Abstract
Background:
Ablation therapy is the treatment of choice in antiarrhythmic drug-refractory atrial fibrillation (AF). It is performed by either cryoballoon ablation (CBA) or radiofrequency abla-tion. CBA is gaining popularity due to simplicity with similar efficacy and complication rate com-pared with RFA. In this meta-analysis, we compare the recurrence rate of AF and the complications from CBA versus RFA for the treatment of AF.
Methods:
We systematically searched PubMed for the articles that compared the outcome of interest. The primary outcome was to compare the recurrence rate of AF between CBA and RFA. We also in-cluded subgroup analysis with complications of pericardial effusion, phrenic nerve palsy and cerebral microemboli following ablation therapy.
Results:
A total of 24 studies with 3527 patients met our predefined inclusion criteria. Recurrence of AF after CBA or RFA was similar in both groups (RR: 0.84; 95% CI: 0.65, 1.07; I2=48%, Cochrane p=0.16). In subgroup analysis, heterogeneity was less in paroxysmal AF (I2=0%, Cochrane p=0.46) compared to mixed AF (I2=72%, Cochrane p=0.003). Procedure and fluoroscopy time was less by 26.37 and 5.94 minutes respectively in CBA compared to RFA. Complications, pericardial effusion, and silent cerebral microemboli, were not different between the two groups, however, phrenic nerve palsy was exclusively present only in CBA group.
Conclusion:
This study confirms that the effectiveness of CBA is similar to RFA in the treatment of AF with the added advantages of shorter procedure and fluoroscopy times.
Keywords: Atrial fibrillation, cryoballoon ablation, meta-analysis, pericardial effusion, phrenic nerve palsy, radiofrequency ablation, silent cerebral microemboli
1. INTRODUCTION
Atrial Fibrillation (AF) is the most common sustained cardiac arrhythmia and is a major healthcare concern worldwide. The prevalence is on the rise and it was estimated that 33.5 million patients had AF in 2010 [1]. Given the increase in stroke, cardiomyopathy, and subsequent heart failure associated with AF, an easy, effective treatment option is in tremendous demand. Catheter ablation is a minimally-invasive treatment strategy and a class I indication to resolve drug-refractory AF by means of isolating the pulmonary veins [2]. Pulmonary vein isolation is usually achieved by two commonly used methods, a radiofrequency ablation [3] or cryoballoon ablation (CBA).
RFA uses heat energy produced by alternating the current to ablate, or burn, a specific tissue portion within the electrical conduction system of the heart [4]. CBA uses energy to freeze cardiac tissue rather than heat energy [5] and has become an alternative approach for ablating AF. Due to its simplicity, relative straightforwardness, and reproducibility, CBA is gaining popularity in the clinical setting. In this meta-analysis, we have reviewed available literature to explore the safety profile, effectiveness as well as the procedure and fluoroscopy time with the use of CBA compared to RFA for AF.
2. METHODS
The current report conforms to standard guidelines according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement [6].
2.1. Literature Search
We systematically searched PubMed using terms “cryosurgery”, “cryo”, “cryosurg”, “cryoballoon” and “atrial fibrillation” in various combinations. The search was conducted in April of 2018. We also manually searched the reference lists of all publications and review articles that would meet inclusion criteria.
2.2. Study Selection
Two authors reviewed all potentially relevant articles in a parallel manner by using a pre-defined criteria. A study was deemed eligible with the following inclusion criteria: (1) evaluated the use of CBA in a study; (2) enrolled patients with either paroxysmal or mixed (combination of paroxysmal and persistent) AF; (3) reported data on the clinical success rate or procedure/fluoroscopy time; and (4) was published as a full manuscript in English.
2.3. Data Abstraction
For each included study, two authors (NP and KP) used a standardized data abstraction tool to independently extract all data with disagreements resolved by consensus. The following information was sought from each study: specific data on study characteristics, patient characteristics, intervention details and outcomes. The primary outcome was clinical success rate including subgroup analysis with different types of AF. The secondary measures were fluoroscopic and procedure time, as well as their complications including phrenic nerve palsy, silent cerebrovascular emboli and pericardial effusion.
2.4. Statistical Analysis
All statistical analyses were performed using Review Manager Version 5.3.5 (Reference 1). Continuous variables were reported as mean (standard deviation) or median [7], and categorical variables as n (%), weighted for a sample size of each study. Funnel plot analysis was used to evaluate potential publication bias, and Cochran’s Q and I2 statistic were used to investigate heterogeneity among studies and interpreted according to Higgins and Thompson criteria. I2 values of 25%, 25-50%, or 50% indicated low, moderate, or high heterogeneity, respectively. We pooled data using the fixed effect model when minimal heterogeneity was observed; otherwise, a Hartung-Knapp method random-effects model was used.
3. RESULTS
3.1. Study Outline and Characteristics
The results of our literature search are shown in Fig. (1). We identified a total of 24 studies [3, 8-30] including 3,527 patients meeting our inclusion criteria. Baseline characteristics of these studies are included in Table 1. Most of the studies were conducted in European nations and in non-randomized fashion. Included patients’ age were 59.1 and 59.3 years in CBA and RFA group, respectively. Left atrial sizes were comparable in both groups with average around 42mm and left ventricular ejection fraction was 61.6% in CBA and 60.6% in RFA group. Duration of follow up is ranging from 6 to 24 hours and most studies had at least 3 months of further follow up after the procedure.
Fig. (1).
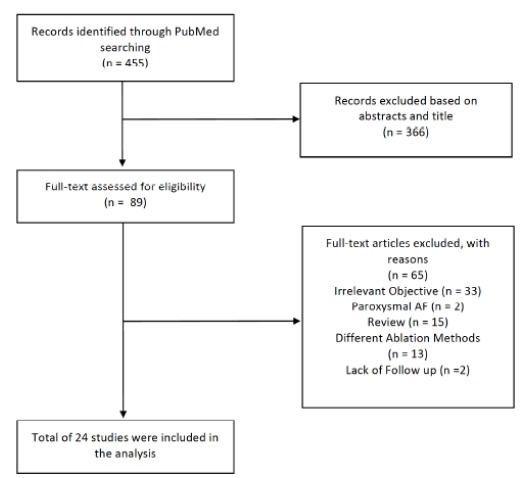
Flow chart of selected studies.
Table 1. Baseline characteristics of analyzed studies.
| Study | Design | CBA Size | n (% Male) | AF Type | Age (years) | LA-D (mm) | LV-EF (%) |
Hypertension
(%) |
Diabetes
(%) |
AF Surveillance Follow up in Months | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CBA | RF | CBA | RF | CBA | RF | |||||||||
| Linhart et al. 2009 [8] | PRS Non-RCT | 23 or 28 mm | 20 (75) | 20 (75) | Paroxysmal | 59.9 | 58.5 | NR | 59.5 | 62.5 | 60 | NR | 6 | |
| Sauren et al. 2009 [9] | PRS Non-RCT | 28 mm | 10 (70) | 10 (100) | Mixed but Paroxysmal in CRYO group | 58 | 53 | NR | NR | NR | NR | NR | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chierchia et al. 2010 [10] |
Non-RCT | 28 mm | 46 (78) | 87 (79) | Paroxysmal | 56 | 56 | 41 | 41 | 64 | 64 | 24 | NR | NR |
| Kojodjojo et al. 2010 [11] |
PRS Non- RCT | 28 mm | 90 (75) | 53 (77) | Mixed* | 57 | 59.3 | 39.6 | 41.6 | 65 | 60.3 | 47 | NR | 14.9 /15.6€ |
| Kuhne et al. 2010 [12] | Non-RCT | 28 mm | 18 (88) | 25 (84) | Paroxysmal | 58 | 59 | 41 | 42 | 60 | 58 | NR | NR | 12 |
| Sorgente et al. 2010 [13] | RSP Non-RCT | 28 mm | 30 (74) | 29 (90) | Mixed | 56 | 56.1 | 40.8 | 42.4 | 63.9 | 64.2 | 29 | NR | 12 |
| Gaita et al. 2011 [14] | PRS Non-RCT | 23 or 28 mm | 36 (69) | 36 (67) | Paroxysmal | 55 | 57 | 41 | 43 | 63 | 64 | 36 | NR | NR |
| Herrera Siklody et al. 2011 [15] | PRS RCT | 23 or 28 mm | 23 (65) | 27 (74) | Mixed | 61 | 61 | 40 | 42 | NR | 61 | NR | NR | |
| Neumann et al. 2011 [16] |
Non-RCT | NR | 45 (53) | 44 (73) | Mixed but Paroxysmal in CRYO group | 56 | 58 | 51 | 53 | 62 | 58 | 51 | NR | NR |
| Herrera Siklody et al. 2012 [17] | PRS RCT | 23 or 28 mm | 30 (83) | 30 (77) | Mixed | 57 | 56 | 41.4 | 40 | NR | 43 | 0 | NR | |
| Schmidt et al. 2012 [18] | Non-RCT | 23 or 28 mm | 37 (76) | 178 (84) | Mixed but Paroxysmal in CRYO group | 60 | 63 | 46 | 46 | 60 | 58 | 58 | 13 | NR |
| Maagh et al. 2013 [19] | RSP Non-RCT | 28 mm | 30 (63) | 42 (69) | Mixed | 59.9 | 60.9 | 38.9 | 37.5 | NR | 20 | NR | 24 | |
| Malmborg et al. 2013 [20] |
PRS RCT | 23 or 28 mm | 54 (79) | 56 (71.4) | Mixed | 59 | 62 | 40 | 42 | NR | 40.7 | NR | 12 | |
| Schmidt et al. 2013 [21] | PRS RCT | 28 mm | 33 (NR) | 33 (NR) | Paroxysmal | 66 | 63 | 40 | 41 | 59 | 58 | 58 | 12 | NR |
| Mugnai et al 2014 [22] | RSP Non-RCT | 28 mm | 136 (NR) | 260 (NR) | Paroxysmal | 57 | 58.3 | 42 | 42.6 | NR | 23 | |||
| Pérez-Castellano et al. 2014 [23] | PRS RCT | 23 or 28 mm | 25 (68) | 25 (88) | Paroxysmal | 58 | 56 | 42 | 42 | NR | 24 | 16 | 12 | |
| Ciconte et al. 2015 [1] | PRS Non-RCT | 28 mm | 50 (72) | 50 (76) | Mixed | 62.4 | 62.4 | 46 | 47.2 | 57.5 | 56.3 | 52 | 8 | 12 |
| Hunter et al. 2015 [24] | PRS RCT | 23 or 28 mm | 78 (56) | 77 (61) | Paroxysmal | 56 | 61 | 42 | 42 | NR | 35 | 5 | 12 | |
| Jourda et al. 2015 [25] | PRS Non-RCT | 28 mm | 75 (74.3) | 75 (76) | Paroxysmal | 59.9 | 62.5 | NR | 64.4 | 65.5 | 34.7 | 8 | 12 | |
| Luik et al. 2015 [26] | PRS RCT | 23 or 28 mm | 156 (64.1) | 159 (52.7) | Paroxysmal | 61 | NR | NR | 62.9 | 9 | 12 | |||
| Wasserlauf et al. 2015 [27] |
PRS Non-RCT | 28 mm | 101 (66) | 100 (69) | Paroxysmal | 62.9 | 60 | 37 | 37 | 58 | 58.9 | 44 | 7 | 12 |
| Kuck et al. 2016 [28] | PRS RCT | NR | 374 (59) | 376 (63) | Paroxysmal | 59.9 | 60.1 | 40.8 | 40.6 | NR | 57.5 | 9.9 | 15 | |
| Yokokava et al. 2017 [29] | PRS Non-RCT | 28 mm | 71 (75) | 75 (56) | Paroxysmal | 63 | 62 | 42 | 42 | 59 | 60 | 56 | NR | 12 |
| Matta et al. 2018 [30] | PRS Non-RCT | NR | 46 (78) | 46 (82) | Paroxysmal | 59 | 59 | 70* | 69* | 61 | 61 | 46 | 7 | 12 |
AF, Atrial Fibrillation; CBA, Cryoballoon ablation; LA-D, Left Atrial Diameter; LV-EF, Left Ventricular Ejection Fraction; mm, millimeters; NR, Not Reported; PRS, Prospective; RFA, Radiofrequency Ablation; RCT, Randomized Control Trial; RSP, Retrospective; The value for the column “n” represents the patients in each study
€: Mean follow up in CBA group was 14.9 months compared to 15.6 months in RF group.
*: Left Atrial volume in milliliters.
3.2. Primary Outcome
As shown in Fig. (2), sixteen studies [3, 8, 11, 13, 19, 20, 22-30] reported the effectiveness of catheter ablation for AF in 2839 patients. The relative risk of experiencing AF post CBA compared with RFA was 0.84 (95% CI: 0.65, 1.07) with medium heterogeneity detected among the studies (I2=48%, p=0.16).
Fig. (2).
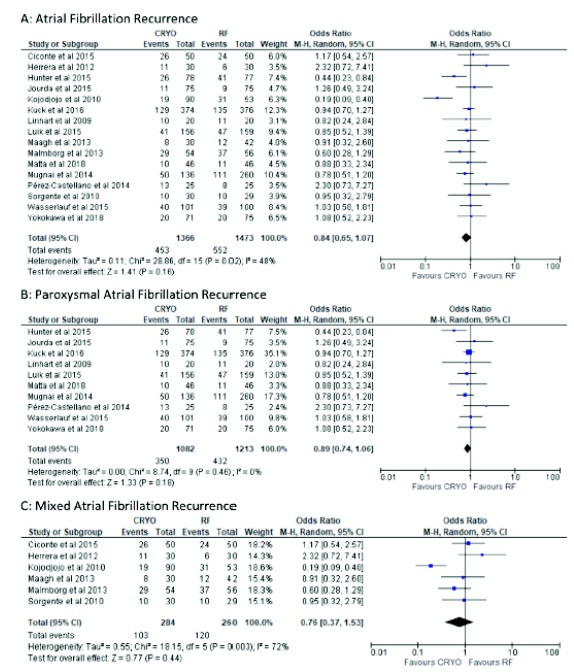
Forest Plot of incidence of recurrence for atrial fibrillation.
Subgroup analysis was performed to confirm the overall effect size and direction. Ten studies [8, 22-30] included patients with paroxysmal AF and six studies [3, 11, 13, 17, 19, 20] included mixed AF patients. The pooled effect did not differ between the two groups. However, sub-grouping was associated with a considerable reduction of the heterogeneity among studies performing paroxysmal AF (I2=0%, p=0.18; Fig. 2) and increase in heterogeneity in studies which included patients with mixed AF (I2=72%, p=0.003; Fig. 2).
3.3. Secondary Outcome
3.3.1. Procedure Time
The procedure time was reported in twenty-four studies [3, 8-30]. Pooling all the results, the procedure time was, on average, around half an hour lower with CBA in comparison to the RFA (MD=26.37, 95% CI: 14.55, 38.20) and the observed heterogeneity among studies was high (I2=96%, p<0.01; Fig. 3).
Fig. (3).
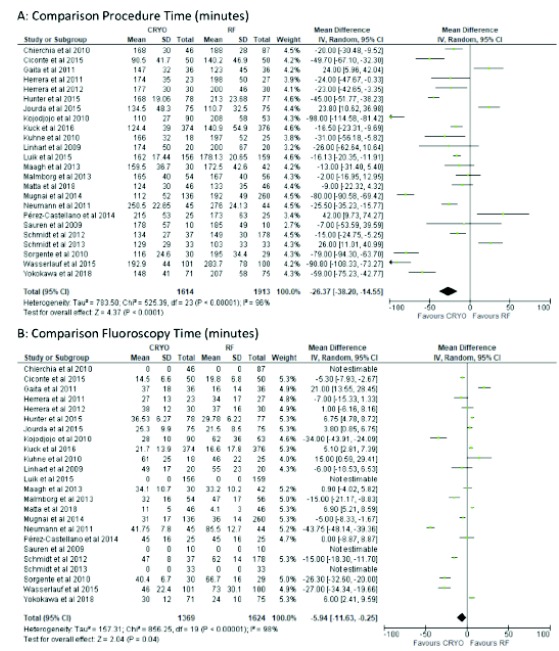
Forest plot of procedure and fluoroscopy time.
3.3.2. Fluoroscopy Time
The fluoroscopic time was reported in Twenty studies [3, 8, 11-20, 22-25, 27-30]. Pooling all the results, the fluoroscopic time was significantly lower in the CBA group compared with the RFA (MD=5.94, 95% CI: 0.25, 11.63) and the observed heterogeneity among studies was high (I2=98%, p<0.01; Fig. 3).
Complications:
3.3.3. Phrenic Nerve Palsy
Thirteen studies [3, 8, 11, 13, 16, 19, 20, 22, 24-27, 29] reported phrenic nerve palsy (PNP) in 1976 patients. The pooled relative risk of PNP was RR: 9.02 (95% CI: 3.92, 20.74) time higher in the CBA group, with no heterogeneity detected among the studies (I2=0%, p=<0.01; Fig. 4).
Fig. (4).
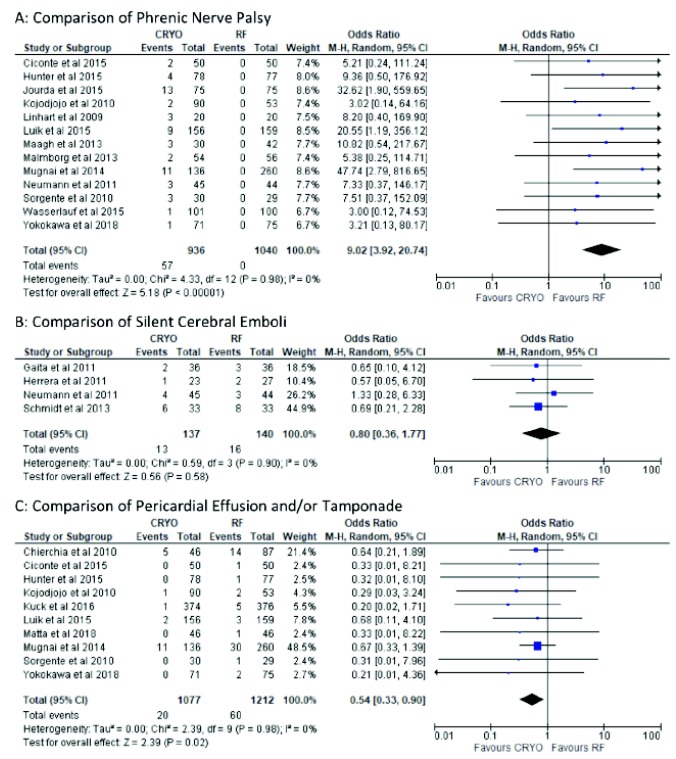
Forest plot of procedure related complications.
3.3.4. Silent Cerebral Emboli
Only four studies [14-16, 21] reported silent cerebrovascular emboli in 277 patients. Overall, 13 of 137 (9%) patients allocated to the CBA had silent cerebrovascular emboli compared with 16 of 140 patients (11%) allocated to RFA. The relative risk with CBA compared to RF was RR: 0.80 (95% CI: 0.36, 1.77), with no heterogeneity detected among the studies (I2=0%, p=0.58; Fig. 4).
3.3.5. Pericardial Effusion and/or Tamponade
Ten studies [10, 11, 13, 22, 24, 26, 28-30] reported pericardial effusion or tamponade in 2489 patients. The pooled relative risk of experiencing pericardial effusion with RFA compared with CBA was statistically significant (RR=0.54; 95% CI: 0.33, 0.90) with no heterogeneity detected among the studies (𝐼2=0%, p=0.98; Fig. 4).
Publication Bias:
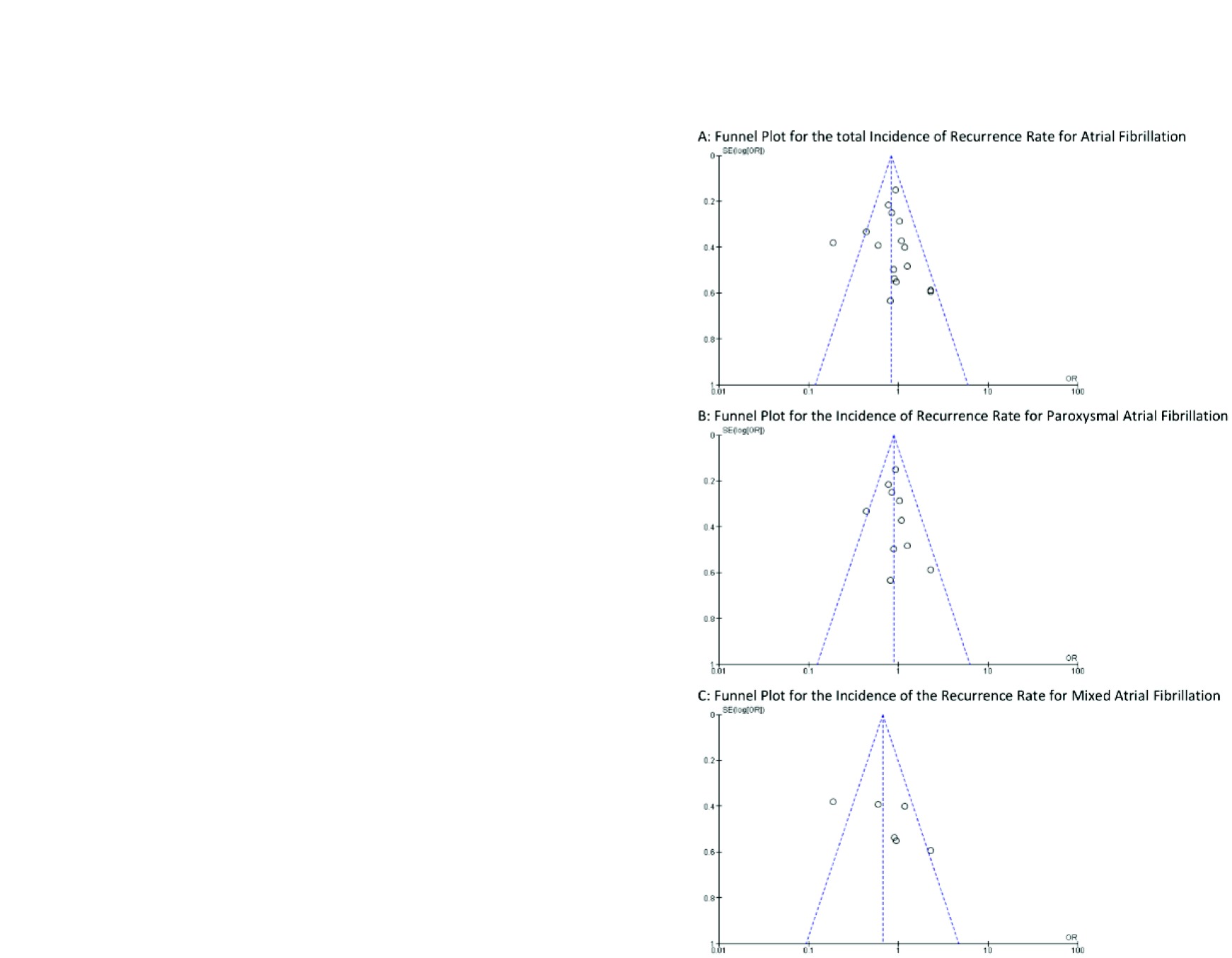
For primary outcome with CBA effectiveness we conducted a funnel plot for publication bias for each study (Fig. 5).
Fig. (5).
Funnel plot evaluating publication bias.
4. DISCUSSION
Catheter ablation therapy is the treatment of choice in drug-resistant AF [2]. It is achieved by pulmonary vein isolation (PVI) by one of two energy sources - the conventional RFA or the newer CBA. A number of studies have demonstrated similar effectiveness and safety of both of these approaches [31, 32]. In this meta-analysis, we have demonstrated that CBA is non-inferior in treating AF compared to RF with a lesser procedure and fluoroscopy time. This study demonstrated that there is no significant difference in recurrence rate of AF between the two groups with median follow up of 12 months.
CBA catheters are larger than conventional RFA catheters, almost as large as the pulmonary vein. They can apply energy in single application compared to multiple application by RFA catheters by covering larger areas with a more homogeneous ice cap formation resulting in faster achievement of PVI [33]. Due to its technical simplicity, the procedure and fluoroscopy time is significantly less with CBA, this is represented in our analysis as well. The novel third-generation CBA catheters have significantly shorter tips which making the procedure even more simplified with mean procedure time of 71 minutes with similar success rate, as it achieves PVI with a “single shot” [34]. In addition to the catheter features, operator experience also plays a vital role. Since RFA has been around for a longer duration and CBA is a relatively newer procedure, with greater operator experience over time, the procedure and fluoroscopy time will be even lesser among electrophysiologists using CBA.
A common complication of CBA is PNP with a rate of 13.5%, due to the proximity of phrenic nerve to pulmonary vein [35]; however, it is usually transient and not associated with increased mortality, morbidity or hospital stay [36]. In our analysis, PNP was exclusively present in the CBA group. In addition, pericardial effusion is a common complication of catheter ablations with an incidence of 14%. This complication increases with larger CBA catheters [36, 37]. However, Chierchia et al. showed that the occurrence of pericardial effusion after ablation is not significantly different between RFA and 28mm CBA. The higher incidence of pericardial effusion in some studies were not associated with an increased hospital stay or mortality [10]. We found that the frequency of pericardial effusion and cardiac tamponade were comparable between both groups.
The most disabling complication of the catheter ablation procedure is cerebral ischemia or stroke, however, asymptomatic or silent cerebrovascular emboli is common with an incidence of 11-14% and symptomatic events can occur in up to 1.8%-2.0% [16, 38, 39]. We did not find any significant difference in the incidence of silent cerebrovascular emboli in both groups; however, Gaita el al. showed that the risk of 1.48 times higher with duty-cycled radiofrequency generator than irrigated RFA or CBA [14].
A serious complication of artrioesophageal fistula can occur with an incidence of 0.1% to 0.25% after AF ablation [38], more commonly observed in RFA although it has been also reported with CBA [40, 41]. Pulmonary vein stenosis can also occur due to energy application, relatively with lower incidence in CBA of 3.1% [42].
5. LIMITATIONS
Our meta-analysis has several limitations. First, only seven trials were randomized which raises the question of selection bias and methods. Second, nearly all the studies were conducted in European countries and in predominantly males, so the conclusions may not be able to be generalized. Third, follow-up methods and surveillance of AF varied among the studies, therefore it is difficult to draw conclusions about the long-term effectiveness of the procedures in said studies. Fourth, the studies were significantly heterogeneous due to multiple factors. Some studies included mixed AF population but paroxysmal AF was predominant in CBA group compared to RFA, hence same outcome should not be applied to persistent AF. The operator experience varied between the studies and also limited information was available on structural heart disease which could greatly influence the success rate.
CONCLUSION
Our meta-analysis confirms that the effectiveness of CBA is similar to RFA in the treatment of AF with the added advantages of shorter procedure and fluoroscopy times. CBA is a viable alternative to RFA for the definitive treatment for drug-refractory AF.
ACKNOWLEDGEMENTS
The authors have no financial relationship with a commercial entity that has an interest in the subject of the presented manuscript or other conflicts to disclose.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
REFERENCES
- 1.Chugh S.S., Havmoeller R., Narayanan K., et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calkins H, Kuck KH, Cappato R, et al. Heart Rhythm Society Task Force on C and Surgical Ablation of Atrial F. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for patient selection, procedural techniques, patient management and followup, definitions, endpoints, and research trial design: A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012;9 doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 3.Ciconte G., Baltogiannis G., de Asmundis C., et al. Circumferential pulmonary vein isolation as index procedure for persistent atrial fibrillation: a comparison between radiofrequency catheter ablation and second-generation cryoballoon ablation. Europace. 2015;17:559–565. doi: 10.1093/europace/euu350. [DOI] [PubMed] [Google Scholar]
- 4.Pratola C., Baldo E., Notarstefano P., Toselli T., Ferrari R. Radiofrequency ablation of atrial fibrillation: is the persistence of all intraprocedural targets necessary for long-term maintenance of sinus rhythm? Circulation. 2008;117:136–143. doi: 10.1161/CIRCULATIONAHA.106.678789. [DOI] [PubMed] [Google Scholar]
- 5.Georgiopoulos G., Tsiachris D., Manolis A.S. Cryoballoon ablation of atrial fibrillation: A practical and effective approach. Clin. Cardiol. 2017;40:333–342. doi: 10.1002/clc.22653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Leblais V., Delannoy E., Fresquet F., et al. Beta-adrenergic relaxation in pulmonary arteries: Preservation of the endothelial nitric oxide-dependent beta2 component in pulmonary hypertension. Cardiovasc. Res. 2008;77:202–210. doi: 10.1093/cvr/cvm008. [DOI] [PubMed] [Google Scholar]
- 8.Linhart M., Bellmann B., Mittmann-Braun E., et al. Comparison of cryoballoon and radiofrequency ablation of pulmonary veins in 40 patients with paroxysmal atrial fibrillation: A case-control study. J. Cardiovasc. Electrophysiol. 2009;20:1343–1348. doi: 10.1111/j.1540-8167.2009.01560.x. [DOI] [PubMed] [Google Scholar]
- 9.Sauren L.D., Vanb Y., Der L., et al. Transcranial measurement of cerebral microembolic signals during endocardial pulmonary vein isolation: Comparison of three different ablation techniques. J. Cardiovasc. Electrophysiol. 2009;20:1102–1107. doi: 10.1111/j.1540-8167.2009.01509.x. [DOI] [PubMed] [Google Scholar]
- 10.Chierchia G.B., Capulzini L., Droogmans S., et al. Pericardial effusion in atrial fibrillation ablation: A comparison between cryoballoon and radiofrequency pulmonary vein isolation. Europace. 2010;12:337–341. doi: 10.1093/europace/eup422. [DOI] [PubMed] [Google Scholar]
- 11.Kojodjojo P., O’Neill M.D., Lim P.B., et al. Pulmonary venous isolation by antral ablation with a large cryoballoon for treatment of paroxysmal and persistent atrial fibrillation: Medium-term outcomes and non-randomised comparison with pulmonary venous isolation by radiofrequency ablation. Heart. 2010;96:1379–1384. doi: 10.1136/hrt.2009.192419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhne M., Suter Y., Altmann D., et al. Cryoballoon versus radiofrequency catheter ablation of paroxysmal atrial fibrillation: Biomarkers of myocardial injury, recurrence rates, and pulmonary vein reconnection patterns. Heart Rhythm. 2010;7:1770–1776. doi: 10.1016/j.hrthm.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 13.Sorgente A., Chierchia G.B., de Asmundis C., Capulzini L., Sarkozy A., Brugada P. Cryoballoon ablation of atrial fibrillation: State of the art 10 years after its invention. Recent Pat. Cardiovasc. Drug Discov. 2010;5:177–183. doi: 10.2174/157489010793351917. [DOI] [PubMed] [Google Scholar]
- 14.Gaita F., Leclercq J.F., Schumacher B., et al. Incidence of silent cerebral thromboembolic lesions after atrial fibrillation ablation may change according to technology used: Comparison of irrigated radiofrequency, multipolar nonirrigated catheter and cryoballoon. J. Cardiovasc. Electrophysiol. 2011;22:961–968. doi: 10.1111/j.1540-8167.2011.02050.x. [DOI] [PubMed] [Google Scholar]
- 15.Herrera Siklody C., Deneke T., Hocini M., et al. Incidence of asymptomatic intracranial embolic events after pulmonary vein isolation: Comparison of different atrial fibrillation ablation technologies in a multicenter study. J. Am. Coll. Cardiol. 2011;58:681–688. doi: 10.1016/j.jacc.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Neumann T., Kuniss M., Conradi G., et al. MEDAFI-Trial (Micro-embolization during ablation of atrial fibrillation): Comparison of pulmonary vein isolation using cryoballoon technique vs. radiofrequency energy. Europace. 2011;13:37–44. doi: 10.1093/europace/euq303. [DOI] [PubMed] [Google Scholar]
- 17.Herrera Siklody C., Arentz T., Minners J., et al. Cellular damage, platelet activation, and inflammatory response after pulmonary vein isolation: A randomized study comparing radiofrequency ablation with cryoablation. Heart Rhythm. 2012;9:189–196. doi: 10.1016/j.hrthm.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt M., Dorwarth U., Straube F., et al. A novel double cryoballoon strategy in persistent atrial fibrillation: A pilot study. Clin. Res. Cardiol. 2012;101:777–785. doi: 10.1007/s00392-012-0456-y. [DOI] [PubMed] [Google Scholar]
- 19.Maagh P., Butz T., Plehn G., Christoph A., Meissner A. Pulmonary vein isolation in 2012: Is it necessary to perform a time consuming electrophysical mapping or should we focus on rapid and safe therapies? A retrospective analysis of different ablation tools. Int. J. Med. Sci. 2013;10:24–33. doi: 10.7150/ijms.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malmborg H., Christersson C., Lonnerholm S., Blomstrom-Lundqvist C. Comparison of effects on coagulation and inflammatory markers using a duty-cycled bipolar and unipolar radiofrequency pulmonary vein ablation catheter vs. a cryoballoon catheter for pulmonary vein isolation. Europace. 2013;15:798–804. doi: 10.1093/europace/eus411. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt M., Dorwarth U., Straube F., et al. Cryoballoon in AF ablation: Impact of PV ovality on AF recurrence. Int. J. Cardiol. 2013;167:114–120. doi: 10.1016/j.ijcard.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Mugnai G., Chierchia G.B., de Asmundis C., et al. Comparison of pulmonary vein isolation using cryoballoon versus conventional radiofrequency for paroxysmal atrial fibrillation. Am. J. Cardiol. 2014;113:1509–1513. doi: 10.1016/j.amjcard.2014.01.425. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Castellano N., Fernandez-Cavazos R., Moreno J., et al. The COR trial: A randomized study with continuous rhythm monitoring to compare the efficacy of cryoenergy and radiofrequency for pulmonary vein isolation. Heart Rhythm. 2014;11:8–14. doi: 10.1016/j.hrthm.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 24.Hunter R.J., Baker V., Finlay M.C., et al. Point-by-point radiofrequency ablation versus the cryoballoon or a novel combined approach: A randomized trial comparing 3 methods of pulmonary vein isolation for paroxysmal atrial fibrillation (The Cryo Versus RF Trial). J. Cardiovasc. Electrophysiol. 2015;26:1307–1314. doi: 10.1111/jce.12846. [DOI] [PubMed] [Google Scholar]
- 25.Jourda F., Providencia R., Marijon E., et al. Contact-force guided radiofrequency vs. second-generation balloon cryotherapy for pulmonary vein isolation in patients with paroxysmal atrial fibrillation-a prospective evaluation. Europace. 2015;17:225–231. doi: 10.1093/europace/euu215. [DOI] [PubMed] [Google Scholar]
- 26.Luik A., Radzewitz A., Kieser M., et al. Cryoballoon versus open irrigated radiofrequency ablation in patients with paroxysmal atrial fibrillation: the prospective, randomized, controlled, noninferiority freezeAF study. Circulation. 2015;132:1311–1319. doi: 10.1161/CIRCULATIONAHA.115.016871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wasserlauf J., Pelchovitz D.J., Rhyner J., et al. Cryoballoon versus radiofrequency catheter ablation for paroxysmal atrial fibrillation. Pacing Clin. Electrophysiol. 2015;38:483–489. doi: 10.1111/pace.12582. [DOI] [PubMed] [Google Scholar]
- 28.Kuck K.H., Brugada J., Furnkranz A., et al. Investigators ICE. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N. Engl. J. Med. 2016;374:2235–2245. doi: 10.1056/NEJMoa1602014. [DOI] [PubMed] [Google Scholar]
- 29.Yokokawa M., Chugh A., Latchamsetty R., et al. Ablation of paroxysmal atrial fibrillation using a second-generation cryoballoon catheter or contact-force sensing radiofrequency ablation catheter: A comparison of costs and long-term clinical outcomes. J. Cardiovasc. Electrophysiol. 2018;29:284–290. doi: 10.1111/jce.13378. [DOI] [PubMed] [Google Scholar]
- 30.Matta M., Anselmino M., Ferraris F., Scaglione M., Gaita F. Cryoballoon vs. radiofrequency contact force ablation for paroxysmal atrial fibrillation: A propensity score analysis. J. Cardiovasc. Med. (Hagerstown) 2018;19:141–147. doi: 10.2459/JCM.0000000000000633. [DOI] [PubMed] [Google Scholar]
- 31.Khairy P., Chauvet P., Lehmann J., et al. Lower incidence of thrombus formation with cryoenergy versus radiofrequency catheter ablation. Circulation. 2003;107:2045–2050. doi: 10.1161/01.CIR.0000058706.82623.A1. [DOI] [PubMed] [Google Scholar]
- 32.Sarabanda A.V., Bunch T.J., Johnson S.B., et al. Efficacy and safety of circumferential pulmonary vein isolation using a novel cryothermal balloon ablation system. J. Am. Coll. Cardiol. 2005;46:1902–1912. doi: 10.1016/j.jacc.2005.07.046. [DOI] [PubMed] [Google Scholar]
- 33.Knecht S., Sticherling C., von Felten S., et al. Long-term comparison of cryoballoon and radiofrequency ablation of paroxysmal atrial fibrillation: A propensity score matched analysis. Int. J. Cardiol. 2014;176:645–650. doi: 10.1016/j.ijcard.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 34.Aryana A., Kowalski M., O’Neill P.G., et al. Catheter ablation using the third-generation cryoballoon provides an enhanced ability to assess time to pulmonary vein isolation facilitating the ablation strategy: Short- and long-term results of a multicenter study. Heart Rhythm. 2016;13:2306–2313. doi: 10.1016/j.hrthm.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Packer D.L., Kowal R.C., Wheelan K.R., et al. Investigators SAC. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: First results of the North American Arctic Front (STOP AF) pivotal trial. J. Am. Coll. Cardiol. 2013;61:1713–1723. doi: 10.1016/j.jacc.2012.11.064. [DOI] [PubMed] [Google Scholar]
- 36.Van Belle Y., Jordaens L. Reflections on reconduction after pulmonary vein isolation. Europace. 2009;11:400–401. doi: 10.1093/europace/eup057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaer B., Kuhne M., Koller M.T., Sticherling C., Osswald S. Therapy with an implantable cardioverter defibrillator (ICD) in patients with coronary artery disease and dilated cardiomyopathy: Benefits and disadvantages. Swiss Med. Wkly. 2009;139:647–653. doi: 10.4414/smw.2009.12668. [DOI] [PubMed] [Google Scholar]
- 38.Petersen P., Madsen E.B., Brun B., Pedersen F., Gyldensted C., Boysen G. Silent cerebral infarction in chronic atrial fibrillation. Stroke. 1987;18:1098–1100. doi: 10.1161/01.str.18.6.1098. [DOI] [PubMed] [Google Scholar]
- 39.Epstein M.R., Knapp L.D., Martindill M., et al. Embolic complications associated with radiofrequency catheter ablation. Atakr Investigator Group. Am. J. Cardiol. 1996;77:655–658. doi: 10.1016/s0002-9149(97)89327-7. [DOI] [PubMed] [Google Scholar]
- 40.Kawasaki R., Gauri A., Elmouchi D., Duggal M., Bhan A. Atrioesophageal fistula complicating cryoballoon pulmonary vein isolation for paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2014;25:787–792. doi: 10.1111/jce.12426. [DOI] [PubMed] [Google Scholar]
- 41.Barbhaiya C.R., Kumar S., John R.M., et al. Global survey of esophageal and gastric injury in atrial fibrillation ablation: Incidence, time to presentation, and outcomes. J. Am. Coll. Cardiol. 2015;65:1377–1378. doi: 10.1016/j.jacc.2014.12.053. [DOI] [PubMed] [Google Scholar]
- 42.Kuck K.H., Furnkranz A. Cryoballoon ablation of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2010;21:1427–1431. doi: 10.1111/j.1540-8167.2010.01944.x. [DOI] [PubMed] [Google Scholar]