Abstract
Background
To examine the effects of the DPP-4i sitagliptin on CV outcomes during and after incident MI in the Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS).
Methods
TECOS randomized 14,671 participants with type 2 diabetes and atherosclerotic cardiovascular disease (ASCVD) to sitagliptin or placebo, in addition to usual care. For those who had a within-trial MI, we analyzed case fatality, and for those with a nonfatal MI, we examined a composite cardiovascular (CV) outcome (CV death or hospitalization for heart failure [hHF]) by treatment group, using Cox proportional hazards models left-censored at the time of the first within-trial MI, without and with adjustment for potential confounders, in intention-to-treat analyses.
Results
During TECOS, 616 participants had ≥ 1 MI (sitagliptin group 300, placebo group 316, HR 0.95, 95% CI 0.81–1.11, P = 0.49), of which 25 were fatal [11 and 14, respectively]). Of the 591 patients with a nonfatal MI, 87 (15%) died subsequently, with 66 (11%) being CV deaths, and 57 (10%) experiencing hHF. The composite outcome occurred in 58 (20.1%; 13.9 per 100 person-years) sitagliptin group participants and 50 (16.6%; 11.7 per 100 person-years) placebo group participants (HR 1.21, 95% CI 0.83–1.77, P = 0.32, adjusted HR 1.23, 95% CI 0.83–1.82, P = 0.31). On-treatment sensitivity analyses also showed no significant between-group differences in post-MI outcomes.
Conclusions
In patients with type 2 diabetes and ASCVD experiencing an MI, sitagliptin did not reduce subsequent risk of CV death or hHF, contrary to expectations derived from preclinical animal models.
Trial registration clinicaltrials.gov no. NCT00790205
Keywords: Acute myocardial infarction, Cardiovascular outcomes, Sitagliptin, Type 2 diabetes
Background
Dipeptidyl peptidase-4 inhibitors (DPP-4is) lower plasma glucose and glycated hemoglobin in people with type 2 diabetes by inhibiting degradation of endogenous glucagon-like peptide-1 (GLP-1) [1]. They have a low risk for hypoglycemia and are weight neutral [2]. Although two GLP-1 receptor agonists, once-daily liraglutide [3] and once-weekly semaglutide [4], have been shown to reduce cardiovascular (CV) events in patients with type 2 diabetes at high CV risk, four CV outcome trials that evaluated the once-daily DPP-4i agents saxagliptin [5], alogliptin [6], sitagliptin [7, 8], and linagliptin [9, 10] versus placebo showed no impact on CV death, myocardial infarction (MI), or stroke outcomes.
GLP-1 receptors are expressed on cells in CV tissues [11], and multiple CV effects of GLP-1 receptor agonism have been demonstrated with administration of native GLP-1, with administration of GLP-1 receptor agonists, and with DPP-4i treatment in preclinical studies [11–14]. Among these well-documented effects is a substantial (30–50%) reduction in the extent of myocardial necrosis after experimentally induced MI in rodents pretreated with native GLP-1 [15, 16] or with a GLP-1 receptor agonist [17, 18]. Similar experimental approaches with a DPP-4i in mice [19], rats [20], pigs [21], and dogs [22] produced largely similar results. Regarding potential mechanisms, sitagliptin seems to improve tolerance to ischemia as demonstrated by an improved regional contractility in ischemic segments of the left ventricle [23, 24]. These effects of DPP-4 inhibition may be mediated by protection of mitochondrial function and preventing cardiomyocyte apoptosis, and by interfering with oxidative stress during reperfusion [20, 21]. Theoretically, a smaller infarct size in humans could result in lower incident case-fatality, less post-MI arrhythmogenic risk, and higher residual left-ventricular function with a lower future risk of heart failure or CV death [25, 26].
The Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) randomized patients with type 2 diabetes and atherosclerotic cardiovascular disease (ASCVD) to double-blind therapy with sitagliptin or placebo, in addition to usual care, aiming for glycemic equipoise [7, 8]. In a post hoc analysis, we evaluated the effects of sitagliptin on a composite outcome defined as CV death or hospitalization for heart failure (hHF) in TECOS participants who experienced a within-trial MI.
Methods
Study design
The TECOS design [8] and primary results [7] and heart failure outcomes [27] have been published previously. Briefly, 14,671 participants from 38 countries were enrolled between December 2008 and July 2012. Eligible participants were ≥ 50 years old (no upper age limit) with type 2 diabetes, ASCVD, and glycated hemoglobin (HbA1c) values of 6.5–8.0% (48–64 mmol/mol) on stable dose mono- or dual-combination therapy with metformin, pioglitazone, sulfonylurea or insulin (with or without metformin). Participants were randomized double-blind to sitagliptin or placebo at doses appropriate for their eGFR [7, 8]. During follow-up, treatment for hyperglycemia and for type 2 diabetes comorbidities was provided by usual care providers according to their local guidelines with addition of any open-label glucose-lowering agent permitted, apart from a GLP-1 receptor agonist or DPP-4i. All reported events of death, MI, stroke, and hospitalization for unstable angina or heart failure were adjudicated by an independent committee masked to randomized treatment assignment. Adjudicated event definitions have been published previously [7, 8].
Objectives
The analyses presented here examine only those participants who experienced an MI during the trial. We evaluated potential differences between the randomized groups in case-fatality and for those with a non-fatal MI the time to a composite outcome defined as CV death or hHF. Secondary outcomes were post-MI time to CV death, hHF, and all-cause death. We also examined hHF in patients not known to have heart failure at baseline, and an extended composite outcome defined as CV death, hHF, a further MI, stroke, or new-onset atrial fibrillation.
Statistical analysis
Baseline characteristics for continuous variables were summarized as median and interquartile range (IQR), and categorical variables as count (percentage).
Primary analyses were performed on the intention-to-treat population in the subset who experienced an MI during the trial. Secondary on-treatment sensitivity analyses were performed with participants classified as “DPP-4i treated” if they were taking double-blind sitagliptin study medication or if they were taking an open-label DPP-4i. Similarly, they were classified as “not DPP-4i treated” if they were taking double-blind placebo study medication or had discontinued double-blind sitagliptin study medication and were not taking an open-label DPP-4i.
The two treatment groups were compared using Cox proportional hazards models, without and with adjustment for potential confounders. Adjustment factors applied were those previously identified in the large Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research (NAVIGATOR) clinical trial [28, 29]. The assumptions of linearity and proportional hazards had been previously evaluated for the set of confounders considered and appropriate adjustments applied when violations were noted. The list of covariates is provided in Additional file 1: Table S1. The proportional hazards assumption was tested for the treatment factor in these new models, and time-varying models would have been applied had violations been noted. Follow-up began (day 0) at the date of the first within-trial MI and continued until the date of the first occurrence of each type of endpoint considered here or the date of last contact when no event occurred. The analyses were performed twice in consideration of fatal MIs. In one case (primary analyses), only patients with nonfatal MIs were considered; in the second, the fatal MIs were in the cohort and included as endpoints.
All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Participant characteristics
Baseline characteristics of all participants at entry to TECOS are listed in Table 1 according to whether or not they had experienced an MI. Those with, compared without, an MI were more likely to be male (77.9% vs. 70.4%, P < 0.0001), to have prior coronary artery disease (89.4% vs. 73.4%, P < 0.0001), prior MI (57.8% vs. 42.0%, P < 0.0001) or prior hHF (21.4% vs. 17.9%, P = 0.024); and to be treated less commonly with metformin (75.5% vs. 81.8%, P < 0.0001) and more commonly with insulin (33.5% vs. 22.8%, P < 0.0001).
Table 1.
Baseline characteristics of TECOS participants who did not have a within-trial nonfatal myocardial infarction (MI), and for those participants with a nonfatal MI, split by sitagliptin or placebo treatment
| Characteristic | Patients without a nonfatal MI during the trial randomized to sitagliptin or placebo N = 14,055 |
Patients with a nonfatal MI during the trial | P-value* | |
|---|---|---|---|---|
| Sitagliptin N = 289 |
Placebo N = 302 |
|||
| Age at randomization (years)a | 65.0 (60.0, 71.0) | 67.0 (62.0, 74.0) | 66.0 (60.0, 72.0) | 0.1414 |
| Female | 4161 (29.6%) | 56 (19.4%) | 75 (24.8%) | 0.1103 |
| Hispanic or Latino | 1754 (12.5%) | 17 (5.9%) | 22 (7.3%) | 0.4924 |
| Race | 0.4603 | |||
| White | 9472 (67.4%) | 235 (81.3%) | 234 (77.5%) | |
| Black | 422 (3.0%) | 10 (3.5%) | 14 (4.6%) | |
| Asian | 3184 (22.7%) | 38 (13.1%) | 42 (13.9%) | |
| Other | 977 (7.0%) | 6 (2.1%) | 12 (4.0%) | |
| Region | 0.5023 | |||
| Latin America | 1445 (10.3%) | 10 (3.5%) | 11 (3.6%) | |
| Asia Pacific and other | 4377 (31.1%) | 98 (33.9%) | 84 (27.8%) | |
| Western Europe | 1977 (14.1%) | 48 (16.6%) | 50 (16.6%) | |
| Eastern Europe | 3822 (27.2%) | 63 (21.8%) | 68 (22.5%) | |
| North America | 2434 (17.3%) | 70 (24.2%) | 89 (29.5%) | |
| Durationb of type 2 diabetes (years) | 10.0 (5.0, 16.0) | 11.0 (6.0, 18.0) | 11.0 (6.0, 16.0) | 0.3233 |
| Diabetes therapy at baseline (alone or in combination) | ||||
| Sulfonylurea | 6394 (45.5%) | 110 (38.1%) | 125 (41.4%) | 0.4085 |
| Metformin | 11,501 (81.8%) | 212 (73.4%) | 235 (77.8%) | 0.2069 |
| Thiazolidinedione | 376 (2.7%) | 9 (3.1%) | 9 (3.0%) | 0.9245 |
| Insulin | 3208 (22.8%) | 98 (33.9%) | 98 (32.5%) | 0.7063 |
| Preexisting vascular disease | 13,975 (99.4%) | 288 (99.7%) | 302 (100.0%) | 0.4890 |
| Coronary artery disease | 10,312 (73.4%) | 261 (90.3%) | 269 (89.1%) | 0.6208 |
| Cerebrovascular disease | 3445 (24.5%) | 72 (24.9%) | 65 (21.5%) | 0.3289 |
| Peripheral arterial disease | 2348 (16.7%) | 40 (13.8%) | 42 (13.9%) | 0.9814 |
| Prior MI | 5899 (42.0%) | 171 (59.2%) | 170 (56.3%) | 0.4790 |
| Prior congestive heart failure | 2511 (17.9%) | 62 (21.5%) | 61 (20.2%) | 0.7072 |
| Previous atrial fibrillation/flutter | 1086 (7.7%) | 32 (11.1%) | 45 (14.9%) | 0.1670 |
| NYHA classification | 0.3241 | |||
| 1 | 510 (20.3%) | 16 (25.8%) | 8 (13.1%) | |
| 2 | 1256 (50.0%) | 24 (38.7%) | 30 (49.2%) | |
| 3 | 339 (13.5%) | 6 (9.7%) | 10 (16.4%) | |
| 4 | 11 (0.4%) | 1 (1.6%) | 1 (1.6%) | |
| Not available | 395 (15.7%) | 15 (24.2%) | 12 (19.7%) | |
| Qualifying HbA1c (mmol/mol) | 55.2 (50.8, 60.7) | 56.1 (51.0, 61.7) | 55.2 (51.9, 59.6) | 0.3239 |
| Qualifying HbA1c (%) | 7.2 (6.8, 7.7) | 7.3 (6.8, 7.8) | 7.2 (6.9, 7.6) | 0.3239 |
| eGFR (mL/min/1.73 m2) | 73.0 (60.0, 88.0) | 68.5 (55.0, 84.0) | 69.0 (56.0, 88.0) | 0.2918 |
| Urine albumin creatinine ratio (g/mol creatinine) | 10.6 (3.5, 35.0) | 12.2 (5.3, 52.7) | 13.8 (5.3, 43.9) | 0.9026 |
| Heart rate (bpm) | 72.0 (65.0, 79.0) | 70.0 (62.0, 78.0) | 71.0 (62.0, 80.0) | 0.0595 |
| Body mass index (kg/m2) | 29.5 (26.3, 33.2) | 29.8 (26.6, 33.5) | 30.4 (27.2, 34.3) | 0.1480 |
| Weight (kg) | 83.0 (71.0, 96.0) | 85.0 (75.0, 98.0) | 88.5 (75.0, 100.0) | 0.1517 |
| Height (cm) | 168.0 (160.0, 174.2) | 169.4 (163.2, 175.3) | 170.0 (162.6, 176.0) | 0.5036 |
| Cigarette smoking status | 0.9049 | |||
| Current | 1589 (11.3%) | 44 (15.2%) | 43 (14.2%) | |
| Former | 5575 (39.7%) | 129 (44.6%) | 133 (44.0%) | |
| Never | 6891 (49.0%) | 116 (40.1%) | 126 (41.7%) | |
| Systolic blood pressure (mmHg) | 133.0 (124.0, 145.0) | 136.0 (124.0, 146.0) | 135.0 (124.0, 148.0) | 0.9803 |
| Diastolic blood pressure (mmHg) | 79.0 (70.0, 84.0) | 77.0 (68.0, 82.0) | 76.0 (68.0, 85.0) | 0.3050 |
| LDL-C | 84.0 (65.0, 109.0) | 81.0 (63.0, 99.6) | 82.1 (65.6, 108.1) | 0.4128 |
| Medications taken at time of randomization | ||||
| Statins | 11,213 (79.8%) | 238 (82.4%) | 248 (82.1%) | 0.9408 |
| ACE inhibitors or angiotensin receptor blockers | 11,040 (78.5%) | 238 (82.4%) | 255 (84.4%) | 0.4959 |
| Diuretics | 5727 (40.7%) | 127 (43.9%) | 150 (49.7%) | 0.1633 |
| Calcium channel blockers | 4730 (33.7%) | 104 (36.0%) | 118 (39.1%) | 0.4386 |
| Beta blockers | 8876 (63.2%) | 210 (72.7%) | 221 (73.2%) | 0.8881 |
| Aspirin | 11,027 (78.5%) | 244 (84.4%) | 235 (77.8%) | 0.0403 |
Data shown are median (interquartile range) or N (%)
ACE angiotensin-converting enzyme, eGFR estimated glomerular filtration rate, HbA1c glycated hemoglobin, LDL-C low-density lipoprotein cholesterol, NYHA New York Heart Association, TECOS Trial Evaluating Cardiovascular Outcomes with Sitagliptin
*P-value is for placebo vs sitagliptin in patients with a nonfatal MI
aAge is missing among patients enrolled in Lithuania because the entire birth date including year was not available
bDuration = (year of randomization − year of diagnosis) + 1
Fatal and nonfatal MI
A total of 616 (4.2%) of the 14,671 TECOS participants had a within-trial fatal or nonfatal MI (300 [49%] randomized to sitagliptin and 316 [51%] to placebo), with no significant difference in the time to first event by randomized therapy (HR 0.95, 95% CI 0.81–1.11, P = 0.49) as reported previously [7]. Outcome information was missing for one participant for hHF and for two other participants for atrial fibrillation and stroke, limiting the number of participants who could be analyzed for these outcomes to 615 and 614, respectively. Twenty-five of these first MI events were fatal, 11 in the sitagliptin group and 14 in the placebo group, leaving 289 and 302 participants respectively with nonfatal MIs. Of the 591 participants who had a within-trial nonfatal MI, 87 (15%) died subsequently (66 [11%] classified as CV death), 57 (10%) experienced hHF, 109 (18%) had a second MI, 20 (3%) had a stroke, and 37 (6%) had incident atrial fibrillation.
CV events after nonfatal MI
The composite outcome of CV death or hHF following a nonfatal MI occurred in 58 of 289 sitagliptin group participants (20.1%; 13.9 events per 100 person-years) and in 50 of 302 placebo group participants (16.6%; 11.7 per 100 person-years), with no significant difference between groups (HR 1.21, 95% CI 0.83–1.77, P = 0.32; adjusted HR 1.23, 95% CI 0.83–1.82, P = 0.31) (Fig. 1a and Table 2). Similar results were seen for the individual outcomes of CV death, hHF, incident heart failure, recurrent MI, and all-cause death, and for the extended composite (CV death, hHF, incident heart failure, recurrent MI, stroke, or incident atrial fibrillation), with no significant differences also seen after adjustment for potential confounders (Table 2). Results were also similar when fatal MI was included in the cohort of interest (Additional file 1: Table S2, Figure S1).
Fig. 1.
Unadjusted event curves by randomized assignment to sitagliptin or placebo (Kaplan–Meier plots) for the composite outcome of cardiovascular (CV) death or heart failure hospitalization (hHF) (a) and for CV death (b), both occurring after the first within-trial nonfatal myocardial infarction (MI) (defining day 0 on the x-axis). Intention-to-treat analysis
Table 2.
Cardiovascular outcomes occurring after a first within-trial non-fatal myocardial infarction in those randomized previously to sitagliptin or placebo treatment (intention-to-treat analysis)
| Sitagliptin n = 289 |
Placebo n = 302 |
Unadjusted hazard ratio (95% CI) | P-value | Adjusted hazard ratio (95% CI) | P-value | |||
|---|---|---|---|---|---|---|---|---|
| No. (%) | Events per 100 patient-years | No. (%) | Events per 100 patient-years | |||||
| Cardiovascular death or hospitalization for heart failure | 58 (20.1) | 13.9 | 50 (16.6) | 11.7 | 1.21 (0.83–1.77) | 0.32 | 1.23 (0.83–1.82) | 0.31 |
| Cardiovascular death | 34 (11.8) | 7.6 | 32 (10.6) | 7.1 | 1.11 (0.68–1.81) | 0.67 | 1.12 (0.67–1.86) | 0.67 |
| Hospitalization for heart failure | 31 (10.7) | 7.5 | 26 (8.6) | 6.1 | 1.26 (0.75–2.12) | 0.39 | 1.40 (0.80–2.42) | 0.23 |
| New onset heart failure | 19 (6.6) | 4.3 | 17 (5.6) | 3.8 | 1.25 (0.64–2.44) | 0.51 | 1.49 (0.72–3.09) | 0.28 |
| Cardiovascular death, hospital admission for heart failure, new heart failure, acute myocardial infarction, stroke or new-onset atrial fibrillation | 108 (37.4) | 33.0 | 100 (33.1) | 28.4 | 1.16 (0.89–1.53) | 0.27 | 1.21 (0.91–1.60) | 0.20 |
| Further acute myocardial infarction | 54 (18.7) | 7.4 | 55 (18.2) | 7.1 | 1.01 (0.69–1.48) | 0.95 | 0.99 (0.67–1.46) | 0.95 |
| All-cause death | 50 (17.3) | 11.0 | 37 (12.3) | 8.1 | 1.40 (0.92–2.15) | 0.12 | 1.41 (0.90–2.21) | 0.13 |
On-treatment sensitivity analyses
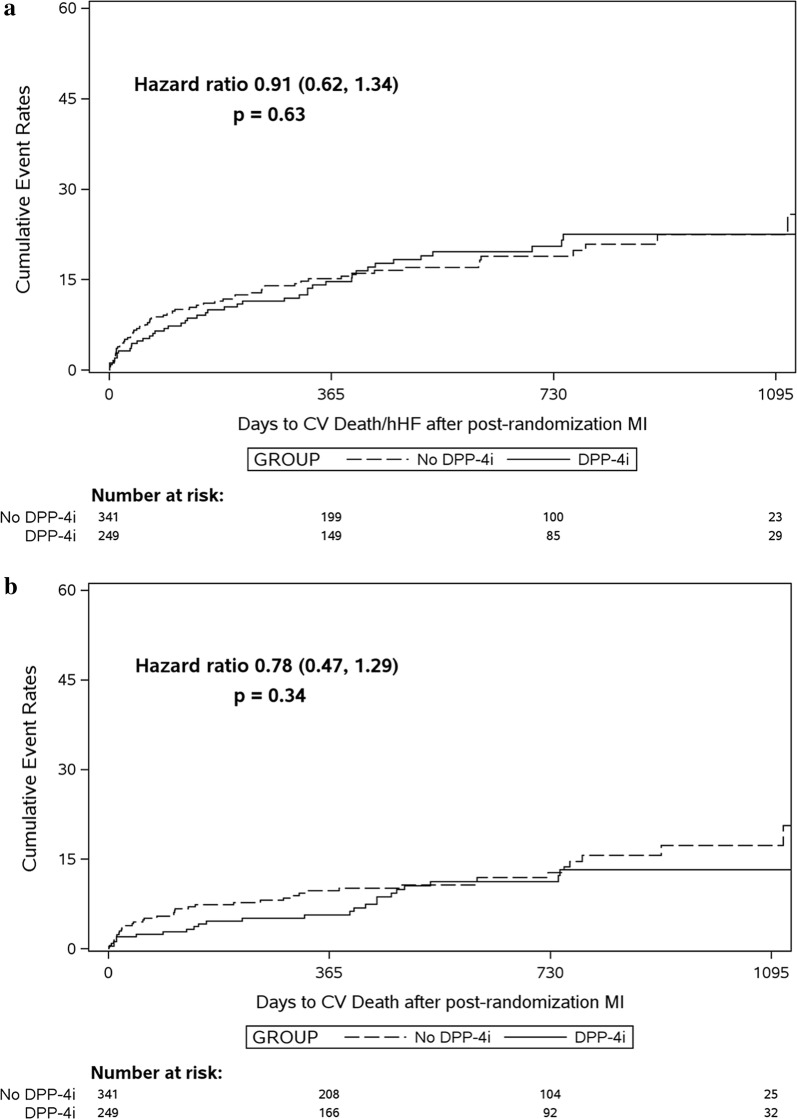
At the time of the first nonfatal MI, 249 (42%) participants were taking a DPP-4i and 341 (58%) were not. There was no significant difference in the composite outcome of CV death or hHF for those treated or not treated with a DPP-4i (Fig. 2a and Table 3) for either unadjusted analyses (HR 0.91, 95% CI 0.62–1.34, P = 0.63) or adjusted analyses (HR 0.95, 95% CI 0.64–1.43, P = 0.82). All results were consistent with those for the intention-to-treat analyses, although CV deaths were numerically less in those treated with a DPP4i (HR 0.75). Results were also consistent when first fatal MI was included in the analysis (Additional file 1: Table S3, Figure S2).
Fig. 2.
Unadjusted event curves by dipeptidyl peptidase-4 inhibitor (DPP-4i) treatment received versus no treatment (Kaplan–Meier plots) for the composite outcome of cardiovascular (CV) death or heart failure hospitalization (hHF) (a) and for CV death (b), both occurring after the first within-trial nonfatal myocardial infarction (MI) (defining day 0 on the x-axis). On-treatment sensitivity analysis
Table 3.
Cardiovascular outcomes occurring after a first within-trial nonfatal myocardial infarction in those pretreated or not pretreated with a dipeptidyl peptidase-4 inhibitor (DPP-4i) (on-treatment sensitivity analysis)
| DPP-4i treated n = 249 |
Not DPP-4i treated n = 341 |
Unadjusted hazard ratio (95% CI) | P-value | Adjusted hazard ratio (95% CI) | P-value | |||
|---|---|---|---|---|---|---|---|---|
| No. (%) | Events per 100 patient-years | No. (%) | Events per 100 patient-years | |||||
| Cardiovascular death or hospitalization for heart failure | 45 (18.1) | 11.9 | 62 (18.2) | 13.3 | 0.91 (0.62–1.34) | 0.63 | 0.95 (0.64–1.43) | 0.82 |
| Cardiovascular death | 25 (10.0) | 6.2 | 40 (11.7) | 8.1 | 0.78 (0.47–1.29) | 0.34 | 0.75 (0.44–1.26) | 0.27 |
| Hospitalization for heart failure | 27 (10.8) | 7.2 | 30 (8.8) | 6.4 | 1.15 (0.68–1.94) | 0.60 | 1.34 (0.77–2.33) | 0.31 |
| New onset heart failure | 16 (6.4) | 4.0 | 20 (5.9) | 4.1 | 1.05 (0.54–2.05) | 0.88 | 1.34 (0.64–2.79) | 0.44 |
| Cardiovascular death, hospital admission for heart failure, new heart failure, acute myocardial infarction, stroke or new-onset atrial fibrillation | 87 (34.9) | 28.8 | 120 (35.2) | 31.8 | 0.92 (0.70–1.22) | 0.56 | 0.95 (0.71–1.27) | 0.72 |
| Further acute myocardial infarction | 46 (18.5) | 7.2 | 63 (18.5) | 7.3 | 0.97 (0.66–1.42) | 0.89 | 0.99 (0.67–1.46) | 0.95 |
| All-cause death | 37 (14.9) | 8.9 | 49 (14.4) | 9.9 | 0.94 (0.61–1.44) | 0.77 | 0.91 (0.58–1.43) | 0.68 |
Discussion
Although preclinical data provided theoretical support [19–22], these post hoc TECOS analyses found no evidence that treatment with sitagliptin, compared with placebo, given prior to a first within-trial nonfatal MI had any impact on subsequent CV outcomes. Similar results were obtained when previous use of any DPP-4i was examined, and in sensitivity analyses that included fatal as well as nonfatal MIs.
Possible explanations for the discordance between human and animal observations include the following: (1) all TECOS participants had established ASCVD versus the lack of disease in experimental animals; (2) our study had only modest statistical power with just 123 composite outcome events analyzed; (3) experimentally induced MI is typically the consequence of total occlusion of a large coronary vessel, leading to a rather large area of myocardial necrosis, associated with adverse clinical consequences and significant mortality in the animal models—in contrast, spontaneous acute MI in humans is more variable in terms of the size of the relevant coronary vessel and the corresponding size of the subtended myocardium, whether complete occlusion of the coronary occurs, and marked variability in the timing from MI onset to clinical presentation, all of which translates into highly variable sizes of the area at risk, i.e. receiving blood supply from the infarct-related vessel, and of the necrotic area [25, 26]; (4) the doses of sitagliptin used in the animal studies are roughly twofold or more higher [19–22]; and (5) not all TECOS participants may have been adherent with respect to their study medication, and the GLP-1 receptor agonism augmented by DPP-4is does not have the same CV consequences in humans that has been demonstrated in animal studies [15–22]. Our results, however, are supported by negative results reported from a similar analysis of the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial examining effects of liraglutide versus placebo pretreatment on CV events following MI occurring during the trial [30].
Controversy persists regarding the effects of DPP-4is on heart failure risk, originating from the observation of an increased risk of hHF with saxagliptin in the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus-Thrombolysis in Myocardial Infarction 53 (SAVOR-TIMI) 53 trial [31] with a similar non-significant trend in the Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care (EXAMINE) trial with alogliptin [32], but no hHF signal observed with sitagliptin [27] or linagliptin [33]. On the other hand, results from observational studies have yielded counter-observations, reporting lower hHF risk associated with DPP-4i use compared with GLP-1 receptor agonists, with no significant difference in patients with a history of heart failure [34], and no difference in the risk of hHF when DPP-4i use was compared with sulfonylurea [35]. If DPP-4i treatment increases heart failure risk, the mechanism remains elusive. By echocardiographic criteria, a trend toward worsening diastolic ventricular function was slowed with sitagliptin treatment [36]. As a potential reason for a heterogeneity in effects between different DPP-4is, a suppression of renal sodium-hydrogen exchanger 3 activity with agents that are excreted in the urine (sitagliptin, alogliptin and linagliptin) has been proposed to protect from DPP-4i–induced heart failure [37]. In the present analysis, in accord with prior results of no heart failure effects of sitagliptin in the overall TECOS cohort, no association between sitagliptin and heart failure events was observed post-MI [7, 8, 27]. Thus, sitagliptin seems to be safe in patients during and after acute MI. Whether this applies to other DPP-4is needs to be studied in dedicated analyses from the respective CV outcomes trials [5, 6, 10]. Along these lines, a meta-analysis of other CV outcomes trials with DPP-4is (e.g. SAVOR TIMI-53 [5], EXAMINE [6], CArdiovascular safety and Renal Microvascular outcomE study with LINAgliptin [CARMELINA] [9, 10], and CARdiovascular Outcome Trial of LINAgliptin Versus Glimepiride in Type 2 Diabetes [CAROLINA] [38]) could provide further clarification.
Limitations of the present analyses include the non-randomized selection of the subset with MI for analysis [7, 8]. In addition, incomplete adherence to randomized treatment that could have occurred selectively post-MI could further confound comparative analyses. These analyses had limited power given the relatively few patients with MI with subsequent outcomes of interest. However, this data set is larger than most available with an ability to explore such associations.
Conclusions
In summary, these post hoc analyses of data from TECOS participants who had type 2 diabetes and ASCVD do not support the preclinically derived hypothesis that DPP-4i treatment prior to an MI can reduce the subsequent risk of CV death or hHF.
Supplementary information
Additional file 1: Table S1. Factors included in adjustment models for each clinical endpoint. Table S2. Cardiovascular outcomes occurring after a first within-trial fatal or nonfatal myocardial infarction in those randomized previously to sitagliptin or placebo treatment (intention-to-treat analysis). Table S3. Cardiovascular outcomes occurring after a first within-trial fatal or nonfatal myocardial infarction in those pretreated or not pretreated with a DPP-4i (on-treatment sensitivity analysis). Figure S1. Unadjusted event curves by randomized assignment to sitagliptin or placebo (Kaplan–Meier plots) for the composite outcome of cardiovascular death or heart failure hospitalization (A) and for cardiovascular death (B), both occurring after the first within-trial myocardial infarction during the TECOS trial (defining day 0 on the x-axis). Intention-to-treat analysis. Figure S2. Unadjusted event curves by treatment received, DPP-4i versus no DPP-4i, Kaplan-Meier plots for the composite outcome of cardiovascular death or heart failure hospitalization (A), and for cardiovascular death (B), both occurring after the first nonfatal within-trial myocardial infarction (defining day 0 on the x-axis). On-treatment sensitivity analysis.
Acknowledgements
The authors acknowledge John Schibler for his help in generating graphics and formatting tables of this study. The authors thank Peter Hoffmann for excellent editorial support. RRH is an NIHR Senior Investigator.
Previous presentation
Poster at the 2017 Annual Meeting of the European Association for the Study of Diabetes.
Abbreviations
- ASCVD
atherosclerotic cardiovascular disease
- CV
cardiovascular
- DPP-4is
dipeptidyl peptidase-4 inhibitors
- hHF
hospitalization for heart failure
- MI
myocardial infarction
- TECOS
Trial Evaluating Cardiovascular Outcomes with Sitagliptin
Authors’ contributions
MAN, KSP, and RRH designed the study. YL and KSP performed the analyses. MAN wrote the initial draft of the manuscript, and all authors were involved in revising it and in deciding to submit the final version for publication. All authors read and approved the final manuscript.
Funding
The TECOS trial was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. The study was designed and run independently by the Duke Clinical Research Institute (DCRI) and the University of Oxford Diabetes Trials Unit (DTU) in an academic collaboration with the sponsor. All analyses were performed by DCRI and DTU independent of the sponsor. The authors are solely responsible for the design and conduct of this study, all analyses, the drafting and editing of the paper, and its final contents. All authors agreed to submit the report for publication; the funder had no role in this decision.
Availability of data and materials
Requests to access the data for this study from qualified researchers trained in human subject confidentiality protocols may be submitted at dcri.org/data-sharing.
Ethics approval and consent to participate
The trial was designed and overseen by a steering committee, and an independent data and safety monitoring committee performed regular safety surveillance. All patients provided written informed consent. Institutional review board approval was required at all participating institutions.
Consent for publication
Not applicable.
Competing interests
MAN has been a member of advisory boards or consulted for AstraZeneca (moderate), Boehringer Ingelheim (moderate), Eli Lilly & Co. (significant), Fractyl (moderate), GlaxoSmithKline (moderate), Intarcia (moderate), Menarini/Berlin Chemie (moderate), Merck, Sharp & Dohme (significant), and NovoNordisk (significant). His institution has received grant support from AstraZeneca, Boehringer Ingelheim, Eli Lilly & Co., GlaxoSmithKline, Intarcia, Menarini/Berlin-Chemie, Merck, Sharp & Dohme, Novartis Pharma, and Novo Nordisk A/S. He has also served on the speakers’ bureau of AstraZeneca, Boehringer Ingelheim, Eli Lilly & Co., GlaxoSmithKline, Menarini/Berlin Chemie (all moderate), Merck, Sharp & Dohme, and Novo Nordisk A/S (both significant). DKM has provided clinical trial leadership for AstraZeneca, Sanofi Aventis, Janssen, Boehringer Ingelheim, Merck & Co, Pfizer, Lilly US, Novo Nordisk, Lexicon, Eisai, GlaxoSmithKline, and Esperion, and consultancy for AstraZeneca, Sanofi Aventis, Lilly US, Boehringer Ingelheim, Merck & Co, Novo Nordisk, Applied Therapeutics, Afimmune and Metavant. KSP has no disclosures. YL has received grants from Merck, Janssen Research & Development, AstraZeneca, GlaxoSmithKline, and Bayer HealthCare AG. TES reports personal fees from several companies (incl. Amgen, AstraZeneca, Merck, NovoNordisk, Pfizer, Orion, Bayer, Boehringer-Ingelheim) and owns a minor amount of stock in OrionPharma. AR has no disclosures. TD has no disclosures. EDP has received grants from Janssen, Merck, Sanofi, AstraZeneca, Genentech, and Amgen, and has consulting associations with Janssen, Bayer, Merck, and Sanofi. HDW reports research grants from GlaxoSmithKline, Sanofi-Aventis, Eli Lilly and Company, National Institute of Health, Merck Sharp & Dohme, George Institute, Omthera Pharmaceuticals, Pfizer New Zealand, Intarcia Therapeutics Inc., Elsai Inc., Daiichi-Sankyo, DalCor Pharmaceuticals; Advisory board/lecture fees from AstraZeneca, Acetelion, Sirte and he is a Steering Board member for Luitpold Pharmaceuticals Ltd and CSL Behring LLC. RS has no disclosures. RRH reports receiving grants from AstraZeneca during the conduct of the study and grants and personal fees from Bayer, Boehringer Ingelheim and Merck Sharp&Dohme Corp., a subsidiary of Merck & Co., Inc.; personal fees from Novartis, Amgen, and Servier; and financial support from Elcelyx, GlaxoSmithKline, Janssen, and Takeda outside the submitted work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Michael A. Nauck and Darren K. McGuire contributed equally to this article
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12933-019-0921-2.
References
- 1.Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007;117:24–32. doi: 10.1172/JCI30076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 3.Marso SP, Daniels GH, Brown-Frandsen K, LEADER Steering Committee. LEADER Trial Investigators et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/nejmoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marso SP, Bain SC, Consoli A, SUSTAIN-6 Investigators et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/nejmoa1607141. [DOI] [PubMed] [Google Scholar]
- 5.Scirica BM, Bhatt DL, Braunwald E, SAVOR-TIMI 53 Steering Committee and Investigators et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/nejmoa1307684. [DOI] [PubMed] [Google Scholar]
- 6.White WB, Cannon CP, Heller SR, EXAMINE Investigators et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/nejmoa1305889. [DOI] [PubMed] [Google Scholar]
- 7.Green JB, Bethel MA, Armstrong PW, TECOS Study Group et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–242. doi: 10.1056/nejmoa1501352. [DOI] [PubMed] [Google Scholar]
- 8.Green JB, Bethel MA, Paul SK, et al. Rationale, design, and organization of a randomized, controlled Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) in patients with type 2 diabetes and established cardiovascular disease. Am Heart J. 2013;166(983–9):e7. doi: 10.1016/j.ahj.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Rosenstock J, Perkovic V, Alexander JH, CARMELINA® Investigators et al. Rationale, design, and baseline characteristics of the CArdiovascular safety and Renal Microvascular outcomE study with LINAgliptin (CARMELINA®): a randomized, double-blind, placebo-controlled clinical trial in patients with type 2 diabetes and high cardio-renal risk. Cardiovasc Diabetol. 2018;17:39. doi: 10.1186/s12933-018-0682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenstock J, Perkovic V, Johansen OE, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321:69–79. doi: 10.1001/jama.2018.18269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drucker DJ. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 2016;24:15–30. doi: 10.1016/j.cmet.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Nauck M, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ. Cardiovascular actions and clinical outcomes with GLP-1R agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors. Circulation. 2017;136:849–870. doi: 10.1161/circulationaha.117.028136. [DOI] [PubMed] [Google Scholar]
- 13.Pujadas G, Drucker DJ. Vascular biology of glucagon receptor superfamily peptides: complexity, controversy, and clinical relevance. Endocr Rev. 2016;37:554–583. doi: 10.1210/er.2016-1078. [DOI] [PubMed] [Google Scholar]
- 14.Katakami N, Mita T, Irie Y, et al. Effect of sitagliptin on tissue characteristics of the carotid wall in patients with type 2 diabetes: a post hoc sub-analysis of the sitagliptin preventive study of intima-media thickness evaluation (SPIKE) Cardiovasc Diabetol. 2018;17:24. doi: 10.1186/s12933-018-0666-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54:146–151. doi: 10.2337/diabetes.54.1.146. [DOI] [PubMed] [Google Scholar]
- 16.Bose AK, Mocanu MM, Carr RD, Yellon DM. Glucagon like peptide-1 is protective against myocardial ischemia/reperfusion injury when given either as a preconditioning mimetic or at reperfusion in an isolated rat heart model. Cardiovasc Drugs Ther. 2005;19:9–11. doi: 10.1007/s10557-005-6892-4. [DOI] [PubMed] [Google Scholar]
- 17.Noyan-Ashraf MH, Momen MA, Ban K, et al. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58:975–983. doi: 10.2337/db08-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Timmers L, Henriques JP, de Kleijn DP, et al. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol. 2009;53:501–510. doi: 10.1016/j.jacc.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 19.Ye Y, Keyes KT, Zhang C, Perez-Polo JR, Lin Y, Birnbaum Y. The myocardial infarct size-limiting effect of sitagliptin is PKA-dependent, whereas the protective effect of pioglitazone is partially dependent on PKA. Am J Physiol Heart Circ Physiol. 2010;298:H1454–H1465. doi: 10.1152/ajpheart.00867.2009. [DOI] [PubMed] [Google Scholar]
- 20.Chinda K, Sanit J, Chattipakorn S, Chattipakorn N. Dipeptidyl peptidase-4 inhibitor reduces infarct size and preserves cardiac function via mitochondrial protection in ischaemia-reperfusion rat heart. Diabetes Vasc Dis Res. 2014;11:75–83. doi: 10.1177/1479164113516134. [DOI] [PubMed] [Google Scholar]
- 21.Chinda K, Palee S, Surinkaew S, Phornphutkul M, Chattipakorn S, Chattipakorn N. Cardioprotective effect of dipeptidyl peptidase-4 inhibitor during ischemia-reperfusion injury. Int J Cardiol. 2013;167:451–457. doi: 10.1016/j.ijcard.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Ihara M, Asanuma H, Yamazaki S, et al. An interaction between glucagon-like peptide-1 and adenosine contributes to cardioprotection of a dipeptidyl peptidase 4 inhibitor from myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2015;308:H1287–H1297. doi: 10.1152/ajpheart.00835.2014. [DOI] [PubMed] [Google Scholar]
- 23.McCormick LM, Kydd AC, Read PA, et al. Chronic dipeptidyl peptidase-4 inhibition with sitagliptin is associated with sustained protection against ischemic left ventricular dysfunction in a pilot study of patients with type 2 diabetes mellitus and coronary artery disease. Circ Cardiovasc Imaging. 2014;7:274–281. doi: 10.1161/CIRCIMAGING.113.000785. [DOI] [PubMed] [Google Scholar]
- 24.Read PA, Khan FZ, Heck PM, Hoole SP, Dutka DP. DPP-4 inhibition by sitagliptin improves the myocardial response to dobutamine stress and mitigates stunning in a pilot study of patients with coronary artery disease. Circ Cardiovasc Imaging. 2010;3:195–201. doi: 10.1161/CIRCIMAGING.109.899377. [DOI] [PubMed] [Google Scholar]
- 25.Hartman MHT, Eppinga RN, Vlaar PJJ, et al. The contemporary value of peak creatine kinase-MB after ST-segment elevation myocardial infarction above other clinical and angiographic characteristics in predicting infarct size, left ventricular ejection fraction, and mortality. Clin Cardiol. 2017;40:322–328. doi: 10.1002/clc.22663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roes SD, Kelle S, Kaandorp TA, et al. Comparison of myocardial infarct size assessed with contrast-enhanced magnetic resonance imaging and left ventricular function and volumes to predict mortality in patients with healed myocardial infarction. Am J Cardiol. 2007;100:930–936. doi: 10.1016/j.amjcard.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 27.McGuire DK, Van de Werf F, Armstrong PW, Trial Evaluating Cardiovascular Outcomes With Sitagliptin (TECOS) Study Group et al. Association between sitagliptin use and heart failure hospitalization and related outcomes in type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016;1:126–135. doi: 10.1001/jamacardio.2016.0103. [DOI] [PubMed] [Google Scholar]
- 28.Preiss D, Thomas LE, Sun JL, et al. Predictors of cardiovascular events in a contemporary population with impaired glucose tolerance: an observational analysis of the Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research (NAVIGATOR) trial. BMJ Open. 2012 doi: 10.1136/bmjopen-2012-001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong YW, Thomas L, Sun JL, et al. Predictors of incident heart failure hospitalizations among patients with impaired glucose tolerance: insight from the Nateglinide and Valsartan in Impaired Glucose Tolerance 21 Outcomes Research study. Circ Heart Fail. 2013;6:203–210. doi: 10.1161/CIRCHEARTFAILURE.112.000086. [DOI] [PubMed] [Google Scholar]
- 30.Nauck MA, Tornøe K, Rasmussen S, Bach Treppendahl M, Marso SP, LEADER Publication Committee on behalf of the LEADER Trial Investigators Cardiovascular outcomes in patients who experienced a myocardial infarction while treated with liraglutide versus placebo in the LEADER trial. Diabetes Vasc Dis Res. 2018;15:465–468. doi: 10.1177/1479164118783935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scirica BM, Braunwald E, Raz I, et al. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014;130:1579–1588. doi: 10.1161/CIRCULATIONAHA.114.010389. [DOI] [PubMed] [Google Scholar]
- 32.Zannad F, Cannon CP, Cushman WC, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067–2076. doi: 10.1016/S0140-6736(14)62225-X. [DOI] [PubMed] [Google Scholar]
- 33.McGuire DK, Alexander JH, Johansen OE, et al. Linagliptin effects on heart failure and related outcomes in individuals with type 2 diabetes mellitus at high cardiovascular and renal risk in CARMELINA. Circulation. 2019;139:351–361. doi: 10.1161/CIRCULATIONAHA.118.038352. [DOI] [PubMed] [Google Scholar]
- 34.Dawwas GK, Smith SM, Park H. Risk of heart failure hospitalization among users of dipeptidyl peptidase-4 inhibitors compared to glucagon-like peptide-1 receptor agonists. Cardiovasc Diabetol. 2018;17:102. doi: 10.1186/s12933-018-0746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim YG, Yoon D, Park S, et al. Dipeptidyl peptidase-4 inhibitors and risk of heart failure in patients with type 2 diabetes mellitus: A population-based cohort study. Circ Heart Fail. 2017;10:e003957. doi: 10.1161/CIRCHEARTFAILURE.117.003957. [DOI] [PubMed] [Google Scholar]
- 36.Yamada H, Tanaka A, Kusunose K, et al. Effect of sitagliptin on the echocardiographic parameters of left ventricular diastolic function in patients with type 2 diabetes: a subgroup analysis of the PROLOGUE study. Cardiovasc Diabetol. 2017;16:63. doi: 10.1186/s12933-017-0546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sano M. Mechanism by which dipeptidyl peptidase-4 inhibitors increase the risk of heart failure and possible differences in heart failure risk. J Cardiol. 2019;73:28–32. doi: 10.1016/j.jjcc.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Marx N, Rosenstock J, Kahn SE, et al. Design and baseline characteristics of the cardiovascular outcome trial of linagliptin versus Glimepiride in type 2 diabetes (CAROLINA®) Diabetes Vasc Dis Res. 2015;12:164–174. doi: 10.1177/1479164115570301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Factors included in adjustment models for each clinical endpoint. Table S2. Cardiovascular outcomes occurring after a first within-trial fatal or nonfatal myocardial infarction in those randomized previously to sitagliptin or placebo treatment (intention-to-treat analysis). Table S3. Cardiovascular outcomes occurring after a first within-trial fatal or nonfatal myocardial infarction in those pretreated or not pretreated with a DPP-4i (on-treatment sensitivity analysis). Figure S1. Unadjusted event curves by randomized assignment to sitagliptin or placebo (Kaplan–Meier plots) for the composite outcome of cardiovascular death or heart failure hospitalization (A) and for cardiovascular death (B), both occurring after the first within-trial myocardial infarction during the TECOS trial (defining day 0 on the x-axis). Intention-to-treat analysis. Figure S2. Unadjusted event curves by treatment received, DPP-4i versus no DPP-4i, Kaplan-Meier plots for the composite outcome of cardiovascular death or heart failure hospitalization (A), and for cardiovascular death (B), both occurring after the first nonfatal within-trial myocardial infarction (defining day 0 on the x-axis). On-treatment sensitivity analysis.
Data Availability Statement
Requests to access the data for this study from qualified researchers trained in human subject confidentiality protocols may be submitted at dcri.org/data-sharing.