Abstract
Sub-Saharan Africa (SSA) is facing a growing co-epidemic of chronic HIV-infection and diabetes. Hemoglobin A1c (A1c) may underestimate glycemia among people living with HIV (PLWH). We estimated the validity of A1c to diagnose diabetes among PLWH and HIV-uninfected persons in rural Uganda. Data were derived from a cohort of PLWH and age and gender-matched HIV-uninfected comparators. We compared A1c to fasting plasma glucose (FPG) using Pearson correlations, regression models, and estimated the sensitivity and specificity of A1c for detecting diabetes with FPG ≥126mg/dl as reference standard. Approximately half (48%) of the 212 participants were female, mean age of 51.7 (SD=7.0) at enrollment. All PLWH (n=118) were on antiretroviral therapy for a median of 7.5 years with mean CD4 count of 442 cells/μl. Mean FPG (89.7mg/dl) and A1c (5.6%) were not different between PLWH and HIV-uninfected (P>0.50) groups, but the HIV-uninfected group had a higher prevalence of A1c >5.7% (33% vs 20%, p=0.024). We found a relatively strong correlation between A1c and FPG (r=0.67). An A1c ≥6.5% had a poor sensitivity (46%, 95% CI 26–67%) but high specificity (98%, 95% CI 96–99%) for detecting diabetes. More work is needed to define an optimal A1c for screening diabetes in SSA.
Search terms: Hemoglobin A1c, fasting plasma glucose, diabetes, HIV, sub-Saharan Africa
Introduction
By 2040, one in ten adults (642 million) are predicted to have diabetes, with large increases in disease burden expected in countries transitioning from low to middle-income status (1). Africa is home to populations with the highest rates of undiagnosed diabetes and many regions are grappling with concurrent infectious and non-communicable disease epidemics (1,2). For example, the sub-Saharan Africa (SSA) region accounts for nearly 67% of people living with HIV (PLWH) globally (3); and although treatment scale-up has been successful, the prevalence of diabetes mellitus (DM), cardiovascular disease, and other metabolic disorders are elevated in PLWH (4,5).
Several factors have been implicated as drivers for the increased risk of diabetes among PLWH, including the increasing lifespan of those infected (4). Yet, data from the 2009–10 Medical Monitoring Project (n=8610), a nationally representative surveillance study of HIV-infected adults in the United States and National Health and Nutrition Survey (n=5604 general population adults), a nationally representative surveillance study of adults in the general population in the United States, indicate that PLWH had higher unadjusted prevalence of diabetes (10.3%) compared with the general population (8.3%), with that difference doubling after adjusting for covariates (6–8). Evidence from Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) cohort and other studies has implicated the use of protease inhibitors and nucleoside reverse transcriptase inhibitors (NRTIs) such as zidovudine, which are still widely used in SSA, as contributors to increased diabetes risk (9,10).
The American Diabetes Association (ADA) now recommends A1c as a screening test for diabetes, in addition to fasting plasma glucose (FPG) or Oral Glucose Tolerance Test (OGTT) (11). In 2011, the World Health Organization (WHO) also began recommending A1c as test for diagnosis of diabetes (12). However, there are concerns that A1c underestimates or overestimates glycemia in different ethnic or racial groups with different A1c-genetic variants (13). Further, A1c can be affected by several factors such as age, shortened red blood cell lifespan, cirrhosis, renal failure and hemolysis, all of which have been associated with HIV-infection (14). Other factors associated with low sensitivity of A1c among PLWH include use of nucleoside analog reverse transcriptase inhibitor (NRTI)-based therapy, macrocytosis and/or abacavir use (15).
In contrast, FPG has been used as a primary test for DM in many low resource settings, owing to its ease of use and low cost. A strong linear relationship between A1c and FPG has been demonstrated in multiple ethnic groups and geographic regions outside of SSA(16–18). However, some studies have also reported that A1c underestimates blood glucose among PLWH (15,19). Yet, there are few studies from SSA on the accuracy of using A1c compared with FPG in the general population or among PLWH (15). This gap in the literature persists despite the high prevalence of HIV in the SSA region and points to a need for targeted research to identify optimal methods for screening diabetes (3). We aimed to estimate the relationship and diagnostic accuracy of A1c compared to FPG in a cohort of PLWH on ART and community based, HIV-uninfected comparators. Our overarching aim was to assess the utility of A1c testing in this population as it becomes increasingly available in the region.
Methods
Study Population
Data for this analysis was collected as part of the Ugandan Non-Communicable Diseases and Aging Cohort (UGANDAC) Study (), a cohort of PLWH and age and gender-matched HIV-uninfected persons in rural Uganda (20,21). Study participants were comprised of [1] PLWH aged 40 years and older, in ambulatory care at the HIV Clinic at Mbarara Regional Referral Hospital, and on ART for a minimum of three years; and [2] HIV-uninfected persons recruited from the catchment area of the hospital, who were age- (by quartile) and sex-matched to PLWH (22). We first enrolled PLWH who met the above inclusion criteria and who were actively in care at the Mbarara Regional Referral Hospital Immune Suppression Syndrome. We then used the population census of Nyakabare Parish, which is a cluster of eight villages approximately 20 kilometers from the clinic, to identify HIV-uninfected comparators. We randomly selected a sample of individuals who were age- and sex-matched (by quartile of PLWH). All HIV-uninfected individuals underwent confirmatory HIV testing on the day of each study visit.
Variables
Participants completed annual visits to collect data on sociodemographic, anthropometric measurements, blood pressure, smoking history [using the WHO Tobacco questionnaire (20), diet, physical activity, body mass index (BMI) and self-reported history and treatment for diabetes and hypertension. Blood was collected for A1c testing using Siemens Vantage A1c testing kits (Siemens Medical Solutions USA, Malvern, PA). A1c testing was done at the time of blood collection using the point-of-care Siemens assay. Serum was stored at −80°C for up to one year before shipment to the United States for serologic testing of glucose and other metabolic parameters. For PLWH, CD4 T-cell counts and HIV-1 RNA viral load were abstracted from medical records. For FPG, whole blood was collected into serum separator tubes and centrifuged and stored at −80°C until testing. Cryopreserved serum samples were tested at LabCorp clinical laboratories for comprehensive metabolic panel (LabCorp, Burlington, NC, USA). Participants were requested to fast after midnight on the days of their procedures, and fasting status is recorded on the date of each visit. All study questionnaires, specimen collection, and A1c testing was performed by study nurses.
Statistical Analysis
We included data from all study visits with paired A1c and FPG. We first summarized the cohort and assessed for differences in sociodemographic and clinical characteristics by HIV serostatus. We used Pearson correlation to determine the relationship between A1c and FPG both for the total cohort and by HIV serostatus. We then fit univariable and multivariable linear regression models with robust standard errors to account for repeated measure clustering, with FPG as the dependent variable, and A1c as the primary independent variable, with and without A1c*HIV serostatus product terms to assess for a modification of the relationship between A1c and FPG by HIV serostatus. We used forward stepwise regression with an alpha threshold of 0.05 to select variables that may influence the relationship between A1c and FPG among PLWH. Such variables included age, sex, BMI or waist circumference, hemoglobin level, MCV, albumin, asset ownership index, and C-reactive protein. Finally, we estimated the sensitivity and specificity of A1c to detect diabetes using ADA thresholds of ≥6.5% for A1c and FPG ≥126mg/dL as indicative of diabetes (23) and fit a receiver operating characteristic curve (ROC) and calculated the area under ROC (AUC) to determine the optimal threshold of A1c to detect FPG ≥126mg/dL. For prediabetes, we estimated the sensitivity and specificity of A1c cut off ≥5.7% and FPG ≥100mg/dL. We used the rocreg command in Stata to account for clustering and used bootstrapping with 1000 repetitions to estimate the standard errors and confidence intervals for the AUC estimations. Statistical analysis was done using Stata version 14 (StataCorp, College Station, TX).
Ethical Considerations
Study procedures were approved by institutional review committees at Mbarara University of Science in Uganda and Technology and Partners Healthcare in the United States. Written informed consent was provided by all study participants.
Results
The study enrolled a total of 309 participants, of which 212 (HIV-positive n=118; HIV-uninfected n=94) had both FPG and A1c measured simultaneously at least once during observation and were therefore included in this analysis. The remaining participants were excluded due to absence of a visit with FPG and A1c during the study. Participants with missing A1c and FPG values were slightly older (53.6 vs 52.6, P=0.03); however, no statistically significant differences were found in sex or BMI among those with missing and non-missing A1c and FPG measurements (Supplemental Table 1). Included participants had a mean age of 51.7 years (SD=7.0) at enrollment, and approximately half (48%) were female (Table 1). Few individuals reported a history of diabetes [n=12 (5.8%)] or current therapy for diabetes [n=5 (2.3%)]. At enrollment, PLWH (n=118) were on ART for a median of 7.5 years and the mean CD4 count was 442 cells/uL (SD=179). The prevalence of elevated blood sugar was 6.1% determined by the A1c threshold ≥6.5% and 6.2% determined by the FPG threshold ≥126mg/dL. PLWH and HIV-uninfected individuals had comparable mean FPG (88.5 vs 91.2, P=0.55) and comparable mean A1c (5.5% vs 5.6%, P= 0.69). However, HIV-uninfected individuals had a higher prevalence of elevated of FPG ≥100mg/dL (18.1% vs 12.7%, P=0.56) and A1c >5.7% (33% vs 20%, p=0.02), despite having a lower prevalence of overweight (18.9% vs 26.3%, P=0.10). At enrollment, all PLWH (n=118) were on ART, the majority of whom (91%) were on nevirapine or efavirenz.
Table 1.
Cohort characteristics
| Characteristic (n%) | Total Cohort (n=212) | HIV-Negative (n=94) | HIV-Positive (n=118) | p-value |
|---|---|---|---|---|
| Age (mean/SD) | 51.7 (7) | 52.2(6.9) | 51.3(7.1) | 0.324 |
| Female | 102 (48.1) | 45 (47.8) | 57 (48.3) | 0.95 |
| BMI (Mean/SD) | 22.7 (4.2) | 22.4 (4.5) | 22.8 (3.9) | 0.431 |
| <18.5 | 21(9.9) | 14 (14.8) | 7 (6.0) | 0.099 |
| 18.5 – 24.9 | 142 (67.0) | 62 (66.0) | 80 (67.8) | |
| 25.0 – 29.9 | 34 (16.0) | 11 (11.7) | 23 (19.5) | |
| ≥30 | 15 (7.1) | 7 (7.5) | 8 (6.8) | |
| FPG (mean/SD) | 89.7(33.2) | 91.2 (34.2) | 88.5 (32.5) | 0.554 |
| <100mg/dl | 180 (84.9) | 77 (81.9) | 103 (87.3) | 0.553 |
| 100–125 mg/dl | 19 (9.0) | 10 (10.6) | 9 (7.6) | |
| ≥126 mg/dl | 13 (6.2) | 7 (7.45) | 6 (5.1) | |
| A1c | 5.6 (1.1) | 5.6 (0.96) | 5.5 (1.2) | 0.687 |
| <5.7% | 156 (73.6) | 62 (66.0) | 94 (79.7) | 0.024* |
| 5.7–6.4% | 43 (20.3) | 27 (28.7) | 16 (13.6) | |
| ≥6.5% | 13 (6.1) | 5 (5.3) | 8 (6.8) | |
| ART Therapy | ||||
| AZT/3TC/NVP | 100 (64.5) | |||
| AZT/3TC/EFV | 41 (26.5) | |||
| TDF/3TC/LPV/R | 10 (6.5) | |||
| Other | 4 (2.5) | |||
| CD4 count (Median/IQR) | 485 (837) | |||
| Nadir CD4 (Median/IQR) | 131 (432) |
Statistically significant at 0.05 level
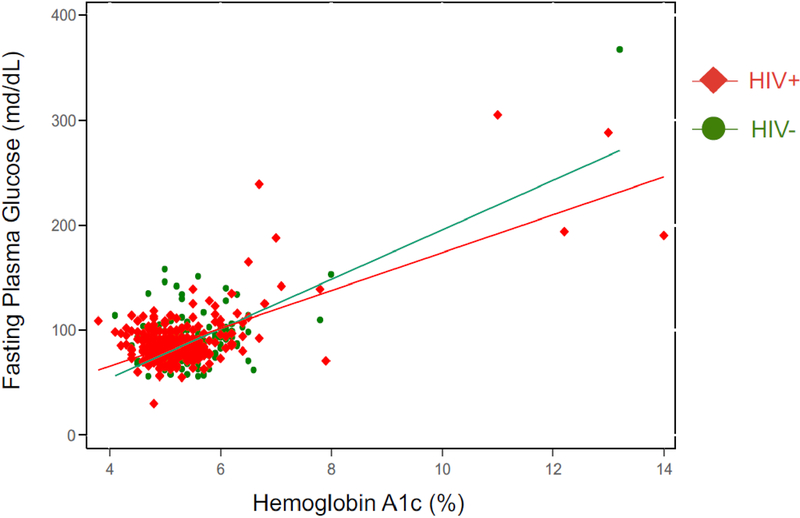
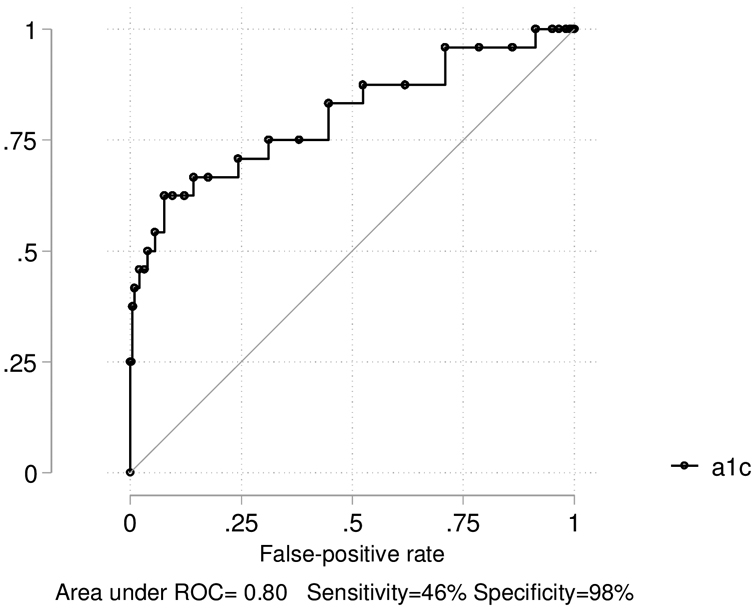
The overall correlation between A1c and FPG was high (r=0.67, P<0.001) and was similar between PLWH and HIV-uninfected individuals (r=0.69 vs r=0.66, P=0.70). In regression analyses, we estimated a linear relationship between A1c and FPG (see Figure 1): the estimated mean increase in FPG for each 1 unit in A1c was 18 mg/dL (95% CI 10.7–25.0) for the entire cohort, 16 mg/dL (95% CI 9.2–23.2) among PLWH, and 23 mg/dL (95% CI 9. 3–37.2) among HIV-uninfected individuals (P-value for interaction =0.349). No statistically significant differences were noted comparing PLWH and HIV-uninfected individuals on A1c and FPG after adjusting for age, sex, and BMI. We estimated sensitivity and specificity for range of A1c cutoff points (see Supplemental Table 2). To detect an elevated FPG for the total cohort at a threshold of ≥126mg/dL, A1c had an AUC of 0.80 (95% CI 0.69–0.92), sensitivity of 46% (95% CI 26–67%) and specificity of 98% (95% CI 96–99%) (Figure 2) at the ≥6.5% cutoff. Given the cohort prevalence of diabetes at 6%, A1c had a corresponding positive predictive value of 55% (95% CI 32–77%) and negative predictive value of 97% (95% CI 95–98%). At an FPG threshold of ≥126mg/dL, A1c performed better among PLWH (75% sensitivity, 95%CI 43–95%; 99% specificity, 95% CI 96–100%, AUC 0.97, 95% CI 0.93–1.00) compared with HIV-uninfected persons (17% sensitivity, 95% CI 2–48%; 97% specificity, 95%CI 93–99%, AUC 0.58, 95% CI 0.38–0.79). We observed a low prevalence of elevated A1c in sub-groups, resulting in large confidence intervals for estimates of sensitivity. At the cutoff of ≥5.7%, A1c had a sensitivity for detecting FPG ≥100mg/dL of 43% (95% CI 32–55%) and a specificity of 88% (95% CI 85–91%).
Figure 1.
Scatter plot and predictive regression line demonstrating relationship between A1c and FPG by HIV serostatus
Figure 2.
Area under receiver operating curve for A1c predicting FPG values for both cohorts
Discussion
In this mixed cohort of community dwelling PLWH on ART and HIV-uninfected comparators, we found a strong correlation between A1c and FPG. However, an A1c threshold of ≥6.5% had relatively low sensitivity but a high specificity using a criterion FPG of ≥126 mg/dL. We found no evidence of decreased sensitivity of A1c among PLWH versus HIV-uninfected individuals. Our results suggest that A1c may be an acceptable screening test for diabetes in rural SSA settings, but also a that there is a need to further evaluate the most appropriate threshold for diagnosis, and the possible role for repeated screening to augment sensitivity.
Contrary to our hypothesis, PLWH had similar correlations between A1c and FPG compared to HIV-uninfected persons. Correlations between A1c and FPG were strong overall in this cohort, which is in keeping with prior work across different ethnic groups and geographic regions in North America, South America, Europe, and Asia that have demonstrated a predictably linear relationship between A1c and FPG levels across a FPG range of 100.8–162mg/dL (16,18). For instance, a meta-analysis of 14 studies reported a pooled correlation of r=0.61 (95 % CI; 0.48–0.72) (17), remarkably similar to our finding (r=0.67), and adds support for the use of A1c in principle as a screening test in SSA.
However, we identified a low sensitivity for A1c threshold of ≥6.5% in our study as well, raising questions about the optimal A1c threshold for diabetes screening in rural SSA(13,24). Data elsewhere have demonstrated a lower A1c threshold may increase overall sensitivity of A1c to screen for diabetes. For example, a large study in the United States suggested an A1c of ≥6.0% was the optimal screening threshold to correspond to an FPG≥126mg/dL, with a sensitivity of 69.8% and specificity of 91.9% (25). Using that same A1c threshold of ≥6.0%, we found similar sensitivity of 63% and specificity of 91% in our cohort.
In contrast, the WHO recommends using A1c threshold of 6.5% to diagnose diabetes (11), noting insufficient evidence to make recommendations at levels below that. The low sensitivity but high specificity of a single A1c to diagnose diabetes in our study supports that recommendation, as do other studies in the field both in the general population and among PLWH. For example, the National Health and Nutrition Examination Survey reported a sensitivity of only 43% for an A1c ≥ 6.5% to detect elevated FPG at ≥ 126mg/dL (25). Others have reported that women with HIV and diabetes in the US had lower A1c compared with HIV-uninfected comparators with the same FPG values, and that this difference was attenuated after controlling for mean corpuscular volume (MCV) among PLWH (26). In the Multicenter AIDS Cohort Study, A1c was an average of 0.21% lower among men with HIV compared with HIV-uninfected men at FPG of 125mg/dL (19).
In summary, findings from our study suggest that A1c correlates well with FPG but the diagnostic threshold of A1c might need to be altered in SSA to improve sensitivity for diagnosing diabetes. Additional considerations about A1c as a primary screening assay remain, including issues related to its reliance on red cell indices in malaria-endemic regions, and comparative costs versus fasting glucose and oral glucose tolerance tests. Future work should explore these issues with longitudinal observation of individuals, inclusion of malaria and red cell index tests, and repeated measures of glucose testing to better clarify the optimal diagnostic approach to using A1c in this setting.
This study had a number of important limitations. We could not compare A1c to average 2 to 3-month glucose since our data consisted of single fasting glucose measures at annual study visits. Similarly, we were not able to assess the contribution of MCV, hemoglobin, hemoglobinopathies or malaria co-infections to A1c-FPG relationships in the cohort because the measures were not taken among most participants. In addition, we did not have OGTT measures. It is known that A1c and FPG may have limitations of moderate sensitivity compared to OGTT which is known at times to have higher sensitivity. Comparing the accuracy of A1c, FPG and OGTT would be necessary to determine which test has highest diagnostic accuracy. We observed a relatively small sample size of individuals with high FPG, and therefore were underpowered to make strong conclusions about the sensitivity of A1c in sub-groups. We also acknowledge that clustered data can affect interpretation of our correlation coefficients, but this would only impact interpretation if individual-level factors contribute meaningfully to diagnostic validity of hemoglobin A1c in comparison to fasting glucose. Finally, our results should be considered within the context of our study population. For example, our participants were characterized by a rural population, and certain characteristics, such as mean BMI were relatively low in this cohort.
In conclusion, we found a strong correlation between A1c and FPG among individuals in Uganda and no evidence of a difference in this relationship by HIV serostatus. However, an A1c ≥6.5% had a poor sensitivity (46%) but high specificity (98%) to detect elevated FPG in this population. Further studies are needed to validate the optimal strategy of diabetes screening and monitoring in SSA.
Supplementary Material
Sources of Support:
This study was funded by the U.S. National Institutes of Health R21 HL124712, P30 AI060354, R24 AG044325, P30AG024409, MGH Executive Committee on Research, and Friends of a Healthy Uganda. The authors acknowledge the following additional sources of support: R01MH113494–01, K23 MH099916 and K43 TW010715.
Footnotes
Conflicts of Interest: All authors report no conflicts of interest
References
- 1.International Diabetes Federation. IDF diabetes atlas −7th Edition [Internet]. 2017. [cited 2017 Apr 23]. Available from: http://www.diabetesatlas.org/ [PubMed]
- 2.Institute for Health Metrics and Evaluation. The Global Burden of Disease: Generating Evidence, Guiding Policy – Sub-Saharan Africa Regional Edition [Internet]. 2018. [cited 2018 Jan 23]. Available from: http://www.healthdata.org/policy-report/global-burden-disease-generating-evidence-guiding-policy-%E2%80%93-sub-saharan-africa-regional
- 3.World Health Organization. HIV/AIDS [Internet]. 2017. [cited 2018 Jan 23]. Available from: http://www.afro.who.int/health-topics/hivaids
- 4.UNAIDS. UNAIDS. Chronic care of HIV and non-communicable diseases: How to leverage the HIV experience [Internet]. 2011. [cited 2018 Jan 23]. Available from: http://files.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2011/20110526_JC2145_Chronic_care_of_HIV.pdf
- 5.NCD Risk Factor Collaboration (NCD-RisC) – Africa Working Group. Trends in obesity and diabetes across Africa from 1980 to 2014: an analysis of pooled population-based studies. Int J Epidemiol. 2017. 01;46(5):1421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernandez-Romieu AC, Garg S, Rosenberg ES, Thompson-Paul AM, Skarbinski J. Is diabetes prevalence higher among HIV-infected individuals compared with the general population? Evidence from MMP and NHANES 2009–2010. BMJ Open Diabetes Res Care. 2017. January 1;5(1):e000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frankel MR, McNaghten A, Shapiro MF, Sullivan PS, Berry SH, Johnson CH, et al. A probability sample for monitoring the HIV-infected population in care in the U.S. and in selected states. Open AIDS J. 2012;6:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Prevention and Control. About the National Health and Nutrition Examination Survey [Internet]. 2017. [cited 2018 Nov 22]. Available from: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm
- 9.De Wit S, Sabin CA, Weber R, Worm SW, Reiss P, Cazanave C, et al. Incidence and risk factors for new-onset diabetes in HIV-infected patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. Diabetes Care. 2008. June;31(6):1224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalra S, Kalra B, Agrawal N, Unnikrishnan A. Understanding diabetes in patients with HIV/AIDS. Diabetol Metab Syndr. 2011. January 14;3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Diabetes Association. ADA Diabetes Management Guidelines [Internet]. 2016. [cited 2017 Apr 25]. Available from: http://www.ndei.org/ADA-diabetes-management-guidelines-diagnosis-A1C-testing.aspx.html
- 12.World Health Organization. Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus [Internet]. 2011. [cited 2018 Apr 15] p. 1–25. Available from: http://www.who.int/diabetes/publications/report-hba1c_2011.pdf?ua=1 [PubMed]
- 13.Wheeler E, Leong A, Liu C-T, Hivert M-F, Strawbridge RJ, Podmore C, et al. Impact of common genetic determinants of Hemoglobin A1c on type 2 diabetes risk and diagnosis in ancestrally diverse populations: A transethnic genome-wide meta-analysis. PLoS Med. 2017. September;14(9):e1002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim S-Y, Friedmann P, Seth A, Fleckman AM. Monitoring HIV-infected Patients with Diabetes: Hemoglobin A1c, Fructosamine, or Glucose? Clin Med Insights Endocrinol Diabetes. 2014. December 4;7:41–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim PS, Woods C, Georgoff P, Crum D, Rosenberg A, Smith M, et al. A1C Underestimates Glycemia in HIV Infection. Diabetes Care. 2009. September 1;32(9):1591–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan X, Zheng L, Sun G, Guo X, Li Y, Song H, et al. The changing relationship between HbA1c and FPG according to different FPG ranges. J Endocrinol Invest. 2016. May;39(5):523–8. [DOI] [PubMed] [Google Scholar]
- 17.Ketema EB, Kibret KT. Correlation of fasting and postprandial plasma glucose with HbA1c in assessing glycemic control; systematic review and meta-analysis. Arch Public Health [Internet]. 2015. September 25;73 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4582842/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramachandran A, Riddle MC, Kabali C, Gerstein HC, ORIGIN Investigators. Relationship between A1C and fasting plasma glucose in dysglycemia or type 2 diabetes: an analysis of baseline data from the ORIGIN trial. Diabetes Care. 2012. April;35(4):749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slama L, Palella FJ, Abraham AG, Li X, Vigouroux C, Pialoux G, et al. Inaccuracy of haemoglobin A1c among HIV-infected men: effects of CD4 cell count, antiretroviral therapies and haematological parameters. J Antimicrob Chemother. 2014. December;69(12):3360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feinstein MJ, Kim J-H, Bibangambah P, Sentongo R, Martin JN, Tsai AC, et al. Ideal Cardiovascular Health and Carotid Atherosclerosis in a Mixed Cohort of HIV-Infected and Uninfected Ugandans. AIDS Res Hum Retroviruses. 2017. January;33(1):49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siedner MJ, Kim J-H, Nakku RS, Hemphill L, Triant VA, Haberer JE, et al. HIV infection and arterial stiffness among older-adults taking antiretroviral therapy in rural Uganda. AIDS Lond Engl. 2016. February 20;30(4):667–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ClinicalTrials.gov. Ugandan Non-Communicable Diseases and Aging Cohort (UGANDAC) [Internet]. 2017. [cited 2018 Feb 16]. Available from: https://clinicaltrials.gov/ct2/show/NCT02445079
- 23.American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018. January 1;41(Supplement 1):S13–27. [DOI] [PubMed] [Google Scholar]
- 24.Bonora E, Tuomilehto J. The Pros and Cons of Diagnosing Diabetes With A1C. Diabetes Care. 2011. May 1;34(Supplement 2):S184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karnchanasorn R, Huang J, Ou H-Y, Feng W, Chuang L-M, Chiu KC, et al. Comparison of the Current Diagnostic Criterion of HbA1c with Fasting and 2-Hour Plasma Glucose Concentration. J Diabetes Res. 2016;2016:6195494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glesby MJ, Hoover DR, Shi Q, Danoff A, Howard A, Tien P, et al. Glycated Hemoglobin in Diabetic Women with and Without HIV Infection: Data from the Women’s Interagency HIV Study. Antivir Ther. 2010;15(4):571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.