Abstract
Introduction:
Despite minimal evidence, public concerns that the human papillomavirus (HPV) vaccine can cause autoimmune diseases (AD) persist. We evaluated whether HPV vaccine is associated with a long-term increased risk of diabetes mellitus type 1 (DM1).
Methods:
This was a retrospective cohort study in which we identified all potential DM1 cases from Kaiser Permanente Northern California (KPNC) members who were between 11 and 26 years old any time after June 2006 through December 2015. We chart reviewed a random sample of 100 DM1 cases to confirm diagnosis and to develop a computer algorithm that reliably determined symptom onset date. Our DM1 Analysis Population comprised all individuals who met membership criteria and who were age and sex eligible to have received HPV vaccine. We adjusted for age, sex, race, Medicaid, and years of prior KPNC membership by stratification using a Cox multiplicative hazards model with a calendar timeline.
Results:
Our DM1 analysis included 911,648 individuals. Of 2613 DM1 cases identified, 338 remained in the analysis after applying our algorithm, HPV vaccine eligibility and membership criteria. Over the 10 years of the study period, comparing vaccinated with unvaccinated persons, we did not find an increased risk of DM1 associated with HPV vaccine receipt (hazard ratio 1.21, 95% Confidence Interval0.94, 1.57).
Conclusions:
We found no increased risk for development of DM1 following HPV vaccination. Our study provides reassurance that during the 10-year time period after HPV vaccine was introduced, there was no substantial increased risk for DM1 following HPV vaccination.
Keywords: HPV vaccine, Diabetes, Vaccine safety, DM1
1. Introduction
The U.S. Food and Drug Administration approved the quadrivalent HPV vaccine (4vHPV) for females between the ages of 9 and 26 years for prevention of HPV-related cancers and warts in June 2006 [1]. The vaccine was approved for use in males in 2009. A nine-valent HPV vaccine (9vHPV) was approved for use in males and females in 2014 and for an expanded age range in males 19–26 years in 2015 [2,3]. Clinical trials and post-licensure studies have shown the 4vHPV vaccine to be safe and well-tolerated [4–6]. Routine vaccination with HPV vaccine at age 11 or 12 years has been recommended by the Advisory Committee on Immunization Practices (ACIP) since 2006 for females and since 2011 for males [7,8].
Routine use of HPV vaccine among girls and boys in early adolescence combined with heightened parental worries regarding adverse events has led to persistent public concerns that this vaccine can cause autoimmune diseases (AD). Multiple studies have evaluated several AD and to date there is minimal evidence to suggest an association between HPV vaccine and any AD [9–15]. These studies have provided reassurance, although data with long-term follow-up after vaccination have been limited.
In response to lingering public concerns and to provide longer term data, we conducted a single site study within the Vaccine Safety Datalink (VSD) to assess potential long-term associations between HPV vaccine and multiple AD. The objective of this current study, conducted as part of this larger AD investigation, was to evaluate whether receipt of HPV vaccine is associated with a subsequent increased risk of diabetes mellitus type 1 (DM1).
2. Methods
2.1. Study setting
We conducted this study in Kaiser Permanente Northern California (KPNC), an integrated healthcare delivery system, which has more than 4 million members and an annual birth cohort of approximately 40,000. KPNC owns all their hospitals and clinics, and their electronic medical record captures all outpatient, emergency department, and inpatient visits, as well as all immunizations, laboratory, pharmacy, radiology and demographic data. All vaccines, including HPV, are provided at no additional cost and members and providers are electronically prompted for appropriate immunizations. Because the study period included use of both the 4vHPV and the 9vHPV (KPNC did not use the 2 valent HPV vaccine), the term “HPV vaccine” refers to both 4vHPV and 9vHPV vaccines throughout.
2.2. Autoimmune disease (AD) study population
The larger retrospective AD cohort study evaluated for longterm risk of 18 AD following HPV vaccination (manuscript in preparation). In brief, this AD study population included all members of KPNC who were age-eligible to receive HPV vaccine beginning either July 2006 for females (when it first became available at KPNC for females) or October 2011 for males (when it was first recommended for males) through December 31, 2015. Since KPNC routinely targets HPV immunization at 11 years of age (in line with ACIP recommendations), we identified all HPV vaccines administered to individuals after their 10th birthday and searched for potential AD first diagnosed after their 11th birthday. The AD study population served as the underlying population for the 18 AD analyses, applying criteria specific for each AD. The AD study population was comprised of 1,580,797 individuals who contributed follow-up time during the study period for all 18 AD, including for the DM1 analyses described below.
2.3. Diabetes mellitus type 1 (DM1) case identification and chart review sample
We identified potential DM1 cases using internal diagnostic codes specific for DM1, and included the terms DM1, type 1 diabetes mellitus, “with” any other insulin dependent condition and excluded the terms DM2, type 2, non-insulin, and any “history of”. We verified completeness by cross-checking the mapping to ICD 9 diagnostic codes 250.XX. Irrespective of HPV exposure status, we selected the first lifetime diagnosis to ensure that it was an incident case of DM1.
2.3.1. Chart review
We chart reviewed a sample of cases to assess whether a subsequent analysis using all electronically identified DM1 cases without chart review would be feasible based on the following: (1) a sufficiently high chart confirmation rate of electronically identified cases; (2) the ability to determine an onset date of DM1 symptoms; and (3) the ability to estimate symptom onset date with acceptable accuracy based on the specificity and consistency with which symptom onset timing was described in the chart (e.g., “3–4 weeks ago” vs “1–2 years ago”).
To assure that all ages and both genders were represented in the chart review (i.e., we included older male adolescents), we identified all DM1 cases from members who were between 11 and 26 years old at any time after June 2006, regardless of eligibility to have received HPV vaccine. We then selected a random sample for chart review.
Considering these criteria together allowed us to assess whether we could define a time period between symptom onset and diagnosis (“lag period”) which could be applied to all cases in the analysis prior to the diagnosis date, regardless of chart review status. We carefully defined a lag period as described in the results below to ensure that we did not misclassify HPV vaccine status at the time of DM1 symptom onset.
2.3.2. KPNC membership criteria
We also assessed the length of KPNC membership prior to DM1 diagnosis that would be required to assure that cases were incident.
Taking into account the feasibility assessment results and KPNC membership requirements, as described in the results, we determined that an analysis using electronically identified DM1 cases without chart review would be feasible and reliable.
2.4. DM1 analysis population
All individuals in the cohort who were age and sex eligible to have received HPV vaccine, and who met the KPNC membership criterion described in the results below comprised the DM1 analysis population.
2.5. Statistical analysis
This was a cohort analysis that included all individuals in the DM1 analysis population. Both incidence of DM1 and HPV vaccination rate varied by age, sex and other factors during the study time period (Tables 1, 2 and Supplemental Figure). We adjusted for these and other factors by stratification using a Cox multiplicative hazards model with a calendar timeline. We stratified by age in 6-month categories, sex, race, Medicaid status, and years of prior KPNC membership; the calendar timeline provided stratification by calendar day.
Table 1.
Vaccination status and sex for diabetes mellitus type 1 analysis population, 2006–2015.
| Sex | Ever HPV vaccinated during study period N = 330,200 |
No record of HPV vaccination during study period N = 581,448 |
Total* N = 911,648 |
|---|---|---|---|
| Female | 207,561 (62.9%) | 292,145 (50.2%) | 499,706 |
| Male | 122,639 (37.1%) | 289,303 (49.8%) | 411,942 |
Included all individuals who met membership criteria and who were age and sex eligible to have received HPV vaccine during the study period.
Table 2.
Demographic characteristics and length of unvaccinated and vaccinated follow-up time by sex, age, medicaid status and race, diabetes mellitus type 1 analysis population 2006–2015.
| Unvaccinateda | Vaccinated (%)a | ||||
|---|---|---|---|---|---|
| Total N (%)b | Person-years (%) | Total N (%)b | Person-years (%) | Proportion of follow-up that was after HPV vaccination | |
| Total | 907,300 | 2,325,930 | 330,200 | 1,026,066 | 0.31 |
| Sex | |||||
| Female (all ages)c | 496,465 (54.7) | 1,383,178 (59.5) | 207,561 (62.9) | 804,599 (78.4) | 0.37 |
| 11–15 years | 290,708 | 477,408 | 148,969 | 303,230 | |
| 16–20years | 158,361 | 333,008 | 119,376 | 340,392 | |
| 21–25 years | 168,655 | 337,203 | 57,434 | 135,812 | |
| 26–30 years | 60,079 | 181,925 | 9856 | 21,692 | |
| 31–35 years | 22,412 | 53,634 | 1882 | 3474 | |
| Male (all ages)c | 410,835 (45.3) | 942,751 (40.5) | 122,639 (37.1) | 221,467 (21.6) | 0.19 |
| 11–15 years | 195,015 | 267,835 | 86,615 | 121,672 | |
| 16–20 years | 142,396 | 281,273 | 56,734 | 93,052 | |
| 21–25 years | 169,955 | 327,710 | 7450 | 5946 | |
| 26–30 years | 38,021 | 65,932 | 869 | 798 | |
| 31–35 years | 0 | 0 | 0 | 0 | |
| Medicaid | |||||
| Yes | 311,104 (13.4) | 184,059 (17.9) | 0.37 | ||
| No | 2,014,826 (86.6) | 842,008 (82.1) | 0.30 | ||
| Race | |||||
| Asian/Pacific Islander | 420,367 (18.1) | 199,438 (19.4) | 0.32 | ||
| African American | 224,601 (9.7) | 98,919 (9.6) | 0.31 | ||
| Hispanic | 551,723 (23.7) | 286,121 (27.9) | 0.34 | ||
| Mixed/Other | 202,593 (8.7) | 40,318 (3.9) | 0.17 | ||
| White | 926,646 (39.8) | 401,270 (39.1) | 0.30 | ||
Individuals contribute to unvaccinated follow-up time until they receive a dose of HPV vaccine, after which they contribute to vaccinated follow-up time. Therefore, the same individual may contribute to both.
Individuals who contributed at least 1 person-day of follow-up.
Total is number of people who contributed at least one day of follow-up in any age group.
We did not include the “lag period” prior to the DM1 diagnosis in the analyses and excluded DM1 cases who were vaccinated during the “lag period”. Thus, all analyses compared the risk of DM1 in people who were beyond the lag period after their 1st HPV vaccine with people who were as yet unvaccinated.
We performed sensitivity analyses to evaluate the possible impact of discrete classification of risk factors, sparse data, and outliers. We performed separate analyses in males and females and in age groups where vaccine delivery patterns differed over time. We assessed sparse data by performing stratified exact analysis. We assessed level of classification for age by performing a multiplicative hazards model with an age timeline (restricted to comparing people who were born within 14 days of each other). We assessed influence of outliers by performing analyses with and without the most extreme risk sets (those with extremely low or high proportions of vaccinated individuals, where a single DM1 case could potentially cause a large change in our effect estimates). Each of these sensitivity analyses reduced various potential biases, but at the expense of reduced precision and power. We conducted all analyses using SAS version 9.3 (SAS Institute, Inc., Cary, NC).
3. Results
3.1. Feasibility assessment of an analysis using non-chart reviewed DM1 cases
We identified 2613 potential DM1 cases from the larger chart review population, and randomly selected 100 for review. Chart review confirmed that 98 (98%) were true cases of DM1, with the remaining 2% being diabetes mellitus Type 2. For the 98 confirmed cases, everyone had a symptom onset date documented, and the onset date of symptoms could be reliably estimated in most cases. In this sample, the symptom onset date was within 6 months prior to 1st DM1 diagnosis for everyone who had at least 1 year of membership. Based on clinical judgment and chart review findings, we therefore considered that a lag period of 180 days between symptom onset and diagnosis could reliably and consistently be applied to all DM1 cases who have had at least one year of prior KPNC membership.
3.2. DM1 cases for analysis
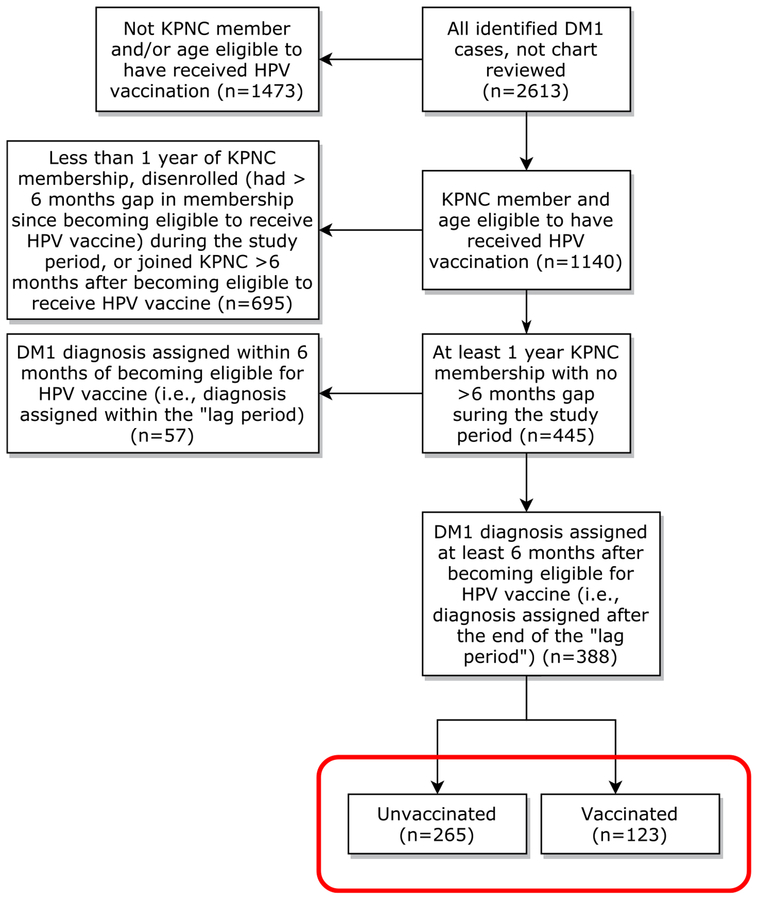
Of the 2613 identified DM1 cases, 1140 (43.6%) occurred in those who were eligible to receive HPV vaccine (Fig. 1). Of these 1140 cases, 445 (39.0%) had at least 1 year of KPNC membership before diagnosis and no disenrollment of 6 months or longer since becoming eligible for HPV vaccine. Of these 445 DM1 cases, 388 (87.2%) were diagnosed at least 6 months after becoming eligible to receive HPV vaccine (i.e., after the lag period) and were therefore included in the analysis. Of these, 123 (31.7%) had received an HPV vaccine and 265 (68.3%) were unvaccinated at time of symptom onset.
Fig. 1.
Flow chart of DM1 case disposition, Kaiser Permanente Northern California (KPNC) 2006–2015.
3.3. DM1 analysis
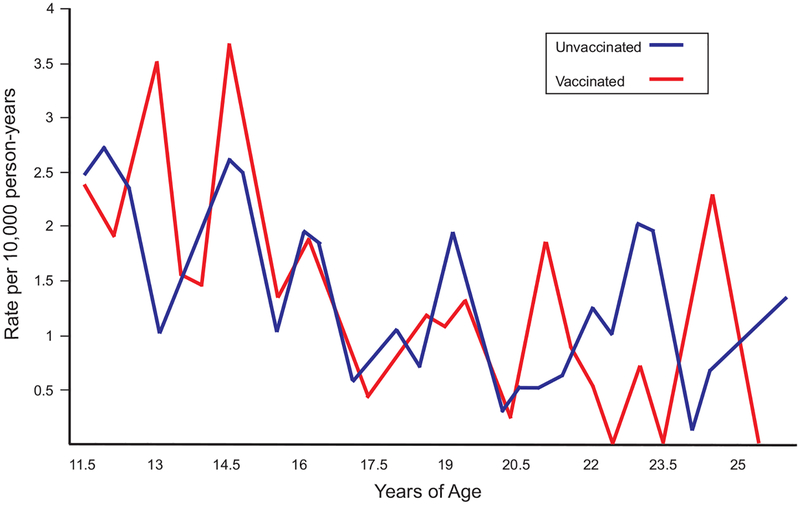
The 1.58 million individuals in the AD study population decreased to 911,648 after we applied all DM1 analysis population criteria. Of the 911,648 in the DM1 analysis population, 330,200 (36.2%) received ≥1 dose of HPV vaccine by the end of their follow up and 581,448 (63.8%) did not (Table 1). The proportion of all follow-up time that was after vaccination was 0.31 (Table 2). As expected, more females than males received 1 dose of HPV vaccine (62.9% vs 37.1%, Table 1) and females also had a larger proportion of post-vaccination follow-up time (0.37 vs 0.19; Table 2). Regardless of vaccination status, overall DM1 incidence in the analysis population was 1.2 per 10,000 person-years and generally decreased with increasing age (Fig. 2). DM1 incidence trended higher among males in our study population, although it was not statistically significantly different (hazard ratio [HR] 1.24, 95% confidence interval [CI] 0.92, 1.65. P = 0.154 and Supplemental Figure).
Fig. 2.
Diabetes mellitus type I incidence by age and HPV vaccination status after applying lag period and membership criteria, Kaiser Permanente Northern California 2006–2015.
Multiplicative hazards analysis anchored on calendar date and stratified on age, membership, race, Medicaid status and controlling for sex comparing vaccinated with unvaccinated did not demonstrate an increased risk of DM1 associated with HPV vaccine (HR 1.21, 95% CI 0.94, 1.57; Table 3).
Table 3.
Hazard ratios for new onset diabetes mellitus type 1 following HPV vaccination.
| Risk of DM1 after HPV vaccine | Hazard ratio | 95% CI | P-value | ||
|---|---|---|---|---|---|
| Primary analysis | HPV vaccinated vs. unvaccinateda | 1.21 | 0.94, 1.57 | 0.15 | |
| Sensitivity analyses assessed | Sensitivity analyses interpretation | ||||
| Potential confounding by sex | Stratified by sexb | 1.18 | 0.91, 1.53 | 0.22 | Model was more restrictive because there had to be variation of HPV vaccination status among people who were the same sex as each DM1 case. It would have yielded different results if there was a risk of DM1 after HPV vaccine and if the risk differed by sex |
| Females onlyc | 1.23 | 0.90, 1.67 | 0.20 | Most of the vaccinated study population was female. | |
| Males onlyc | 1.07 | 0.64, 1.77 | 0.80 | These analyses confirmed that inclusion ofmales did not drive the primary result | |
| Impact of sparse data | Stratified by sexd | 1.18 | 0.91, 1.53 | 0.22 | If sparse strata mattered to the size of the confidence intervals, computing with exact methods would have yielded different results |
| Females onlyd | 1.23 | 0.90, 1.67 | 0.20 | ||
| Potential confounding by age | Stratified by 6-month instead of 1-year age categoriese | 1.21 | 0.93, 1.57 | 0.15 | All sensitivity analyses were also done using age in 6- month categories and results were all similar |
| Analysis used an age timeline instead of a calendar timelinef | 1.21 | 0.92, 1.59 | 0.17 | These analyses controlled very finely for the effects of age. They demonstrate that the primary results were not strongly confounded by age | |
| Analysis used an age timeline instead of a calendar timeline, females onlyg | 1.27 | 0.92, 1.76 | 0.14 | ||
| Influence of age groups for whom vaccine delivery differed over time | Included only people aged <26 yearsh | 1.17 | 0.92, 1.49 | 0.21 | This analysis showed that the primary analysis results were not unduly influenced by the small number of DM1 cases which occurred in people >26years of age, all of whom were female |
| Influence of outliers | Analysis restricted to risk sets where at least 2% were vaccinated with HPV | 1.18 | 0.91, 1.53 | 0.22 | This analysis showed that outliers did not have undue influence on the primary results and that results were not driven by a small number of overly-influential DM1 cases |
Cox regression stratified by age in years, calendar year, years of prior membership, race and Medicaid status. HPV vaccination status and sex were independent variables in the model.
Cox regression stratified by sex, age in years, calendar year, years of prior membership, race and Medicaid status. HPV vaccination status was an independent variable in the model.
Same as b, but restricted to females/males only.
Same as b, but confidence intervals and p-value computed with exact methods.
Cox regression stratified by age in 6-month categories, calendar year, years of prior membership, race and Medicaid status. HPV vaccination status and male sex were independent variables in the model.
Same as b, except analysis used an age timeline instead of a calendar timeline. In this analysis, all individuals in each risk set were born within two weeks of each other.
Same as g, but restricted to females only.
Same as b, but restricted to those <26 years of age.
Sensitivity analyses stratified by sex and exact analyses were similar, as were analyses based on an age timeline, those limited to those less than 26 years of age, and analyses leaving out the most unbalanced risk sets. All sensitivity analyses produced relative risk estimates near 1, but with wider confidence intervals, as would be expected (Table 3). In addition, analyses which included a utilization measure the year before HPV eligibility did not change the results (HR 1.21, 95% CI 0.93, 1.57, P = 0.15).
4. Discussion
In this study, we evaluated risk of new onset DM1 following HPV vaccination during the first 10 years of routine use of HPV vaccine and found no evidence of an association between HPV vaccine receipt and development of DM1. Despite DM1 occurring more commonly among males in our study population, a previously observed phenomenon, our analysis limited to males did not detect an increased risk for DM1 after HPV vaccination [16,17]. Our study provides several important advantages over prior studies. First, we confirmed via chart review sample that nearly all identified DM1 cases were true cases. We applied a 6-month period prior to DM1 diagnosis to account for the timing of symptom onset and the lag time to diagnosis to ensure that we correctly assigned vaccination status at the time DM1 symptoms would have begun. Finally, and importantly, our study assessed for new onset DM1 over a decade of real-world use of the HPV vaccine. Using these rigorous analytic criteria, this study found no association between HPV vaccination and long-term development of DM1.
In general, our findings are consistent with prior studies. We previously conducted an observational study assessing the general safety of 4vHPV in more than 180,000 vaccinees from both Northern and Southern California Kaiser Permanente and did not note any association between DM1 and receipt of 4vHPV [10]. Autoimmune-specific safety analyses performed separately as part of this larger 4vHPV safety study noted a decreased association between 4vHPV and new onset DM1 (incidence rate ratio 0.57, 95% CI 0.47, 0.73). This finding was based on small numbers of cases in which the timing of disease onset had no clear relationship with vaccination [9]. A French case-control study similarly found no association between DM1 and 4vHPV vaccine receipt (odds ratio 1.2, 95% CI 0.4, 3.6), nor did another French cohort study (HR 1.07, 95% CI 0.87, 1.31), while a recent Danish study among males did not identify an association between HPV vaccination and DM1 (rate ratio 0.80, 95% CI 0.40, 1.6) [13,15,18].
A large study in Denmark and Sweden evaluating AD among females during the 180 days following HPV vaccination found a statistically significant association with DM1 (rate ratio [RR]1.29, 95% CI 1.03, 1.62), as well as with Behcet’s disease (RR 3.37, 95% CI 1.05, 10.80) and Raynaud’s disease (RR 1.67, 95% CI 1.14,2.44) [12]. However, after the authors applied three predefined criteria (“reliability of analysis”, “strength of association” and “consistency”), none met all three (DM1 only met the “reliability” criteria), and they concluded that there was no consistent evidence for a causal association. Our study differs from this study in several important ways. Our population included males and was racially and ethnically diverse. We also had a longer exposure period and looked for DM1 any time after vaccination within the study period. In addition, we excluded the 6-month time-period prior to diagnosis because during that time period, it would be unclear if the onset of disease came before or after the vaccine. We conducted medical record review to confirm cases and onset date, thus we had high confidence that our vaccinated cases had symptom onset after vaccination. Finally, background incidence of diabetes varies by age, for which we controlled very finely.
In our study of DM1 we were able to address a number of challenges inherent in studying AD. First, we found that specific, welldefined initial symptoms of DM1 were nearly always documented in our medical records. From our sample chart review, we found that we could consistently determine when symptoms began with a reasonable degree of accuracy. The initial presentation of DM1 in adolescents is typically acute and can be severe, thus patients usually seek care soon after onset of symptoms [19,20]. We were therefore able to reliably estimate a lag time between symptom onset and DM1 diagnosis which we could confidently apply to all cases, regardless of chart review. These methods of DM1 case detection should be useful in future studies. Finally, because these specific factors meant that we could conduct a credible analysis using nonchart reviewed cases, we were able to analyze a larger number of cases.
However, this study faced unusual and specific challenges related both to the changing recommendations for adolescent HPV vaccine use and changes to KPNC family memberships during the study period. In our study population, the likelihood of having received HPV vaccine varied dramatically depending on calendar year, age, and sex. Studies of AD following adolescent vaccination must consider that during the late teen years, many adolescents leave their parent’s health plan to go to school and work and these individuals could no longer be included in the study population. For this reason, we lost a large fraction of our potential follow up time after HPV vaccination. Since females began receiving HPV 5 years earlier than males, many of them had 5 more years during which they could have a gap in membership and be excluded from the study population. Consequently, since 2011, there were many more males in the older teenage groups than there were females of similar age. Together, these factors created a dynamic variation in the age-sex composition of our study population over time, and it was critical that we correctly adjusted for age and sex in our analyses so that we did not produce spurious results.
4.1. Limitations
We acknowledge that our study had limitations. Many males could not be included in the analysis because HPV was only recommended for males starting late in 2011. It is also possible that people who received HPV vaccine as a catch-up (i.e., at ages older than the recommended 11 or 12 years) may have been different in unmeasured ways than people who received HPV vaccine as part of routine scheduled well care. Similarly, our comparison group of people who never received HPV vaccine may have also differed in unmeasured ways from vaccinated people. Finally, our study population suffered from informative censoring (i.e., receipt of HPV vaccine was correlated with continued membership, dropout rates differed for vaccinated and unvaccinated persons). We attempted to mitigate this by close matching on years of prior membership, but this does not fully eliminate the problem. Finally, the results of all the sensitivity analyses suggest that there was residual confounding that we were unable to address.
5. Conclusions
Our large retrospective cohort study found no increased longterm risk for development of DM1 following HPV vaccination during the 10-year study period. Based on our DM1 case ascertainment in this study, it is feasible to monitor the safety of HPV vaccine with regard to DM1 using automated data. While safety assessments of HPV vaccine, as for all vaccines, are ongoing, our study provides reassurance that there is no substantial increased risk for DM1 after HPV vaccination.
Supplementary Material
Acknowledgements
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of CDC.
Funding
This work was supported by grant funding from the Centers for Disease Control and Prevention (CDC). Contract Number: 200-2012-53581/0008.
Footnotes
Potential conflicts of interest
N.K. has received research support for unrelated studies from Sanofi Pasteur, GlaxoSmithKline, Pfizer, Merck, Protein Sciences (now Sanofi Pasteur), MedImmune and Dynavax. All other authors have no conflicts or financial disclosures.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2019.02.051.
References
- [1].Prescribing information. Gardasil (Human Papillomavirus Quadrivalent Vaccine [types 6, 11, 16 and 18]); 2015. Available at http://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm111263.pdf [accessed February 8, 2018].
- [2].Prescribing information. Gardasil 9 (Human Papillomavirus 9-valent Vaccine, Recombinant); 2018. Available at http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM426457.pdf [accessed June 20, 2018]. [PubMed]
- [3].Food and Drug Administration. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2015. Available at https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM477341.pdf [accessed June 20, 2018]. [Google Scholar]
- [4].Slade BA, Leidel L, Vellozzi C, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 2009;302:750–7. [DOI] [PubMed] [Google Scholar]
- [5].Gee J, Naleway A, Shui I, et al. Monitoring the safety of quadrivalent human papillomavirus vaccine: findings from the Vaccine Safety Datalink. Vaccine 2011;29:8279–84. [DOI] [PubMed] [Google Scholar]
- [6].Klein NP et al. Safety of quadrivalent human papillomavirus vaccine administered routinely to females. Arch Pediatr Adolesc Med 2012;166(12):1140–8. [DOI] [PubMed] [Google Scholar]
- [7].CDC. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2007;56(No. RR-2). [PubMed] [Google Scholar]
- [8].CDC. Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP). MMWR 2011;60(50):1705–8. [PubMed] [Google Scholar]
- [9].Karussis D, Petrou P. The spectrum of post-vaccination inflammatory CNS demyelinating syndromes. Autoimmun Rev 2014;13(3):215–24. [DOI] [PubMed] [Google Scholar]
- [10].Pellegrino P et al. On the relationship between human papilloma virus vaccine and autoimmune diseases. Autoimmun Rev 2014;13(7):736–41. [DOI] [PubMed] [Google Scholar]
- [11].Chao C et al. Surveillance of autoimmune conditions following routine use of quadrivalent human papillomavirus vaccine. J Intern Med 2012;271(2):193–203. [DOI] [PubMed] [Google Scholar]
- [12].Arnheim-Dahlstrom L et al. Autoimmune, neurological, and venous thromboembolic adverse events after immunisation of adolescent girls with quadrivalent human papillomavirus vaccine in Denmark and Sweden: Cohort study. BMJ 2013;347:f5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Grimaldi-Bensouda L et al. Autoimmune disorders and quadrivalent human papillomavirus vaccination of young female subjects. J Intern Med 2014;275(4):398–408. [DOI] [PubMed] [Google Scholar]
- [14].Klein NP et al. Rates of autoimmune diseases in Kaiser Permanente for use in vaccine adverse event safety studies. Vaccine 2010;28(4):1062–8. [DOI] [PubMed] [Google Scholar]
- [15].Miranda S, Chaignot C, Collin C, et al. Human papillomavirus vaccination and risk of autoimmune diseases: a large cohort study of over 2 million young girls in France. Vaccine 2017;35(36):4761–8. [DOI] [PubMed] [Google Scholar]
- [16].Holman DM et al. Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr 2014;168(1):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jacobson DL et al. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol 1997;84(3):223–43. [DOI] [PubMed] [Google Scholar]
- [18].CDC Human Papillomavirus Vaccination Coverage Among Adolescents, 2007–2013, and postlicensure vaccine safety monitoring, 2006–2014—United States. MMWR 2014;63(29):620–4. [PMC free article] [PubMed] [Google Scholar]
- [19].Frisch M et al. Quadrivalent human papillomavirus vaccination in boys and risk of autoimmune diseases, neurological diseases and venous thromboembolism. Int J Epidemiol 2018. April 1;47(2):634–41. [DOI] [PubMed] [Google Scholar]
- [20].Merger SR, Leslie RD, Boehm BO. The broad clinical phenotype of Type 1 diabetes at presentation. Diabet Med 2013;30(2):170–8. 10.1111/dme.12048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.