Abstract
Objective
Vagus nerve stimulation (VNS) is a treatment option for patients with drug-resistant seizures, but it is also associated with sleep-disordered breathing (SDB). We present four patients with VNS who underwent polysomnography (PSG) concurrently with VNS stimulation monitoring and adjustment, and positive airway pressure (PAP) treatment. We demonstrate the importance of sleep apnea screening prior to VNS placement and the dilemma of optimizing VNS settings.
Background
VNS is a common adjunct therapy for refractory epilepsy. Despite its low side effect profile, complications of VNS include delayed arrhythmias, laryngopharyngeal dysfunction, obstructive sleep apnea, and tonsillar pain mimicking glossopharyngeal neuralgia. Risk of developing or exacerbating existing obstructive sleep apnea (OSA) limits the VNS settings, as there appears to be a dose dependent effect. OSA can further cause sleep fragmentation and cause hypoxia, potentially worsening seizures.
Methods
Four patients with drug-resistant epilepsy with VNS underwent PSG with concurrent VNS leads to monitor correlation of SDB and VNS. AHI was calculated to quantify SDB, and it was scored as non-VNS related when the VNS was off, and VNS-induced when the onset of SDB corresponded to VNS activation. Subsequent PAP and VNS adjustment was performed to treat the SDB episodes.
Results
Three out of four patients had non-VNS associated SDB, which improved with PAP treatment. All four patients had VNS-induced SDB episodes but none improved with PAP. The VNS-induced SDB events decreased in a dose dependent manner, when VNS was adjusted down and disappeared when turned off completely.
Conclusion
Our case series provides further evidence of VNS-induced SDB secondary to VNS. PAP treatment alone is ineffective for VNS-induced SDB. Screening for OSA before VNS implant is crucial; further research is needed to establish optimal VNS parameters for prevention andminimization of VNS-induced SDB along with other possible treatments.
Keywords: Epilepsy, Vagus nerve stimulation, Sleep apnea, Positive airway pressure, Titration
Highlights
-
•
Further evidence of VNS-induced SDB as a side effect of VNS
-
•
PAP treatment alone is not effective in eliminating VNS-induced SDB
-
•
VNS setting titration showed dose-dependent effect on SDB
-
•
Screening of primary OSA before and after VNS implant is crucial
Introduction
Vagus nerve stimulator (VNS) has become a common adjunct therapy for individuals with drug-resistant epilepsy [1], [2]. However, its complications include delayed arrhythmias, laryngopharyngeal dysfunction, tonsillar pain mimicking glossopharyngeal neuralgia, and sleep disordered breathing (SDB) [3]. The rates of these adverse effects were reported to be low, ranging from ≪ 2% to 10.7% with the exception of hoarseness which was reported to 32% in one study. [4] According to a case-controlled study by Zambrelli, the development of obstructive sleep apnea (OSA) may be related to intermittent obstruction caused by VNS-associated laryngeal dysfunction associated with fixed vocal cord adduction [5]. VNS-associated SDB was also reported in children, and vocal cord adduction was resolved when VNS setting was adjusted in two children in another study [6], [7]. While breakthrough seizures and frequency can be controlled better at higher VNS settings, risk of developing obstructive and central sleep apnea [8] or exacerbating existing OSA limits the high settings, as there appears to be a dose-dependent effect. OSA itself, or as an adverse effect, is particularly concerning since it is known to cause sleep fragmentation and increased hypoxia putting patients at risk for subsequent seizures. [9], [10], [11], [12] The prevalence of VNS-associated SDB is not clear, as most of the studies were small [13] or case reports [14]. While treatment of primary OSA with positive airway pressure in patients with epilepsy can reduce seizure frequency, [15] the association of VNS and therefore sleep disordered breathing (SDB) deserves a closer look, especially since several cases have been described in a recent review [16] and there is no established treatment. [1], [2], [17], [18] We report 4 subjects who experienced marked SDB during VNS activation and discuss the dilemma of VNS titration.
Material and methods
This study was approved by the Institutional Review Committee, and written consent was obtained from each patient or their guardian. Four subjects (2 males and 2 females), ages ranging from 15 to 53 with drug-resistant epilepsy and VNS underwent polysomnography (PSG) for symptoms of SDB. PSG included 8 channels of electroencephalography (EEG), 2 channels for electrooculography (EOG), electromyography (EMG) for chin, right intercostal, right lower rectus abdominis, tibialis anterior and gastrocnemius muscles bilaterally, airflow recording by oronasal thermistor and pressure transducer, thoracoabdominal respiratory bands, SaO2 by finger oximetry, and video monitoring. VNS electrodes were placed in the left neck below the angle of mandible and above the anterior border of the sternocleidomastoideus muscle. The baseline VNS settings for each subject were documented and the apnea hypopnea index was used as a measurement of SDB. SDB was scored as non-VNS related when VNS was off and VNS-induced, when the onset of SDB corresponded to VNS activation. Subsequent PAP titration was performed in all 4 subjects with VNS settings adjusted in 3 of the 4 subjects from baseline settings to the lowest effective settings or until it was completely turned off. Obstructive apnea was scored by the following: cessation of airflow ≫ 10 s with effort; central apnea, cessation of airflow ≫ 10 s without effort; hypopnea, 30% or more reduction in airflow for at least 10 s accompanied by decrement in SaO2 ≫ 4% according to the Medicare American Academy of Sleep Medicine guidelines [19]. The entire polysomnogram was scored for both apnea hypopnea index and non-apnea hypopnea index induced events by three independent scorers (XM, SB and BS).
VNS parameters were lowered during the PSG studies by two of the authors (XM and SB) for all four cases. The frequency was lowered to 10 Hz as the first step of titration in all cases, followed by serial down titration of amplitudes. None of the subjects had awakening for more than 3 min as results of the procedure for parameter changes. We did not alter the pulse width, as we did not want to have too many permutations that altered VNS settings within the limited time of the PSG. Another report suggested a combination of output current and pulse width changes for VNS-induced cortical plasticity. [20] None of the subjects had awakening for more than 3 min as a result of the parameter changes.
Case results
Case 1: both non-VNS and VNS-induced SDB
A 28-year-old man with drug-resistant focal to bilateral tonic–clonic seizures, morbid obesity, and hypothyroidism had complaints of snoring, witnessed apneas, and daytime somnolence. His BMI was 34 and he was on valproic acid, topiramate and lamotrigine, as well as treatment with VNS. The subject underwent a diagnostic PSG, followed by continuous positive airway pressure (CPAP) titration and VNS setting titration. His original VNS settings were: current 2 mA/ frequency 25 Hz/ pulse width 500 ms/ on-time 30 s/off-time 3 min. Moderate OSA [Apnea Hypopnea Index (AHI) of 27.3/h] was detected during VNS off time. These apneas and hypopneas were reduced to 12/h with CPAP treatment of 12 cm H2O, but the subject could not tolerate the higher pressure. Independent apneas and hypopneas were documented during VNS monitoring, and the duration of the apneas or hypopneas correlated grossly with the VNS on time; AHI was 22.6/h for these VNS-induced SDB. When current was reduced to 1.5 mA, AHI responsively decreased to 6.4/h. With reduction of current further to 1 mA and frequency to 10 Hz, VNS-induced SDB decreased; AHI down-trended to 1.4/h. When VNS was turned off, the AHI remained low at 1.3/h. However, VNS-induced SDB did not respond to CPAP.
Case 2: both non-VNS and VNS-induced SDB
A 24-year-old woman with developmental delay, craniofacial abnormalities, morbid obesity, well-controlled asthma, and drug-resistant focal to bilateral tonic–clonic seizures had snoring and daytime somnolence. Her BMI was 43. She was on levetiracetam, phenytoin, divalproex sodium ER and felbamate. In addition, her original VNS settings were: current 2.25 mA/frequency 20 Hz/ pulse width 250 ms/on-time 30 s/ off-time 3 min. She underwent a diagnostic PSG and CPAP treatment. Both VNS-induced and non-VNS related respiratory events were documented. During VNS off time, her AHI was 13.8/h which was improved by an optimal CPAP pressure of 14 cm H2O to AHI of 4/h. Her VNS-induced SDB AHI was 22.3/h, which were not changed in its manifestation at the highest CPAP setting of 14 cm H2O. There were hyperpneas immediately following the VNS-induced hypopneas. This subject did not have her VNS adjusted.
Case 3: Predominantly VNS-induced SDB
A 15-year-old girl with history of a left middle cerebral artery stroke and nocturnal focal to bilateral tonic–clonic seizures had increasing hyper somnolence despite reduction in number of antiseizure drugs from three drugs to two (lamotrigine and topiramate). Her BMI was 33. A PSG with CPAP and VNS titration was performed following a diagnostic recording. Her original VNS settings were: current 2.25 mA/frequency 30 Hz /pulse width 250 ms/on-time 30 s/off-time 3 min. She had no significant SDB (AHI of 0.8/h) during her VNS off period. The vast majority of the respiratory events (AHI 13.8/h) were concurrent with VNS stimulation that did not respond to final settings of CPAP at 8 cm H2O. However, the subject could not resume sleep with this CPAP pressure and could only tolerate CPAP settings of 5 cm H2O, possibly due to nasal obstruction. Lowering the current to 1.75 mA improved the VNS-induced SDB and AHI to 5.9/h, and further reduction to 1.25 mA showed a decrease of AHI to 2.4/h. This subject could not tolerate the lower VNS setting as her seizures were exacerbated. The prior study of this case was reported previously without detailed calculation of AHI [2].
Case 4: both non-VNS and VNS-induced SDB
A 53-year-old man with drug-resistant focal epilepsy –complained of snoring for the past several years. His BMI was 40. He was on divalproex and levetiractam. He had a PSG for diagnosis, followed by CPAP and VNS titration. Original VNS settings were: current 1.75 mA/frequency 30 Hz/pulse width 300 ms/on-time 30s/off-time 3 min. He had moderate OSA (AHI of 23.5/h) when VNS was off. Despite titration with bi-level positive airway pressure up to 20/16 cm H2O, the non-VNS associated AHI was reduced only to 17.8/h only. Even on BIPAP, subject had cyclical respiratory events every 3 min and lasted for 30 s (AHI 20/h), which correlated with the VNS stimulations. When VNS current was lowered from 1.75mAmp to 1.25 mA, VNS-induced SDB events decreased to an AHI of 7.8. When VNS was eventually turned off, VNS-induced SDB disappeared.
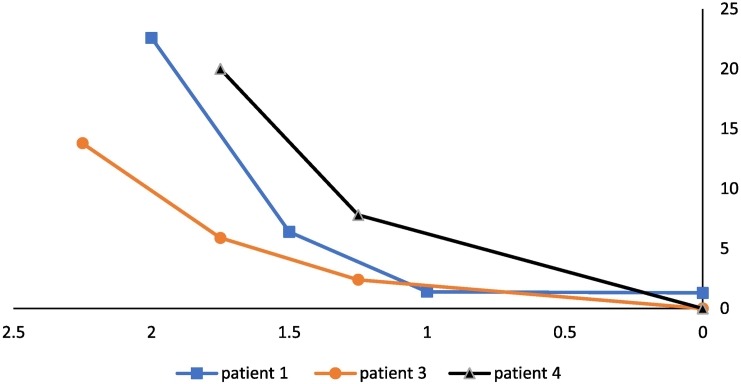
In 3 subjects who had their VNS adjusted, SDB decreased in a dose-dependent manner, as summarized in Fig. 1. The VNS-induced events were not sleep stage or position dependent. N1, N2, N3 or REM were not a variable of VNS AHI. We paid particular attention to the VNS AHI during rapid eye movement sleep. Position had no impact on VNS AHI in any of the four subjects studied. (See Fig. 2.) (See Table 1.)
Fig. 1.
Graph of VNS adjustment vs. SDB in 3 patients. The graph shows dose-dependent effects of VNS current (mA) on SDB as shown on Table 2. On the horizontal axis VNS settings are listed in mA, and on the vertical axis, SDB is quantified by the AHI (#/hr). Note the VNS-induced SDB decreased and disappeared when VNS was adjusted from initial settings to 0 mAs in 3 subjects.
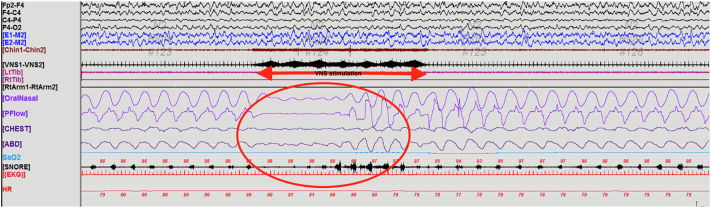
Fig. 2.
PSG showing VNS-induced SDB. Example of artifact in the EKG lead during VNS activation (red arrow) and induced apnea (red circle) followed by hyperpnea.
Table 1.
Subject' demographics and polysomnographic parameters.
| Subject | Age (Years) Gender BMI |
Seizure type | AED/Medications | Comorbidities | TST Sleep Efficiency SaO2: Nadir, Mean |
|---|---|---|---|---|---|
| 1 | 28 M BMI: 34 |
focal impaired awareness, drug-resistant | Valproate Topiramate Lamotrigine Levothyroxine |
Developmental delay, hypothyroidism | TST: 306 min SE: 86.1% SaO2: 70%, 96.7% |
| 2 | 24 F BMI: 49.9 |
focal impaired awareness, drug-resistant | Levetiracetam Felbamate Phenytoin Folic acid |
Enlarged tonsils and adenoids | TST: 365 min SE:91.5% SaO2: 91%, 99% |
| 3 | 14 F BMI: 30 |
focal impaired awareness | Lamotrigine Topiramate Clonidine |
HTN, ADHD, left hemiparesis, developmental delay | TST: 149 mina SE: 40.9% SaO2: 96%, 100% |
| 4 | 53 M BMI: 40 |
focal impaired awareness, drug-resistant | Valproate Levetiracetam Paroxetine |
Developmental delay, depression | TST: 299 min SE: 61.4% SaO2: 85%, 90% |
Subject 3 could only resume sleep when CPAP pressure was at 5 cm H2O or lower, thus the low sleep efficiency.
Discussion
Our case series demonstrates the intricate balance involved in managing epilepsy patients with VNS and sleep apnea. We report VNS-induced SDB in four subjects, three of whom had both VNS and non-VNS associated SDB, and the remaining one who had predominantly VNS-induced SDB. VNS on-time correlated with the SDB, although not every VNS stimulation during sleep induced a respiratory event. Hyperpnea at the end of VNS-induced events was noted, suggesting a compensatory response upon arousal similar to conventional apneas/hypopneas. All the VNS-induced apneas or hypopneas were clinically significant, as the criteria to score them was the same as non-VNS induced respiratory events (i.e. arousals or desaturation of 4% or greater). [19] Interestingly, neither specific sleep stages such as rapid eye movement sleep nor positions such as remaining supine showed an increased likelihood of worsening VNS-induced respiratory events in these four subjects. We do not believe the SDB during VNS activation was by chance alone, as these events did not respond to treatment with positive airway pressure support as evidenced in Fig. 3, while the SDB during VNS off time did respond.
Table 2.
VNS and response to positive airway pressure adjusstmenta
| Subject | VNS activationa |
PAP treatmenta |
||
|---|---|---|---|---|
| VNS Amplitude (mA), Frequency (Hz) | VNS AHI | Non VNS AHI Baseline | Non VNS AHI with PAP | |
| 1 | 2 mA, 10 Hz | 22.6 | 27.3 | 12 |
| 1.5 mA, 10 Hz | 6.4 | |||
| 1 mA, 10 Hz | 1.4 | |||
| Off | 1.3 | |||
| 2 | Not titrated | 13.8 | 4 | |
| 3 | 2.25 mA, 10 Hz | 13.8 | 0.8 | n/a |
| 1.75 mA, 10 Hz | 5.9 | |||
| 1.25 mA, 10 Hz | 2.4 | |||
| Off | 0 | |||
| 4 | 1.75 mA, 10 Hz | 20 | 23.5 | 17.8 |
| 1.25 mA, 10 Hz | 7.8 | |||
| Off | 0 | |||
Abbreviations: VNS: vagus nerve stimulation; PAP; positive airway pressure.
VNS titration and PAP treatment were performed on the same sleep studies but sequential in timing.
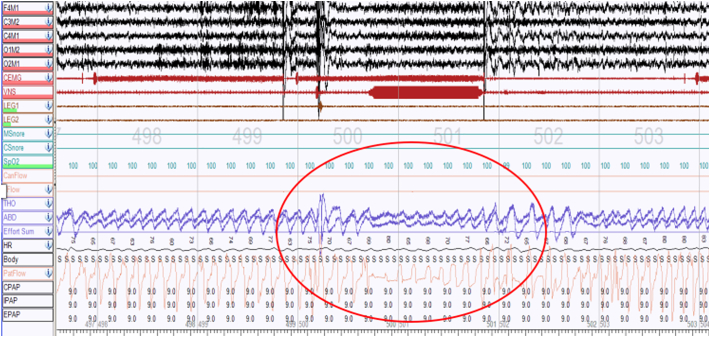
Fig. 3.
PSG showing a lack of responsiveness to positive airway pressure adjustment for VNS-induced hypopnea. Persistent VNS-induced hypopneas persisted (red circle) despite PAP adjustment.
In this case series, the use of CPAP or BIPAP did not improve the VNS-induced SDB events in any of the 4 subjects, despite improving the non-VNS related events in three out of four subjects. Our results aligns with literature that report minimal effectiveness of PAP in eliminating SDB during VNS stimulation. [1], [2], [9], [11] Tonsillectomy and adenotonsillectomy are the first line of treatment for OSA in pediatric populations but has shown variable levels of success in VNS-induced sleep apnea. [11], [15], [17] This suggests that airway splint or positive airway pressure may not overcome the inhibitory action of vagal nerve on respiration in our subjects. Most of the respiratory events associated with VNS stimulation were hypopneas which were obstructive at least in subject 3 who had an intercostal EMG lead monitoring muscle activation. It is unclear why positive airway pressure could not overcome the obstruction, as none of the 4 subjects had any improvement in airflow, even at high pressure in subject 4. However we did not use positive airway pressure at lower VNS settings. As such, we could not comment on whether there is an optimal VNS setting that facilitates a response from positive airway pressures to treat VNS-induced SBD. Others reported that VNS settings did not appear to be a factor in the unresponsiveness to PAP, while VNS frequency was reported to be a cofactor in lowering VNS-induced SDB [21]. Our subjects' VNS frequencies ranged from 20 to 30 Hz. The frequency did not correlate to the severity of an AHI or response to positive airway pressure. Reducing frequency to 10 Hz had minimal effects on VNS-induced AHI while the on and off time of the stimulators were the same in all four subjects. Another possibility which was not tested but potentially viable is the stepwise VNS parameter adjustment during positive airway pressure titration, as suggested by another investigator [22]. Perhaps there exists a medium whereby VNS parameters are reduced sufficiently and safely so that PAP may become effective.
Changing the VNS settings is a reasonable solution for VNS-induced SDB, although it must be balanced with seizure control. [1], [2], [9], [17] However, determining ideal VNS parameters for each individual subject while maintaining therapeutic levels of stimulation is challenging. Three of our subjects showed improvement in their VNS-induced SDB after titrations of their specific VNS settings. VNS-induced SDB in subject 1 substantially decreased once the intensity decreased from 2 mA and 25 Hz to 1 mA and 10 Hz. However, he had oxygen desaturations once the level was readjusted to 1.5 mA. Subject 3 and 4 showed decreasing VNS-induced SDB with lower VNS settings, and when eventually turned off, SDB resolved. There was a dilemma in finding the optimal effective VNS setting that prevented breakthrough seizures as seizures resumed in subject 3 until VNS was reset to the original setting. There are no uniformed guidelines of VNS setting for VNS-induced SDB. Our limited number of subjects precludes generalization to a greater population. Furthermore, we believe VNS settings should be individualized by in laboratory VNS titration to suit a specific subject's need. Large scale studies on a sleep-related VNS settings to guide those patients with VNS-induced SDB would be most helpful.
In our case series some subjects had OSA not associated with VNS. But we do not know, however, whether our subjects had OSA prior to the implantation of VNS. Patients often do not go through PSG testing to receive a diagnosis of OSA prior to VNS implantation. Three of our 4 subjects had an underlying primary OSA that was only revealed when PSG was performed after VNS implantation. It is possible that many patients with VNS implants could have undiagnosed primary OSA and that the addition of VNS-induced SDB exacerbates the underlying symptoms. Currently, the long-term effects of VNS-induced SDB episodes on breakthrough seizure frequency is unclear. A feared complication includes breakthrough seizures due to sleep fragmentation. [23], [24]
With a growing number of epilepsy patients treated with VNS implants, multicenter prospective study is warranted on patients wtih SDB and OSA. However, as our cases show, the VNS-induced SDB episodes may not be recognized clinically. This presents a challenge for patients and caregivers when it comes to identifying potential symptoms of sleep apnea. We suggest that patients who are being considered for a VNS implanation be screened for primary OSA and be treated appropriately before receiving a VNS, as the condition may worsen after VNS implantation.
Lastly, research in sleep-related treatment for VNS-induced SDB is critical for this patient group to standardize care. Some interventions may be able to dampen the effects of VNS on laryngeal and other upper respiratory muscles which could be determined in future trials. A second, follow-up sleep study with titration of VNS settings during sleep should be considered, as three of our subjects showed improvement in SDB with lowered intensity. A close analysis to measure the risk and benefits for seizure occurrence during sleep will be necessary to design the individualized ‘smart’ VNS adjustment. VNS with lower frequency mitigating the degree of SDB has been previously reported by Malow et al., [25] to reiterate the importance of addressing VNS settings in patients with severe SDB.
Conclusions
We describe the effect of VNS-induced SDB in our case series. Positive airway pressure did not help with treatment of SDB in our subjects, while VNS adjustment titrating parameters to optimal parameters was effective. We recommend VNS candidates be screened for OSA. Optimal treatment includes a balance between VNS parameters and positive airway pressure to maximize treatment for SDB and minimize risk of worsening seizures.
Financial disclosures
All authors, Daniel M. Oh, Jacklyn Johnson, Bankim Shah, Sushanth Bhat, Rolla Nuoman, and Xue Ming disclose no conflict of interest.
Data availability
Request corresponding author for figures and data.
Declaration of Competing Interest
None.
Acknowledgments
Acknowledgement
This study is approved by the Institutional Review Committee. We thank the research participants who made this study possible.
Ethical publication statement
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Contributor Information
Daniel M. Oh, Email: daniel.oh@rutgers.edu.
Jacklyn Johnson, Email: jaj176@njms.rutgers.edu.
Sushanth Bhat, Email: SBhat@JFKhealth.org.
Xue Ming, Email: mingxu@njms.rutgers.edu.
Appendix 1. Authors
| Name | Location | Role | Contribution |
|---|---|---|---|
| Daniel Oh, MD | Rutgers New Jersey Medical School, Department of Neurology, Newark, NJ | Author | Performed data analysis and interpretation; drafting of manuscript for content synthesis and critical revision |
| Jacklyn Johnson, BS | Rutgers New Jersey Medical School, Department of Neurology, Newark, NJ | Author | Conducted literature review; performed data analysis; assisted with drafting of manuscript |
| Bankim Shah, MD | Riverside Medical Group, Bayonne Sleep Medicine, Bayonne, NJ | Author | Data collection and analysis |
| Sushanth Bhat, MD | Seton Hall University, New Jersey Neuroscience Institute, Sleep Medicine Center, Edison, NJ | Author | Data collection and analysis |
| Rolla Nuoman, MD | Rutgers New Jersey Medical School, Department of Neurology, Newark, NJ | Author | Drafting and revision of manuscript |
| Xue Ming, MD, PhD | Rutgers New Jersey Medical School, Department of Neurology, Newark, NJ | Author, Investigator | Data collection and interpretation; conceptualization of study; critical revision of manuscript. |
References
- 1.Parhizgar F., Nugent K., Raj R. Obstructive sleep apnea and respiratory complications associated with vagus nerve stimulators. J Clin Sleep Med. 2011;7(4):401–407. doi: 10.5664/JCSM.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Upadhyay H., Bhat S., Gupta D., Mulvey M., Ming S. The therapeutic dilemma of vagus nerve stimulator-induced sleep disordered breathing. Ann Thorac Med. 2016;11(2):151–154. doi: 10.4103/1817-1737.180025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giordano F., Zicca A., Barba C., Guerrini R., Genitori L. Vagus nerve stimulation: surgical technique of implantation and revision and related morbidity. Epilepsia. 2017;58(Suppl. 1):85–90. doi: 10.1111/epi.13678. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez H.F.J., Yengo-Kahn A., Englot D.J. Vagus nerve stimulation for the treatment of epilepsy. Neurosurg Clin N Am. 2019;30(2):219–230. doi: 10.1016/j.nec.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zambrelli E., Saibene A.M., Furia F., Chiesa V., Vignoli A., Pipolo C. Laryngeal motility alteration: a missing link between sleep apnea and vagus nerve stimulation for epilepsy. Epilepsia. 2016;57(1):e24–e27. doi: 10.1111/epi.13252. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh T., Chen M., McAfee A., Kifle Y. Sleep-related breathing disorder in children with vagal nerve stimulators. Pediatr Neurol. 2008;38(2):99–103. doi: 10.1016/j.pediatrneurol.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Aron M., Vlachos-Mayer H., Dorion D. Vocal cord adduction causing obstructive sleep apnea from vagal nerve stimulation: case report. J Pediatr. 2012;160(5):868–870. doi: 10.1016/j.jpeds.2012.01.045. [DOI] [PubMed] [Google Scholar]
- 8.Papathanasion E.S., Papacostas S.S. Sleep-related breathing disorders in children with vagal nerve stimulators. Pediatr Neurol. 2008;39(2):142. doi: 10.1016/j.pediatrneurol.2008.04.009. [author reply] [DOI] [PubMed] [Google Scholar]
- 9.Bhat S., Lysenko L., Neiman E.S., Rao G.K., Chokroverty S. Increasing off-time improves sleep-disordered breathing induced by vagal nerve stimulation. Epileptic Disord. 2012;14(4):432–437. doi: 10.1684/epd.2012.0534. [DOI] [PubMed] [Google Scholar]
- 10.Ebben M.R., Sethi N.K., Conte M., Pollak C.P., Labar D. Vagus nerve stimulation, sleep apnea, and CPAP titration. J Clin Sleep Med. 2008;4(5):471–473. [PMC free article] [PubMed] [Google Scholar]
- 11.El-Kersh K., Senthilvel E. An 18-year-old woman with snoring and refractory epilepsy. Chest. 2015;148(2):e48–e51. doi: 10.1378/chest.14-3114. [DOI] [PubMed] [Google Scholar]
- 12.Khurana D.S., Reumann M., Hobdell E.F., Neff S., Valencia I., Legido A. Vagus nerve stimulation in children with refractory epilepsy: unusual complications and relationship to sleep-disordered breathing. Childs Nerv Syst. 2007;23(11):1309–1312. doi: 10.1007/s00381-007-0404-8. [DOI] [PubMed] [Google Scholar]
- 13.Salvade A., Ryvlin P., Rossetti A.O. Impact of vagus nerve stimulation on sleep-related breathing disorders in adults with epilepsy. Epilepsy Behav. 2018;79:126–129. doi: 10.1016/j.yebeh.2017.10.040. [DOI] [PubMed] [Google Scholar]
- 14.Vollono C., Fuggetta F., Cioni B., Della Marca G. Teaching NeuroImages: obstructive sleep apnea triggered by vagus nerve stimulation. Neurology. 2015;85(18):e140. doi: 10.1212/WNL.0000000000002083. [DOI] [PubMed] [Google Scholar]
- 15.Vendrame M., Auerbach S., Loddenkemper T., Kothare S., Montouris G. Effect of continuous positive airway pressure treatment on seizure control in patients with obstructive sleep apnea and epilepsy. Epilepsia. 2011;52(11):e168–e171. doi: 10.1111/j.1528-1167.2011.03214.x. [DOI] [PubMed] [Google Scholar]
- 16.Romero-Osorio O., Gil-Tamayo S., Narino D., Rosselli D. Changes in sleep patterns after vagus nerve stimulation, deep brain stimulation or epilepsy surgery: systematic review of the literature. Seizure. 2018;56:4–8. doi: 10.1016/j.seizure.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 17.Kelts G., O'Connor P.D., Hussey R.W., Maturo S. An electrical cause of stridor: pediatric vagal nerve stimulators. Int J Pediatr Otorhinolaryngol. 2015;79(2):251–253. doi: 10.1016/j.ijporl.2014.10.037. [DOI] [PubMed] [Google Scholar]
- 18.Marzec M., Edwards J., Sagher O., Fromes G., Malow B.A. Effects of vagus nerve stimulation on sleep-related breathing in epilepsy patients. Epilepsia. 2003;44(7):930–935. doi: 10.1046/j.1528-1157.2003.56202.x. [DOI] [PubMed] [Google Scholar]
- 19.Berry R.B., Gamaldo C.E., Harding S.M., Brooks R., Lloyd R.M., Vaughn B.V. AASM scoring manual version 2.2 updates: new chapters for scoring infant sleep staging and home sleep apnea testing. J Clin Sleep Med. 2015;11(11):1253–1254. doi: 10.5664/jcsm.5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loerwald K.W., Borland M.S., Rennaker R.L., 2nd, Hays S.A., Kilgard M.P. The interaction of pulse width and current intensity on the extent of cortical plasticity evoked by vagus nerve stimulation. Brain Stimul. 2018;11(2):271–277. doi: 10.1016/j.brs.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McPherson-Baker S., Malow R.M., Penedo F., Jones D.L., Schneiderman N., Klimas N.G. Enhancing adherence to combination antiretroviral therapy in non-adherent HIV-positive men. AIDS Care. 2000;12(4):399–404. doi: 10.1080/09540120050123792. [DOI] [PubMed] [Google Scholar]
- 22.St Louis E.K., Faber K. Reversible sleep-related stridor during vagus nerve stimulation. Epileptic Disord. 2010;12(1):76–80. doi: 10.1684/epd.2010.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boudewyns A., Abel F., Alexopoulos E., Evangelisti M., Kaditis A., Miano S. Adenotonsillectomy to treat obstructive sleep apnea: is it enough? Pediatr Pulmonol. 2017;52(5):699–709. doi: 10.1002/ppul.23641. [DOI] [PubMed] [Google Scholar]
- 24.Kataria L., Vaughn B.V. Sleep and epilepsy. Sleep Med Clin. 2016;11(1):25–38. doi: 10.1016/j.jsmc.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Malow B.A., Edwards J., Marzec M., Sagher O., Fromes G. Effects of vagus nerve stimulation on respiration during sleep: a pilot study. Neurology. 2000;55(10):1450–1454. doi: 10.1212/wnl.55.10.1450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Request corresponding author for figures and data.