Abstract
The accumulation of misfolded proteins in the endoplasmic reticulum (ER) causes ER stress that initiates the unfolded protein response (UPR). UPR activates both adaptive and apoptotic pathways, which contribute differently to disease pathogenesis. To further understand the functional mechanisms of UPR, we identified 12 commonly UPR-upregulated genes by expression microarray analysis. Here, we describe characterization of Armet/MANF, one of the 12 genes whose function was not clear. We demonstrated that the Armet/MANF protein was upregulated by various forms of ER stress in several cell lines as well as by cerebral ischemia of rat. Armet/MANF was localized in the ER and Golgi and was also a secreted protein. Silencing Armet/MANF by siRNA oligos in HeLa cells rendered cells more susceptible to ER stress-induced death, but surprisingly increased cell proliferation and reduced cell size. Overexpression of Armet/MANF inhibited cell proliferation and improved cell viability under glucose-free conditions and tunicamycin treatment. Based on its inhibitory properties for both proliferation and cell death we have demonstrated, Armet is, thus, a novel secreted mediator of the adaptive pathway of UPR.
Keywords: UPR, Armet, MANF, Secretion, Endoplasmic reticulum, ER stress, Cell proliferation, Cell death, Cell size, ERAD
Introduction
The endoplasmic reticulum (ER) is the organelle responsible for folding and modification of proteins destined for the secretory pathway and endosomal compartment. It also serves as a site for Ca2+ storage and synthesis of sterols and lipids [1–3]. These important functions are susceptible to perturbation by various pathogenic insults, such as genetic mutations, aging, oxidative stress, hypoxia, and viral infection [2,4]. The converging consequence of these perturbations is protein misfolding and accumulation in the ER [4]. Cells utilize a protective mechanism, the ER-associated degradation (ERAD), to remove misfolded proteins, thereby preventing proteins from accumulating [5]. However, if protein accumulation exceeds the capacity of ERAD, ER stress is induced to initiate the unfolded protein response (UPR) and restore ER homeostasis [2,4,6].
Misfolded proteins activate UPR through three ER stress sensors: PERK (PKR-like ER kinase), Ire1, and ATF6 [2,4,6]. All three sensors are single ER transmembrane proteins and share similar mechanisms for initial activation. Their intraluminal domains sense the condition of misfolded protein load and their cytosolic domains transduce the information to elicit responses. Under physiological conditions, the chaperone Bip/grp78 binds to the intraluminal domains of the stress sensors to keep the sensors in their inactive states [7,8]. When accumulated in the ER, misfolded proteins compete with the stress sensors to bind Bip/grp78, thereby releasing the inhibition and activating UPR [7,8].
Although our understanding of UPR has advanced significantly in recent years, the functions of many genes regulated by UPR as revealed by expression microarray studies have not been fully elucidated. Different types of ER stress inducers perturb ER homeostasis in different ways. Therefore, it is likely that each type of stressor may preferentially regulate certain sets of gene expression to respond to the specific perturbation [9–13]. Using microarray analysis of changes in global gene expression under various forms of ER stress in different cell types, we identified 12 commonly upregulated genes that potentially play central roles in UPR. While the majority of these genes have been previously reported and characterized, one of these genes, known as Armet (Arginine-Rich, Mutated in Early stage Tumors) [14–18] or MANF (Mesencephalic Astrocyte-derived Neurotrophic Factor) [19], has not been fully characterized in terms of its functional role in UPR. Several microarray studies have previously identified Armet as a UPR-upregulated gene [12,13]. Armet was so named because it was initially thought to be 50 amino acids longer at the N-terminus and to contain an arginine-rich region [14,15,17]. Later studies showed that Armet did not contain the arginine tract [16,18–20]. Armet was later isolated from the conditioned medium of a rat mesencephalic type-1 astrocyte cell line and was renamed MANF [19]. Functionally, MANF was shown to selectively protect cultured nigral dopaminergic neurons but not GABAergic or serotonergic neurons [19]. In the present study, we demonstrated that Armet/MANF is a UPR-upregulated and secreted protein. Armet/MANF inhibits cell proliferation and ER stress-induced cell death and also affects cell size and morphology. Therefore, the previously reported function of MANF may be part of its general role in cytoprotection [19]. Based on the protein’s functions discovered by the present study, Armet/MANF is, thus, a novel secreted mediator of the adaptive pathway of UPR.
Materials and methods
Cell culture
HeLa, U2OS, SHSY-5Y, HEK293 cells were cultured in Dulbecco’s modified essential medium (DMEM) supplemented with 10% fetal bovine serum.
Antibodies
Antibodies from commercial sources used for immunostaining, immunoblotting (IB) and immunoprecipitation (IP) include: mouse monoclonal anti-6xHis (Applied Biological Materials Inc. Vancouver, Canada), mouse monoclonal anti-GAPDH antibody was purchased from Ambion (Austin, TX), anti-Actin (Sigma-Aldrich, St. Louis, MO), anti-calnexin (BD Biosciences, San Jose, CA), anti-Golgin-97 (Invitrogen Corporation, Carlsbad, CA), anti-Caspase-3 (Cell Signaling Technology Inc., Danvers, MA), anti-FLAG (Clone M2, Sigma-Aldrich, St. Louis, MO), and anti-CHOP (Santa Cruz Inc., Santa Cruz, CA) as well as rabbit polyclonal anti-Bip/grp78 (Sigma-Aldrich), eIF2α (Santa Cruz Inc.), phospho-eIF2α (Cell Signaling Technology Inc.). Secondary antibodies were purchased from PIERCE (Rockford, IL) and mitotracker from Invitrogen.
Microarray
Expression microarray experiments were designed to identify genes that are commonly upregulated by different UPR inducers. Two cell lines, U2OS and HEK293, were treated with three UPR inducers (tunicamycin, thapsigargin, and DTT (dithiothreitol)) for 5 h and 9 h (12 treated samples in total) or with solvents for the same time periods (2 untreated controls for each cell line). RNA extraction, labeling, hybridization, image analysis, and data analysis were performed according to the methods described previously [21]. Briefly, total RNA extracted from 16 test samples (12 treated samples and 4 untreated controls) were amplified using the MessageAmp RNA kit (Ambion, Austin, TX) using an input of 500 ng of total RNA, and labeled by direct incorporation Cy5-dUTP (Amersham Pharmacia, Piscataway, NJ) in a reverse transcription (RT) reaction using random primers and Superscript II reverse transcriptase (Invitrogen, Carlsbad, California). Printed glass cDNA microarrays containing 20344 human ESTs (expression sequence tags) were used for microarray analysis [21]. Expression profiles were generated by co-hybridization of each of the Cy5-labeled test samples with a Cy3-labeled common reference prepared by pooling of all the test samples. The expression profile for each sample was represented as normalized ratios of sample/reference for all genes represented on the array. To select genes whose expression was induced commonly by different UPR inducers, we applied stringent filtration criteria of “at least 2 fold induction in at least 10 of the 12 treatment conditions when compared to the average of untreated controls in the corresponding cell type”, yielding a total of 12 genes.
RNA interference
For transient knockdown of Armet, siRNA1: GGA CCU CAA AGA CAG AGA UTT (sense), siRNA2: GCA GAU CGA CCU GAG CAC ATT (sense), and the negative control siRNA #1 were purchased from Ambion (Austin, TX). HeLa or HEK293 cells were transfected with the appropriate siRNA using Dharma-FECT reagent 1 according to the protocol provided by the manufacturer (Dharmacon, Lafayette, CO). 48 to 72 hours (h) after transfection cells were harvested and processed for further experiments.
RT-PCR
U2OS cells were treated with tunicamycin (2.5 μg/ml) for 0, 1, 2, 5, or 7 h. Total RNA was extracted using TRIzol Reagent (Invitrogen) following the protocol provided by the manufacturer. RT was performed using M-MLV reverse transcriptase using oligo dT as primers. PCR for amplification of Armet mRNA was performed in a reaction mix containing Taq-Pro RED Complete (PCR Master Mix from Denville Scientific Inc., Metuchen, NJ) and equal amounts of RT reaction mix as well as forward and reverse primers: 5’ GGA ATT CAC CAT GGG GAT GTG GGC CAC GCA GGG (forward) and 5’CAA ATC GGT CCG TGC ACT GGC TGC CTT GG 3’ (reverse). To amplify Armet-L1 mRNA, the following primers were used: 5’GTT CTC GAG ACC ATG GCA TGG TGC GCG AGC CCA GTT G 3′ (forward) and 5’GAG CTC TGT TTT GGG GTG TGTC 3’ (reverse). GAPDH mRNA was amplified using primers: 5’TCC CAT CAC CAT CTT CCA G3’ (forward) and 5’GTC CTT CCA CGA TAC CAA A3’ (reverse).
Expression of Armet for antibody production
Human Armet cDNA was amplified by RT-PCR of cDNA from HEK293 cells using primers 5’-CAT GAC CAT GGG GCT GCG GCC GGG CGA CTG C-3’ and 5’-GGC CTC GAG CAA ATC GGT CCG TGC ACT GGC-3’. The PCR product was cloned into pET28a(+) vector using NcoI and XhoI sites. Cloned Armet cDNA was confirmed by sequencing. To express recombinant N-terminal 6xHis-tagged Armet. Escherichia coli strain BL21 was transformed with the pET28a(+)-Armet and grown at 37 °C until OD 600 reached 0.6. Induction of the recombinant protein was performed with 0.2 mM isopropyl 1-thio-β-D-galactoside for 2 h at 37 °C. Cells were harvested and lysed on ice using Lysis buffer containing 50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, pH 8.0 and 1 μg/ml lysozyme followed by sonication. The resulting lysates were cleared by centrifugation at 15,000×g for 30 min. The supernatant was incubated with nickel resin for 1.5 h to isolate 6xHis-tagged Armet. The nickel resin-bound Armet was then washed 3 times with wash buffer (50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole, pH 8.0) and then eluted using elution buffer (50 mM NaH2PO4, 300 mM NaCl, 250 mM imidazole, pH 8.0) followed by dialysis overnight at 4 °C with 1× PBS. The purified recombinant 6xHis-Armet was used to produce rabbit polyclonal antibodies against Armet by Cocalico Biologicals, Inc.
Immunoprecipitation and immunoblotting
For IP, HEK293 cells were transfected with pCIneo-Armet-6xHis, pCIneo-Armet-L1–6xHis or pCIneo vector as control using calcium phosphate precipitation procedure. Transfected cells were harvested and lysed with 1% Triton X-100 lysis buffer and cleared by centrifugation as previously described [22]. 500 μg of total proteins were immunoprecipitated with 1 μg anti-6xHis antibody and 20 μl Protein A bead slurry rotating for 2 h at 4 °C. 5% of total proteins used for IP were analyzed as input. IB for the indicated proteins was performed as previously reported [22].
Cellular fractionation and proteinase K digestion assay
HEK293 cells transfected as indicated in the figure legends were fractionated into microsomes and cytosol as we previously reported [23]. Proteinase K digestion assay was conducted by incubating the microsome membranes with increasing concentrations of proteinase K in fractionation buffer (0.32 M sucrose, 10 mM triethanolamine, 1 mM EDTA, 1 mM 2-mercaptoethanol) for 30 min on ice. The reactions were stopped by addition of 5 mM phenylmethylsulfonyl fluoride [22].
Density gradient fractionation was done as reported [22]. Briefly, HeLa cells werehomogenized in 1ml bufferB (0.25M sucrose, 1 mM EDTA, 10 mM HEPES-NaOH, pH 7.4), then centrifuged at 3000 g for 10 min at 4 °C to remove the nuclei and unbroken cells. The post-nuclear supernatant was layered on top of a 9 ml preformed 0–25% iodixanol gradient in buffer B. The layered gradients were then centrifuged at 200,000 g at 4 °C for 2.5 h in a Beckman SW41Ti rotor. After centrifugation, fractions (0.65 ml) were collected starting from the bottom of the tube. An equal volume of each fraction was processed for IB for the indicated proteins.
Immunofluorescent microscopy
U2OS and HeLa cells were cultured on coverslips in 24-well dishes and transfected with the indicated plasmids using calcium phosphate precipitation. 24 h post-transfection cells were fixed with 4% paraformaldehyde for 30 min at room temperature. For double immunofluorescent staining, Armet and endogenous Golgin-97 were labeled using rabbit anti-Armet and mouse anti-Golgin-97 antibodies in conjunction with anti-mouse IgG-Alexa Fluor-594 and anti-rabbit IgG-Alexa Fluor-488. Mitotracker (Invitrogen Corporation, Carlsbad, CA) was used for the mitochondria staining as described by the manufacturer. Fluorescent microscopy was performed using a Zeiss Axiovert 200 M fluorescent microscope.
Flow cytometry
Control or Armet siRNA-transfected HeLa cells were collected and fixed in 70% ethanol. Cells were then washed in 1× PBS and resuspended in 1 ml propidium iodide/Triton X-100 staining solution (0.2 mg DNase-free RNase A, 20 μl of 1 mg/ml Propidium iodide to 1 ml of 0.1% (v/v) Triton X-100) for 30 min at room temperature before being analyzed by flow cytometry. The resulting data were analyzed using ModFit LT 3.1 SP2.
Armet secretion
Cells were transfected with pCIneo-Armet-6xHis or pCIneo-Armet-L1–6xHis. Twenty-four hours after transfection, the culture medium was changed to DMEM containing 2% FBS. Sixteen hours later, conditioned medium was harvested from the transfected cells. For blocking the ER to Golgi trafficking, Brefeldin A (Epicentre Biotechnologies, Madison, WI) was added to the medium at 5 μg/ml. Armet-6xHis or Armet-L1–6xHis was precipitated from conditioned medium using 20 μl nickel-agarose beads by rotating for 4 h at 4 °C. To detect endogenous Armet secretion, HeLa cells were seeded to a confluency of 70–80% with DMEM with 10% FBS. Medium was changed to serum free DMEM. Sixteen hours later, conditioned medium was harvested from the cells and was concentrated using Amicon-Ultra (Millipore) before being processed for SDS-PAGE and immunoblotting for Armet.
Experimental cerebral ischemia
Animal procedures were done according to the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Adult male Sprague-Dawley rats weighing 220–250 g were anesthetized with 10% chloral hydrate (3 ml/kg). Focal cerebral ischemia was induced by using the middle cerebral artery occlusion (MCAO) model. A length of 19.0–21.0 mm heparinized intraluminal surgical suture (Ø 0.235 mm) with the tip rounded by heating near a flame was introduced from the external carotid artery into the lumen of the internal carotid artery to block the origin of the MCA. The suture was removed after 2 h of occlusion. The animals were sacrificed after 2 h of reperfusion. Then, the brain tissues were processed for immunohistochemical staining for Armet using an avidin-biotin-peroxidase complex (ABC) kit (PIERCE, Rockford, IL) following the manufacturer’s protocol.
Results
Identification of Armet as a commonly upregulated gene by ER stress
To identify genes that are commonly upregulated by ER stress, we analyzed upregulated genes in response to ER stress induced by three inducers (tunicamycin, thapsigargin, and dithiothreitol) in two cell lines (HeLa and HEK293) at two time points (5 and 9 h) after the treatment. This results in a total of 12 treatment conditions. By comparing to the untreated control cells, we identified 12 genes using the criteria “at least 2 fold induction in at least 10 of the 12 treatment conditions when compared to the average of untreated controls in the corresponding cell type” (Table 1). Among the 12 genes, seven have previously been reported to be UPR-upregulated genes, including cystathionase [24], stanniocalcin 2 [25], Armet/MANF [12,13], CHOP [12,13], HERP [12,13,26], and Tribbles homolog 3 (TRB3) [27]. Five new UPR targets were also identified, including TPX2, elongation factor RNA polymerase II (ELL), Sec24D, contactin associated protein-like 2 (CNTNAP2), and kinasin family member C3 (KIFC3). We decided to further characterize Armet, a highly conserved protein whose function in UPR has not been elucidated. We generated affinity-purified rabbit polyclonal anti-Armet antibodies and demonstrated that the antibody recognized both endogenous and overexpressed Armet (Fig. 1A). Armet has a homolog that will be called Armet-like protein 1 (Armet-L1) (see an alignment of Armet and Armet-L1 in Fig. s1). Despite the homology shared between Armet and Armet-L1, the anti-Armet antibodies are highly specific for Armet. Anti-Armet did not recognize Armet-L1–6xHis despite its higher levels of overexpression (Fig. 1B, lanes 1–3). The specificity of the antibodies was further confirmed by immunoblotting the precipitates of Armet-6xHis and Armet-L1–6xHis (Fig. 1B, lanes 4–6). Consistent with the results of sequence analysis that human Armet-L1 but not Armet contains an N-linked glycosylation site (aa57NSFL60, labeled by the red square in Fig. s1), Armet-L1 had a slower migrating band (Fig. 1B, arrowhead). Pretreatment of the cells with tunicamycin, a specific inhibitor of N-linked glycosylation, abolished the slower migrating band (Fig. 1C, lane 8). Brefeldin A (BFA), an inhibitor of ER to Golgi trafficking, did not affect the modification of Armet-L1 (Fig. 1C, lane 9), indicating that, as expected, the modification occurred in the ER. BFA treatment caused a substantial increase of Armet, suggesting that Armet might actively traffic from the ER to Golgi for secretion and/or may be caused by BFA-induced ER stress.
Table 1 –
List of commonly upregulated UPR target genes
| Image ID | Symbol | Name |
|---|---|---|
| 773278 | DNAJB9 | DnaJ (Hsp40) homolog, subfamily B, member 9 |
| 823578 | STC2 | Stanniocalcin 2 |
| 196501 | ARMET | Arginine-rich, mutated in early stage tumors |
| 361456 | DDIT3 | chop/Gadd153 |
| 1533695 | HERPUP1 | Herp/Homocysteine-inducible, endoplasmic reticulum stress-inducible, ubiquitin-like domain member 1 |
| 2055807 | TRIB3 | TRB3/Tribbles homolog 3 (Drosophila) |
| 126795 | CTH | Cystathionase (cystathionine gamma-lyase) |
| 2308994 | TPX2 | Microtubule-associated, homolog (Xenopus laeuis)* |
| 2308153 | ELL | Elongation factor RNA polymerase II* |
| 785840 | SEC24D | SEC24 related gene family, member D (Saccharomyces cerevisiae)* |
| 27404 | CNTNAP2 | Contactin associated protein-like 2* |
| 753038 | KIFC3 | Kinesin family member C3* |
New UPR target genes.
Fig. 1 –
Characterization of Armet. A. The endogenous and transfected non-tagged Armet have the same size. Increasing amounts of plasmid encoding non-tagged Armet were transfected into HEK293 cells. Transfected cells were processed for IB for Armet using affinity-purified rabbit anti-Armet. B. Anti-Armet antibody specifically recognizes Armet-6xHis but not Armet-L1–6xHis. pCIneo-Armet-6xHis, pCIneo-Armet-L1–6xHis or pCIneo as control were transfected in HEK293 cells. Twenty-four hours post-transfection cells were processed for IP with anti 6xHis antibody and the precipitates were further analyzed by IB. The input represent 5% of the total proteins used for IP. Arrowhead indicates modified Armet-L1. Laddering bands in lane 4 (upper panel) are non-specific staining. C. Effects of Brefeldin A and tunicamycin on levels and migration of transfected Armet and Armet-L1. HEK293 cells transfected with Armet or Armet-L1 were treated with Tunicamycin (2.5 μg/ml) or Brefeldin A (5 μg/ml.) for 5 h before processed for IB. Arrowhead indicates glycosylated Armet-L1.
Consistent with the microarray data, Armet protein was upregulated in U2OS, HEK293, and SHSY-5Y cells by various ER stress inducing agents, including a proteasome inhibitor, lactacystin; an N-glycosylation inhibitor, tunicamycin; and a SERCA (sarco/endoplasmic reticulum Ca2+ ATPase) inhibitor, thapsigargin (Fig. 2A). Armet protein was induced in a time-dependent manner (Figs. 2B and C). Interestingly, RT-PCR shows that the transcription of Armet but not Armet-L1 was upregulated by ER stress (Fig. 2D), indicating that Armet-L1 acts constitutively.
Fig. 2 –
Induction of Armet expression by chemical ER stress inducers in cells and by ischemia in rat brain. A. Armet is upregulated by different ER stress inducers in different cell lines. U2OS, HEK293 and SHSY-5Y were treated with tunicamycin (Tun, 2.5 μg/ml), thapsigargin (Tha, 500 nM), or proteasome inhibitor lactacystin (Lac, 10 μM) for 7 h. B and C. Armet is upregulated by ER stress in a time-dependent manner. D. Armet but not Armet-L1 is upregulated by tunicamycin. U2OS cells were treated with Tunicamycin for 0, 1, 2, 5 and 7 h. Total RNA was extracted and RT-PCR was performed. E. Anti-Armet antibody is specific for Armet in immunostaining. U2OS cells were transfected with pCIneo-Armet-FLAG. Twenty-four hours after transfection cells were fixed and stained with rabbit polyclonal anti-Armet and mouse monoclonal anti-FLAG antibodies in conjunction with Alexa Fluor-488 (green) and Alexa fluor-594 (red). F and G. Induction of Armet by experimental cerebral ischemia. Armet was revealed by immunohistochemical staining by ABC method of ischemic cerebral cortex (G) or contralateral side (F, negative control). Nuclei were counterstained by hematoxylin.
We next determined whether Armet is induced by cerebral ischemia, a condition known to cause ER stress [28], using immunohistochemical staining. To determine whether our affinity-purified rabbit polyclonal anti-Armet antibodies were suitable for immunostaining, U2OS cells expressing Armet-FLAG were doubly labeled with rabbit polyclonal anti-Armet and mouse monoclonal anti-FLAG antibodies. Both antibodies stained identical structures in cells, and the staining clearly revealed a network-like pattern concentrated in the juxta-nuclear region (Fig. 2E), indicating that our anti-Armet antibodies are highly specific for labeling Armet. Two hours of middle cerebral artery occlusion followed by 2 h of reperfusion significantly induced Armet expression in cortical neurons compared to those in contralateral side as a negative control (Figs. 2G vs. F), demonstrating that Armet is also inducible by ER stress under pathogenic conditions.
Armet is localized in ER and Golgi and also secreted
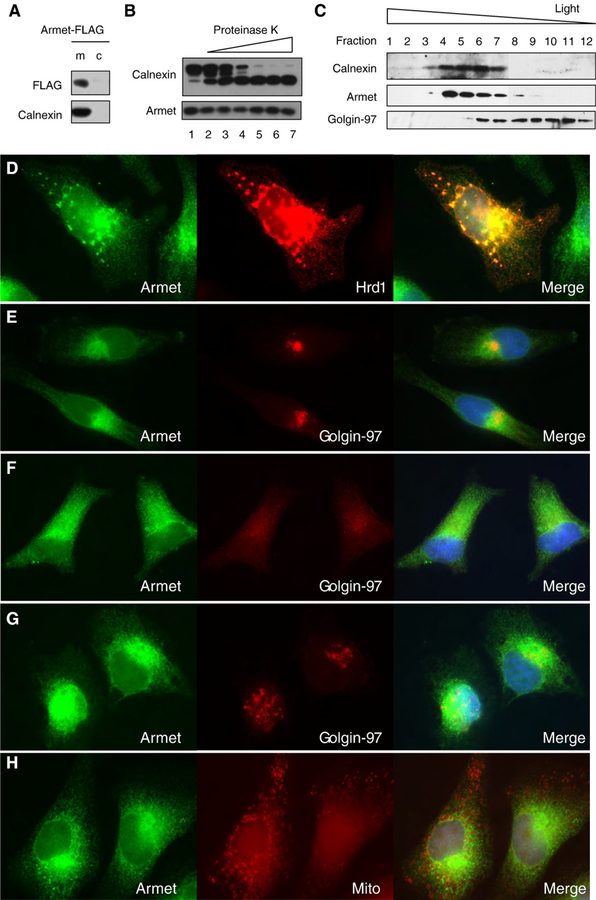
Armet has a predicted cleavable signal peptide, suggesting it is a secreted protein. Consistently, previous study has isolated Armet known as MANF from the conditioned medium of an astrocyte-derived cell line [19], but whether it was actually secreted to the conditioned medium was not addressed. To approach this issue, we first determined whether it is localized in the microsomes. HEK293 cells transfected with C-terminal FLAG-tagged Armet were fractionated into microsomes and cytosol as we previously described [29]. As predicted, Armet was associated with the microsomal fraction and not found in the cytosol (Fig. 3A). To determine the topology of Armet, we carried out a proteinase K digestion assay of isolated microsomes. Armet was totally protected from proteinase K digestion (Fig. 3B). As a control, the intraluminal domain of calnexin, an ER-anchored single transmembrane protein, was protected from proteinase K while its cytosolic domain was digested (Fig. 3B). To investigate with which cellular membrane Armet associates, we performed a density gradient fractionation of the post-nuclear fraction of HeLa cell homogenates. The results showed that Armet was cofractionated with ER membranes and partially with Golgi membranes (Fig. 3C), supporting the notion that Armet is a secreted protein.
Fig. 3 –
Armet is localized in the ER and Golgi. A. Armet is associated with the microsomal fraction. HEK293 cells were transfected with pCIneo-Armet-FLAG. Twenty-four hours post-transfection cells were fractionated into microsomes (m) and cytosol (c) and analyzed by IB for the indicated proteins. B. Armet is localized in the lumen of the ER. Microsomes isolated from HeLa cells were subjected to digestion by increasing concentrations of proteinase K for 30 min on ice. Following the digestion, microsomes and supernatants were separated by centrifugation and analyzed by IB. C. Armet is cofractionated with the ER marker calnexin and partially with the Golgi marker Golgin-97. The post-nuclear fraction of HeLa cell homogenates were subjected to density gradient fractionation as described in Materials and Methods. An equal volume from each fraction was analyzed by IB for the indicated proteins. D, E, F, and G. Armet is localized in the ER and Golgi. D. Armet colocalizes with Hrd1, an ER resident ubiquitin ligase. HeLa cells were transfected with plasmid encoding Hrd1. Transfected cells were then processed for double immunofluorescent staining of Armet (green) and Hrd1 (red). E. Colocalization of Armet (green) and Golgin-97 in the juxta-nuclear region of HeLa cells. F and G. Treatment with BFA but not tunicamycin causes accumulation of Armet in the periphery of HeLa cells. HeLa cells were treated with BFA (C) or tunicamycin (D) for 5 h before processing for staining for endogenous Armet and Golgin-97 as a Golgi marker. H. Armet is not colocalized with mitochondrial marker. Live HeLa cells were labeled with red mitotracker for 15 min and then fixed and stained with anti-Armet antibody and Alexa Fluor-488.
To further define the subcellular localization of Armet, we utilized double immunofluorescent staining of Armet and an ER or Golgi marker. Endogenous Armet colocalized well with ectopically expressed Hrd1, an established ER resident ubiquitin ligase, as an ER marker in HeLa cells (Fig. 3D). The aggregates of Hrd1 were caused by Hrd1 overexpression (Fig. 3D). The staining of the endogenous Armet was weaker in the periphery of the cells, but primarily concentrated in the juxta-nuclear region (Fig. 3E) where it colocalized with Golgin-97, a trans-Golgi marker, indicating that Armet is also localized to the Golgi (Fig. 3E). BFA treatment for 6 h increased the intensity of cellular peripheral staining, suggesting an accumulation of Armet in the ER (Fig. 3F). As a positive control, trafficking of Golgin-97 to Golgi was also blocked by BFA (Fig. 3F). To exclude the possibility that accumulation of Armet in the ER caused by BFA treatment was due to Armet upregulation, we stained tunicamycin-treated cells, which resulted in Armet localization primarily in the juxta-nuclear region (Fig. 3G). Armet staining did not overlap with mitotracker, a mitochondria marker (Fig. 3H). Taken together, these results suggest that Armet is actively trafficking from the ER to the Golgi, probably for secretion.
To determine whether Armet is a secreted protein, we transfected HEK293 cells with plasmid encoding Armet-6xHis. Transfected cells were treated with tunicamycin, BFA, or grown under glucose-free condition for 16 h. Secreted Armet-6xHis was precipitated from the conditioned medium by nickel-agarose beads and analyzed by IB. Significant amounts of Armet-6xHis were detected in the conditioned medium (Fig. 4A, lane 7), indicating that Armet-6xHis is a secreted protein. This notion was supported by the fact that BFA almost completely blocked the appearance of Armet in the medium (Fig. 4A, lane 9). Importantly, tunicamycin- and glucose-free-induced ER stress had minimal effects on Armet secretion (Fig. 4A, lanes 8 and 10). In order to demonstrate that the overexpression of Armet had no effect on its secretion, conditioned medium from HeLa cells was analyzed. Endogenous Armet secretion was indeed observed (Fig. 4B). Armet-L1 known as CDNF (conserved dopamine neurotrophic factor) has also been reported to be a secreted protein. We used the same assay as for Armet secretion and confirmed that Armet-L1 is indeed secreted from cells (Fig. 4C, lane 4). Similar to Armet, tunicamycin-induced ER stress did not significantly affect Armet-L1–6xHis secretion (Fig. 4B, lane 5). As seen for Armet-6xHis, BFA completely blocked Armet-L1 secretion and caused a significant accumulation of Armet-L1–6xHis in cells (Fig. 4B, lanes 3 and 6). Thus, both Armet and Armet-L1 are secreted proteins and their secretion is intact under ER stress, suggesting that they may be important regulators of UPR.
Fig. 4 –
Armet and Armet-L1 are secreted proteins. A. Armet is a secreted protein. HEK293 cells were transfected with pCIneo-Armet-6xHis. Twenty-four hours after transfection cells were treated overnight with tunicamycin (2.5 μg/ml), Brefeldin A or grown in glucose-free medium. Then, the conditioned medium was harvested and precipitated using nickel-agarose beads. Armet-6xHis in precipitates was determined by IB using anti-6xHis antibody. Arrowhead indicates a non-specific band pulled down by nickel-beads, because it also presents in non-transfected control (lane 6). B. Endogenous Armet is a secreted protein. Conditioned medium was collected from HeLa cells after 6 h. Medium was concentrated using Amicon-4 Ultra centrifugal filter device. Equal volume of control and conditioned medium was analyzed by IB with anti-Armet antibody.C. Armet-L1 is a secreted protein. Experiment was done similarly to a. Intracell = Intracellular.
Effects of Armet on cell proliferation, morphology, size, and viability
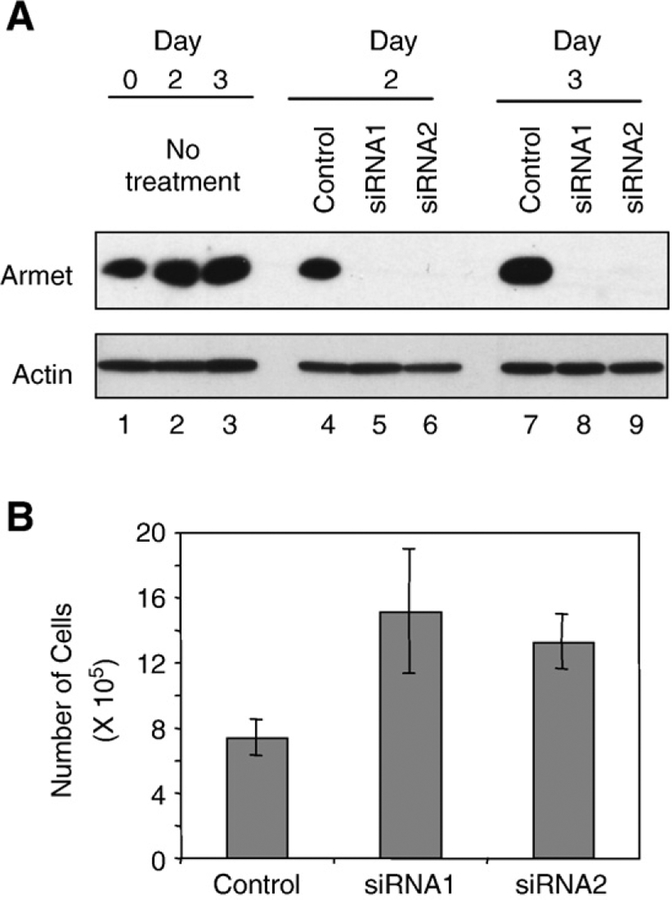
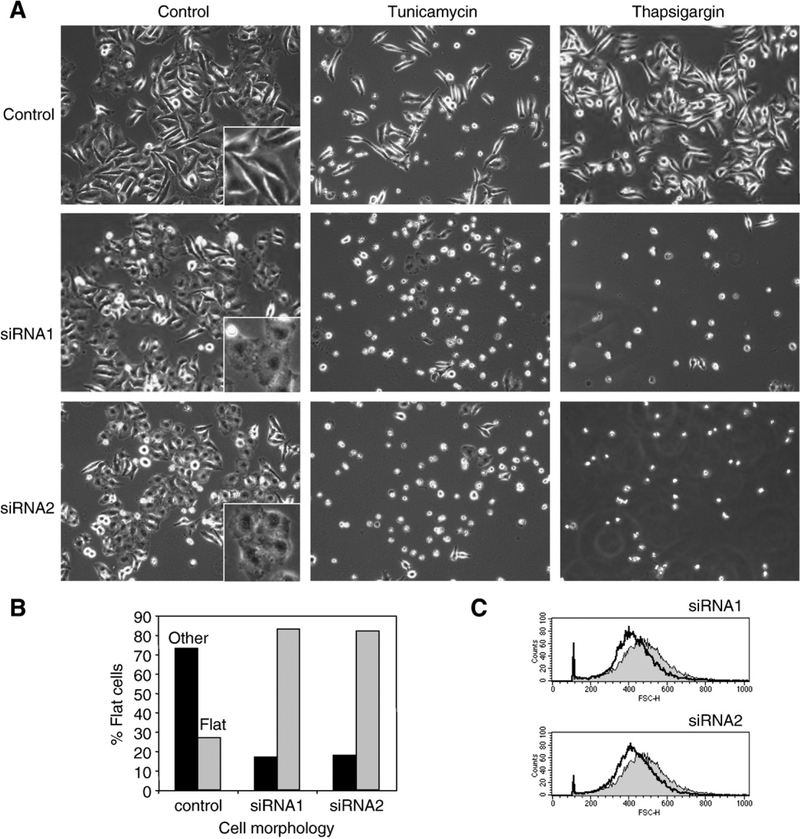
To explore the cellular function of Armet, we silenced the expression of endogenous Armet by two different siRNA oligos that target different regions of Armet mRNA. Both siRNAs markedly knocked down the expression of Armet protein in HeLa cells at day 2 and day 3 after transfection (Fig. 5A, lanes 5, 6, 8 and 9). We chose HeLa cells because they constitutively express high levels of Armet. To determine the effects of Armet silencing on cell proliferation, equal numbers of Armet or control siRNA oligo-transfected HeLa cells were seeded in culture 24 h after the transfection. After additional 2 days in culture, cell numbers were counted. Compared with the control, knockdown of Armet by siRNA1 and siRNA2 increased cell numbers by 105 and 80%, respectively (Fig. 5B), suggesting a surprising role for Armet in inhibiting cell proliferation. Armet silencing also induced a morphological change of HeLa cells. HeLa cells changed from polygon-shaped to a flattened and round morphology (Fig. 6A). The number of flattened and round cells increased from 20% to 85% (Fig. 6B). Flow cytometry analysis revealed that Armet silencing also leads to a decrease in cell size (Fig. 6C). Thus, Armet is involved in the regulation of cell proliferation and affects cell size and morphology.
Fig. 5 –
Armet knockdown increases cell proliferation. A. siRNA efficiently silences Armet protein expression. Control or two siRNA oligos (siRNA1 and siRNA2) targeting Armet were transfected into HeLa cells. Transfected cells were harvested at days 2 and 3 post-transfection and Armet expression was determined by IB. Actin was blotted as loading control. B. Armet knockdown increases cell proliferation. HeLa cells were transfected with siRNA1, siRNA2 or control oligos. Twenty-four hours after transfection, equal numbers of transfected cells from each group were seeded in culture. After an additional 2 days in culture, cell numbers were counted. The results were expressed as mean ±SD, n=3.
Fig. 6 –
Transient knockdown of Armet decreases cell size, changes cell morphology, and renders cells more susceptible to ER stress-induced cell death. A. Armet knockdown cells are more sensitive to ER stress-induced cell death. Armet silencing was done as described in Fig. 5. At day 3 cells were treated for 24 h with tunicamycin or thapsigargin and the images were taken under phase contrast microscope. A and B. Armet knockdown changes cell morphology from polygon-like to flattened and round shape. At day 3 of Armet knockdown, 200 cells from each group were counted and separated into polygon-shaped and flattened-round morphology. The relative percentages of these two morphologies were expressed in the graph. C. Armet knockdown decreases cell size. At day 3 of Armet knockdown, cells were processed for flow cytometry as described in the Materials and Methods. The X-axis indicates relative cell size. Cell size distribution curve for the control is shaded in gray and the Armet-targeting siRNA curve is not shaded.
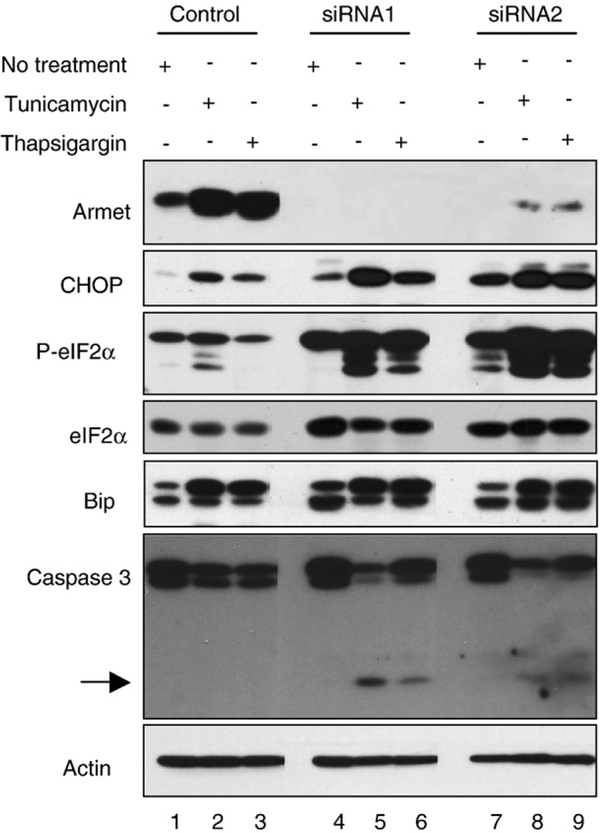
Next, we examined the effects of Armet silencing on ER stress-induced cell death. Three days after transfection with Armet siRNA, HeLa cells were treated with tunicamycin or thapsigargin for 24 h. As shown in Fig. 6A, round and detached cells, suggestive of cell death, were dramatically increased in Armet knockdown cells. Importantly, tunicamycin and thapsigargin upregulated Armet in control cells, but the upregulation was markedly inhibited by Armet siRNA transfection (Fig. 7), indicating that Armet upregulation plays a protective role against ER stress-induced cell death. To determine the effects of Armet silencing on UPR, we examined several established UPR responsive genes. We found that silencing Armet was sufficient to induce UPR as manifested by increased expression of CHOP, Bip/grp78, and eIF2α, as well as increased eIF2α phosphorylation, which was further aggravated by tunicamycin and thapsigargin treatment (Fig. 7). This result explains the hypersensitivity to ER stress-induced death of Armet-silenced cells (Fig. 6A). In agreement with this, tunicamycin and thapsigargin treatment generated more active caspase-3 in Armet knockdown cells (Fig. 7).
Fig. 7 –
Armet knockdown causes UPR that is aggravated by ER stress inducers. Armet knockdown causes UPR (lane 1 vs. 4 and 7) that is enhanced by the treatment with tunicamycin (lanes 5, 8) and thapsigargin (lanes 6, 9). Armet was knocked down in HeLa cells using siRNA1 and 2 as described in Fig. 6. At day 3 cells were treated with tunicamycin (2.5 μg/ml) or thapsigargin (500 nM) for 24 h. The indicated proteins were examined by IB.
To assess the effects of Armet overexpression on cell proliferation and ER stress-induced cell death, we stably overexpressed Armet in U2OS cells by lentivirus-mediated transfection. We chose U2OS cells because it expresses lower endogenous levels of Armet, which may help to observe the effects of overexpression. Two positive clones were established and confirmed by immunofluorescent staining for Armet. Immunoblotting shows that the two clones show overexpressed Armet levels (Fig. 8A). The large fold increase was due to the low endogenous Armet levels in U2OS cells and strong ectopic expression driven by the CMV promoter. Consistent with what we see in knockdown cells, overexpression of Armet inhibited cell proliferation in both clones (Fig. 8B). Moreover, Armet overexpression renders cells more resistant to cell death under glucose-free condition and in response to tunicamycin treatment (Fig. 8C). However, we did not observe changes in cell size in overexpressing clones (data not shown). These results support the notion that Armet plays an inhibitory role in cell proliferation and ER stress-induced cell death.
Fig. 8 –
Overexpression of Armet inhibits cell proliferation and protects cells from ER stress-induced death. A. Armet protein overexpression. Control or two overexpression clones that stably express Armet were established in U2OS cells. Armet expression was determined by IB. GAPDH was blotted as loading control. B. Armet overexpression inhibits cell proliferation. Control and two clones (#2 and 11) that overexpress Armet were established by lentiviral vector-mediated transfection. C. Armet-overexpressing cells are resistant to cell death. Control and clone 2 and 11 cells were cultured under glucose-free condition (upper panel) or treated with tunicamycin (lower panel) for 17 h.
Discussion
In this study, we identified Armet as one of the 12 commonly UPR-upregulated genes. We established Armet as a secreted protein. We demonstrated that Armet plays an important role in protecting cells against tunicamycin and thapsigargin-induced cell death. Loss of Armet renders cells more susceptible to those drugs, but surprisingly, significantly increases cell proliferation and decreases cell size. Previously, another UPR-upregulated and secreted protein, stanniocal-cin2, has been characterized [25]. Although stanniocalcin2 is induced by various stresses, it appears to selectively protect cells against thapsigargin, but not tunicamycin, -induced cell death. Armet and stanniocalcin2 are differentially regulated by UPR. Armet induction requires either ATF6 or XBP1 [13], whereas stanniocalcin2 expression is activated by PERK-ATF4 pathway [25]. Thus, all arms of UPR signal transducers are capable of induce expression of secreted factors, such as Armet and stanniocalcin2, to cope with different forms of ER stress and regulate other cellular activities.
Previous microarray studies have identified Armet as a UPR-upregulated gene [12,13]. Initial characterization by others demonstrated that Armet was localized in the ER and present in the conditioned medium of a rat mesencephalic type-1 astrocyte cell line [19,20]. However, neither study provided sufficient data to establish Armet as a secreted protein. We demonstrated in this study that Armet is not only localized in the ER, but also found in the Golgi and secreted into the culture medium. Inhibition of ER to Golgi trafficking by BFA blocked Armet secretion. Functionally, Armet (MANF) was shown to selectively protect cultured nigral dopaminergic neurons but not GABAergic or serotonergic neurons [19]. However, we provided evidence that Armet plays a general cytoprotective role. It is not clear if the protection of neurons is related to the general cytoprotective role of Armet that we discovered. Another puzzle is that we did not find Armet being glycosylated, whereas MANF was shown to be modified by sialic acid [19]. This discrepancy was not due to the protein expression system, since we also used HEK293 cells. Moreover, sialyation occurs on N- or O-glycans [30,31], but MANF contains neither N- nor O-glycan. It is, therefore, puzzling that how any sialyation of MANF could proceed. Human Armet-L1 is predicted to have a N-linked glycosylation site (aa57NSFL60, Fig. s1). Consistently, we indeed saw a modified form of Armet-L1 that could be abolished by treatment with tunicamycin, an inhibitor of N-glycosylation. However, this modification site is not highly conserved among species (Fig. s1), suggesting that the modification may not be essential for its function.
While this manuscript was in preparation, Armet-L1 was reported as CDNF (conserved dopamine neurotrophic factor) based on its neuroprotective role in 6-OHDA-induced Parkinson’s disease model [32]. When rats were given recombinant CDNF (Armet-L1) unilaterally into the striatum 6 h before injection of 6-OHDA into the same location, CDNF (Armet-L1) prevents the 6-OHDA-induced degeneration of dopaminergic neurons. Interestingly, 6-OHDA is known to induce ER stress and upregulates Armet [12,26,33,34]. Moreover, ER stress has been proposed to play an important role in Parkinson’s disease development [12,26,33,35,36]. Thus, upregulation of Armet may have a protective role against 6-OHDA-induced neuronal cell death. This notion is consistent with what we have found that Armet plays a general role against ER stress-induced cell death. CDNF (Armet-L1) is highly homologous to Armet. Therefore, it is reasonable to speculate that, in addition to its neurotrophic effects, the pre-injected CDNF (Armet-L1) might protect neurons against ER stress-induced neurodegeneration caused by the treatment of 6-OHDA. In other words, CDNF (Armet-L1) may also have a protective role against ER stress, as does Armet.
Although previous studies have suggested a neuroprotective role for Armet (MANF) and Armet-L1 (CDNF) in Parkinson’s disease [19,32], as a highly ER stress-upregulated gene with a cytoprotective role and other cellular activities, Armet is likely to be broadly involved in many disease processes. For example, protein aggregation is the pathological hallmark of many neurodegenerative disorders, including Parkinson’s disease, Huntington’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis, and many others [37–41]. Protein aggregation irrespective of its localization in cells inevitably leads to inhibition of proteasomal degradation [42,43], which further leads to build up of misfolded proteins in the ER and triggers ER stress and UPR. It would be interesting to determine if Armet is upregulated and has a protective role against ER stress-induced neurodegeneration in those neurodegenerative diseases. We showed that Armet is highly inducible in neurons by cortical ischemia, a condition known to cause ER stress [28]. Therefore, Armet is likely to play a general neuroprotective role in neurodegenerative diseases, but this role could be eventually overwhelmed by the continuous buildup of misfolded proteins in neurons. ER stress is also a significant contributing factor to tumor pathogenesis [44–46]. Hypoxia is commonly present and triggers ER stress in solid tumors [47]. However, instead of inducing cell death as seen in neurodegenerative diseases, ER stress appears to promote tumor progression and resistance to radiotherapy and chemotherapy [46,48]. Armet may be a mediator of these tumor responses. We have shown that silencing Armet increases, whereas overexpression of Armet inhibits, cell proliferation. It is possible that Armet may be induced under hypoxia condition to inhibit tumor cell proliferation and protect tumor cells from ER stress-induced death. Once the blood supply is restored, Armet may be downregulated, which allows tumors to grow. Decreasing cell size and changing cell morphology by Armet silencing are also intriguing. Armet may be a mediator of tissue remodeling under ER stress, such as cardiac myocyte hypertrophy. mTOR-S6K pathway transduces signals for cell size regulation in tissue hypertrophy of several organs [49]. It would be very interesting to determine if the effects of Armet on cell size involves the mTOR-S6K pathway.
The mechanisms underlying Armet functions are not known. Our data are in agreement with previous reports that both Armet and Armet-L1 are secreted proteins. We further show that their secretion follows the classic ER-Golgi pathway by using BFA treatment. Thus, Armet and Armet-L1 may act as both autocrine and paracrine factors to exert their effects via a receptor-mediated mechanism. Importantly, unlike Armet, Armet-L1 is not upregulated by ER stress. This difference suggests that Armet acts in an inducible fashion and Armet-L1 functions constitutively. We have also shown that UPR is hyperactive in Armet knockdown cells, suggesting that Armet may play a role of alleviating ER stress. Armet may also play a role in facilitating the removal of misfolded proteins from the ER by enhancing protein folding and/or degradation. It is also possible that Armet may be involved in inhibition of the activation of ER stress sensors. The hyperactive UPR in Armet knockdown cells may render the cells more sensitive to ER stress-induced death. Future studies will be directed to understand the mechanisms underlying Armet’s functions and the roles of Armet in physiology and disease pathogenesis.
Supplementary Material
Acknowledgments
We thank Dr. Martin Flajnik and Ms. Pamela Wright for critical editing and comments of the manuscript. This work was supported by a faculty startup fund from the University of Maryland Biotechnology Institute and in part by Public Health Service grant R01 GM69967 from the National Institute of General Medical Sciences.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.yexcr.2008.05.001.
REFERENCES
- [1].Kaufman RJ, Scheuner D, Schroder M, Shen X, Lee K, Liu CY, Arnold SM, The unfolded protein response in nutrient sensing and differentiation, Nat. Rev. Mol. Cell. Biol 3 (2002) 411. [DOI] [PubMed] [Google Scholar]
- [2].Malhotra JD, Kaufman RJ, The endoplasmic reticulum and the unfolded protein response, Semin. Cell. Dev. Biol 18 (2007) 716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ron D, Walter P, Signal integration in the endoplasmic reticulum unfolded protein response, Nat. Rev. Mol. Cell. Biol 8 (2007) 519. [DOI] [PubMed] [Google Scholar]
- [4].Marciniak SJ, Ron D, Endoplasmic reticulum stress signaling in disease, Physiol. Rev 86 (2006) 1133. [DOI] [PubMed] [Google Scholar]
- [5].McCracken AA, Brodsky JL, Evolving questions and paradigm shifts in endoplasmic-reticulum-associated degradation (ERAD), Bioessays 25 (2003) 868. [DOI] [PubMed] [Google Scholar]
- [6].Rutkowski DT, Kaufman RJ, A trip to the ER: coping with stress, Trends Cell Biol. 14 (2004) 20. [DOI] [PubMed] [Google Scholar]
- [7].Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D, Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response, Nat. Cell Biol. 2 (2000) 326. [DOI] [PubMed] [Google Scholar]
- [8].Shen J, Chen X, Hendershot L, Prywes R, ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals, Dev. Cell 3 (2002) 99. [DOI] [PubMed] [Google Scholar]
- [9].Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P, Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation, Cell 101 (2000) 249. [DOI] [PubMed] [Google Scholar]
- [10].Tinhofer I, Anether G, Senfter M, Pfaller K, Bernhard D, Hara M, Greil R, Stressful death of T-ALL tumor cells after treatment with the anti-tumor agent Tetrocarcin-A, FASEB J. 16 (2002) 1295. [DOI] [PubMed] [Google Scholar]
- [11].Okada T, Yoshida H, Akazawa R, Negishi M, Mori K, Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response, Biochem. J 366 (2002) 585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Holtz WA, O’Malley KL, Parkinsonian mimetics induce aspects of unfolded protein response in death of dopaminergic neurons, J. Biol. Chem 278 (2003) 19367. [DOI] [PubMed] [Google Scholar]
- [13].Lee AH, Iwakoshi NN, Glimcher LH, XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response, Mol. Cell. Biol 23 (2003) 7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shridhar V, Rivard S, Shridhar R, Mullins C, Bostick L, Sakr W, Grignon D, Miller OJ, Smith DI, A gene from human chromosomal band 3p21.1 encodes a highly conserved arginine-rich protein and is mutated in renal cell carcinomas, Oncogene 12 (1996) 1931. [PubMed] [Google Scholar]
- [15].Shridhar R, Shridhar V, Rivard S, Siegfried JM, Pietraszkiewicz H, Ensley J, Pauley R, Grignon D, Sakr W, Miller OJ, Smith DI, Mutations in the arginine-rich protein gene, in lung, breast, and prostate cancers, and in squamous cell carcinoma of the head and neck, Cancer Res. 56 (1996) 5576. [PubMed] [Google Scholar]
- [16].Evron E, Cairns P, Halachmi N, Ahrendt SA, Reed AL, Sidransky D, Normal polymorphism in the incomplete trinucleotide repeat of the arginine-rich protein gene, Cancer Res. 57 (1997) 2888. [PubMed] [Google Scholar]
- [17].Shridhar V, Rivard S, Wang X, Shridhar R, Paisley C, Mullins C, Beirnat L, Dugan M, Sarkar F, Miller OJ, Vaitkevicius VK, Smith DI, Mutations in the arginine-rich protein gene (ARP) in pancreatic cancer, Oncogene 14 (1997) 2213. [DOI] [PubMed] [Google Scholar]
- [18].Tanaka H, Shimada Y, Harada H, Shinoda M, Hatooka S, Imamura M, Ishizaki K, Polymorphic variation of the ARP gene on 3p21 in Japanese esophageal cancer patients, Oncol. Rep 7 (2000) 591. [DOI] [PubMed] [Google Scholar]
- [19].Petrova P, Raibekas A, Pevsner J, Vigo N, Anafi M, Moore MK, Peaire AE, Shridhar V, Smith DI, Kelly J, Durocher Y, Commissiong JW, MANF: a new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons, J. Mol. Neurosci 20 (2003) 173. [DOI] [PubMed] [Google Scholar]
- [20].Mizobuchi N, Hoseki J, Kubota H, Toyokuni S, Nozaki J, Naitoh M, Koizumi A, Nagata K, ARMET is a soluble ER protein induced by the unfolded protein response via ERSE-II element, Cell Struct. Funct 32 (2007) 41. [DOI] [PubMed] [Google Scholar]
- [21].Dunn TA, Chen S, Faith DA, Hicks JL, Platz EA, Chen Y, Ewing CM, Sauvageot J, Isaacs WB, De Marzo AM, Luo J, A novel role of myosin VI in human prostate cancer, Am. J. Pathol 169 (2006) 1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ballar P, Zhong Y, Nagahama M, Tagaya M, Shen Y, Fang S, Identification of SVIP as an endogenous inhibitor of ER-associated degradation, J. Biol. Chem 282 (2007) 33908. [DOI] [PubMed] [Google Scholar]
- [23].Ballar P, Shen Y, Yang H, Fang S, The role of a novel p97/valosin-containing protein-interacting motif of gp78 in endoplasmic reticulum-associated degradation, J. Biol. Chem 281 (2006) 35359. [DOI] [PubMed] [Google Scholar]
- [24].Cook JA, Chuang EY, Tsai MH, Coffin D, Degraff W, Sowers AL, Mitchell JB, Radiation-induced changes in gene-expression profiles for the SCC VII tumor cells grown in vitro and in vivo, Antioxid. Redox. Signal 8 (2006) 1263. [DOI] [PubMed] [Google Scholar]
- [25].Ito D, Walker JR, Thompson CS, Moroz I, Lin W, Veselits ML, Hakim AM, Fienberg AA, Thinakaran G, Characterization of stanniocalcin 2, a novel target of the mammalian unfolded protein response with cytoprotective properties, Mol. Cell. Biol 24 (2004) 9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ryu EJ, Harding HP, Angelastro JM, Vitolo OV, Ron D, Greene LA, Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson’s disease, J. Neurosci 22 (2002) 10690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H, TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death, EMBO J. 24 (2005) 1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].DeGracia DJ, Montie HL, Cerebral ischemia and the unfolded protein response, J. Neurochem 91 (2004) 1. [DOI] [PubMed] [Google Scholar]
- [29].Zhong X, Shen Y, Ballar P, Apostolou A, Agami R, Fang S, AAA ATPase p97/valosin-containing protein interacts with gp78, a ubiquitin ligase for endoplasmic reticulum-associated degradation, J. Biol. Chem 279 (2004) 45676. [DOI] [PubMed] [Google Scholar]
- [30].Jaeken J, Matthijs G, Congenital disorders of glycosylation, Annu. Rev. Genomics Hum. Genet 2 (2001) 129. [DOI] [PubMed] [Google Scholar]
- [31].Angata K, Fukuda M, Polysialyltransferases: major players in polysialic acid synthesis on the neural cell adhesion molecule, Biochimie 85 (2003) 195. [DOI] [PubMed] [Google Scholar]
- [32].Lindholm P, Voutilainen MH, Lauren J, Peranen J, Leppanen VM, Andressoo JO, Lindahl M, Janhunen S, Kalkkinen N, Timmusk T, Tuominen RK, Saarma M, Novel neurotrophic factor CDNF protects and rescues midbrain dopamine neurons in vivo, Nature. 448 (2007) 73. [DOI] [PubMed] [Google Scholar]
- [33].Chen G, Bower KA, Ma C, Fang S, Thiele CJ, Luo J, Glycogen synthase kinase 3beta (GSK3beta) mediates 6-hydroxydopamine-induced neuronal death, FASEB J. 18 (2004) 1162. [DOI] [PubMed] [Google Scholar]
- [34].Yamamuro A, Yoshioka Y, Ogita K, Maeda S, Involvement of endoplasmic reticulum stress on the cell death induced by 6-hydroxydopamine in human neuroblastoma SH-SY5Y cells, Neurochem. Res 31 (2006) 657. [DOI] [PubMed] [Google Scholar]
- [35].Imai Y, Soda M, Hatakeyama S, Akagi T, Hashikawa T, Nakayama KI, Takahashi R, CHIP is associated with Parkin, a gene responsible for familial Parkinson’s disease, and enhances its ubiquitin ligase activity, Mol. Cell 10 (2002) 55. [DOI] [PubMed] [Google Scholar]
- [36].Sekine Y, Takeda K, Ichijo H, The ASK1-MAP kinase signaling in ER stress and neurodegenerative diseases, Curr. Mol. Med 6 (2006) 87. [DOI] [PubMed] [Google Scholar]
- [37].Kopito RR, Aggresomes, inclusion bodies and protein aggregation, Trends Cell Biol. 10 (2000) 524. [DOI] [PubMed] [Google Scholar]
- [38].Taylor JP, Hardy J, Fischbeck KH, Toxic proteins in neurodegenerative disease, Science. 296 (2002) 1991. [DOI] [PubMed] [Google Scholar]
- [39].Giasson BI, Lee VM, Are ubiquitination pathways central to Parkinson’s disease? Cell. 114 (2003) 1. [DOI] [PubMed] [Google Scholar]
- [40].Ciechanover A, Brundin P, The ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg, Neuron 40 (2003) 427. [DOI] [PubMed] [Google Scholar]
- [41].Berke SJ, Paulson HL, Protein aggregation and the ubiquitin proteasome pathway: gaining the UPPer hand on neurodegeneration, Curr. Opin. Genet. Dev 13 (2003) 253. [DOI] [PubMed] [Google Scholar]
- [42].Bence NF, Sampat RM, Kopito RR, Impairment of the ubiquitin-proteasome system by protein aggregation, Science 292 (2001) 1552. [DOI] [PubMed] [Google Scholar]
- [43].Bennett EJ, Bence NF, Jayakumar R, Kopito RR, Global impairment of the ubiquitin-proteasome system by nuclear or cytoplasmic protein aggregates precedes inclusion body formation, Mol. Cell 17 (2005) 351. [DOI] [PubMed] [Google Scholar]
- [44].Feldman DE, Chauhan V, Koong AC, The unfolded protein response: a novel component of the hypoxic stress response in tumors, Mol. Cancer Res. 3 (2005) 597. [DOI] [PubMed] [Google Scholar]
- [45].Lee AS, Hendershot LM, ER stress and cancer, Cancer Biol. Ther 5 (2006) 721. [DOI] [PubMed] [Google Scholar]
- [46].Koumenis C, ER stress, hypoxia tolerance and tumor progression, Curr. Mol. Med 6 (2006) 55. [DOI] [PubMed] [Google Scholar]
- [47].Koumenis C, Bi M, Ye J, Feldman D, Koong AC, Hypoxia and the unfolded protein response, Methods Enzymol. 435 (2007) 275. [DOI] [PubMed] [Google Scholar]
- [48].Koumenis C, Wouters BG, “Translating” tumor hypoxia: unfolded protein response (UPR)-dependent and UPR-independent pathways, Mol. Cancer Res. 4 (2006) 423. [DOI] [PubMed] [Google Scholar]
- [49].Lee CH, Inoki K, Guan KL, mTOR pathway as a target in tissue hypertrophy, Annu. Rev. Pharmacol. Toxicol 47 (2007) 443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.