cancers, including ovarian, lung, and colorectal cancers (22–24). Furthermore, McSorley et al clarified that low radiation attenuation in muscle was correlated with GPS in colorectal cancer (25). However, there are no reports of an association between GPS and skeletal muscle mass in OC.
Multi-detector CT scanning is widely accessed in routine practice, and 3D-CT enables volumetric data to be obtained. Volumetric measurements on a 3D scale are more accurate compared with conventional measurements on a one- or two-dimensional scale (14,15). We examined body composition using either cross-sectional CT measurement or 3D-CT measurement. In fact, because only one slice was examined for SMA and PA on a CT sagittal view, it was hard to confirm that it was precisely evaluated (20). To our knowledge, this is the first study to evaluate SMA, PA, and PV and the possible prognostic roles of these values in the evaluation of OC.
With respect to body composition, the median pre-treatment SMA, PA, and PV were 92.92 cm2, 9.96 cm2, and 195.6 cm3, respectively. We investigated the association between pre-treatment GPS and body composition such as SMA, PA, and PV with OC. Therefore, PV was more related to pre-treatment GPS than SMI and PA.
We examined whether SMA, PA, and PV were correlated with clinical parameters, using CT for the first two factors and 3D-CT for the latter. We found that PA was significantly associated with stage, histology, lymph node metastasis, ascites, neo-adjuvant chemotherapy, and GPS. Furthermore, PV was significantly associated with age, stage, histology, lymph node metastasis, ascites, neo-adjuvant chemotherapy, and GPS. Our analyses revealed that patients with low PV had significantly poorer PFS and OS than patients with high PV. Interestingly, PV was found to be superior to SMA and PA in prognosis prediction. Univariate analysis showed that high PV was significantly associated with PFS and OS in our study population. However, the multivariate analysis showed that PV was not an independent predictor of recurrence and survival in patients with OC.
This study has some limitations. For example, the number of analyzed samples was small and was obtained from a retrospective cohort in a single hospital. Thus, further confirmation in a prospective trial would help to validate our findings.
In conclusion, this report shows that PV could be a potential imaging biomarker to predict poor prognosis in patients with OC. Based on these results, further sarcopenia research is required to predict prognosis.
Acknowledgements
The authors would like to thank all those who contributed to this study, particularly the statisticians and colleagues of Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences. We appreciate their help with data management and statistical support. We would also like to thank H. Nikki March, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
KN and YM contributed to the conception, design and conduction of the study and analysis and interpretation of the data. KN contributed to the conception of the study and interpretation of the data. H. Mat, CO and H. Mas contributed to data collection and the conduction of the study. All the authors have read and approved the final version of this manuscript.
Ethics approval and consent to participate
This study was performed according to the principles set out in the Declaration of Helsinki 1964 and all subsequent revisions and was approved by the Institutional Review Board of Okayama University Hospital (IRB approval no. 1704-012).
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
References
- 1.American Cancer Society. American Cancer Society; Atlanta, GA: 2009. Cancer Facts & Figures 2009. [Google Scholar]
- 2.Ushijima K. Current status of gynecologic cancer in Japan. J Gynecol Oncol. 2000;20:67–71. doi: 10.3802/jgo.2009.20.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fathalla MF. Factors in the causation and incidence of ovarian cancer. Obstet Gynecol Surv. 1972;27:751–768. doi: 10.1097/00006254-197211000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Vergote IB, Kaern J, Abeler VM, Pettersen EO, De Vos LN, Tropé CG. Analysis of prognostic factors in stage I epithelial ovarian carcinoma: Importance of degree of differentiation and deoxyribonucleic acid ploidy in predicting relapse. Am J Obstet Gynecol. 1993;169:40–52. doi: 10.1016/0002-9378(93)90129-7. [DOI] [PubMed] [Google Scholar]
- 5.Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB, Baracos VE. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 6.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 7.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 8.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, Heymsfield SB, Heshka S. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image. J Appl Physiol (1985) 2004;97:2333–2338. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 9.Moses AW, Slater C, Preston T, Barber MD, Fearon KC. Reduced total energy expenditure and physical activity in cachectic patients with pancreatic cancer can be modulated by an energy and protein dense oral supplement enriched with n-3 fatty acids. Br J Cancer. 2004;90:996–1002. doi: 10.1038/sj.bjc.6601620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joglekar S, Nau PN, Mezhir JJ. The impact of sarcopenia on survival and complications in surgical oncology: A review of the current literature. J Surg Oncol. 2015;112:503–509. doi: 10.1002/jso.24095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 12.Sabel MS, Lee J, Cai S, Englesbe MJ, Holcombe S, Wang S. Sarcopenia as a prognostic factor among patients with stage III melanoma. Ann Surg Oncol. 2011;18:3579–3585. doi: 10.1245/s10434-011-1976-9. [DOI] [PubMed] [Google Scholar]
- 13.Harimoto N, Shirabe K, Yamashita YI, Ikegami T, Yoshizumi T, Soejima Y, Ikeda T, Maehara Y, Nishie A, Yamanaka T. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg. 2013;100:1523–1530. doi: 10.1002/bjs.9258. [DOI] [PubMed] [Google Scholar]
- 14.Yankelevitz DF, Reeves AP, Kostis WJ, Zhao B, Henschke CI. Small pulmonary nodules: Volumetrically determined growth rates based on CT evaluation. Radiology. 2000;217:251–256. doi: 10.1148/radiology.217.1.r00oc33251. [DOI] [PubMed] [Google Scholar]
- 15.Jennings SG, Winer-Muram HT, Tarver RD, Farber MO. Lung tumor growth: Assessment with CT-comparison of diameter and cross-sectional area with volume measurements. Radiology. 2004;231:866–871. doi: 10.1148/radiol.2313030715. [DOI] [PubMed] [Google Scholar]
- 16.Malietzis G, Aziz O, Bagnall NM, Johns N, Fearon KC, Jenkins JT. The role of body composition evaluation by computerized tomography in determining colorectal cancer treatment outcomes: A systematic review. Eur J Surg Oncol. 2015;41:186–196. doi: 10.1016/j.ejso.2014.10.056. [DOI] [PubMed] [Google Scholar]
- 17.Sarkozy C, Camus V, Tilly H, Salles G, Jardin F. Body mass index and other anthropometric parameters in patients with diffuse large B-cell lymphoma: Physiopathological significance and predictive value in the immunochemotherapy era. Leuk Lymphoma. 2015;56:1959–1968. doi: 10.3109/10428194.2014.979412. [DOI] [PubMed] [Google Scholar]
- 18.Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, Nakagomi R, Kondo M, Nakatsuka T, Minami T, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. 2015;63:131–140. doi: 10.1016/j.jhep.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 19.McMillan DC, Watson WS, O'Gorman P, Preston T, Scott HR, McArdle CS. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer. 2001;39:210–213. doi: 10.1207/S15327914nc392_8. [DOI] [PubMed] [Google Scholar]
- 20.Wang CS, Sun CF. C-reactive protein and malignancy: Clinico-pathological association and therapeutic implication. Chang Gung Med J. 2009;32:471–482. [PubMed] [Google Scholar]
- 21.Funovics PT, Edelhauser G, Funovics MA, Laux C, Berzaczy D, Kubista B, Kotz RI, Dominkus M. Pre-operative serum C-reactive protein as independent prognostic factor for survival but not infection in patients with high-grade osteosarcoma. Int Orthop. 2011;35:1529–1536. doi: 10.1007/s00264-011-1208-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omichi C, Nakamura K, Haraga J, Masuyama H, Hiramatsu Y. Glasgow prognostic score is an independent marker for poor prognosis with all cases of epithelial ovarian cancer. Cancer Med. 2016;5:1074–1080. doi: 10.1002/cam4.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2003;89:1028–1030. doi: 10.1038/sj.bjc.6601242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leitch EF, Chakrabarti M, Crozier JE, McKee RF, Anderson JH, Horgan PG, McMillan DC. Comparison of the prognostic value of selected markers of the systemic inflammatory response in patients with colorectal cancer. Br J Cancer. 2007;97:1266–1270. doi: 10.1038/sj.bjc.6604027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McSorley ST, Black DH, Horgan PG, McMillan DC. The relationship between tumour stage, systemic inflammation, body composition and survival in patients with colorectal cancer. Clin Nutr. 2018;37:1279–1285. doi: 10.1016/j.clnu.2017.05.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.