Abstract
Elevated homocysteine (Hcy), i.e., hyperhomocysteinemia (HHcy), causes skeletal muscle myopathy. Among many cellular and metabolic alterations caused by HHcy, oxidative and endoplasmic reticulum (ER) stress are considered the major ones; however, the precise molecular mechanism(s) in this process is unclear. Nevertheless, there is no treatment option available to treat HHcy-mediated muscle injury. Hydrogen sulfide (H2S) is increasingly recognized as a potent anti-oxidant, anti-apoptotic/necrotic/pyroptotic, and anti-inflammatory compound and also has been shown to improve angiogenesis during ischemic injury. Patients with CBS mutation produce less H2S, making them vulnerable to Hcy-mediated cellular damage. Many studies have reported bidirectional regulation of ER stress in apoptosis through JNK activation and concomitant attenuation of cell proliferation and protein synthesis via PI3K/AKT axis. Whether H2S mitigates these detrimental effects of HHcy on muscle remains unexplored. In this review, we discuss molecular mechanisms of HHcy-mediated oxidative/ER stress responses, apoptosis, angiogenesis, and atrophic changes in skeletal muscle and how H2S can restore skeletal muscle homeostasis during HHcy condition. This review also highlights the molecular mechanisms on how H2S could be developed as a clinically relevant therapeutic option for chronic conditions that are aggravated by HHcy.
Keywords: angiogenesis, apoptosis, atrophy, endoplasmic reticulum stress, ischemia, myopathy, redox imbalance (ROS), inflammation, JNK, cystathionine-β synthase
Mots-clés: angiogenèse, apoptose, atrophie, stress du réticulum endoplasmique, ischémie, myopathie, déséquilibre redox (dérivés réactifs de l’oxygène), inflammation, JNK, cystathionine-β synthase
Résumé:
La hausse des taux d’homocystéine (Hcy) (c.-à-d. l’hyperhomocystéinémie (HHcy)) provoque une myopathie du muscle squelettique. Des nombreuses modifications cellulaires et métaboliques que provoque l’HHcy, on compte le stress oxydatif et du réticulum endoplasmique (RE) parmi les plus importantes, mais le(s) mode(s) d’action moléculaire(s) précis de ce processus demeurent à clarifier. Néanmoins, il n’existe aucune option thérapeutique qui soit accessible pour traiter les lésions musculaires médiées par l’HHcy. On considère de plus en plus le sulfure d’hydrogène (H2S) comme étant un puissant composé antioxydant, anti-apoptotique/nécrotique/pyroptotique et anti-inflammatoire, aussi décrit comme pouvant améliorer l’angiogenèse au cours des lésions ischémiques. Les patients porteurs de la mutation CBS produisent moins de H2S, ce qui les rend vulnérables aux dommages cellulaires médiés par l’Hcy. De nombreuses études ont rapporté la régulation bidirectionnelle du stress du RE dans l’apoptose par l’intermédiaire de l’activation du gène JNK, ainsi que l’atténuation concomitante de la prolifération cellulaire et de la synthèse protéique passant par l’axe PI3K/AKT. On n’a pas étudié si l’H2S permet de mitiger ces effets délétères de l’HHcy dans le muscle. Dans cet article de synthèse, nous discutons des modes d’action moléculaires des réactions de stress oxydatif et du RE, d’apoptose, d’angiogenèse et de changements atrophiques médiés par l’HHcy dans le muscle squelettique, ainsi que sur la manière dont l’H2S peut permettre de rétablir l’homéostasie du muscle squelettique en situation d’HHcy. Cet article de synthèse met aussi en lumière les modes d’action moléculaires qui pourraient sous-tendre le développement de l’H2S en tant qu’option thérapeutique pertinente sur le plan clinique en cas de maladies chroniques aggravées par l’HHcy. [Traduit par la Rédaction]
Introduction
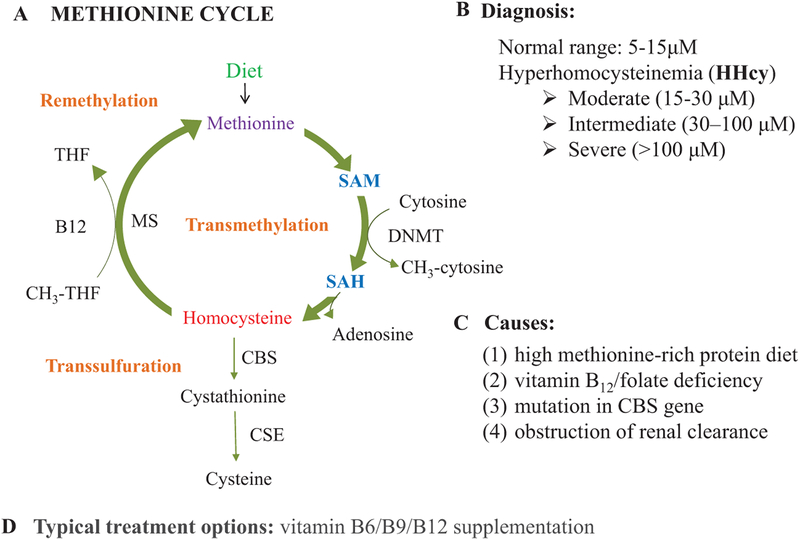
Homocysteine (Hcy) has been studied extensively for over 30 years for its unique involvement in an increasing number of human diseases (Hankey and Eikelboom 1999; Narayanan et al. 2014; Stipanuk and Ueki 2011). It is generated via methionine cycle (MET-cycle), and its level is controlled by 2 processes: around 50% of Hcy goes to transsulfuration pathway to produce cysteine and the remaining 50% is re-methylated back to methionine (MET) via folate cycle (Cascella et al. 2015; Majumder et al. 2017; Veeranki and Tyagi 2013). Normal total plasma Hcy concentration in our body is 5–10 μmol/L, however in a diseased condition, i.e., in hyperhomocysteinemia (HHcy), total plasma Hcy levels increases (>15 μmol/L) (Lehotský et al. 2016). HHcy can be classified as moderate (15–30 μmol/L), intermediate (30–100 μmol/L), and severe (>100 μmol/L) (Lehotský et al. 2016; Majumder et al. 2017). There are 4 main ways that people can develop HHcy: (i) methionine-rich protein diet, (ii) vitamin B12 and (or) folate deficiency, (iii) heterozygous/homozygous for cystathionine-β synthase (CBS+/−/CBS−/−), (iv) obstruction of renal clearance (Sen et al. 2010).
HHcy has been associated with severe skeletal muscle dysfunction, but the precise mechanism(s) is still unknown (Brustolin et al. 2010; Kanwar et al. 1976; Kolling et al. 2013; Majumder et al. 2017; Miller et al. 2000; Valentino et al. 2010; Veeranki et al. 2015; Voskoboeva et al. 2018; Zoccolella et al. 2008). Children born with very high levels of Hcy, i.e., HHcy due to deficiency of functional CBS gene (CBS−/−), show severe muscle myopathy and die shortly after birth, but heterozygous for CBS mutation (CBS+/−) children can survive (Voskoboeva et al. 2018). Previous reports showed that HHcy could cause disruption of Z-discs, fiber size, banding pattern, and excessive collagen deposition in muscle (Kalra et al. 1985; Kanwar et al. 1976; Veeranki et al. 2015), but which pathway triggers such pathological effects in the skeletal muscles are not fully understood. Skeletal muscle is the largest organ in our body and constitutes 50%–75% of all the body’s proteins. Loss of skeletal muscle mass (muscle wasting) is mediated mainly by 3 processes, apoptosis, poor vasculature, and atrophy (protein degradation exceeds protein synthesis over time) (Dupont-Versteegden 2005; Powers et al. 2016). Therefore identification of the precise molecular mechanism that regulates these processes during HHcy condition is essential to devise proper treatment strategies.
Findings from our group and others groups determined that HHcy induces oxidative stress in the cardiac microvascular endothelial cells (Tyagi et al. 2005), skeletal tissue (Behera et al. 2018), vascular smooth muscle cells (Yan et al. 2006), skeletal muscle (Majumder et al. 2018a), liver tissue (Matté et al. 2009), and brain (George et al. 2018a). Oxidative stress has been implicated in many diseases associated with protein misfolding and reduction of efficiency of protein folding pathways (Merad-Boudia et al. 1998; Nakamura and Lipton 2007; Plaisance et al. 2016; Uehara et al. 2006; Werstuck et al. 2001; Zhang and Kaufman 2008), but the cellular pathways that are involved in these stress-related conditions have not been elucidated. During severe ER stress conditions, activated IRE1 recruits TNF receptor-associated factor 2 (TRAF2) and apoptosis signal-regulating kinase 1 (ASK–1), which further activate c-Jun N-terminal kinase (JNK) (Deng et al. 2001; Lei and Davis 2003; Sano and Reed 2013). However whether HHcy can compromise cell survival via activation JNK is not known. AKT is a major survival factor promoting cell proliferation, and its dys-regulation has been detected in muscle wasting (Bodine et al. 2001; Manning and Cantley 2007; Romanino et al. 2011). A previous study showed that activated JNK could induce inhibitory phosphorylation of IRS-1 at Ser307 (Aguirre et al. 2000). Since Ser307 phosphorylation promotes general inhibition of IRS-1 signaling, it suggests that JNK-activation may impair PI3/AKT axis (Aguirre et al. 2002).
JNK controls cell response to the harmful extracellular stimulus (Ogata et al. 2006; Xie et al. 2011). JNK/c-Jun pathway regulates pro-apoptotic genes expression (TNF-a, Fas-L, and Bak) (Dhanasekaran and Reddy 2008). Whether HHcy-mediated oxidative/ER stress can induce apoptosis has not been studied in muscle yet. HHcy is implicated in vascular toxicity, causing endothelial cell damage and dysfunction (McCully 2007). Previously, our lab showed that HHcy antagonizes the angiogenic signals via reduced expression of HIF1-[H9251], VEGF, and eNOS in skeletal muscle (Chen et al. 2014; Veeranki et al. 2014, 2015). Although several markers of ER stress including CHOP have been shown to increase in a model of skeletal muscle atrophy, whether HHcy-mediated ER stress can play a role in muscle atrophy is still unclear (Yu et al. 2011). Additionally, several models of atrophy showed that inhibition of PI3K/Akt signaling induces nuclear import of forkhead box protein O (FOXO) regulating Atrogin-1, MurF-1 expression (Clavel et al. 2010; Shimizu et al. 2017). Some studies have already demonstrated that HHcy inhibits Akt activation in endothelial cells, but whether FOXO could remain un-phosphorylated during HHcy condition and allow it to translocate to the nucleus and upregulate MuRF-1 and Atrogin-1 in muscle cells is not studied yet (Manning and Cantley 2007).
H2S is increasingly being recognized as an important signaling molecule in the cardiovascular and nervous systems via its ability to neutralize a variety of ROS (Kimura et al. 2010; Mironov et al. 2017; Yang et al. 2015), as well as via increased cellular glutathione levels through activation/expression of γ-glutamylcysteine synthetase, and also via reduced disulfide bonds in proteins (Calvert et al. 2010; Elsey et al. 2010; Fiorucci et al. 2006; Gadalla and Snyder 2010; Kimura 2010; Predmore and Lefer 2010; Szabó 2007; Wang 2003). Cystathionine γ-lyase (CSE) and CBS are main H2S producing enzymes, which produce H2S from Hcy in transsulfuration reactions (Kabil et al. 2011). Patients with CBS gene mutation lead to dysfunction in endogenous H2S biosynthesis (Beard and Bearden 2011), suggesting that these patients are more prone to oxidative stress-mediated damages due to malfunction in Hcy metabolism and inefficient H2S biosynthesis (Szabó 2007). CBS is a crucial enzyme in the transsulfuration pathway, and heterozygous CBS deficient (CBS+/−) has proved a useful model for HHcy, which shows mild to a severe endogenous elevation of Hcy in several tissues in mice (Familtseva et al. 2014; Nandi and Mishra 2017; Narayanan et al. 2013; Tyagi et al. 2011, 2012; Watanabe et al. 1995; Winchester et al. 2018; Yang et al. 2018). Studies have been performed using the CBS model to show that these mice have less H2S levels in their blood and showed oxidative stress-mediated damages (Ishihara et al. 2008; John et al. 2017).
In this review we discuss HHcy-mediated oxidative/ER stress responses, apoptosis, angiogenesis, atrophy in skeletal muscle, and potential mechanisms and whether H2S has any beneficial effects on mitigating these conditions. We also highlight the molecular mechanisms of how H2S could be developed as a clinically relevant therapeutic option for chronic conditions like HHcy that are implicated in a host of cellular stress conditions, including the apparent defect in skeletal muscle functions.
Metabolism of homocysteine and hyperhomocysteinemia
Homocysteine (Hcy) is a sulfur-containing nonproteinogenic amino acid that is produced during the metabolism of methionine (MET)viathe1-carbonmetabolism(intheMET-cycle)(Stipanuk and Ueki 2011). Post absorption, MET is converted into S-adenosylmethionine (SAM) by ATP and catalyzed by methionine adenosyltransferase (MAT), leaving it with a transferrable methyl group. This methyl group is donated for cellular methylation requirements and becomes S-adenosyl-homocysteine (SAH). SAH hydrolase is an enzyme that reversibly hydrolyzes SAH, in turn, that produces Hcy (Lan et al. 2018). Hcy is present in blood in 3 different forms: around 1% as free thiol, 70%–80% as a disulfide bound to a swath of plasma proteins, and the remaining 20%–30% as a homodimer/heterodimer along with other thiols (Hankey and Eikelboom 1999). After generation of Hcy by MET-cycle, around 50% goes to the transsulfuration pathway for production of glutathione (an antioxidant), and the rest is re-methylated back to MET via the folate cycle (Fig. 1) (Cascella et al. 2015; Stipanuk and Ueki 2011; Veeranki and Tyagi 2013).
Fig. 1.
Etiology of hyperhomocysteinemia (HHcy). (A) Methionine cycle is a source of homocysteine, which is further metabolized via transsulfuration and remethylation pathways. Around 50% homocysteine is cycled via transsulfuration pathway to produce cysteine, while the remaining 50% is re-methylated back to methionine via the folate 1-carbon cycle. (B) The range of homocysteine levels in healthy vs. disease states. (C) Major causes of hyperhomocysteinemia. (D) Management of hyperhomocysteinemic conditions via supplementation with vitamins B6, B9, and B12. CBS, cystathionine-β-synthase; CSE, cystathionine γ-lyase.
In healthy individuals, the generation and elimination of Hcy stay in the balance; however, once this homeostasis is disturbed, it leads to HHcy (Tiahou et al. 2009). HHcy can be developed via consumption of an excess amount of the methionine-rich diet, vitamin B12/folate deficiency, the occurrence of heterozygous/homozygous CBS gene mutation, and the obstruction of renal clearance (Sen et al. 2010). Besides these, there can be genetic mutations in any Hcy metabolism pathway-regulated genes; the most common one are the variants such as 677C > T and 1298A > C in the MTHFR gene that can also lead to HHcy (Brustolin et al. 2010; Iqbal et al. 2009; Kharb et al. 2016; Verhoef et al. 2005). Other determinants such as age, sex, physical activity, alcohol intake, certain medications, and even different disease conditions can offset the rate of methionine cycle, thereby influencing the corresponding increase in the total Hcy levels in the blood (Diakoumopoulou et al. 2005). During HHcy conditions, the methylation cycle is generally dysregulated (Maddocks et al. 2016). This leads to disruption of multiple signaling pathways, because it is the only pathway that gives rise to the production of essential methyl groups needed for the subsequent biosynthesis of cellular compounds such as creatine, epinephrine, carnitine, phospholipids, proteins, and polyamines and also the epigenetically governed cellular processes like methylation of DNA, RNA, and histones that are dependent upon Hcy metabolism (Laha et al. 2018; Majumder et al. 2017). Although HHcy conditions can cause multi-organ pathologies via oxidative and ER stress systems (Ishii et al. 2010; Majumder et al. 2017; Veeranki and Tyagi 2015a; Veeranki et al. 2015; Winchester et al. 2014), the precise mechanisms of multi-organ pathology due to HHcy remain elusive.
Metabolism of Hcy depends on several factors, the level of methionine in the diet, SAM level, and the type of cells in which methionine metabolism takes place (Laster et al. 1965). High SAM levels can act as an allosteric inhibitor for methylenetetrahydrofolate reductase (MTHFR). MTHFR catalyzes the conversion of 5,10-MTHF to 5-MTHF, which is a co-substrate of Hcy in the remethylation reaction (Finkelstein and Martin 1984). Therefore, high SAM levels prevent Hcy from entering the remethylation pathway. High SAM levels also act as an allosteric activator for CBS of the transsulfuration pathway (Finkelstein et al. 1975; Stabler et al. 2002). Altogether it appears that the presence of high levels of SAM favors Hcy entering the transsulfuration pathway. However, only a few organs in our body such as the liver, kidney, pancreas, brain, adipose tissue, and small intestine synthesize CBS (Finkelstein 1990; Mudd et al. 1965). Vascular muscle tissues do not express CBS, so high SAM levels lead to transient accumulation of Hcy (Veeranki and Tyagi 2015a). Hcy metabolism also depends on dietary MET load, which can affect the rate of SAM synthesis, which in turn will determine the pathway Hcy takes up at the remethylation and transsulfuration junctions (Selhub 1999; Selhub et al. 1999; Stolzenberg-Solomon et al. 1999). When diet contains a basal methionine level, Hcy cycles through remethylation pathway about 1.5–2.0 times before being directed towards the transsulfuration pathway, but when dietary methionine content is half the basal level, the cycling of Hcy via the remethylation pathway increases 2-fold. Conversely, when the dietary MET level is high, Hcy cycling through the remethylation is reduced by 1.5-fold (Eloranta et al. 1990). High levels of intracellular Hcy is exported out into the circulation (Gupta et al. 1998), however the exact mechanism of Hcy export is not identified yet. Although HHcy is found in ~5% of the general population and is associated with increased risk of cardiovascular disease, osteoporosis, skeletal muscle myopathy, and other metabolic complications (Ishii et al. 2010; Majumder et al. 2017; Veeranki and Tyagi 2015a; Veeranki et al. 2015; Winchester et al. 2014), precise molecular pathways that lead to multi-organ dysfunction remain mostly unexplored.
Structural aspects of skeletal muscle functioning
Skeletal muscle is the largest organ in our body (roughly 40% to 50% of the body’s total mass in average human), which constitutes 50%–75% of the body’s proteins (Frontera and Ochala 2015; Pedersen 2013; Smith et al. 2013). Muscle contains very long multi-nucleated cells (myocytes) that are bundled up and surrounded by connective tissues. Skeletal muscle has 2 primary functions: (i) production of contractile force that provides breathing, locomotion, and postural support and (ii) thermogenesis during periods of cold stress. Force production, depending on the primary function of the muscle, may consist of either fast twitch (type I) or slow twitch (type II) fibers (Talbot and Maves 2016). Type I fibers are used for repetitive, low-intensity functions such as postural support. These fibers provide less force generation but are fatigue resistant and have a higher reliance on oxidative metabolism and therefore have more density of mitochondria and oxidative enzymes (Talbot and Maves 2016). Type II fibers are divided into two fiber subsets: type IIx and type IIa (Talbot and Maves 2016). Type IIx fibers are very glycolytic and produce high contractile force but are susceptible to fatigue due to a lower mitochondrial density (Talbot and Maves 2016). Type IIa fibers are an intermedi ate fiber type (Talbot and Maves 2016). They provide a moderate contractile force and are more fatigue resistant than Type IIx fibers (Talbot and Maves 2016).
Myocytes, unlike most other cell types, contain multiple nuclei and are striated because of contractile segments called sarcomeres (Talbot and Maves 2016). Within each myocyte are structures called myofibrils, which contain the contractile proteins of the muscle, called actin and myosin (Talbot and Maves 2016). On the actin filamentthere are 2 additional proteins known as troponin and tropomyosin, which are used for contractile regulation (Talbot and Maves 2016). The striated regions, called sarcomeres, are within the myofibrils and consist of areas of myosin/actin overlap, as well as an area where myosin does not overlap with actin called the H zone (Talbot and Maves 2016). Sarcomeres are divided by a wall of structural proteins, which makes up a region of the sarcomere called the Z line (Talbot and Maves 2016). Based on “sliding filament theory,” during contraction, the required energy comes from the de-phosphorylation of adenosine triphosphate (ATP), wherein myosin heads “cock” form cross-bridges with actin and then pivot inward towards the H zone (Allen and Westerblad 2007), which ultimately decrease the sarcomere length from Z line to Z line (Mi-Mi and Pruyne 2015).
Surrounding each myofibril is a network of channels that serve as a storage site for calcium, which is crucial for regulation of muscle contraction (Gehlert et al. 2015). This structure is called the sarcoplasmic reticulum (Gehlert et al. 2015). The transverse tubules are another set of channels that pass through the muscle fiber (Gehlert et al. 2015). Each myocyte is connected to a branch of a motor neuron through a neuromuscular junction serving as the site of electrochemical transmission (Deschenes 2011). Collectively, all the myocytes that are innervated by a single motor neuron are termed a motor unit (Deschenes 2011). Upon generation of an action potential high enough to cause contraction, the neural impulse is transmitted to the myocyte through the neuromuscular junction and is propagated down the transverse tubules to the sarcoplasmic reticulum, causing Ca++ to be released (Kuo and Ehrlich 2015). Ca++ binds to the troponin molecule, which creates a shift in tropomyosin position (Kuo and Ehrlich 2015). This allows for exposure of the actin active sites and myosin-actin cross bridge formation, resulting in contraction (Kuo and Ehrlich 2015).
In adults, repair of degenerated muscles relies on a small population of skeletal muscle stem cells known as satellite cells (Mauro 1961). Satellite cells, a popular of quiescent muscle precursor cells that reside beneath the basal lamina, provide the predominant source of additional myonuclei for muscle growth (Mitchell and Pavlath 2001; Rosenblatt et al. 1994). Once activated, satellite cells give rise to myoblasts that proliferate, differentiate, and fuse to form new muscle fibers or to repair damaged muscle fibers (Chargé and Rudnicki 2004; Hawke and Garry 2001). The availability of a pool of myoblasts for myogenesis is essential for the development of muscle (Miyake et al. 2011). A decreased number of muscle fibers could be due to the reduced myoblast proliferation or cytotoxicity. Moreover in most cases, the apoptosis of myoblasts serves as a physiological behavior to remove excess myoblasts during myogenesis or muscle regeneration, while inappropriate apoptosis will pathologically lead to degeneration that is associated with various muscular dystrophies and atrophies (Tews and Goebel 1997; Tidball et al. 1995). Therefore, identification of toxicants that regulate myoblast cytotoxicity is essential in understanding skeletal muscle growth, disease, and regeneration.
Hyperhomocysteinemia-mediated skeletal muscle pathophysiology
HHcy is a known risk factor for vascular diseases (Ganguly and Alam 2015), however several studies also reported that HHcy leads to skeletal muscle weakness and functional impairment (Kalra et al. 1985; Kanwar et al. 1976; Veeranki et al. 2014, 2015; Veeranki and Tyagi 2013, 2015a; Winchester et al. 2018). Children born with severe homocystinuria due to CBS deficiency exhibit reduced body weight, skeletal muscle myopathy, and die in teenage years (Majumder et al. 2017). A clinical manifestation of HHcy displays decreased body mass and loss of skeletal muscle mass, which ultimately lead to myopathy (Kalra et al. 1985; Kanwar et al. 1976; Veeranki et al. 2014; 2015; Veeranki and Tyagi 2013, 2015a; Winchester et al. 2018). Results from our laboratory previously showed that HHcy mice (CBS+/−gene) have lower body mass and less fatigue resistance and produce less contractile force, have lower muscle ATP levels, low dystrophin, and mitochondrial transcription factor A (mtTFA) compared to control mice (C57BL/6J) (Veeranki and Tyagi 2015a). In a study, Kanwar et al. (1976) found that HHcy can cause focal fragmentation, disruption, and smearing of the Z-discs and disorganization of the myofilaments in the skeletal muscles (Kanwar et al. 1976). They showed that HHcy could reduce cellular metabolic activity and induce energy imbalance in gastrocnemius rat skeletal muscle by decreasing the activity of pyruvate kinase, creatine kinase, and noticed increased activity of succinate dehydrogenase (Kolling et al. 2013). Similarly, Veeranki and Tyagi showed that Hcy affects fatty acid oxidation affecting energy metabolism in skeletal muscle via PGC-1αspecific protein nitrotyrosylation and a concomitant reduction in association with PPAR-γ (Veeranki and Tyagi 2015a). On the other hand, many neurological disorders such as amyotrophic lateral sclerosis (ALS) and multiple sclerosis affect muscle degeneration, and these medical conditions are connected to HHcy (Valentino et al. 2010). HHcy also significantly lowers physical functions by deteriorating skeletal muscle functions in older people compared to age-matched healthy subjects (Kado et al. 2002), which suggests that aging is one of the potential risk factors to be considered in this process (Westerblad et al. 2010).
Disorders such as muscular dystrophy, sarcopenia, and immobilization have been extensively researched, however there is not enough data available that consider the effects of HHcy on these conditions. Swart et al. demonstrated that in elderly individuals (~75.6 years of age), plasma Hcy levels had a strong inverse correlation with grip strength and functional capabilities (Swart et al. 2013). High Hcy levels resulted in poor grip strength in men and lowered functional ability including walking, climbing stairs, and rising from a chair (Swart et al. 2013). In heterozygous CBS-deficient mice (CBS+/−), a model of mild to moderate HHcy, it was observed that 28 days post induction of hindlimb ischemia CBS+/− mice displayed blunted hind limb perfusion and lower collateral blood vessel development when compared to WT mice (Majumder et al. 2018b; Singh et al. 2018; Veeranki et al. 2014). This study also found that CBS+/− mice had lower vascular endothelial growth factor (VEGF), hypoxia-inducible factor (HIF1-α), and peroxisome proliferator-activated receptor coactivator 1-α (PGC1-α) after 28 days of ischemic recovery, when compared to control (Veeranki et al. 2014). We also showed that excessive circulating Hcy could cause oxidative and endoplasmic reticulum (ER) stress in skeletal muscle that may induce skeletal muscle atrophy (Majumder et al. 2018a).
Hyperhomocysteinemia mediates oxidative/ER stress responses in skeletal muscle
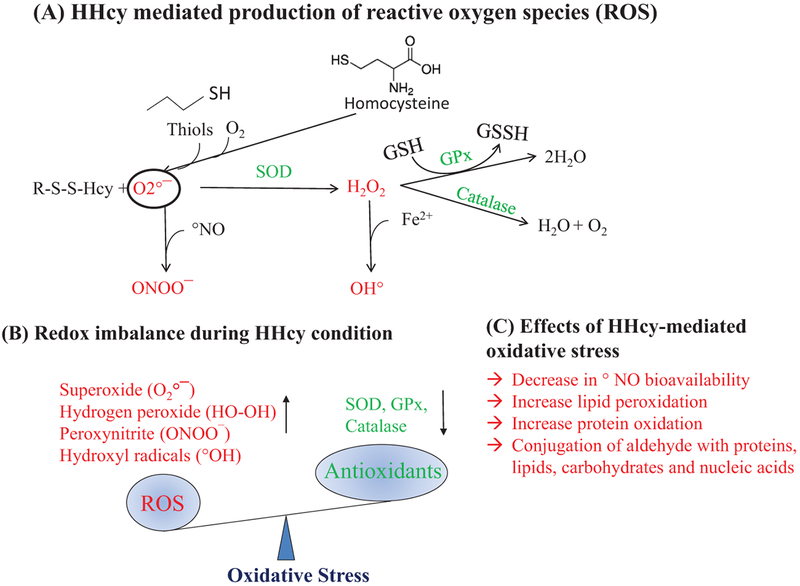
During metabolic processes, a certain amount of pro-oxidative ROS are formed. Intracellular environment is predominantly a reducing environment, first due to the presence of enzymes like glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase, which breakdown ROS into water and oxygen, and second due to a series of built-in redox defense systems that inactivate ROS, which include reduced glutathione (GSH), NADH, thioredoxin, and free radical scavengers such as vitamins C and E (Gasparetto et al. 2005; Yamamoto et al. 2003; Zima et al. 2004). Oxidative stress occurs when there is an imbalance between free radical production and antioxidant capacity in a given cell, as shown in Fig. 2. Previous studies revealed that Hcy contains -SH group-like thiols (RSH), which can undergo oxidation to form a disulfide (RSSR) even at physiological pH in the presence of metal catalysts and molecular oxygen [O °2] (Kalra et al. 1985). Further, Hcy can also produce hydrogen peroxide (H2O2, a pro-oxidant molecule) during the metal-catalyzed oxidation step and peroxynitrite (ONOO−, a potent oxidant) in the presence of nitric oxide (NO) and superoxide anion (·O−2) (Lubos et al. 2008). Findings from our laboratory and others demonstrated HHcy effects on oxidative stress in cardiac microvascular endothelial cells (Tyagi et al. 2005), vascular smooth muscle cells (Yan et al. 2006), and the liver tissue (Matté et al. 2009). Although these phenomena have been studied in multiple tissue types, whether HHcy exerts its detrimental effects on muscle through similar mechanisms is not yet elucidated.
Fig. 2.
Redox imbalance during hyperhomocysteinemia (HHcy). (A) Homocysteine can homo/hetero-dimerize in the presence of transition metal catalysts and molecular oxygen to form superoxide radicals that are converted to H2O2 by superoxide dismutase (SOD) and which are in turn neutralized to H2O and O2 by glutathione peroxidase (GPx) and catalase. (B) HHcycauses redox imbalance via excessive production of reactive oxygen species (ROS) and dysregulation of oxidative defense mechanisms (SOD, GPx, and catalase). (C) Overall effects of oxidative stress in the cellular environment.
Several studies have reported that HHcy creates oxidative stress that promotes apoptosis, inflammation, insulin resistance, and dysregulation of lipid metabolism in different organs (Hayden and Tyagi 2004; Homme et al. 2018; Lentz 2005; Majumder et al. 2017; Veeranki and Tyagi 2013). Increased ROS level due to HHcy were also found to be involved in lipid peroxidation, protein denaturation, and DNA damage, which ultimately damage cellular components, modulates gene expression, alters cell signaling pathways, and produce energy imbalance. Besides, Hcy can also form Hcy-thiolactone and acetylates free amino groups in proteins, which further intensify the exaggerated oxidative stress condition (Hayden and Tyagi 2004). DiBello et al. showed that proteins (PRDX1, PRDX2, and HSP90AA), which are produced as a response to oxidative stress, were significantly upregulated in the liver during HHcy (Mishra et al. 2010). One study showed that production of ROS was ameliorated by PPAR-γ activation in ECs (Hayden and Tyagi 2004), as previously reported by our laboratory, indicating that PPARγ was reduced in HHcy condition (Laha et al. 2018; Mishra et al. 2010), suggesting that HHcy can induce ROS levels via PPAR-γ mediated pathway. Furthermore, Hcy also uses the cysteine transporter (ASC transporter: alanine–serine–cysteine transporter) to enter into the endothelial cells (Büdy et al. 2006), so maybe Hcy also uses the same transporter while entering muscle cells. Hence during HHcy, Hcy competitively inhibits cysteine entrance inside the cells (Veeranki et al. 2017). As cysteine is indis pensable for the synthesis of glutathione (GSH), lack of cysteine inside cells in HHcy conditions can compromise the cellular anti-oxidant potential in skeletal muscle (Brustolin et al. 2010). Since HHcy leads to oxidative stress, which interferes with different cellular signaling pathways in various organs other than muscle, it suggests that HHcy may have similar pathological effect in muscle tissue.
Oxidative stress has been implicated in many diseases associated with protein misfolding. Indeed, in a study by Malhotra et al. showed that antioxidants reduce endoplasmic reticulum stress and improve protein secretion in Chinese hamster ovary (CHO)-H9 cells (Malhotra et al. 2008). Similarly, a study using differential display analysis in HUVECs found that GRP78 (78-kDa glucose-regulated protein-an ER stress marker) was upregulated in Hcy treated cells compared to controls (Kokame et al. 1996). GRP78, also known as immunoglobulin heavy-chain binding protein (BiP), is a chaperone protein belonging to the HSP70 family and predominantly resides in the lumen of the ER (Panayi and Corrigall 2014). HHcy can induce ER stress (Werstuck et al. 2001), but which pathways are affected by this condition is poorly understood. It is known that after translation, protein folding occurs inside ER, but that during stress conditions, misfolded proteins accumulate inside the ER lumen and induce unfolded protein response (UPR) (Oslowski and Urano 2011). UPR increases transcription of chaperones genes to facilitate protein folding, helps to attenuate translation process, and finally it degrades misfolded proteins accumulated in ER (Kaufman 2002; Mori 2000; Ron 2002). In the mammalian system, UPR is mediated through inositol-requiring protein 1 (IRE1), PRKR-like ER kinase (PERK), and activating transcription factor 6 (ATF6) (Rutkowski and Kaufman 2004). IRE1 promotes X-box binding protein 1 (XBP1) mRNA splicing, which finally activates ER chaperones (e.g., BiP/GRP78 (BiP), EDEM and ERdj4). PERK phosphorylates eukaryotic translation initiation factor 2α (eIF2α) and that stops translation. ATF6 regulates the expression of chaperones genes and ER-associated degradation (ERAD) genes (Adham et al. 2005; Kondo et al. 2005; Zhang et al. 2006). Several studies showed that HHcy could worsen ER stress by reducing the efficiency of protein folding pathways and increasing the production of misfolded proteins (Plaisance et al. 2016). ER stress might play a key role in mediating adverse effects during HHcy in muscle (Cao 2015).
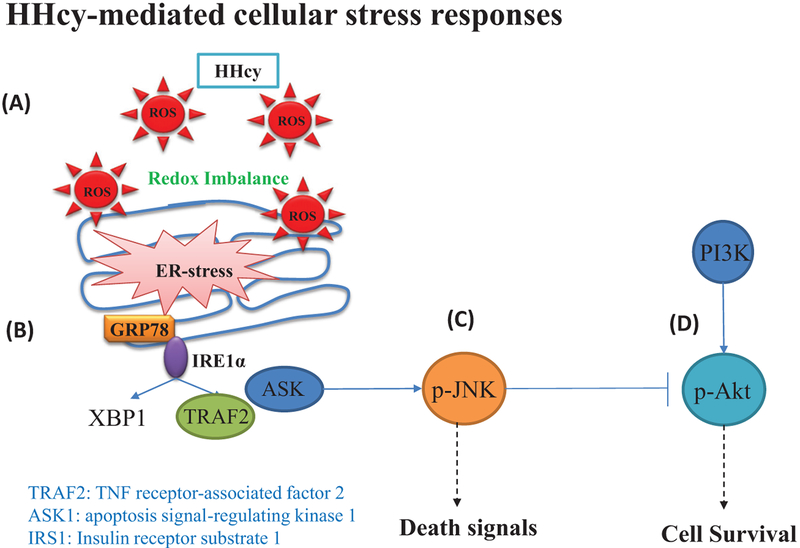
In the mammalian system, there are 2 isoforms of IRE1: IRE1γ and IRE1β. Both of these proteins are transmembrane proteins and contain a Ser/Thr kinase domain and an endoribonuclease domain (Hassler et al. 2012; Shamu and Walter 1996). In a stress-free environment, IRE1 remains in an inactivated form bound with GRP78, however in stress conditions, its dissociates from GRP78 and becomes auto-phosphorylated (Gardner et al. 2013). Then the endonuclease activity of activated IRE1 cleaves a 26 bp intron from ubiquitously expressed XBP1u mRNA. Removal of this intron causes a frameshift in the XBP1 coding sequence resulting in the translation of XBP-1s isoform (Oslowski and Urano 2011). XBP-1s encodes a specific basic leucine zipper-containing transcription factor, known as X-Box Binding Protein-1 (XBP1), to promote cell survival (Gardner et al. 2013; Hollien et al. 2009; Maurel et al. 2014). However, during severe ER stress conditions, IRE1 recruits TNF receptor-associated factor 2 (TRAF2) and apoptosis signal-regulating kinase 1 (ASK-1), then activates c-Jun N-terminal kinase (JNK) and induces apoptosis (Deng et al. 2001; Lei and Davis 2003; Sano and Reed 2013). Although many studies also reported bidirectional regulation of ER stress in apoptosis through JNK activation, and concomitant attenuation of cell proliferation and protein synthesis via PI3K/AKT axis, whether HHcy exerts its detrimental effect in muscle through a similar mechanism(s) remains unexplored (Fig. 3).
Fig. 3.
Hyperhomocysteinemia (HHcy) is a non-inducer of cellular stress responses. (A) During oxidative stress conditions, the biogenesis as well as protein folding process are severely compromised leading to unfolded protein response (UPR). (B) Activation of inositol-requiring enzyme-1α (IRE1α) during endoplasmic reticulum (ER) stress response causes X-box binding protein-1 (XBP1) mRNA splicing (induces the production of chaperon proteins) and during severe ER stress condition it recruits TNF receptor-associated factor-2 (TRAF2) along with apoptosis signal-regulating kinase-1 (ASK1), which further leads to activation of c-Jun N-terminal kinase (JNK). (C) Activated-JNK phosphorylates c-Jun (Ser63 and 73 residues) that leads to the recruitment of activator protein-1 (AP-1) to the promoter sequences of various pro-apoptotic genes, and induce apoptosis. (D) JNK is also involved in the phosphorylation of IRS-1 at Ser307 which in turn inhibits IRS-1 signaling thus impairing the PI3K/AKT survival axis.
Hyperhomocysteinemia mediated cell death pathways
Apoptosis is programmed cell death and is required for normal development; however, very higher amounts of apoptotic cell death due to environmental stress can be detrimental (Renehan et al. 2001). Excessive apoptosis is observed in many well-known diseases such as ischemic heart disease, AIDS, Alzheimer’s, and Parkinson’s, etc. (Kerr et al. 1994; Lockshin and Zakeri 2007; Thompson 1995). Apoptosis occurs in 2 phases; namely the death decision phase and execution phase. Death decision phase is controlled by 2 proteins, pro-apoptotic proteins (Bax and Bak) and anti-apoptotic proteins (BCL-XL and BCL-2). The execution phase is regulated by caspases (proteolytic enzymes), responsible for the execution of apoptosis after the death decision is confirmed (Elmore 2007). Unlike necrosis, apoptosis occurs at the single cell level (Elmore 2007). Studies showed that an increase in oxidative stress could lead to caspase activation (Green and Reed 1998; Morel and Barouki 1999; Richter 1993; Zamzami et al. 1996). This oxidative stress-mediated apoptosis was further supported by other studies demonstrating that antioxidants such as N-acetylcysteine (NAC) treatment could block apoptosis in a similar way that caspase inhibitors do (Kannan and Jain 2000; Okamoto et al. 2016). Several mechanisms have been proposed pointing to the fact that oxidative stress generated by HHcy can lead to multi-organ pathologies. This argues that no stimulus other than redox imbalance during HHcy is capable of inducing apoptotic cell death. Even though findings suggested that HHcy could be causal in a wide range of disorders, including skeletal muscle myopathy, the action mechanism of Hcy in different types of cell deaths (apoptotic, necrotic, and pyroptotic) is yet to be fully explained.
A study showed that Hcy reduced DNA synthesis and induced apoptotic EC death (Bessede et al. 2001). Similarly, another finding revealed that Hcy causes apoptosis in HUVECs via activation of UPR, which was mediated through IRE1 activation (Zhang et al. 2001). A similar finding suggested that Hcy can induce trophoblast cell death during embryonic development (Di Simone et al. 2003). Hcy was also found to induce apoptosis in bone marrow mesenchymal stem cells (BMSCs) via oxidative stress mechanism, and it was mediated through JNK activation (Cai et al. 2013). Folate deficiency could induce apoptotic cell death in RINm5F pancreatic Islet β-cells, through oxidative-nitrosative stress (Hsu et al. 2013); as folate deficiency is one of the causes of HHcy, this suggests that during HHcy apoptosis remains a favored outcome in various organs. Mesangial cell apoptosis via inducing ROS and p38-MAPK activation had also been reported (Shastry et al. 2007). Hcy induces apoptosis via upregulation of DNA damage response in neurons, and this process is mediated through PARP activation and p53 induction (Kruman et al. 2000). Our lab, using genetically engineered heterozygous CBS deficiency mouse model (CBS+/−), reported that HHcy causes cell death via upregulation of mitochondria-mediated cell death pathway (BAX, caspase-9, and caspase-3) (Familtseva et al. 2016). Hydrogen sulfide (H2S) is known to involved in modulation of many physiological pathways. A study found that H2S-releasing sildenafil (ACS6) treatment could protect HHcy-mediated apoptosis in PC12 cells (Tang et al. 2011).
Accumulating data indicate that ER stress is a potent trigger of autophagy (Bernales et al. 2006; Criollo et al. 2007; Fujita et al. 2007; Høyer-Hansen and Jäättelä 2007; Kamimoto et al. 2006; Kouroku et al. 2007; Yorimitsu et al. 2006). Autophagy is a process that degrades misfolded proteins and plays a role in cell survival (Ding et al. 2007; Hart et al. 2012; Høyer-Hansen et al. 2007). This process is initiated by class III PI3-kinase complex and ATG proteins, where a microtubule-associated protein light chain 3 II (LC3-II) is formed due to conjugation of phosphatidylethanolamine (PC) and LC3-I (Fujita et al. 2007). A double membrane vacuole is formed called autophagosome, which finally fuses with the lysosomal membrane to deliver the contents into the autolysosome to degrade and recycle macromolecules (Nixon 2006). In addition to UPR, ER stress also leads to release of Ca2+ from the ER and possibly may have some role in autophagy signaling (Høyer-Hansen et al. 2007; Høyer-Hansen and Jäättelä 2007). Although autophagy occurs at basal levels in most tissues and in addition to turnover of cellular components, it plays a role in development, differentiation, and tissue remodeling. It is unclear how high homocysteine can modulate autophagy process in different tissues (Momoi 2006).
Hyperhomocysteinemia mediated dysregulation of angiogenesis
Although many studies have been conducted to identify the mechanism(s) of skeletal muscle myopathy, fewer studies have demonstrated the potential risk factors that inhibit skeletal muscle adaptability in response to chronic ischemia. Poor angiogenic capacity is known to play a vital role in skeletal muscle adaptability (Olfert et al. 2016; Yan et al. 2011). Lack of revascularization is already reported in elderly frailty and in many metabolic disorders, where metabolic factors and associated signaling pathways are found to be involved in a variety of skeletal muscle dysfunction (Evans et al. 2010). In addition, HHcy is well studied in the cardiovascular system, where it has been shown to increase HHcy-mediated increased oxidative stress causing dysfunction of endothelial cells (ECs), swelling and vacuolization of ECs, fibrin deposition, and even clot formation in vessels (Loscalzo 2009; Refsum et al. 1998).
Angiogenesis is a physiological process, where new blood vessels form from pre-existing ones. It is vital for growth and development, which depend on supply of nutrients, oxygen, and waste disposal (Logsdon et al. 2014). Previous studies demonstrated that HHcy impairs angiogenesis in multiple tissues (Duan et al. 2000; Jacovina et al. 2009; Nagai et al. 2001; Oosterbaan et al. 2012; Zhang et al. 2012). During angiogenesis, the main angiogenic signal is that vascular endothelial growth factor (VEGF) binds to VEGF receptor on EC surface. This promotes EC growth and migration towards the source of VEGF. An early study showed that Hcy treatment on chicken embryos (embryonic day 3.5) causes inhibition of early extraembryonic vascular development, altered composition of the vascular beds, and reduction of VEGF-A and VEGFR-2 expression (Oosterbaan et al. 2012). Similarly, when HUVEC cells were treated with Hcy, it reduced the cell numbers, viability, and induced a G1/S arrest as well as attenuate the cell migration capacity and inhibited tube-like formation on Matrigel (Zhang et al. 2012). This study showed that this effect is mediated through VEGF/VEGFR, Akt, and ERK½ inhibition (Zhang et al. 2012). Similarly, a study demonstrated that Hcy inhibits angiogenesis by preventing proliferation and migration of endothelial cells in a dose-dependent manner (Nagai et al. 2001). In an in vivo rat model, it was reported that HHcy impaired ischemia-induced angiogenesis and collateral vessel formation, which is partly mediated through the reduction in bioactivity of endogenous NO (Duan et al. 2000). Also, many groups showed that Hcy-mediated defective angiogenesis is largely due to decreasing in glutathione peroxidase (GPx) expression and consequent increase in oxidant stress, leading to endothelial progenitor cell dysfunction (Galasso et al. 2006; Handy et al. 2005), reduction of the bioavailability of NO (Eberhardt et al. 2000; Upchurch et al. 1997), and dysregulation of matrix metalloproteinases (MMPs) (Kundu et al. 2009, 2015).
Our lab showed that HHcy inhibits expression of HIF1α and VEGF after hindlimb ischemia; however the molecular mechanisms were not precisely studied. Previous studies already showed that high Hcy can be toxic to endothelial cells and can induce endothelium dysfunction (Harker et al. 1983; Lee and Wang 1999; Lentz et al. 1996; Wang et al. 1997). Along these lines, a study proposed that this effect may be mediated through a hypomethylation-related mechanism (Wang et al. 1997). It also noticed that Hcy affects ECs via inhibiting cyclin A (Jamaluddin et al. 2007). When they transduced cyclin A in ECs, the inhibitory effect of ECs was rescued. These studies have been conducted in an in vitro system; however there is much less evidence reported for in vivo system. Nevertheless there is no treatment strategy to improve endothelium dysfunction during HHcy-mediated myocardial/skeletal muscle ischemia, peripheral arterial disease, and organ transplantation, where endothelial damage is a major characteristic.
Hyperhomocysteinemia mediated skeletal muscle atrophy
As mentioned earlier skeletal muscle is the largest organ in our body, containing 50%–75% of the body’s proteins. Loss of skeletal muscle mass is commonly referred to as muscle atrophy. Several E3 ubiquitin ligases such as MuRF1, MAFBx (Atrogin-1), Nedd4.1, TRAF6, and MUSA1 have been identified, which mediate proteolytic degradation of both thick and thin filament proteins in skeletal muscle (Bodine and Baehr 2014; Bonaldo and Sandri 2013). Although several markers of ER stress including CHOP have been shown to increase in a model of skeletal muscle atrophy, the mechanisms are unclear (Yu et al. 2011). The purpose of capillaries is to serve as the interface for delivery of oxygen and removal of metabolites to/from tissues. Angiogenesis plays a vital role, and it is required during tissue injury/wound repair, the endometrial cycle, and striated muscle adaptation to stress/exercise, etc. (Olfert et al. 2016). Hcy has been implicated in vascular toxicity, causing endothelial cell damage and dysfunction (McCully 2007). Our group showed that HHcy antagonizes the angiogenic signals via reduced expression of HIF1-α, VEGF, and eNOS in skeletal muscle (Chen et al. 2014; Veeranki et al. 2014, 2015). Although HHcy inhibits Akt (also known as protein kinase B) activation in endothelial cells that has been linked to improving angiogenesis, but whether these 2 processes potentiate myopathy is not studied yet (Manning and Cantley 2007).
It has been reported that HHcy is associated with a decline in physical performance and skeletal muscle dysfunction in genetically engineered CBS deficient mice (CBS+/−) (Veeranki and Tyagi 2013, 2015a; Veeranki et al. 2015). Skeletal muscle dysfunction and poor physical performance can result from a reduction of muscle mass and (or) structural and metabolic alteration (Filler et al. 2014; Myhill et al. 2009; Westerblad et al. 2010). Indeed, a study found that cystathionine γ-Lyase-deficient mice have lethal myopathy and oxidative injury, wherein dietary cysteine supplementation could mitigate this effect (Ishii et al. 2010). Another group reported patients with HHcy due to methyltetrahydrofolate reductase (MTHFR) mutations (C677T and A1298C) showed poor survival and muscle pain (Osian et al. 2009). Similarly, a study from our lab concluded that vasodilation in skeletal muscle arterioles is decreased during HHcy because of reduced expression of gap junction proteins (connexins 37, 40, and 43) and increased expression of myostatin in the skeletal muscle (Givvimani et al. 2013).
Mitigation of skeletal muscle dysfunction by H2S
H2S is increasingly being recognized as a novel endogenous gasotransmitter like nitric oxide (NO) and carbon monoxide (CO) (Gadalla and Snyder 2010; Polhemus and Lefer 2014; Szabó 2007). Being a potent anti-apoptotic/anti-necrotic (Jiang et al. 2016; Sun et al. 2017), anti-inflammatory (Sağlam et al. 2017), and cytoprotective agent (Gemici et al. 2015), H2S plays crucial roles in physiological homeostasis, wherein it reduces oxidative damage (George et al. 2018b; Mironov et al. 2017; Xie et al. 2016). H2S is produced endogenously from Hcy in various mammalian tissues by 2 main enzymes (CBS and CSE) in the transsulfuration pathway (Asimakopoulou et al. 2013). As mentioned earlier, high SAM levels can act as an allosteric inhibitor for MTHFR of remethylation pathway (Finkelstein and Martin 1984) and allosteric activator for CBS of the transsulfuration pathway (Finkelstein et al. 1975; Stabler et al. 2002). Together these results suggest that the presence of a high level of SAM favors Hcy entering the transsulfuration pathway, however in patients with CBS mutation, a high SAM level builds up (due to methionine load) leading to transient accumulation of Hcy and reduction of H2S levels. The endogenous H2S production ranges from 1–10 pmoles per second per mg protein (Doeller et al. 2005). Patients with CBS mutation have a high amount of Hcy and tend to produce a lesser amount of H2S (Beard and Bearden 2011; Kozich ˇ et al. 2016), suggesting that these patients are likely more prone to oxidative stress-mediated damage (Szabó 2007). Studies revealed that endogenous H2S could modulate multiple signaling pathways, upregulate endogenous antioxidant systems, and exert additive effects with known antioxidants (Xie et al. 2016), suggesting that exogenous supplementation of H2S could be employed as a beneficial strategy to improve redox imbalance owing to HHcy.
H2S and oxidative/ER stress
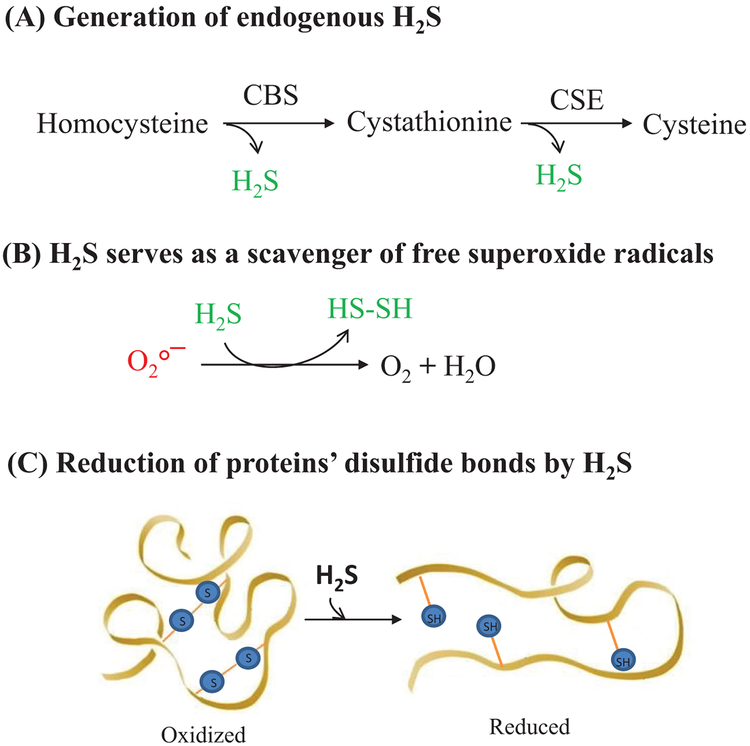
Multiple studies demonstrated the cytoprotective (anti-necrotic/apoptotic/pyroptotic) effects of H2S in different in vitro models, all relating to its ability to neutralize a variety of reactive species (Whiteman et al. 2004, 2005; Yan et al. 2006) and reduction of a disulfide bond in proteins (Greiner et al. 2013; Szabó 2007). H2S in water dissociates into H+, HS−, and S2− ions. HS− has a capacity to scavenge ROS (Fig. 4). H2S itself has also been recognized to be a reducing agent, as it can react directly with and quench the super-oxide anion (O2−) (Al-Magableh et al. 2014; Predmore et al. 2012) and free radicals like peroxynitrite (Whiteman et al. 2006) as well as other ROS in vitro. Micro concentrations of H2S generated from Na2S/NaHS were found to neutralize free oxyradicals (Geng et al. 2004), peroxynitrite (Whiteman et al. 2004), hypochlorous acid (Whiteman et al. 2005), and homocysteine (Yan et al. 2006) in in vitro conditions. There is no sulfide receptor in mammalian cells that is responsible for the biological actions of sulfide, hence sulfide, as a thiol with strong reducing activities, may also be a redox-controlling molecule similar to other small thiols, such as cysteine and glutathione (Kimura and Kimura 2004; Whiteman et al. 2004). A study by Kimura and Kimura (2004) using primary cultures of neurons, found that H2S increases the cellular glutathione levels by enhancing the activity of gamma-glutamylcysteine synthetase upregulating cystine transport (Kimura and Kimura 2004). Similarly, another study reported that 100 μmol/L NaHS induces glutamate uptake by assisting glial glutamate transporter-1 (GLT-1) and enhances cysteine transport and GSH synthesis (Lu et al. 2008). In support of this effect, multiple studies demonstrated that H2S induces cellular GSH in the brain (Tyagi et al. 2009), spinal cord (Kesherwani et al. 2013), heart (Huang et al. 2013), lung (Wang et al. 2011), kidney (Sen et al. 2009), liver (Wang et al. 2011), and gastrointestinal tract. Moreover, recent reports suggested that H2S could attenuate cellular oxidative stress by improving activities of CAT (Fu et al. 2008; Huang et al. 2013; Wen et al. 2013; Zhu et al. 2013) and GPx (Benetti et al. 2013; Liu et al. 2013; Su et al. 2009). In addition to oxidative stress, HHcy also induces ER stress in multiple tissues. Many studies showed that H2S attenuates ER stress in numerous tissue types (Majumder et al. 2018a). A study found that H2S attenuated HHcy-induced cardiomyocytic ER stress in rats (Wei et al. 2010). Li et al. (2015) demonstrated that H2S protected against myocardial ischemia/reperfusion injury in rats by inhibiting ER stress (Li et al. 2015). Likewise, another study also reported that H2S exerts its protection against the neurotoxicity of formal-dehyde by overcoming ER stress in PC12 cells (Li et al. 2014).
Fig. 4.
Salubrious effects of H2S on cellular biochemistry. (A) Being an important antioxidant molecule H2S helps synthesize cysteine, which gets converted into GSH via cystathionine-β-synthase (CBS) and cystathionine γ-lyase (CSE) enzymatic pathways. (B) H2S is a potent scavenger for a variety of reactive oxygen species (ROS). (C) H2S is known to reduce disulfide bonds in proteins, thereby reversing the homocysteinylation of proteins during hyperhomocysteinemia conditions.
H2S and cell death pathways
In addition to an antioxidant effect of H2S, several studies also proposed that it has the ability to reduce cell death in multiple tissues. An in vitro study using nucleus pulposus (NP) from patients with lumbar disc herniation showed that exogenous H2S donor attenuated hypoxia (generated by CoCl2 treatment) induced apoptosis in primary rat NP cells (Sun et al. 2018). Similarly, a study using a rat model found that H2S mitigates cigarette smoke-induced apoptosis via reducing ER stress (Lin et al. 2017). H2S also decreased Hcy-mediated ER stress and apoptosis in the hippocampus of rats via enhancing the BDNF-TrkB pathway (Wei et al. 2014). In support of this, a study showed that NaHS supplementation could significantly inhibit hypoxia-induced neuronal apoptosis through inhibition of ROS (mainly H2O2)-activated Ca2+ signaling pathway in mouse hippocampal neurons (Luo et al. 2012). In another study, it was observed that H2S attenuated gentamicin-induced apoptosis via reducing ROS moiety in normal rat kidney-52E cells, where they found the protein levels of Bax, Caspase-3, and cleaved-caspase-3 were decreased, and the expression of Bcl-2 was increased (Wu et al. 2017). Similarly, NaHS (H2S donor) administration significantly inhibited the early phosphorylation of JNK and decreased the number of apoptotic cells, lowered cytochrome C release, and enhanced Bcl-2 expression in cardiomyocyte after ischemia-reperfusion (I/Re) injury (Shi et al. 2009). H2S also attenuated 1-methyl-4-phenylpyridinium ion (MPP(+))-induced apoptosis in PC12 cells by attenuating an overproduction of intracellular ROS. Exogenous NaHS could ameliorate early brain injury after subarachnoid hemorrhage (SAH) by inhibiting neuronal apoptosis by reducing the activity of the MST1 protein (Yin et al. 2009).
H2S and angiogenic defect
Apart from antioxidative effects of H2S, it has been noticed that H2S is also involved in vasodilation, vascular protection, regulation of blood pressure, and many other functions (Calvert et al. 2010; Elsey et al. 2010; Gadalla and Snyder 2010; Predmore and Lefer 2010; Szabó 2007). Like other gasotransmitters (CO and NO), H2S can rapidly travel to vascular tissues without any transporter exerting a host of biological responses. Two independent groups reported, using either transformed endothelial cell line (RF/6A) or HUVECs, that H2S induces proliferation and migration of ECs (Cai et al. 2007; Papapetropoulos et al. 2009). The pro-angiogenic effect of H2S is further supported by the increase in tube-like structure formation in ECs in in vitro Matrigel assays (Cai et al. 2007; Papapetropoulos et al. 2009). H2S exerts angiogenic effects via releasing VEGF in smooth muscle cells, ECs (Szabó and Papapetropoulos 2011;Wang et al. 2015). Apart from in vitro studies, some in vivo studies demonstrated that H2S induced a concentration-dependent increase in the length and complexity of the vascular network (Cai et al. 2007; Papapetropoulos et al. 2009). When ECs were exposed to H2S donors, it activated multiple signaling pathways such as PI-3K/Akt and MAPK pathways during neovascularization (Cai et al. 2007; Papapetropoulos et al. 2009). Further evidence for H2S-mediated VEGF induction during angiogenesis was reported in an ex vivo mouse aorta sprouting assay, where the VEGF-induced angiogenesis was markedly suppressed in aortic rings of the CSE−/− mice (Papapetropoulos et al. 2009). In an alternative pathway, exposure to H2S increased calcium levels in ECs, which would lead to eNOS activation and increased NO release during angiogenesis (Bauer et al. 2010; Minamishima et al. 2009; Yusof et al. 2009).
H2S and muscle atrophy
Skeletal muscles are now considered to be an endocrine organ, synthesizing and secreting a variety of bioactive molecules including inflammatory cytokines, growth factors, adipokines, carnitine, and more recently H2S (Du et al. 2013). In this context, H2S appears to protect against low oxygen and nutrient supply as well as ischemic injury in multiple organs including skeletal muscles (Du et al. 2013; Suzuki et al. 2011; Veeranki and Tyagi 2015b). Similarly, recent studies have confirmed that H2S plays an anti-atherosclerotic role, and its deficiency leads to early development and progression of atherosclerosis (Wang et al. 2009). In agreement with this view, it was hypothesized that dysregulation of H2S metabolism is involved in chronic fatigue syndrome, also called myalgic encephalomyelitis (Lemle 2009). Among the known cellular targets of H2S, potassium channels have been the first to be discovered (Tang et al. 2005; Zhao et al. 2001). KATP channel activation has been suggested to be involved in the prevention of calcium overload and preservation of myofibre integrity during exercise, as well as recovery from muscle fatigue, rather than in normal muscle contractility and excitability (MacIntosh et al. 2012; Matar et al. 2000). Similarly, a significant decrease in H2S content in muscle fibers, together with a reduction in SOD1 expression, was presented in a rat model of skeletal muscle ischemiareperfusion (I-R) injury (Du et al. 2013; Majumder et al. 2019).
Conclusions
So far, we and others have discovered apparent beneficial effects of H2S on skeletal muscle dysfunction during chronic HHcy conditions, and the potential mechanistic role that H2S plays during oxidative/ER stress responses, apoptosis, angiogenesis, and muscle atrophy (Fig. 5). However there is still a lot left to clarify with regards to skeletal muscle functions. (i) Most of the previous research has been done studying the effects of HHcy on skeletal muscle not exactly simulating the physiological conditions observed in HHcy patients, so a suitable animal model is still lacking. (ii) As the half-life of most of the H2S donors is much less, most of the effects of H2S vary in different studies. (iii) There are many more hurdles to overcome in regard to identifying a proper delivery method for H2S, as skeletal muscle dysfunction ranges from general muscle weakness and soreness to severe myopathy, muscle wasting, and cachexia. (iv) These complex dysfunctions do not even necessarily originate from skeletal muscle; even though many studies have been devoted to clarifying the molecular mechanisms involved in E-C coupling in vitro, the real impact of these mechanisms are very difficult to assess in vivo settings because of the intrinsic complexity of this phenomenon.
Fig. 5.
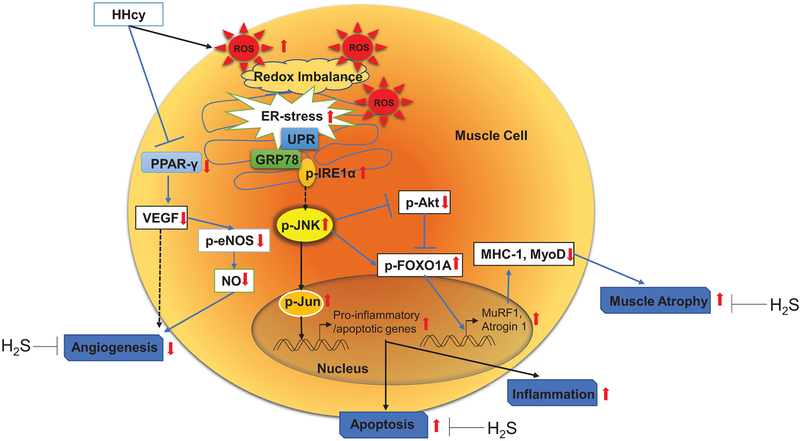
Schematic diagram highlighting hyperhomocysteinemia (HHcy)-mediated skeletal muscle dysfunction via induction of apoptosis, endoplasmic reticulum (ER), oxidative stress responses, and inhibition of angiogenesis that can lead to skeletal muscle atrophy as a result of JNK-activation and concurrent impairment of PI3K/AKT survival axis. H2S, by virtue of it multi-farious beneficial properties, could mitigate these harmful effects successfully. eNOS, endothelial nitric oxide synthase; FOXO, forkhead box protein O; IRE1α, inositol-requiring enzyme-1α; JNK, c-Jun N-terminal kinase; MuRF1, muscle RING-finger protein-1; PPAR-γ, peroxisome proliferator-activated receptor-γ; UPR, unfolded protein response; VEGF, vascular endothelial growth factor.
Acknowledgements
The work was supported by grants from the National Institute of Health (Heart, Lung, and Blood Institute; No. HL-74815, HL-107640) and the Institute of Neurological Disorders and Stroke (No. NS-084823).
Footnotes
This paper is part of a Special Issue of selected papers from Cardiology 2018 held in Havana, Cuba, on 5–8 June 2018.
Conflict of interest
The authors declare that there is no conflict of interest associated with this work.
Contributor Information
Avisek Majumder, Department of Physiology, University of Louisville School of Medicine, Louisville, KY 40202, USA; Biochemistry and Molecular Genetics, University of Louisville School of Medicine, Louisville, KY 40202, USA..
Mahavir Singh, Department of Physiology, University of Louisville School of Medicine, Louisville, KY 40202, USA; Eye and Vision Science Laboratory, University of Louisville School of Medicine, Louisville, KY 40202, USA..
Akash K. George, Department of Physiology, University of Louisville School of Medicine, Louisville, KY 40202, USA; Eye and Vision Science Laboratory, University of Louisville School of Medicine, Louisville, KY 40202, USA.
Suresh C. Tyagi, Department of Physiology, University of Louisville School of Medicine, Louisville, KY 40202, USA.
References
- Adham IM, Eck TJ, Mierau K, Müller N, Sallam MA, Paprotta I, et al. 2005. Reduction of spermatogenesis but not fertility in Creb3l4-deficient mice. Mol. Cell. Biol 25(17): 7657–7664. doi: 10.1128/MCB.25.17.7657-7664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre V, Uchida T, Yenush L, Davis R, and White MF 2000. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J. Biol. Chem 275(12): 9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, and White MF 2002. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J. Biol. Chem 277(2): 1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- Allen DG, and Westerblad H 2007. Understanding muscle from its length.J. Physiol 583(Pt. 1): 3–4. doi: 10.1113/jphysiol.2007.137067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Magableh MR, Kemp-Harper BK, Ng HH, Miller AA, and Hart JL 2014. Hydrogen sulfide protects endothelial nitric oxide function under conditions of acute oxidative stress in vitro. Naunyn Schmiedebergs Arch. Pharmacol 387(1): 67–74. doi: 10.1007/s00210-013-0920-x. [DOI] [PubMed] [Google Scholar]
- Asimakopoulou A, Panopoulos P, Chasapis CT, Coletta C, Zhou Z, Cirino G, et al. 2013. Selectivity of commonly used pharmacological inhibitors for cystathionine beta synthase (CBS) and cystathionine gamma lyase (CSE). Br. J. Pharmacol 169(4): 922–932. doi: 10.1111/bph.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CC, Boyle JP, Porter KE, and Peers C 2010. Modulation of Ca(2+) signalling in human vascular endothelial cells by hydrogen sulfide. Atherosclerosis, 209(2): 374–380. doi: 10.1016/j.atherosclerosis.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Beard RS Jr., and Bearden SE 2011. Vascular complications of cystathionine beta-synthase deficiency: future directions for homocysteine-to-hydrogen sulfide research. Am. J. Physiol. Heart Circ. Physiol 300(1): H13–H26. doi: 10.1152/ajpheart.00598.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behera J, George AK, Voor MJ, Tyagi SC, and Tyagi N 2018. Hydrogen sulfide epigenetically mitigates bone loss through OPG/RANKL regulation during hyperhomocysteinemia in mice. Bone, 114: 90–108. doi: 10.1016/j.bone.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetti LR, Campos D, Gurgueira SA, Vercesi AE, Guedes CE, Santos KL, et al. 2013. Hydrogen sulfide inhibits oxidative stress in lungs from allergic mice in vivo. Eur. J. Pharmacol 698(1–3): 463–469. doi: 10.1016/j.ejphar.2012.11.025. [DOI] [PubMed] [Google Scholar]
- Bernales S, McDonald KL, and Walter P 2006. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol 4(12): e423. doi: 10.1371/journal.pbio.0040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessede G, Miguet C, Gambert P, Neel D, and Lizard G 2001. Efficiency of homocysteine plus copper in inducing apoptosis is inversely proportional to gamma-glutamyl transpeptidase activity. FASEB J 15(11): 1927–1940. doi: 10.1096/fj.00-0848com. [DOI] [PubMed] [Google Scholar]
- Bodine SC, and Baehr LM 2014. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab 307(6): E469–E484. doi: 10.1152/ajpendo.00204.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, et al. 2001. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol 3(11): 1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- Bonaldo P, and Sandri M 2013. Cellular and molecular mechanisms of muscle atrophy. Dis. Model. Mech 6(1): 25–39. doi: 10.1242/dmm.010389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustolin S, Giugliani R, and Félix TM 2010. Genetics of homocysteine metabolism and associated disorders. Braz. J. Med. Biol. Res 43(1): 1–7. doi: 10.1590/S0100-879X2009007500021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büdy B, O’Neill R, DiBello PM, Sengupta S, and Jacobsen DW 2006. Homo-cysteine transport by human aortic endothelial cells: identification and properties of import systems. Arch. Biochem. Biophys 446(2): 119–130. doi: 10.1016/j.abb.2005.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai B, Li X, Wang Y, Liu Y, Yang F, Chen H, et al. 2013. Apoptosis of bone marrow mesenchymal stem cells caused by homocysteine via activating JNK signal. PLoS One, 8(5): e63561. doi: 10.1371/journal.pone.0063561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai WJ, Wang MJ, Moore PK, Jin HM, Yao T, and Zhu YC 2007. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc. Res 76(1): 29–40. doi: 10.1016/j.cardiores.2007.05.026. [DOI] [PubMed] [Google Scholar]
- Calvert JW, Coetzee WA, and Lefer DJ 2010. Novel insights into hydrogen sulfide–mediated cytoprotection. Antioxid. Redox Signal 12(10): 1203–1217. doi: 10.1089/ars.2009.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao SS 2015. Endoplasmic reticulum stress and unfolded protein response in inflammatory bowel disease. Inflamm. Bowel Dis 21(3): 636–644. doi: 10.1097/MIB.0000000000000238. [DOI] [PubMed] [Google Scholar]
- Cascella M, Arcamone M, Morelli E, Viscardi D, Russo V, De Franciscis S, et al. 2015. Erratum to: Multidisciplinary approach and anesthetic management of a surgical cancer patient with methylene tetrahydrofolate reductase deficiency: a case report and review of the literature. J. Med. Case Rep 9: 218. doi: 10.1186/s13256-015-0706-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chargé SB, and Rudnicki MA 2004. Cellular and molecular regulation of muscle regeneration. Physiol. Rev 84(1): 209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhao S, Wang Y, Li Y, Bai L, Liu R, et al. 2014. Homocysteine reduces protein S-nitrosylation in endothelium. Int. J. Mol. Med 34(5): 1277–1285. doi: 10.3892/ijmm.2014.1920. [DOI] [PubMed] [Google Scholar]
- Clavel S, Siffroi-Fernandez S, Coldefy AS, Boulukos K, Pisani DF, and Dérijard B 2010. Regulation of the intracellular localization of Foxo3a by stress-activated protein kinase signaling pathways in skeletal muscle cells. Mol. Cell. Biol 30(2): 470–480. doi: 10.1128/MCB.00666-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criollo A, Maiuri MC, Tasdemir E, Vitale I, Fiebig AA, Andrews D, et al. 2007. Regulation of autophagy by the inositol trisphosphate receptor. Cell Death Differ 14(5): 1029–1039. doi: 10.1038/sj.cdd.4402099. [DOI] [PubMed] [Google Scholar]
- Deng X, Xiao L, Lang W, Gao F, Ruvolo P, and May WS Jr. 2001. Novel role for JNK as a stress-activated Bcl2 kinase. J. Biol. Chem 276(26): 23681–23688. doi: 10.1074/jbc.M100279200. [DOI] [PubMed] [Google Scholar]
- Deschenes MR 2011. Motor unit and neuromuscular junction remodeling with aging. Curr. Aging Sci 4(3): 209–220. doi: 10.2174/1874609811104030209. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran DN, and Reddy EP 2008. JNK signaling in apoptosis. Onco-gene, 27(48): 6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Simone N, Maggiano N, Caliandro D, Riccardi P, Evangelista A, Carducci B, and Caruso A 2003. Homocysteine induces trophoblast cell death with apoptotic features. Biol. Reprod 69(4): 1129–1134. doi: 10.1095/biolreprod.103.015800. [DOI] [PubMed] [Google Scholar]
- Diakoumopoulou E, Tentolouris N, Kirlaki E, Perrea D, Kitsou E, Psallas M, et al. 2005. Plasma homocysteine levels in patients with type 2 diabetes in a Mediterranean population: relation with nutritional and other factors. Nutr. Metab. Cardiovasc. Dis 15(2): 109–117. doi: 10.1016/j.numecd.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Ding WX, Ni HM, Gao W, Hou YF, Melan MA, Chen X, et al. 2007. Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J. Biol. Chem 282(7): 4702–4710. doi: 10.1074/jbc.M609267200. [DOI] [PubMed] [Google Scholar]
- Doeller JE, Isbell TS, Benavides G, Koenitzer J, Patel H, Patel RP, et al. 2005. Polarographic measurement of hydrogen sulfide production and consumption by mammalian tissues. Anal. Biochem 341(1): 40–51. doi: 10.1016/j.ab.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Du JT, Li W, Yang JY, Tang CS, Li Q, and Jin HF 2013. Hydrogen sulfide is endogenously generated in rat skeletal muscle and exerts a protective effect against oxidative stress. Chin. Med. J 126(5): 930–936. [PubMed] [Google Scholar]
- Duan J, Murohara T, Ikeda H, Sasaki K, Shintani S, Akita T, et al. 2000. Hyperhomocysteinemia impairs angiogenesis in response to hindlimb ischemia. Arterioscler. Thromb. Vasc. Biol 20(12): 2579–2585. doi: 10.1161/01.ATV.20.12.2579. [DOI] [PubMed] [Google Scholar]
- Dupont-Versteegden EE 2005. Apoptosis in muscle atrophy: relevance to sarcopenia. Exp. Gerontol 40(6): 473–481. doi: 10.1016/j.exger.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Eberhardt RT, Forgione MA, Cap A, Leopold JA, Rudd MA, Trolliet M, et al. 2000. Endothelial dysfunction in a murine model of mild hyperhomo-cyst(e)inemia. J. Clin. Invest 106(4): 483–491. doi: 10.1172/JCI8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore S 2007. Apoptosis: a review of programmed cell death. Toxicol. Pathol 35(4): 495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eloranta TO, Martikainen V, and Smith TK 1990. Adaptation of adenosylmethionine metabolism and methionine recycling to variations in dietary methionine in the rat. Proc. Soc. Exp. Biol. Med 194(4): 364–371. doi: 10.3181/00379727-194-43110. [DOI] [PubMed] [Google Scholar]
- Elsey DJ, Fowkes RC, and Baxter GF 2010. Regulation of cardiovascular cell function by hydrogen sulfide (H(2)S). Cell. Biochem. Funct 28(2): 95–106. doi: 10.1002/cbf.1618. [DOI] [PubMed] [Google Scholar]
- Evans WJ, Paolisso G, Abbatecola AM, Corsonello A, Bustacchini S, Strollo F, and Lattanzio F 2010. Frailty and muscle metabolism dysregulation in the elderly. Biogerontology, 11(5): 527–536. doi: 10.1007/s10522-010-9297-0. [DOI] [PubMed] [Google Scholar]
- Familtseva A, Kalani A, Chaturvedi P, Tyagi N, Metreveli N, and Tyagi SC 2014. Mitochondrial mitophagy in mesenteric artery remodeling in hyperhomocysteinemia. Physiol. Rep 2(4): e00283. doi: 10.14814/phy2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Familtseva A, Chaturvedi P, Kalani A, Jeremic N, Metreveli N, Kunkel GH, and Tyagi SC 2016. Toll-like receptor 4 mutation suppresses hyperhomocysteinemia-induced hypertension. Am. J. Physiol. Cell Physiol 311(4): C596–C606. doi: 10.1152/ajpcell.00088.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filler K, Lyon D, Bennett J, McCain N, Elswick R, Lukkahatai N, and Saligan LN 2014. Association of mitochondrial dysfunction and fatigue: a review of the literature. BBA Clin 1: 12–23. doi: 10.1016/j.bbacli.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein JD 1990. Methionine metabolism in mammals. J. Nutr. Biochem 1(5): 228–237. doi: 10.1016/0955-2863(90)90070-2. [DOI] [PubMed] [Google Scholar]
- Finkelstein JD, and Martin JJ 1984. Methionine metabolism in mammals. Distribution of homocysteine between competing pathways. J. Biol. Chem 259(15): 9508–9513. [PubMed] [Google Scholar]
- Finkelstein JD, Kyle WE, Martin JL, and Pick AM 1975. Activation of cystathionine synthase by adenosylmethionine and adenosylethionine. Biochem. Biophys. Res. Commun 66(1): 81–87. doi: 10.1016/S0006-291X(75)80297-X. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Distrutti E, Cirino G, and Wallace JL 2006. The emerging roles of hydrogen sulfide in the gastrointestinal tract and liver. Gastroenterology, 131(1): 259–271. doi: 10.1053/j.gastro.2006.02.033. [DOI] [PubMed] [Google Scholar]
- Frontera WR, and Ochala J 2015. Skeletal muscle: a brief review of structure and function. Calcif. Tissue Int 96(3): 183–195. doi: 10.1007/s00223-014-9915-y. [DOI] [PubMed] [Google Scholar]
- Fu Z, Liu X, Geng B, Fang L, and Tang C 2008. Hydrogen sulfide protects rat lung from ischemia-reperfusion injury. Life Sci 82(23–24): 1196–1202. doi: 10.1016/j.lfs.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Fujita E, Kouroku Y, Isoai A, Kumagai H, Misutani A, Matsuda C, et al. 2007. Two endoplasmic reticulum-associated degradation (ERAD) systems for the novel variant of the mutant dysferlin: ubiquitin/proteasome ERAD(I) and autophagy/lysosome ERAD(II). Hum. Mol. Genet 16(6): 618–629. doi: 10.1093/hmg/ddm002. [DOI] [PubMed] [Google Scholar]
- Gadalla MM, and Snyder SH 2010. Hydrogen sulfide as a gasotransmitter.J. Neurochem 113(1): 14–26. doi: 10.1111/j.1471-4159.2010.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galasso G, Schiekofer S, Sato K, Shibata R, Handy DE, Ouchi N, et al. 2006. Impaired angiogenesis in glutathione peroxidase-1-deficient mice is associated with endothelial progenitor cell dysfunction. Circ. Res 98(2): 254–261. doi: 10.1161/01.RES.0000200740.57764.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly P, and Alam SF 2015. Role of homocysteine in the development of cardiovascular disease. Nutr. J 14: 6. doi: 10.1186/1475-2891-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner BM, Pincus D, Gotthardt K, Gallagher CM, and Walter P 2013. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb. Perspect. Biol 5(3): a013169. doi: 10.1101/cshperspect.a013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparetto C, Malinverno A, Culacciati D, Gritti D, Prosperini PG, Specchia G, and Ricevuti G 2005. Antioxidant vitamins reduce oxidative stress and ventricular remodeling in patients with acute myocardial infarction. Int. J. Immunopathol. Pharmacol 18(3): 487–496. doi: 10.1177/039463200501800308. [DOI] [PubMed] [Google Scholar]
- Gehlert S, Bloch W, and Suhr F 2015. Ca2+-dependent regulations and signaling in skeletal muscle: from electro-mechanical coupling to adaptation. Int. J. Mol. Sci 16(1): 1066–1095. doi: 10.3390/ijms16011066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemici B, Elsheikh W, Feitosa KB, Costa SK, Muscara MN, and Wallace JL 2015. H2S-releasing drugs: anti-inflammatory, cytoprotective and chemopreventative potential. Nitric Oxide, 46: 25–31. doi: 10.1016/j.niox.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Geng B, Chang L, Pan C, Qi Y, Zhao J, Pang Y, et al. 2004. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem. Biophys. Res. Commun 318(3): 756–763. doi: 10.1016/j.bbrc.2004.04.094. [DOI] [PubMed] [Google Scholar]
- George AK, Behera J, Kelly KE, Mondal NK, Richardson KP, and Tyagi N 2018a. Exercise mitigates alcohol induced endoplasmic reticulum stress mediated cognitive impairment through ATF6-Herp signaling. Sci. Rep 8(1): 5158. doi: 10.1038/s41598-018-23568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George AK, Singh M, Homme RP, Majumder A, Sandhu HS, and Tyagi SC 2018b. A hypothesis for treating inflammation and oxidative stress with hydrogen sulfide during age-related macular degeneration. Int. J. Ophthalmol 11(5): 881–887. doi: 10.18240/ijo.2018.05.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givvimani S, Narayanan N, Armaghan F, Pushpakumar S, and Tyagi SC 2013. Attenuation of conducted vasodilation in skeletal muscle arterioles during hyperhomocysteinemia. Pharmacology, 91(5–6): 287–296. doi: 10.1159/000350394. [DOI] [PubMed] [Google Scholar]
- Green DR, and Reed JC 1998. Mitochondria and apoptosis. Science, 281(5381):1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Greiner R, Pálinkás Z, Bäsell K, Becher D, Antelmann H, Nagy P, and Dick TP 2013. Polysulfides link H2S to protein thiol oxidation. Antioxid. Redox Signal 19(15): 1749–1765. doi: 10.1089/ars.2012.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Moustapha A, Jacobsen DW, Goormastic M, Tuzcu EM, Hobbs R, et al. 1998. High homocysteine, low folate, and low vitamin B6 concentrations: prevalent risk factors for vascular disease in heart transplant recipients. Transplantation, 65(4): 544–550. doi: 10.1097/00007890-199802270-00016. [DOI] [PubMed] [Google Scholar]
- Handy DE, Zhang Y, and Loscalzo J 2005. Homocysteine down-regulates cellular glutathione peroxidase (GPx1) by decreasing translation. J. Biol. Chem 280(16): 15518–15525. doi: 10.1074/jbc.M501452200. [DOI] [PubMed] [Google Scholar]
- Hankey GJ, and Eikelboom JW 1999. Homocysteine and vascular disease. Lancet, 354(9176): 407–413. doi: 10.1016/S0140-6736(98)11058-9. [DOI] [PubMed] [Google Scholar]
- Harker LA, Harlan JM, and Ross R 1983. Effect of sulfinpyrazone on homocysteine-induced endothelial injury and arteriosclerosis in baboons. Circ. Res 53(6): 731–739. doi: 10.1161/01.RES.53.6.731. [DOI] [PubMed] [Google Scholar]
- Hart LS, Cunningham JT, Datta T, Dey S, Tameire F, Lehman SL, et al. 2012. ER stress-mediated autophagy promotes Myc-dependent transformation and tumor growth. J. Clin. Invest 122(12): 4621–4634. doi: 10.1172/JCI62973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassler J, Cao SS, and Kaufman RJ 2012. IRE1, a double-edged sword in pre-miRNA slicing and cell death. Dev. Cell, 23(5): 921–923. doi: 10.1016/j.devcel.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawke TJ, and Garry DJ 2001. Myogenic satellite cells: physiology to molecular biology. J. Appl. Physiol 91(2): 534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- Hayden MR, and Tyagi SC 2004. Homocysteine and reactive oxygen species in metabolic syndrome, type 2 diabetes mellitus, and atheroscleropathy: the pleiotropic effects of folate supplementation. Nutr. J 3: 4. doi: 10.1186/1475-2891-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollien J, Lin JH, Li H, Stevens N, Walter P, and Weissman JS 2009.. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells.J. Cell Biol 186(3): 323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homme RP, Singh M, Majumder A, George AK, Nair K, Sandhu HS, et al. 2018. Remodeling of Retinal architecture in diabetic retinopathy: disruption of ocular physiology and visual functions by inflammatory gene products and pyroptosis. Front. Physiol 9: 1268. doi: 10.3389/fphys.2018.01268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høyer-Hansen M, and Jäättelä M 2007. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ 14(9): 1576–1582. doi: 10.1038/sj.cdd.4402200. [DOI] [PubMed] [Google Scholar]
- Høyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, et al. 2007. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol. Cell, 25(2): 193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Hsu HC, Chiou JF, Wang YH, Chen CH, Mau SY, Ho CT, et al. 2013. Folate deficiency triggers an oxidative-nitrosative stress-mediated apoptotic cell death and impedes insulin biosynthesis in RINm5F pancreatic islet beta-cells: relevant to the pathogenesis of diabetes. PLoS One, 8(11): e77931. doi: 10.1371/journal.pone.0077931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Kan J, Liu X, Ma F, Tran BH, Zou Y, et al. 2013. Cardioprotective effects of a novel hydrogen sulfide agent-controlled release formulation of S-propargyl-cysteine on heart failure rats and molecular mechanisms. PLoS One, 8(7): e69205. doi: 10.1371/journal.pone.0069205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal MP, Lindblad BS, Mehboobali N, Yusuf FA, Khan AH, and Iqbal SP 2009. Folic acid and vitamin B6 deficiencies related hyperhomocysteinemia in apparently healthy Pakistani adults; is mass micronutrient supplementation indicated in this population? J. Coll. Physicians Surg. Pak 19(5): 308–312. [PubMed] [Google Scholar]
- Ishihara H, Kubota H, Lindberg RL, Leppert D, Gloor SM, Errede M, et al. 2008. Endothelial cell barrier impairment induced by glioblastomas and transforming growth factor beta2 involves matrix metalloproteinases and tight junction proteins. J. Neuropathol. Exp. Neurol 67(5): 435–448. doi: 10.1097/NEN.0b013e31816fd622. [DOI] [PubMed] [Google Scholar]
- Ishii I, Akahoshi N, Yamada H, Nakano S, Izumi T, and Suematsu M 2010. Cystathionine gamma-Lyase-deficient mice require dietary cysteine to protect against acute lethal myopathy and oxidative injury. J. Biol. Chem 285(34): 26358–26368. doi: 10.1074/jbc.M110.147439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacovina AT, Deora AB, Ling Q, Broekman MJ, Almeida D, Greenberg CB, et al. 2009. Homocysteine inhibits neoangiogenesis in mice through blockade of annexin A2-dependent fibrinolysis. J. Clin. Invest 119(11): 3384–3394. doi: 10.1172/jci39591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamaluddin MD, Chen I, Yang F, Jiang X, Jan M, Liu X, et al. 2007. Homo-cysteine inhibits endothelial cell growth via DNA hypomethylation of the cyclin A gene. Blood, 110(10): 3648–3655. doi: 10.1182/blood-2007-06-096701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Xiao J, Kang B, Zhu X, Xin N, and Wang Z 2016. PI3K/SGK1/GSK3beta signaling pathway is involved in inhibition of autophagy in neonatal rat cardiomyocytes exposed to hypoxia/reoxygenation by hydrogen sulfide. Exp. Cell Res 345(2): 134–140. doi: 10.1016/j.yexcr.2015.07.005. [DOI] [PubMed] [Google Scholar]
- John A, Kundu S, Pushpakumar S, Fordham M, Weber G, Mukhopadhyay M, and Sen U 2017. GYY4137, a hydrogen sulfide donor modulates miR194-dependent collagen realignment in diabetic kidney. Sci. Rep 7(1): 10924. doi: 10.1038/s41598-017-11256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabil O, Vitvitsky V, Xie P, and Banerjee R 2011. The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid. Redox Signal 15(2): 363–372. doi: 10.1089/ars.2010.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado DM, Bucur A, Selhub J, Rowe JW, and Seeman T 2002. Homocysteine levels and decline in physical function: MacArthur Studies of Successful Aging. Am. J. Med 113(7): 537–542. doi: 10.1016/S0002-9343(02)01269-X. [DOI] [PubMed] [Google Scholar]
- Kalra BR, Ghose S, and Sood NN 1985. Homocystinuria with bilateral absolute glaucoma. Indian J. Ophthalmol 33(3): 195–197. [PubMed] [Google Scholar]
- Kamimoto T, Shoji S, Hidvegi T, Mizushima N, Umebayashi K, Perlmutter DH, and Yoshimori T 2006. Intracellular inclusions containing mutant alpha1-antitrypsin Z are propagated in the absence of autophagic activity. J. Biol. Chem 281(7): 4467–4476. doi: 10.1074/jbc.M509409200. [DOI] [PubMed] [Google Scholar]
- Kannan K, and Jain SK 2000. Oxidative stress and apoptosis. Pathophysiology,7(3): 153–163. doi: 10.1016/S0928-4680(00)00053-5. [DOI] [PubMed] [Google Scholar]
- Kanwar YS, Manaligod JR, and Wong PW 1976. Morphologic studies in a patient with homocystinuria due to 5,10-methylenetetrahydrofolate reductase deficiency. Pediatr. Res 10(6): 598–609. doi: 10.1203/00006450-197606000-00008. [DOI] [PubMed] [Google Scholar]
- Kaufman RJ 2002. Orchestrating the unfolded protein response in health and disease. J. Clin. Invest 110(10): 1389–1398. doi: 10.1172/JCI0216886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JF, Winterford CM, and Harmon BV 1994. Apoptosis. Its significance in cancer and cancer therapy. Cancer, 73(8): 2013–2026. [DOI] [PubMed] [Google Scholar]
- Kesherwani V, Nelson KS, and Agrawal SK 2013. Effect of sodium hydrosulphide after acute compression injury of spinal cord. Brain Res 1527: 222–229. doi: 10.1016/j.brainres.2013.06.023. [DOI] [PubMed] [Google Scholar]
- Kharb S, Aggarwal D, Bala J, and Nanda S 2016. Evaluation of homocysteine, vitamin B12 and folic acid levels during all the trimesters in pregnant and preeclamptic womens. Curr. Hypertens. Rev 12(3): 234–238. doi: 10.2174/1573402112666161010151632. [DOI] [PubMed] [Google Scholar]
- Kimura H 2010. Hydrogen sulfide: from brain to gut. Antioxid. Redox Signal 12(9): 1111–1123. doi: 10.1089/ars.2009.2919. [DOI] [PubMed] [Google Scholar]
- Kimura Y, and Kimura H 2004. Hydrogen sulfide protects neurons from oxidative stress. FASEB J 18(10): 1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Goto Y, and Kimura H 2010. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid. Redox Signal 12(1): 1–13. doi: 10.1089/ars.2008.2282. [DOI] [PubMed] [Google Scholar]
- Kokame K, Kato H, and Miyata T 1996. Homocysteine-respondent genes in vascular endothelial cells identified by differential display analysis. GRP78/BiP and novel genes. J. Biol. Chem 271(47): 29659–29665. [DOI] [PubMed] [Google Scholar]
- Kolling J, Scherer EB, Siebert C, Hansen F, Torres FV, Scaini G, et al. 2013. Homocysteine induces energy imbalance in rat skeletal muscle: is creatine a protector? Cell. Biochem. Funct 31(7): 575–584. doi: 10.1002/cbf.2938. [DOI] [PubMed] [Google Scholar]
- Kondo S, Murakami T, Tatsumi K, Ogata M, Kanemoto S, Otori K, et al. 2005. OASIS, a CREB/ATF-family member, modulates UPR signalling in astrocytes. Nat. Cell Biol 7(2): 186–194. doi: 10.1038/ncb1213. [DOI] [PubMed] [Google Scholar]
- Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H, et al. 2007. ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ 14(2): 230–239. doi: 10.1038/sj.cdd.4401984. [DOI] [PubMed] [Google Scholar]
- Kožich V, Krijt J, Sokolová J, Melenovská P, Ješina P, Vozdek R, et al. 2016. Thioethers as markers of hydrogen sulfide production in homocystinurias. Biochimie, 126: 14–20. doi: 10.1016/j.biochi.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Kruman II, Culmsee C, Chan SL, Kruman Y, Guo Z, Penix L, and Mattson MP 2000. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J. Neurosci 20(18): 6920–6926. doi: 10.1523/JNEUROSCI.20-18-06920.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu S, Kumar M, Sen U, Mishra PK, Tyagi N, Metreveli N, et al. 2009. Nitrotyrosinylation, remodeling and endothelial-myocyte uncoupling in iNOS, cystathionine beta synthase (CBS) knockouts and iNOS/CBS double knockout mice. J. Cell. Biochem 106(1): 119–126. doi: 10.1002/jcb.21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu S, Pushpakumar S, and Sen U 2015. MMP-9- and NMDA receptor-mediated mechanism of diabetic renovascular remodeling and kidney dys-function: hydrogen sulfide is a key modulator. Nitric Oxide, 46: 172–185. doi: 10.1016/j.niox.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo IY, and Ehrlich BE 2015. Signaling in muscle contraction. Cold Spring Harb. Perspect. Biol 7(2): a006023. doi: 10.1101/cshperspect.a006023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laha A, Majumder A, Singh M, and Tyagi SC 2018. Connecting homocysteine and obesity through pyroptosis, gut microbiome, epigenetics, peroxi-some proliferator-activated receptor gamma, and zinc finger protein 407. Can. J. Physiol. Pharmacol 96(10): 971–976. doi: 10.1139/cjpp-2018-0037. [DOI] [PubMed] [Google Scholar]
- Lan X, Field MS, and Stover PJ 2018. Cell cycle regulation of folate-mediated one-carbon metabolism. Wiley Interdiscip. Rev. Syst. Biol. Med 10(6): e1426. doi: 10.1002/wsbm.1426. [DOI] [PubMed] [Google Scholar]
- Laster L, Mudd SH, Finkelstein JD, and Irreverre F 1965. Homocystinuria due to cystathionine synthase deficiency: the metabolism of L-methionine.J. Clin. Invest 44(10): 1708–1719. doi: 10.1172/JCI105278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ME, and Wang H 1999. Homocysteine and hypomethylation. A novel link to vascular disease. Trends Cardiovasc. Med 9(1–2): 49–54. [DOI] [PubMed] [Google Scholar]
- Lehotský J, Tothová B, Kovalská M, Dobrota D, Beňová A, Kalenská D, and Kaplán P 2016. Role of homocysteine in the ischemic stroke and development of ischemic tolerance. Front. Neurosci 10: 538. doi: 10.3389/fnins.2016.00538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, and Davis RJ 2003. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc. Natl. Acad. Sci. U.S.A 100(5): 2432–2437. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemle MD 2009. Hypothesis: chronic fatigue syndrome is caused by dysregulation of hydrogen sulfide metabolism. Med. Hypotheses, 72(1): 108–109. doi: 10.1016/j.mehy.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Lentz SR 2005. Mechanisms of homocysteine-induced atherothrombosis.J. Thromb. Haemost 3(8): 1646–1654. doi: 10.1111/j.1538-7836.2005.01364.x. [DOI] [PubMed] [Google Scholar]
- Lentz SR, Sobey CG, Piegors DJ, Bhopatkar MY, Faraci FM, Malinow MR, and Heistad DD 1996. Vascular dysfunction in monkeys with diet-induced hyperhomocyst(e)inemia. J. Clin. Invest 98(1): 24–29. doi: 10.1172/JCI118771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Hu M, Wang Y, Lu H, Deng J, and Yan X 2015. Hydrogen sulfide preconditioning protects against myocardial ischemia/reperfusion injury in rats through inhibition of endo/sarcoplasmic reticulum stress. Int. J. Clin. Exp. Pathol 8(7): 7740–7751. [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang KY, Zhang P, Chen LX, Wang L, Xie M, et al. 2014. Hydrogen sulfide inhibits formaldehyde-induced endoplasmic reticulum stress in PC12 cells by upregulation of SIRT-1. PLoS One, 9(2): e89856. doi: 10.1371/journal.pone.0089856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Liao C, Sun Y, Zhang J, Lu W, Bai Y, et al. 2017. Hydrogen sulfide inhibits cigarette smoke-induced endoplasmic reticulum stress and apoptosis in bronchial epithelial cells. Front. Pharmacol 8: 675. doi: 10.3389/fphar.2017.00675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YY, Nagpure BV, Wong PT, and Bian JS 2013. Hydrogen sulfide protects SH-SY5Y neuronal cells against d-galactose induced cell injury by suppression of advanced glycation end products formation and oxidative stress. Neurochem. Int 62(5): 603–609. doi: 10.1016/j.neuint.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Lockshin RA, and Zakeri Z 2007. Cell death in health and disease. J. Cell. Mol. Med 11(6): 1214–1224. doi: 10.1111/j.1582-4934.2007.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon EA, Finley SD, Popel AS, and Mac Gabhann F 2014. A systems biology view of blood vessel growth and remodelling. J. Cell. Mol. Med 18(8): 1491–1508. doi: 10.1111/jcmm.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscalzo J 2009. Homocysteine-mediated thrombosis and angiostasis in vascular pathobiology. J. Clin. Invest 119(11): 3203–3205. doi: 10.1172/jci40924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Hu LF, Hu G, and Bian JS 2008. Hydrogen sulfide protects astrocytes against H(2)O(2)-induced neural injury via enhancing glutamate uptake. Free Radic. Biol. Med 45(12): 1705–1713. doi: 10.1016/j.freeradbiomed.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Lubos E, Handy DE, and Loscalzo J 2008. Role of oxidative stress and nitric oxide in atherothrombosis. Front. Biosci 13: 5323–5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Liu X, Zheng Q, Wan X, Ouyang S, Yin Y, et al. 2012. Hydrogen sulfide prevents hypoxia-induced apoptosis via inhibition of an H2O2-activated calcium signaling pathway in mouse hippocampal neurons. Biochem. Biophys. Res. Commun 425(2): 473–477. doi: 10.1016/j.bbrc.2012.07.131. [DOI] [PubMed] [Google Scholar]
- MacIntosh BR, Holash RJ, and Renaud JM 2012. Skeletal muscle fatigue – regulation of excitation-contraction coupling to avoid metabolic catastrophe. J. Cell Sci 125(Pt. 9): 2105–2114. doi: 10.1242/jcs.093674. [DOI] [PubMed] [Google Scholar]
- Maddocks OD, Labuschagne CF, Adams PD, and Vousden KH 2016. Serine metabolism supports the methionine cycle and DNA/RNA methylation through de novo ATP synthesis in cancer cells. Mol. Cell, 61(2): 210–221. doi: 10.1016/j.molcel.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder A, Behera J, Jeremic N, and Tyagi SC 2017. Hypermethylation: causes and consequences in skeletal muscle myopathy. J. Cell. Biochem 118(8): 2108–2117. doi: 10.1002/jcb.25841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder A, Singh M, Behera J, Theilen NT, George AK, Tyagi N, et al. 2018a. Hydrogen sulfide alleviates hyperhomocysteinemia-mediated skeletal muscle atrophy via mitigation of oxidative and endoplasmic reticulum stress injury. Am. J. Physiol. Cell Physiol 315(5): C609–C622. doi: 10.1152/ajpcell.00147.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder A, Singh M, George AK, Behera J, Tyagi N, and Tyagi SC 2018b. Hydrogen sulfide improves postischemic neoangiogenesis in the hind limb of cystathionine-beta-synthase mutant mice via PPAR-gamma/VEGF axis. Physiol. Rep 6(17): e13858. doi: 10.14814/phy2.13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder A, Singh M, George AK, Homme RP, Laha A, and Tyagi SC 2019. Remote ischemic conditioning as a cytoprotective strategy in vasculopathies during hyperhomocysteinemia: an emerging research perspective.J. Cell. Biochem 120(1): 77–92. doi: 10.1002/jcb.27603. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, and Kaufman RJ 2008. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc. Natl. Acad. Sci. U.S.A 105(47): 18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, and Cantley LC 2007. AKT/PKB signaling: navigating downstream. Cell, 129(7): 1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matar W, Nosek TM, Wong D, and Renaud J 2000. Pinacidil suppresses contractility and preserves energy but glibenclamide has no effect during muscle fatigue. Am. J. Physiol. Cell Physiol 278(2): C404–C416. doi: 10.1152/ajpcell.2000.278.2.C404. [DOI] [PubMed] [Google Scholar]
- Matté C, Stefanello FM, Mackedanz V, Pederzolli CD, Lamers ML, Dutra-Filho CS, et al. 2009. Homocysteine induces oxidative stress, inflam matory infiltration, fibrosis and reduces glycogen/glycoprotein content in liver of rats. Int. J. Dev. Neurosci 27(4): 337–344. doi: 10.1016/j.ijdevneu.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Maurel M, Chevet E, Tavernier J, and Gerlo S 2014. Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem. Sci 39(5): 245–254. doi: 10.1016/j.tibs.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Mauro A 1961. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol 9: 493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully KS 2007. Homocysteine, vitamins, and vascular disease prevention. Am. J. Clin. Nutr 86(5): 1563S–1568S. doi: 10.1093/ajcn/86.5.1563S. [DOI] [PubMed] [Google Scholar]
- Merad-Boudia M, Nicole A, Santiard-Baron D, Saillé C, and Ceballos-Picot I 1998. Mitochondrial impairment as an early event in the process of apoptosis induced by glutathione depletion in neuronal cells: relevance to Parkinson’s disease. Biochem. Pharmacol 56(5): 645–655. doi: 10.1016/S0006-2952(97)00647-3. [DOI] [PubMed] [Google Scholar]
- Miller A, Mujumdar V, Shek E, Guillot J, Angelo M, Palmer L, and Tyagi SC 2000. Hyperhomocyst(e)inemia induces multiorgan damage. Heart Vessels, 15(3): 135–143. doi: 10.1007/s003800070030. [DOI] [PubMed] [Google Scholar]
- Mi-Mi L, and Pruyne D 2015. Loss of sarcomere-associated formins disrupts Z-line organization, but does not prevent thin filament assembly in Caenorhabditis elegans muscle. J. Cytol. Histol 6(2): 318. doi: 10.4172/2157-7099.1000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamishima S, Bougaki M, Sips PY, Yu JD, Minamishima YA, Elrod JW, et al. 2009. Hydrogen sulfide improves survival after cardiac arrest and cardiopulmonary resuscitation via a nitric oxide synthase 3-dependent mechanism in mice. Circulation, 120(10): 888–896. doi: 10.1161/CIRCULATIONAHA.108.833491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov A, Seregina T, Nagornykh M, Luhachack LG, Korolkova N, Lopes LE, et al. 2017. Mechanism of H2S-mediated protection against oxidative stress in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A 114(23): 6022–6027. doi: 10.1073/pnas.1703576114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra PK, Tyagi N, Sen U, Joshua IG, and Tyagi SC 2010. Synergism in hyperhomocysteinemia and diabetes: role of PPAR gamma and tempol. Cardiovasc. Diabetol 9: 49. doi: 10.1186/1475-2840-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PO, and Pavlath GK 2001. A muscle precursor cell-dependent pathway contributes to muscle growth after atrophy. Am. J. Physiol. Cell Physiol 281(5): C1706–C1715. doi: 10.1152/ajpcell.2001.281.5.C1706. [DOI] [PubMed] [Google Scholar]
- Miyake M, Hayashi S, Iwasaki S, Uchida T, Watanabe K, Ohwada S, et al. 2011. TIEG1 negatively controls the myoblast pool indispensable for fusion during myogenic differentiation of C2C12 cells. J. Cell. Physiol 226(4): 1128–1136. doi: 10.1002/jcp.22434. [DOI] [PubMed] [Google Scholar]
- Momoi T 2006. Conformational diseases and ER stress-mediated cell death: apoptotic cell death and autophagic cell death. Curr. Mol. Med 6(1): 111–118. doi: 10.2174/156652406775574596. [DOI] [PubMed] [Google Scholar]
- Morel Y, and Barouki R 1999. Repression of gene expression by oxidative stress. Biochem. J 342(Pt. 3): 481–496. doi: 10.1042/bj3420481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K 2000. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell, 101(5): 451–454. doi: 10.1016/S0092-8674(00)80855-7. [DOI] [PubMed] [Google Scholar]
- Mudd SH, Finkelstein JD, Irreverre F, and Laster L 1965. Transsulfuration in mammals. Microassays and tissue distributions of three enzymes of the pathway. J. Biol. Chem 240(11): 4382–4392. [PubMed] [Google Scholar]
- Myhill S, Booth NE, and McLaren-Howard J 2009. Chronic fatigue syndrome and mitochondrial dysfunction. Int. J. Clin. Exp. Med 2(1): 1–16. [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Tasaki H, Takatsu H, Nihei S, Yamashita K, Toyokawa T, and Nakashima Y 2001. Homocysteine inhibits angiogenesis in vitro and in vivo. Biochem. Biophys. Res. Commun 281(3): 726–731. doi: 10.1006/bbrc.2001.4400. [DOI] [PubMed] [Google Scholar]
- Nakamura T, and Lipton SA 2007. Molecular mechanisms of nitrosative stress-mediated protein misfolding in neurodegenerative diseases. Cell. Mol. Life Sci 64(13): 1609–1620. doi: 10.1007/s00018-007-6525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi SS, and Mishra PK 2017. H2S and homocysteine control a novel feedback regulation of cystathionine beta synthase and cystathionine gamma lyase in cardiomyocytes. Sci. Rep 7(1): 3639. doi: 10.1038/s41598-017-03776-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan N, Tyagi N, Shah A, Pagni S, and Tyagi SC 2013. Hyperhomocysteinemia during aortic aneurysm, a plausible role of epigenetics. Int. J. Physiol. Pathophysiol. Pharmacol 5(1): 32–42. [PMC free article] [PubMed] [Google Scholar]
- Narayanan N, Pushpakumar SB, Givvimani S, Kundu S, Metreveli N, James D, et al. 2014. Epigenetic regulation of aortic remodeling in hyperhomocysteinemia. FASEB J 28(8): 3411–3422. doi: 10.1096/fj.14-250183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon RA 2006. Autophagy in neurodegenerative disease: friend, foe or turn-coat? Trends Neurosci 29(9): 528–535. doi: 10.1016/j.tins.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, et al. 2006. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol. Cell. Biol 26(24): 9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto A, Tanaka M, Sumi C, Oku K, Kusunoki M, Nishi K, et al. 2016. The antioxidant N-acetyl cysteine suppresses lidocaine-induced intracellular reactive oxygen species production and cell death in neuronal SH-SY5Y cells. BMC Anesthesiol 16(1): 104. doi: 10.1186/s12871-016-0273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfert IM, Baum O, Hellsten Y, and Egginton S 2016. Advances and challenges in skeletal muscle angiogenesis. Am. J. Physiol. Heart Circ. Physiol 310(3): H326–H336. doi: 10.1152/ajpheart.00635.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterbaan AM, Steegers EA, and Ursem NT 2012. The effects of homocysteine and folic acid on angiogenesis and VEGF expression during chicken vascular development. Microvasc. Res 83(2): 98–104. doi: 10.1016/j.mvr.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Osian G, Procopciuc L, Vlad L, Iancu C, Mocan T, and Mocan L 2009. C677T and A1298C mutations in the MTHFR gene and survival in colorectal cancer.J. Gastrointestin. Liver Dis 18(4): 455–460. [PubMed] [Google Scholar]
- Oslowski CM, and Urano F 2011. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol 490: 71–92. doi: 10.1016/B978-0-12-385114-7.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayi GS, and Corrigall VM 2014. Immunoglobulin heavy-chain-binding protein (BiP): a stress protein that has the potential to be a novel therapy for rheumatoid arthritis. Biochem. Soc. Trans 42(6): 1752–1755. doi: 10.1042/BST20140230. [DOI] [PubMed] [Google Scholar]
- Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, et al. 2009. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc. Natl. Acad. Sci. U.S.A 106(51): 21972–21977. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK 2013. Muscle as a secretory organ. Compr. Physiol 3(3): 1337–1362. doi: 10.1002/cphy.c120033. [DOI] [PubMed] [Google Scholar]
- Plaisance V, Brajkovic S, Tenenbaum M, Favre D, Ezanno H, Bonnefond A, et al. 2016. Endoplasmic reticulum stress links oxidative stress to impaired pancreatic beta-cell function caused by human oxidized LDL. PLoS One, 11(9): e0163046. doi: 10.1371/journal.pone.0163046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polhemus DJ, and Lefer DJ 2014. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ. Res 114(4): 730–737. doi: 10.1161/CIRCRESAHA.114.300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SK, Lynch GS, Murphy KT, Reid MB, and Zijdewind I 2016. Disease-induced skeletal muscle atrophy and fatigue. Med. Sci. Sports Exerc 48(11): 2307–2319. doi: 10.1249/MSS.0000000000000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Predmore BL, and Lefer DJ 2010. Development of hydrogen sulfide-based therapeutics for cardiovascular disease. J. Cardiovasc. Transl. Res 3(5): 487–498. doi: 10.1007/s12265-010-9201-y. [DOI] [PubMed] [Google Scholar]
- Predmore BL, Lefer DJ, and Gojon G 2012. Hydrogen sulfide in biochemistry and medicine. Antioxid. Redox Signal 17(1): 119–140. doi: 10.1089/ars.2012.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refsum H, Ueland PM, Nygård O, and Vollset SE 1998. Homocysteine and cardiovascular disease. Annu. Rev. Med 49: 31–62. doi: 10.1146/annurev.med.49.1.31. [DOI] [PubMed] [Google Scholar]
- Renehan AG, Booth C, and Potten CS 2001. What is apoptosis, and why is it important? Br. Med. J. (Clin. Res. Ed.), 322(7301): 1536–1538. doi: 10.1136/bmj.322.7301.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter C 1993. Pro-oxidants and mitochondrial Ca2+: their relationship to apoptosis and oncogenesis. FEBS Lett 325(1–2): 104–107. doi: 10.1016/0014-5793(93)81423-W. [DOI] [PubMed] [Google Scholar]
- Romanino K, Mazelin L, Albert V, Conjard-Duplany A, Lin S, Bentzinger CF, et al. 2011. Myopathy caused by mammalian target of rapamycin complex 1 (mTORC1) inactivation is not reversed by restoring mitochondrial function. Proc. Natl. Acad. Sci. U.S.A 108(51): 20808–20813. doi: 10.1073/pnas.1111448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D 2002. Translational control in the endoplasmic reticulum stress response. J. Clin. Invest 110(10): 1383–1388. doi: 10.1172/JCI0216784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt JD, Yong D, and Parry DJ 1994. Satellite cell activity is required for hypertrophy of overloaded adult rat muscle. Muscle Nerve, 17(6): 608–613. doi: 10.1002/mus.880170607. [DOI] [PubMed] [Google Scholar]
- Rutkowski DT, and Kaufman RJ 2004. A trip to the ER: coping with stress. Trends Cell Biol 14(1): 20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Sağlam K, Alhan E, Türkyılmaz S, Vanizor BK, and Erçin C 2017. The anti-inflammatory effect of hydrogen sulphide on acute necrotizing pancreatitis in rats. Turk. J. Surg 33(3): 158–163. doi: 10.5152/UCD.2017.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano R, and Reed JC 2013. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta Mol. Cell Res 1833(12): 3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selhub J 1999. Homocysteine metabolism. Annu. Rev. Nutr 19: 217–246. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- Selhub J, Jacques PF, Rosenberg IH, Rogers G, Bowman BA, Gunter EW, et al. 1999. Serum total homocysteine concentrations in the third National Health and Nutrition Examination Survey (1991–1994): population reference ranges and contribution of vitamin status to high serum concentrations. Ann. Intern. Med 131(5): 331–339. doi: 10.7326/0003-4819-131-5-199909070-00003. [DOI] [PubMed] [Google Scholar]
- Sen U, Basu P, Abe OA, Givvimani S, Tyagi N, Metreveli N, et al. 2009. Hydrogen sulfide ameliorates hyperhomocysteinemia-associated chronic renal failure. Am. J. Physiol. Renal Physiol 297(2): F410–F419. doi: 10.1152/ajprenal.00145.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen U, Mishra PK, Tyagi N, and Tyagi SC 2010. Homocysteine to hydrogen sulfide or hypertension. Cell Biochem. Biophys 57(2–3): 49–58. doi: 10.1007/s12013-010-9079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamu CE, and Walter P 1996. Oligomerization and phosphorylation of the Ire1p kinase during intracellular signaling from the endoplasmic reticulum to the nucleus. EMBO J 15(12): 3028–3039. doi: 10.1002/j.1460-2075.1996.tb00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shastry S, Ingram AJ, Scholey JW, and James LR 2007. Homocysteine induces mesangial cell apoptosis via activation of p38-mitogen-activated protein kinase. Kidney Int 71(4): 304–311. doi: 10.1038/sj.ki.5002031. [DOI] [PubMed] [Google Scholar]
- Shi S, Li QS, Li H, Zhang L, Xu M, Cheng JL, et al. 2009. Anti-apoptotic action of hydrogen sulfide is associated with early JNK inhibition. Cell Biol. Int 33(10): 1095–1101. doi: 10.1016/j.cellbi.2009.06.029. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Langenbacher AD, Huang J, Wang K, Otto G, Geisler R, et al. 2017. The calcineurin-FoxO-MuRF1 signaling pathway regulates myofibril integrity in cardiomyocytes. eLife, 6: e27955. doi: 10.7554/eLife.27955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, George AK, Homme RP, Majumder A, Laha A, Sandhu HS, and Tyagi SC 2018. Circular RNAs profiling in the cystathionine-beta-synthase mutant mouse reveals novel gene targets for hyperhomocysteinemia induced ocular disorders. Exp. Eye Res 174: 80–92. doi: 10.1016/j.exer.2018.05.026. [DOI] [PubMed] [Google Scholar]
- Smith IC, Bombardier E, Vigna C, and Tupling AR 2013. ATP consumption by sarcoplasmic reticulum Ca(2)(+) pumps accounts for 40–50% of resting metabolic rate in mouse fast and slow twitch skeletal muscle. PLoS One, 8(7): e68924. doi: 10.1371/journal.pone.0068924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabler SP, Steegborn C, Wahl MC, Oliveriusova J, Kraus JP, Allen RH, et al. 2002. Elevated plasma total homocysteine in severe methionine adenosyltransferase I/III deficiency. Metabolism, 51(8): 981–988. doi: 10.1053/meta.2002.34017. [DOI] [PubMed] [Google Scholar]
- Stipanuk MH, and Ueki I 2011. Dealing with methionine/homocysteine sulfur: cysteine metabolism to taurine and inorganic sulfur. J. Inherit. Metab. Dis 34(1): 17–32. doi: 10.1007/s10545-009-9006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzenberg-Solomon RZ, Miller ER 3rd, Maguire MG, Selhub J, and Appel LJ 1999. Association of dietary protein intake and coffee consumption with serum homocysteine concentrations in an older population. Am. J. Clin. Nutr 69(3): 467–475. doi: 10.1093/ajcn/69.3.467. [DOI] [PubMed] [Google Scholar]
- Su YW, Liang C, Jin HF, Tang XY, Han W, Chai LJ, et al. 2009. Hydrogen sulfide regulates cardiac function and structure in adriamycin-induced cardiomyopathy. Circ. J 73(4): 741–749. doi: 10.1253/circj.CJ-08-0636. [DOI] [PubMed] [Google Scholar]
- Sun H, Qi L, Wang S, Li X, and Li C 2018. Hydrogen sulfide is expressed in the human and the rat cultured nucleus pulposus cells and suppresses apoptosis induced by hypoxia. PLoS One, 13(2): e0192556. doi: 10.1371/journal.pone.0192556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Wang W, Dai J, Jin S, Huang J, Guo C, et al. 2017. A long-term and slow-releasing hydrogen sulfide donor protects against myocardial ischemia/reperfusion injury. Sci. Rep 7(1): 3541. doi: 10.1038/s41598-017-03941-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Olah G, Modis K, Coletta C, Kulp G, Gerö D, et al. 2011. Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc. Natl. Acad. Sci. U.S.A 108(33): 13829–13834. doi: 10.1073/pnas.1105121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swart KM, van Schoor NM, Heymans MW, Schaap LA, den Heijer M, and Lips P 2013. Elevated homocysteine levels are associated with low muscle strength and functional limitations in older persons. J. Nutr. Health Aging, 17(6): 578–584. doi: 10.1007/s12603-013-0047-2. [DOI] [PubMed] [Google Scholar]
- Szabó C 2007. Hydrogen sulphide and its therapeutic potential. Nat. Rev. DrugDiscov 6(11): 917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- Szabó C, and Papapetropoulos A 2011. Hydrogen sulphide and angiogenesis: mechanisms and applications. Br. J. Pharmacol 164(3): 853–865. doi: 10.1111/j.1476-5381.2010.01191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot J, and Maves L 2016. Skeletal muscle fiber type: using insights from muscle developmental biology to dissect targets for susceptibility and resistance to muscle disease. Wiley Interdiscip. Rev. Dev. Biol 5(4): 518–534. doi: 10.1002/wdev.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Wu L, Liang W, and Wang R 2005. Direct stimulation of K(ATP) channels by exogenous and endogenous hydrogen sulfide in vascular smooth muscle cells. Mol. Pharmacol 68(6): 1757–1764. doi: 10.1124/mol.105.017467. [DOI] [PubMed] [Google Scholar]
- Tang XQ, Chen RQ, Ren YK, Soldato PD, Sparatore A, Zhuang YY, et al. 2011. ACS6, a Hydrogen sulfide-donating derivative of sildenafil, inhibits homocysteine-induced apoptosis by preservation of mitochondrial function. Med. Gas Res 1(1): 20. doi: 10.1186/2045-9912-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tews DS, and Goebel HH 1997. DNA-fragmentation and expression of apoptosis-related proteins in muscular dystrophies. Neuropathol. Appl. Neurobiol 23(4): 331–338. doi: 10.1111/j.1365-2990.1997.tb01304.x. [DOI] [PubMed] [Google Scholar]
- Thompson CB 1995. Apoptosis in the pathogenesis and treatment of disease. Science, 267(5203): 1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Tiahou G, Dupuy AM, Jaussent I, Sees D, Cristol JP, and Badiou S 2009. Determinants of homocysteine levels in Ivorian rural population. Int. J. Vitam. Nutr. Res 79(5–6): 319–327. doi: 10.1024/0300-9831.79.56.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidball JG, Albrecht DE, Lokensgard BE, and Spencer MJ 1995. Apoptosis precedes necrosis of dystrophin-deficient muscle. J. Cell Sci 108(Pt. 6): 2197–2204. [DOI] [PubMed] [Google Scholar]
- Tyagi N, Sedoris KC, Steed M, Ovechkin AV, Moshal KS, and Tyagi SC 2005. Mechanisms of homocysteine-induced oxidative stress. Am. J. Physiol. Heart Circ. Physiol 289(6): H2649–H2656. doi: 10.1152/ajpheart.00548.2005. [DOI] [PubMed] [Google Scholar]
- Tyagi N, Moshal KS, Sen U, Vacek TP, Kumar M, Hughes WM Jr., et al. 2009. H2S protects against methionine-induced oxidative stress in brain endothelial cells. Antioxid. Redox Signal 11(1): 25–33. doi: 10.1089/ars.2008.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi N, Qipshidze N, Sen U, Rodriguez W, Ovechkin A, and Tyagi SC 2011. Cystathionine beta synthase gene dose dependent vascular remodeling in murine model of hyperhomocysteinemia. Int. J. Physiol. Pathophysiol. Pharmacol 3(3): 210–222. [PMC free article] [PubMed] [Google Scholar]
- Tyagi N, Qipshidze N, Munjal C, Vacek JC, Metreveli N, Givvimani S, and Tyagi SC 2012. Tetrahydrocurcumin ameliorates homocysteinylated cytochrome-c mediated autophagy in hyperhomocysteinemia mice after cerebral ischemia.J. Mol. Neurosci 47(1): 128–138. doi: 10.1007/s12031-011-9695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y, et al. 2006. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature, 441(7092): 513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- Upchurch GR Jr., Welch GN, Fabian AJ, Freedman JE, Johnson JL, Keaney JF Jr., and Loscalzo J 1997. Homocyst(e)ine decreases bioavailable nitric oxide by a mechanism involving glutathione peroxidase. J. Biol. Chem 272(27): 17012–17017. doi: 10.1074/jbc.272.27.17012. [DOI] [PubMed] [Google Scholar]
- Valentino F, Bivona G, Butera D, Paladino P, Fazzari M, Piccoli T, et al. 2010. Elevated cerebrospinal fluid and plasma homocysteine levels in ALS. Eur. J. Neurol 17(1): 84–89. doi: 10.1111/j.1468-1331.2009.02752.x. [DOI] [PubMed] [Google Scholar]
- Veeranki S, and Tyagi SC 2013. Defective homocysteine metabolism: potential implications for skeletal muscle malfunction. Int. J. Mol. Sci 14(7): 15074–15091. doi: 10.3390/ijms140715074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeranki S, and Tyagi SC 2015a. Mechanisms of hyperhomocysteinemia induced skeletal muscle myopathy after ischemia in the CBS–/+ mouse model. Int. J. Mol. Sci 16(1): 1252–1265. doi: 10.3390/ijms16011252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeranki S, and Tyagi SC 2015b. Role of hydrogen sulfide in skeletal muscle biology and metabolism. Nitric Oxide, 46: 66–71. doi: 10.1016/j.niox.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeranki S, Givvimani S, Pushpakumar S, and Tyagi SC 2014. Hyperhomocysteinemia attenuates angiogenesis through reduction of HIF-1alpha and PGC-1alpha levels in muscle fibers during hindlimb ischemia. Am. J. Physiol. Heart Circ. Physiol 306(8): H1116–H1127. doi: 10.1152/ajpheart.00003.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeranki S, Winchester LJ, and Tyagi SC 2015. Hyperhomocysteinemia associated skeletal muscle weakness involves mitochondrial dysfunction and epigenetic modifications. Biochim. Biophys. Acta Mol. Basis Dis 1852(5): 732–741. doi: 10.1016/j.bbadis.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeranki S, Gandhapudi SK, and Tyagi SC 2017. Interactions of hyperhomocysteinemia and T cell immunity in causation of hypertension. Can. J. Physiol. Pharmacol 95(3): 239–246. doi: 10.1139/cjpp-2015-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoef P, van Vliet T, Olthof MR, and Katan MB 2005. A high-protein diet increases postprandial but not fasting plasma total homocysteine concentrations: a dietary controlled, crossover trial in healthy volunteers. Am. J. Clin. Nutr 82(3): 553–558. doi: 10.1093/ajcn/82.3.553. [DOI] [PubMed] [Google Scholar]
- Voskoboeva E, Semyachkina A, Yablonskaya M, and Nikolaeva E 2018. Homocystinuria due to cystathionine beta-synthase (CBS) deficiency in Russia: Molecular and clinical characterization. Mol. Genet. Metab. Rep 14: 47–54. doi: 10.1016/j.ymgmr.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Wang HY, Liu ZW, Fu Y, and Zhao B 2011. Effect of endogenous hydrogen sulfide on oxidative stress in oleic acid-induced acute lung injury in rats. Chin. Med. J 124(21): 3476–3480. [PubMed] [Google Scholar]
- Wang H, Yoshizumi M, Lai K, Tsai JC, Perrella MA, Haber E, and Lee ME 1997. Inhibition of growth and p21 ras methylation in vascular endothelial cells by homocysteine but not cysteine. J. Biol. Chem 272(40): 25380–25385. doi: 10.1074/jbc.272.40.25380. [DOI] [PubMed] [Google Scholar]
- Wang R 2003. The gasotransmitter role of hydrogen sulfide. Antioxid. Redox Signal 5(4): 493–501. doi: 10.1089/152308603768295249. [DOI] [PubMed] [Google Scholar]
- Wang R, Szabó C, Ichinose F, Ahmed A, Whiteman M, and Papapetropoulos A 2015. The role of H2S bioavailability in endothelial dys-function. Trends Pharmacol. Sci 36(9): 568–578. doi: 10.1016/j.tips.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhao X, Jin H, Wei H, Li W, Bu D, et al. 2009. Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol 29(2): 173–179. doi: 10.1161/ATVBAHA.108.179333. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Osada J, Aratani Y, Kluckman K, Reddick R, Malinow MR, and Maeda N 1995. Mice deficient in cystathionine beta-synthase: animal models for mild and severe homocyst(e)inemia. Proc. Natl. Acad. Sci. U.S.A 92(5): 1585–1589. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Zhang R, Jin H, Liu D, Tang X, Tang C, and Du J 2010. Hydrogen sulfide attenuates hyperhomocysteinemia-induced cardiomyocytic endo plasmic reticulum stress in rats. Antioxid. Redox Signal 12(9): 1079–1091. doi: 10.1089/ars.2009.2898. [DOI] [PubMed] [Google Scholar]
- Wei HJ, Xu JH, Li MH, Tang JP, Zou W, Zhang P, et al. 2014. Hydrogen sulfide inhibits homocysteine-induced endoplasmic reticulum stress and neuronal apoptosis in rat hippocampus via upregulation of the BDNF-TrkB pathway. Acta Pharmacol. Sin 35(6): 707–715. doi: 10.1038/aps.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen YD, Wang H, Kho SH, Rinkiko S, Sheng X, Shen HM, and Zhu YZ 2013. Hydrogen sulfide protects HUVECs against hydrogen peroxide induced mitochondrial dysfunction and oxidative stress. PLoS One, 8(2): e53147. doi: 10.1371/journal.pone.0053147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werstuck GH, Lentz SR, Dayal S, Hossain GS, Sood SK, Shi YY, et al. 2001. Homocysteine-induced endoplasmic reticulum stress causes dysregulation of the cholesterol and triglyceride biosynthetic pathways. J. Clin. Invest 107(10): 1263–1273. doi: 10.1172/JCI11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Place N, and Yamada T 2010. Mechanisms of skeletal muscle weakness. Adv. Exp. Med. Biol 682: 279–296. doi: 10.1007/978-1-4419-6366-6_16. [DOI] [PubMed] [Google Scholar]
- Whiteman M, Armstrong JS, Chu SH, Jia-Ling S, Wong BS, Cheung NS, et al. 2004. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite ‘scavenger’? J. Neurochem 90(3): 765–768. doi: 10.1111/j.1471-4159.2004.02617.x. [DOI] [PubMed] [Google Scholar]
- Whiteman M, Cheung NS, Zhu YZ, Chu SH, Siau JL, Wong BS, et al. 2005. Hydrogen sulphide: a novel inhibitor of hypochlorous acid-mediated oxidative damage in the brain? Biochem. Biophys. Res. Commun 326(4): 794–798. doi: 10.1016/j.bbrc.2004.11.110. [DOI] [PubMed] [Google Scholar]
- Whiteman M, Li L, Kostetski I, Chu SH, Siau JL, Bhatia M, and Moore PK 2006. Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Biochem. Biophys. Res. Commun 343(1): 303–310. doi: 10.1016/j.bbrc.2006.02.154. [DOI] [PubMed] [Google Scholar]
- Winchester L, Veeranki S, Givvimani S, and Tyagi SC 2014. Exercise mitigates the adverse effects of hyperhomocysteinemia on macrophages, MMP-9, skeletal muscle, and white adipocytes. Can. J. Physiol. Pharmacol 92(7): 575–582. doi: 10.1139/cjpp-2014-0059. [DOI] [PubMed] [Google Scholar]
- Winchester LJ, Veeranki S, Pushpakumar S, and Tyagi SC 2018. Exercise mitigates the effects of hyperhomocysteinemia on adverse muscle remodeling. Physiol. Rep 6(6): e13637. doi: 10.14814/phy2.13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Luo N, Wang L, Zhao Z, Bu H, Xu G, et al. 2017. Hydrogen sulfide ameliorates chronic renal failure in rats by inhibiting apoptosis and inflammation through ROS/MAPK and NF-kappaB signaling pathways. Sci. Rep 7(1): 455. doi: 10.1038/s41598-017-00557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie CM, Chan WY, Yu S, Zhao J, and Cheng CH 2011. Bufalin induces autophagy-mediated cell death in human colon cancer cells through reactive oxygen species generation and JNK activation. Free Radic. Biol. Med 51(7): 1365–1375. doi: 10.1016/j.freeradbiomed.2011.06.016. [DOI] [PubMed] [Google Scholar]
- Xie ZZ, Liu Y, and Bian JS 2016. Hydrogen sulfide and cellular redox homeostasis. Oxid. Med. Cell. Longev 2016: 6043038. doi: 10.1155/2016/6043038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Yang G, Hong C, Liu J, Holle E, Yu X, et al. 2003. Inhibition of endogenous thioredoxin in the heart increases oxidative stress and cardiac hypertrophy. J. Clin. Invest 112(9): 1395–1406. doi: 10.1172/JCI200317700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SK, Chang T, Wang H, Wu L, Wang R, and Meng QH 2006. Effects of hydrogen sulfide on homocysteine-induced oxidative stress in vascular smooth muscle cells. Biochem. Biophys. Res. Commun 351(2): 485–491. doi: 10.1016/j.bbrc.2006.10.058. [DOI] [PubMed] [Google Scholar]
- Yan Z, Okutsu M, Akhtar YN, and Lira VA 2011. Regulation of exercise-induced fiber type transformation, mitochondrial biogenesis, and angiogenesis in skeletal muscle. J. Appl. Physiol 110(1): 264–274. doi: 10.1152/japplphysiol.00993.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, Jiao Y, Yang S, Deng M, Yang X, Mao C, et al. 2018. Homocysteine activates autophagy by inhibition of CFTR expression via interaction between DNA methylation and H3K27me3 in mouse liver. Cell Death Dis 9(2): 169. doi: 10.1038/s41419-017-0216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, An SS, Ji Y, Zhang W, and Pei Y 2015. Hydrogen sulfide signaling in oxidative stress and aging development. Oxid. Med. Cell. Longev 2015: 357824. doi: 10.1155/2015/357824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin WL, He JQ, Hu B, Jiang ZS, and Tang XQ 2009. Hydrogen sulfide inhibits MPP(+)-induced apoptosis in PC12 cells. Life Sci 85(7–8): 269–275. doi: 10.1016/j.lfs.2009.05.023. [DOI] [PubMed] [Google Scholar]
- Yorimitsu T, Nair U, Yang Z, and Klionsky DJ 2006. Endoplasmic reticulum stress triggers autophagy. J. Biol. Chem 281(40): 30299–30304. doi: 10.1074/jbc.M607007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Wang AM, Adachi H, Katsuno M, Sobue G, Yue Z, et al. 2011. Macroautophagy is regulated by the UPR-mediator CHOP and accentuates the phenotype of SBMA mice. PLoS Genet 7(10): e1002321. doi: 10.1371/journal.pgen.1002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusof M, Kamada K, Kalogeris T, Gaskin FS, and Korthuis RJ 2009. Hydrogen sulfide triggers late-phase preconditioning in postischemic small intestine by an NO- and p38 MAPK-dependent mechanism. Am. J. Physiol. Heart Circ. Physiol 296(3): H868–H876. doi: 10.1152/ajpheart.01111.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamzami N, Susin SA, Marchetti P, Hirsch T, Gómez-Monterrey I, Castedo M, and Kroemer G 1996. Mitochondrial control of nuclear apoptosis. J. Exp. Med 183(4): 1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Cai Y, Adachi MT, Oshiro S, Aso T, Kaufman RJ, and Kitajima S 2001. Homocysteine induces programmed cell death in human vascular endothelial cells through activation of the unfolded protein response. J. Biol. Chem 276(38): 35867–35874. doi: 10.1074/jbc.M100747200. [DOI] [PubMed] [Google Scholar]
- Zhang K, and Kaufman RJ 2008. From endoplasmic-reticulum stress to the inflammatory response. Nature, 454(7203): 455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, et al. 2006. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell, 124(3): 587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Li Q, Chen Y, Huang X, Yang IH, Cao L, et al. 2012. Homocysteine-impaired angiogenesis is associated with VEGF/VEGFR inhibition. Front. Biosci. (Elite Ed.), 4: 2525–2535. [DOI] [PubMed] [Google Scholar]
- Zhao W, Zhang J, Lu Y, and Wang R 2001. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J 20(21): 6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Tang Z, Cong B, Du J, Wang C, Wang L, et al. 2013. Estrogens increase cystathionine-gamma-lyase expression and decrease inflammation and oxidative stress in the myocardium of ovariectomized rats. Menopause, 20(10): 1084–1091. doi: 10.1097/GME.0b013e3182874732. [DOI] [PubMed] [Google Scholar]
- Zima AV, Copello JA, and Blatter LA 2004. Effects of cytosolic NADH/NAD(+) levels on sarcoplasmic reticulum Ca(2+) release in permeabilized rat ventricular myocytes. J. Physiol 555(Pt. 3): 727–741. doi: 10.1113/jphysiol.2003.055848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccolella S, Simone IL, Lamberti P, Samarelli V, Tortelli R, Serlenga L, and Logroscino G 2008. Elevated plasma homocysteine levels in patients with amyotrophic lateral sclerosis. Neurology, 70(3): 222–225. doi: 10.1212/01.wnl.0000297193.53986.6f. [DOI] [PubMed] [Google Scholar]