Abstract
Objective:
Neurological and medical complications are major causes of morbidity and mortality after ischemic stroke. This study aimed to identify the incidence of stroke-related complications following large hemisphere infarction (LHI) and to explore their influence on unfavorable outcome in LHI patients.
Methods:
We prospectively enrolled consecutive patients with LHI. The unfavorable outcome was defined as an modified Rankin Scale (mRS) score of 4–6 at 3 months. Multivariate logistic regression analysis was employed to identify the stroke-related complications associated with unfavorable outcome.
Results:
Of the 256 cases with LHI included, 41 (16.0%) died during hospitalization, 94 (36.7%) died and 140 (55.3%) patients had unfavorable outcome at 3 months. A total of 194 (75.8%) had at least one complication. The three most common medical complications were pneumonia (53.5%), electrolyte disorder (30.9%), and urinary incontinence (18.4%), and the three most common neurological complications were malignant brain edema (31.2%), hemorrhagic transformation (27.7%), and poststroke seizures (7.0%). Overall, LHI patients with unfavorable outcome had more frequent stroke-related complications (91.4% versus 55.8%, p < 0.001) than patients with favorable outcome. After adjusting for age, baseline National Institutes of Health Stroke Scale score, and other confounders, only malignant brain edema [odds ratios (OR) 19.76, 95% confidence interval (CI) 4.73–82.45] and pneumonia (OR 2.45, 95% CI 1.11–5.40) were independently associated with 3-month unfavorable outcome in patients with LHI.
Conclusions:
More than three-quarters of LHI patients have at least one stroke-related complication. LHI patients with the unfavorable outcome had stroke-related complications more frequently, whereas only malignant brain edema and pneumonia are independently associated with 3-month unfavorable outcome.
Keywords: complications, large hemispheric infarction, outcome
Introduction
Large hemispheric infarction (LHI), which constitutes up to 10% of all supratentorial ischemic strokes,1 is a devastating condition with high mortality and poor functional outcome in most conservatively treated patients.2 Several pharmacological strategies have been proposed, but none has been proved by adequate evidence from clinical trials. Until recently, treatment of LHI remained a major unsolved problem in neurocritical care.3 On account of the limitations of medical therapies, decompressive hemicraniectomy (DHC) within 48 h after stroke onset has been proposed as a therapeutic choice for LHI patients with malignant brain edema that is characterized as malignant middle cerebral artery infarction (mMCAI).4
It is known that poststroke complications are the leading cause of death, constituting 23–50% of total deaths in ischemic stroke patients.5 It has also been reported that poststroke medical and neurological complications may influence not only mortality but also functional outcome.6,7 Roth and colleagues have indicated that greater neurological deficit is closely related to a higher frequency of complications in stroke patients, and neurological impairment level is the most substantial factor predicting the rate of complications.8 As a result, it is reasonable to suspect that stroke-related complications might play an important role in the development of unfavorable outcome in LHI patients.
Nowadays, limited data exist regarding the incidence of stroke-related complications after LHI, and the relationship between stroke-related complications and unfavorable outcome in patients with LHI has not been systematically investigated. One of our published studies mainly discussed the factors associated with favorable outcome in LHI patients.9 The present study is a follow-up study aimed at describing the incidence of medical and neurological complications in an LHI cohort, and exploring the impact of these complications on unfavorable outcome in LHI patients.
Methods
Study design and subjects
From 1 October 2011 to 30 September 2014, patients who were admitted to the Department of Neurology, People’s Hospital of Deyang City with either a first-ever or recurrent stroke were consecutively registered. We enrolled patients who were admitted within 30 days from symptom onset and diagnosed with LHI. LHI was defined as an ischemic stroke in the territory of the middle cerebral artery (MCA), with computed tomography (CT) or magnetic resonance imaging (MRI) evidence of infarction involving more than 50% of MCA region, with or without the involvement of the adjacent territories.10 All patients had an initial brain CT scan before treatment. A second CT scan or MRI was performed within the first 7 days of hospitalization. Other CT scans were performed if patients had neurological deterioration, to determine brain edema or hemorrhagic transformation. Cases with incomplete hospital records or missing imaging that would prevent complete data collection were excluded. We also excluded cases with a pre-existing score of more than 2 on the modified Rankin scale (mRS, a scale of 0–6, with 0 indicating no symptoms and 6 indicating death) and who lived dependently.11
The study protocol was approved by the Ethics Committee of People’s Hospital of Deyang City (Reference No. 2011-04-134). Written informed consent was obtained from all patients before they were enrolled or from their legal representative if the patient had lost the capacity to give informed consent.
Data collection and outcome
Data were collected at the time of assessment via a standardized structured form. Detailed methods for data collection were described in our previous study.9,12 We collected baseline data including age, gender, living environment (rural or urban), admission delay, baseline systolic and diastolic blood pressure, and serum glucose on admission. Initial stroke severity was assessed by using the National Institutes of Health Stroke Scale (NIHSS) score. Vascular risk factors surveyed in the present study included hypertension, diabetes mellitus, dyslipidemia, coronary heart disease, atrial fibrillation, rheumatic heart disease, previous stroke/transient ischemic attack (TIA), and current smoking and alcohol consumption, which have been elaborated upon in a previous study.9,12 In-hospital treatments analyzed in our study included thrombolysis, DHC, mechanical ventilation, osmotic agents (such as mannitol), antiplatelet agents, anticoagulants, antihypertensives, statins, and antidiabetics. DHC was conducted according to the eligibility and exclusion criteria from the pooled analysis of three European randomized trials.4 Stroke-related complications, including both neurological and medical complications during hospitalization, were reviewed by data collectors who were not aware of the study from hospital records when the patient was discharged. Neurological complications included brain edema, hemorrhagic transformation, poststroke seizures, central hyperthermia, and recurrent stroke, while medical complications included pneumonia, urinary tract infection, gastrointestinal bleeding, electrolyte disorder, urinary incontinence, acute renal failure, deep venous thrombosis, bedsore, and falls.6 Pneumonia was diagnosed according to the criteria of the Centers for Disease Control and Prevention (CDC) criteria or diagnosed as hospital-acquired pneumonia (HAP) or ventilator-associated pneumonia by respiratory physicians.13 Electrolyte disturbances were defined as abnormal serum potassium or sodium concentration (normokalemia was defined as serum K+ concentration 3.5–5.5 mmol/l and normonatremia was defined as serum Na+ concentration 135–150 mmol/l, respectively). Gastrointestinal bleeding was defined as an episode of upper or lower gastrointestinal bleeding, documented by data collectors from the patient’s hospital records, regardless of the cause of bleeding. We have not included subacute and chronic neurological and medical complications such as depression and dementia, because these complications are beyond the scope of our study.
Patients were followed up 3 months after stroke onset by telephone interview or letter inquiries. The primary outcomes measure in our study was 3-month unfavorable outcome (defined as an mRS score of 4–6).11
Statistical analyses
Baseline characteristics were compared between LHI patients with unfavorable and favorable outcomes. Intergroup differences in categorical variables were assessed for significance using the χ2 tests or Fisher’s exact tests, while differences in continuous variables were assessed using Student’s t tests or the Mann–Whitney U test. Univariate analysis was performed to test variables that may affect the outcome. The included variables were: age, baseline NIHSS score, vascular risk factors surveyed in our study, in-hospital treatments, and stroke-related complication. The odds ratios (ORs) for variables associated with unfavorable outcomes were identified via multivariable logistic regression analyses by using the forced entry method adjusted for variables with p < 0.05 in univariate analyses. The 95% confidence intervals (CI) were calculated to describe the precision of the estimates. All statistical analysis was performed using SPSS v21.0 (SPSS, Chicago, IL, USA). Two-sided p < 0.05 was considered to be statistically significant.
Results
During the study period, 1542 patients suffering first-ever or recurrent ischemic stroke were consecutively registered. Of these patients, 256 (16.6%) with LHI were enrolled [mean age: 61.6 ± 15.3 years; 133 (52.0%) female; median NIHSS score on admission: 14]. Among the enrolled cohort, 24 (9.4%) cases received DHC and 30 (11.7%) received mechanical ventilation. None of the patients administered intra-arterial revascularization procedures. All LHI patients received a cranial CT examination at least once and 161 (62.9%) patients received MRI; 41 (16.0%) patients died during hospitalization. At the end of 3 months, three (1.2%) patients were lost to follow up. Among the entire cohort, 94 (36.7%) patients died and 140 (55.3%) patients had unfavorable outcome at 3 months.
Baseline characteristics by outcome groups are shown in Table 1. Compared with patients with favorable outcome, patients with unfavorable outcomes were older (66.2 ± 14.1 versus 55.8 ± 14.7, p < 0.001), had more frequent history of hypertension (59.3% versus 38.9%, p = 0.002), higher blood pressure on admission (systolic 145.1 ± 26.1 versus 134.7 ± 24.9 mmHg, p = 0.001; diastolic 86.9 ± 16.2 versus 80.2 ± 14.9 mmHg, p = 0.001; respectively), more elevated baseline serum glucose (8.2 ± 3.3 versus 7.2 ± 3.3mmol/l, p = 0.015), and shorter onset to admission time (24 versus 26 h, p = 0.014). The median baseline NIHSS score was 17 in patients with unfavorable outcomes and 11 in those with favorable outcomes (p < 0.001). For the in-hospital treatments of LHI, patients with unfavorable outcomes less frequently received antiplatelets (57.1% versus 93.8%, p < 0.001) and statins (25.7% versus 46.9%, p < 0.001) in the acute phase of stroke, but nevertheless more frequently used osmotic agents (89.3% versus 69.9%, p < 0.001), mechanical ventilation (20.0% versus 1.8%, p < 0.001), and DHC (15.7% versus 1.8%, p < 0.001).
Table 1.
Baseline characteristics and in-hospital treatment of LHI patients with unfavorable and favorable outcome.
| Unfavorable (mRS score 4–6) (n = 140) | Favorable (mRS score 0–3) (n = 113) | p value | |
|---|---|---|---|
| Age (years) | |||
| Mean ± SD | 66.2 ± 14.1 | 55.8 ± 14.7 | <0.001* |
| Median(range) | 69 (17–99) | 57 (15–88) | <0.001† |
| Female, n (%) | 76 (54.3) | 56 (49.6) | 0.527‡ |
| Rural population, n (%) | 43 (30.7) | 41 (36.3) | 0.421‡ |
| Time from onset (hours), median (range) | 24 (1–720) | 26 (3–720) | 0.014† |
| NIHSS score on admission, median (range) | 17 (5–33) | 11 (4–31) | <0.001† |
| SBP on admission (mm Hg) | 145.1 ± 26.1 | 134.7 ± 24.9 | 0.001* |
| DBP on admission (mm Hg) | 86.9 ± 16.2 | 80.2 ± 14.9 | 0.001* |
| Serum glucose on admission (mmol/l) | 8.2 ± 3.3 | 7.2 ± 3.3 | 0.015* |
| Risk factors, n (%) | |||
| Hypertension | 83 (59.3) | 44 (38.9) | 0.002‡ |
| Diabetes mellitus | 32 (22.9) | 20 (17.7) | 0.350‡ |
| Dyslipidemia | 22 (15.7) | 25 (22.1) | 0.198‡ |
| Coronary heart disease | 21 (15.0) | 9 (8.0) | 0.117‡ |
| Atrial fibrillation | 69 (49.3) | 42 (37.2) | 0.057‡ |
| Rheumatic heart disease | 27 (19.3) | 31 (27.4) | 0.135‡ |
| Current smoking | 30 (21.4) | 27 (23.9) | 0.653‡ |
| Alcohol consumption | 23 (16.4) | 18 (15.9) | 0.915‡ |
| Previous all strokes/TIA | 27 (19.3) | 15 (13.3) | 0.236‡ |
| Stroke in dominant hemisphere, n(%) | 65 (46.4) | 60 (53.1) | 0.313‡ |
| In-hospital Treatments, n(%) | |||
| Thrombolysis§ | 5 (3.6) | 2 (1.8) | 0.466‡ |
| Decompressive surgery§ | 22 (15.7) | 2 (1.8) | <0.001‡ |
| Mechanical ventilation§ | 28 (20.00) | 2 (1.8) | <0.001‡ |
| osmotic agents§ | 125 (89.3) | 79 (69.9) | <0.001‡ |
| Statins in acute phase§ | 36 (25.7) | 53 (46.9) | <0.001‡ |
| Antiplatelets§ | 80 (57.1) | 106 (93.8) | <0.001‡ |
| Anticoagulants‖ | 6 (4.3) | 12 (10.6) | 0.083‡ |
| Antihypertensives‖ | 35 (25.0) | 25 (22.1) | 0.657‡ |
| Antidiabetic drugs‖ | 19 (13.6) | 16 (14.2) | 0.893‡ |
DBP, diastolic blood pressure; LHI, large hemisphere infarction; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; SBP, systolic blood pressure; TIA, transient ischemic attack.
Student’s t test.
Mann–Whitney U test.
χ2 test.
Acute phase treatment.
Percentage is calculated for patients with an indication of the treatment.
The incidence of stroke-related complications in LHI patients is displayed in Figure 1. Among the entire cohort, 194 (75.8%) cases had at least one stroke-related complication. The three most common medical complications in our cohort were pneumonia (53.5%), electrolyte disorder (30.9%), and urinary incontinence (18.4%), followed by gastrointestinal bleeding (11.7%), urinary tract infection (7.4%), acute renal failure (7.0%), bedsore (5.1%), deep venous thrombosis (3.5%), and falls (1.2%). The three most common neurological complications were malignant brain edema (31.2%), hemorrhagic transformation (27.7%), and poststroke seizures (7.0%), followed by central hyperthermia (4.3%) and recurrent stroke (1.2%).
Figure 1.
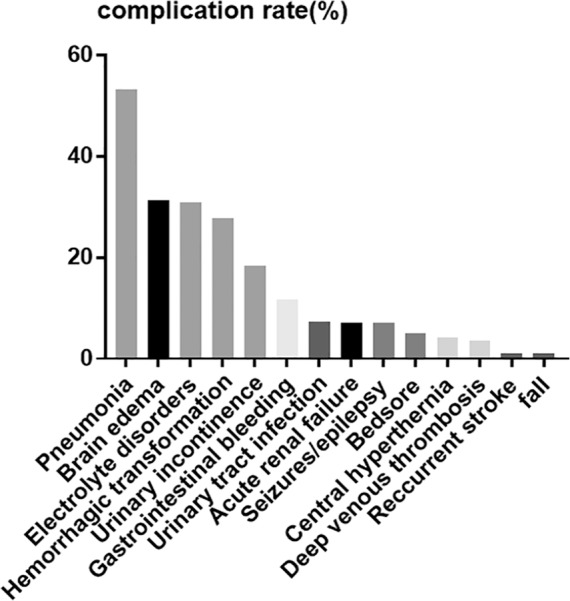
The incidence of stroke-related complications in the entire cohort of LHI patients.
LHI, large hemisphere infarction.
Stroke-related complications during hospitalization in LHI patients with and without unfavorable outcomes are compared in Table 2. Overall, patients with unfavorable outcome had more frequent stroke-related complications (91.4% versus 55.8%, p < 0.001). When neurological complications were concerned, patients with unfavorable outcomes had a significantly higher incidence of malignant brain edema (53.6% versus 4.4%, p < 0.001) than those with favorable outcomes. However, there was no significant difference in the event rates of hemorrhagic transformation, seizures/epilepsy, central hyperthermia, and recurrent stroke between LHI patients with unfavorable outcomes and those without (all p > 0.05). Concerning medical complications, pneumonia was the most common medical complication in LHI patients, whether there was an unfavorable outcome or not (67.1% and 36.3%, respectively). Moreover, the event rates of pneumonia, electrolyte disturbance, gastrointestinal bleeding, acute renal failure, urinary incontinence, and bedsore were significantly higher in LHI patients with unfavorable outcome (all p < 0.05).
Table 2.
Stroke-related complication during hospitalization of LHI patients with unfavorable and favorable outcome<.
| Unfavorable (mRS score 4–6) (n = 140) | Favorable (mRS score 0–3) (n = 113) | p value | |
|---|---|---|---|
| Stroke-related complications, n (%) | 128 (91.4) | 63 (55.8) | <0.001 |
| Neurological complications, n (%) | |||
| Brain edema | 75 (53.6) | 5 (4.4) | <0.001 |
| Hemorrhagic transformation | 46 (32.9) | 25 (22.1) | 0.068 |
| Seizures/epilepsy | 11 (7.9) | 7 (6.2) | 0.634 |
| Central hyperthermia | 9 (6.4) | 2 (1.8) | 0.118 |
| Recurrent stroke | 3 (2.1) | 0 (0) | 0.256* |
| Medical complications, n (%) | |||
| Pneumonia | 94 (67.1) | 41 (36.3) | <0.001 |
| Urinary tract infection | 14 (10.0) | 5 (4.4) | 0.148 |
| Gastrointestinal bleeding | 25 (17.9) | 4 (3.5) | 0.001 |
| Electrolyte disturbance | 56 (40.0) | 22 (19.5) | <0.001 |
| Acute renal failure | 18 (12.9) | 0 (0) | <0.001* |
| Urinary incontinence | 39 (27.9) | 8 (7.1) | <0.001 |
| Bedsore | 11 (7.9) | 2 (1.8) | 0.042 |
| Deep venous thrombosis | 6 (4.3) | 3 (2.7) | 0.735 |
| Falls | 3 (2.1) | 0 (0) | 0.256* |
mRS, modified Rankin scale.
Fisher exact test.
Variables that had potential confounding effects on the 3-month unfavorable outcome in univariate analysis (p < 0.05) were included in multivariate logistic regression, and the final results are shown in Table 3 with OR and 95% CI. After adjusting for potential confounding factors excluding stroke-related complications (model 1), age (OR 1.06; 95% CI 1.03–1.08), baseline NIHSS score (OR 1.15; 95% CI 1.08–1.21), history of hypertension (OR 2.65; 95% CI 1.28–5.50), DHC (OR 9.60; 95% CI 1.10–83.54), and statins used in acute phase (OR 0.36; 95% CI 0.18–0.74) were all independently associated with 3-month unfavorable outcome in patients with LHI (all p < 0.05). When stroke-related complications were included in the multivariate logistic regression (model 2), history of hypertension and DHC were no longer independent predictors of 3-month unfavorable outcome in LHI patients; nevertheless, brain edema (OR 19.76, 95 %CI 4.73–82.45) and pneumonia (OR 2.45, 95% CI 1.11–5.40) were independent predictors of 3-month unfavorable outcome combined with age (OR 1.05, 95% CI 1.02–1.08), baseline NIHSS score (OR 1.11, 95% CI 1.04–1.19), and statins used in acute phase (OR 0.40, 95% CI 0.18–0.92) (all p < 0.05).
Table 3.
Factors associated with 3-month unfavorable outcome in LHI patients.
| Variables | Univariate analysis | Multivariate analysis (model 1)* |
Multivariate analysis (model 2)* |
|---|---|---|---|
| Age | 1.05 (1.03–1.07) | 1.06 (1.03–1.08) | 1.05 (1.02–1.08) |
| Baseline NIHSS score | 1.16 (1.11–1.22) | 1.15 (1.08–1.21) | 1.11 (1.04–1.19) |
| Hypertension | 2.28 (1.37–3.79) | 2.65 (1.28–5.50) | |
| Baseline serum glucose | 1.10 (1.00–1.21) | ||
| Decompressive surgery | 10.34 (2.37–45.02) | 9.60 (1.10–83.54) | |
| Ventilatory Support | 13.87 (3.22–59.64) | ||
| Antiplatelets | 0.19 (0.08–0.44) | ||
| Statins | 0.39 (0.23–0.66) | 0.36 (0.18–0.74) | 0.40 (0.18–0.92) |
| Pneumonia | 3.58 (2.13–6.04) | 2.45 (1.11–5.40) | |
| Gastrointestinal bleeding | 5.92 (1.99–17.57) | ||
| Electrolyte disturbance | 2.75 (1.55–4.90) | ||
| Bedsore | 4.73 (1.02–21.80) | ||
| Urinary incontinence | 5.06 (2.25–11.37) | ||
| Brain edema | 24.92 (9.58–64.84) | 19.76 (4.73–82.45) |
CI, Confidence intervals; LHI, large hemisphere infarction; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio.
Variables which had a significant association with unfavorable outcome in univariate analysis are listed (p < 0.05). Figures in parentheses are 95% CI. Model 1: adjusted for variables with p < 0.05 in univariate analyses excluding stroke-related complications. Model 2: adjusted for variables with p < 0.05 in univariate analyses including stroke-related complications.
Adjusted OR with p < 0.05 in the multivariate logistic regression analysis.
Discussion
Nowadays, information on the incidence of stroke-related complications after LHI is scarce, and the relationship between stroke-related complications and clinical outcome of LHI patients has not been examined systematically. In our study, we established that more than three-quarters of patients with LHI have at least one stroke-related complication. The three most common stroke-related complications were pneumonia (53.5%), malignant brain edema (31.2%), and electrolyte disorder (30.9%). LHI patients with 3-mouth unfavorable outcome had more frequent stroke-related complications than those without unfavorable outcome. After adjusting for age, baseline NIHSS score and other confounders, only malignant brain edema (OR 19.76, 95% CI 4.73–82.45) and pneumonia (OR 2.45, 95% CI 1.11–5.40) were independent predictors of 3-month unfavorable outcome in LHI patients.
It has been reported that neurological and medical complications are major causes of morbidity and mortality after ischemic stroke.14 Medical complications, such as pneumonia, electrolyte disorder, urinary incontinence, gastrointestinal bleeding, urinary tract infection, acute renal failure, bedsore, deep venous thrombosis, and falls, usually develop in the acute or subacute phase of stroke (within the first few weeks of stroke).15 Pre-existing medical conditions, advanced age, and prestroke disability can increase an individual’s risk for developing these events. Patients with severe neurological deficit are particularly susceptible to many medical complications.6,8,16 Neurological complications, such as brain edema, hemorrhagic transformation, poststroke seizures, central hyperthermia, and recurrent stroke, are less frequent than medical complications.17 However, most neurological complications occur earlier in the course of neurological deterioration, within 48–72 h of stroke onset rather than within the first few weeks of stroke.6,18–20 Results from some studies have indicated that deaths within the early few days after stroke are caused mainly by the direct consequence of brain damage from neurological complications, such as the formation of herniation caused by malignant brain edema.21 In a study of early neurological deterioration in 1964 acute ischemic stroke patients, 33.6% of cases deteriorated because of stroke progression, 27.3% as a result of brain edema, 11.3% owing to recurrent ischemic stroke, 10.5% because of hemorrhagic transformation, and the remaining 17.3% deteriorated because of medical complications.22 Several previous studies have suggested that about 24.2–95% of patients with acute ischemic stroke experienced one or more neurological and medical complications during hospitalization.6,17,18 In the present study, of the total of 256 LHI patients, 75.8% had at least one stroke-related complication. The differences in the incidence of stroke-related complications among different studies are most logically explained by the severity of stroke patients and the definition and scope of the stroke-related complications studied. In the present study, we did not include subacute and chronic neurological and medical complications such as depression and dementia, because these complications are beyond the scope of our study.
Several previous studies have focused on one single medical complication and its management in DHC cohorts, with little discussion of neurological complications.23–27 Moreover, there are few data available to indicate the impact of different stroke-related complications on unfavorable outcome in patients with LHI. In our cohort, we found that LHI patients with unfavorable outcomes had more frequent stroke-related complications than those with favorable outcomes. Meanwhile, patients with unfavorable outcomes more frequently had DHC and mechanical ventilation administered than those with favorable outcomes. Multivariate analysis excluding stroke-related complications showed that DHC is an independent predictor of 3-month unfavorable outcome in LHI patients (model 1). However, when stroke-related complications were included in the multivariate analysis, DHC was no longer an independent predictor of a 3-month unfavorable outcome, while brain edema and pneumonia were independent predictors of unfavorable outcome (model 2). Three randomized clinical trials in Europe had demonstrated the effect of DHC among LHI patients 60 years of age or younger with life-threatening edema.4 However, a meta-analysis of 12 observational studies indicated that DHC reduced mortality with increasing the risk of poor functional outcome among patients older than 60 years in patients with mMCAI.28 The DESTINY II trial published recently indicated that DHC increased survival among mMCAI patients older than 60 years at the cost of persistent morbidity,27 which was also confirmed in a small sample randomized clinical trial conducted in a Chinese population.29 In our study, DHC was not an independent predictor of 3-month unfavorable outcome in LHI patients after adjusting for age, baseline NIHSS score, and stroke-related complications. Moreover, we could reasonably speculate that it was the malignant brain edema and increased risk of pneumonia concomitant with surgery, rather than DHC itself, that were independently associated with unfavorable outcome in LHI patients. Medical complications concomitant with DHC might affect the clinical outcome of LHI patients. A 6-year single-center study in the United States found an incidence of pneumonia after DHC of 11.1% in 252 patients, and this was closely related to the increased rate of mortality.30 In another cohort conducted in LHI patients undergoing DHC for life-threatening edema, 42.4% of patients experienced at least one postoperative complication, with lung infection being the most common cause (39% of all complications).10 Pneumonia was the most common medical complication in our cohort (67.1% in patients with unfavorable outcomes and 36.3% in those without unfavorable outcomes), and, after adjusting for age, stroke severity, and other confounding factors, the risk of unfavorable outcome in LHI patients complicated with pneumonia was 2.45 (95% CI 1.11–5.40) times higher than those without pneumonia. Since most pneumonia is potentially preventable or treatable, we should pay more attention to the prevention, early detection, and treatment of pneumonia in LHI patients especially those undergoing DHC, because of the higher events risk and concomitant unfavorable outcome.
In our study, of patients with unfavorable outcome, 53.6% developed brain edema, while only 15.7% underwent decompressive surgery, which indicated that decompressive surgery for malignant brain edema in LHI patients remains underutilized. In our entire cohort, 31.2% (80/256) patients with LHI had malignant brain edema; of these 80, only 30% (24/80) underwent DHC. This result is in line with data from a published cohort study of decompressive craniectomy for mMCAI conducted in a Chinese population that also indicated that DHC remains underutilized in clinical settings.31 The DHC procedure for malignant edema is also underused in other countries worldwide.32,33 Several reasons account for the low rate of DHC. First, both clinicians and relatives of patients do not consider mRS score 4 as an acceptable outcome and most families could not accept the result of living with a severe disability. Second, most families hesitate to accept brain surgery, and choose palliative care or hospice due to financial concerns and in accordance with religious and other values. The decision-making process should balance evidence, patient preference, and clinical expertise. Moreover, safer and widely available decompressive procedures and postoperative care may be needed as a life-saving measure. In China, some critically ill patients were given up to treatment by their relatives and died within a few days of discharge, especially those patients coming from rural areas. Meanwhile, most survivors with severe disability were taken home directly or opted for hospice care, rather than seeking further speech therapy and physiotherapy, which could explain why the follow-up data obtained over the phone or by mail from LHI patients recovering from such a devastating condition were so close to their discharge in the real world.
The results of the present study should be interpreted with caution because of its limitations. First, this was a single tertiary hospital-based study, which may not represent the whole population. Some severe stroke patients might not be hospitalized, especially those who died before being admitted to hospital, so we cannot exclude inclusion bias. Second, we conducted only a 3-month follow up so the long-term effects remains unclear. Therefore, we cannot advise whether the complications we identified associated with unfavorable outcome in LHI patients also have long-term effects. Finally, follow up in our study was performed by telephone interview or postal questionnaire instead of a clinic visit, which may result in a reporting bias. However, there are few available data to indicate the impact of different stroke-related complications on unfavorable outcome in patients with LHI. In our cohort, we identified that more than three-quarters of patients with LHI have at least one stroke-related complication. LHI patients with the unfavorable outcome more frequently had stroke-related complications than patients without unfavorable outcome. Medical and neurological complications may seriously affect clinical outcomes of LHI patients, whereas only malignant brain edema and pneumonia are independently associated with 3-month unfavorable outcome. Well designed studies focused on the prevention and treatment of stroke-related pneumonia are urgently needed.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and publication of this article: This research was funded by Universal Application Program, Health and Family Planning Commission of Sichuan Province in China (17PJ084) and Key Research and Development Program, Science and Technology Department of Sichuan Province in China (NO.2017SZ0007).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Jie Li  https://orcid.org/0000-0001-9575-4232
https://orcid.org/0000-0001-9575-4232
Contributor Information
Jie Li, Department of Neurology, People’s Hospital of Deyang City, Deyang, PR China.
Ping Zhang, Department of Neurology, People’s Hospital of Deyang City, Deyang, PR China.
Simiao Wu, Stroke Clinical Research Unit, Department of Neurology, West China Hospital, Sichuan University, Chengdu, PR China.
Yanfen Wang, Department of Neurology, People’s Hospital of Deyang City, Deyang, PR China.
Ju Zhou, Department of Neurology, People’s Hospital of Deyang City, Deyang, PR China.
Xingyang Yi, Department of Neurology, People’s Hospital of Deyang City, Deyang, PR China.
Chun Wang, Department of Neurology, People’s Hospital of Deyang City, No.173, North Taishan Road, Deyang 618000, Sichuan Province, PR China.
References
- 1. Huttner HB, Schwab S. Malignant middle cerebral artery infarction: clinical characteristics, treatment strategies, and future perspectives. Lancet Neurol 2009; 8: 949–958. [DOI] [PubMed] [Google Scholar]
- 2. Heiss WD. Malignant MCA infarction: pathophysiology and imaging for early diagnosis and management decisions. Cerebrovasc Dis 2016; 41: 1–7. [DOI] [PubMed] [Google Scholar]
- 3. Steiner T, Ringleb P, Hacke W. Treatment options for large hemispheric stroke. Neurology 2001; 57(Suppl. 2): S61–S68. [DOI] [PubMed] [Google Scholar]
- 4. Vahedi K, Hofmeijer J, Juettler E, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol 2007; 6: 215–222. [DOI] [PubMed] [Google Scholar]
- 5. Weimar C, Roth MP, Zillessen G, et al. Complications following acute ischemic stroke. Eur Neurol 2002; 48: 133–140. [DOI] [PubMed] [Google Scholar]
- 6. Johnston KC, Li JY, Lyden PD, et al. Medical and neurological complications of ischemic stroke: experience from the RANTTAS trial. Stroke 1998; 29: 447–453. [DOI] [PubMed] [Google Scholar]
- 7. Davenport RJ, Dennis MS, Wellwood I, et al. Complications after acute stroke. Stroke 1996; 27: 415–420. [DOI] [PubMed] [Google Scholar]
- 8. Roth EJ, Lovell L, Harvey RL, et al. Incidence of and risk factors for medical complications during stroke rehabilitation. Stroke 2001; 32:523–529. [DOI] [PubMed] [Google Scholar]
- 9. Li J, Zhang P, Wu S, et al. Factors associated with favourable outcome in large hemispheric infarctions. BMC Neurol 2018; 18: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Uhl E, Kreth FW, Elias B, et al. Outcome and prognostic factors of hemicraniectomy for space occupying cerebral infarction. J Neurol Neurosurg Psychiatry 2004; 75: 270–274. [PMC free article] [PubMed] [Google Scholar]
- 11. de Haan R, Limburg M, Bossuyt P, et al. The clinical meaning of Rankin ‘handicap’ grades after stroke. Stroke 1995; 26: 2027–2030. [DOI] [PubMed] [Google Scholar]
- 12. Li J, Zhang P, Tao W, et al. Age-specific clinical characteristics and outcome in patients over 60 years old with large hemispheric infarction. Brain Behav 2018; 8: e01158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008; 36: 309–332. [DOI] [PubMed] [Google Scholar]
- 14. Balami JS, Chen RL, Grunwald IQ, et al. Neurological complications of acute ischaemic stroke. Lancet Neurol 2011; 10: 357–371. [DOI] [PubMed] [Google Scholar]
- 15. Kumar S, Selim MH, Caplan LR. Medical complications after stroke. Lancet Neurol 2010; 9: 105–118. [DOI] [PubMed] [Google Scholar]
- 16. Kalra L, Yu G, Wilson K, et al. Medical complications during stroke rehabilitation. Stroke 1995; 26: 990–994. [DOI] [PubMed] [Google Scholar]
- 17. Dromerick A, Reding M. Medical and neurological complications during inpatient stroke rehabilitation. Stroke 1994; 25: 358–361. [DOI] [PubMed] [Google Scholar]
- 18. Hong KS, Kang DW, Koo JS, et al. Impact of neurological and medical complications on 3-month outcomes in acute ischaemic stroke. Eur J Neurol 2008; 15: 1324–1331. [DOI] [PubMed] [Google Scholar]
- 19. Langhorne P, Stott DJ, Robertson L, et al. Medical complications after stroke: a multicenter study. Stroke 2000; 31: 1223–1229. [DOI] [PubMed] [Google Scholar]
- 20. Karepov VG, Gur AY, Bova I, et al. Stroke-in-evolution: infarct-inherent mechanisms versus systemic causes. Cerebrovasc Dis 2006; 21: 42–46. [DOI] [PubMed] [Google Scholar]
- 21. Vernino S, Brown RD, Sejvar JJ, et al. Cause-specific mortality after first cerebral infarction: a population-based study. Stroke 2003; 34: 1828–1832. [DOI] [PubMed] [Google Scholar]
- 22. Weimar C, Mieck T, Buchthal J, et al. Neurologic worsening during the acute phase of ischemic stroke. Arch Neurol 2005; 62: 393–397. [DOI] [PubMed] [Google Scholar]
- 23. Vahedi K, Vicaut E, Mateo J, et al. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL trial). Stroke 2007; 38: 2506–2517. [DOI] [PubMed] [Google Scholar]
- 24. Georgiadis D, Schwarz S, Aschoff A, et al. Hemicraniectomy and moderate hypothermia in patients with severe ischemic stroke. Stroke 2002; 33: 1584–1588. [DOI] [PubMed] [Google Scholar]
- 25. Chalouhi N, Daou B, Rincon F, et al. Risk of venous thromboembolism in patients with large hemispheric infarction undergoing decompressive hemicraniectomy. Neurocrit Care 2016; 25: 105–109. [DOI] [PubMed] [Google Scholar]
- 26. O’Donnell MJ, Kapral MK, Fang J, et al. Gastrointestinal bleeding after acute ischemic stroke. Neurology 2008; 71: 650–655. [DOI] [PubMed] [Google Scholar]
- 27. Jüttler E, Unterberg A, Woitzik J, et al. Hemicraniectomy in older patients with extensive middle-cerebral-artery stroke. N Engl J Med 2014; 370: 1091–1100. [DOI] [PubMed] [Google Scholar]
- 28. Gupta R, Connolly ES, Mayer S, et al. Hemicraniectomy for massive middle cerebral artery territory infarction: a systematic review. Stroke 2004; 35: 539–543. [DOI] [PubMed] [Google Scholar]
- 29. Zhao J, Su YY, Zhang Y, et al. Decompressive hemicraniectomy in malignant middle cerebral artery infarct: a randomized controlled trial enrolling patients up to 80 years old. Neurocrit Care 2012; 17: 161–171. [DOI] [PubMed] [Google Scholar]
- 30. Alshekhlee A, Horn C, Jung R, et al. In-hospital mortality in acute ischemic stroke treated with hemicraniectomy in US hospitals. J Stroke Cerebrovasc Dis 2011; 20: 196–201. [DOI] [PubMed] [Google Scholar]
- 31. Hao Z, Chang X, Zhou H, et al. A cohort study of decompressive craniectomy for malignant middle cerebral artery infarction: a real-world experience in clinical practice. Medicine (Baltimore) 2015; 94: e1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bar M, Mikulik R, Skoloudik D, et al. Decompressive surgery for malignant supratentorial infarction remains underutilized after guideline publication. J Neurol 2011; 258: 1689–1694. [DOI] [PubMed] [Google Scholar]
- 33. Neugebauer H, Creutzfeldt CJ, Hemphill JC, et al. DESTINY-S: attitudes of physicians toward disability and treatment in malignant MCA infarction. Neurocrit Care 2014; 21: 27–34. [DOI] [PubMed] [Google Scholar]


