Abstract
Background:
Knee osteoarthritis (OA) is a leading cause of disability that is associated with quadriceps weakness. However, strengthening in people with or with risk factors for knee OA can be poorly tolerated.
Objective:
To assess the efficacy of a 12-week low-load exercise program, using a hybrid training system (HTS) that uses the combination of neuromuscular electrical stimulation and volitional contractions, for improving thigh muscle strength, knee pain relief, and physical performance in women with or with risk factors for knee OA.
Design:
Randomized, single-blinded, controlled trial.
Setting:
Exercise training laboratory.
Participants:
Forty-two women 44–85 years old with risk factors for knee OA.
Interventions:
Participants randomized to 12 weeks of biweekly low-load resistance training with the HTS or on an isokinetic dynamometer (control).
Outcomes:
Maximum isokinetic knee extensor torque. Secondary measures included maximum isokinetic knee flexor torque, knee pain (Knee Injury and Osteoarthritis Outcome Score), and timed 20-m walk and chair stand tests.
Results:
The HTS and control treatments resulted in muscle strengthening, decreased knee pain, and improved physical performance. HTS group quadriceps and hamstring strength increased by 0.06 ± 0.04 Nm/kg (P > .05) and 0.05 ± 0.02 Nm/kg (P = .02), respectively. Control group quadriceps and hamstring strength increased by 0.03 ± 0.04 Nm/kg (P > .05) and 0.06 ± 0.02 Nm/kg (P = .009), respectively. Knee pain decreased by 11.9 ± 11.5 points (P < .001) for the HTS group and 14.1 ± 15.4 points (P = .001) for the control group. The 20-m walk time decreased by 1.60 ± 2.04 seconds (P = .005) and 0.95 ± 1.2 seconds (P = .004), and chair stand time decreased by 4.8 ± 10.0 seconds (P > .05) and 1.9 ± 4.7 seconds (P > .05) in the HTS and control groups, respectively. These results did not differ statistically between the HTS and control groups.
Conclusions:
These results suggest the HTS is effective for alleviating pain and improving physical performance in women with risk factors for knee OA. However, the HTS does not appear to be superior to low-load resistance training for improving muscle strength, pain relief, or physical function.
Clinical trial registration number: .
Introduction
Arthritis is one of the most common causes of disability in the United States, and osteoarthritis (OA) is the most common form [1]. The knee is the most commonly affected weight-bearing joint [2]. Approximately 42% of women and 31% of men older than 60 years are diagnosed with knee OA [3]. In women, quadriceps weakness is a risk factor for the development of incident [4] and progressive [5] knee OA and worsening of knee pain [6]. Barriers to reversing muscle strength deficits include disuse atrophy, neuromuscular changes such as neural inhibition from painful joints [7,8], and decreased voluntary muscle activation [9]. In people with risk factors for knee OA (ie, with 1 of the following risk factors for knee OA: knee pain on most days of the month, a history of knee injury or surgery, or overweight or obese), traditional strengthening programs might not be well tolerated because of the torque exerted about the knee joint. Thus, to adequately strengthen thigh muscles, it is important to minimize knee pain and knee joint torques.
Moderate- to high-load exercise is recommended for strengthening, but many adults with knee OA cannot tolerate this level of training [10]. There is a need for well-tolerated interventional studies to build on observational findings and evaluate the relations of thigh muscle strength, pain, and physical function. Neuromuscular electrical stimulation (NMES) is widely used to strengthen muscles and improve physical function in people who cannot exercise at medium to high intensity [11–15]. Moreover, NMES is effective for quadriceps strength, knee pain, and physical function for people with knee OA [16]. Unlike volitional contraction (VC), NMES can activate some fast motor units, in addition to slow units, even at relatively low force levels [17]. This is one of the merits of NMES. However, NMES induces contraction of only superficial muscle fibers in proximity to the stimulating electrodes [17]; therefore, it is ineffective in improving coordination between different agonistic and antagonistic muscles [18]. Moreover, exercise intensity is affected by the electrical stimulation tolerance of the user [16]. For example, because of the high electrical resistance of subcutaneous fat tissue, higher stimulus currents are required to evoke sufficient muscle contraction in obese patients [19]. These limitations of NMES affect generalizability to obese patients with risk factors for knee OA. To overcome these limitations, Paillard [18] combined NMES with VC, resulting in increased quadriceps strength and improved knee pain relief and physical function in adults regardless of obesity level. Moreover, the combined application of NMES and VC might be more effective than NMES or VC alone [18].
Recent studies have suggested that a hybrid training system (HTS) that combines applications of NMES with VCs (NMES-VC) is effective in improving coordination of muscle activation and accelerating rehabilitation programs compared with NMES alone [18,20]. The HTS electrically stimulates antagonist muscles to contract eccentrically, providing resistance for the agonist muscle group during exercise [21]. Eccentric contractions provide a greater stimulus for muscle hypertrophy than concentric contractions [22]. The exercise load of the HTS is regarded as approximately 40%−50% of 1-repetition maximum (1RM), although the intensity level is low [23,24]. This exercise modality could enable an improvement in thigh muscle strength for people with risk factors for knee OA and minimize deleterious joint loading by training at low intensity.
The objective of this research was to assess the efficacy of a 12-week HTS exercise program for improving knee extensor muscle strength and, secondarily, knee flexor muscle strength, knee pain relief, and physical performance in women with 1 of the following risk factors for knee OA: body mass index (BMI) greater than or equal to 25 kg/m2, a history of a knee joint injury or surgery, or knee symptoms (pain, aching, or stiffness) during most of the previous 30 days.
Methods
Participants
Forty-two women 44–85 years old volunteered to participate. Potential participants were recruited through campus e-mail and study advertisements in area clinics, newspapers, and businesses. All potential participants were screened by telephone by a research team member for inclusion and exclusion criteria before the consent process. Potential participants reported having at least 1 of the following risk factors for symptomatic knee OA: BMI greater than or equal to 25 kg/m2, a history of a knee joint injury or surgery, or knee symptoms (pain, aching, or stiffness) during most of the previous 30 days. Exclusion criteria were:
Factors that might alter the response to exercise training (knee injection within 6 weeks before the study, resistance training at any time in the 3 months before the study, known neuropathy or self-report of diabetes, peripheral vascular disease, myocardial infarction or stroke in the previous year, chest pain during exercise or at rest, or use of supplemental oxygen)
Difficulty with participation (bilateral knee replacement; lower limb amputation; lower limb surgery in the previous 6 months that affects walking ability or ability to exercise; back, hip, or knee problems that affect walking ability or ability to exercise; inability to walk without a cane or walker; inflammatory joint or muscle disease such as rheumatoid or psoriatic arthritis or polymyalgia rheumatica; multiple sclerosis or other neurodegenerative disorder; or inability to attend visits or understand instructions)
Suspected difficulty with compliance until the follow- up visit (currently being treated for cancer or having untreated cancer, terminal illness that could not be cured or adequately treated and with a reasonable expectation of death in the near future, or research team concern for participant health such as history of dizziness or faintness or current restrictions on activity)
The study was approved by the investigators’ institutional review board and registered at clinicaltrials.gov (study ID NCT02802878). The study was conducted from June 22, 2016 through December 14, 2016. All participants provided written informed consent after completion of a consent process approved by the institutional review board. The study consisted of 26 visits, including baseline and follow-up visits. The 24 intervention visits took place twice per week for 12 weeks.
Assessments
Anthropometric Measures
Body mass (kilograms) and height (centimeters) were measured at the baseline visit. Body mass was measured using a digital physicians scale (Detecto 6449 Digital Physician Scale, DETECTO Scale Company, Webb City, MO); height was measured using a wall-mounted stadi- ometer (Seca 216 Stadiometer, Seca Corporation, Chino, CA). BMI was calculated by dividing body mass by the square of height (kilograms per meter squared).
Isokinetic Strength
Participants’ isokinetic knee extensor and flexor muscle strength was measured on a HUMAC NORM isokinetic dynamometer (Model 502140, Computer Sports Medicine, Inc, Stoughton, MA) using a previously published protocol [5]. Participants were seated on the HUMAC NORM at an 85° seat tilt with the popliteal fossa 2 fingerbreadths past the end of the seat and their knees positioned in approximately 90° of flexion. The chair back was adjusted to contact each participant’s lumbar curvature. The thigh was strapped tightly to the seat and the shin was strapped to a cushioned shin pad on the dynamometer arm using Velcro straps. Once all straps were secured, the participant’s range of motion (ROM) was assessed and mechanical stops were placed at each endpoint to protect participants from hyperextension and hyperflexion. After the ROM assessment, the research assistants weighed the participant’s limb to compute the maximal gravity eliminated torque. Maximal gravity eliminated torque, limb position, and direction of motion were used to adjust the torque values for the effects of gravity.
The isokinetic strength assessment was performed after completion of the participant’s setup. Starting with the least painful knee, each participant extended her leg against the dynamometer arm at 50% effort and then flexed her knee, pulling the dynamometer arm at 50% effort for 3 practice trials as a warmup. Then, the participant completed 4 alternating repetitions of maximal knee extensor and knee flexor muscle strength testing. A trained and certified research assistant gave standardized instructions before the start of testing and coaching to “push and pull as hard and as fast as you can” during the baseline and follow-up visits. Maximum isokinetic torque (Newton-meters) for extension and flexion was recorded for each limb. The strength testing protocol had a test-retest reliability (intraclass correlation coefficient) of 0.94 (0.82–0.99) and a coefficient of variation of 8% (6%−12%) with a within-subject variation of 6.3 Nm (4.71–9.63 Nm) in a prior study [5].
Self-Reported Pain
The Knee Injury and Osteoarthritis Outcome Score Pain subscale was used at baseline and follow-up to assess participant outcomes [25]. Each participant completed the questionnaire before strength and physical function testing at baseline and follow-up visits. The pain subscale is composed of 9 questions and was scored from 0 to 100, with 0 corresponding to extreme knee problems and 100 corresponding to no knee problems.
Physical Performance
A timed 20-m walk was completed as a measure of lower limb physical performance. Participants were instructed to walk along a straight, uninterrupted 20-m course as quickly as they could. Timing started when the participant initiated foot movement and stopped when both feet crossed the 20-m mark. Times for 2 trials were recorded and then averaged.
The chair stand test is a validated measure of physical performance in adults with knee OA [26]. Participants were instructed to stand from a chair (seat height = 44.45 cm) 5 times without using their arms. Two trials were timed and averaged.
Intervention
Low-Load Resistance Training
Participants completed the exercise protocol performing 5 sets of 10 repetitions of 3-second isokinetic extension and flexion contractions at 40% of the isotonic 1RM on each leg using the HUMAC NORM isokinetic dynamometer. After each set was a 30-second rest period [27]. A single set of arm exercises was completed with participants in the seated position during each rest period to enhance participant compliance and retention (ie, to provide them with sufficient exercise value to retain their interest in participating). Each set included 5 repetitions of 1 of the following exercises completed with a yellow or red elastic band (TheraBand, Akron, OH): bicep curls, lateral triceps extension, posterior rhomboids extension, and anterior deltoid raises. Training load was progressed for each leg on visits 9 and 17. Each 1RM was estimated by finding a challenging weight with which the participant could move through the full ROM.
The examiner asked the participant to rate how difficult the movement was using a numerical rating system and increased the weight according to the examiner’s judgment of the participant’s rating. After sufficient rest, participants continued to complete trials with increased resistance until they could no longer complete the full ROM. The 1RM was determined as the highest load for which full ROM was achieved, which usually required 3–4 trials. The 1RM was used to calculate the 40% 1RM load used for training in subsequent sessions. A research assistant, certified in the research protocol, used a standardized script to verbally encourage participants’ maximal effort in all 1RM testing and resistance training. The research assistant was trained to assess for stopping criteria to ensure participant safety during the intervention; this included participant report or symptoms of chest pain or dizziness and participant discomfort such as back or knee pain.
Hybrid Training
Electrodes (Sekisui Plastics Co, Tokyo, Japan) were placed on the anterior thigh over the vastus medialis, vastus lateralis, rectus femoris, and medial and lateral hamstrings on the posterior thigh (Figure 1). The electrodes were 15 × 6 cm for the quadriceps and 12 × 6 cm for the hamstrings and coated with an oxidation-resistant silver-carbon compound and low-impedance gel coating. Electrical stimulation parameters were based on a standard Russian waveform [28] in which a 5,000-Hz carrier frequency is modulated at 40 Hz (2.4 ms on, 22.6 ms off) to deliver a rectangular voltage biphasic pulse. The burst duty cycle is 10% and pulse duration is 200 μs. Increasing the pulse duration increases the muscle contraction [29] but increases discomfort [30]. Therefore, in the HTS unit, they are set to minimize discomfort as much as possible and to maximize muscle contraction [30,31]. The electrical stimulator (HIZA TRAINER, EU-JLM50S, Panasonic Corporation, Osaka, Japan) provided a constant voltage stimulus to the skin electrodes (regulated voltage). Acceleration sensors, placed on the anterior mid-tibia, were used to detect joint motion (EWTS9PD, Home Appliances Development Center Corporate Engineering Division, Appliances Company Panasonic Corporation, Shiga, Japan). Data from the joint motion sensors were used to signal stimulation of the antagonist muscle for each joint motion (eg, knee extension results in stimulation of the hamstring muscles). Placement of the HTS device is depicted in Figure 2.
Figure 1.
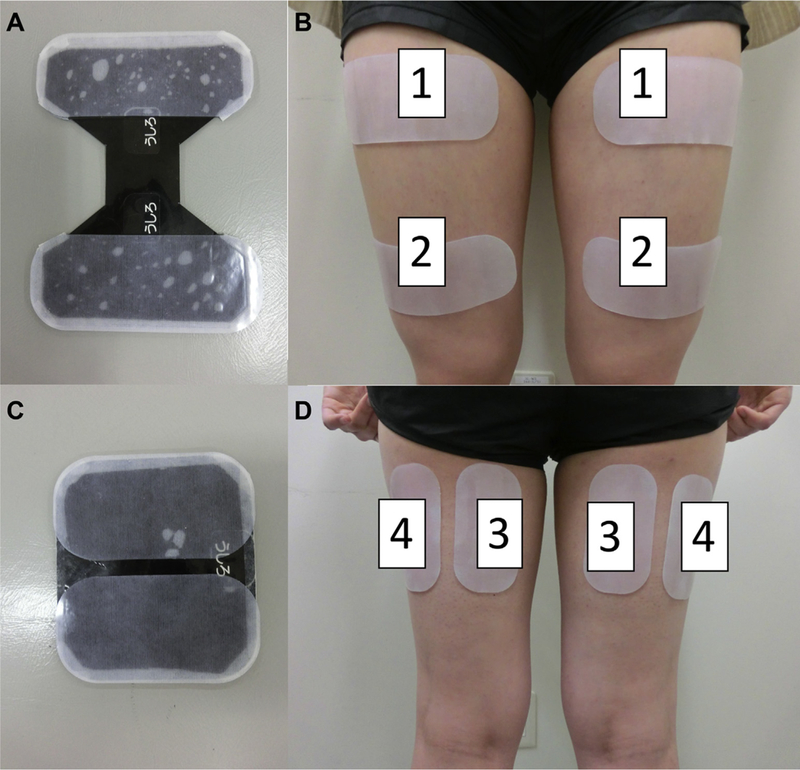
Placement of electrodes for hybrid training system intervention. (A) Electrodes placed over anterior thigh. (B) 1 = Proximal electrode of quadriceps; 2 = distal electrode of quadriceps. (C) Electrodes placed over posterior thigh. (D) 3 = Electrodes for medial hamstrings; 4 = electrodes for lateral hamstrings.
Figure 2.
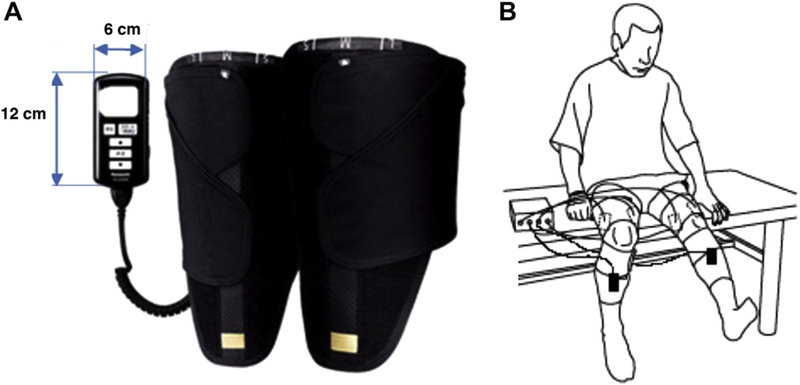
Electrical stimulation unit includes wrapping cloth to fix the device, acceleration sensors, and electrodes for exercise during the hybrid training system intervention. (A) Electrical stimulation unit. (B) Hybrid training system device with placement of the acceleration sensor on the anterior midtibia. The hybrid training system group followed 5 sets of 10 repetitions of 3-second knee extension and 3-second knee flexion with 30- second rest periods between sets.
Training was performed in a seated position with feet not touching the floor. Exercise intensity was set to the maximum tolerable voltage for each participant and measured at visits 1, 9, and 17. Progression of exercise increased as participants’ thigh muscle strength increased owing to the participants’ ability to tolerate increased electrical stimulation, thereby increasing the maximum tolerable voltage. The HTS group followed an exercise protocol analogous to the low-load resistance training (LLRT) group, completing 5 sets of 10 repetitions of 3-second extension and 3-second flexion contractions with 30-second rest periods between sets. During each 30-second rest period, participants completed 5 repetitions of the same arm exercises as described for the LLRT group. A research assistant, who was certified in the research protocol, used a standardized script to verbally encourage participants throughout HTS training. The research assistant was trained to assess for the same stopping criteria as for the LLRT intervention.
Group Assignment
A research assistant, who was not involved in outcome assessments, randomized participants to LLRT (control) or the HTS using a 1:1 random number generator (http://www.randomization.com/). Participants were unaware of which exercise intervention was considered therapeutic and were instructed not to discuss their intervention experience with other study participants if they met them incidentally. Appointments were conducted individually and staggered to avoid interaction between participants.
Before randomization and initiation of the exercise protocol and again after completion of the study, a single assessor collected data for each outcome measure. The assessor was uninvolved with the training and blinded to group assignment. To enhance the quality of the measurements, the staff member who assessed outcome measures was trained and certified in each outcome measure testing protocol and the equipment was calibrated before initiation of the study.
Statistical Analyses
Participant baseline characteristics were described using means ± SDs for continuous variables. Baseline demographics of each intervention group were compared using analysis of variance for continuous variables (eg, age, BMI). For each subject, the difference between the pre-intervention and postintervention measurement values was calculated. Person-based variables were compared with 2-sample t-tests. For limb-based variables (ie, strength), differences in primary outcome variables were compared between groups using models that recognized side as a repeated factor within participants to adjust for incomplete independence between limbs. Extensor and flexor strength gains were divided by body mass to assess changes in strength and account for differences in body size.
A sample size was estimated based on prior data collected for the clinically significant difference in isokinetic quadriceps torque and appropriate SDs within and between groups [32]. At a significance level of .025 (adjusted for 2-sidedness), an SD in the quadriceps strength response variable of 12.2 Nm and a power of 0.80 to detect an intergroup difference in means of 11.4 Nm would require a minimum of 38 subjects for this 2-treatment parallel-design study. To account for up to 10% dropout, 42 subjects were recruited and randomized.
Results
Forty-two women were enrolled in the study. Twenty-one participants were randomized into each intervention group. After enrollment and randomization, 7 participants discontinued the study; data from 18 participants in the control group and 17 participants in the HTS group were analyzed (Figure 3). There were no statistically significant differences in baseline characteristics between the 2 intervention groups (Table 1).
Figure 3.
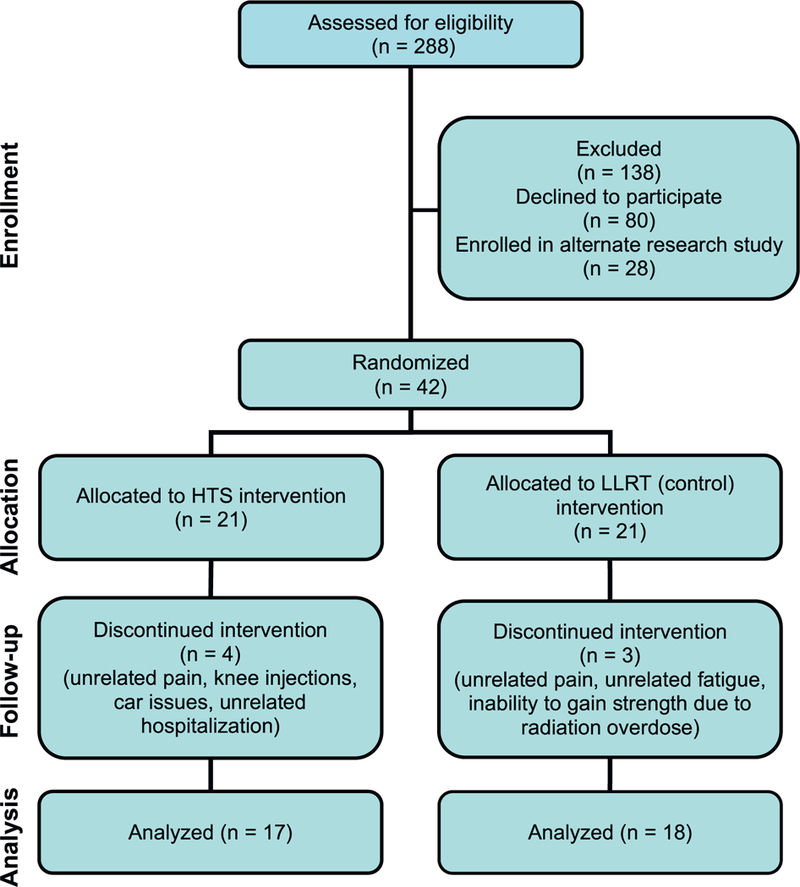
Flow of participants through each stage of the randomized trial. HTS = hybrid training system; LLRT = low-load resistance training.
Table 1.
Baseline characteristics of participants by randomization group
| Variable | Control group (n = 18) | HTS group (n = 17) | P value comparing groups |
|---|---|---|---|
| Age (y), mean ± SD | 65.89 ± 9.39 | 67.29 ± 8.46 | .65 |
| Height (cm), mean ± SD | 162.7 ± 6.89 | 165.4 ± 5.29 | .22 |
| Body mass (kg), mean ± SD | 73.22 ± 14.15 | 75.06 ± 15.92 | .72 |
| BMI (kg/m2), mean ± SE | 27.48 ± 4.06 | 27.50 ± 6.12 | .99 |
| Extensor strength per body mass (Nm/kg), mean ± SE | 0.93 ± 0.06 | 0.97 ± 0.07 | .68 |
| Flexor strength per body mass (Nm/kg), mean ± SE | 0.39 ± 0.03 | 0.44 ± 0.04 | .40 |
| Extensor strength (Nm), mean ± SE | 67.60 ± 3.57 | 69.40 ± 3.67 | .73 |
| Flexor strength (Nm), mean ± SE | 28.36 ± 1.77 | 31.32 ± 1.82 | .25 |
| KOOS Pain score, mean ± SD | 54.9 ± 14.6 | 60.9 ± 15.5 | .25 |
| Average 20-m walk time (s), mean ± SD | 13.36 ± 2.91 | 13.74 ± 4.67 | .78 |
| Average chair stand time (s), mean ± SD | 14.72 ± 4.29 | 18.48 ± 12.66 | .24 |
HTS = hybrid training system; BMI = body mass index; KOOS = Knee Injury and Osteoarthritis Outcome Score.
Quadriceps strength adjusted for participant body mass increased by 0.03 ± 0.04 and 0.06 ± 0.04 Nm/kg for the control and HTS groups, respectively. However, neither increase was statistically significant (P > .05). Hamstring strength increased by 0.06 ± 0.02 Nm/kg (P = .009) and 0.05 ± 0.02 Nm/kg (P = .021) for the control and HTS groups, respectively. The Knee Injury and Osteoarthritis Outcome Score Pain score improved by 14.1 ± 15.4 points (P = .001) for the control group and 11.9 ± 11.5 points (P = .001) for the HTS group. Both measures of physical performance improved for both groups. The 20-m walk time decreased by 0.95 ± 1.21 seconds (P = .004) and 1.60 ± 2.04 seconds (P = .005) for the control and HTS groups, respectively. The 5-time chair stand time decreased by 1.86 ± 4.72 seconds for the control group and 4.81 ± 9.99 seconds for the HTS group. However, neither was statistically significant (P > .05). There were no statistically significant differences between baseline and follow-up for the 2 groups for any outcome measures (Table 2).
Table 2.
Change in outcome measures from baseline to 12-week follow-up
| Variable | Control group (n = 18) | HTS group (n = 17) | P value comparing groups |
|---|---|---|---|
| Extensor strength per body mass (Nm/kg), | 0.03 ± 0.04 (.41) | 0.06 ± 0.04 (.17) | .67 |
| mean ± SE (within-group P) | |||
| Flexor strength per body mass (Nm/kg), | 0.06 ± 0.02 (.009) | 0.05± 0.02 (.02) | .85 |
| mean ± SE (within-group P) | |||
| Extensor strength (Nm), mean ± SE (within-group P) | 1.36 ± 2.86 (.64) | 4.99 ± 2.94 (.09) | .38 |
| Flexor strength (Nm), mean ± SE (within-group P) | 4.09 ± 1.55 (.01) | 4.02 ± 1.59 (.02) | .98 |
| KOOS Pain score, mean ± SD (within-group P) | 14.1 ± 15.4 (.001) | 11.9 ± 11.5 (.001) | .66 |
| Average 20-m walk time (s), mean ± SD (within-group P) | −0.95 ± 1.21 (.004) | −1.60 ± 2.04 (.005) | .26 |
| Average chair stand time (s), mean ± SD (within-group P) | −1.86 ± 4.72 (.11) | −4.81 ± 9.99 (.07) | .29 |
HTS = hybrid training system; KOOS = Knee Injury and Osteoarthritis Score.
Discussion
The results from this research support the secondary hypothesis that an HTS that uses the combination of NMES with VC would be effective for increasing knee flexor strength, decreasing knee pain, and improving physical performance. Although there were no statistically significant differences in outcome measures between the HTS and control groups, the 2 groups exhibited significant improvements in flexor strength, 20-m walk time, and self-reported knee pain. In addition, there were improvements in extensor strength and 5-time chair stand times for the 2 groups; however, these improvements were not statistically significant. These results indicate that the HTS can improve strength and knee symptoms to a similar extent as traditional resistance training.
The HTS has several advantages over traditional strength training, including (1) a simple, small device that does not require joining a gym, being supervised, or maintaining large expensive equipment; (2) requiring minimal external stabilization; (3) efficiency in time required to exercise because of simultaneous contractions of agonist and antagonist muscles; (4) VC of deep layers of muscle; and (5) the ability for patients to use the HTS independently [33]. These features contribute to the advantages of this relatively inexpensive and portable piece of exercise equipment over traditional heavy exercise machinery. This line of research could have a significant positive impact on public health by leading to the introduction of an inexpensive means of well-tolerated exercise that can be completed by adults in different settings, including their home, a nursing facility, recreational gym, outdoor space, and community rehabilitation environments.
The HTS device used in this study was targeted to electrically stimulate muscles at 40%−50% 1RM. The American College for Sports Medicine recommends a minimum of 60%−70% 1RM for novice exercisers to improve strength [34]. Compared with a traditional 60%−70% 1RM exercise program, a 40%−50% 1RM training program with the HTS should induce less strain on the articular surfaces. Furthermore, the torque being resisted during the HTS program derives primarily from internal forces of the hamstrings on the proximal tibia, rather than from external forces applied at the ankle that would occur with traditional strength training.
Previous researchers have reported that the combined application of NMES with VC is effective in overcoming limitations of NMES alone, such as only peripheral stimulation and spatial recruitment of muscle fibers [17,18,20]. Use of NMES-VC, or the HTS, has been shown to improve motor recovery in the forearm extensors and flexors after stroke [35]. Moreover, the HTS has been found to increase central nervous system excitability in the plantar flexors but without effect from NMES or VC alone [36]. The results of these studies are consistent with our findings that the HTS, using NMES and VC simultaneously, is an effective method for muscle strengthening, pain relief, and improving physical performance [21,37]. Mizusaki Imoto et al [38] examined the effect of strengthening with NMES in patients with knee OA. Participants in the intervention group completed loaded quadriceps extension at 50%−60% of 10RM with the addition of NMES, whereas participants in the control group completed quadriceps extension exercises at 50%−60% of 10RM without NMES. After 8 weeks of biweekly exercise, there was a statistically significant improvement in physical performance, self-reported pain, and physical function for the NMES and control groups; however, this did not differ significantly between groups. These results suggest that the application of NMES combined with strengthening exercise is no more effective than conventional low-load strengthening exercise alone. Rosemffet et al [39] completed a pilot study to assess the effects of NMES on pain, muscular strength, and functional capacity in patients with knee OA. Participants were randomized to 1 of 3 groups: (1) NMES in a sitting position for 30 minutes alternating 5 seconds of contraction and relaxation, (2) an exercise program consisting of aerobic exercise followed by isotonic and isometric strengthening exercises and stretching, and (3) combination of an NMES program and an exercise program. Self-reported pain and physical function improved in all 3 groups, whereas strength improved significantly in the exercise group and the NMES plus exercise group. The strength gains of the NMES plus exercise group were significantly greater than for each of the other 2 groups. That study concluded that a combination of an NMES program with an exercise program could be more effective than NMES or exercise alone.
Segal et al [40] found that women in the lowest tertile of peak knee extensor strength (20–60 Nm) were at significantly greater risk for joint space narrowing, a biomarker of radiographic knee OA, over 30 months. A cross-sectional study by Muraki et al [41] found that quadriceps muscle strength was significantly associated with knee pain after adjustment for age, BMI, gender, and knee OA. In another study, Glass et al [6] found that women in the lowest tertile of peak knee extensor strength (20–54 Nm) were at significantly greater risk for worsening of knee pain over 60 months. Baseline peak extensor strength for the women in this study was 67.60 ± 3.57 and 67.40 ± 3.67 Nm for the control and HTS groups, respectively. Therefore, the women in this study were slightly stronger than the women in the lowest tertiles of baseline peak extensor strength in the study by Segal et al [40]. The knee extensor strength gains resulting from the 12-week intervention were 1.36 ± 2.86 Nm for the control group and 4.99 ± 2.94 Nm for the HTS group. If these strength gains were sustained, this could lower the risk of worsening knee pain and joint space narrowing.
Limitations could have prevented statistical significance between groups in this study. The HTS intervention protocol electrically stimulated participants at each individual’s maximum tolerable voltage, leading to a lack of standardization of the magnitude of electrical stimulation. We did not measure maximum voluntary isometric torque (MVIT), or the highest torque achieved during maximal contractions, during electrical stimulation in the HTS intervention. Currier and Mann [42] identified a minimum stimulation intensity of 60% MVIT for strength gains. Therefore, owing to differences in participants’ tolerance, some participants might have been stimulated at intensities less than 60% MVIT for the duration of the study. Future studies ensuring a standard MVIT closer to 60% could improve findings from the present study by suggesting a potential for the HTS to increase thigh muscle strength beyond traditional resistance training.
The 17% dropout rate could have had a negative impact on the between-group analyses for most outcome measures, because we anticipated up to a 10% dropout rate. Larger samples in the intervention and control groups might improve the between-group analyses. Furthermore, the lack of extended follow-up after the completion of the exercise programs precludes assessment of potential lasting effects of the HTS on outcome measures. In the context of previous findings, this study provides evidence that the HTS is an effective method for improving thigh strength, pain relief, and physical function in women with risk factors for knee OA. Although the lack of standardization of the magnitude of stimulation was a scientific limitation, it does improve external validity in generalizing results to the way in which this device is used by individuals for exercise outside the research setting. To clarify inter-subject effects, we recommend a study with sufficient statistical power, using optimized electrical stimulation parameters during the HTS intervention, that includes an extended follow-up assessment to advance understanding of the effects of the HTS for improving thigh muscle strength, relief of knee pain, and physical performance in adults with risk factors for knee OA.
CME Question.
In female patients at risk for knee osteoarthritis (OA), the advantage of a hybrid training system combining neuromuscular electrical stimulation (NMES) and volitional contraction (VC) compared to low-load resistance training (LLRT) is:
Increased knee flexor strength.
Decreased knee pain.
Improved efficiency in exercise time.
Improved physical performance.
Answer online at me.aapmr.org
Acknowledgments
The authors thank the participants who dedicated their time for this study. This study was supported in part by the Milbank Foundation Chronic Pain Management Research Grant through the Foundation for Physical Medicine and Rehabilitation. This work was supported in part by a CTSA grant from the National Center for Research Resources and National Center for Advancing Translational Sciences awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research (UL1RR033179), which is now at the National Center for Advancing Translational Sciences (UL1TR000001). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or National Center for Advancing Translational Sciences.
Footnotes
Disclosure
Disclosure related to this publication: institutional grant from the Foundation for Physical Medicine and Rehabilitation
Disclosure outside this publicaion: institutional grants from the Japan Agency for Medical Research and Development and Grant-in-Aid for Scientific Research
Disclosure related to this publication: institutional grant from the Foundation for Physical Medicine and Rehabilitation
Disclosures related to this publication: institutional grant from the Foundation for Physical Medicine and Rehabilitation;
Disclosure outside this publication: remuneration for board membership on the Springer Editorial Board; pending institutional grants from the National Institutes of Health
Peer reviewers and all others who control content have no financial relationships to disclose.
Contributor Information
Kaitlin G. Rabe, Department of Rehabilitation Medicine, The University of Kansas, Kansas City, KS.
Hiroo Matsuse, Department of Orthopedics, Kurume University School of Medicine, Kurume, Fukuoka, Japan.
Anthony Jackson, Department of Rehabilitation Medicine, The University of Kansas, Kansas City, KS.
Neil A. Segal, Department of Rehabilitation Medicine, University of Kansas Medical Center, 3901 Rainbow Blvd, MS 1046, Kansas City, KS 66160..
References
- 1.Hootman J, Brault M, Helmick C, Theis K, Armour B. Prevalence and most common causes of disability among adults—United States, 2005. MMWR Morb Mortal Wkly Rep 2009;58:421–426. [PubMed] [Google Scholar]
- 2.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum 2008;58:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: Arthritis data from the Third National Health and Nutrition Examination Survey 1991–94. J Rheumatol 2006;33:2271–2279. [PubMed] [Google Scholar]
- 4.Segal NA, Torner JC, Felson DT, et al. Knee extensor strength does not protect against incident knee symptoms at 30 months in the multicenter knee osteoarthritis (MOST) cohort. PM R 2009;1:459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segal NA, Glass NA, Felson DT, et al. Effect of quadriceps strength and proprioception on risk for knee osteoarthritis. Med Sci Sports Exerc 2010;42:2081–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glass NA, Torner JC, Frey Law LA, et al. The relationship between quadriceps muscle weakness and worsening of knee pain in the MOST cohort: A 5-year longitudinal study. Osteoarthritis Cartilage 2013;21:1154–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmieri RM, Ingersoll CD, Edwards JE, et al. Arthrogenic muscle inhibition is not present in the limb contralateral to a simulated knee joint effusion. Am J Phys Med Rehabil 2003;82:910–916. [DOI] [PubMed] [Google Scholar]
- 8.Heroux ME, Tremblay F. Corticomotor excitability associated with unilateral knee dysfunction secondary to anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc 2006;14:823–833. [DOI] [PubMed] [Google Scholar]
- 9.Petterson SC, Barrance P, Buchanan T, Binder-Macleod S, Snyder-Mackler L. Mechanisms underlying quadriceps weakness in knee osteoarthritis. Med Sci Sports Exerc 2008;40:422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang MH, Lin YS, Yang RC, Lee CL. A comparison of various therapeutic exercises on the functional status of patients with knee osteoarthritis. Semin Arthritis Rheum 2003;32:398–406. [DOI] [PubMed] [Google Scholar]
- 11.Kagaya H, Shimada Y, Ebata K, et al. Restoration and analysis of standing-up in complete paraplegia utilizing functional electrical stimulation. Arch Phys Med Rehabil 1995;76:876–881. [DOI] [PubMed] [Google Scholar]
- 12.Balogun JA, Onilari OO, Akeju OA, Marzouk DK. High voltage electrical stimulation in the augmentation of muscle strength: Effects of pulse frequency. Arch Phys Med Rehabil 1993;74:910–916. [PubMed] [Google Scholar]
- 13.Delitto A, Rose SJ, McKowen JM, Lehman RC, Thomas JA, Shively RA. Electrical stimulation versus voluntary exercise in strengthening thigh musculature after anterior cruciate ligament surgery. Phys Ther 1988;68:660–663. [DOI] [PubMed] [Google Scholar]
- 14.Snyder-Mackler L, Delitto A, Bailey SL, Stralka SW. Strength of the quadriceps femoris muscle and functional recovery after reconstruction of the anterior cruciate ligament. A prospective, randomized clinical trial of electrical stimulation. J Bone Joint Surg Am 1995;77:1166–1173. [DOI] [PubMed] [Google Scholar]
- 15.Stein RB, Momose K, Bobet J. Biomechanics of human quadriceps muscles during electrical stimulation. J Biomech 1999;32: 347–357. [DOI] [PubMed] [Google Scholar]
- 16.Giggins O, Fullen B, Coughlan G. Neuromuscular electrical stimulation in the treatment of knee osteoarthritis: A systematic review and meta-analysis. Clin Rehabil 2012;26:867–881. [DOI] [PubMed] [Google Scholar]
- 17.Maffiuletti NA. Physiological and methodological considerations for the use of neuromuscular electrical stimulation. Eur J Appl Physiol 2010;110:223–234. [DOI] [PubMed] [Google Scholar]
- 18.Paillard T Combined application of neuromuscular electrical stimulation and voluntary muscular contractions. Sports Med 2008; 38:161–177. [DOI] [PubMed] [Google Scholar]
- 19.Doheny EP, Caulfield BM, Minogue CM, Lowery MM. The effect of subcutaneous fat thickness on the efficacy of transcutaneous electrical stimulation. Conf Proc IEEE Eng Med Biol Soc 2008;2008: 5684–5687. [DOI] [PubMed] [Google Scholar]
- 20.Dehail P, Duclos C, Barat M. Electrical stimulation and muscle strengthening. Ann Readapt Med Phys 2008;51:441–451. [DOI] [PubMed] [Google Scholar]
- 21.Yanagi T, Shiba N, Maeda T, et al. Agonist contractions against electrically stimulated antagonists. Arch Phys Med Rehabil 2003; 84:843–848. [DOI] [PubMed] [Google Scholar]
- 22.Dudley GA, Tesch PA, Miller BJ, Buchanan P. Importance of eccentric actions in performance adaptations to resistance training. Aviat Space Environ Med 1991;62:543–550. [PubMed] [Google Scholar]
- 23.Takano H, Morita T, Iida H, et al. Hemodynamic and hormonal responses to a short-term low-intensity resistance exercise with the reduction of muscle blood flow. Eur J Appl Physiol 2005;95:65–73. [DOI] [PubMed] [Google Scholar]
- 24.Iwasaki T, Shiba N, Matsuse H, et al. Improvement in knee extension strength through training by means of combined electrical stimulation and voluntary muscle contraction. Tohoku J Exp Med 2006;209:33–40. [DOI] [PubMed] [Google Scholar]
- 25.Collins NJ, Prinsen CA, Christensen R, Bartels EM, Terwee CB, Roos EM. Knee Injury and Osteoarthritis Outcome Score (KOOS): Systematic review and meta-analysis of measurement properties. Osteoarthritis Cartilage 2016;24:1317–1329. [DOI] [PubMed] [Google Scholar]
- 26.Segal NA, Boyer ER, Wallace R, Torner JC, Yack HJ. Association between chair stand strategy and mobility limitations in older adults with symptomatic knee osteoarthritis. Arch Phys Med Rehabil 2013;94:375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baechle TR, Earle RW, National Strength & Conditioning Association (U.S.). Essentials of Strength Training and Conditioning. 3rd ed Champaign, IL: Human Kinetics; 2008. [Google Scholar]
- 28.Ward AR, Shkuratova N. Russian electrical stimulation: the early experiments. Phys Ther 2002;82:1019–1030. [PubMed] [Google Scholar]
- 29.Gorgey AS, Dudley GA. The role of pulse duration and stimulation duration in maximizing the normalized torque during neuromuscular electrical stimulation. J Orthop Sports Phys Ther 2008;38: 508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward AR, Robertson VJ, Ioannou H. The effect of duty cycle and frequency on muscle torque production using kilohertz frequency range alternating current. Med Eng Phys 2004;26:569–579. [DOI] [PubMed] [Google Scholar]
- 31.McLoda TA, Carmack JA. Optimal burst duration during a facilitated quadriceps femoris contraction. J Athl Train 2000;35:145–150. [PMC free article] [PubMed] [Google Scholar]
- 32.Segal NA, Torner JC, Felson D, et al. Effect of thigh strength on incident radiographic and symptomatic knee osteoarthritis in a longitudinal cohort. Arthritis Rheum 2009;61:1210–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiba N, Matsuse H, Takano Y, et al. Electrically stimulated antagonist muscle contraction increased muscle mass and bone mineral density of one astronaut—Initial verification on the International Space Station. PLoS One 2015;10:e0134736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armstrong L, Balady GJ, Berry MJ, et al. ACSM’s Guidelines for Exercise Testing and Prescription. 7th ed Baltimore, MD; Philadelphia, PA: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 35.Santos M, Zahner LH, McKiernan BJ, Mahnken JD, Quaney B. Neuromuscular electrical stimulation improves severe hand dysfunction for individuals with chronic stroke: a pilot study. J Neurol Phys Ther 2006;30:175–183. [DOI] [PubMed] [Google Scholar]
- 36.Lagerquist O, Mang CS, Collins DF. Changes in spinal but not cortical excitability following combined electrical stimulation of the tibial nerve and voluntary plantar-flexion. Exp Brain Res 2012; 222:41–53. [DOI] [PubMed] [Google Scholar]
- 37.Shiba N, inventor; Kurume University, assignee. Apparatus for Strengthening Muscles. US patent 6456885B1; September 24, 2002.
- 38.Mizusaki Imoto A, Peccin S, Gomes da Silva KN, de Paiva Teixeira LE, Abrahao MI. Fernandes Moca Trevisani V. Effects of neuromuscular electrical stimulation combined with exercises versus an exercise program on the pain and the function in patients with knee osteoarthritis: A randomized controlled trial. Biomed Res Int 2013;2013:272018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosemffet MG, Schneeberger EE, Citera G, et al. Effects of functional electrostimulation on pain, muscular strength, and functional capacity in patients with osteoarthritis of the knee. J Clin Rheumatol 2004;10:246–249. [DOI] [PubMed] [Google Scholar]
- 40.Segal NA, Glass NA, Torner J, et al. Quadriceps weakness predicts risk for knee joint space narrowing in women in the MOST cohort. Osteoarthritis Cartilage 2010;18:769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muraki S, Akune T, Teraguchi M, et al. Quadriceps muscle strength, radiographic knee osteoarthritis and knee pain: the ROAD study. BMC Musculoskelet Disord 2015;16:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Currier DP, Mann R. Muscular strength development by electrical stimulation in healthy individuals. Phys Ther 1983;63:915–921. [DOI] [PubMed] [Google Scholar]


