Summary
Background
An intravaginal ring (IVR) that releases the tenofovir prodrug, tenofovir disoproxil fumarate (TDF), provided 100% protection in macaques against simian human immunodeficiency virus and was safe in a 14-day clinical study in sexually abstinent women. We aimed to assess the safety and pharmacokinetics of this TDF IVR over 90 days in sexually active women.
Methods
We conducted a Phase 1, single-blind, randomised, placebo-controlled trial to evaluate safety, pharmacokinetics, and acceptability of a TDF IVR used continuously with monthly ring changes for three months. Sexually active women who were HIV negative were randomised 3:1 to TDF or placebo ring. Primary safety endpoints were the proportion of women who experienced Grade 2 or higher genitourinary adverse events (AEs) judged related to study product and any Grade 2 or higher AE as defined by the Division of AIDS Table for Grading the Severity of Adult and Pediatric AEs. We quantified TDF and tenofovir concentrations in cervicovaginal fluid (CVF), tenofovir in plasma, and tenofovir diphosphate (TFV-DP), the active metabolite, in cervical tissue and dried blood spots one month after each ring insertion. We compared changes over time in CVF cytokine and chemokine concentrations by Luminex assay and vaginal microbiota using 16S rRNA gene PCR and deep sequencing in each group. CVF cytokine and chemokine concentrations were compared with Friedman’s test. Holm-Bonferroni adjustments were applied for post-hoc comparisons between time-points. The study was electively terminated early and is registered with ClinicalTrials.gov, number .
Findings
Between February 24, 2017 and July 20, 2017, 17 of 40 women (12 in the TDF and 5 in the placebo group) were enrolled in Bronx, New York before study termination. Two TDF participants completed 3 months of continuous ring use; eight TDF participants were asked to discontinue ring use early because of ulcerations (Grade 1) near the ring; rings were electively removed by study staff on day 20 and day 23 in the remaining two women in the TDF group. Ulcers were detected on average 32 days after ring use (range 23–56). Four of eight with ulcers were symptomatic with vaginal discharge; four had ulcers identified when examined; three had two ulcers; all ulcers resolved after ring removal. No participant in the placebo arm developed ulcers. There were no Grade 2 product-related AEs in either group and 4 non-product related Grade 2 AEs in the TDF group. CVF tenofovir concentrations did not differ at day 14 (p=0·14) comparing those who did (n=8; median 1·0 × 105 ng/mL [IQR 9·1 × 104–1·1 × 105]), or did not (n=4, 6·0 × 104 ng/mL [IQR 5·6 × 104–1·1 × 105]) develop ulcers. There were no significant changes in vaginal microbiota in either group. Multiple inflammatory cytokines and chemokines were significantly higher at days 14 and 28 compared to baseline in the TDF but not placebo arm.
Interpretations
Future studies are needed to determine if the unanticipated finding of ulcerations is specific to this TDF ring or generalizable to other sustained topical release formulations of tenofovir and/or its prodrugs.
Introduction
Nearly half of the 36·7 million people living with HIV worldwide are female and adolescent girls and young women are among the most vulnerable to HIV.1 While oral PrEP has demonstrated effectiveness in men who have sex with men and in HIV-serodiscordant couples and vaginally delivered PrEP has shown some efficacy in subpopulations of women, clinical trial results in adolescent girls and young women have been disappointing. These discrepant results indicate that there are biological, virological, and/or behavioral factors that limit efficacy. For example, although a 39% reduction in HIV acquisition was observed with pericoital dosing of 1% vaginal tenofovir (TFV) gel2, neither TFV gel, oral tenofovir disoproxil fumarate (TDF), or the oral combination of TDF and emtricitabine (Truvada) were protective in subsequent Phase 3 trials.3,4 Similarly, although a dapivirine intravaginal ring (IVR) provided overall 27% and 31% HIV protection, respectively, in two Phase 3 trials, there was no protection in women below the age of 21 (−27% [−133 to 31], p=0·45).5,6 Poor adherence, which was assessed by quantifying plasma TFV levels or residual dapivirine in used rings, respectively, contributed to the outcomes.3,5,7 However, even with high adherence protection was incomplete.
The ability of PrEP to protect against HIV depends on virological factors (viral load and transmission efficiency), host susceptibility, and drug potency and pharmacokinetics (PK). TFV enters human vaginal/cervical epithelial and immune cells by an endocytic pathway whereas the prodrug, TDF, passively diffuse into cells.8 These differences in transport mechanisms are reflected in the relative potency; TDF inhibits HIV-1 and herpes simplex virus (HSV) at 100-fold lower concentrations than TFV.9,10 Once inside a cell, TDF is hydrolyzed by carboxylesterases to the monoprotected moiety and then by phosphodiesterases into TFV, which is subsequently phosphorylated by kinases to tenofovir diphosphate (TFV-DP). TFV-DP has a long intracellular half-life and competes with cellular 2’-deoxyadenosine triphosphate for incorporation into the viral DNA chain. TDF that is not metabolized intracellularly passively diffuses out of cells where it is hydrolyzed to TFV within vaginal fluid. The PK of dapivrine, a non-nucleoside reverse transcriptase inhibitor that blocks HIV, but not HSV replication, are much simpler. Dapivirine passively diffuses into and out of cells without modifications, and thus has a short intracellular half-life compared to TFV-DP. These pharmacological differences also contribute to the ability of the vaginal environment and its microbiota to modulate drug PK. For example, TFV endocytosis is reduced as the pH increases to >6·0, a pH common in the presence of anaerobic dysbiosis, compared to uptake at a healthy vaginal pH of 3.5–4.5.10 In addition, Gardnerella (G.) vaginalis and other bacteria secrete adenine, which competitively inhibits TFV endocytosis.10. G. vaginalis may also degrade TFV.11 Dapivirine binds irreversibly and nonspecifically to individual bacteria and semen, which renders it less forgiving with intermittent adherence.10,12 If a dapivirine ring is removed, the drug will rapidly diffuse out of cells where it may be sequestered by bacteria or semen leaving the cells unprotected. In contrast, neither pH nor microbiota adversely impact passive diffusion of TDF into cells and intracellular TFV-DP levels persist for days.10
Thus, given the realities of incomplete adherence and these different PK properties, we hypothesized that a sustained release formulation of TDF would provide greater and more consistent HIV protection than TFV or dapivirine, as well as the potential added benefit of HSV prevention. A reservoir polyurethane IVR was engineered to deliver 5–7 mg/day of TDF for one month based on in vitro release studies.13 Macaque-sized rings completely protected non-human primates from repeated low-dose challenges with simian-HIV and provided significant protection in a stringent model of medroxyprogesterone-treated macaques.13,14 In a first-in-human clinical study, the TDF ring was well tolerated in sexually abstinent women with 14 days of continuous use.15 Building on these early results, we initiated a Phase 1 randomised, placebo-controlled trial of three months of continuous TDF versus placebo ring use in sexually active women with monthly ring changes.
Methods
Study design and participants
A placebo-controlled trial was designed to evaluate the safety, PK, and acceptability of a TDF ring in sexually active women (minimum of four vaginal sex acts per month) who were using any form of hormonal contraception exclusive of an IVR. The planned enrollment was 80 women at two sites (Albert Einstein College of Medicine in Bronx, New York, USA and Partners in Health Research and Development site in Thika, Kenya) with an initial review of one-month safety data in 20 US women prior to enrollment at Thika. Women were randomised 3:1 to TDF or placebo ring with monthly ring changes and study visits every two weeks. The study was approved by the Albert Einstein College of Medicine Institutional Review Board and registered in clinicaltrials.gov (). All participants provided written informed consent. Exclusion criteria included pregnancy, breastfeeding, HIV, menopause, intermenstrual bleeding, use of antiretrovirals, sex partner with HIV, sexually transmitted infection (STI) within three months of screening, abnormal Pap test, active hepatitis B, and abnormal renal or liver function.
Procedures
At screening, participants had a urine pregnancy test, gynecological examination, Pap and nucleic acid amplification testing (NAAT) for Neisseria gonorrhoeae, Chlamydia trachomatis and Trichomonas vaginalis (Gen-Probe, Inc., San Diego, CA). Vaginal swabs were collected for pH (Whatman pH paper) and Nugent scoring. Blood was collected for HSV-1 and HSV-2 antibodies (BioPlex 2200, Bio-Rad Laboratories, Inc., Hercules, CA), hepatitis B serologies, complete blood count, kidney and liver function tests. An oral swab was collected for the OraQuick ADVANCE Rapid HIV-1/2 Antibody Test (OraSure Technologies, Inc., Bethlehem, PA).
Enrollment (visit 2) occurred within 28 days of screening. HIV and pregnancy testing were repeated and vaginal swabs were collected for pH, Nugent score, and vaginal microbiota. Additional swabs were individually held at the proximal vaginal walls near the cervix for two minutes for assessment of baseline drug levels and immune mediators. Additional STI testing was performed if women developed symptoms including vaginal discharge or genital ulcers.
Rings were designed as previously described15 and manufactured by Particle Science (Bethlehem, PA). TDF and placebo rings were formulated using hydrophilic elastomer HydroThane AL 25–93A tubing (AdvanSource Biomaterials, Inc., Wilmington, MA). The inner core compartment of TDF IVRs was comprised of 360 mg TDF and 55 mg sodium chloride (NaCl). The placebo rings contained 55 mg of NaCl. The ring dimensions were comparable to those of the commercially available NuvaRing with outer and cross-sectional diameters of 55 and 5.5 mm respectively. The daily TDF in vitro release rate was 5.5 ± 1.5 mg/day in acetate buffer at pH 4.2 and 37°C.
Randomisation and masking
The first ring was inserted at enrollment and scheduled for clinician replacement every 30 days. A block randomization scheme with a block size of four was computer generated by the study statistician. Participants and laboratory staff were blinded to treatment arm, but the study was not double-blinded since the TDF and placebo rings differ in appearance (drug versus only NaCl filled). Study visits 3–8 were scheduled every two weeks to assess safety. Visit 9 was scheduled five to seven days after the third ring removal. Sampling at each of these visits included blood for PK and vaginal swabs for pH, Nugent, PK and immune mediators. Additional swabs for vaginal microbiota were collected at days 28, 56, 84 and days after final ring removal. Two ectocervical tissue biopsies and dried blood spots (DBS) were collected monthly. Participants were randomised to additional PK sampling at one of six time-points (1 hour, 4 hours, 1 day, 1 week, 2 weeks, or 3 weeks after initial ring insertion). Adverse events (AEs) were collected at each visit.
Outcomes
The primary safety endpoints were the proportion of women who experienced Grade 2 or higher genitourinary AEs judged related to study product as defined by the Female Genital Grading Table for Use in Microbicide Studies and any Grade 2 or higher AE as defined by the Division of AIDS Table for Grading the Severity of Adult and Pediatric AEs (https://rsc.niaid.nih.gov/clinical-research-sites/daids-adverse-event-grading-tables). For vaginal lesions, Grade 0 was defined as normal variants (skin tags, moles, scars, etc); Grade 1 AEs were mild ulcerations with no treatment indicated; Grade 2 moderate, treatment indicated; Grade 3 severe epithelial disruption, requiring hospitalization. Secondary PK endpoints included measurement of TDF and TFV levels in cervicovaginal fluid (CVF), TFV in plasma, and TFV-DP in DBS and cervical tissue one month after ring insertion. We assessed ring acceptability as a secondary endpoint, which we plan to report in a separate manuscript. Pre-specified exploratory outcomes included assessment of the vaginal microbiota and CVF immune mediators before, during, and after ring use. Post-hoc analyses included evaluation of changes in ectocervical tissue gene expression (RNA-Seq).
Cervical tissue and swab-collected fluids were weighed, placed in separate cryovials, flash frozen in liquid nitrogen and stored at −80°C prior to analysis. Tenofovir disoproxil and TFV concentrations were quantified using liquid chromatographic-tandem mass spectrometric (LC-MS/MS) methods.16 The lower limits of quantification (LLOQ) for TFV in plasma, tissue, and CVF are 0·31 ng/mL, 0·25 ng/sample, and 0·625 ng/swab, respectively. For tenofovir disoproxil, the LLOQ was 0·0625 ng/swab. For swabs and tissue, concentrations were converted to ng/mg based on the net weight of the specimen swab or biopsy. CVF drug concentrations were converted to ng/mL. Values below the LLOQ were reported as below the limit of quantification. TFV-DP concentrations were also measured in tissue homogenates using validated LC-MS/MS assays with LLOQ of 50 fmol/sample.16 Final concentrations were converted to ng/mg based on the net weight of tissue. TFV-DP levels in DBS were measured as described with LLOQ of 25 fmol/sample.15,17 After ring removal, residual drug was assayed as described.9
Concentrations of IL-1α, IL-1β, IL-8, IP-10 (CXCL10), MIG (CXCL9), MIP-1β (CCL4), RANTES (CCL5), GM-CSF, IL-17, TNF-α, IL-6, IL-10, MCP-1 (CCL2), MIP-3α (CCL20) in vaginal swabs were determined by Luminex (Luminex Corp., Austin, TX) with beads from Chemicon International (Billerica, MA) and analyzed using StarStation (Applied Cytometry Systems, Sacramento, CA). Concentrations below the LLOQ were set at the midpoint between zero and the LLOQ.
DNA was extracted from vaginal swabs using the MoBio Bacteremia extraction kit (MoBio, Carlsbad, CA). Broad-range PCR targeting the V3-V4 hypervariable region of the 16S rRNA gene coupled with sequencing on the Illumina MiSeq instrument (Illumina, San Diego, California) was performed to characterize the vaginal microbiota.18 Sequences were classified using the phylogenetic placement tool pplacer and a curated reference set of vaginal bacteria.19,20 An average of 42,400 reads per sample was generated. Alpha diversity was measured using Shannon Diversity Index and beta diversity is represented using a nonmetric multidimensional scaling (MDS) plot using implementations of the R microbiome package (http://microbiome.github.io/microbiome/).
RNA-Seq and bioinformatics were performed at the Yerkes NHP Genomics Core at Emory University (http://www.yerkes.emory.edu/nhp_genomics_core/). A section of 48 snap frozen ectocervical tissue was cut and placed into 350μl of RLT lysis buffer (QIAGEN) and 1% 2-mercaptoethanol (Sigma-Aldrich). The tissues were bead milled with a stainless-steel bead and Tissuelyser II (QIAGEN) and RNA extracted using RNeasy Micro Kit with DNase digestion (QIAGEN). RNA quantity and quality were assessed using Nanodrop 2000 Spectrophotometer and Agilent’s 4200 Bioanalyzer Capillary electrophoresis. 1ng of total RNA was used for mRNA amplifications using Clontech Smarter V4 chemistry (Takara Bio Inc). Amplified mRNA was fragmented and barcodes appended using Illumina’s Nextera XT kits. Amplified libraries were validated using an Agilent 4200 Tapestation and quantified using a Qubit fluorimeter. Libraries were normalized, pooled and clustered on an Illumina HiSeq 3000/4000 flowcell using an Illumina cBOT. Libraries were sequenced on an Illumina HiSeq 3000 system in 101-base single-read reactions with multiplexing to achieve approximately 20 million reads per sample. Reads were aligned to the Human Reference Genome Sequence and transcripts annotated with Genome Reference Consortium Build 38 (GRCh38) using STAR software (v2·5·2b).21 Unsorted bam files were sorted and indexed using samtools and converted to HTSeq-count format. Estimates of gene-wise and isoform-wise expression levels for individual genes were performed using the R package DESeq2 with R version 3·5·0.22 Differentially expressed transcripts (n=457, p≤0·02, FC>1·5) between TDF and placebo recipients were identified by negative binomial generalized linear models (DESeq2). To identify pathways differentially modulated between TDF and placebo recipients, Gene Set Enrichment Analysis (GSEA) was performed using the desktop module available from the Broad Institute.23 For each contrast, transcripts were ranked by differential expression using the Signal2Noise metric and GSEA was performed on the ranked transcript lists using 1,000 gene set permutations, collapse of duplicates to Max probe, and random seeding. Gene sets used included the MSigDB Hallmark and Gene Ontology Biological Processes23 and co-transcriptional networks (Blood Transcriptome Modules).24
Statistical analysis
Demographic and clinical characteristics were compared between TDF or placebo ring participants using Fisher’s exact tests for categorical variables. Continuous data were not normally distributed, therefore, we tested for differences between groups using the non-parametric Mann Whitney U test. Vaginal pH, Nugent score, cytokine and chemokine concentrations were compared with Friedman’s test to examine changes over time while accounting for repeated measures taken from the same women. Holm-Bonferroni adjustments were applied for post-hoc comparisons between time-points. Alpha diversity between the TDF and placebo groups was compared using the Wilcoxon rank-sum test. Statistical analyses were performed using GraphPad Prism Version 7 (GraphPad Software, La Jolla, CA) and Stata Version 15·1 (StataCorp LLC, College Station, TX).
The sample size of 40 women at each of 2 sites was chosen with the goal of achieving 32 with complete data (24 TDF and 8 placebo). An interim safety analysis was planned when 20 US women completed one month of ring use. A Data Safety Monitoring Committee (DSMC) reviewed all AEs monthly and when requested by the study team.
Role of the funding source
The funder of the study had a role in the study design but had no role in data collection, analysis, and interpretation, or writing of this report. All authors had final responsibility for the decision to submit for publication.
Results
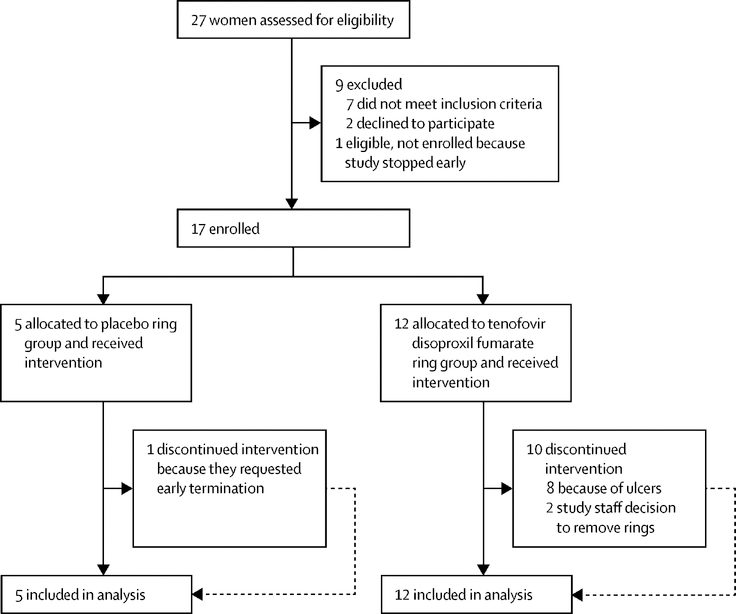
Between February 24, 2017 and July 20, 2017, we assessed 27 women for eligibility and randomly assigned 17 participants to either TDF (n=12) or placebo (n=5) IVR. There were no statistically significant demographic or clinical differences in participants assigned to each arm except the number of reported sex acts in the month prior to screening was higher in the placebo arm (Table 1 and Figure 1). Only two women in the TDF arm (Participant ID 902 and 903) completed three months of ring use. Eight women who were randomised to the TDF IVR developed Grade 1 genital ulcers, which occurred on average 32 days after first ring insertion (range 23–56 days). Because of this unanticipated finding, the study team, in collaboration with the DSMC, elected to remove the rings from the other two actively enrolled participants in the TDF arm (919, 921) on days 20 and 23, respectively, and to discontinue the trial. None of the women randomised to the placebo ring developed ulcers. Four placebo ring participants completed 3 months of continuous ring use and one discontinued the study at day 67 because of unanticipated travel. All participants were followed until October 17, 2017.
Table 1.
Demographic and clinical characteristics of participants
| TDF (n=12) | Placebo (n=5) | |
|---|---|---|
| Age, years | 28·3 (22·2–33·5) | 33·6 (28·8–38·1) |
| Race | ||
| White | 8 (67) | 2 (40) |
| Black | 3 (25) | 0 |
| Asian | 0 | 1 (20) |
| >1 race | 1 (8) | 0 |
| Not reported | 0 | 2 (40) |
| Ethnicity | ||
| Hispanic | 2 (17) | 1 (20) |
| Non-Hispanic | 10 (83) | 4 (80) |
| Relationship status | ||
| Married | 1 (8) | 2 (40) |
| Single | 2 (17) | 1 (20) |
| Cohabitating | 5 (42) | 1 (20) |
| In relationship, not living together | 4 (33) | 1 (20) |
| Contraception | ||
| Oral contraceptive pills | 7 (58) | 3 (60) |
| DMPA | 1 (8) | 1 (20) |
| Intrauterine device | 2 Copper, 1 LNG (25) | 1 LNG (20) |
| Implant | 1 (8) | 0 |
| Self-report of condom use | ||
| Never | 8 (67) | 4 (80) |
| Sometimes | 4 (33) | 1 (20) |
| Sex acts month prior to screening | 6·5 (4·2–10) | 12(10·5–17·5) |
| Partner circumcised | 7 (58) | 4 (80) |
| History of receptive anal intercourse | 6 (50) | 4 (80) |
| History of STI | 4 (33) | 1 (20) |
| HSV- 2 seropositive | 2 (17) | 1 (20) |
| Prior NuvaRing use | 2 (17) | 0 |
| Ever pregnant | 3 (25) | 2 (40) |
| Vaginal pH at enrollment | 4·6 (3·8–5·1) | 4·9 (3·9–5·5) |
| Nugent score at enrollment | 2·5 (1–3·2) | 7(0·5–8) |
n, number; TDF, tenofovir disoproxil fumarate; DMPA; depot medroxyprogesterone acetate; LNG, levonorgestrel; STI, sexually transmitted infection; HSV-2 herpes simplex virus type 2
Categorical variables reported as number (%); continuous variables reported as median (interquartile range [IQR])
Figure 1:
Participant recruitment and follow-up.
All ulcerations occurred near the ring at the apex of the vagina and lateral to the cervix. All ulcers resolved over time after ring removal (Table 2). Three women had two ulcers (one on each vaginal wall), and one had both a vaginal and cervical ulcer. The ulcers ranged in size from 2 mm-2 cm in length, 2 mm-1.5 cm in height, and 1–5 mm in diameter. Four women with ulceration were symptomatic with vaginal discharge (904, 915, 916, 920). The other 4 were asymptomatic and had ulcers identified at scheduled study visit examinations. Participants 908, 915 and 920 had vaginal swabs tested for HSV DNA by PCR at the time ulcers were observed; results were negative. All participants with vaginal discharge had additional testing including wet mount and NAATs for gonorrhea, chlamydia and trichomonas. All results were negative (Table 2).
Table 2.
Participants with premature TDF ring discontinuation
| ID | Ulcer Onset | Day IVR Terminated | Ulcer Resolution | Symptoms | Comment |
|---|---|---|---|---|---|
| 904 | Day 34 | Day 45 | Day 38 | Vaginal discharge | 2nd ring was removed for ulceration; replaced on Day 38; erythema on Day 42; ring electively removed |
| 908 | Day 56 | Day 56 | Day 65 | Vaginal discharge | 2nd ring removed, not replaced at scheduled visit |
| 912 | Day 31 | Day 28 | Day 36 | None | 2nd ring replacement delayed for erythema; ulcer identified on follow up visit; no 2nd ring placed |
| 915 | Day 28 | Day 28 | Day 42 | Vaginal discharge | Ring 1 removed as scheduled, not replaced |
| 9161 | Day 28 | Day 28 | Day 34, Day 44 | Vaginal discharge | Ring 1 removed as scheduled, not replaced |
| 918 | Day 23 | Day 23 | Day 36 | None | Ring 1 removed early for ulcer noted at scheduled visit |
| 919 | No ulcer | Day 23 | Vaginal discharge | Ring removed preemptively by study staff | |
| 920 | Day 29 | Day 29 | Day 72 | Vaginal discharge | Ring 1 removed as scheduled, not replaced |
| 921 | No ulcer | Day 20 | None | Ring removed preemptively by study staff | |
| 922 | Day 29 | Day 29 | Day 43 | None | Ring 1 removed as scheduled, not replaced |
916 had resolution of one ulcer on Day 34 and second ulcer on Day 44
The frequency of vaginal sex reported during the first month of ring use did not differ between women with (median 5, interquartile range [IQR] 3–8) or without (median 6, IQR 4–7) ulcers or between women in the TDF (median 6, IQR 4–8) versus placebo (median 6, IQR 4–6) arm.
Overall, 57 AEs (including the 12 genital ulcers) occurred in 12 women assigned to the TDF ring and 13 AEs occurred in the five placebo ring recipients (Table 3, appendix p 1). Most AEs involved the reproductive tract and 36 were judged to be product-related. One reproductive tract AE was related to study procedures (bleeding after biopsy). All product-related AE were mild (Grade 1). There were four Grade 2 AEs that were judged not to be product-related and no Grade 3, Grade 4 or serious AEs. There were three laboratory abnormalities, which were Grade 1 and judged not to be product-related in one TDF and two placebo participants.
Table 3.
Summary of adverse events
| TDF | Placebo | |
|---|---|---|
| All adverse events | 57/70 (81%) | 13/70 (19%) |
| Grade 1 | 53/70 (76%) | 13/70 (19%) |
| Grade 2 | 4/70 (6%) | 0 |
| Product or procedure-related genitourinary adverse events | 33/70 (47%) | 4/70 (6%) |
| Cervical discharge | 2/33 (6%) | 0 |
| Cervical ulcer | 1/33 (3%) | 0 |
| Dyspareunia | 1/33 (3%) | 0 |
| Postcoital bleeding | 1/33 (3%) | 0 |
| Vaginal bleeding | 1/33 (3%) | 0 |
| Vaginal dryness | 0 | 2/4 (50%) |
| Vaginal discharge* | 7/33 (21%) | 2/4 (50%) |
| Vaginal erythema | 8/33 (24%) | 0 |
| Vaginal itching | 1/33 (3%) | 0 |
| Vaginal ulcer | 11/33 (33%) | 0 |
| Common adverse events not related to study product | 23/70 (33%) | 7/70 (10%) |
| Gastrointestinal disorders | 1/23 (4%) | 2/7 (29%) |
| Respiratory disorders | 5/23 (22%) | 1/7 (14%) |
| Psychiatric disorders | 1/23 (4%) | 0 |
| Reproductive system and breast disorders | 3/23 (13%) | 2/7 (29%) |
| Renal and urinary disorders | 5/23 (22%) | 0 |
| Eye disorders | 3/23 (13%) | 1/7 (14%) |
| Nervous system disorders | 1/23 (4%) | 0 |
| Infections and infestations | 4/23 (17%) | 0 |
| Musculoskeletal disorders | 0 | 1/7 (14%) |
| Laboratory abnormalities | 1/70 (1%) | 2/70 (3%) |
| Elevated ALT | 0 | 1/70 (1%) |
| Elevated non-fasting glucose | 0 | 1/70 (1%) |
| Reduced hemoglobin | 1/70 (1%) | 0 |
TDF=tenofovir disoproxil fumarate. ALT=alanine transferase
5 were self-reported (3 TDF, 2 Placebo) and not observed on exam
Extracellular TDF is hydrolyzed to TFV by vaginal fluid and thus provides a more consistent measure of extracellular drug. CVF TFV concentrations on day 14 and 28 were similar in the current study to levels observed on day 14 in the prior two-week study in sexually abstinent women. The levels did not differ comparing those who did (day 14, n=8; median 1·0 × 105 ng/mL [IQR 9·1 × 104-1·1 × 105] and day 28, n=7; median 6·0 × 104 ng/mL [IQR 3·2 × 104-9·6 × 104]) or did not (day 14, n=4; median 6·0 × 104 ng/mL [IQR 5·6 × 104-1·1 × 105] and day 28, n=2; median 1·1 × 105 ng/mL [IQR 1·1 × 105-1·2 × 105]) develop ulcers (p=0·14 for day 14 and p=0·22 for day 28) (Figure 2A). The tissue TDF-DP levels were more variable, possibly reflecting the smaller sample size of biopsy tissue available at each time-point (Figure 2B) but were also similar to tissue concentrations detected on day 14 in the previous study in sexually abstinent women.15 Based on drug recovered from used rings, the calculated average in vivo release rate was 7·1 mg/day (IQR 5·6–7·9) (appendix p 2). Plasma levels were low but detectable in all 9 women who had a TDF ring in place on day 28 and TFV-DP in RBCs, a potential biomarker of recent and cumulative adherence, was also detected in these 9 participants. Additional PK data is summarized in appendix p 2.
Figure 2: Tenofovir and tenofovir diphosphate levels in vaginal swab fluid and ectocervical tissue, respectively.
(A) Secretions were collected by swabbing the vaginal wall proximal to intravaginal ring and assayed for tenofovir concentrations (ng/ml). (B) Ectocervical tissue was obtained at indicated time-points and assayed for tissue tenofovir-diphosphate (fmol per mg tissue). Each colored symbol represents results from indicated participant. A horizontal black line inside a colored symbol represents participants who developed an ulcer during the study period.
To explore potential mechanisms that may have contributed to ulcerations in women randomised to TDF IVR, differences in genital tract pH, microbiota, cytokines, chemokines and gene expression were assessed. There were significant increases in vaginal pH (p=0.049) and Nugent score (p=0.01) over time in the TDF, but not placebo group, when comparing day 0, 14 and 28. In post-hoc analyses, there were significant differences comparing day 28 to day 0 for vaginal pH (p=0.03) and Nugent score (p=0.01) (appendix p 3). However, the magnitude of these differences was small. For example, the median Nugent score in women randomised to the TDF ring increased from a baseline of 2.5 [1.0–3.2] to 4.0 [3.2–5.0] on day 28. No major shifts in the vaginal microbiota were noted when we compared the relative abundances of bacterial taxa using 16S rRNA gene PCR coupled with deep sequencing on day 28 (range day 26–30) to enrollment in either the TDF or placebo arm (Figure 3) (sequencing data was not available for one TDF participant). Among the TDF ring participants who developed ulcers, two (904, 908) maintained L. iners-dominant and one (912) maintained L. crispatus-dominant communities throughout the study; one (915) shifted from L. iners dominance to a more diverse community; two (916, 920) had higher diversity with the appearance of several BV-associated bacteria, and in one (922), the microbiota shifted from G. vaginalis and Atopobium vaginae dominance to L. crispatus dominance. Among the five placebo participants, three had diverse vaginal bacterial communities at enrollment (reflected in their higher Nugent scores) and maintained these diverse communities and two had Lactobacillus-dominant communities which were maintained throughout the study.
Figure 3: Relative abundances of bacteria detected in vaginal swabs in participants by study arm.
Colored bars represent the top 30 most abundant bacteria across all samples. Column order for each participant includes indicated visits by day (day 0 representing enrollment). Vaginal pH, Nugent scores, and alpha diversity are indicated below the heat map and presence or absence of vaginal ulcer are indicated above the heat map.
The Shannon Diversity Index, which provides a measure of the number of bacterial taxa and the evenness of distribution of these taxa, was not significantly different in either the placebo or the TDF groups between visits 2 and 4 (Placebo group: median 1.5 at visit 2 versus 1.52 at visit 4, p=0.75; TDF group: median 0.6 at visit 2 versus 0.63 at visit 4, p=0.28) (Figure 3, appendix p 18). Large shifts in the bacterial community were noted in two participants in the TDF arm, but the composition shifted in opposite directions. Participant 920 went from a Lactobacillus-dominant to a diverse microbiota, while participant 922 went from a diverse microbiota to a Lactobacillus-dominant microbiota after use of the TDF ring for a month (appendix p 19).
Although there was, as demonstrated in prior studies25, intersubject variability in the baseline levels of cytokines and chemokines in CVF, the median concentrations of IL-1α, IL-1β, TNF-α, GM-CSF, IL-6, IL-8, CXCL9, CXCL10, RANTES, MIP-1β, MIP-3α, MCP-1, IL-10 and IL-17 were significantly increased on day 14 and/or day 28 compared to enrollment in the participants randomised to the TDF (including participants 902 and 903), but not the placebo ring (Figure 4). Most of the mediators returned to baseline by day 84, although IL-8, IL-1β, CXCL9, and RANTES remained significantly higher relative to enrollment.
To further compare the host response to the TDF versus placebo ring, RNA sequencing of available ectocervical tissue was performed. This included tissue from the following time-points in the TDF arm: 1h (n=1), 4h (n=2), 24h (n=1), day 8 (n=1), day 14 (n=2), day 20–21 (n=4), day 26–30 (n=5); day 56–58 (n=3 including participants 902 and 903); and Day 84–86 (n=11), which corresponds to approximately two months after ring removal in nine women and a few days after ring removal in Participants 902 and 903 who never developed ulcers. Tissue was available from the following placebo ring participants: day 28 (n=5), day 56 (n=5), day 84–85 (n=3) and n=1 each at 1h, 24h, day 15 and day 21. Per protocol, ectocervical tissue was intended for PK and tissue was not collected prior to initial ring insertion (baseline) or from ulcerative lesions.
As differences in CVF cytokines were most pronounced at ~day 28 and because of the small samples size at earlier time-points, we conducted a cross-sectional analysis of the RNA-Seq data contrasting day 26–30 samples from TDF with placebo. 457 genes were significantly differentially expressed between TDF and placebo recipients at these time-points (232 higher and 225 lower) (Figure 5A and appendix p 4). However, when the transcriptome was ranked for GSEA, significantly enriched pathways were only identified containing genes expressed at higher levels in TDF compared to placebo recipients. This included genes associated with inflammation, interferon responses and T lymphocyte activation and proliferation (Figure 5B and 5C and appendix p 17). Inflammatory and T cell enrichment gene expression peaked on day 28, were elevated in women who did (908, 920) and did not develop ulcers (902, 903, 919), and generally returned to pre-day 28 levels by day 84–85 except in subject 903 who maintained ring use until the final biopsy (Figure 5D). Genes driving enrichment of inflammatory pathways (Leading Edge Genes) included cytokines such as IL-6 and IL-15, and chemokines such as CXCL10, CCL5, CCL7, and CCL17 (Figure 5D). Genes driving enrichment of T cell activation included T cell receptor components and signaling molecules (CD3D, CD3G, CD3E, TRAC, TRBC1, LCK, ZAP70, ITK, SLAMF1), costimulatory molecules (ICOS, CD28), transcription factors (EOMES), T cell effector molecules, chemokines, and cytokines (PRF1, GZMB, GZMK, GZMA, XCL1) (Figure 5D, bottom panel).
Several genes associated with the NLRP2 inflammasome were found in the leading edge of significantly enriched pathways, including NLRP2, IL1B, CASP1and CASP4. These were expressed at higher levels in TDF as compared to placebo recipients at day 26–30, and NLRP2, CASP1 and CASP4 also showed trends of elevated expression at time- points preceding day 26–30 (appendix p 17). Although IL1B gene expression was not obviously elevated prior to day 26–36, IL-1β cytokine concentrations were elevated in CVF at day 14. These data suggest the observed inflammatory response may be mediated by the NLRP2 inflammasome and activated prior to day 26–30.
Discussion
Despite extensive preclinical safety data, a three-month trial was electively terminated early after enrolling 17 of a planned 80 participant cohort because eight of the twelve women in the TDF arm developed Grade 1 vaginal ulcers. The TDF, but not the placebo ring was associated with increases in inflammatory cytokines/chemokines in vaginal swabs and inflammatory gene expression in tissue in women with and without ulcerations. On average, there was 5–15-fold increase in the majority of cytokines/chemokines detected in the CVF samples in women randomised to the TDF ring. These findings were not predicted by the preclinical macaque studies where no ulcerations and no consistent changes in cytokine/chemokine patterns in genital tract secretions were observed over a six-month period of ring use, although gene expression was not assessed.26 The findings were also not predicted by a previous 14-day study in sexually abstinent women who used this same TDF ring with no safety concerns, although neither CVF cytokines nor genital tissue gene expression were measured.
Precisely why the TDF, but not the placebo ring was associated with the development of vaginal ulcers remains uncertain, but the findings suggest several possibilities. Unlike most Phase 1 studies, this study intentionally recruited sexually active women (minimum of four vaginal sex acts per month). The first-in-human 14-day study was conducted in sexually abstinent participants and the preclinical macaque studies were in the absence of simulated sex.15,26 Thus, one possibility is that microabrasions associated with sex and the physical presence of the ring combined with the drug or one of its metabolites, to inhibit healing or promote an inflammatory response.
The CVF TFV concentrations were comparable on days 14 and 28 and similar to levels observed in the prior 14-day TDF ring study in sexually abstinent women. Moreover, the tissue TFV-DP concentrations on day 28 in this study were similar to tissue concentrations detected on day 14 in the previous study.15 Thus, it seems unlikely that excessively high drug levels contributed to the observed toxicity. Additionally, the CVF TDF levels were similar to the previous study on day 14 and lower on day 28. The reason for this decrease in CVF TDF levels on day 28 is unclear as most other PK measures did not change to this degree in parallel. The drop may reflect a reduction in drug release towards the end of the 30-day period, but would not have contributed to the observed toxicity. TDF contains equimolar amounts of tenofovir disoproxil and fumaric acid and generates two molecules of formaldehyde. The possibility that fumaric acid or formaldehyde, which is rapidly converted to formic acid, contributed to the adverse outcomes cannot be excluded and assays to quantify these molecules are not available. However, one might have expected a decrease in vaginal pH if there was substantial accumulation of these acids.
Several lines of evidence suggest that uninterrupted intracellular exposure to the drugs, and in particular to intracellular TFV-DP, may have contributed to the development of vaginal ulcers. This notion is supported by in vitro studies and clinical experiences with related products, which suggest that sustained intracellular levels of TFV-DP may induce inflammatory responses and/or interfere with epithelial cell repair. For example, a recent study demonstrated that in vitro exposure of primary genital epithelial cells and fibroblasts to TFV or TFV alafenamide (TAF) (at doses designed to yield similar concentrations of intracellular TFV-DP) resulted in significant impairment of wound healing.27 Moreover, changes in gene expression similar to those reported in this study were observed in a prior clinical study of global gene expression by oligo-nucleotide microarray analysis in rectal biopsies obtained after seven daily doses of rectally applied 1% TFV gel.28 Although the time-points and tissues analyzed in the latter study differed from the current study, there was substantial overlap in genes upregulated in response to TFV gel and the TDF ring (appendix p 17). These included cytokine and chemokine genes (CCL2 and CCL19) associated with inflammation, interferon response genes (IFI27, IFIT1, MX1, GBP2) associated with T cell activation and maturation (CD3D, GZMB, SELL, CD7), as well as genes associated with tissue remodeling and wound repair (MMP9 and MMP12). The authors also observed similar differences in vaginal cells treated in vitro with TFV.28 The notion that the toxicity observed in this study may be mediated by TFV-DP is further supported by studies with a related nucleotide analogue, cidofovir. In a clinical trial of 1% topical cidofovir cream for human papillomavirus disease, 13 of 33 participants developed mild to moderate ulcerations.29
Although these findings suggest that the toxicities observed may be linked to sustained intracellular levels of TFV-DP, other possibilities cannot be excluded. It is unlikely that the polymer was directly toxic since the placebo ring, which is comprised of the same material, was not associated with ulcerations. However, an interaction between the polyurethane and TDF or one of its metabolites that was not detected in preclinical studies including a rabbit vaginal irritation study, could have contributed to inflammation and ulceration. Toxicities did not appear to be linked to changes in the vaginal microbiota as no consistent changes in microbiota were observed.
Our data suggest that caution with other sustained release formulations of TFV or its prodrugs (TDF and TAF) is warranted.30 Results also indicate the need for direct comparisons of different TFV-based sustained delivery products to determine the cause of the unanticipated and disappointing outcome with this TDF ring. Further, our data also suggest that while the non-human primate model provides insights into potential efficacy, it may not be optimal for predicting safety. Indeed, the TDF ring was highly protective in rigorous macaque challenge studies and also prevented HSV in murine models of HIV-HSV coinfection.13,14,31 Moreover, safety results obtained in short-term studies with abstinent women may not predict results with more prolonged drug exposure in sexually active women.
Supplementary Material
Research in context.
Evidence before this study
Daily oral pre-exposure prophylaxis (PrEP) with tenofovir disoproxil fumarate (TDF) and emtricitabine is recommended by WHO and the US Centers for Disease Control and Prevention as an additional prevention tool for people at substantial risk for HIV infection, including men who have sex with men, heterosexual men and women, intravenous drug users, and HIV-negative partners in serodiscordant couples. While oral PrEP has proven effective in HIV-1-serodiscordant heterosexual couples, two clinical trials failed to demonstrate efficacy in young high-risk women, which has fostered the development of alternative topical formulations including vaginal rings, films, and fast dissolve tablets.
We searched PubMed and abstracts from major international AIDS conferences with combinations of the terms “tenofovir disoproxil fumarate” or “tenofovir”, “pre-exposure prophylaxis”, and “vaginal ring” for safety analyses of HIV prevention products in women with no restrictions on language or publication date. A previously published Phase 1 study of this polyurethane reservoir-type intravaginal ring designed for 30-day delivery of TDF demonstrated that the ring was safe when used for 14 days in women who were asked to abstain from sexual activity during ring use. A different polyurethane reservoir-type vaginal ring designed to deliver tenofovir (not the prodrug TDF) was also safe in sexually abstinent women for up to one month. Additionally, sustained TDF delivery from a silicone pod-type vaginal ring was safe when used for 7 days in sexually abstinent women.
Added value of this study
We are the first group, to our knowledge, to report safety and pharmacokinetics of vaginal ring delivery of TDF in sexually active women. Our Phase 1 trial was stopped early due to vaginal ulcerations which developed in eight of twelve women in the TDF arm. There were no ulcers in five women who were randomised to a placebo ring. Multiple inflammatory cytokines and chemokines were significantly higher 14 and 28 days after ring insertion in women who received TDF but not placebo vaginal rings. Additionally, an inflammatory gene signature was observed in cervical tissue from women randomised to TDF, but not placebo, which occurred in women with and without ulcers.
Implications of all the available evidence
Our findings were not predicted by extensive preclinical data including macaque studies or the 14-day clinical study in sexually abstinent women. Results suggest that sustained concentrations of intracellular tenofovir diphosphate and/or other metabolites may induce inflammation in sexually active women. The findings underscore the need for more predictive preclinical models to assess topical PrEP safety. Moreover, results suggest caution with ongoing and planned trials of vaginal release formulations of tenofovir and its prodrugs (TDF or tenofovir alafenamide).
Acknowledgments
This work was supported by grants from the National Institute of Allergy and Infectious Diseases and the National Center for Advancing Translational Sciences at the National Institutes of Health (U19 AI03461, P30 AI124414, and UL1 TR002256). The Yerkes NHP Genomics Core is supported in part by ORIP/OD P51OD011132. We acknowledge Gilead Sciences, Inc. for providing tenofovir disoproxil fumarate and regulatory assistance. We thank Jeanna Piper, Cherlynn Mathias and Jenny Stanwix for assisting with protocol development and study operations, Jason McConnell for helping with the manufacture of rings, and Michael Zinaman and Susan Cu-Uvin for serving as members of the Data Safety Monitoring Committee. We also acknowledge the site staff and study participants.
Funding
National Institutes of Health
Declaration of interests
MJK reports grants from NIH and non-financial support from Gilead, during the conduct of the study. PLA reports grants from Gilead Sciences, outside the submitted work. CWH reports grants from NIH, Viiv, GlaxoSmithKlein, outside the submitted work. In addition, CWH has a patent: United States Patent Application 15/120,852; Hypotonic Microbicidal Formulations and Methods of Use. BCH, JM, MM, and SS report grants from NIH during the conduct of the study.
Footnotes
Data Sharing
The study protocol, informed consent document, and deidentified participant data will be made available to others upon request to marla.keller@einstein.yu.edu following publication. RNA-Seq reads and the normalized expression table were deposited in the Gene Expression Omnibus (GEO) database at the National Center for Biotechnology Information (NCBI) (accession number GEO GSE122702). Sequencing reads for 16S rRNA gene sequences have been deposited to the NCBI Short Read Archive (SRA) (accession number PRJNA529191).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Marla J Keller, Department of Medicine, Albert Einstein College of Medicine, Bronx, NY.
Lianna Wood, Department of Pediatrics, Albert Einstein College of Medicine, Bronx, NY.
James M Billingsley, Yerkes National Primate Research Center, Atlanta, GA.
Laurie L Ray, Department of Medicine, Albert Einstein College of Medicine, Bronx, NY.
Jessica Goymer, Department of Pediatrics, Albert Einstein College of Medicine, Bronx, NY.
Shada Sinclair, Department of Pediatrics, Albert Einstein College of Medicine, Bronx, NY.
Aileen P McGinn, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY.
Mark A Marzinke, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD.
Bruce Frank, Particle Sciences, Bethlehem, PA.
Sujatha Srinivasan, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Research Center, Seattle, WA.
Congzhou Liu, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Research Center, Seattle, WA.
Jessica M Atrio, Department of Obstetrics & Gynecology and Women’s Health, Albert Einstein College of Medicine, Bronx, NY.
Lilia Espinoza, Department of Medicine, Albert Einstein College of Medicine, Bronx, NY.
Nelly Mugo, Centre for Clinical Research, Kenya Medical Research Institute, Nairobi, Kenya.
Hans M L Spiegel, Division of AIDS, Kelly Government Solutions, Contractor to National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, Rockville, MD.
Peter L Anderson, Department of Pharmaceutical Sciences, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora, CO.
David N Fredricks, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Research Center, Seattle, WA.
Craig W Hendrix, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD.
Jeanne Marrazzo, Department of Medicine, University of Alabama at Birmingham School of Medicine, Birmingham, AL.
Steven E Bosinger, Yerkes National Primate Research Center, Atlanta, GA.
Betsy C Herold, Department of Pediatrics, Albert Einstein College of Medicine, Bronx, NY.
References
- 1.UNAIDS. Ending AIDS Progress towards the 90–90-90 Targets, 2017.
- 2.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010; 329(5996): 1168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 2015; 372(6): 509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delany-Moretlwe S, Lombard C, Baron D, Bekker LG, et al. Tenofovir 1% vaginal gel for prevention of HIV-1 infection in women in South Africa (FACTS-001): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 2018; 18(11): 1241–1250. [DOI] [PubMed] [Google Scholar]
- 5.Baeten JM, Palanee-Phillips T, Brown ER, et al. Use of a vaginal ring containing Dapivirine for HIV-1 prevention in women. N Engl J Med 2016; 375(22): 2121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nel A, van Niekerk N, Kapiga S, et al. Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N Engl J Med 2016; 375(22): 2133–43. [DOI] [PubMed] [Google Scholar]
- 7.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012; 367(5): 411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taneva E, Crooker K, Park SH, et al. Differential mechanisms of tenofovir and tenofovir disoproxil fumarate cellular transport and implications for topical preexposure prophylaxis. Antimicrob Agents Chemother 2015; 60(3): 1667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mesquita PM, Rastogi R, Segarra TJ, et al. Intravaginal ring delivery of tenofovir disoproxil fumarate for prevention of HIV and herpes simplex virus infection. J Antimicrob Chemother 2012; 67(7): 1730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taneva E, Sinclair S, Mesquita PM, et al. Vaginal microbiome modulates topical antiretroviral drug pharmacokinetics. JCI Insight 2018; 3(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klatt NR, Cheu R, Birse K, et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science 2017; 356(6341): 938–45. [DOI] [PubMed] [Google Scholar]
- 12.Mesquita PM, Srinivasan P, Johnson TJ, et al. Novel preclinical models of topical PrEP pharmacodynamics provide rationale for combination of drugs with complementary properties. Retrovirology 2013; 10(1): 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith JM, Rastogi R, Teller RS, et al. Intravaginal ring eluting tenofovir disoproxil fumarate completely protects macaques from multiple vaginal simian-HIV challenges. Proc Natl Acad Sci U S A 2013; 110(40): 16145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith JM, Srinivasan P, Teller RS, et al. Tenofovir disoproxil fumarate intravaginal ring protects high-dose depot medroxyprogesterone acetate-treated macaques from multiple SHIV exposures. J Acquir Immune Defic Syndr 2015; 68(1): 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keller MJ, Mesquita PM, Marzinke MA, et al. A phase 1 randomised placebo-controlled safety and pharmacokinetic trial of a tenofovir disoproxil fumarate vaginal ring. AIDS 2016; 30(5): 743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendrix CW, Chen BA, Guddera V, et al. MTN-001: randomised pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PLoS One 2013; 8(1): e55013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castillo-Mancilla JR, Zheng JH, Rower JE, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses 2013; 29(2): 384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golob JL, Pergam SA, Srinivasan S, et al. Stool microbiota at neutrophil recovery Is predictive for severe acute graft vs host disease after hematopoietic cell transplantation. Clin Infect Dis 2017; 65(12): 1984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srinivasan S, Hoffman NG, Morgan MT, et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One 2012; 7(6): e37818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsen FA, Kodner RB, Armbrust EV. pplacer: linear time maximum-likelihood and Bayesian phylogenetic placement of sequences onto a fixed reference tree. BMC Bioinformatics 2010; 11: 538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 2013; 29(1): 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014; 15(12): 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 2005; 102(43): 15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li S, Rouphael N, Duraisingham S, et al. Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nat Immunol 2014; 15(2): 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keller MJ, Madan RP, Torres NM, et al. A randomised trial to assess anti-HIV activity in female genital tract secretions and soluble mucosal immunity following application of 1% tenofovir gel. PLoS One 2011; 6(1): e16475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srinivasan P, Dinh C, Zhang J, et al. Pharmacokinetic evaluation of tenofovir disoproxil fumarate released from an intravaginal ring in pigtailed macaques after 6 months of continuous use. J Med Primatol 2014; 43(5): 364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez-Garcia M, Patel MV, Shen Z, Bodwell J, Rossoll RM, Wira CR. Tenofovir inhibits wound healing of epithelial cells and fibroblasts from the upper and lower human female reproductive tract. Sci Rep 2017; 8: 45725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hladik F, Burgener A, Ballweber L, et al. Mucosal effects of tenofovir 1% gel. eLife 2015; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stier EA, Goldstone SE, Einstein MH, et al. Safety and efficacy of topical cidofovir to treat high-grade perianal and vulvar intraepithelial neoplasia in HIV-positive men and women. AIDS 2013; 27(4): 545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thurman AR, Schwartz JL, Brache V, et al. Randomised, placebo controlled phase I trial of safety, pharmacokinetics, pharmacodynamics and acceptability of tenofovir and tenofovir plus levonorgestrel vaginal rings in women. PLoS One 2018; 13(6): e0199778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seay K, Khajoueinejad N, Zheng JH, et al. The vaginal acquisition and dissemination of HIV-1 infection in a novel transgenic mouse model Is facilitated by coinfection with herpes simplex virus 2 and is inhibited by microbicide treatment. J Virol 2015; 89(18): 9559–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.