Abstract
A systematic review was conducted to identify best practices for increasing linkage, retention and re-engagement in HIV care (LRC) for persons living with HIV (PLWH). Our search strategy consisted of automated searches of electronic databases and hand searches of journals, reference lists and listservs. We developed two sets of criteria: evidence-based to identify evidence-based interventions (EBIs) tested with a comparison group and evidence-informed to identify evidence-informed interventions (EIs) tested with a one-group design. Eligible interventions included being published between 1996 and 2014, U.S.-based studies with a comparison or one-group designs with pre-post data, international randomized controlled trials, and having objective measures of LRC-relevant outcomes. We identified 10 best practices: 5 EBIs and 5 EIs. None focused on re-engagement. Providers and prevention planners can use the review findings to identify best practices suitable for their clinics, agencies, or communities to increase engagement in care for PLWH, ultimately leading to viral suppression.
Keywords: linkage to HIV care, retention in HIV care, engagement in HIV care, systematic review, evidence-based interventions, best practices
Palabras clave: vinculación con la atención médica del VIH, la permanencia en la atención médica del VIH, la participación en la atención médica del VIH, revisión sistemática, las intervenciones basadas en la evidencia, las mejores prácticas
Resumen
Una revisión sistemática se realizó para identificar las mejores prácticas para aumentar la vinculación, la permanencia y el regreso hasta atención médica del VIH (VPR) para las personas que viven con el VIH (PVVS). La estrategia de búsqueda consistió en búsquedas automatizadas de bases de datos electrónicas y búsquedas manuales en revistas, listas de referencias y listas de correo electrónico. Hemos desarrollado dos juegos de criterios: “basadas en evidencias” para identificar las intervenciones basadas en la evidencia y probadas con un grupo de comparación (IBEs), y “informadas por evidencias” para identificar las intervenciones informadas por evidencias y probadas con un diseño empleando un solo grupo (IIEs). Intervenciones elegibles incluyeron siendo publicados entre 1996 y 2014, estudiados en los Estados Unidos con un grupo de comparación o uno grupo con datos pre-post, ensayos internacionales controlados aleatorios, y que tienen medidas objetivas de resultados VPR-relevantes. Se identificaron 10 mejores prácticas: 5 IBEs y 5 IIEs. Ninguno se centró en un regreso hasta atención médica. Los proveedores y los planificadores de prevención pueden utilizar los resultados de la revisión para identificar las mejores prácticas adecuadas para sus clínicas, agencias, o comunidades para aumentar la participación en la atención médica para las PVVS, en última instancia conduciendo a la supresión viral.
INTRODUCTION
Being engaged in HIV medical care (e.g., linked to and retained in HIV medical care) is a crucial step in achieving viral suppression for persons living with HIV (PLWH) [1]; however, recent estimates have indicated only 40% of PLWH are engaged in care [2]. Engagement in HIV care has been associated with better health outcomes for PLWH [3, 4] and the population as a whole [5, 6] while not being in care or consistent care may contribute to poor health outcomes [7, 8], increased mortality [9, 10] and a large percentage of new HIV infections [11]. Identifying best practices that may help to increase the percentage of PLWH who are linked to, retained and re-engaged in HIV care is urgently needed and may contribute to reaching national HIV prevention goals in the National HIV/AIDS Strategy [1].
Previous systematic reviews on U.S.-based literature on engagement in HIV care interventions have synthesized intervention characteristics and study aspects across studies [12, 13]. These reviews have been useful for providing an overview of the types of interventions tested and used study design to rank studies for strength of evidence. A more comprehensive approach that rigorously evaluates individual interventions by examining additional domains such as study quality, study implementation, data analysis, as well as strength of evidence extends the findings from these previous reviews. Identifying “best practices” for linkage to, retention, or re-engagement in HIV care (LRC) using this approach provides the field with model programs that have demonstrated low risk of bias and evidence of effectiveness.
This article describes the systematic review process implemented by the Centers for Disease Control and Prevention’s (CDC) Prevention Research Synthesis Project (PRS) for identifying LRC best practices including the evaluation criteria and review methods. The paper summarizes the LRC best practices identified as well as discusses LRC interventions not meeting best practices criteria. Findings, limitations, and recommendations for future research are also discussed.
METHODS
PRS Best Practices Criteria for LRC Interventions
PRS staff conducted a series of activities to develop LRC best practices criteria within the context of an emerging intervention research literature. These activities included multiple consultations with CDC scientists, non-federal researchers with expertise in HIV care engagement, and a key federal partner – the National Institute of Mental Health (NIMH). PRS also used existing efficacy criteria for identifying evidence-based HIV-related risk reduction (RR) and medication adherence (MA) interventions as an initial framework and adapted the criteria to address issues relevant to engagement in HIV care. PRS criteria are consistent with evaluation components used or recommended by groups such as the Agency for Healthcare Research and Quality [14], the Cochrane Collaboration [15], The Community Guide [16], and Grade of Recommendation, Assessment, Development and Evaluation (GRADE) [17].
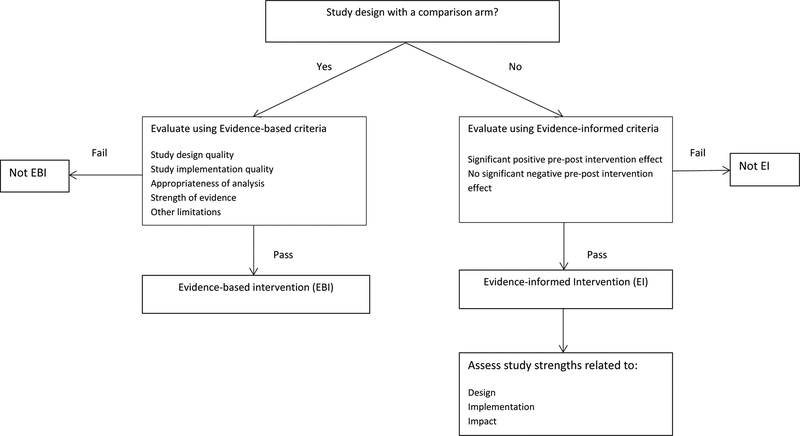
Given the limited number of controlled trials and the urgent need for identifying best practices, PRS developed two sets of criteria to evaluate LRC interventions: evidence-based (EB) and evidence-informed (EI) criteria. Figure 1 illustrates the process for deciding which set of criteria is used for evaluating studies. EB criteria are used to evaluate LRC interventions that have been tested with a comparison group. The EB criteria assess four domains: study design quality, study implementation quality, data analysis quality, and strength of evidence (specific EB criteria are listed in http://www.cdc.gov/hiv/pdf/prs_compendium_criteria_for_ebis.pdf, also Appendix 1). LRC interventions meeting all EB criteria are identified as Evidence-Based Interventions (EBIs) and provide the strongest evidence of efficacy.
Figure 1:
Process for identifying best practices for linkage to, retention and re-engagement in HIV medical care
http://www.cdc.gov/hiv/prevention/research/compendium/lrc/bestpractices.html
The second set of evaluation criteria were developed to evaluate LRC interventions tested with one-group study designs that are often used in evaluations of LRC-related programs. We labeled this set of criteria as evidence-informed (EI). EI criteria are not as rigorous as evidence-based criteria because bias related to selection/allocation, history, or differential attribution cannot be appropriately assessed given the one-group study design (specific EI criteria are listed in http://www.cdc.gov/hiv/pdf/prs_compendium_criteria_for_eis.pdf, also Appendix 2). LRC interventions meeting all EI criteria are identified as Evidence-Informed Interventions (EIs).
Systematic Search Strategy
Two librarians developed and conducted a comprehensive and systematic search strategy, including both annual automated and quarterly manual searches, to identify all relevant engagement in HIV care intervention reports for the PRS cumulative database. The annual automated search component focused on literature published from January 1996 using the following electronic databases and platforms: CINAHL (EBSCOhost), EMBASE (OVID), MEDLINE (OVID), and PsycINFO (OVID). We selected 1996 as the start date for our search to be consistent with the year that ART was made more available to persons living with HIV in the U.S. The automated search component used indexing and keyword terms, cross-referenced employing Boolean logic, in three areas: (a) HIV/AIDS; (b) intervention and prevention evaluation; (c) and engagement in care related terms (e.g., linkage, retention). Indexing terms for the electronic searches were varied according to each database, but keywords remained constant across all databases and searches. The search was not restricted by country or language. The last automated search for this review was conducted in May 2014 and covered citations published from January 1996 to December 2013. The full search strategy of the MEDLINE database is provided in Appendix 3. The searches of the other databases are available from the corresponding author.
The quarterly manual search component involved reviewing all articles published in the previous 3 months of 60 journals to identify potentially relevant articles not yet indexed in electronic databases and examining the reference lists of relevant published articles, HIV/AIDS Internet listservs, various research databases (i.e., ISI Web of Knowledge, RePORTER, Cochrane), and unpublished manuscripts of study authors. The last quarterly manual search for this review was conducted in and covered citations published up to October 2014. Further details of the quarterly manual search can be obtained from the corresponding author.
Study Selection
We searched the PRS database for eligible reports. Reports were included for the LRC best practices review if they (1) were published or accepted for publication in a peer-reviewed journal between 1996 and October 2014; (2) were conducted in the U.S. or a U.S. territory and had a comparison arm or if one-group study design, had pre-post intervention data; (3) were conducted outside the U.S. and used a randomized controlled trial study design, (4) used relevant measures for LRC outcomes (i.e., HIV medical visits documented in medical or agency records, or surveillance reports; HIV viral loads or CD4 counts as proxies for HIV medical visits; self-reports validated by medical or agency records, or surveillance reports) and (5) reported relevant LRC intervention outcome data (i.e., linkage to, retention in, or re-engagement in HIV medical care). Linkage to care was defined as the initial HIV medical visit for newly- or recently-diagnosed PLWH. Retention in care was broadly defined as having two or more HIV medical visits within a specified time period. Re-engagement in care was defined as the first HIV medical visit for PLWH who were in care previously, but fell out of care. All types of interventions that reported the above outcomes were considered (e.g., individual, couple, group, agency, community, structural, policy). Given the LRC intervention research literature in the U.S. is still emerging, we increased the pool of eligible studies by also including international RCTs, but not international studies with one-group pre-post designs. Interpreting findings from one-group pre-post designs conducted in international settings is challenging because the results are more susceptible to historical events and contextual factors such as local laws and policy changes. We included international RCTs because these interventions were evaluated with the most rigorous methodology and a comparison arm to reduce potential bias that may result from confounders. Exclusion criteria included reports that: a) focused on health care utilization (e.g., focused on persons with unknown HIV care histories or reports with outcomes that could not be clearly classified as linkage, retention, or re-engagement); b) were non-specific to HIV primary care (e.g., emergency room visits, hospitalizations); c) exclusively relied on self-reported outcomes; d) did not have pre-intervention data for U.S.-based one-study designs; and e) described pilot studies of full-scale interventions that were included in the review.
Qualitative Data Coding
Pairs of trained coders independently evaluated each eligible intervention against the newly established best practices criteria on study design, implementation, analysis, and strength of findings. The reliability between coders on best practices coding was not calculated. All PRS coders go through standardized and stringent coding training and on average, the overall percentage agreement among the trained coders is 96% with a kappa rate of 80% on our regular citation-level coding, indicating a high inter-rater reliability. All discrepancies were reconciled between paired coders. Linked reports, defined as publications providing additional information on the same study, were included if they provided relevant intervention evaluation information. The first author of individual studies was contacted to provide missing data or clarification as needed. Final best practices determination for each study was reached by PRS group consensus.
RESULTS
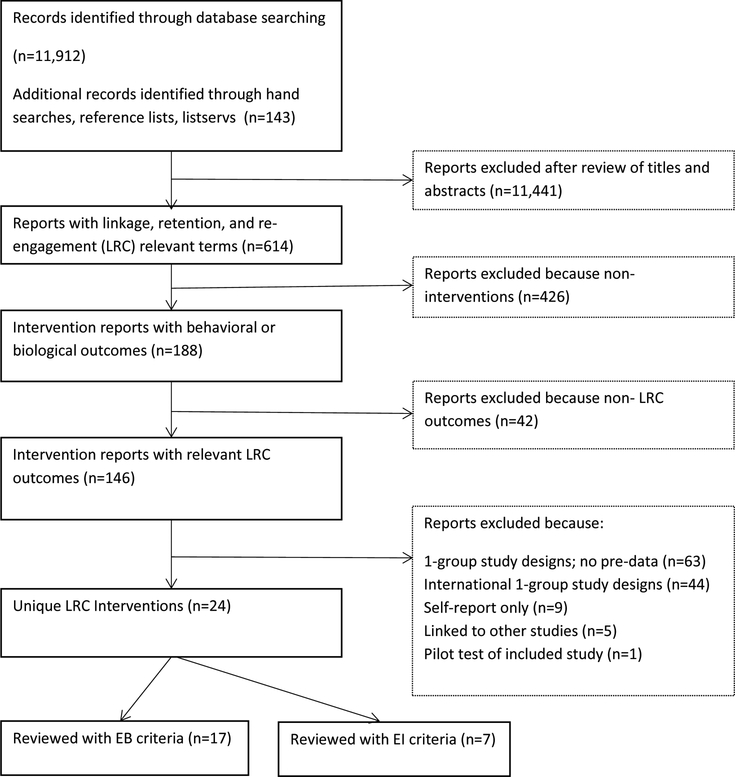
Figure 2 presents the systematic search results. The automated and manual searches yielded 12,055 reports. After screening titles, abstracts and full reports, we identified 146 intervention reports with relevant LRC outcomes. Of these, we excluded 122 reports and identified 24 eligible interventions. We evaluated 17 interventions (2 for linkage to care, 12 for retention in care, 3 for both linkage and retention) with evidence-based criteria and 7 studies with evidence-informed criteria (3 for linkage, 4 for retention). None of the eligible studies reported re-engagement outcomes. Ten best practices were identified – five EBIs and 5 EIs.
Figure 2.
Study Selection Process and Results
Evidence-based Interventions (EBIs)
Study characteristics of the 5 EBIs are presented in Table 1. One EBI found effects for linkage [18], one for linkage and retention [19], and three for retention [20–22]. None of the EBIs reported re-engagement outcomes. The linkage EBI focused on linking newly diagnosed persons to medical care using trained counselors and home visits from community support workers in Uganda [18]. The remaining 4 EBIs were U.S.-based. The Antiretroviral Treatment Access Study (ARTAS) that provided up to 5 strengths-based case management counseling sessions showed significant intervention effects for both linkage and retention outcomes among recently diagnosed patients [19]. The other three EBIs that demonstrated intervention effects on retention outcomes used different primary intervention strategies including co-location of services [21], an interactive notification system to alert providers when patients missed appointments [22], and encouraging patients to keep their medical visits via in-person and telephone contact [20]. The retention-in-care EBIs, including ARTAS, varied in how they measured retention in care. The most common ways retention in care was measured were visit constancy (at least one visit in consecutive intervals over a specified time period) and kept visits over a specified time period.
Table 1.
Evidence-Based Interventions (EBIs) for Linkage to, Retention and Re-engagement in HIV Care
| Author (Year) Locationa | Intervention Name | LRC Outcome | Key Population | Nb | Sexc M/F/T % |
PoCd % | Agee | Intervention Delivererf: Intervention Strategies; Intervention Duration |
Comparison Strategies |
|---|---|---|---|---|---|---|---|---|---|
| Gardner [19] (2005) U.S. |
ARTAS | Linkage to care (1st visit in 6 months post enrollment) Retention (at least 1 visit in each of 2 consecutive 6-month follow-up periods) |
Recently-diagnosed | 273 | 71/29 | 93 | 18–39 (63%) >40 (37%) |
Linkage Coordinator:
|
|
| Gardner [20](2014) U.S. |
Retention through Enhanced Personal Contacts | Retention (visit constancy-keeping at least 1 visit with HIV primary doctor in 3 consecutive 4-month intervals; visit adherence - % kept visits; mean # of kept visits; mean # of missed visits in 12 months) |
Clinic patients | 1838 | 63/36/1 | 88 | 18–29 (11%) 30–39 (20%) 40–49 (34%) 50–59 (29%) >60 (7%) |
Interventionist:
|
Automated and personal appointment reminder calls |
| Lucas [21](2010) U.S. |
Buprenorphine Treatment | Retention (number of kept visits in 12-months) |
Opioid-dependent clinic patients | 96 | 78/22 | 98 | 46 median | Physician, nurse with substance use training:
|
Drug treatment referrals Case management |
| Muhamadi [18](2011) Uganda |
Extended Counseling | Linkage (1st visit within 5 months post enrollment) |
Recently-diagnosed | 400 | 36/64 | NR | 18–24 (17%) 25–34 (31%) 35–44 (28%) 45–70 (23%) |
Trained counselor, community support workers:
|
Non-trained counselor provides post-test counseling |
| Robbins [22](2012) U.S. |
Virology Fasttrack | Retention (no arrived appointment for > 6 months) measured as events per 100 patient-years in 12 months) |
HIV care providers and their clinic patients | 1011 | 72/28 | 46 | >40 (75%) |
Clinical decision support system
|
Static alerts to patient-specific EMR |
Study location
Total sample size
M=Males; F=Females; T=Transgendered persons
Persons of Color
Mean age unless specified as median or category. Totals may not sum to 100% due to rounding.
If Intervention not delivered by a person, intervention deliverer is not listed
NR = Not reported
Evidence-Informed Interventions (EIs)
Five U.S.-based interventions were identified as EIs (Table 2). One EI focused on linkage [23] while the remaining four EIs focused on retention [24–27]. No EIs reported re-engagement in care outcomes although one study did include previously-in-care participants in the study [27]. The EI that demonstrated intervention effects on the linkage outcome implemented a policy of scheduling an orientation visit when new clinic patients called for an appointment. Among the four EIs that demonstrated intervention effects on the retention outcomes, three exclusively focused on special populations within racial/ethnic minority groups such as MSM or young persons. These EIs intervened at both the individual and clinic level. At the individual level, examples of strategies included counseling, motivational interviewing, case management, providing brief messages on the importance of staying in care, and help with appointment scheduling. At the clinic level, strategies included displaying posters about the importance of keeping medical appointments in exam and waiting rooms, and adding staff that had expertise in or represented the clinic population.
Table 2.
Evidence-Informed Interventions (EIs) for Linkage to, Retention and Re-engagement in HIV Care
| Author (Year) Locationa |
Intervention Name | LRC Outcome | Key Population | n (pre)b | n (post)c | Sexd M/F/T % | PoCe % | Agef | Intervention Deliverer: Intervention Strategies Intervention Duration |
|---|---|---|---|---|---|---|---|---|---|
| Davila [24](2013) U.S. |
Centralized HIV Services | Retention visit constancy (had 3 or more quarters with at least 1 HIV primary care visit in year after diagnosis); gaps in care - ≥ 180 days between any 2 consecutive HIV primary care visit in year after diagnosis |
Young black or African American and Hispanic/Latino clinic patients | 90 | 36 | 62/38 | 100 | <18 (13%) 18–20 (40%) 21–23 (47%) |
Youth-focused health care provider, social worker, case manager:
|
| Enriquez [25](2008) U.S. |
Bilingual Bicultural Care Team |
Retention (number of scheduled and kept HIV clinic visits in 12 months) |
Hispanic/Latino clinic patients | 43 | 43g | 79/21 | 100 | 18–25 (2%) 26–39 (60%) 40–55 (30%) >55 (7%) |
Bilingual/bicultural nurse, social worker, case manager:
|
| Gardner [26](2012) U.S. |
Stay Connected | Retention (kept at least 2 consecutive visits within 12 months after anchor visit; proportion of all kept scheduled HIV primary care visits within 12 months after anchor visit |
Clinic patients | 11039 | 10018 | 65/35 | 83 | 16–29 (6%) 30–39 (17%) 40–49 (36%) >50 (41%) |
Clinic staff:
|
| Hightow-Weidman [27](2011) U.S. |
STYLE | Retention (Percent of medical visits-number of attended visits/total number of scheduled HIV care visits every 4 months over a 2 year period) |
Recently-diagnosed or lost to-care black or African American and Hispanic/Latino young MSM | 81 | 31 | 100 | 100 | 21 | Peer Outreach Worker, physician, medical case manager, research staff:
|
| Mugavero [23](2008) U.S. |
Project CONNECT | Linkage (attending primary HIV visit within 6 months of orientation visit) |
Recently-diagnosed | 361 | 522 | 76/24 | 56 | 40 | Social worker:
|
Study Location
Sample size of pre-intervention group
Sample size of post-intervention group
M=Male, F=Female, T=Transgendered persons
Persons of Color
Mean age unless specified as a median or category. Totals may not sum to 100% due to rounding.
Cohort study
Characteristics of Studies not identified as Best Practices
Fourteen studies were evaluated, but did not meet the best practices criteria. Of these, twelve studies did not meet evidence-based criteria (non-EBIs) and two did not meet evidence-informed criteria (non-EIs). For the non-EBIs, one was a linkage study [28], nine focused on retention [29–37] and two reported both linkage and retention outcomes [38, 39]. The most common reason for not being identified as an EBI was not having a significant positive intervention effect [31–38]. Additional reasons for not meeting evidence-based criteria included having outcomes and follow-up assessments that did not occur at a required time point [29, 38] or were unclear[28, 30, 39], biased allocation of participants to study arms (e.g., comparing two pre-existing groups, researcher pre-selecting a clinic to be an intervention arm and another to be the comparison arm) [30, 31, 33, 39], retrospective study designs [30, 33, 39], baseline sample sizes less than 40 per arm [29, 33, 34], and non-appropriate comparison arms (e.g., same intervention, different delivery method) [32, 36]. The non-EBIs and EBIs were similar in baseline sample characteristics and study characteristics (Table 3). Baseline sample sizes for non-EBIs had a larger range than EBIs. A slightly higher percent of non-EBIs focused on persons of color and women compared to non-EBIs.
Table 3.
Comparison of characteristics between EBIs and non-EBIs, EIs and non-EIs included in PRS Best Practices Review
| Characteristics | 5 EBIs n (%) |
12 Non-EBIs n (%) |
5 Els n (%) |
2 Non-EIs n (%) |
|---|---|---|---|---|
| Outcomes | ||||
| Linkage to HIV care | 1 (20) | 1 (8) | 1 (20) | 2 (100) |
| Retention in HIV care | 3 (60) | 9 (75) | 4 (80) | 0 |
| Both | 1 (20) | 2 (17) | 0 | 0 |
| Re-engagement | 0 | 0 | 0 | 0 |
| Location | ||||
| U.S. | 4 (80) | 9 (75) | 5 (100) | 2 (100) |
| International | 1 (20) | 3 (25) | NA | NA |
| Primary Setting | ||||
| Clinic only | 4 (80) | 11 (92) | 4 (80) | 2 (100) |
| Clinic & Communityb | 1 (20) | 1 (9) | 1 (20) | 0 |
| Specific Population Focusc | ||||
| African American/black | 0 | 0 | 2 (40) | 0 |
| Hispanic/Latino | 0 | 0 | 3 (60) | 0 |
| Youth or young persons | 0 | 1(9) | 2 (40) | 0 |
| MSM | 0 | 0 | 1 (20) | 0 |
| Women only | 0 | 1(9) | 0 | 0 |
| Persons with substance use or mental health issues | 1(20) | 1(9) | 0 | 0 |
| Recently incarcerated | 0 | 1(9) | 0 | 0 |
| Veterans | 0 | 1(9) | 0 | 0 |
| Study-Related | ||||
| Baseline sample size (min, max) | 96,1838 | 60,10095 | 43,11039 | 6, 28a |
| Percent Persons of Color (mean) | 81% | 86% | 88% | NR |
| Percent Women (mean) | 36% | 48% | 22% | NR |
Sample sizes reflect the number of persons testing HIV positive.
Community-based organizations, field settings
Multiple responses possible
EBI = Evidence-based intervention
EI = Evidence-informed intervention
NR = Not reported
With regard to the non-EIs, the two interventions were HIV testing-related interventions in emergency room settings [40, 41]. Neither study found significant intervention effects on the change in the number of newly diagnosed persons who were linked to care from pre to post implementation of the intervention. One intervention tested routinely recommending HIV testing [40] while the other examined a tracking system for HIV testing to flag emergency room patients who were at risk for HIV [41]. The most noteworthy difference between the two non-EIs and 5 EIs was baseline sample sizes: non-EIs had much smaller sample sizes compared to EIs due to the subset of persons testing HIV positive within the larger sample of persons tested for HIV.
DISCUSSION
Given the importance of engaging PLWH in HIV medical care, it is encouraging that we identified 10 best practices for the first LRC review. The majority of LRC best practices were designed to improve retention in HIV care while a few best practices addressed linkage to care. We did not identify any intervention specifically designed for re-engagement in HIV care. Re-engaging PLWH who have been in care previously or linking those who may have been diagnosed with HIV, but never linked to care may be particularly challenging and require intensive intervention strategies. CDC recommends the use of surveillance data to identify people who are out of care and re-engage them into HIV care (see Data to Care - https://www.effectiveinterventions.org/en/HighImpactPrevention/PublicHealthStrategies/DatatoCare.aspx). Promising public health strategies may be available for guiding re-engagement of care activities; however, identifying best practices that successfully link never-in-care PLWH or re-engage lost-to-care PLWH remains a crucial need.
Programs that are considering adopting LRC best practices need to consider their resources and capacity for successful adoption and implementation. Three EBIs may be especially promising for a wider adoption. ARTAS has been successfully implemented and found to be effective in most field settings [42]. Intervention materials, training and technical assistance for ARTAS are readily available (https://effectiveinterventions.cdc.gov/en/HighImpactPrevention/PublicHealthStrategies/ARTAS.aspx). The Enhanced Personal Contacts intervention has been shown to be relatively low cost to implement [43] and interventions can be delivered by trained, non-professional staff. FastTrack, the provider notification system that alerts providers when patients miss appointments [22], may be viable for clinical settings that have existing patient reminder systems.
Evidence-informed interventions (EIs) can also be considered for implementation in clinic settings, but ideally, should be further tested with more rigorous study designs (e.g., using a comparison group) or replicated. A majority of the EIs we identified focused on special populations of PLWH and included interventionists with expertise in or from special populations. Having staff with expertise in or who represent specific patient populations in the clinic or other intervention settings may be important in reducing barriers to staying in care. Patients may feel more comfortable with their health care providers and agency staff and thus, be more likely to keep their medical appointments.
Fourteen studies did not meet best practices criteria, but these studies offer valuable insights. For interventions not meeting EB criteria, almost all did not find a significant positive intervention effect. Using seemingly “strong” comparison arms in which participants receive potent intervention components that go beyond the common standard of clinical care may be a plausible reason for this. Minimizing the variability in standard care provided to comparison groups may produce more comparable intervention effects across studies [44]. Several non-EBIs also had other issues besides not finding significant positive intervention effects, such as small sample sizes or not meeting PRS assessment indicators (e.g., within 6 months for linkage to care and at least 6 months or longer for retention in care). Using national indicators for assessing linkage and retention [45] as well as implementing strategies to reduce the risk of bias related to study design [46] would further facilitate evaluation of the LRC studies.
Finally, we noted that LRC-related outcome measures varied among the best practices that we identified, particularly for retention in care. Although no gold standard yet exists for measuring retention in care [47], we encourage researchers and programs to use the Human and Health Services (HHS) indicator for retention [45] and follow-up for at least 12 months, but strive for a 24-month measurement period. In addition, using other measures of retention in care such as missed visits or gaps in care may be important as these measures have been found to be highly correlated with viral suppression [47]. The updated NHAS for 2020 outlines the most recent indicators that the field should consider to meet national HIV prevention and care goals [1].
Limitations
Limitations of this review should be mentioned. First, similar to our risk reduction and medication adherence efficacy criteria, our best practices criteria for LRC primarily focused on internal validity with less emphasis on external validity, cost, and scalability. These additional criteria are important parts of high-impact prevention (HIP) [48] and critical for deciding which interventions could yield the most impact when they are implemented and scaled-up. Although these HIP criteria are not currently part of the best practice criteria, we abstracted the information from studies and found few studies that reported the information. Measuring and reporting the information about generalizability, cost, and scalability could be an important quest. We also found limited information from the published reports to provide specific guidelines for implementation. Although general recommendations for implementing linkage to and retention in care strategies in clinical and non-clinical settings are available in the Updated “Recommendations for HIV prevention with adults and adolescents with HIV in the United States”, we strongly encourage more publications and information sharing regarding the successful implementation of best practices [49]. Another limitation is that we only reviewed studies that meet our inclusion criteria: studies with a comparison arm or evaluated with a pre-post design. Observational and correlational studies may highlight potential correlates of intervention effects or barriers and facilitators of LRC outcomes. Further synthesis of these data could be informative. We also limited this review by excluding international one-group studies with pre-post intervention data due to concerns about historical factors that are not often properly controlled for in one-group designs and may impact the interpretation of study findings. We encourage others to conduct systematic reviews of all LRC interventions to identify best practices that are suitable for international settings. Finally, we primarily relied on the published literature to identify EBIs and EIs. Exploring the grey literature to identify best practices from non-peer reviewed sources would potentially expand the portfolio of LRC best practices.
Implications for Future Research
Our best practices review, despite the above limitations, has several implications for further research. Mentioned earlier, the most glaring research gap is the lack of best practices focused on re-engagement in care. While efforts must be improved to link and retain PLWH in care, developing and testing strategies to locate PLWH lost to care and retaining them once they are located needs to be prioritized. We also noted a lack of agreement in measuring LRC outcomes, particularly for retention in care. Although no gold standard exists for measuring retention in care, we hope the field will adopt a common set of indicators to measure LRC outcomes to facilitate evaluation of studies [45, 47]. Another implication for future research emerges from our review exclusion criteria. We excluded reports that relied only on self-reported data or lacked pre-intervention data. Given the low reliability of self-reported LRC outcomes [50], using objective measures of completed medical visits in lieu of or in addition to self-report are recommended when evaluating programs and conducting future research studies. We also encourage researchers to collect pre-intervention data when testing interventions in one-group study designs. Pre-intervention data allow for some kind of comparison when assessing the impact of an intervention. Finally, although randomized controlled trials may be the gold standard for determining intervention efficacy, assessing an intervention’s effectiveness in “real world” settings is also important. ARTAS has demonstrated effectiveness when implemented in clinical settings [42], but may be less effective with ex-offenders [37]. This kind of information is critical for assessing the impact of best practices and strengthening the evidence of best practices. We also recommend building research and evaluation capacity for agencies or prevention providers doing LRC-related work so they can evaluate their programs that have shown some promise, but not yet scientifically evaluated.
Conclusions
The LRC Best Practices review extends previous systematic reviews on engagement in HIV care by identifying model programs and promising intervention strategies for promoting linkage to, retention and re-engagement in HIV medical care. Providers and other prevention planners may use the review findings to identify best practices suitable for their clinics, agencies, or communities to increase engagement in care for PLWH, ultimately leading to viral suppression. Research gaps identified in our review can further inform the design and implementation of LRC studies. Although much work still needs to be done to reach national prevention goals related to engaging PLWH in HIV care, this review has identified best practices and potential research directions that may help to achieve these goals.
Supplementary Material
Acknowledgements
The authors would like to acknowledge current and former members of the Prevention Research Synthesis Project (PRS) who helped with the review (alphabetical order: Adebukola Adegbite, Brittney Baack, Terrika Barham, Julia Deluca, Linda Kay, Cindy Lyles, Khiya Marshall, Theresa Sipe, Malu Tungol, H. Wavery Vosburgh, & Christina White) and the consultants who helped to develop the LRC best practices criteria (alphabetical order: Rivet Amico, Jason Craw, Arin Freeman, Lytt Gardner, Thomas Giordano, Cyndi Grossman, Janet Heitgerd, Lisa Hightow-Weidman, Linda Koenig, Gary Marks, Lisa Metsch, Michael Mugavero, David Purcell, Stephen Safren, Luke Shouse, Michael Stirratt, Raekiela Taylor, Amy Wohl).
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Reference List
- 1.Office of National AIDS Policy. National HIV/AIDS Strategy for the United States: updated to 2020. Washington, DC: office of National AIDS Policy; https://www.aids.gov/federal-resources/national-hiv-aidsstrategy/nhas-update.pdf (2015). Accessed 30 July 2015. [Google Scholar]
- 2.Bradley H, Hall HI, Wolitski RJ, et al. Vital Signs: HIV diagnosis, care, and treatment among persons living with HIV--United States, 2011. MMWR Morb Mortal Wkly Rep. 2014;63(47):1113–7. [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yehia BR, French B, Fleishman JA, et al. Retention in care is more strongly associated with viral suppression in HIV-infected patients with lower versus higher CD4 counts. J Acquir Immune Defic Syndr. 2014;65(3):333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das M, Chu PL, Santos GM, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5(6):e11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montaner JS, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376(9740):532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen SM, Hu X, Sweeney P, Johnson AS, Hall HI. HIV viral suppression among persons with varying levels of engagement in HIV medical care, 19 US jurisdictions. J Acquir Immune Defic Syndr. 2014;67(5):519–27. [DOI] [PubMed] [Google Scholar]
- 8.Crawford TN. Poor retention in care one-year after viral suppression: a significant predictor of viral rebound. AIDS Care. 2014;26(11):1393–9. [DOI] [PubMed] [Google Scholar]
- 9.Mugavero MJ, Westfall AO, Cole SR, et al. Beyond core indicators of retention in HIV care: missed clinic visits are independently associated with all-cause mortality. Clin Infect Dis. 2014;59(10):1471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mugavero MJ, Lin HY, Willig JH, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis. 2009;48(2):248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skarbinski J, Rosenberg E, Paz-Bailey G, et al. Human Immunodeficiency Virus Transmission at Each Step of the Care Continuum in the United States. JAMA Intern Med. 2015;175(4):588–96 [DOI] [PubMed] [Google Scholar]
- 12.Higa DH, Marks G, Crepaz N, Liau A, Lyles CM. Interventions to improve retention in HIV primary care: a systematic review of U.S. studies. Curr HIV/AIDS Rep. 2012;9(4):313–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liau A, Crepaz N, Lyles CM, et al. Interventions to promote linkage to and utilization of HIV medical care among HIV-diagnosed persons: a qualitative systematic review, 1996–2011. AIDS Behav. 2013;17(6):1941–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Methods Guide for Effectiveness and Comparative Effectiveness Reviews AHRQ Publication No. 10(14)-EHC063-EF. Rockville, MD: agency for healthcare research and quality; www.effectivehealthcare.ahrq.gov (2014). Accessed 10 Feb 2015. [PubMed] [Google Scholar]
- 15.Higgins JPT, Altman DG, Sterne JAC, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: assessing the risk of bias in included studies Higgins JPT, Green S, editors. Cochrane Handbook of Systematic Reviews of Interventions Version 5.1.0. http://handbook.cochrane.org/chapter_8/8_assessing_risk_of_bias_in_included_studies.htm (2011). Accessed 10 Feb 2015. [Google Scholar]
- 16.The Guide to Community Preventive Services (The Community Guide). http://www.thecommunityguide.org/index.html (2015). Accessed 12 Feb 2015.
- 17.Grading of Recommendations, Assessment, Development and Evaluation (GRADE). http://www.gradeworkinggroup.org/index.htm (2015). Accessed 10 Feb 2015.
- 18.Muhamadi L, Tumwesigye NM, Kadobera D, et al. A single-blind randomized controlled trial to evaluate the effect of extended counseling on uptake of pre-antiretroviral care in Eastern Uganda. Trials. 2011;12:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardner LI, Metsch LR, Anderson-Mahoney P, et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS. 2005;19(4):423–31. [DOI] [PubMed] [Google Scholar]
- 20.Gardner LI, Giordano TP, Marks G et al. Enhanced personal contact with HIV patients improves retention in primary care: a randomized trial in 6 US HIV clinics. Clin Infect Dis. 2014;59(5):725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lucas GM, Chaudhry A, Hsu J, et al. Clinic-based treatment of opioid-dependent HIV-infected patients versus referral to an opioid treatment program: A randomized trial. Ann Intern Med. 2010;152(11):704–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robbins GK, Lester W, Johnson KL, et al. Efficacy of a clinical decision-support system in an HIV practice: a randomized trial. Ann Intern Med. 2012;157(11):757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mugavero MJ. Improving engagement in HIV care: what can we do? Top HIV Med. 2008;16(5):156–61. [PubMed] [Google Scholar]
- 24.Davila JA, Miertschin N, Sansgiry S, Schwarzwald H, Henley C, Giordano TP. Centralization of HIV services in HIV-positive African-American and Hispanic youth improves retention in care. AIDS Care. 2013;25(2):202–06. [DOI] [PubMed] [Google Scholar]
- 25.Enriquez M, Farnan R, Cheng AL, et al. Impact of a bilingual/bicultural care team on HIV-related health outcomes. J Assoc Nurses AIDS Care. 2008;19(4):295–301. [DOI] [PubMed] [Google Scholar]
- 26.Gardner LI, Marks G, Craw JA, et al. A low-effort, clinic-wide intervention improves attendance for HIV primary care. Clin Infect Dis. 2012;55(8):1124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hightow-Weidman LB, Smith JC, Valera E, Matthews DD, Lyons P. Keeping them in “STYLE”: finding, linking, and retaining young HIV-positive black and Latino men who have sex with men in care. AIDS Patient Care STDS. 2011;25(1):37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wanyenze RK, Kamya MR, Fatch R, et al. Abbreviated HIV counselling and testing and enhanced referral to care in Uganda: a factorial randomised controlled trial. Lancet Glob Health. 2013;1(3):e137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersen M, Tinsley J, Milfort D, et al. HIV health care access issues for women living with HIV, mental illness, and substance abuse. AIDS Patient Care STDS. 2005;19(7):449–59. [DOI] [PubMed] [Google Scholar]
- 30.Bocour A, Renaud TC, Udeagu CC, Shepard CW. HIV partner services are associated with timely linkage to HIV medical care. AIDS. 2013;27(18):2961–63. [DOI] [PubMed] [Google Scholar]
- 31.Henry SR, Goetz MB, Asch SM. The effect of automated telephone appointment reminders on HIV primary care no-shows by veterans. J Assoc Nurses AIDS Care. 2012;23(5):409–18. [DOI] [PubMed] [Google Scholar]
- 32.Keitz SA, Box TL, Homan RK, Bartlett JA, Oddone EZ. Primary care for patients infected with human immunodeficiency virus: a randomized controlled trial. J Gen Intern Med. 2001;16(9):573–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keller S, Jones J, Erbelding E. Choice of Rapid HIV testing and entrance into care in Baltimore City sexually transmitted infections clinics. AIDS Patient Care STDS. 2011;25(4):237–43. [DOI] [PubMed] [Google Scholar]
- 34.Konkle-Parker DJ, Erlen JA, Dubbert PM, May W. Pilot testing of an HIV medication adherence intervention in a public clinic in the Deep South. J Am Acad Nurse Pract. 2012;24(8):488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kunutsor S, Walley J, Katabira E, et al. Improving clinic attendance and adherence to antiretroviral therapy through a treatment supporter intervention in Uganda: a randomized controlled trial. AIDS Behav. 2011;15(8):1795–802. [DOI] [PubMed] [Google Scholar]
- 36.Naar-King S, Outlaw A, Green-Jones M, Wright K, Parsons JT. Motivational interviewing by peer outreach workers: a pilot randomized clinical trial to retain adolescents and young adults in HIV care. AIDS Care. 2009;21(7):868–73. [DOI] [PubMed] [Google Scholar]
- 37.Wohl DA, Scheyett A, Golin CE, et al. Intensive case management before and after prison release is no more effective than comprehensive pre-release discharge planning in linking HIV-infected prisoners to care: a randomized trial. AIDS Behav. 2011;15(2):356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perron NJ, Dao MD, Kossovsky MP, et al. Reduction of missed appointments at an urban primary care clinic: a randomised controlled study. BMC Fam Pract. 2010;11:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willis S, Castel AD, Ahmed T, Olejemeh C, Frison L, Kharfen M. Linkage, engagement, and viral suppression rates among HIV-infected persons receiving care at medical case management programs in Washington, DC. J Acquir Immune Defic Syndr. 2013;64 Suppl 1:S33–S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention. Routinely recommended HIV testing at an urban urgent-care clinic--Atlanta, Georgia, 2000. MMWR Morb Mortal Wkly Rep. 2001;50(25):538–541. [PubMed] [Google Scholar]
- 41.Schrantz SJ, Babcock CA, Theodosis C, et al. A targeted, conventional assay, emergency department HIV testing program integrated with existing clinical procedures. Ann Emerg Med. 2011;58(1 Suppl 1):S85–8. [DOI] [PubMed] [Google Scholar]
- 42.Craw JA, Gardner LI, Marks G, et al. Brief strengths-based case management promotes entry into HIV medical care: results of the antiretroviral treatment access study-II. J Acquir Immune Defic Syndr. 2008;47(5):597–606. [DOI] [PubMed] [Google Scholar]
- 43.Shrestha RK, Gardner L, Marks G, et al. Estimating the cost of increasing retention in care for HIV-infected patients: results of the CDC/HRSA retention in care trial. J Acquir Immune Defic Syndr. 2014. doi: 10.1097/QAI.0b013e3182a99b67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Bruin M, Viechtbauer W, Schaalma HP, Kok G, Abraham C, Hospers HJ. Standard care impact on effects of highly active antiretroviral therapy adherence interventions: A meta-analysis of randomized controlled trials. Arch Intern Med. 2010;170(3):240–50. [DOI] [PubMed] [Google Scholar]
- 45.Valdiserri RO, Forsyth AD, Yakovchenko V, Koh HK. Measuring what matters: development of standard HIV core indicators across the U.S. Department of Health and Human Services. Public Health Rep. 2013;128(5):354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Bruin M, McCambridge J, Prins JM. Reducing the risk of bias in health behaviour change trials: improving trial design, reporting or bias assessment criteria? A review and case study. Psychol Health. 2015;30(1):8–34. [DOI] [PubMed] [Google Scholar]
- 47.Mugavero MJ, Westfall AO, Zinski A, et al. Measuring retention in HIV care: the elusive gold standard. J Acquir Immune Defic Syndr. 2012;61(5):574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Centers for Disease Control and Prevention. High impact HIV-prevention: CDC’s approach to reducing HIV infections in the United States. Available at: http://www.cdc.gov/hiv/pdf/policies_NHPC_Booklet.pdf. Accessed 13 Mar 2015.
- 49.Centers for Disease Control and Prevention, Health Resources and Services Administration, National Institute of Health et al. Recommendations for HIV prevention with adults and adolescents with HIV in the United States, 2014. http://stacks.cdc.gov/view/cdc/26062 (2014).
- 50.Cunningham CO, Li X, Ramsey K, Sohler NL. A comparison of HIV health services utilization measures in a marginalized population: self-report versus medical records. Med Care. 2007;45(3):264–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.