Abstract
Three-dimensional (3D) printing, also known as additive manufacturing, was developed originally for engineering applications. Since its early advancements, there has been a relentless de-velopment in enthusiasm for this innovation in biomedical research. It allows for the fabrication of structures with both complex geometries and heterogeneous material properties. Tissue engineering using 3D bio-printers can overcome the limitations of traditional tissue engineering methods. It can match the complexity and cellular microenvironment of human organs and tissues, which drives much of the interest in this technique. However, most of the preliminary evaluations of 3D-printed tissues and organ engineering, including cardiac tissue, relies extensively on the lessons learned from tradi-tional tissue engineering. In many early examples, the final printed structures were found to be no bet-ter than tissues developed using traditional tissue engineering methods. This highlights the fact that 3D bio-printing of human tissue is still very much in its infancy and more work needs to be done to realise its full potential. This can be achieved through interdisciplinary collaboration between engi-neers, biomaterial scientists and molecular cell biologists. This review highlights current advance-ments and future prospects for 3D bio-printing in engineering ex vivo cardiac tissue and associated vasculature, such as coronary arteries. In this context, the role of biomaterials for hydrogel matrices and choice of cells are discussed. 3D bio-printing has the potential to advance current research signif-icantly and support the development of novel therapeutics which can improve the therapeutic out-comes of patients suffering fatal cardiovascular pathologies.
Keywords: Cardiac tissue engineering, vasculature network, 3D bio-printing, biomaterials, micro-environment, cardiovascular diseases
1. INTRODUCTION
Adult cardiac muscle cells (cardiomyocytes) are considered to be terminally differentiated and are not capable of proliferation [1]. Hence, mammalian cardiac tissue has limited regenerative capabilities following any damage caused by a myocardial infarction. This is due to the limited capability of reactivating the cellular functions required for normal proliferation. However, several studies have shown that cardiomyocytes have the ability to dedifferentiate and proliferate into active cardiomyocytes. To date, natural tissue regenerative capacity has shown to be insufficient to repair scar damage in a severely damaged heart. Reports also suggest that the adult mammalian heart contains its own bank of progenitor cells, which could be used for tissue regeneration [2]. Adult heart cells do not multiply and are seen to exit the cell multiplication machinery immediately after birth. Furthermore, the proteins essential for cellular specialisation are considered to be accumulated inside the cells which drive them to their final form and required functions. In general, reciprocity exists between cell proliferation and differentiation i.e. cells, which rapidly proliferate but do not differentiate, and terminally differentiated cells that cannot proliferate [2].
Cardiac regeneration is a complex biological process, which not only requires the structural and functional recreation of myocardial tissue, including stroma, vessels, extracellular matrix and the conduction system, but also needs strict control over the various signalling cascades and check points. Development of thick cardiac tissue in vitro without vessels is the biggest challenge. However, the recent use of 3D printers in tissues and organs allows for the fabrication of structures with complex spatial arrangements with various biomaterials (cellular and non-cellular), which have revolutionised the field of tissue regeneration. The application of this technology to biological structures that matches the native tissue complexity is of great interest to researchers. Research work in 3D cardiac tissue and vessel regeneration could extend our capabilities of developing multi-material 3D cardiac tissue with vessels. In the future, this will not only bring us one step closer to creating complex 3D cardiac tissue architecture for tissue repair and regeneration, but also for transplantation. In this review, current progress of 3D printing for fabricating artificial cardiac tissues and vessels has been discussed. Here, we reviewed the most recent information concerning the capacity of 3D bio-printers in managing cardiovascular disorders.
2. 3D BIO-PRINTING
3D printing, also known as additive manufacturing, was initially developed as an industrial application, but it is now an important technology used for tissue engineering (3D bio-printing). Bio-printing for 3D biofabrication is a process of arranging cellular and extra-cellular matrices in space to mimic the anatomy and physiology of tissue. 3D bio-printing possesses the promise to fabricate physiologically complex tissues or organs with digital control. Computerised control over the printing process with a high throughput outcome makes 3D bio-printing unique when compared with classical 2D tissue engineering. Specificity and selectivity makes this technology ideal for basic and translational research. Other than tissue engineering, it has the potential application for drug delivery, clinical and preclinical research and drug discovery [3]. 3D bio-printing possess the capacity for fabricating tissues and organs through bottom up or top-down approaches.
In top-down approaches, cells are homogenously suspended in biomaterials often shaped to look like biological morphologies. On the other hand, in bottom-up approaches, singular units of cells and biomaterials are combined to form the desired tissue morphology. Although a top-down approach has been in use for many years, it not only often fails to control cell distribution in the scaffold, but it has also been seen to be inappropriate for arranging extracellular matrix in the scaffold [4]. In a top-down approach, cells are expected to populate in the scaffold and develop their own extracellular matrix. However, an extracellular matrix, which is the key for the survival of the cell, is often inappropriately organized and hence the cell cannot function as tissue fabricated by a top-down approach. The bottom down method has a much greater potential to address this issue by building up the tissues in a layer by layer approach. A bottom down method focuses on engineering nanoscale tissue building blocks with a predesigned microarchitecture and assembling all nano unites to form larger tissue constructs [5].
3. BIO-PRINTING TECHNIQUES
To date no bio-printing technique enables the production of tissues and organs with anatomical and physiological complexities. Four major bio-printing techniques viz inkjet, laser-assisted, extrusion bio-printing and stereolithography are available with their specific strengths, weaknesses, and limitations.
3.1. Inkjet Bio-printers
Inkjet bio-printers, also known as the drop-on-demand printers, are the modification of a conventional 2D inkjet printer. 3D injet printers use a thermal, piezoelectric or electromagnetic force and non-contact technique to deposit droplets of bioinks on the surface [6]. It was observed that inkjet bio-printing methods could reduce cell viability due to the high localised temperature at the nozzle which sometimes increases to 300 °C [7]. However the exposure of bioink to a higher temperature is extremely short (∼2 μs) and could only increase the temperature to not more than 10°C in extreme cases. Studies have demonstrated that short exposure to the high temperature does not significantly affect the mammalian cell viability [8]. High speed and relatively low cost are the key advantages whereas; a lack of uniformity in droplet size and their placement are the key disadvantages. The need for less viscous bioink in an injet printer eliminates the possibility of it being used with several viscous bioinks which offer the advantage of better cell survival.
3.2. Microextrusion 3D Bio-printing
Microextrusion 3D bio-printing uses mechanical or pneumatic forces to deposit cell leaden bioink through a nozzle [9]. Unlike injet bio-printers which produce droplets, microextrusion bio-printers produce a continuous stream of materials. This stream of materials (bioink) is controlled by the computer program connected to the microextrusion 3D bio-printers. The key advantages of microextrusion 3D bio-printers are the compatibility with viscous bioinks and they can also print with very high cell densities. One of the major disadvantages is the use of high pressure to expel the bioinks which sometimes could distort the cellular morphology and even reduce the cell viability [10].
3.3. Laser-assisted 3D Printing
Laser-assisted 3D printing was initially developed for high resolution metals patterning required for electronic chip manufacturing. Laser-assisted 3D bio-printing is a nozel free printing with no surface contact [11]. Laser-assisted 3D bio-printing utilized a lazer pulse to create and deposit the bioink droplet on to the surface. The key benefit of this printing method is the high degree of precision and resolution which enables the patterning of peptides, DNA, and cell arrays [11]. Other major advantages are the ability to print tissues of very high cell density; no viscosity limitations or clogging issues; and finally, the retention of cellular phenotypes during and after printing. One of the major limitations of laser bio-printing is its relatively lower cellular viability when compared with other printing methods.
3.4. Stereolithography
Stereolithography works by focusing an ultraviolet (UV) light or laser beam on to a vat of photopolymer resin [12]. In stereolithography, objects are created by selectively photocuring the photosensitive polymer layer by layer. UV light or a laser beam follows the computer directed patterns programmed on CAD software onto the surface of a light sensitive polymer vat. This technique is particularly useful when curable acrylics and epoxies are used as the photopolymerizable material. The main drawback of stereolithography is the use of intense UV radiation for the cross-linking process. Other limitations are the lengthy post-processing time requirement and the relative few materials that are compatible for use with Stereolithography [12]. A comparative analysis of these approaches is also provided in Table 1 [13, 14].
Table 1. A comparative analysis of 3d-bioprinting techniques.
| Parameter | Bio-printing Technique | |||
|---|---|---|---|---|
| Inkjet | Laser assisted | Extursion | Stereolithography | |
| Cost | Low | High | Moderate | Low |
| Print speed | 1 to 10000 droplets /sec | 200 – 1600 mm /sec | 10-50 µ/s | 0.3 – 0.7 in/hr |
| Accuracy | Medium | High | Medium–low | High |
| Resolution | 50 μm | 10 μm | 100 μm | 100 μm |
| Cell viability | >85% | > 95% | 40% – 80% | > 85% |
| Material Viscosity | <10 mPa s* | 1-300 mPa s* | 30–6 × 107 mPa s* | No limitation |
| Advantages | High speed. Availability. Low cost. Ability to introduce concentration gradients in 3D constructs. |
High degree of precision and resolution. Ability to use high viscosity bioink and print high cell density. A nozzle-free, noncontact process; cells are printed with high activity and high resolution. High degree of control of ink droplets and precise delivery. |
Ability to use high viscosity bioink. Print high cell density. |
High degree of fabrication accuracy, and low printing time. Solid freeform and nozzle-free technology. Highest fabrication accuracy. Compatibility with an increasing number of materials. Light-sensitive hydrogels can be printed layer-by-layer. |
| Disadvantages | Lack of precision in droplet placement and size, need for low viscosity bioink. | Time consuming, and high cost. | Distortion of cell structure. | Use of high intensity UV light, lengthy post-processing, lack of compatible materials. |
| Operating Principle | Thermal, piezoelectric, or electromagnetic forces expel successive drops of bioink onto a substrate. | Bioink and cells are suspended on the bottom of a ribbon and when vaporized by a laser pulse, they are propelled onto a receiving substrate. | Mechanical or pneumatic forces dispense bioink through a nozzle. | Use digital light to cure bioink in a layer by layer fashion. |
*Millipascal-second.
4. 3D BIO-PRINTING OF CARDIAC TISSUES
Inefficiencies in both the contraction and relaxation of the cardiac muscle leads to ineffective cardiac output. The resultant decreased output gives rise to cardiac ischemia which is now one of the leading causes of death worldwide. As discussed above, restricted cardiomyocyte regeneration and shortage of suitable organs for allotransplant is a major cause of concern. Cardiac tissue regeneration has been an intense area of research for tissue engineers. One of the major concerns of cardiac tissue regeneration is the formation of a complex micro-vasculature within the tissue for efficient blood and nutrient supply.
Moreover, in cellular therapy, the damaged heart tissue could be repaired by implanting cells at the damaged site. However, the effectiveness of this approach depends upon cell survival at the site of implementation and how well the cell tissues integrate with the implanted cells and vice versa. Cell therapy alone is not sufficient to regenerate the physiological function of the heart tissue, and hence techniques of tissue engineering and more recently 3D bio-printing are being explored.
Efficient transport of the oxygen to the cells of in vitro generated tissue could be achieved by either limiting the thickness to <200 μm and/or by integrating open pores or channels to facilitate the oxygen to reach the distant cells by simple diffusion or by convective mass transport [15]. One of the major disadvantages of restricting the thickness in vitro, is that several such constructs are needed to implant surgically in time gaps. A time gap is also essential because of the development of the proper vascularisation by the hosts which needs time in terms of days [16]. This issue was tried to be solved by integrating the vascular system inside the tissue before implantation and the method was able to develop tissue of 100 μm thickness [17].
The method of integrating pores or channels into the engineered tissue looks promising but ultimately the cellular density equal to natural tissues is still not achievable. Less cellular density in the construct is one of the limiting causes of achieving the desired physiological heart function. Increasing the cellular density is not the solution to the problem, as increased cellular density increases the cellular metabolism for which an increased supply of oxygen, nutrients and blood supply is required. Higher cellular densities also lead to the increased metabolic products in the surrounding area which needs to be relocated to avoid cell damage. However, this is difficult to achieve. Firstly due to the vascular system needed to move oxygen and nutrients to the cells and metabolic products away from the cells that are embedded inside the tissue. Secondly, cardiomyocytes do not proliferate, so the use of stem cells is needed if sufficient densities of cardiomyocytes are not seeded initially. 3D bio-printing has the ability to overcome this issue by virtue of its capability to uniformly disperse the cells to match the density of the natural tissues. Current methods are not efficient enough to recapitulate the complex vascularisation, geometry and self-renovation of the organ. Kolesky et al, in order to overcome the drawbacks associated with traditional tissue engineering methods, proposed the novel 3D printing of the thick tissue with a complex vascular pattern [18]. Kolesky et al. were able to arrange cells, extracellular matrices, vascular systems in a growth stimulated micro-environment which helped the engineered tissue to survive for an extended period of time [18]. The tissue constructed was of 1 cm in thickness and could be perfused onto a chip for long periods of time (>6 weeks). Notably Kolesky et al. was able to assimilate hMSCs and human neonatal dermal fibroblasts (hNDFs) within a tailored extracellular matrix along with an embedded vascular system, which was subsequently lined with human umbilical vein endothelial cells (HUVECs). Furthermore, the cell (hMSCs to an osteogenic) of this thick tissue was actively stimulated with growth factors to get the sufficient survival time in vitro [18].
Unlike Newt and Zebrafish hearts, which could regenerate cardiac tissue, mammal cardiomyocytes cannot regenerate after being damaged. The damage caused was tried to be reversed by using the reactivation of fetal genes in the adult failing heart. As transcriptional, posttranscriptional and epigenetic factors regulated the fetal genes, reactivation using a manipulation of these factors to date has shown limited success. However, as discussed, cardiomyocytes can proliferate and regenerate by dedifferentiation, which is somehow negatively regulated by various proteins. For example, p38 MAP kinase is one of the most important negative growth regulators of cardiac tissue [19]. MKK3bE which is an activator of the p38 has been found to hamper the fetal cardiomyocyte proliferation whereas cardiac-specific p38 knockout mice have shown an increase in neonatal cardiomyocyte mitoses. Both, growth factors and p38 inhibitors could be used in in vitro (in a traditional way) or a 3D bio-printed cardiac tissue engineering method.
This approach could be further supplemented with the proper maintenance of a microenvironment [19]. Similarly, cell cycle regulators/promotors like Cyclin D2 were exploited to enhance the DNA synthesis in cardiomycytes [20]. Although these studies presented indirect proof of proliferation (increased DNA synthesis and mitosis), the clear evidence suggests that adult cardiomyocytes proliferate is still missing [20]. Integration of molecular biology techniques to block p38 or an addition of cycle promoters to the cardiac cells, or use of stem or progenitor cells along with ultra-modern additive printing techniques for scaffold preparation could be the future of cardiac tissue regeneration. Biomaterial mesh devices such as a scaffold act as a mechanical support for the cells of the heart tissue. For example, Yeong et al. used a selective laser sintering printing (SLS) technique to fabricate a porous scaffold with poly(caprolactone) (PCL). This scaffold was porous enough (85% porosity) to ensure enough mass transport inside the tissue [21].
Recently, for spontaneous and synchronous muscle contraction, Wang et al, constructed dense cardiac tissue that could mimic the structural, physiological, and functional features of native myocardium [22]. Neonatal rat ventricular cardiomyocytes were isolated and used to construct cardiac tissue using an in-house developed integrated tissue-organ printing system. For the cardiac tissue constructs, cell-laden fibrin-based composite hydrogel, sacrificial hydrogel, and poly(ε-caprolactone) (PCL) was used separately. The most important observation of the study is the formation of dense and uniformly aligned heart muscle bundles, elongated α-actinin positive cells and the formation of a clear sarcomere structure [22].
Another recently reported approach for 3D bio-printed cardiac tissue includes the use of HUVECs and iPSC-derived cardiomyocytes [23]. A HUVECs and iPSC-derived cardiomyocytes approach is exciting since bone marrow, adipose tissue derived-mesenchymal cells, skeletal myoblasts, embryonic stem cells and resident cardiac stem cells have been tested to treat myocardium injuries with limited success. The major limitation observed was that of the inadequate physiological integration of immature cardiomyocytes which is essential for the rhythmic and spontaneous contraction of the cardiac tissue [24, 25]. Pluripotency of iPSC cells offers the distinct advantage of overcoming the ethical concerns. On the clinical front, iPSC can be derived from adult patients’ own cells, have unlimited proliferation capacity, and they can be differentiated into any type of tissue which could be crucial for future research.
Biomaterials are an important component of 3D bio-printing. Blending of several biomaterials for the better biological and mechanical properties is the recent trend. Jia et al. presented a unique strategy for generating a properly organized, perfusable vascular construct using a simple one-step advance extrusion based 3D bio-printing technique. The combination of polymers used were gelatin methacryloyl, sodium alginate, and 4-arm poly(ethylene glycol)-tetra-acrylate along with a multi-layered coaxial extrusion system. This blend of polymers was then crosslinked using calcium ion to form the stable construct. Cell leaden bioink that was based on these polymers was found to have the required rheological properties with enough mechanical strength sufficient for the bio-printing process [26]. The bioink was also found to support the proliferation of loaded endothelial and stem cells in the construct, leading to the formation of mechanically and biologically relevant and highly perfusable vessels.
The use of biomaterials faces some serious challenges, such as the toxicity of degraded biomaterials, fibrous tissue formation and immunogenicity, that could affect the long-term viability of the fabricated tissue. To address these challenges, Ong et al. developed a unique biomaterial-free 3D bio-printing of cardiac tissue using human iPSC derived cardiomyocytes, fibroblasts and endothelial cells [27]. All these cells were aggregated to form the mix cell spheroids. Cardiac patches were created later using a 3D bio-printing technique that had different cell ratios. Cardiac patches were not only found to beat spontaneously, but were also found to exhibit the proper generation of electrochemical waveforms and conduction systems. The most important was the immunofluorescence observation which confirmed the presence of the cardiac gap junction protein Cx43. This could be considered as a major step forward towards the generation of more versatile cardiac tissue that has limited drawbacks. A few other notable works in cardiac tissue patches which are worthy of mention here are first, the cardiac patch composed of cardiac-specific extracellular matrix and progenitor cells for heart repair; and second, a UV-assisted 3d bio-printing of nanoreinforced hybrid cardiac patch for myocardial tissue engineering [28, 29].
The replacement of necrotic heart tissue with the cardiac tissue patch is theoretically a viable alternative. Such a patch could assist in contraction; however, their functionality can be improved further with the help of contractile cells. Unfortunately, contractile cardiomyocytes have negligible proliferation rates. Stem cell solutions are currently being sought to generate sufficient functional cardiomyocytes for this application. One important feature, along with the use of stem cells which could improve the functions of the cardiac tissue, are the channels and grooves on the bio-printed tissues. Channels and grooves could positively influence the morphology and physiological functions of cells; such an effect is known as topological or surface guidance. Tijore et al. prepared a cell aligning hydrogel microchannel scaffold using a 3D extrusion bio-printing method (Fig. 1) [30]. This team also studied the effect of different widths on cell morphology and its functions. The channels and the groves on the surface were found to positively affect the synchronized beating heart. A possible application of this technique not only includes the in vitro cardiac tissue modelling for myocardial regeneration, but also for cardiac cytotoxicity studies.
Fig. (1).
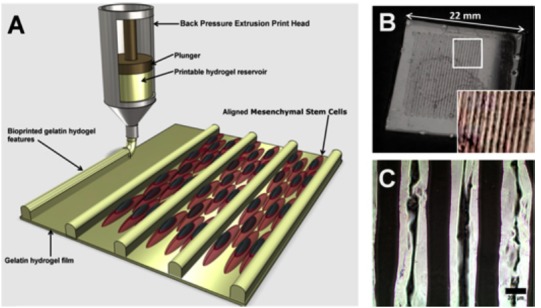
3D bioprinting and characterization of a gelatin hydrogel features. (A) Schematic representation of the process. (B) Image of 3D patterned gelatin hydrogel. (C) Bright field microscope image of bioprinted gelatin pattern on gelatin film coated coverslip [30].
One other challenge associated with polymeric biomaterials used in cardiac tissue bio-printing is the poor conduction of electrochemical signals between the cells. Zhu et al. developed a gold nanorod that incorporated a gelatin methacryloyl polymer based bioink for printing functional cardiac tissue [31]. This low density bioink was not only found to support cell integration, but was also found to improve the cell-to-cell coupling and support synchronized tissue contraction.
Oxygen and nutrient supply to the cells of construct is another major challenge [32, 33]. Proper vascularization could be the key to the development of 3D bio-printed tissues and organs. To solve the issue of nutrient and blood supply, scientists have turned their attention to nature and first evidence of this comes from the work recently published by Gershlak et al. [34]. In this work spinach leaves were converted to a beating human heart tissue. This ground breaking work, has exploited the similarities between the vascular structure of plant and animal tissues and replaced the plant cells (spinach leaves) with the heart and vessel cell to get the beating heart [34]. Spinach leaves were first decellularised leaving behind the cellular scaffold made up of the cellulose. The cellulose skeleton remained after decellurisation was used as a prevascularised natural scaffold for cardiac tissue engineering and was found to conduct the micro-particles (Fig. 2). The cellulose scaffold thus formed was then used to form the vasculature network by recellularising it with human endothelial cells. After recellularising the scaffold with human endothelial cells, vacant spaces were filled with cardiomyocytes obtained from human mesenchymal stem cells and human pluripotent stem cells. This study shows the potential for a new branch of science that investigates lessons learned from the plant kingdom and its application in improving the overall health of the patients. Overall, all of these new approaches cannot only help to improve our understanding of the pathophysiological conditions, but could serve the most important need of tissue engineering.
Fig. (2).
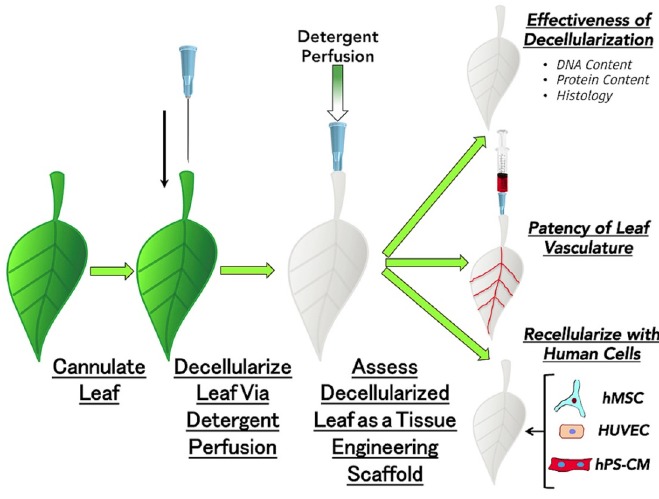
Recellularization of spinach cellulose skeleton vasculature network with human endothelial cells [34].
At present, bio-printed cardiac tissue is nowhere near the structural and functional substitute of the natural cardiac tissue. Scaffold and vasculature related research which are discussed here indicate a gradual progression in 3D printing of cardiac tissue with uniformity in the use of materials and printing methodology. Advancement in biomaterials and printing methodology could ultimately lead to the enhanced synchronous contraction, relaxation and optimum oxygen supply in the later stages of cardiac tissue development.
5. 3D BIO-PRINTING OF BLOOD VESSELS
Irrespective of the size and shape, almost all tissues and organs need blood vessels to work properly. A perfusable blood vascular networking 3D dense cardiac tissue is a prerequisite for the maintenance of the proper physiological function and extended survival. If cardiac tissue engineering has to reach the fruitful therapeutic application, the thickness of the engineered tissue must cross the limit of 100–200 μm. Tissue engineering must overcome the challenge of creating the blood vasculature network to supply cells with oxygen and nutrients and to remove the waste products. One of the possible solutions and a common technique for the introduction of a vascular network into the engineered tissue is co-culturing the progenitors of heart cells / cardiomyocytes with vascular endothelial cells [35]. Vascular endothelial cells could form a capillary network similar to that of an in vivo heart when they are co-cultured with cardiac cells or cardiac progenitor cells, fibroblast and myoblast. Levenberg et al. demonstrated the transport system by engineering 3D vascularised skeletal muscle constructs from myoblasts, fibroblasts and endothelial cells [36].
A large number of the thick tissue engineering is based on research that took place in the angiogenesis. Angiogenesis could be stimulated in the engineered tissue by using the angiogenesis stimulation molecules like vascular endothelial growth factor (VEGF) at the required site. However, when VEGF was used alone it was observed that the newly formed vascular network was largely disorganised and leaky [37]. Such leaky and unorganised vessels are not suitable to meet the physiological and metabolic demands. To overcome this serious issue, a cocktail of the molecules essential for the angiogenesis and vasculature network maturations needs to be delivered at the required site. The molecules which could support the angiogenesis process include VEGF, placental growth factor (PlGF), Ang1, platelet-derived growth factor-BB (PDGFBB), and transforming growth factor β (TGF- β) [38]. The sequence of the administration of these molecules could also be vital for the angiogenesis process, for example. Richardson et al. has shown that the PDGF-BB delivery after the VEGF using a controlled released device inside the skin and in a hind limb ischemia model induces the formation of the new mature blood vasculature with a coat of smooth muscle cells of the appropriate thickness [39]. Delivery of such molecules in the specific quantity is one of the barriers in therapeutic angiogenesis. One possible solution to overcome this issue is the site specific generation of such molecules by regulation of the transcription factors which controls the expression of the gene. For example, regulation of the transcription factor hypoxia-inducible factor 1α is now under consideration for the ultimate regulation of the angiogenicgenes [38].
Vascularisation, as discussed above, could be achieved by providing the essential cellular components (endothelial cell) directly onto the engineered construct. The cellular component could then induce the host vasculature to generate the new blood capillaries from it. Survival of the vascular system is found to be increased after the introduction of the human telomerase reverse transcriptase or the anti-apoptotic Bcl2 gene into endothelial cells [40]. Such manipulations are sometimes oncogenic in nature. Complementation of the endothelial cells with other cells like perivascular cells in the constructs can lead to the formation of a more robust blood vasculature network [41]. Perivascular cells are important because several studies have shown that they express various angiogenic factors [38].
Levenberg et al. combined the co-culture of myoblasts, endothelial cells and embryonic fibroblasts which allows for the creation of vascularised skeletal muscle tissue. In another experiment, Levenberg et al. pursued the importance of embryonic fibroblasts, a perivascular cell precursor, by adding it to the myoblasts and endothelial cells where it was observed that the perivascular cell is a crucial partner of the endothelial cell in forming healthy blood vessels which was mediated via VEGF expression. This finding proves that perivascular cells not only provide the physical support for endothelial cells, but also release growth factors essential for angiogenesis. One other important notion concluded that the pre-vascularisation is not only essential to reduce apoptosis, but it is also essential for increased blood supply. Collectively, Levenberg et al. demonstrated the benefits of pre-vascularisation, micro-environment, co-culturing and tri-culturing for engineering larger pieces of tissue [36].
Tissue engineering is moving closer to being investigated in clinical trials. A notable challenge now is the selection of the proper stem cell and its source. Human embryonic stem cell–derived endothelial cells and HUVECs could be the potential stem cells for the vascular network. HUVECs are terminally differentiated vascular endothelial cells with a limited proliferation capacity, whereas embryonic stem cells may be a rich source of easily expandable bipotential vascular—endothelial and perivascular cell—precursors [42]. Adult tissues, such as bone marrow, blood or fat, may be additional sources of vascular precursor cells [43]. The obvious question is which types of endothelial cells and endothelial or perivascular cell precursors can perform in engineered-tissue experiments? It is known that several phenotypes of endothelial cells exist depending on vessel type and organ. This essentially makes it compulsory to select the tissue-specific endothelial cell progenitors. One important thing to be noted here is the therapeutic angiogenesis and pathological angiognesis that have striking similarities. A better understanding of vessel stabilisation and maturation in the context of malignant tissues will likely benefit both the delivery of therapeutics to tumours and tissue engineering for regenerative medicine [44].
The tissue engineering approach of Levenberg et al. is essential to fabricate the thicker cardiac tissue which can be coupled with a 3D printer to arrange the desired spatial arrangement of various cells in a 3D construct. For example, Bertassoni et al. has made progress towards the fabrication of blood vessels for tissue engineering using 3D bio-printing technology and have tried to address the challenge of vascularisation of the complex bioengineered tissue by presenting an exclusive approach for vascularisation of a hydrogel construct that combines advances in 3D printing technology and biomaterials [45].
The common manufacturing approach of blood vessels includes many aspects such as, induced formation, the removal of sacrificial materials and direct construction. Induced blood vessel formation is based on the angiogenesis principle. This could however sometimes lead to unlimited proliferation [46]. Direct formation allows the vessel printing directly from bioinks but lacks the precision. On the other hand a sacrificial method is more flexible with better control over the operation [46]. In this method, vessels that are formed from the sacrificial materials, are coated with hydeogels loaded with the cells. After crosslinking, sacrificial materials are then removed using the appropriate solvents [47]. One of the major limitations of this approach is that it is difficult to control the precise distribution of the cells in thick tissues.
Higher blood vessels are more complicated as they are formed with up to 3 different layers. Several different approaches for the manufacturing of blood vessels are reported in the literature. Notably the drop-on-demand approach proposed by Schöneberg et al. to generate a blood vessel model was unique in terms of the presence of all three layers of higher vessels i.e. tunica intima, tunica media and tunica adventitia [48]. Similarly, in order to address the manufacturing challenges of blood vessels, Xu et al. developed a novel strategy that employs a custom built 3D bio-printer that used bio-compatible polymers. This team biofabricated blood vessels with a hierarchal arrangement that had more flexibility and a more precise control over the thickness of the wall [46]. Whereas the Qia et al. approach of fabricating the blood vessel was based on the sacrificial method. Sucrose was used as the sacrificial material to make the vascular base. A spraying process was then used to coat the sodium alginate and polyvinyl alcohol gel on the vascular base and later it was cross-linked in a CaCl2 solution. Sucrose was then sacrificed in the aqueous solution. Good internal connectivity of the blood vessel was the highlight of this work [49].
Finally, extensive application of the 3D bio-printed vessels is not only limited to the increase in the viability of 3D bio-printed tissues and organs, but in the near future such blood vessels could play a crucial role in the drug development process. For example, such blood vessels could serve as the thrombus model in blood pathological studies and for clinical and preclinical screening of drugs in vitro.
6. 3D BIO-PRINTING OF CORONARY ARTERIES
Plaques in a coronary artery reduce the blood flow to the heart muscles and increase the risk of a heart attack or a stroke. Depending upon the percentage of the artery blockage, health care providers manage the symptoms by lifestyle modification, prescriptions (e.g. antiplatelet agents, statins, PCSK9 antibodies, etc.), angioplasty or by coronary artery bypass grafting (CABG) [50]. CABG is a type of surgery that improves blood flow to the heart by diverting the blood flow around a section of the severely narrowed or the blocked artery using grafts harvested from other parts of the body (e.g. internal thoracic arteries, radial arteries, or saphenous veins). CABG elevates the symptoms associated with blocked arteries and improves the survival rate. However, many patients are not eligible for the procedure because of the unavailability of the suitable autologous vessels. Artificially grown human arteries known as Tissue Engineered Vascular Grafts (TEVGs) could be the big breakthrough that could revolutionise CABG [51]. TEVGs not only have the potential to meet the clinical challenges of the coronary artery bypass and peripheral vascular surgery, but they could be equally useful to patients on haemodialysis who are often on the grafts made of plastics.
The main synthetic material used to fabricate the graft is expanded poly(tetrafluoroethylene) (ePTFE) and woven poly(ethylene terephthalate) (PET), commonly known as Dacron. PTFE is the non-biodegradable inert fluorocarbon polymer made more microporous by extrusion and sintering to form an expanded PTFE (ePTFE) [52]. One of the major disadvantages associated with a graft made up of ePTFE is the poor compliance and the lack of endothelial cells on the inner lining of the lumen which contributes to its poor patency. Dacron on the other hand is a type of multi filament poly(ester) either woven or knitted into vascular grafts [53]. In vivo results of the grafts made up of these classic materials, particularly low flow grafts are not encouraging. Some reports have shown that the human umbilical vein can be used as a biological graft with patency rates of 46% between 3— 13 months [54]. Along the same line Vrandecic et al. has used a Bovine Internal Mammary Artery (BIMA) graft in the surgical treatment of small arteries [55]. Perloff et al. implanted two glutaraldehyde-tanned poly(ester) mesh supported sheep connective tissue tubes (6mm in diameter) into the left and right main coronary artery bypasses in one patient [56]. They confirmed the patency angiographically at 19 months. The patency rate of the biological grafts were observed to be no better than PTFE grafts and were also found to undergo dilatation and degenerative changes [57].
After limited success with the PTFE, PET and biological grafts, the research focus has now shifted towards the development of more bio-compatible materials. The current research is now mostly focused on embedding synthetic grafts with endothelial cells, antithrombic drugs (heparin coated grafts) or the development of new biomaterials. This approach also includes embedding synthetic grafts with dipyridamole, hirudin, tissue factor pathway inhibitors or non-thrombogenic phospholipid polymers [58]. Traditionally, synthetic materials could not satisfy the requirements of the ideal prosthetic conduit. In the search for synthetic biomaterials, poly(urethane)s were studied for their utility in designing the artificial coronary graft. Poly(urethane)s were found to be more compatible than the PTFE and Dacron, due to their better mechanical and flow parameters [59].
Over the next few years, improved biomaterials will almost certainly be available for the optimised graft function. Improved biomaterials could be best supplemented with modern tissue engineering and 3D bio-printing techniques. 3D bio-printing is a promising approach to fabricate the patient specific grafts. Vascular networks, made from stem cells with the help of 3D bio-printers, will undoubtedly be the major breakthrough in vascular system generation. In this approach stem cell bioink made of the adipose mesenchymal stem cells, for example, can be used to print out the vessels designed using imaging techniques like CT or MRI scans. Current research in 3D bio-printing of vascular systems is mostly focused on the in vitro generation of patient specific vascular networks with the inner lining of the endothelial cells. Another especially important focus of the 3D bio-printing vascular system engineering is the formation of microvascular networks to study the pathology involved in the plaque and thrombus formation.
Vascular networks within engineered tissues can be generated using either “bottom-up” or “top-down” approaches. In addition to this, another popular approach for the construction of the vessels is the sacrificial template approach. Using this approach Lee et al. developed 3D microvascular networks within gelatin hydrogels using thermo responsive sacrificial microfibers [60]. Lee et al. used solvent-spun poly(n-isopropylacrylamide) (PNIPAM) fibres as the sacrificial material to produce 3D microvascular networks in cell-laden gelatin hydrogels (Fig. 3). The typical physio-chemical character of PNIPAM allows for an aqueous fabrication process thereby avoiding the use of organic solvents and extreme temperatures. Diameters of the capillaries formed after high speed spinning of PNIPAM was found to range from 3 to 55 μm. This study confirmed that the perfusion of the tissue using these microfibers causes considerable improvement of the cells when compared with the non-perfused counterparts [60]. Kolesky et al. reported a similar approach in which a 3D printed sacrificial template was printed in the presence of a cell-laden hydrogel by exploiting the thermoresponsive behaviour of Pluronic F127 (Poloxamer 407). However, removing Pluronic F127 requires cooling the scaffold to 4 °C, which potentially damages the encapsulated cells [61].
Fig. (3).
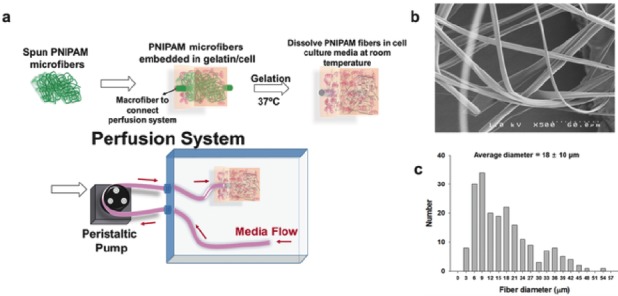
a) Schematic diagram of device fabrication and the perfusion system. b) SEM image and c) diameter distribution of PNIPAM microfibers [60].
Wu et al. used the approach of direct ink writing to print the 3D microvascular networks [62]. In this approach an escapable ink is patterned, which then encapsulates into a curable resin and is then removed by a liquefaction process. Escape of the ink generates a network of 3D microvesicles. A similar approach was used by Hansen et al. in which they used dual escapable inks in conjugation with vertical printing to create 3D interpenetrating microvascular networks [63]. Wu et al. demonstrated the new omnidirectional printing, a new variant of direct ink writing. In this approach, fugitive ink filaments are printed within a curable reservoir, which physically supports the patterned features thereby allowing truly omnidirectional freeform fabrication (Fig. 4) [62].
Fig. (4).
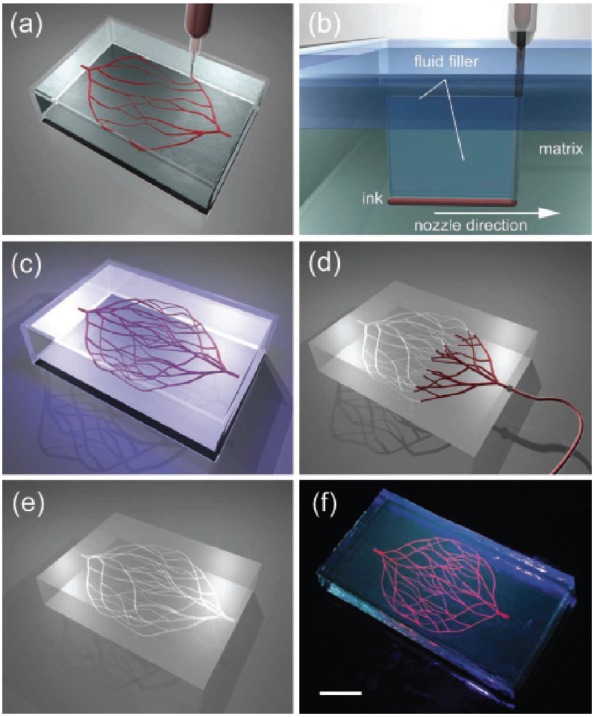
a–e) Schematics of omnidirectional printing of 3D microvascular networks within a hydrogel reservoir. a) Deposition of a fugitive ink into a physical gel reservoir allows hierarchical, branching networks to be patterned. b) Voids induced by nozzle translation are filled with liquid that migrates from the fluid capping layer. c) Subsequent photopolymerization of the reservoir yields a chemically cross-linked, hydrogel matrix. d,e) The ink is liquefied and removed under a modest vacuum to expose the microvascular channels. f) Fluorescent image of a 3D microvascular network fabricated via omnidirectional printing of a fugitive ink (dyed red) within a photopolymerizedPluronic F127-diacrylate matrix. (scale bar 10 mm) [62]. (The color version of the figure is available in the electronic copy of the article).
To enable omnidirectional printing of a 3D biomimetic microvascular networks, Wu et al. developed a fluorescent dye and a fugitive ink made up of Pluronic F127 a triblock copolymer with a hydrophobic poly(propylene oxide) (PPO) segment and two hydrophilic poly(ethylene oxide) (PEO) segments arranged in a PEO-PPO-PEO configuration and a diacrylate-functionalised Pluronic F127 solution of varying concentration as the fluid filler and physical gel reservoir, respectively. Using this system Wu et al., developed microchannel diameters ranging from 200–600 µm, in which two large parent channels are subdivided into several smaller microchannels. The ink from the system can be subsequently removed by liquefaction under a modest vacuum to yield the desired microvascular network within the solidified gel matrix [62].
Miller et al. used the template sacrificial approach for rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues [64]. In a template sacrificial approach, the template base of the network is fabricated from fugitive material, which is subsequently removed forming a fluidic network into a bulk material [65]. In their approach, carbohydrate glass was used to print a robust 3D filament into an engineered tissue containing live cells. Carbohydrate filament thus formed was then sacrificed to generate a vascular system. This network was then lined with the cells of interest such as endothelial cells. The endothelial cells lined network was later perfused with high pressure pulsated blood flow. The authors reported that the proposed network sustained the metabolic function of rat liver cells in a tissue construct that otherwise exhibited suppressed function.
Miller et al. printed a rigid pattern of vascular networks in fabricated tissues using extrusion through a syringe mounted on a custom-modified Rep Rap Mendel 3D printer [64]. Using a sacrificial approach, a network of channels can be fabricated by creating a rigid 3D lattice of filaments and its subsequent sacrifice leads to the formation of a microfluidic network. Originally this method requires organic solvents of cytotoxic nature or adverse processing to remove the sacrificial material. The Miller et al. approach however, utilises simple bio-compatible, economical and easily available carbohydrates as a sacrificial template material. To demonstrate the flexibility of the carbohydrate glass approach, they patterned vascular channels in the presence of living cells with a wide range of natural and synthetic extracellular materials (ECM) [64].
Golden et al. also used the sacrificial approach to form the interconnected microfluidic networks. In this approach, Golden et al. used gelatin as a sacrificial material. A gelatin network mesh was first formed and then encapsulated into hydrogel. Gelatin was then subsequently melted and flushed out to form the channels in the hydrogel [66]. Zhao et al. has fabricated uniaxial meso-scale fluidic channels in hydrogels using gelatin as a sacrificial template. Collagen and gelatin were co-extruded such that a fibre of gelatin (~1 mm) was enclosed in a bulk collagen matrix [67]. Subsequent removal of the gelatin template at 37 °C resulted in the formation of the microfluidic network.
As most of the organs, including the heart, are made from more than one type of cell, organising different cell types and extracellular matrices in the same tissue construct is a promising approach to bio-fabricate 3D tissues and organs. Li et al. has fabricated a hybrid cell/hydrogel construct by two-nozzle assembly technique [68]. Li et al. first constructed digital models to mimic a liver anatomy. Hepatocytes were first dispersed in the hydrogel composed of gelatin/alginate/chitosan (2:1:1 v/v/v at 37 °C) (final density being 1 x 106 cellsml-1) and adipose-derived stromal cells (ADSC) were dispersed in the hydrogel constructed of gelatin/alginate/fibrinogen (2:1:1 v/v/v.) (final density being 1 x 06 cellsml-1). Both the solution of the hydrogel were then put into a sterile syringe and mounted onto the nozzle fixer. Later the mixtures were extruded from the respective syringes into the chamber with a low temperature to get the pattern of the liver designed digitally. The construct was stabilised in a thrombin/CaCl2/Na5P3O10 solution after assembly. ADSC were subsequently differentiated into endothelial cells using an endothelial growth factor. After a 2-week culture, an albumin secretion level was found to be increased whereas the level of urea and alanine transaminase was decreased. The result of this experiment confirms the potential of the two nozzle assembling technique in complex bio-fabrication of cardiac tissue.
As VEGF alone and in combination with other growth factors are not enough to give rise to complete microvascular networks, Cui et al. used human microvascular endothelial cells in conjugation with fibrin as a scaffold to fabricate microvasculature using thermal inkjet printing technology [69]. In fact, Cui et al., was among the first few scientists who utilised the commercial (HP and/or Cannon) printers to print cells and biomaterials to fabricate 3D cellular scaffolds. In this experiment, it was observed that cells not only aligned themselves on the inner side of the channel but were also found to proliferate well. Another important part of this study is the modification of the HP Deskjet 500 thermal inkjet printer which was used simultaneously to deposit human microvascular endothelial cells and fibrin to form the digitally designed microvasculature.
Analyzing the overall trend, the sacrificial approach is also becoming popular along with 3D printing (extrusion-based printing and stereolithography) of fluidic based networks. However, extrusion-based printing and stereolithography have their own limitations. For example, small channels formed from direct extrusion printing have the tendency to collapse under their own weight. This issue can be overcome by printing in the presence of a support material (Sacrificial template). Stereolithography utilises light as a patterning tool for additive fabrication and hence conventional stereolithography (light-based 3D printing) requires the photopolymerisable materials (e.g. poly(ethylene glycol) acrylates), and printing protocols must be designed to minimise phototoxicity or excessive exposure to photoinitiator [70].
The above results indicate the future applications of 3D printing technology in bio-fabrication of vasculature networks. Nevertheless, it is also clear that several challenges need to be overcome in order to translate the success in the laboratory to patients who are waiting for a coronary bypass graft. The most important challenge in 3D printing of the autologous blood vascular network is the construction of various layers of the blood vascular system, which are composed of different cell types. Seeing the present resolution and scale of the latest technology, it appears to be challenging to scaffold all layers of the vasculature network into a single construct. Second, and the most important limitation in the bio-fabrication of the autologous blood vascular system, is the blood supply to the coronary bypass graft, because to keep the cells of the coronary arteries alive, a continuous supply of oxygen and other nutrients is essential. In normal physiological conditions, vasa vasorum (small capillaries) provide the necessary nutrients for the coronary arteries. Taking this into account in the future, graft engineering essentially needs to not only consider the bigger arteries, but it should also give due consideration to the microvascular network.
7. BIOMATERIALS
Biomaterials are undoubtedly the primary limitation for 3D bio-printing. Several biocompatible synthetic and natural polymers are available and have been developed for the scaffold formation. Synthetic materials provide the mechanical strength and natural polymers are more favourable for cell attachment, proliferation and differentiation. Crosslinking methodology and viscosity determines its printability. Various cross-linking mechanisms to obtain stable scaffold include photocross linking, pH based crosslinking, and temperature- based cross-linking. The selection between natural and synthetic polymers is critically important for the bio-printing process and for the final construct.
7.1. Natural Polymers
Natural sources have been used to improve the biological features of printed constructs. Natural polymers can be classified according to their composition as proteins, polysaccharides or glycosaminoglycans. Natural polymers can withstand harsh fabrication conditions like temperature and organic solvents, thereby allowing for the printing of cells and bioactive components. However, there are several key limitations associated with natural biopolymers. One of them is the inconsistent viscosity and moisture content which make printing reproducible scaffolds an immediate challenge. Owing to the selection criteria and challenges, the number of natural polymers that have been successfully used for bio-printing are limited. Commonly used natural polymers include collagen, gelatin, alginate, hyaluronic acid, dextran, fibrin, etc. For better rheological properties and durability, combinations of these polymers have been used.
7.2. Synthetic Polymers
While natural polymers provide a positive cell environment through mimicking native components of the extra cellular matrix, synthetic polymers allows for the chemical manipulation of the structure to make it biocompatible and more durable. However, the lack of active binding sites, activates various cellular signalling pathways, which could hamper the cell adhesion and adversely affect the cellular viability. To impart the biological recognition found in natural materials, these synthetic polymers can be equipped with molecular agents to enhance bioactivity. Moreover, they can be spatially deposited to stimulate cellular responses via mechanotransduction pathways. Some of these synthetic polymers that are used for bio-printing include polyethylene glycol (PEG), poly(lactic-co-glycolic acid) (PLGA), poly(ε-caprolactone), and Poly(lactic acid) (PLLA).
Before the use of 3D bio-printing for scaffold designing, several materials and methods were studied for the preparation of the biocompatible cardiac scaffold. Cardiac scaffold, perfectly mimicking the natural proteins, was fabricated using the materials sourced from natural sources such as alginate, collagen or by using synthetic materials like poly(glycolic acid), poly(lactic acid), poly(p-dioxanone), poly(glycerol sebacate), poly(urethane), etc. For example, Chen et al. prepared a fibrous scaffold for cardiac tissue engineering using poly(urethane)/cellulose. Uniform fibrous nanostructures, and high mechanical and three dimensional porous networks is the highlight of this work [71]. Park et al. used a mixture of poly(DL-lactide-co-caprolactone), poly(DL-lactide-co-glycolide) (PLGA), and type I collagen to fabricate a re-absorbable novel scaffold compatible with neonatal rat heart cells suspended in Matrigel and seeded into the scaffolds at a density similar to that of natural cardiac tissue. In this scaffold, PCL provided the mechanical stability and elasticity, collagen provided the sites for cell attachment and PLGA lowered the degradation rate of PCL [72].
Chitosan, a polymer of natural origin, has also been studied for its effectiveness in cardiac tissue regeneration [73]. It is not only biodegradable, bio-compatible and non-immunogenic but also has antithrombogenic properties and supports neo-vascularisation which makes it one of the best suitable candidates for scaffold fabrication in tissue engineering [74]. Recently, research interest has been growing towards developing the scaffold, not only with the desired mechanical properties, but also with electrical properties. A lot of work has already been done to generate the scaffold with appropriate mechanical and electrical properties [75]. According to this, a Chitosan-based scaffold can combine almost all the property of interest which are essential for cardiac tissue engineering including electrical conduction. Although, Chitosan is non-conductive, its electrical properties could be enhanced by adding materials with good conductivity. Martins et al. has done the same modification by combining chitosan with carbon [76].
It was observed that electrically conductive scaffolds improved the cardiomyocyte function by increasing the expression of cardiac genes. In a similar line of research, scaffold based on poly(urethane) (PU), collagen, PU/collagen, PU/CNT and PU/collagen/CNT was fabricated by electrospinning and spray methods. The scaffolds were seeded with H9c2 cardiac myoblasts and cultured for 8 days to investigate cell viability, adhesion and morphology in scaffolds. The important finding of this study was that the carbon nanotube and collagen presence in the scaffold not only provided the mechanical strength, but also helped to increase the electrical conductivity [77, 78]. Patra et al. has investigated the suitability of a non-mulberry silk protein fibroin as a scaffold for engineering a cardiac patch obtained from the Indian tropical Tasar silkworm Antheraea mylitta [79]. Moreover, they have tested cell adhesion, cellular metabolic activity, response to extracellular stimuli, cell to cell communication and contractility of 3-days postnatal rat cardiomyocytes on silk fibroin. Their data demonstrates that A. mylitta silk fibroin exhibits similar properties as that of fibronectin, a component of the natural matrix for cardiomyocytes. In comparison to the mulberry, a fibroin obtained from A. mylittais is superior probably due to its RGD domains (Arg-Gly-Asp). 3D scaffolds made up of this protein can be efficiently loaded with cardiomyocytes resulting in contractile patches [80].
Poly(urethane) is an important and widely trusted elastomer for cardiac tissue engineering because of its favourable mechanical properties [81]. However, few of the major drawbacks associated with the fibres made from poly(urethane) include intertwining and stickiness. This issue has been solved by the Wei-Fang Su et al. approach in which they blended rigid cellulose into poly(urethane) made from aromatic diisocyanate and polyether diol [82]. Poly(urethane) fibres are non-biodegradable. Various bio-degradable poly(urethane)s analogues made from aromatic isocyanate have been previously reported [83]. Aliphatic analogues like 1,4-diisocyanatobutane and 1,6-diisocyanato hexane are prepared and used instead of aromatic isocyanate, but they come with the drawback of crystallisation [84, 85]. Their approach was successful in synthesising nontoxic, biodegradable, non-crystalline poly(urethane)s that can be fabricated into a 3D scaffold. It was concluded that the scaffold made from this polymer blend has the potential to be used in myocardium engineering [82].
In addition, the scaffold made from the autologous materials does not have the risk of immune response foreign body reaction. One such material could be the fibrin which was exploited by Jockenhoevel et al. to fabricate the biodegradable scaffold for cardiovascular tissue engineering [86]. One other non-immunogenic material used for scaffold fabrication is the small intestinal submucosa which is a naturally derived bio-polymer material and cellular xenogeneic extracellular matrix material. Small intestinal submucosa does not trigger the immunologic reactions as confirmed by testing in several species and by direct immunogenic challenge testing [87]. To reduce undesired immune reactions, the use of a bio-polymer from blood proteins is a desirable approach because such proteins are biodegradable and non-immunogenic. A proof of concept was given by Kuhn et al. who developed blood protein based scaffolds for cardiovascular tissue engineering using anti-coagulated porcine blood and an electrospinning method [88].
8. CELL SOURCE
Cells are the structural and functional unit of the tissue. Cells exhibit an intrinsic ability to self-assemble into tissue-like structures if placed in a similar geometry to that of the native tissue. Exogenous electrochemical stimulus from extracellular matrices helps cells to get organized into the anatomical and physiological unit. This makes the cell source selection an important issue. Autologous or allogeneic stem cells are the two typical cell sources in tissue engineering and regenerative medicine. Important properties like a high proliferation rate, paracrine stimulation and pluripotency of stem cells makes them the most favoured source.
Embryonic, mesenchymal, and induced pluripotent stem cells are the most commonly used cell source. Each has their distinct advantages and disadvantages. Embryonic stem cells have the highest degree of multi-potency, but drawbacks include difficult procurement, ethical issues, and immunogenicity. Mesenchymal stem cells are less difficult to procure, and they have been shown to stimulate immunotolerance in target tissue, but they do not possess the same degree of multi-potency as embryonic stem cells. Induced pluripotent stem cells have increased multi-potency, but they have been linked to tumorigenesis in some studies.
Patient-derived hPSC-derived cardiovascular cells, which can present the genetic, environmental, and physical differences of individuals, are particularly interesting in cardiac tissue engineering. Wang et al, using iPSC derived cardiomyocytes, first developed the in vitro testing 2D model for the Barth syndrome which is a mitochondria related genetic disorder [89]. This model was useful in studying the pathophysiology involved in the Barth syndrome.
In traditional tissue engineering, seeding cells in a biodegradable scaffold without proper arrangement for the oxygen supply, limits the thickness of the tissue. As an alternative approach, Masuda, et al. has developed cell sheet engineering as a scaffold-free approach for cardiac tissue engineering. Layered cardiac cell sheets were found to be efficiently conducting the electrical stimulus with improved functions in the damaged heart. Pre-vascularised scaffolds within cardiac tissue and multi-step transplantation methods could further facilitate the fabrication of the thick vascularised tissues in vivo. Vascular beds further allow for the fabrication of thick 3D tissues within the functional transport system.
Carrier et al. in a report has shown that optimum oxygen perfusion not only improves the cell culture condition, but also allows for the uniform distribution of the cells and enhanced expression of heart specific proteins [90]. Dvir et al. developed a well perfused bioreactor which allowed for the thorough flow of the medium and oxygen to ultimately enhance the survival of the cell [91]. Improvement of the cell culture was observed when cells are grown in the porous scaffold having separate compartments for growth medium supplemented with an oxygen carrier [92]. Three dimensional cardiac tissues fabricated by cell sheet engineering and the provision of oxygen and medium supply may be applied to treat heart disease and tissue model construction [93].
De-cellularised cardiac tissue is another approach where 3D complex natural architecture and vasculature can be preserved. A De-cellularised heart was seeded with a human iPSC and was found to conduct the electrical stimulus and has also shown an intended response towards the drugs acting on the cardiovascular system [94].
Cells inside the tissue must not only replicate the electrical conduction system of the cardiac tissue, but they must also have the equivalent mechanical forces for efficient relaxation and contraction of the myocardium. These properties can be re-orchestrated by the precise arrangement of the cardiomyocytes along with other components. Taking this into account, Choi et al. has evaluated the potential for generating human cardiac muscle cells in vivo from adipose-derived stem cells (ASC) by co-implanting it in a vascularised tissue engineering chamber with rat cardiomyocytes (rCM) [95]. hESC is another important cell source for tissue engineers. They are capable of proliferating indefinitely and can be differentiated into many clinically relevant cell types. In response to angiogenic factors, early differentiation of hESCs in an in vivo microenvironment that induces vascular network formation has already been demonstrated [96]. Scaffolds derived from biodegradable materials could be used to support vascular development from hESCs. One other possible source of the cells for cardiomyocyte regeneration is the embryonic cardiac progenitor cells (CPCs) [97]. CPCs have already demonstrated their ability to differentiate into smooth muscle cells, endothelial cells and cardiomyocytes and hence they are considered to have greater potential to repair the damaged heart tissue and in tissue engineering [97].
Adult cells reprogramming to stem cells by Takahashi et al. has had a dramatic impact on the field of regenerative medicine, especially in the field of tissue engineering [98]. Takahashi used Oct3/4, Sox2, Klf4 and c-Myc genes to transform the skin cells of adult mice into stem cells, known as induced pluripotent stem cells (iPS). iPS cells have the potential to transform the regeneration of the cardiac tissue for therapeutic and transplantation applications. As iPS cells are reprogrammed from the patient’s own cells, the issue of tissue rejection associated with solid organ transplantation is automatically circumvented [99]. iPS cells can be expanded in vitro, while maintaining their capacity to undergo differentiation towards endothelial cells, vascular smooth muscle cells and cardiomyocytes [100]. Testing these cells in cardiovascular injury models and in 3D printing of the cardiac tissue would help to gain a fundamental understanding of their functional properties. Several methods are available to differentiate the iPS to cardiomyocytes and most of them are very similar to that of the generation of cardiomyocytes from human embryonic stem cells [101]. Zhang et al. generated the cardiomyocytes from iPS [102]. In this study the authors compared the differentiation potential of iPS with embryonic stem cell and concluded that their differentiation into cardiomyocytes is almost similar. iPS was also differentiated into the cardiovascular tissue lineages and has shown comparable properties to those of the cardiomyocytes generated from the embryonic stem cells [103]. Similarly, Martinez-Fernandez et al. developed the method for the differentiation of the iPS into cardiomyocytes without c-Myc [104].
The results of the experiments with various stem cells, scaffolds and extracellular matrices are promising; more studies are needed in vitro, animal models and in 3D orientation to gain insights into the potential benefits. As evident from the literature discussed above, 3D bio-printing is useful for building porous constructs that can maintain the viability of cells, however, to date this technology has not been found to be successful in printing tissues that have clinical relevance. To meet the scientific demand, scientists have used 3D printing technology in tissue engineering, however so far none of the available technology is able to bio fabricate the functional heart. As cited before the biggest problem in engineering heart muscle is getting blood flow to all of the cells, and in particular the cells which are very densely arranged in thick heart tissue. None of the currently available technology is close enough to construct the tissue dense enough so as to match the natural cardiac muscle. Moreover, none of the available technology is sufficiently advanced enough to correspond with the dense micro vasculature network of blood supply to the cells.
9. FUTURE OF 3D BIO-PRINTING IN CARDIOVASCULAR DISORDER
3D bio-printing technology certainly has a lot of advantages over the traditional tissue culture approach, but it still has several hurdles to overcome to become a technology of choice for biomedical research and clinical applications. Several universities and companies like Organovo are investing heavily in 3D bio-printing. For example, Organovo’s 3D bio-printer has delivered a liver using in-house developed 3D printing technology and it has seen the potential for the transplantation and in toxicity screening [105]. Similarly, bio-printing of a human kidney, bones, skin, cartilage, ears, tumour models, using 3D bio-printing technology has already been reported in the literature. The future of 3D bio-printing is just not limited to mimicking the anatomical structures, but 3D bio-printed tissue and organs have the potential to overcome the issues related with organ transplantation and also with clinical and preclinical testing of the drugs in animal models. The present scenario indicates that 3D bio-printing has revolutionised the field of nano-medicine, pharmaceuticals and prostate organs [106, 107]. 3D organ and dense tissue fabrication is the ultimate goal in tissue engineering. A few of the major challenges involved are the development of an organised system to transport nutrients and oxygen to all of the cells; the integrity of the 3D printed scaffold; a lack of formal protocols for cell isolations and polymer selections for the scaffold; longer production timelines; and finally, ethical issues.
The use of 3D bio-printers in cardiac tissue and vasculature engineering is in its early stages of development. However, 3D bio-printing has the potential for the fabrication of the functional cardiac tissue because of its intrinsic capability of building the structure of the complex geometries using various biomaterials. Since vascularisation is the major challenge, one of the solutions to this is to employ the biology of the cells themselves and use the endothelial cell for the development of the vasculature network. This is best supported by our basic knowledge of tissue architecture, development and its component. This knowledge would be useful to reprogram the printers according to the tissue geometry, complexity and biological components. Research findings discussed in our review have shown that porous structures, proper oxygen and nutrient supply through micro blood vascular systems not only preserves the viability of the cells in the constructs but also allows for the fabrication of the tissue thicker than 200nm.
Since 3D bio-printing involves layer by layer deposition of the cells, integrity of the construct and cell survival inside it is another major challenge. The issue of cell survival and integrity of construct has already been attempted to be resolved by developing bio-inks which become stiff after being deposited and by employing an extrusion base deposition. Many of these approaches require the use of photo or thermal sensitive materials which need light or heat for curing, for example, hydrogel crosslinking. These materials and extrusion base deposition requires heat to lower the viscosity which could affect the survival of the cell. Alternatively, strength to the scaffold could be provided by using additives such as nanofibres. Cell survival within the scaffold is the function of the deposition method, ECM and materials used in its construct. Development of bio-inks that are biocompatible, non-toxic, stable and which degrade into non-toxic metabolite over a period of time is certainly a difficult task. This will be the crucial area of research which could affect the success of 3D bio-printing. In cardiac tissue engineering the materials used in the construction of the cardiac tissue should essentially capture the essence of the cardiac physiology such as electrical properties. The material should be compatible with the various types of cells and should increase the viability of the cells inside the construct. Hydrogels and the microenvironment should be tuned in such a way that it should promote the stem cell differentiation and proliferation [108].
Nanoscale printing is possible using 3D printers with two-photon lithography, however this printer cannot translate it into thicker tissue as it is not compatible with cells and they cannot be built big enough to build the larger tissues. Therefore, the priority at this moment is the development of 3D printers which should be compatible with various kinds of cells and materials and should also be able to construct hierarchical structures of at least the 7 orders of magnitude. Therefore, an ideal bio-printer for cardiac tissue should be able to produce an ECM at a microscale level and then should immediately switch to printing of the cells.
In cardiac tissue regeneration, adult cardiac progenitor cells, iPS’s, embryonic stem cells, adipose-derived stem cells are some of the most promising cell sources for regeneration because of their natural cardiac commitment and for their high proliferative capability. After isolation, spatial arrangement of the cells requires more sophisticated 3D bio-printers of increased resolution, high speed and compatibility with cells and other biomaterials used in ECM and scaffold preparation. Collaboration of 3D bio-printing technology, polymer science and biotechnology with proper stem cells source is the ultimate need of cardiac tissue engineering. Various reports discussed in this review have demonstrated that stem cells can be printed and cultured in biocompatible scaffolds composed of synthetic or of a natural origin. Cell viability is also found to be dependent on the various factors present in the extracellular matrix. Matching the component of the ECM with cardiomyocyte proliferation represents another step forward in the in vitro development of the fully functional cardiac tissue.
Despite the potential of 3D bio-printing, it would appear that most of the particle applications of the 3D bio-printing of the cardiac tissues are developing slowly compared to the traditional tissue engineering approach. Several limitations appear to prevent this technology being applicable in clinical settings. For example, the ideal cell source for the cardiomyocytes and vasculature generation has not yet been identified and standardised. Similarly, the protocol essential for the isolation of the cardiomyocyte progenitors or the stem cells has not yet been properly established. As discussed before, vascularisation of the construct is essential to provide proper nourishment and oxygen, however due to the limitation in the development of proper vascular network, development of functional thick cardiac tissue is still a matter of intense research. One other important concern is the choice of the material and the approach for the vessel construction. It is very important to note that the vasculature network should not collapse under its own weight, and at the same time it should not be so stiff that it hinders the transport of the oxygen and other nutrients.
Most of the limitation and problems of 3D bio-printers discussed here are associated with fundamental issues like cell source and cell extraction protocols which need to be improved. It is noteworthy that advances in the area of bio-printing will improve the living standard of the patients which depends on a timely, synergistic approach from the field of material science, engineering and biology.
CONCLUSION
Bio-printing, despite certain shortcomings and technical challenges, will remain a powerful technology for bioengineers to construct organs and tissues from digital files. In future, in vitro cardiovascular tissue engineering will amalgamate decade old lessons of cellular differentiation and de-differentiation, basic biology of animal and plant cells, material science and 3D bio-printing to fabricate tissue mimicking the native cardiac tissue. Innovations, especially in the field of material science and cardiac tissue with electrical conduction systems, are important and are the major challenges in the near future. 3D bio-printing based advances made in the medical field are very exciting but 3D organ printing using bio-printers and bio-materials will need time and investment to realise their full potential.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Ahuja P., Sdek P., Maclellan W.R. Cardiac myocyte cell cycle control in development, disease, and regeneration. Physiol. Rev. 2007;87:521–544. doi: 10.1152/physrev.00032.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Studzinski G.P., Harrison L.E. Differentiation-related changes in the cell cycle traverse. Int. Rev. Cytol. 1999;189:1–58. doi: 10.1016/s0074-7696(08)61384-4. [DOI] [PubMed] [Google Scholar]
- 3.Charbe N., McCarron P.A., Tambuwala M.M. Three-dimensional bio-printing: A new frontier in oncology research. World J. Clin. Oncol. 2017;8(1):21. doi: 10.5306/wjco.v8.i1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy S.V., Atala A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014;32(8):773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 5.Mandrycky C., Wang Z., Kim K., et al. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016;43(4):422–434. doi: 10.1016/j.biotechadv.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dababneh A.B., Ozbolat I.T. Bioprinting technology: A current state-of-the-art review. J. Manuf. Sci. Eng. 2014;136(6):061016 [Google Scholar]
- 7.Wolinsky H. Printing organs cell-by-cell: 3-D printing is growing in popularity, but how should we regulate the application of this new technology to health care? EMBO Rep. 2014;15(8):836–838. doi: 10.15252/embr.201439207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui X., Dean D., Ruggeri Z.M., et al. Cell damage evaluation of thermal inkjet printed chinese hamster ovary cells. Biotechnol. Bioeng. 2010;106(6):963–969. doi: 10.1002/bit.22762. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X., Zhang Y. Tissue engineering applications of three-dimensional bioprinting. Cell Biochem. Biophys. 2015;72(3):777–782. doi: 10.1007/s12013-015-0531-x. [DOI] [PubMed] [Google Scholar]
- 10.Peltola S.M., Melchels F.P.W., Grijpma D.W., et al. A review of rapid prototyping techniques for tissue engineering purposes. Ann. Med. 2008;40(4):268–280. doi: 10.1080/07853890701881788. [DOI] [PubMed] [Google Scholar]
- 11.Skardal A., Atala A. Biomaterials for integration with 3-D bioprinting. Ann. Biomed. Eng. 2015;43(3):730–746. doi: 10.1007/s10439-014-1207-1. [DOI] [PubMed] [Google Scholar]
- 12.Wang J., Goyanes A., Gaisford S., et al. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int. J. Pharm. 2016;503(1-2):207–212. doi: 10.1016/j.ijpharm.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Dai G., Lee V. Three-dimensional bioprinting and tissue fabrication: Prospects for drug discovery and regenerative medicine. Adv. Health Care Technol. 2015;1:23. [Google Scholar]
- 14.Lee V., Singh G., Trasatti J.P., et al. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng. Part C Methods. 2014;20(6):473–484. doi: 10.1089/ten.tec.2013.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caspi O., Lesman A., Basevitch Y., et al. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ. Res. 2007;100(2):263–272. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu T., Sekine H., Yang J., et al. Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. FASEB J. 2006;20(6):1–20. doi: 10.1096/fj.05-4715fje. [DOI] [PubMed] [Google Scholar]
- 17.Sakaguchi K., Shimizu T., Horaguchi S., et al. In vitro engineering of vascularized tissue surrogates. Sci. Rep. 2013;3:1316. doi: 10.1038/srep01316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolesky D.B., Homan K.A., Skylar-Scott M.A., et al. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. USA. 2016;113(12):3179–3184. doi: 10.1073/pnas.1521342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engel F.B., Schebesta M., Duong M.T., et al. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 2005;19(10):1175–1187. doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaudhry H.W., Dashoush N.H., Tang H., et al. Cyclin A2 mediates cardiomyocyte mitosis in the postmitotic myocardium. J. Biol. Chem. 2004;279(34):35858–35866. doi: 10.1074/jbc.M404975200. [DOI] [PubMed] [Google Scholar]
- 21.Yeong W.Y., Sudarmadji N., Yu H.Y., et al. Porous polycaprolactone scaffold for cardiac tissue engineering fabricated by selective laser sintering. Acta Biomater. 2010;6(6):2028–2034. doi: 10.1016/j.actbio.2009.12.033. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z., Lee S.J., Cheng H.J., et al. 3D bioprinted functional and contractile cardiac tissue constructs. Acta Biomater. 2018;70:48–56. doi: 10.1016/j.actbio.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maiullari F., Costantini M., Milan M., et al. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci. Rep. 2018;8(1):1–15. doi: 10.1038/s41598-018-31848-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez-Ilzarbe M., Agbulut O., Pelacho B., et al. Characterization of the paracrine effects of human skeletal myoblasts transplanted in infarcted myocardium. Eur. J. Heart Fail. 2008;10(11):1065–1072. doi: 10.1016/j.ejheart.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Oh H., Bradfute S.B., Gallardo T.D., et al. Cardiac progenitor cells from adult myocardium: Homing, differentiation, and fusion after infarction. Proc. Natl. Acad. Sci. USA. 2003;100(21):12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia W., Gungor-Ozkerim P.S., Zhang Y.S., et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials. 2016;106:58–68. doi: 10.1016/j.biomaterials.2016.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ong C.S., Fukunishi T., Zhang H., et al. Biomaterial-free three-dimensional bioprinting of cardiac tissue using human induced pluripotent stem cell derived cardiomyocytes. Sci. Rep. 2017;7(1):1–11. doi: 10.1038/s41598-017-05018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bejleri D., Streeter B.W., Nachlas A.L.Y., et al. A bioprinted cardiac patch composed of cardiac-specific extracellular matrix and progenitor cells for heart repair. Adv. Healthc. Mater. 2018;7(23):e1800672. doi: 10.1002/adhm.201800672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Izadifar M., Chapman D., Babyn P., et al. UV-assisted 3D bioprinting of nano-reinforced hybrid cardiac patch for myocardial tissue engineering. Tissue Eng. Part C Methods. 2017;24(2):74–88. doi: 10.1089/ten.TEC.2017.0346. [DOI] [PubMed] [Google Scholar]
- 30.Tijore A., Irvine S.A., Sarig U., et al. Contact guidance for cardiac tissue engineering using 3D bioprinted gelatin patterned hydrogel. Biofabrication. 2018;10(2):025003. doi: 10.1088/1758-5090/aaa15d. [DOI] [PubMed] [Google Scholar]
- 31.Zhu K., Shin S.R., van Kempen T., et al. Gold nanocomposite bioink for printing 3D cardiac constructs. Adv. Funct. Mater. 2017;27(12):1605352. doi: 10.1002/adfm.201605352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan Y., Wang X., Pan Y., et al. Fabrication of viable tissue-engineered constructs with 3D cell-assembly technique. Biomaterials. 2005;26(29):5864–5871. doi: 10.1016/j.biomaterials.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 33.Kim J.J., Hou L., Huang N.F. Vascularization of three-dimensional engineered tissues for regenerative medicine applications. Acta Biomater. 2016;41:17–26. doi: 10.1016/j.actbio.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gershlak J.R., Hernandez S., Fontana G., et al. Crossing kingdoms: Using decellularized plants as perfusable tissue engineering scaffolds. Biomaterials. 2017;125:13–22. doi: 10.1016/j.biomaterials.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirkpatrick C.J., Fuchs S., Unger R.E. Co-culture systems for vascularization - Learning from nature. Adv. Drug Deliv. Rev. 2011;63(4):291–299. doi: 10.1016/j.addr.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Levenberg S., Rouwkema J., Macdonald M., et al. Engineering vascularized skeletal muscle tissue. Nat. Biotechnol. 2005;23(7):879–884. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 37.Blau H.M., Banfi A. The well-tempered vessel. Nat. Med. 2001;7(5):532–534. doi: 10.1038/87850. [DOI] [PubMed] [Google Scholar]
- 38.Jain R.K. Molecular regulation of vessel maturation. Nat. Med. 2003;9(6):685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 39.Richardson T.P., Peters M.C., Ennett A.B., et al. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 2001;19(11):1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 40.Schechner J.S., Nath K., Zheng L., et al. In vivo formation of complex microvessels lined by human endothelial cells in an immunodeficient mouse. Proc. Natl. Acad. Sci. USA. 2000;97(16):9191–9196. doi: 10.1073/pnas.150242297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koike N., Fukumura D., Gralla O., et al. Tissue engineering: Creation of long-lasting blood vessels. Nature. 2004;428(6979):138–139. doi: 10.1038/428138a. [DOI] [PubMed] [Google Scholar]
- 42.Yamashita J., Itoh H., Hirashima M., et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408(6808):92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 43.Jiang Y., Jahagirdar B.N., Reinhardt R.L., et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 44.Jain R.K. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 45.Bertassoni L.E., Cecconi M., Manoharan V., et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip. 2014;14(13):2202–2211. doi: 10.1039/c4lc00030g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu Y., Hu Y., Liu C., et al. A novel strategy for creating tissue-engineered biomimetic blood vessels using 3D bioprinting technology. Materials (Basel) 2018;11(9):1–15. doi: 10.3390/ma11091581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarker M.D., Naghieh S., Sharma N.K., et al. 3D biofabrication of vascular networks for tissue regeneration: A report on recent advances. J. Pharm. Anal. 2018;8(5):277–296. doi: 10.1016/j.jpha.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schöneberg J., De Lorenzi F., Theek B., et al. Engineering biofunctional in vitro vessel models using a multilayer bioprinting technique. Sci. Rep. 2018;8(1):10430. doi: 10.1038/s41598-018-28715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qi J., Li J., Zheng S., Liu J. A vascular fabrication method based on sacrificial material and spraying process. IOP Conf Ser Mater Sci Eng. 2018;394(2):022061. [Google Scholar]
- 50.Hannan E.L., Racz M.J., Walford G., et al. Long-term outcomes of coronary-artery bypass grafting versus stent implantation. N. Engl. J. Med. 2005;352(21):2174–2183. doi: 10.1056/NEJMoa040316. [DOI] [PubMed] [Google Scholar]
- 51.Dahl S.L.M., Kypson A.P., Lawson J.H., et al. Readily available tissue-engineered vascular grafts. Sci. Transl. Med. 2011;3(68):1–11. doi: 10.1126/scitranslmed.3001426. [DOI] [PubMed] [Google Scholar]
- 52.Matsuda H., Miyazaki M., Oka Y., et al. A polyurethane vascular access graft and a hybrid polytetrafluoroethylene graft as an arteriovenous fistula for hemodialysis: Comparison with an expanded polytetrafluoroethylene graft. Artif. Organs. 2003;27(8):722–727. doi: 10.1046/j.1525-1594.2003.07031.x. [DOI] [PubMed] [Google Scholar]
- 53.Van Damme H., Deprez M., Creemers E., et al. Intrinsic structural failure of polyester (Dacron) vascular grafts. A general review. Acta Chir. Belg. 2005;105(3):249–255. doi: 10.1080/00015458.2005.11679712. [DOI] [PubMed] [Google Scholar]
- 54.Gary M., Silver G.E.K., Stutzman F.L., et al. Umbilical vein for aortocoronary bypass. Angiology. 1982;33(7):450–453. doi: 10.1177/000331978203300704. [DOI] [PubMed] [Google Scholar]
- 55.Vrandecic M.O. New graft for the surgical treatment of small vessel diseases. J. Cardiovasc. Surg. (Torino) 1987;28(6):711–714. [PubMed] [Google Scholar]
- 56.Perloff L.J., Christie B.A., Ketharanathan V., et al. A new replacement for small vessels. Surgery. 1981;89(1):31–41. [PubMed] [Google Scholar]
- 57.Tomizawa Y, Moon MR, DeAnda A, et al. Coronary bypass grafting with biological grafts in a canine model. Circulation. 1994;90(5 II):II160–6. [PubMed] [Google Scholar]
- 58.Engbers G.H., Feijen J. Current techniques to improve the blood compatibility of biomaterial surfaces. Int. J. Artif. Organs. 1991;14(4):199–215. [PubMed] [Google Scholar]
- 59.Brothers T.E., Stanley J.C., Burkel W.E., et al. Small-caliber polyurethane and polytetrafluoroethylene grafts: A comparative study in a canine aortoiliac model. J. Biomed. Mater. Res. 1990;24(6):761–771. doi: 10.1002/jbm.820240610. [DOI] [PubMed] [Google Scholar]
- 60.Lee J.B., Wang X., Faley S., et al. Development of 3D microvascular networks within gelatin hydrogels using thermoresponsive sacrificial microfibers. Adv. Healthc. Mater. 2016;5(7):781–785. doi: 10.1002/adhm.201500792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kolesky D.B., Truby R.L., Gladman A.S., et al. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 2014;26(19):3124–3130. doi: 10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- 62.Wu W., Deconinck A., Lewis J.A. Omnidirectional printing of 3D microvascular networks. Adv. Mater. 2011;23(24):H178–H183. doi: 10.1002/adma.201004625. [DOI] [PubMed] [Google Scholar]
- 63.Hansen C.J., Wu W., Toohey K.S., et al. Self-healing materials with interpenetrating microvascular networks. Adv. Mater. 2009;21(41):4143–4147. [Google Scholar]
- 64.Miller J.S., Stevens K.R., Yang M.T., et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 2012;11(7):768–774. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kinstlinger I.S., Miller J.S. 3D-printed fluidic networks as vasculature for engineered tissue. Lab Chip. 2016;16(11):2025–2043. doi: 10.1039/c6lc00193a. [DOI] [PubMed] [Google Scholar]
- 66.Golden A.P., Tien J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip. 2007;7(6):720–725. doi: 10.1039/b618409j. [DOI] [PubMed] [Google Scholar]
- 67.Zhao L., Lee V.K., Yoo S-S., et al. The integration of 3-D cell printing and mesoscopic fluorescence molecular tomography of vascular constructs within thick hydrogel scaffolds. Biomaterials. 2012;33(21):5325–5332. doi: 10.1016/j.biomaterials.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li S., Zhuo X., Xiaohong W., et al. Direct fabrication of a hybrid cell/hydrogel construct by a double-nozzle assembling technology. J. Bioact. Compat. Polym. 2009;24(3):249–265. [Google Scholar]
- 69.Cui X., Boland T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials. 2009;30(31):6221–6227. doi: 10.1016/j.biomaterials.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 70.Yang Y., Urbas A., Gonzalez-Bonet A., et al. A composition-controlled cross-linking resin network through rapid visible-light photo-copolymerization. Polym. Chem. 2016;7(31):5023–5030. [Google Scholar]
- 71.Chen P.H., Liao H.C., Hsu S.H., et al. A novel polyurethane/cellulose fibrous scaffold for cardiac tissue engineering. RSC Advances. 2015;5(9):6932–6939. [Google Scholar]
- 72.Park H., Radisic M., Lim J.O., et al. A novel composite scaffold for cardiac tissue engineering. In Vitro Cell. Dev. Biol. Anim. 2005;41(7):188–196. doi: 10.1290/0411071.1. [DOI] [PubMed] [Google Scholar]
- 73.Pok S., Vitale F., Eichmann S.L., et al. Biocompatible carbon nanotube-chitosan scaffold matching the electrical conductivity of the heart. ACS Nano. 2014;8(10):9822–9832. doi: 10.1021/nn503693h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu W.N., Lü S.H., Wang H.B., et al. Functional improvement of infarcted heart by co-injection of embryonic stem cells with temperature-responsive chitosan hydrogel. Tissue Eng. Part A. 2009;15(6):1437–1447. doi: 10.1089/ten.tea.2008.0143. [DOI] [PubMed] [Google Scholar]
- 75.Borriello A., Guarino V., Schiavo L., et al. Optimizing PANi doped electroactive substrates as patches for the regeneration of cardiac muscle. J. Mater. Sci. Mater. Med. 2011;22(4):1053–1062. doi: 10.1007/s10856-011-4259-x. [DOI] [PubMed] [Google Scholar]
- 76.Peter M.G. Applications and environmental aspects of chitin and chitosan. J Macromol Sci Part A. 1995;32(4):629–640. [Google Scholar]
- 77.Lee J.H., Lee J.Y., Yang S.H., et al. Carbon nanotube-collagen three-dimensional culture of mesenchymal stem cells promotes expression of neural phenotypes and secretion of neurotrophic factors. Acta Biomater. 2014;10(10):4425–4436. doi: 10.1016/j.actbio.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 78.Agarwal S., Wendorff J.H., Greiner A. Use of electrospinning technique for biomedical applications. Polymer (Guildf.) 2008;49(26):5603–5621. [Google Scholar]
- 79.Patra C., Talukdar S., Novoyatleva T., et al. Silk protein fibroin from Antheraea mylitta for cardiac tissue engineering. Biomaterials. 2012;33(9):2673–2680. doi: 10.1016/j.biomaterials.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 80.Naskar D., Nayak S., Dey T., et al. Non-mulberry silk fibroin influence osteogenesis and osteoblast-macrophage cross talk on titanium based surface. Sci. Rep. 2014;4:1–9. doi: 10.1038/srep04745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mackay T.G., Wheatley D.J., Bernacca G.M., et al. New polyurethane heart valve prosthesis: Design, manufacture and evaluation. Biomaterials. 1996;17(19):1857–1863. doi: 10.1016/0142-9612(95)00242-1. [DOI] [PubMed] [Google Scholar]
- 82.Su W.F., Ho C.C., Shih T.H., et al. Exceptional biocompatibility of 3D fibrous scaffold for cardiac tissue engineering fabricated from biodegradable polyurethane blended with cellulose. Int J Polym Mater Polym Biomater. 2016;65(14):703–711. [Google Scholar]
- 83.Guelcher S.A. Biodegradable polyurethanes: Synthesis and applications in regenerative medicine. Tissue Eng. Part B Rev. 2008;14(1):3–17. doi: 10.1089/teb.2007.0133. [DOI] [PubMed] [Google Scholar]
- 84.Cohen A., Yan Q.L., Shlomovich A., et al. Novel nitrogen-rich energetic macromolecules based on 3,6-dihydrazinyl-1,2,4,5-tetrazine. RSC Advances. 2015;5(129):106971–106980. [Google Scholar]
- 85.Fujimoto K.L., Tobita K., Merryman W.D., et al. An elastic, biodegradable cardiac patch induces contractile smooth muscle and improves cardiac remodeling and function in subacute myocardial infarction. J. Am. Coll. Cardiol. 2007;49(23):2292–2300. doi: 10.1016/j.jacc.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jockenhoevel S., Zund G., Hoerstrup S.P., et al. Fibrin gel -- advantages of a new scaffold in cardiovascular tissue engineering. Eur. J. Cardiothorac. Surg. 2001;19(4):424–430. doi: 10.1016/s1010-7940(01)00624-8. [DOI] [PubMed] [Google Scholar]
- 87.Cheng E.Y., Kropp B.P. Urologic tissue engineering with small-intestinal submucosa: Potential clinical applications. World J. Urol. 2000;18(1):26–30. doi: 10.1007/PL00007071. [DOI] [PubMed] [Google Scholar]
- 88.Kuhn A.I., Müller M., Knigge S., et al. Novel blood protein based scaffolds for cardiovascular tissue engineering. Curr. Dir. Biomed. Eng. 2016;2(1):5–9. [Google Scholar]
- 89.Wang G., McCain M.L., Yang L., et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat. Med. 2014;20(6):616–623. doi: 10.1038/nm.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carrier R.L., Rupnick M., Langer R., et al. Perfusion improves tissue architecture of engineered cardiac muscle. Tissue Eng. 2002;8(2):175–188. doi: 10.1089/107632702753724950. [DOI] [PubMed] [Google Scholar]
- 91.Dvir T., Benishti N., Shachar M., et al. A novel perfusion bioreactor providing a homogenous milieu for tissue regeneration. Tissue Eng. 2006;12(10):2843–2852. doi: 10.1089/ten.2006.12.2843. [DOI] [PubMed] [Google Scholar]
- 92.Radisic M., Marsano A., Maidhof R., et al. Cardiac tissue engineering using perfusion bioreactor systems. Nat. Protoc. 2008;3(4):719–738. doi: 10.1038/nprot.2008.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Masuda S., Shimizu T. Three-dimensional cardiac tissue fabrication based on cell sheet technology. Adv. Drug Deliv. Rev. 2016;96:103–109. doi: 10.1016/j.addr.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 94.Lu T.Y., Lin B., Kim J., et al. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nat. Commun. 2013;4:2307. doi: 10.1038/ncomms3307. [DOI] [PubMed] [Google Scholar]
- 95.Choi Y.S., Matsuda K., Dusting G.J., et al. Engineering cardiac tissue in vivo from human adipose-derived stem cells. Biomaterials. 2010;31(8):2236–2242. doi: 10.1016/j.biomaterials.2009.11.097. [DOI] [PubMed] [Google Scholar]
- 96.Levenberg S., Golub J.S., Amit M., et al. Endothelial cells derived from human embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2002;99(7):4391–4396. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu S.M., Chien K.R., Mummery C. Origins and fates of cardiovascular progenitor cells. Cell. 2008;132(4):537–543. doi: 10.1016/j.cell.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 99.Pei D., Xu J., Zhuang Q., Tse H.F., et al. Induced pluripotent stem cell technology in regenerative medicine and biology. Adv. Biochem. Eng. Biotechnol. 2010;123:127–141. doi: 10.1007/10_2010_72. [DOI] [PubMed] [Google Scholar]
- 100.Narazaki G., Uosaki H., Teranishi M., et al. Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation. 2008;118(5):498–506. doi: 10.1161/CIRCULATIONAHA.108.769562. [DOI] [PubMed] [Google Scholar]
- 101.Burridge P.W., Keller G., Gold J.D., et al. Production of de novo cardiomyocytes: Human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell. 2012;10(1):16–28. doi: 10.1016/j.stem.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang J., Wilson G.F., Soerens A.G., et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res. 2009;104(4):e30–e41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Van Laake L.W., Qian L., Cheng P., et al. Reporter-based isolation of induced pluripotent stem cell-and embryonic stem cell-derived cardiac progenitors reveals limited gene expression variance. Circ. Res. 2010;107(3):340–347. doi: 10.1161/CIRCRESAHA.109.215434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Martinez-Fernandez A., Nelson T.J., Ikeda Y., et al. c-MYC independent nuclear reprogramming favors cardiogenic potential of induced pluripotent stem cells. J. Cardiovasc. Transl. Res. 2010;3(1):13–23. doi: 10.1007/s12265-009-9150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Molitch-Hou M. Organovo Delivers its 3D Printed Livers - 3D Printing Industry [Internet]. 3D printing industry. 2014. Available from: http: //3dprintingindustry.com/news/organovo-delivers-its-3d-printed-livers-36648/
- 106.Velasquillo C. Skin 3D bioprinting. Applications in cosmetology. J Cosmet Dermatological Sci Appl. 2013;03(01):85–89. [Google Scholar]
- 107.Wang C., Tang Z., Zhao Y., et al. Three-dimensional in vitro cancer models: A short review. Biofabrication. 2014;6(2):022001. doi: 10.1088/1758-5082/6/2/022001. [DOI] [PubMed] [Google Scholar]
- 108.Roy A., Saxena V., Pandey L.M. 3D printing for cardiovascular tissue engineering: A review. Mater. Technol. 2018;33(6):433–442. [Google Scholar]


