Abstract
Interactions between the germ line and the soma help optimize reproductive success. We discovered a phenomenon linking reproductive status to longevity: In both hermaphroditic and gonochoristic Caenorhabditis, mating leads to female shrinking and death, compressing postreproductive life span. Male sperm induces germline- and DAF-9/DAF-12-dependent shrinking, osmotic stress susceptibility, and subsequent life-span decrease, whereas seminal fluid induces DAF-16-dependent life-span decrease and fat loss. Our study provides insight into the communication between males and the female germ line and soma to regulate reproduction and longevity, revealing a high-reproduction, low-life-span state induced by mating. Postmating somatic collapse may be an example of the sexually antagonistic influence that males in many species exert on female behavior to maximize their own reproductive success.
Mating is an elaborately regulated process with critical individual and population consequences (1, 2). The development of sexual mating resulted in a now ancient conflict: Although the mother’s genome is always propagated, the father is driven to maximize his genomic contribution to the exclusion of other males, often effected through manipulation of the mother’s behavior or physiology. This tension leads to a war between the sexes that plays out in different ways in different species; for example, many insects display sexual antagonism, in which males receive benefits of mating (increased offspring, decreased chance of female remating) by inflicting damage on the female (3).
Reproduction and longevity are intimately linked, with signals between the germ line and soma coordinating the rate of aging of both tissues (4–8). Reproductive aging is regulated by many of the same pathways that control somatic aging (7, 9, 10), via signals from the soma that coordinate sensing of nutrient conditions with reproductive status (7, 8). Conversely, signals from the reproductive system regulate somatic aging through DAF-16/FOXO and the nuclear hormone DAF-12, extending life span when germline proliferation is arrested (5, 6).
Caenorhabditis elegans hermaphrodites can reproduce either by self-fertilization or by mating with males (11 ), whose sperm outcompete hermaphroditic sperm (12). Mating increases brood size, extends reproductive span, exacerbates matricide (“bagging,” or internal hatching), and shortens life span (9, 10, 13) (fig. S1; see supplementary materials and methods). While studying reproductive aging, we discovered a drastic change in C. elegans hermaphrodite physiology after mating that results in shrinking and death of the mother shortly after completion of reproduction. Although unmated worms continued to grow through day 4 of adulthood, mated N2 [wild-type (WT)] hermaphrodites began to shrink 2 days after mating and shrank by up to 30% by day 7 of adulthood (Fig. 1A and figs.S2 and S3). Unsupervised clustering by percent body-size change separated the worms into two distinct groups, unmated and mated N2 worms (fig. S4A), suggesting that mating is tightly correlated with shrinking. The mean life span of mated WT worms also decreased significantly (>40%) (Fig. 1B and fig. S4C) (13, 14), and experiments modulating mating efficacy indicated that shrinking is a good predictor of postmating death (fig. S4, B and C). Direct resource utilization, mechanical tension, and matricide were eliminated as the cause of postmating shrinking and death (figs. S5 to S7).
Fig. 1. Postmating somatic collapse: Hermaphrodites shrink and die after mating.
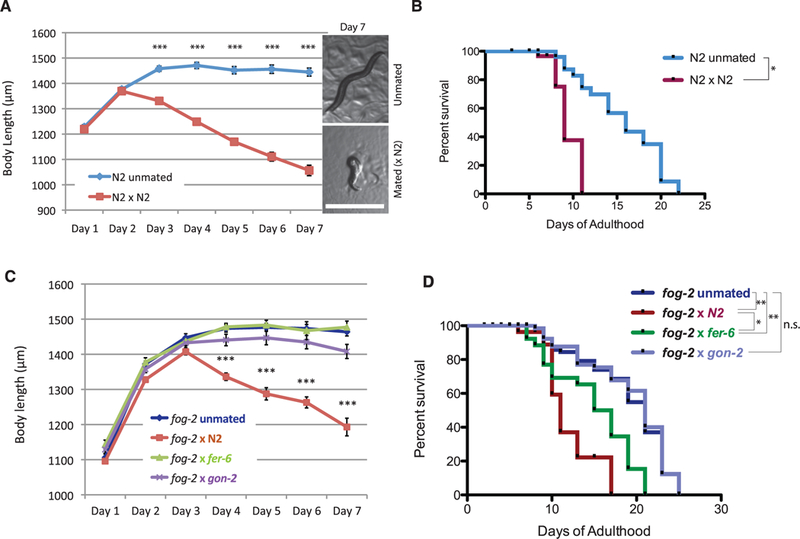
Statistical analysis: survival curves, log-rank test, body size, and others. t test, *P < 0.05, **P < 0.01, ***P < 0.001 for all graphs. Error bars represent SEM unless noted. n.s., not significant. (A) Length of unmated and mated N2 worms. (Inset) Representative pictures of unmated and mated N2 on day 7. Scale bar, 1 mm. (B) Life spans of unmated and mated N2 worms. Unmated N2:16.0 ± 1.0 days, n = 30 worms; mated N2:9.6 ± 0.2 days, n = 36, P < 0.05. (C) Length of unmated fog-2(q71) hermaphrodites (n = 35), and fog-2(q71) mated with N2 (n = 42), fer-6(hc6) (n = 24), and gon-2(q362) males (n = 29). (D) Mean life spans of unmated and mated fog-2. Unmated: 19.0 ± 0.6 days (n = 94); fog-2 × N2:12.0 ± 0.8 days (n = 83); fog-2 × fer-6: 15.0± 0.9 days (n = 28); fog-2 x gon-2:19.2 ± 0.6 days (n = 73).
To identify signals triggered by mating, we tested the roles of male sperm and seminal fluid. gon-2(q362) males lack gonads and, therefore, have no seminal fluid or sperm, but they copulate normally (15), whereas fer-6(hc6) males do not transfer sperm (13, 16), spe-9(hc88) males’ sperm fail to fertilize oocytes (17), and glp-1(e2141) males lack germ line, but these three mutants have normal seminal fluid and copulation. Mating spermless fog-2 hermaphrodites with gon-2 males prevented both shrinking and life-span decrease (Fig. 1, C and D), suggesting that physical copulation plays no role. By contrast, fog-2 hermaphrodites mated with fer-6 (13), spe-9, or glp-1 males lived for a significantly shorter time than unmated worms but did not shrink (Fig. 1, C and D, and fig. S8), indicating that functional sperm, but not seminal fluid, is required for shrinking, whereas seminal fluid contributes to postmating death.
The somatic breakdown of worms after mating is slowed but ultimately not prevented by reduced insulin-like signaling: As in the wild type, daf-2(e1370) hermaphrodites shrank significantly (25%) after mating with N2 males and lived 45% shorter than unmated daf-2 worms (Fig. 2, A and B, and fig. S9A) (14). Germline proliferation (glp-1) mutants are long-lived in a daf-16- and daf-9/daf-12-dependent manner (5, 6). Like daf-2 worms, glp-1(e2141) hermaphrodites lived ~55% shorter lives after mating (Fig. 2B), but they did not shrink (Fig. 2A), suggesting that the germ line is required for shrinking but not for life-span decrease. Similarly, the steroid hormone receptor DAF-12 and the cytochrome P450 that synthesizes DAF-12’s ligand, DAF-9, which are both required for germline-mediated longevity (5, 6), are required for postmating shrinking, but less so (16%) for life-span shortening (Fig. 2, A and B). [Shrinking is not mediated by the sma-9 transforming growth factor-β (TGF-β) body-size pathway (fig. S9B).] Mating decreased Pdaf-9::daf-9::gfp expression in the spermatheca in a sperm-dependent manner (Fig. 2C and fig. S10). Thus, shrinking acts through sperm-induced reduction of DAF-9 levels and requires DAF-12 nuclear hormone signaling and a functional germ line.
Fig. 2. Postmating shrinking is mediated by the germline pathway.
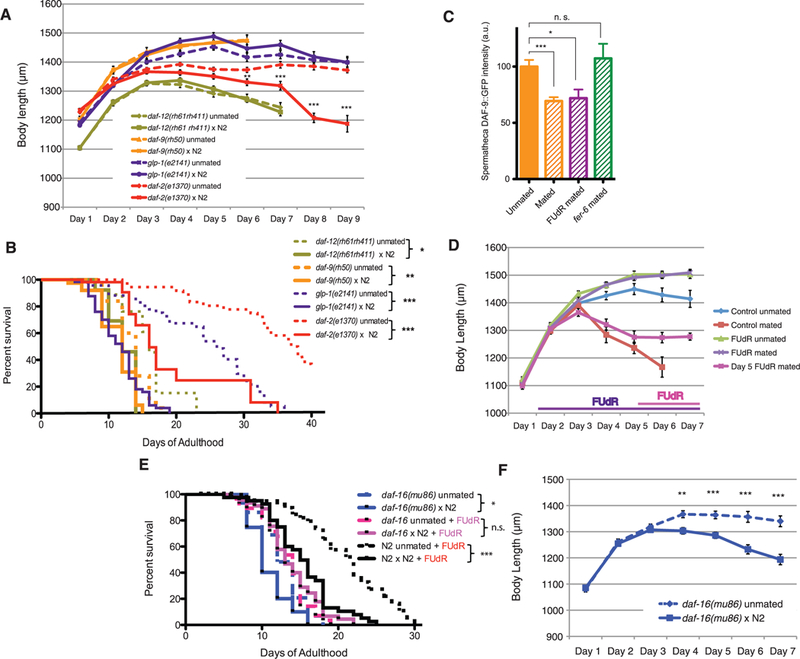
(A) Mated daf-2(e1370) worms shrink up to 25% compared with unmated daf-2, g whereas germline pathway mutants [glp-1(e2141), daf-12(rh61rh411), and daf-9(rh50)] do not shrink after mating. (B) Mated daf-2 (19.4 ± 2.2 days, n = 60) live 45% shorter than unmated daf-2 (37.0 ± 1.9 days, n = 47), P < 0.001. Mated glp-1 (11.6 ± 0.5 days, n = 60) live 55% shorter than unmated glp-1 (24.1 ± 1.3 days, n = 48), P < 0.001. Mated daf-12 (12.1 ± 1.0 days, n = 26) live 20% shorter than unmated daf-12 (16.0 ± 1.4 days, n = 20), P < 0.05; mated daf-9 (11.3 ± 0.4 days, n = 45) live 16% shorter than unmated daf-9 (13.4 ± 0.5 days, n = 36), P < 0.01. (C) Pdaf-9::daf-9::gfp expression in the spermatheca is decreased after mating with N2 males, but not with fer-6 males, and is not affected by FUdR. Error bars denote SD. a.u., arbitrary units. (D) 50 μM FUdR treatment dynamically prevents shrinking in mated N2s. (E) Mated daf-16 (10.83 ± 0.6 days, n = 53) live 15% shorter than unmated (12.62 ± 0.5 days, n = 40), P < 0.05. Adult treatment with FUdR eliminates the difference between mated and unmated daf-16(mu86) life span [unmated, 13.2 ± 0.6 days (n = 36); mated, 13.8 ± 0.5 days (n = 48), P = 0.385]. By contrast, FUdR does not prevent postmating life-span decrease in the wild type. Unmated, 21.0 ± 0.9 days (n = 41); mated, 15.3 ± 0.7 days (n = 43); P < 0.001. (F) Mated daf- 16(mu86) worms shrink by 15%. *P < 0.05, **P < 0.01, ***P < 0.001 for all graphs. Error bars represent SEM unless noted. n.s., not significant.
Using 4’,6-diamidino-2-phenylindole (DAPI) staining and GLD-1::GFP (GFP, green fluorescent protein) to identify the distal proliferative mitotic region (18), we found that mating reduced mitotic zone size and nuclei number (by 30 and 40%, respectively) (fig. S11, A to E). Adult treatment with the DNA replication inhibitor 5-fiuorodeoxyruridine (FUdR), which has little effect on un- or self-mated life span or meiosis at low doses (50 μM) (10) (fig. S11, F and G), reduced the number of germ cell nuclei but had no effect on self-mated brood size (fig. S11, H to J), suggesting that self-mated worms do not require newly proliferating germline cells for progeny production. By contrast, there was a further reduction in the mitotic zone when treated with FUdR after mating (fig. S11D). Adult FUdR treatment prevented shrinking after mating, as in glp-1 mutants (Fig. 2D), but these worms still died prematurely (27%) (Fig. 2E). Shifting worms onto FUdR 4 days after mating prevented further shrinking (Fig. 2D), and movement off of FUdR plates delayed the onset but did not prevent shrinking (fig. S11K). Thus, shrinking is actively regulated throughout the postmating period and is tightly coupled with DNA synthesis in the germ line, implicating the sperm-stimulated proliferation of germline stem cells in generating the signal that induces shrinking.
Longevity of daf-2 and glp-1 worms requires DAF-16/FOXO (5, 6,19). Mating daf-16(mu86) mutants with N2 males caused shrinking (Fig. 2F) but had a smaller effect on life span (15%) (Fig. 2E) than that observed for WT or daf-2 worms (45%). FUdR treatment or loss of daf-12 eliminated daf-16’s life-span decrease altogether (Fig. 2E and fig. S12), suggesting that germline-mediated shrinking itself causes significant daf-16-independent life-span shortening.
In both daf-2 and glp-1 mutants, DAF-16 localizes to the nucleus, where it activates the expression of longevity-promoting genes (5, 6, 19, 20). Mating counteracts this effect, inducing a notable DAF-16::GFP translocation out of the nucleus of both glp-1 and daf-2 hermaphrodites (Fig. 3A and fig. S13, A to C), explaining their delayed but significant life-span decreases. Intestinal expression of Pins-7::gfp, a reporter for the INS-7 insulin-like peptide DAF-2 agonist that regulates FOXO-to-FOXO signaling (21), increased significantly after mating (Fig. 3B and fig. S13, D and E), likely promoting cytoplasmic localization of DAF-16.
Fig. 3. Postmating life-span decrease is mediated by DAF-16, and shrinking correlates with osmotic stress sensitivity.
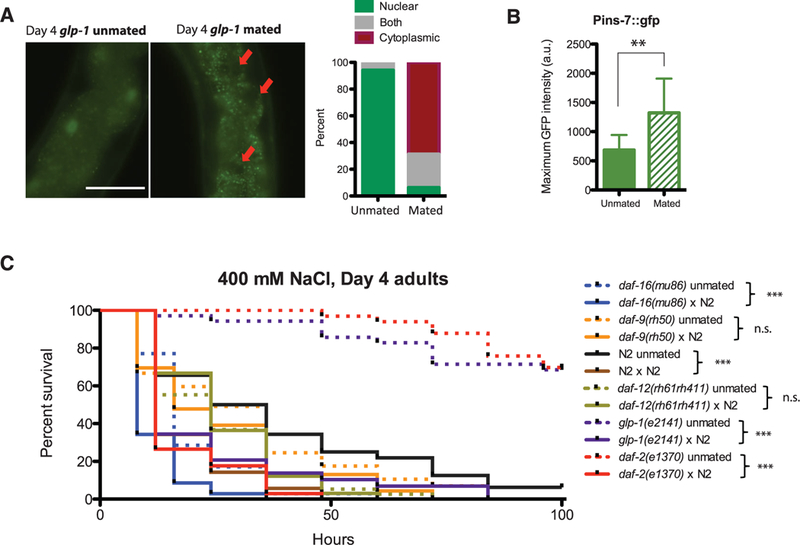
(A) DAF-16::GFP in glp-1 translocates from the nucleus to the cytoplasm after mating. GFP channel images of glp-1 unmated (left) and mated worms (middle); red arrows point to dark nuclei. Scale bar, 100 μm. (Right) Quantification of GFP localization. (B) Pins-7::gfp expression [maximum ± SD (error bars)] increases significantly in the intestine postmating. a.u., arbitrary units. (C)Survival curves under osmotic stress (400 mM NaCl): Day 1 mated N2 and daf-16(mu86) died faster than the unmated controls put under osmotic stress starting on day 4. Unmated N2: 40.3 ± 6.0 hours, n = 31; mated N2:18.5 ± 1.8 hours, n = 35, P <0.001. Unmated daf-16:19.2 ± 1.9 hours, n = 35; mated daf-16:11.8 ± 1.1 hours, n = 35, P < 0.001. Mated daf-9(rh50) and daf-12(rh61rh411) survived as long as the unmated controls under osmotic stress. Unmated daf-9: 31.2 ± 4.3 hours, n = 30; mated daf-9: 25.7 ± 3.8 hours, n = 23, P = 0.3242. Unmated daf-12: 25.6 ± 2.5 hours, n = 38; mated daf-12: 26.2 ± 2.3 hours, n = 33, P = 0.9714. glp-1 and daf-2 mated worms survive longer than wild type on NaCl, but still die prematurely. glp-1 unmated (n = 35): 131.7 ± 10.4 hours; glp-1 mated (n = 29): 23.2 ± 3.8 hours; P < 0.0001. daf-2 unmated (n = 33): 250.5 ± 30.0; daf-2 mated (n = 34): 17.7 ± 1.8 hours; P < 0.0001. *P < 0.05, **P < 0.01, ***P < 0.001 for all graphs.
Fat levels increase in daf-2 and glp-1 mutants; this increase is associated with germline-mediated longevity (22–25). Mating induced ~50% fat decrease but was independent of shrinking (figs. S14 and S15). By contrast, hypertonic stress susceptibility correlated well with shrinking (correlation coefficient r2 = 0.76) (Fig. 3C and fig. S16): Worms that shrink after mating (N2, daf-2, daf-16) die earlier than their unmated counterparts when placed on high-salt plates on day 4, whereas nonshrinking worms (daf-12, daf-9) do not, implying that water loss causes shrinking. Although daf-16 is required for osmotic stress resistance through its regulation of osmotic stress protection genes (20, 26), daf-16 mutants are even shorter-lived on high salt after mating, suggesting that there is a second, daf-16-independent, daf-9/daf-12- dependent regulation of osmotic stress resistance that correlates with shrinking (Fig. 3C). daf-12 also regulates osmotic stress resistance genes (27, 28). Although DAF-16 regulates osmotic stress resistance (20, 26), mating induces cytoplasmic localization of DAF-16 in daf-2- and glp-1-mated animals, decreasing survival on high salt plates (Fig. 3C and fig. S16).
Together, our data suggest that an active signaling process in mothers originates from males, causing an endocrine signaling decision in mated hermaphrodites that causes shrinking and death (Fig. 4A). Sperm both decreases DAF-9 levels and enhances a signal from the germ line through increased mitotic stem cell proliferation. This signal controls a daf-12-dependent pathway that induces water loss, shrinking, osmotic stress sensitivity, and life-span shortening. A separate, germline-independent decrease in life span that is mediated by DAF-16 cytoplasmic localization and reinforced by INS-7 feedback may be induced by seminal fluid. Together, the two branches shorten life span by up to 40% after mating. Eliminating both the daf-12 and daf-16 pathways removes the effect of mating on both body size and life span (fig. S12). Sperm and seminal fluid maximally activate pathways that are blocked under low-reproduction, high-longevity conditions, resulting in shrinking, fat loss, and death.
Fig. 4. Evolutionary conservation of mating-induced shrinking and death.
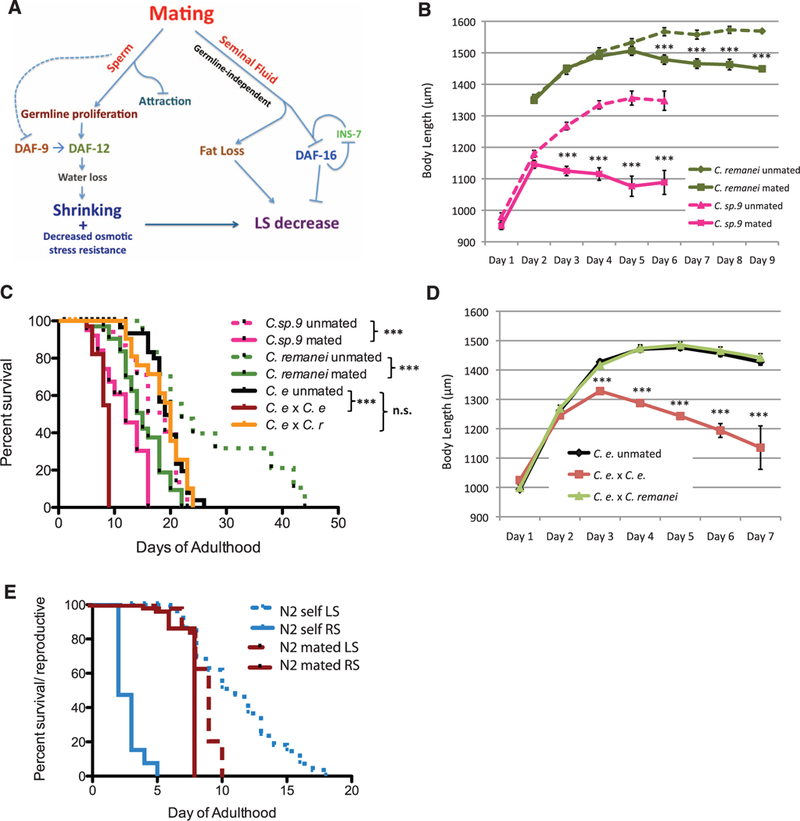
(A) Sperm decreases DAF-9 activity and induces germline proliferation, which in turn emits a DAF-12-dependent signal that results in shrinking and subsequent life-span shortening, probably due to reduced osmotic stress resistance. Seminal fluid induces a germline-independent signal that causes both fat loss and DAF-16 cytoplasmic translocation (possibly amplified by INS-7), further reducing life span (LS). (B to D) Mated C. remanei and C. sp.9 shrink (B) and die prematurely (C). C. remanei: unmated: 26.9 ± 2.8 days, n = 48; mated:15.1 ± 0.8 days, n = 48, P < 0.001. C sp.9: unmated: 17.3 ± 1.2 days, n = 42; mated: 11.9 ± 0.8 days, n = 41, P < 0.01. An interspecies cross between C. remanei (C.r) males and C. elegans (C.e) hermaphrodites does not reduce life span (C) or induce shrinking (D). C.e unmated: 19.4 ± 0.6 days, n = 33; C.e × C.e:8.2 ± 0.4 days, n = 36; C.e × C.r. 18.8 ± 0.9 days; n = 27. (E) Postreproductive life span is significantly reduced in mated worms. Unmated N2 worms (n = 60): mean LS = 11.2 ± 0.5 days; reproductive span (RS) = 3.1 ± 0.1 days; postreproductive LS = ~8.1 days. Mated N2 worms (n = 62): LS = 8.7 ± 0.3 days; P = 0.131 (compared with unmated LS); RS = 7.7 ± 0.1 days, P < 0.001 (compared with unmated RS); postreproductive LS = ~1.0 day. *P < 0.05, **P < 0.01, ***P < 0.001 for all graphs. Error bars represent SEM unless noted. n.s., not significant.
Our data argue against direct resource exhaustion or a life span-versus-reproduction trade off (14), because (i) brood size does not correlate with shrinking, (ii) mated daf-12 mutants have more progeny than unmated daf-12 but do not shrink, and (iii) animals with no germ line (and, thus, no progeny) are also short-lived after mating. The fact that mutants without a germ line also lose fat in response to mating suggests that fat depletion is a programmed response anticipating future resource allocation.
Like C. elegans, the females of the gono-choristic (male or female) species C. remanei and C. sp9 JU1422 shrank significantly and died prematurely after mating (Fig. 4, B and C). However, C. elegans hermaphrodites crossed with C. remanei, which are not cross-fertile, did not shrink or die early (Fig. 4, C and D). Thus, postmating somatic collapse is evolutionarily conserved, but its signals are species-restricted.
Mating increases reproductive span and decreases life span, eliminating the postreproductive life span of mated mothers (Fig. 4E). C. elegans are hermaphroditic, and the male population is normally low (0.1%). Under stress, however, male offspring production increases (7), and mating results in 50% male production, increasing the male population and thus the possibility of sperm competition. Mating also causes a sperm-dependent decrease in attractiveness to males (fig. S17), suggesting that a programmed response to sperm levels modulates a signal from the hermaphrodites that affects male attraction, increasing the chances of successful mating (29). The mother’s death immediately after the last eggs are laid prevents subsequent mating, maximizing the father’s reproductive success. C. elegans males hijack the very pathways that the hermaphrodite employs to slow down reproduction and aging in times of low nutrients, reversing these processes to accelerate the mother’s death, perhaps for the male’s benefit.
Supplementary Material
Acknowledgments:
We thank the Caenorhabditis Genetics Center for strains, J. Ashraf for assistance, M. Barr for useful discussion, and Z. Gitai and members of the Murphy laboratory for critically reading the manuscript. This work was supported by an NIH Innovator award (DP20D004402) to C.T.M.
References and Notes
- 1.Kokko H, Brooks R, Jennions MD, Morley J, Proc. Biol. Sci. 270, 653–664 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villella A, Hall JC, Adv. Genet. 62, 67–184 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Wolfner MF, Soc. Reprod. Fertil. Suppl. 65, 183–199 (2007). [PubMed] [Google Scholar]
- 4.Kenyon CJ, Nature 464, 504–512 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Hsin H, Kenyon C, Nature 399, 362–366 (1999). [DOI] [PubMed] [Google Scholar]
- 6.Berman JR, Kenyon C, Cell 124, 1055–1068 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Luo S, Kleemann GA, Ashraf JM, Shaw WM, Murphy CT, Cell 143, 299–312 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo S, Murphy CT, Genesis 49, 53–65 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Hughes SE, Evason K, Xiong C, Kornfeld K, PLOS Genet. 3, e25 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo S, Shaw W, Ashraf J, Murphy C, PLOS Genet. 5, e1000789 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brenner S, Genetics 77, 71–94 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaMunyon CW, Ward S, Proc. Biol. Sci. 265, 1997–2002 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gems D, Riddle DL, Nature 379, 723–725 (1996). [DOI] [PubMed] [Google Scholar]
- 14.Wu D, Tedesco PM, Phillips PC, Johnson TE, Exp. Gerontol. 47, 759–763 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun AY, Lambie EJ, Genetics 147, 1077–1089 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Argon Y, Ward S, Genetics 96, 413–433 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singson A, Mercer KB, L’Hernault SW, Cell 93, 71–79 (1998). [DOI] [PubMed] [Google Scholar]
- 18.Kimble J, Crittenden SL, “Germline proliferation and its control,” in WormBook, The C. elegans Research Community, Ed. (WormBook, 2005); doi: 10.1895/wormbook.1.13.1; www.wormbook.org/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R, Nature 366, 461–464 (1993). [DOI] [PubMed] [Google Scholar]
- 20.Murphy CT et al. , Nature 424, 277–283 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Murphy CT, Lee SJ, Kenyon C, Proc. Natl. Acad. Sci. U.S.A. 104, 19046–19050 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen M, Flatt T, Aguilaniu H, Cell Metab. 17, 10–19 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Rourke EJ, Soukas AA, Carr CE, Ruvkun G, Cell Metab. 10, 430–435 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lapierre LR, Gelino S, Meléndez A, Hansen M, Curr. Biol. 21, 1507–1514 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yen K et al. , PLOS ONE 5, e12810 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamitina ST, Strange K, Am. J. Physiol. Cell Physiol. 288, C467–C474 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Fisher AL, Lithgow GJ, Aging Cell 5, 127–138 (2006). [DOI] [PubMed] [Google Scholar]
- 28.McCormick M, Chen K, Ramaswamy P, Kenyon C, Aging Cell 11, 192–202 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morsci NS, Haas LA, Barr MM, Genetics 189, 1341–1346 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


