Graphical abstract
Haematological (right) and biochemical (left) parameter.
Keywords: Black caraway (Bunium persicum), Essential oil, Acute toxicity, Sub-acute toxicity, Rat
Highlights
-
•
The black caraway essential oil was considered as practically low-toxic.
-
•
Sub-acute study the results demonstrate histological damages and no biochemical and hematological changes.
-
•
The black caraway essential oil exhibited no acute toxicity.
-
•
The black caraway essential oil as an alternative to synthetic drug can be used.
Abstract
The aim of the present study was to evaluate the acute toxicity as lethal dose 50% (LD50) and sub-acute toxicity of the black caraway Bunium persicum (Bioss) seed essential oil in male Wistar rats. The compounds of B. persicum were identified by GC/MS and amount of each compound was evaluated. 21 different compounds were determined in the essential oil and the main components were: carvone, p-cymene, gamma-terpinene, p-cymene-8-ol, limonene, isoterpinolene, and 2-beta pinene. For acute toxicity evaluation, the animals were randomly divided into nine group (n = 6) and received 500, 1000, 1500, 2000, 2500, 3000, 3500 and 4000 mg/kg seed essential oil, respectively and the LD50 value for black caraway seed essential oil was obtained above 4000 mg/kg body weight. According to data, treatment with the black caraway seed essential oil sub-acute toxicity study attenuated histopathological changes in lung, liver, kidney, testes and spleen tissues and the results of this study show that the black caraway essential oil can not affect the immune and blood system, important enzymes and vital organs of the body..
1. Introduction
The plants are natural chemical compound synthesis factory, and medicinal plants are known to be important resources of bioactive molecules [[1], [2], [3], [4]]. The use of herbs and herbal formulation for medication is rapidly on the increase [5,6]. Essential oils (EO) have attracted considerable worldwide attention over the last few decades. These natural products have wide-ranging pharmacological activities and biotechnological applications. However, incidence of renal and hepatic toxicity has been reported with the consumption of some medicinal herbs, particularly at high doses [[7], [8], [9]].
Bunium persicum Bioss (black caraway or black cumin) is a two-year herbaceous plant of the Apiaceae family called in Persian as “Zireh Kohi” which widely grows in the southeast of Iran. The extract of the fruit of this plant has 3–9% of the EO depending on environment and species. The main EOs of this plant is carvone, gama-terpinene, para-cymene a-ol, beta-pinene, alpha-pinene, myrcene, and limonene [[10], [11], [12]]. Carvone and limonene are the main part of the B. persicum essential oils with antibacterial and antioxidant properties [13]. The antimicrobial effect of B. persicum EO has been proven in numerous studies [[14], [15], [16], [17], [18], [19], [20], [21], [22]]. B. persicum is principally used in medicine as power source, digestive and diuretic agent, flatulence and indigestion treatment in babies and other items. According to FDA, many essential oils are known as GRAS (Generally Recognized as Safe) substances [23]. Many researchers have evaluated the beneficial properties of B. persicum, and some of them reported various side effects such as allergy, emaciation, and jaundice [[24], [25]].
Ingredients found in EOs are organic compounds with low molecular weights and various properties [24]. These compounds are divided into three major classes of terpenes, terpenoids and phenylpropanoids (hemi-terpenes). Phenylpropanoids or phenolic compounds are responsible for making antimicrobial properties. [26]. Furthermore, the presence of hydroxyl functional groups within the structure of these materials will increase their antimicrobial properties [27]. After hemi-terpenes, terpenoids have the highest antimicrobial properties. The most B. persicum EO compounds are terpenes and terpenoids. Terpenes (e.g. Gama-terpinene, limonene or terpinene) have no or little antimicrobial and antifungal properties. Thus, use them alone may have no significant antimicrobial effects. Terpenoids (such as carvone) have more antibacterial properties than terpenes, since terpenoids have functional group like hydroxyl which can scatter electrons, and so, promotes antimicrobial properties. Carvone and gama-terpinene belong to terpenes group with slight antimicrobial properties [[28], [29], [30], [31]]. A number of EO compounds which do not have health-risk have been registered by the European Commission for use as flavors in foods. These compounds include carvacrol, carvone, cinnamaldehyde, citral, picemene, eugenol, simonene, menthol and thymol. Essential oils are obtained as plant secondary metabolites by various methods such as distillation with steam, or water or both. Today, with the increased awareness of the risks and side effects of chemicals on human health and possibility of the residues in animals used for humans, a tendency to use herbal remedies and EOs as an alternative to synthetic drugs is on the rise. Thus accurate and numerous toxicological studies should be carried out on these plants for medicinal and therapeutic purposes.
In this study, acute lethal effect and determination of LD50 concentration of B. persicum EO and sub-acute toxicity evaluation were performed via histopathological, haematological, and biochemical analysis.
2. Material and method
2.1. Plant preparation, extraction and identification
Two kg of B. persicum was prepared and approved by the Herbarium Center of University of Tehran. To begin the study, 300 g of B. persicum seeds was turned into smaller pieces with a mixer. The obtained plant seeds powder was extracted by passing steam from seeds generate from 350 mL of distilled water for 12 h in a 1000 mL volumetric flask in Clevenger apparatus (steam distillation method). The oily liquid was collected without use of organic solvents in the tank.
The extracted compounds were analyzed on a 6890 N Agilent gas chromatograph coupled to a 5975 C Agilent mass-selective detector (Agilent Technologies, Avondale, PA, USA) with a 7683 Agilent autosampler and 1.0 μL of the sample were injected in the splitless mode at 250 °C into a 30 m ×0.25 mm ×0.5 μm DB-5 MS capillary column and operated by MSD Chemstation Software (Agilent Technologies). The temperature program used for the chromatographic separation was as follows: 50 °C for 2 min, temperature increase at 25 °C min−1 to 100 °C and hold for 2 min, then temperature increase at 5 °C min−1 to 290 °C where it was finally held for 5 min. The carrier gas was helium (99.999%) and was kept at a constant flux of 1.0 mL min−1. The mass spectrometer was operated in the electron impact ionization mode and the energy of the electrons was kept at 70 eV. After injection of sample to GC/MS several unknown peaks were observed. The impact mass spectra of these obtained peaks were searched for in our computer library.
2.2. Preparation of animals
Seventy-eight Wistar male rats with eight weeks old and an average weight of 225–250 g were purchased from Baqiyatallah University of Medical Sciences. Animals were kept at the temperature of 22 ± 2 and 12 h of dark/light cyclic programs and free access to food and water for 2 weeks for spending the compatibility course according to OECD guideline for the testing of chemicals (OECD 407).
2.3. Acute toxicity study
Fifty-four Wistar rats were randomly and equally divided into nine groups (one control group and eight treated groups) (n = 6). After depriving the animals of food overnight, the control group received 1 mL of 2% Tween 80 solution in normal saline intraperitoneally, while each treated group received the EO prepared by dispersing 1 mL in 100 mL volume of 2% tween 80 and normal saline in the doses as follows: 500, 1000, 1500, 2000, 2500, 3000, 3500, and 4000 mg/kg body weight of B. persicum.
2.4. Subacute toxicity study
The rats were randomly divided into four groups (n = 6). Group I (control) served as a vehicle and received 1 mL of 2% Tween 80 solution and normal saline intraperitoneally. Groups I, II, III, and IV received the essential oil solution intraperitoneally at doses of 0, 250, 500, and 1000 mg/kg respectively for 14 days. During this period, all the animals were observed daily for signs of toxicity and mortality. The changes in body weight, food and water intake and clinical signs were recorded.
2.5. Hematological and biochemical evaluation
On day 15, after anesthesia, blood samples were taken from the heart of animals. The hematological parameters such as white blood cell count (WBC), red blood cell count (RBC), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and platelet count were determined using automatic cell counter (Hospitex, Italy). Serum was also obtained for biochemical analysis after centrifugation of the collected blood at 2500 rpm for 15 min. The biochemical indices such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total protein, blood urea nitrogen (BUN), creatinine phosphokinase (CPK), creatinine, and urea were analyzed using Elitech (SElectra prom, France).
2.6. Histopathological examination
After necropsy, samples from liver, heart, spleen, lung, testis, and kidney were obtained and fixed in buffered formalin (10%) for at least 24 h. Then, the samples were dehydrated in a graded series of ethanol, cleared in xylene, embedded in paraffin wax, sectioned at 4–5 μm and stained with the Hematoxylin and Eosin (H&E).
2.7. Statistical analysis
Results were expressed as mean ± standard deviation of mean (SD). The differences between groups of sub-acute toxicity were determined by analysis of variance (one-way ANOVA) followed by Tukey post hoc test. P value <0.05 was considered statistically significant. Statistics were performed using SPSS Software (IBM, v.22; CA, USA).
3. Results and Discussion
The LD50 value for black caraway essential oil was above 4000 mg/kg body weight. Identification of essential oil compounds by GC/MS revealed 21 different combinations that included a total of 93.95% of the total essential oil. Major compositions identified including: carvone, p-cymene, gama- terpinene, p-cymene-8-ol, limonene, 3-isoterpinelene, and beta-pinene.
There is little information about toxicity of B. persicum essential oil in human and animals. In the book of “Essential Oil Safety: A Guide for Herbal Care Professional” written by Robert Tisserand and Rodney young, was reported LD50 of B. persicum essential oil in rats was considered 3500 mg/kg, when it administrated orally. According to “CRC handbook of medicinal species” written by James A. Duke, the LD50 of limonene (one of the most important components) is 4600 mg/kg orally, but not mentioned about caraway essential oil.
3.1. Acute intra-peritoneally toxicity
The LD50 of acute toxicity of B. persicum essential oil was determined and revealed that the LD50 of black cumin essential oil was > 4000 mg/kg. There were not significant changes in behavior of the treated groups which received B. persicum essential oil up to 4000 mg/kg. According to classification, chemical substance with a LD50 in the range of 1000–5000 mg/kg is considered as practically low-toxic [29].
3.2. Subacute intra-peritoneally toxicity
It is important to be note that no significant change in body weight statistically was shown at the end of the experiment compared with the control.
3.3. Hematological analysis
The changes in the blood parameters in animals have been considered as a useful indicator for predicting animal toxicity. In the present study, hematological parameters in treated groups showed significant differences in comparison with the control group. The administration of B. persicum essential oil caused no significant change in WBC, HGB, MCHC, MCV, HCT, PLT and RDW, when compared with the control group and each other (P < 0.05), except for RBC, MPV, and MCV were observed significant changes. The anomaly levels of MCV and MPV indicated that the morphology and osmotic fragility of the red blood cells were affected with inoculated of B. persicum essential oil (Table 1).
Table 1.
Effect of Bunium persicum Bioss essential oil on hematological parameters after 14 days' treatment.
| The black Caraway essential oil (mg/kg body weight) |
||||
|---|---|---|---|---|
| Parameters | 0 | 250 | 500 | 1000 |
| Total RBC (1012/L) | 8.5 ± 0.1 a | 8.9 ± 0.1ab | 8.0 ± 0.2 a | 7.6 ± 0.8ab |
| HGB (g/dL) | 14.9 ± 0.5a | 14.6 ± 0.4 a | 17.1 ± 0.5 a | 14.9 ± 2.0 a |
| MPV (%) | 7.8 ± 0.4 ab | 6.6 ± 0.2 ab | 6.4 ± 0.8 ab | 4.5 ± 0.3 ab |
| RDW (%) | 12.9 ± 0.6 a | 12.7 ± 1.6 a | 14.3 ± 0.9 a | 13.0 ± 2.5 a |
| MCV (fl) | 46.9 ± 1.7 ab | 57.4 ± 0.7 ab | 50.6 ± 2.1 ab | 55.5 ± 2.7 ab |
| MCH (pg) | 22.8 ± 3.1 a | 22.8 ± 3.2 a | 26.9 ± 7.0 a | 25.9 ± 6.4 a |
| MCHC (%) | 45.9 ± 1.7 a | 42.0 ± 1.6 a | 43.7 ± 2.1 a | 46.5 ± 8.4 a |
| WBC (109/L) | 8.1 ± 0.3 a | 8.2 ± 1.0 a | 9.5 ± 1.1 a | 9.4 ± 3.1 a |
| HCT (%) | 41.9 ± 1.1 a | 40.0 ± 2.0 a | 43.0 ± 1.2 a | 37.4 ± 3.5 a. |
| PLT(109/L) | 818.4 ± 61.2 a | 786.5 ± 72.6 a | 744.7 ± 100.8 a | 525.0 ± 213.9 a |
RBC, red blood cell count; WBC, white blood cell count; RDW, Red cell distribution width; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MPV, Mean platelet volume; HGB, hemoglobin; HCT, hematocrit; fL, femtolitre; pg, pictogram.
*Each value represents the mean ± SD.
aNot significant; when compared with control P < 0.05).
In the same row, mean values with different superscript letters differ significantly (P<0.05).
3.4. Histopathological and Biochemical Analysis
Biochemical assessment indicated was no statistical difference in liver or renal parameters in ALT, ALP, AST, CPK, albumin, creatinine, total proteine and urea while changes in BUN was significant between the treated and control groups (Table 2 and has no clinical significance.
Table 2.
Effect of Bunium persicum Bioss essential oil on biochemical parameters after 14 days' treatment.
| The black Caraway essential oil (mg/kg body weight) |
||||
|---|---|---|---|---|
| Parameters | 0 | 250 | 500 | 1000 |
| Total Protein (g/dL) | 7.1 ± 0.1 a | 6.9 ± 0.2 a | 6.9 ± 0.3 a | 6.6 ± 0.4 a |
| Creatinine (mg/dL) | 0.9 ± 0.1 a | 0.9 ± 0.1 a | 1.0 ± 0.2 a | 1.4 ± 0.2 a |
| Urea (mg/dL) | 2.0 ± 0.2 a | 2.9 ± 0.4 a | 3.6 ± 0.6 a | 4.1 ± 1.2 a |
| ALT (IU/L) | 40.4 ± 1.0 a | 38.7 ± 1.7 a | 41.4 ± 5.0 a | 46.9 ± 6.4 a |
| ALP (IU/L) | 98.6 ± 24.2 a | 146.2 ± 18.2 a | 162.2 ± 28.7 a | 177.0 ± 48.1 a |
| AST (IU/L) | 77.9 ± 18.6a | 88.4 ± 15.1 a | 93.0 ± 32.5 a | 132.5 ± 51.1 a |
| CPK (IU/L) | 342. ± 28.0 a | 293.0 ± 22.6 a | 337.7 ± 29.3 a | 355.0 ± 65.0 a |
| Albumin (g/dL) | 3.8 ± 0.1 a | 3.8 ± 0.2 a | 3.9 ± 0.1 a | 3.5 ± 0.2 a |
| BUN (mg/dL) | 27.0 ± 3.3 ab | 31.3 ± 4.7 ab | 38.1 ± 4.2 ab | 47.0 ± 4.4 ab |
ALT, alanine transaminase; AST, aspartate transaminase; ALP: Alkaline phosphatase, CPK: Creatinine phosphokinase; BUN: blood urea nitrogen.
*Each value represents the mean ± SD.
aNot significant; when compared with control P < 0.05).
In the same row, mean values with different superscript letters differ significantly (P<0.05).
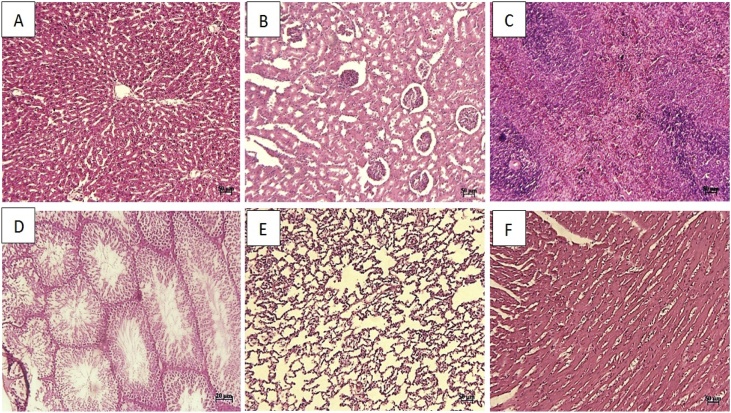
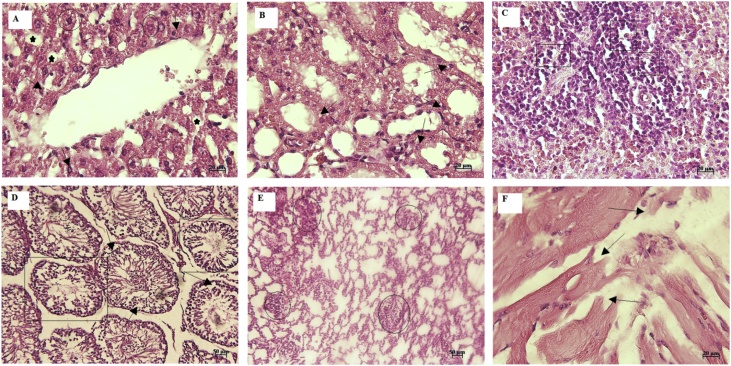
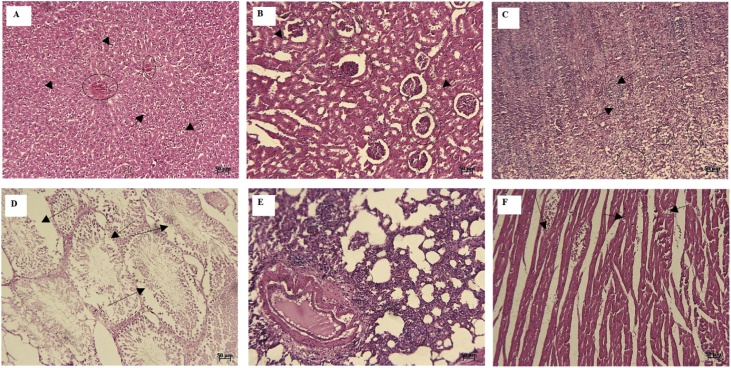
We named the groups as I (control), II (250 mg/kg) and III (1000 mg/kg). In hepatic tissue of group I, microscopic examination showed normal histological structure with cords of large polygonal hepatocyte with prominent round nuclei and eosinophilic cytoplasm. Normal sinusoidal space was noted between the cords. The central vein and portal area appeared normal (Fig. 1A). In group II, moderate diffuse vacuolar degeneration, atrophy of hepatocytes, dilated sinusoidal space, mild portal hepatitis, and slight oval cell proliferation were detected (Fig. 2A). In group III, moderate to severe diffuse vacuolar degeneration, mild to moderate granularity of hepatocytes and fatty changes were observed in these animals. In some area, severe empty vacuoles associated with strands of necrotic hepatocytes especially in sub capsular area were detected. Dilatation of sinusoids, some foci of coagulative necrotic areas with infiltration of inflammatory cells, slight cholangitis, oval cell proliferation and mild bile duct hyperplasia were the other detected features (Fig. 3A).
Fig. 1.
Group I (control): Note to normal structure of liver (A), kidney (B), spleen (C), lung (D), testis(E), and heart (F) tissue (H & E; A-E: 50 μm and F: 20 μm).
Fig. 2.
Group II (250 mg/kg body weight): Diffuse vacuolar degeneration (circle), atrophy of hepatocytes (arrow), and dilated sinusoidal space (star) (A), diffuse degenerative change in cells of renal tubules (arrow) (B), Mild depletion in the lymphocyte population of white pulp (rectangle) (C), testicular tubular atrophy (rectangle) and degenerative changes (arrow) (D), mild interstitial inflammation in lung tissue (circle) (E), and fragmentation in the cardiac muscle fibers (arrow) (F) (H & E; A–C, F: 20 μm and D-E: 50 μm).
Fig. 3.
Group II (1000 mg/kg body weight): moderate to severe diffuse vacuolar degeneration (arrow) and vascular congestion (circle) (A), moderate degenerative changes (arrow) and atrophic glomeruli (circle) (B), increase in the number of inflammatory cell components (arrow) and depletion of white pulp (circle) (C), degenerative and destructive sings of seminiferous tubules (arrow) (D), moderate infiltration of mononuclear inflammatory cells in the interstitium (E), and note to some foci of disrupted cardiac muscle fibers (arrow) (F) (H & E; A-F: 50 μm).
The level of ALT and AST has been established as markers of hepatocellular injury while ALP is considered as a marker of cholestasis [32]. These enzymes are also involved in amino acid metabolism and are useful in assessing the functional integrity of the liver. ALT is produced within liver cells, and increases in conditions where liver cells have been inflamed or undergone cell death. As the cells are damaged, the ALT leaks lead to a rise in the serum levels. It is the most sensitive marker for liver cell damage. AST, ALT and ALP are released in the liver and an elevation in its plasma concentration explains dysfunction of the liver. The liver and heart release AST and ALT and an elevation in their plasma concentrations are indicators of hepatic and cardiac damage [33,34]. In this study, there was no statistical difference (p ≤ 0.05) in ALT, AST, and ALP level.
Renal function indices such as urea and creatinine can be used for evaluation of the functional capacity of the nephrons and are considered as good indicators of kidney function. The kidney is the primary organ for clearance and excretion of xenobiotic including: drugs and drug products from the body. Damage to the kidney may arise due to the administration of EO’s. The normal levels of serum urea and creatinine, total protein and albumin indicate that the EO did not interfere with renal function, and renal integrity.
In group I, histological examination of the kidney sections revealed normal architecture showing the normal renal tubules and glomeruli (Fig. 1B). In group II, slight vacuolar change and some single cell necrosis were detected (Fig. 2B). In group III, moderate degenerative changes, cell swelling, desquamation of epithelial cells, atrophic glomeruli, tubules dilatation, and vascular congestion were the prominent features. The intensity of tubular necrosis was mild (Fig. 3B). The decrease in the plasma levels of albumin and increase in creatinine levels occurred in animals treated with higher doses. In rats administered 1000 mg/kg essential oil may be a sign of impaired renal function. Further biochemical changes were observed in creatinine concentration at higher doses which suggest possible kidney damage, especially by renal infiltration mechanism.
In histopathological examination of spleen tissue, in group I, apparent normal structures for white and red pulps were observed (Fig. 1C). Mild decrease in the lymphocyte population was seen in group B (Fig. 2C). In group C, increased hemosiderosis and an increase in the number of inflammatory cell components, mild depletion of white pulp was seen in group C. In addition, an increase in the number of the reticular cells and macrophages was detected in some of cases in this group (Fig. 3C).
In testes tissue, in group I, the thick seminiferous epithelium with different stages of the spermatogenic cells and well-developed Leydig cells in the interstitial spaces were detected (Fig. 1D). In group B, some disorganization and distortion of the seminiferous tubules were detected. Some affected seminiferous tubules were lost typical spherical shape and had wrinkled with irregular basement membrane, which indicated tubular atrophy. Degeneration of spermatogonia and decreased number of spermatids and spermatocytes were seen in affected tubules. Sloughed germ cells were filled the lumens of tubules (Fig. 2D). In group C, moderate atrophy of the seminiferous tubules was detected. Degenerative and destructive sings were more prominent in spermiogenic than interstitial cells. The Seminiferous tubules were not closely arranged and also, seminiferous epithelium was thinner compared to the control group, so, the cavity of the seminiferous tubule was larger than that in the control. Some sertoli cells showed vacuolization and swelling. Some vascular congestion was noted under the tunica albuginea. Exfoliated germ cells and multinucleated giant were accumulated in the lumen of the tubules. The interstitial tissue was loosely packed around the seminiferous tubules (Fig. 3D).
In lung tissue, in group I, normal pulmonary architecture was detected (Fig. 1E). In group II, the lung sections showed mild interstitial inflammation. The predominant inflammatory cells infiltrated within the lungs consisted of mononuclear cells; however, diffuse eosinophil infiltration was also noted. Some patchy peribronchial consolidations with mild necrotic bronchiolitis were seen in some cases (Fig. 2E). In group III, accumulation of alveolar edema, mild hyperemia, moderate infiltration of mononuclear inflammatory cells in the interstitial and perivascular areas was seen. There was also evidence of necrotic bronchiolitis (especially terminal airway epithelium) and prebronchiolitis with slight and moderate intensity respectively. In some portion, mild patchy thickening, which was created by epithelial hyperplasia, was noted. Smooth muscle hypertrophy of the airways was also seen. In some case, hyperplasia of BALT also noted. In addition, emphysema and atelectasis were detected in the microscopic evaluation (Fig. 3E).
The heart sections from the control group (group I) displayed normal appearance and cardiac muscle fibers were well arranged with centrally nuclei (Fig. 1F). Examination of the heart sections in group II and III revealed focal areas of disrupted cardiac muscle fibers, mild degenerative changes, and fragmentation in the cardiac muscle fibers (Fig. 2F and 3 F).
4. Conclusions
The results of this study show that the black caraway essential oil can not affect the immune and blood system, important enzymes and vital organs of the body. This study showed LD50 of black caraway essential oil was more than 4000 mg/kg and no mortality on those levels was observed that prove relative safety of the black caraway essential oil in acute exposure. Although in sub-acute study the result demonstrates histological damages and exhibited no acute toxicity and no significant change were observed between groups in (p ≤ 0.05) in biochemical (except BUN) and hematological assessment (except total RBC, MPV, and MCV).
Animals received animal care, and all the experiments were performed in accordance with the Guidelines of the Institutional Animal Care and Use Committee and were approved by the Animal Ethics Committee of the Faculty of Veterinary Medicine, University of Tehran, Iran (approval No of 7506023.6.17).
Declaration of Competing Interest
Animals received animal care, and all the experiments were performed in accordance with the Guidelines of the Institutional Animal Care and Use Committee and were approved by the Animal Ethics Committee of the Faculty of Veterinary Medicine, University of Tehran, Iran (approval No of 7506023.6.17).
References
- 1.Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol. Aspects Med. 2006;27(1):1–93. doi: 10.1016/j.mam.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Mahmoudvand H., Asadi A. In vitro lethal effects of various extracts of Nigella sativa seed on hydatid cyst protoscoleces. Iran J basic Med Sci. 2014;17(12):1001. [PMC free article] [PubMed] [Google Scholar]
- 3.Mazaki M., Kataoka K. Inhibitory effects of caraway (Carum carvi L.) and its component on N-methyl-N’-nitro-N-nitrosoguanidine-induced mutagenicity. J. Med. Invest. 2006;53(1-2):123–133. doi: 10.2152/jmi.53.123. [DOI] [PubMed] [Google Scholar]
- 4.Micklefield G., Greving I. Effects of peppermint oil and caraway oil on gastroduodenal motility. Phytother. Res. 2000;14(1):20–23. doi: 10.1002/(sici)1099-1573(200002)14:1<20::aid-ptr542>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 5.Mirghazanfari S.M., Hosseinzadeh L. Acute and subchronic toxicological evaluation of Echinophora platyloba DC (Apiaceae) total extract in Wistar rats. Clinics. 2012;67(5):497–502. doi: 10.6061/clinics/2012(05)15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Awotunde O.S., Adewoye S.O., Dhanabal P.S., Hawumba J. Subacute toxicity study of aqueous root extract of Terminalia schimperiana in male Wistar rats. Toxicology Reports. 2019 doi: 10.1016/j.toxrep.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navarro V., Khan I. Liver Injury from Herbal and Dietary Supplements. Hepatology. 2017;65(1):363–373. doi: 10.1002/hep.28813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asif Mohammad. A brief study of toxic effects of some medicinal herbs on kidney. Adv Biomed Res. 2012;44:1–5. doi: 10.4103/2277-9175.100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mashour N.H., Lin G.I. Herbal Medicine for the Treatment of Cardiovascular DiseaseArch Intern. Arch. Intern. Med. 1998;158(20):2225–2234. doi: 10.1001/archinte.158.20.2225. [DOI] [PubMed] [Google Scholar]
- 10.Das N., Goshwami D. Evaluation of acute and subacute toxicity induced by methanol extract of Terminalia citrina leaves in Sprague Dawley rats. J Acute Dis. 2015;4(4):316–321. [Google Scholar]
- 11.Benhouda A., Yahya M. Toxicity and anti-inflammatory effects of methanolic extract of Umbilicus rupestris L. leaves (Crassulaceae) Int J Pharma Bio Sci. 2015;6(1):395–408. [Google Scholar]
- 12.Muthuraman A., Singh N. Acute and sub-acute oral toxicity profile of Acorus calamus (Sweet flag) in rodents. Asian Pac J Trop Biomed. 2012;2(2):1017–1023. [Google Scholar]
- 13.Peixoto M.G., Costa-Júnior L.M. Acaricidal activity of essential oils from Lippia alba genotypes and its major components carvone, limonene, and citral against Rhipicephalus microplus. Vet. Parasitol. 2015;210(1):118–122. doi: 10.1016/j.vetpar.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Iacobellis N.S., Lo Cantore P. Antibacterial activity of Cuminum cyminum L. And Carum carvi L. essential oils. J Agric Food Chem. 2005;53(1):57–61. doi: 10.1021/jf0487351. [DOI] [PubMed] [Google Scholar]
- 15.Kumar P., Singh D.K. Molluscicidal activity of Ferula asafoetida, Syzygium aromaticum and Carum carvi and their active components against the snail Lymnaea acuminata. Chemosphere. 2006;63(9):1568–1574. doi: 10.1016/j.chemosphere.2005.08.071. [DOI] [PubMed] [Google Scholar]
- 16.Johri R.K. Cuminum cyminum and Carum carvi: An updatePharmacogn. Pharmacogn. Rev. 2011;5(9):63–72. doi: 10.4103/0973-7847.79101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seo S.M., Kim J., Lee S.G. Fumigant antitermitic activity of plant essential oils and components from ajowan (Trachyspermum ammi), allspice (Pimenta dioica), caraway (Carum carvi), dill (Anethum graveolens), geranium (Pelargonium graveolens), and litsea (Litsea cubeba) oils against Japanese termite (Reticulitermes speratus Kolbe) J Agric Food Chem. 2009;57(15):6596–6602. doi: 10.1021/jf9015416. [DOI] [PubMed] [Google Scholar]
- 18.Fang R., Jiang C.H. Insecticidal activity of essential oil of Carum carvi fruits from China and its main components against two grain storage insects. Molecules. 2010;15(12):9391–9402. doi: 10.3390/molecules15129391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simic A., Rančic A. Essential oil composition of Cymbopogon winterianus. and Carum carvi. and their antimicrobial activities. Pharm. Biol. 2008;46(6):437–441. [Google Scholar]
- 20.Razzaghi-Abyaneh M., Shams-Ghahfarokhi M. Chemical composition and antiaflatoxigenic activity of Carum carvi L., Thymus vulgaris and Citrus aurantifolia essential oils. Food Control. 2009;20(11):1018–1024. [Google Scholar]
- 21.Thompson Coon J., Ernst E. Herbal medicinal products for non‐ulcer dyspepsia. Alimentary pharmacology & therapeutics. 2002;16(10):1689–1699. doi: 10.1046/j.1365-2036.2002.01339.x. [DOI] [PubMed] [Google Scholar]
- 22.Manso S., Pezo D. Diminution of aflatoxin B1 production caused by an active packaging containing cinnamon essential oil. Food Control. 2014;45:101–108. [Google Scholar]
- 23.Evandri M., Battinelli L., Daniele C. The antimutagenic activity of Lavandula angustifolia (lavender) essential oil in the bacterial reverse mutation assay. Food Chem. Toxicol. 2005;43(9):1381–1387. doi: 10.1016/j.fct.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Bajpai V.K., Baek K.H. Control of Salmonella in foods by using essential oils: A review. Food Res. Int. 2012;45(2):722–734. [Google Scholar]
- 25.Pourbakhsh H., Taghiabadi E. Effect of Nigella sativa fixed oil on ethanol toxicity in rats. Iran. J. Basic Med. Sci. 2014;17(12):1020–1031. [PMC free article] [PubMed] [Google Scholar]
- 26.Rhayour K., Bouchikhi T. The mechanism of bactericidal action of oregano and clove essential oils and of their phenolic major components on Escherichia coli and Bacillus subtilis. J Essent Oil Res. 2003;15(4):286–292. [Google Scholar]
- 27.Veldhuizen E.J., Tjeerdsma-van Bokhoven J.L. Structural requirements for the antimicrobial activity of carvacrol. J Agric Food Chem. 2006;54(5):1874–1879. doi: 10.1021/jf052564y. [DOI] [PubMed] [Google Scholar]
- 28.de Assis Oliveira F., Nalone Andrade L. Review Anti-Ulcer Activity of Essential Oil Constituents. Molecules. 2014;19:5717–5747. doi: 10.3390/molecules19055717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dorman H., Deans S.G. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 30.Adokoh C.K., Asante D.B. Chemical profile and invivo toxicity evaluation of unripe Citrus aurantifolia essential oil. Toxicology Reports. 2019;6:692–702. doi: 10.1016/j.toxrep.2019.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31."40 CFR 156.64: Toxicity Category" (PDF). Code of Federal Regulations. Office of the Federal Register. Retrieved 2009-04-30.
- 32.Giannini E.G., Testa R. Liver enzyme alteration: a guide for clinicians. CMAJ. 2005;172(3):367–379. doi: 10.1503/cmaj.1040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasan K.M., Tamanna N. Biochemical and histopathological profiling of Wistar rat treated with Brassica napus as a supplementary feed. Food Science and Human Wellness. 2018;7(1):77–82. [Google Scholar]
- 34.Gowda S., Desai P.B. A review on laboratory liver function tests. Pan Afr Med J. 2009;3(17):1–11. [PMC free article] [PubMed] [Google Scholar]