Abstract
Background:
Chronic kidney disease (CKD) is a significant health problem in Canada. Understanding the capacity of the Canadian health-care system to deliver kidney care is important to provide optimal care.
Objective:
To compare Canada’s position in relation to countries of similar economic standing.
Design:
Cross-sectional electronic survey.
Setting:
Member countries of the Organisation for Economic Co-operation and Development (OECD) that participated in the survey.
Participants:
Nephrologists, other physicians, policymakers, and other professionals with relevant expertise in kidney care.
Measurements:
Not applicable.
Methods:
A survey administered by the International Society of Nephrology assessed the global capacity of kidney care delivery. Data from participating OECD countries were analyzed using descriptive statistics to compare Canada’s position.
Results:
Of the participating countries, most funded kidney care services (non-medication) by government (transplantation: 85%, dialysis: 81%, acute kidney injury (AKI): 77%). Most countries covered medication. Canada reported a public funding model for kidney services and a mix of public and private sources for medication. Nephrologists and nephrology trainee densities were lower in Canada compared to the median (15.33 vs. 25.82 and 1.74 vs. 3.94, respectively). CKD was recognized as a health priority in five countries, but not in Canada. Registries for CKD did not exist in most (24/26) countries. Canada followed a national strategy for noncommunicable diseases, but this was not specific to CKD care, dialysis, or transplantation.
Limitations:
Risks of recall bias or social desirability bias are present. Differences in a number of factors could influence discrepancies among countries and were not explored. Responses reflected the existence of practices, policies, and strategies, and may not necessarily describe action or impact. Capacity of care is not equal across all regions and provinces within Canada; however, the findings are reported on a national level and therefore may not appropriately address variability.
Conclusions:
This study describes the capacity for kidney care at a national level within the context of the Canadian health system. The Canadian health-care system is well funded by the government; however, there are areas that could be improved to increase the optimization of kidney care provided.
Keywords: kidney care structure, survey, Canada, OECD, capacity
Abrégé
Contexte:
L’insuffisance rénale chronique (IRC) est un problème de santé important au Canada. Comprendre la capacité du système de santé canadien à fournir des soins en néphrologie est essentiel pour les optimiser.
Objectif:
Situer la position du Canada par rapport aux pays de même statut économique.
Type d’étude:
Une étude transversale menée par sondage en ligne.
Cadre:
Les pays membres de l’Organisation de coopération et de développement économique (OCDE) ayant participé au sondage.
Participants:
Des néphrologues, des médecins, des décideurs et d’autres professionnels possédant une expertise pertinente en santé rénale.
Mesures:
Sans objet
Méthodologie:
Un sondage administré par la Société internationale de néphrologie a évalué la capacité de prestation de soins en néphrologie au niveau mondial. La statistique descriptive a été utilisée pour analyser les données des pays de l’OCDE participants et situer la position du Canada.
Résultats:
Dans la plupart des pays participants, les services de néphrologie (outre les médicaments) étaient financés par le gouvernement (transplantation: 85%, dialyse: 81%, IRA: 77%); et la plupart couvraient les médicaments. Le Canada présente un modèle de financement public pour les soins rénaux et une combinaison de financement public et privé pour les médicaments. Les densités de néphrologues et de stagiaires en néphrologie étaient plus faibles au Canada comparativement à la médiane (respectivement 15,33 contre 25,82 et 1,74 contre 3,94). L’IRC était désignée comme une priorité de santé dans cinq pays, mais pas au Canada. La très grande majorité des pays participants (24/26) ne possédaient pas de registre d’IRC. Le Canada suit une stratégie nationale pour les maladies non transmissibles, mais celle-ci n’est pas spécifique aux soins en IRC, à la dialyse ou à la transplantation.
Limites:
Des risques de biais de rappel ou de biais de désirabilité sociale sont présents. Des différences observées dans un certain nombre de facteurs pourraient influencer les divergences entre les pays; ces différences n’ont pas été explorées. Les réponses obtenues reflètent l’existence de pratiques, de politiques et de stratégies, mais ne décrivent pas nécessairement d’actions ou d’impacts. Enfin, la capacité de prestation de soins n’est pas équivalente dans toutes les régions et provinces du Canada; les résultats sont cependant rapportés au niveau national, ce qui pourrait ne pas traiter la variabilité de manière appropriée.
Conclusion:
Cette étude décrit la capacité du système de santé canadien d’offrir des soins de santé rénale au niveau national. Le système de santé canadien bénéficie d’un bon financement de la part du gouvernement. Certains domaines gagneraient toutefois à être améliorés si l’on souhaite optimiser les soins offerts en néphrologie.
What was known before
The capacity of kidney care delivery in Canada has never before been documented. Health-care systems are structured differently, even in countries with similar economic standing. The current capacity for kidney care based on established domains of health systems delivery in this setting remains unknown.
What this adds
This work reports the current capacity of Canada (in terms of health service delivery, health workforce, health information systems, access to essential medicines, health systems finance, and leadership and governance) for kidney care in comparison to other OECD (Organisation for Economic Co-operation and Development) countries. This information has policy implications for monitoring progress and trends over time.
Introduction
Chronic kidney disease (CKD) is recognized as a major health-care problem due to its association with cardiovascular mortality,1 costs to the health system,2 and patient well-being.3 An estimated 1 230 200 people die annually due to CKD, a 33% increase over the past decade.4 In Canada (2013), an estimated 12.5% of the adult population has CKD.5 Furthermore, the incidence of end-stage kidney disease (ESKD) appears to be increasing in Canada over the last decade,6 and in 2014 there were 193 ESKD patients per million population (PMP).6 This increase is worrisome due to the high financial costs of dialysis2 and impact on patient quality of life.7 The progression of CKD to ESKD can be reduced through appropriate care management. Despite the existence of guidelines for the management of CKD,8 gaps in kidney care remain in Canada.9
Similarly, the burden of acute kidney injury (AKI), coupled with its associated adverse health consequences,10-12 warrants attention. Among patients hospitalized in Alberta, Canada, a presence of AKI during hospitalization has been associated with an increased length of hospitalization stay and increased costs during hospitalization, compared to patients without a diagnosis of AKI.13 Furthermore, AKI has been suggested as a risk factor for the development of CKD14 and deserves focus with respect to optimal kidney care delivery goals.
As kidney care is a global problem and international frameworks exist to guide the evaluation of health systems delivery,19 comparing Canada’s current capacity of kidney care with other countries of similar economic standing may help identify barriers and opportunities. This report describes Canada’s current capacity for kidney care in comparison to other OECD countries.20
Methods
Setting—Health-care System in Canada (General)
Canada is an industrialized nation characterized by a vast geography, high economy indices, and a well-developed health-care system (Table 1). In Canada, health-care delivery is governed by the principles of the Canada Health Act of 1984,21 with roles divided between federal, provincial, and territorial governments.
Table 1.
| National statistics | Area | 9 984 670 sq km |
| Total population | 35 623 680 (2017) | |
| CKD care plan | National plan/strategy for NCDs | Yes |
| National plan/strategy: CKD specific | No | |
| Guideline/service framework | Yes | |
| CKD (non-KRT) registry | No | |
| Planned actionsa | Yes | |
| ESKD data | Incidence/PMP | 200.2 (2016) |
| CKD data | Prevalence/PMP | 1346.4 (2016) |
| Prevalence | 12.5% (2013)5 | |
| Costs data | GDP (PPP) | USD 1.764 trillion (2017) |
| Total health expenditures (% of GDP)17 | 10.4 (2014) | |
| CKD costs data (as % total health expenditure) | Not available | |
| Maintenance HD | USD 73 789 (per person)18 | |
| Maintenance PD | USD 44 434 (per person)18 | |
| Kidney transplantation (first-year) | USD 82 852 (per person)18 |
Note. CKD = chronic kidney disease; NCDs = noncommunicable diseases; ESKD = end-stage kidney disease; KRT = kidney replacement therapy; PMP = per million population; PPP = purchasing power parity; HD = hemodialysis; PD = peritoneal dialysis; GDP = gross domestic product; Can-SOLVE CKD = Canadians Seeking Solutions and Innovations to Overcome Chronic Kidney Disease.
Deliberate policies or strategies by the government at national level toward optimal kidney care (eg, Can-SOLVE CKD funded by the government of Canada).
Setting—Health-care System in Canada (Kidney Care)
Each province has a distinct structure for kidney care (both for AKI and CKD), which varies from a single organization for a whole province (as in British Columbia, Manitoba, New Brunswick, Newfoundland and Labrador, Nova Scotia, Prince Edward Island, Quebec, and Saskatchewan), two organizations (Alberta), or to multiple organizations (Local Health Integration Networks in Ontario). Oversight of CKD care is by individual hospitals (Quebec, Manitoba, Saskatchewan, and the Atlantic provinces) or provincial/regional programs (Alberta, British Columbia, and Ontario). Dialysis is delivered in renal hubs (in-center) across the country, mostly based in university and city hospitals.
Data Collection—Global Kidney Health Atlas (GKHA) Survey
An online survey was administered in 2016 by the International Society of Nephrology (ISN), as described elsewhere.22,23 In brief, a questionnaire was developed to (1) align with the World Health Organization (WHO) Health Systems Framework19 and (2) assess response of the nephrology community through existing strategies and policies and capacity for research and development. Data were analyzed following the WHO framework for assessing national capacity for the prevention and control of noncommunicable diseases (NCDs).24 A convenience sample of stakeholders (nephrologists, other physicians, policymakers, or other professionals) with relevant expertise in kidney care were invited to participate in the survey.23 Three stakeholders were invited from each country based on their knowledge and expertise and therefore ability to represent the country at large. The survey was cross-sectional with the questionnaires administered online. Participation was voluntary assuming implied consent.
Participants were emailed a description of the study and provided a link to access the online survey. Responses were de-identified but tracked using a unique ID, stored in a separate database for administrative purposes. Survey items were mandatory as appropriate (flagged when left empty), and branching logic was used so that questions appeared when relevant (eg, if peritoneal dialysis [PD] was not available in a country, all questions pertaining to PD were hidden). The entire questionnaire was 40 pages, averaging 5 questions per page.23 Participants were able to change responses while they were completing the survey (save & return later function), but responses were not editable following submission. All questionnaires were analyzed. In cases with complete data, we followed up with survey respondents and/or regional boards to clarify. Respondents were allotted 4 weeks to complete the survey, followed by an additional 4 weeks with two reminder emails in 2-week intervals. No monetary incentives or any other incentives were provided to the participants. As described elsewhere,23 data from all individual questionnaires were subsequently extracted and checked for inconsistencies, missing data, duplications, and formatting errors. The data were then merged into a single file to create the global database. Any major inconsistencies that remained following the reviews were systematically addressed by follow-up of individuals who responded to the survey.
The survey was repeated in 2018, using the same methods as described above. The second iteration, however, placed stronger focus on ESKD, particularly related to quality indicators for kidney replacement therapy (KRT) delivery and conservative kidney management (CKM). Data from both the 2016 and 2018 surveys are used additively to report on selected indicators relevant for CKD and ESKD management. For the purposes of this sub-study, only data from participating OECD countries are reported.
Results
Survey Participation
In 2016, 35 OECD countries20 received a survey invitation and 26 (76%) responded (Table 2). In 2018, 36 OECD countries received a survey invitation and all (100%) responded. Overall, participants represented Europe, Latin America, North America, North and East Asia, and Oceania & South East Asia (Table 2). Respondents included nephrologists, other physicians, policymakers, or other (nongovernment organization, nurse, etc.).
Table 2.
Description of Respondents From Participating OECD Countries in the 2016 and 2018 Global Kidney Health Atlas Surveys.
| OECD country | Participation in the 2016 survey? | Total no. of respondents (2016 survey) | Participation in the 2018 survey? | Total no. of respondents (2018 survey) |
|---|---|---|---|---|
| Australia | Yes | 2 | Yes | 3 |
| Austria | No | 0 | Yes | 1 |
| Belgium | Yes | 1 | Yes | 3 |
| Canada | Yes | 4 | Yes | 5 |
| Chile | Yes | 5 | Yes | 2 |
| Czech Republic | Yes | 2 | Yes | 1 |
| Denmark | Yes | 1 | Yes | 3 |
| Estonia | Yes | 1 | Yes | 2 |
| Finland | No | 0 | Yes | 1 |
| France | Yes | 3 | Yes | 2 |
| Germany | Yes | 2 | Yes | 1 |
| Greece | Yes | 4 | Yes | 6 |
| Hungary | Yes | 2 | Yes | 1 |
| Iceland | No | 0 | Yes | 1 |
| Ireland | No | 0 | Yes | 1 |
| Israel | Yes | 2 | Yes | 1 |
| Italy | No | 0 | Yes | 7 |
| Japan | Yes | 8 | Yes | 3 |
| Korea | Yes | 6 | Yes | 4 |
| Latvia | Yes | 1 | Yes | 1 |
| Lithuania | No | 0 | Yes | 2 |
| Luxembourg | No | 0 | Yes | 1 |
| Mexico | Yes | 3 | Yes | 1 |
| Netherlands | Yes | 2 | Yes | 5 |
| New Zealand | Yes | 7 | Yes | 3 |
| Norway | Yes | 1 | Yes | 3 |
| Poland | Yes | 2 | Yes | 1 |
| Portugal | No | 0 | Yes | 3 |
| Slovakia | Yes | 1 | Yes | 4 |
| Slovenia | Yes | 1 | Yes | 1 |
| Spain | Yes | 3 | Yes | 1 |
| Sweden | No | 0 | Yes | 1 |
| Switzerland | No | 0 | Yes | 2 |
| Turkey | Yes | 3 | Yes | 3 |
| United Kingdom | Yes | 1 | Yes | 4 |
| United States | Yes | 3 | Yes | 2 |
Note. OECD = Organisation for Economic Co-operation and Development.
2016 Findings
Financing of kidney care
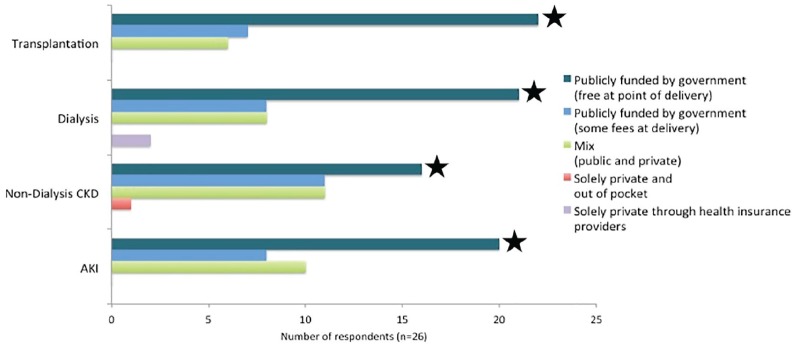
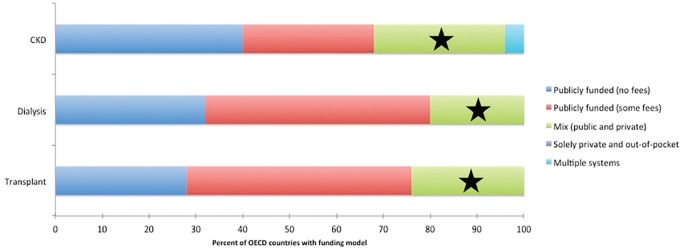
Of the 26 participating OECD countries, most funded kidney care services (excluding medication) exclusively by government, particularly for transplantation (85%), dialysis (81%), and AKI (77%) (Figure 1). Canada reported that non-medication costs were publicly funded and free at point of delivery for people with kidney disease. More than half of OECD countries provided full coverage of medication (Figure 2). Medications were publicly funded (either with no or minimal fees) most often for patients receiving dialysis (80% of countries), those who received a transplant (76%), and lastly with CKD (68%). Canada reported a mix of public and private sources for funding medication.
Figure 1.
Funding of kidney care services (excluding medication) across participating OECD countries (n = 26).
Note. The survey respondents for Canada (★) selected “Publicly funded by government” with no fees at the point of delivery. AKI = acute kidney injury; CKD = chronic kidney disease; OECD = Organisation for Economic Co-operation and Development.
Figure 2.
Funding models for medications of CKD, dialysis, and transplantation patients in participating OECD countries (n = 25).
Note. The survey respondents for Canada (★) selected a “mixed-funding model,” a combination of public and private for the three indicator kidney services. Response from Belgium missing from analysis. CKD = chronic kidney disease; OECD = Organisation for Economic Co-operation and Development.
Health information systems and guidelines
Only 2 out of 26 countries (United Kingdom and Norway) reported a non-dialysis CKD registry. Nearly half (43%; 9/21) of OECD countries used national guidelines for CKD, whereas 57% (12/21) relied on international guidelines. Survey respondents reported that the awareness and adoption of these guidelines were high among nephrologists however, that low among non-nephrologist physicians.
Government prioritization and policies
CKD was recognized as a health priority by the government in 5 of the 25 countries (Chile, Estonia, France, Spain, and the United Kingdom). While Canada lacked national strategies for CKD care, dialysis, and transplantation, the presence of a national policy for NCDs in general was reported. Among other OECD countries, strategies for kidney care were minimal. Strategies for CKD, dialysis, and transplantation were reported in 36% (9/25), 40% (10/25), and 48% (12/25) of countries, respectively.
2018 Findings
Workforce capacity
The median nephrologist density (calculated per country as the number of nephrologists/total population) of the 36 OECD countries was 25.82 PMP with large variance (interquartile range [IQR] = 12.0; Figure 3). Canada ranked 28th (from highest to lowest) with a density of 15.33 PMP. The median density of nephrology trainees was 3.94 PMP (IQR = 4.7) and similarly, Canada fell below with a density of 1.74 PMP. The highest reported workforce shortages were among nephrologists (17/32 countries reported a shortage), surgeons for hemodialysis (HD) access (17/32), and dialysis nurses (16/32) followed by dietitians (14/32), vascular access coordinators (13/32), and counselors/psychologists (13/32). Six countries (Australia, Canada, Finland, France, Spain, and the United Kingdom) reported no shortages of health-care providers relevant for ESKD management.
Figure 3.
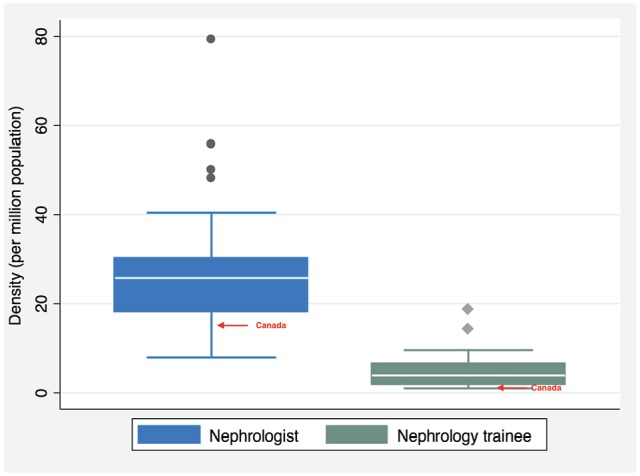
Per million population (PMP) densities of nephrologists (n = 33) and nephrology trainees (n = 31) in participating OECD countries.
Note. Canada reported a nephrology trainee density of 3.94 PMP and a nephrologist density of 15.33 PMP. OECD = Organisation for Economic Co-operation and Development.
Quality indicators of KRT
Countries were asked whether common quality KRT indicators were measured and reported in most centers (Table 3). The majority of countries measured and reported quality indicators across all KRT types, with the exception of patient-reported outcome measures (PROMS). In Canada, PROMS were measured in most (51%-75%) HD centers but in few (1%-10%) PD centers. All other dialysis quality indicators (blood pressure, small solute clearance, hemoglobin/hematocrit, bone mineral markers, technique survival, and patient mortality) were reported in almost all centers. In transplantation, Canada reported that no centers measure and report PROMS in patients. Other transplantation indicators (delayed graft function, rejection rates, renal allograft function, graft survival, and patient mortality) were reported in almost all centers that perform kidney transplantation (Table 3).
Table 3.
Presence of Measurement and Reporting Systems of Common Quality Indicators for KRT Among Participating OECD Countries.
| Total no. of countries with KRT option available | None N (%) |
Few N (%) |
Some N (%) |
Most N (%) |
Almost All N (%) |
Not reported N (%) |
|
|---|---|---|---|---|---|---|---|
| HD | |||||||
| PROMS | 36 | 2 (6) | 2 (6) | 7 (19) | 5 (14) | 18 (50) | 2 (6) |
| Blood pressure | 36 | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 33 (92) | 2 (6) |
| Small solute clearance | 36 | 1 (3) | 0 (0) | 0 (0) | 2 (6) | 31 (86) | 2 (6) |
| Hemoglobin/hematocrit | 36 | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 33 (92) | 2 (6) |
| Bone mineral markers | 36 | 1 (3) | 0 (0) | 1 (3) | 1 (3) | 31 (86) | 2 (6) |
| Technique survival | 36 | 2 (6) | 0 (0) | 1 (3) | 3 (8) | 28 (78) | 2 (6) |
| Patient mortality | 36 | 1 (3) | 0 (0) | 0 (0) | 3 (8) | 30 (83) | 2 (6) |
| PD | |||||||
| PROMS | 36 | 2 (6) | 3 (8) | 8 (22) | 9 (25) | 13 (36) | 1 (3) |
| Blood pressure | 36 | 1 (3) | 0 (0) | 0 (0) | 1 (3) | 33 (92) | 1 (3) |
| Small solute clearance | 36 | 1 (3) | 0 (0) | 1 (3) | 4 (11) | 29 (81) | 1 (3) |
| Hemoglobin/hematocrit | 36 | 1 (3) | 0 (0) | 0 (0) | 1 (3) | 33 (92) | 1 (3) |
| Bone mineral markers | 36 | 1 (3) | 0 (0) | 1 (3) | 1 (3) | 32 (89) | 1 (3) |
| Technique survival | 36 | 1 (3) | 0 (0) | 0 (0) | 3 (8) | 31 (86) | 1 (3) |
| Patient mortality | 36 | 1 (3) | 0 (0) | 0 (0) | 1 (3) | 33 (92) | 1 (3) |
| Transplantation | |||||||
| PROMS | 36 | 6 (17) | 2 (6) | 4 (11) | 3 (8) | 19 (53) | 2 (6) |
| Delayed graft function | 36 | 2 (6) | 0 (0) | 1 (3) | 1 (3) | 30 (83) | 2 (6) |
| Rejection rates | 36 | 2 (6) | 0 (0) | 0 (0) | 3 (8) | 29 (81) | 2 (6) |
| Renal allograft function | 36 | 1 (3) | 0 (0) | 0 (0) | 2 (6) | 31 (86) | 2 (6) |
| Graft survival | 36 | 1 (3) | 0 (0) | 0 (0) | 2 (6) | 31 (86) | 2 (6) |
| Patient mortality | 36 | 1 (3) | 0 (0) | 0 (0) | 1 (3) | 32 (89) | 2 (6) |
Note. Rows may not total 100% due to rounding. None = 0% of centers; few = 1%-10% of centers; some = 11%-50% of centers; most = 51%-75% of centers; and almost all = more than 75% of centers. HD = hemodialysis; KRT = kidney replacement therapy; N = number of countries; OECD = Organization for Economic Co-operation and Development; PD = peritoneal dialysis; PROMS = patient-reported outcome measures.
Canada reported:
HD: PROMS were reported in most centers; blood pressure, small solute clearance, hemoglobin/hematocrit, bone mineral markers, technique survival, and patient mortality were reported in almost all.
PD: PROMS were reported in few centers; blood pressure, small solute clearance, hemoglobin/hematocrit, bone mineral markers, technique survival, and patient mortality were reported in almost all.
Transplant: PROMS were reported in no centers; delayed graft function, rejection rates, renal allograft function, graft survival, and patient mortality were reported in almost all.
CKM delivery
In total, 30 (83%) of the 36 countries reported that CKM is generally available (ie, in 50% or more centers, hospitals, or clinics), in which 28 (93%) offer the service as medically advised or chosen by patients, providers, and families (as opposed to opting for CKM as KRT is unavailable). Availability of important elements of CKM varied: multidisciplinary teams were present in 21 (70%) of the 30 countries; shared decision-making in 19 (63%); active symptom management of common complaints of ESKD in 28 (93%); psychological, cultural, and spiritual support in 18 (60%); and training of relevant health-care providers on CKM delivery in 14 (47%) of the 30 countries with CKM. Canada reported that all elements of CKM were generally available. Easy access to conservative care across settings (eg, home, hospital, hospice, and nursing home) was reported in 22 (73%) countries, including Canada.
Discussion
This study describes the capacity for kidney care at a national level within the context of the Canadian health-care system. The Canadian health-care system offers universal access to most health-care services and is well funded by the government; however, there are areas that could be improved to increase the optimization of kidney care provided. For example, there is a current lack of a CKD-specific policy at national or provincial levels, variable implementation of national guidelines across provinces, no system for monitoring the uptake of guidelines, no organized system for detecting or managing early CKD, and perceived as having generally low recognition of CKD as a public health priority. This is likely to change over the coming years due to the efforts of multiple initiatives and advocacy groups, including (a) the Canadian Society of Nephrology (CSN); (b) the Kidney Foundation of Canada (KFOC) that works to increase awareness of kidney disease, develop and share resources, and guide programs to help support people living with kidney disease; and (c) the Canadians Seeking Solutions and Innovations to Overcome Chronic Kidney Disease (Can-SOLVE CKD), the largest-ever initiative targeted to achieving optimal kidney care in Canada.25
As was reported in the majority of OECD countries, Canada funded most kidney-related health services (excluding medications) through a public funding model. However, medication coverage in Canada was limited. Across the country, medications are funded through mixed sources, where most OECD countries fully cover medication costs. Excluding medication from health coverage is a common issue in Canada.26 Lack of coverage has been suggested as a key reason behind poor adherence to prescription medications in Canada,27 which further appears to correspond to an increase in emergency department visits or hospitalizations.28 Cost-related nonadherence to medication is high in Canada,29 despite cost-sharing mechanisms. Secondly, not all medications are included in government drug plans and therefore, are not always accessible to patients. Including essential medicines to public drug plans in Canada has been estimated to cost the government CAD 1.23 billion per year, but result in savings of over CAD 4 billion.30 Identifying how other OECD countries, for example, Australia, New Zealand, Norway, Sweden, and the United Kingdom, addresses these barriers30 may inform policy change on this issue in Canada.
Nephrology workforce shortages (including nephrologists) were high across most OECD countries. Possible explanations may include a lack of adequate exposure to nephrology among students during training, work schedules, among others.31 Furthermore, the nature of the field is evolving (eg, it is only of recent that multidisciplinary team building has been introduced in the field of nephrology), and in many places this is still under-recognized and/or not seen as a priority31 The GKHA survey reported no shortages in Canada of relevant health-care providers, including nephrologists; however, the density of both nephrologists and nephrology trainees in Canada were below the median reported for OECD countries. However, density in this survey was estimated using the total population as the denominator (not the number of patients). Using the total number of patients with kidney disease requiring nephrologist care, for example, may increase the utility of this metric. Furthermore, understanding how nephrology care is distributed among multidisciplinary teams may also help with the interpretation of the density score. Improving adoption of CKD guidelines among non-nephrologists who are heavily involved with supporting CKD patients (primary care physicians, for example) may help increase capacity of these multidisciplinary teams.
Considering the prevalence of ESKD, Canada has ratio of nephrologists to patients with ESKD of 14:1000,31 which is lower than several countries worldwide. Understanding how the ratio impacts quality of care and health outcomes, and additionally, factors that affect the appropriate nephrologist density (burden of disease, distribution of services across multiple health-care providers, etc.) may help identify appropriate benchmarks for workforce densities. Studies in the United States have suggested that while nephrologist density may be related to demand, process measures such as timing of dialysis initiation do not appear to be better with an increased density of nephrologists.32 Multidisciplinary teams may be an optimal approach for delivering kidney care, allowing for appropriate delegation of care across multiple providers. In Canada, a wide variation in multidisciplinary CKD clinics structure has been observed and a better understanding of how clinics should be organized may help guide care.33 Furthermore, as most nephrologists in Canada are based in urban centers, patients in rural settings may have reduced access to health care. Geographic dispersion and remoteness of certain areas in Canada has limited access to care and is a major issue for patients in some provinces and territories. This has resulted in an increased use of telehealth, which allows clinicians to interact with patients from remote sites.34-36
Access to information on disease burden is important for predicting resource allocation, such as workforce availability, and to improve quality of care. Despite the importance of monitoring early-stage CKD, few OECD countries reported a non-dialysis CKD registry. While there are some provincial registries in Canada, a single national system is lacking. Learning from the experiences of countries that do have a national registry37 may help guide the development of a pre-dialysis CKD registry in Canada. Initiatives like Can-SOLVE CKD25 can also help to share information and resources to link nephrologists and other health-care providers across Canada.
We have identified key opportunities to improve CKD care in Canada along with obstacles currently limiting these services (Table 4). Development of national policies could significantly improve the care of patients with kidney failure and therefore could be utilized to coordinate the care of people with earlier stages of CKD and help them manage their disease and prevent progression to ESKD.38,39 The fact that kidney care (like all health care) is a provincial responsibility hampers attempts to achieve a national quality standard. Effectively implementing a national strategy to standardize the quality of care (including the development of common evaluation metrics), enable comprehensive disease surveillance, and enhance the dissemination of and adherence to guidelines may improve the capacity of kidney care in Canada.
Table 4.
Opportunities, Challenges, and Potential Solutions to Delivering Optimal Kidney Care in Canada.
| Opportunities | Challenges | Potential solutions |
|---|---|---|
| Solid health system: UHC system and well-developed infrastructures for care delivery | Lack of a national surveillance strategy for CKD burden and care facilities. Limited coverage of drugs for conditions that may lead to CKD, or for patients with CKD. |
National CKD registry needed to facilitate better comparative studies across provinces and care facilities. CORR capacity could be extended to cover this important gap. Improve coverage for adults aged 18-65 years, particularly those with lower income or limited insurance coverage. Furthermore, negotiate lower drug prices when purchasing products from pharmaceutical companies. |
| National initiatives led by patient organizations (eg, the SeeKD targeted screening program by the Kidney Foundation of Canada has produced the first national targeted screening program in Canada for CKD). Its goal is to collect data and information about screening and prevention, early detection, and management of CKD across Canada | Limited workforce planning and guideline in terms of how hemodialysis units and CKD clinics are staffed and operated, and what intensity of resource should be applied. | National Kidney Care Policy addressing the key domains of UHC. |
| National initiatives led by professional organizations, and collaborative networks of patients, providers, and policymakers. For example, existing networks to share information and resources and tackle national problems collaboratively at a country level through the Canadians Seeking Solutions and Innovations to Overcome Chronic Kidney Disease (Can-SOLVE CKD) initiative | Variations in kidney care and workforce structures. The health-care systems of all provinces function in silos, and even within provinces huge variation exists between hospitals. | Development of a national strategy and standardization of care organization to monitor quality targets, structures, workforce needs. |
Note. CKD = chronic kidney disease; CORR = Canadian Organ Replacement Registry; UHC = Universal Health Care; SeeKD = See Kidney Disease.
Limitations
Although this study surveyed knowledgeable experts in the field of kidney care and included responses from most OECD countries, there are limitations. Due to the method of data collection (survey), risks of recall bias or social desirability bias are present. In addition, this study compared the capacity of care in Canada compared to other OECD countries. Differences in a number of factors (competing government priorities, economy, funding models, etc.) could influence these discrepancies and were not explored. Furthermore, survey items were high-level and lacked detail to explore how the quality of care differs across regions within Canada and also across different population characteristics (Indigenous peoples, for example). Similarly, survey items were designed to document only the existence of processes (eg, measuring quality indicators), policies and strategies, or the number of nephrologists. This does not explain action or effectiveness, which is important when exploring overall capacity of care.
Conclusions
The findings from this study provide health-care providers and policymakers in Canada with an overview of the current national capacity of kidney care, with reference to other countries of similar economic standing. Through understanding current barriers and limitations of kidney care delivery in Canada, action plans can be appropriately generated and existing strategies in other countries can be considered for adoption in Canada. Together, this work demonstrates the need for a more coordinated health system to allow for standardization and improvement of CKD care across Canada.
Acknowledgments
We thank Drs Marcello Tonelli and Valerie Luyckx for their contributions to the Global Kidney Health Atlas project and the manuscript. We thank Sandrine Damster, research project manager at the International Society of Nephrology (ISN), and Alberta Kidney Disease Network staff (Ghennete Houston, Sue Szigety, and Sophanny Tiv) for their support with the organization and conduct of the survey and project management. We thank the ISN staff (Louise Fox and Charu Malik) for their support. We thank the executive committee of the ISN, the ISN regional leadership, and the leaders of the ISN affiliate societies at regional and country levels for their support toward the success of this initiative.
Footnotes
Ethics Approval and Consent to Participate: The project was approved by the University of Alberta Research Ethics Committee (Protocol number: PRO00063121). Participation was voluntary assuming implied consent.
Consent for Publication: All co-authors reviewed this final manuscript and consented to its publication.
Availability of Data and Materials: Data and materials may be made available upon written request to the corresponding author. Reasonable requests for data access will be assessed in consultation with the appropriate Research Ethics Boards.
Author Contributions: D.W.J., A.L., and A.K.B. contributed to the conception or design; F.Y., M.L., and A.K.B. contributed to the analysis and interpretation of data; and M.L. and A.K.B. drafted the manuscript. All authors provided intellectual content of critical importance and approved the final version.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was funded by the International Society of Nephrology (award no. RES0033080).
ORCID iDs: Meaghan Lunney  https://orcid.org/0000-0003-4954-0529
https://orcid.org/0000-0003-4954-0529
Natasha Wiebe  https://orcid.org/0000-0002-5613-1582
https://orcid.org/0000-0002-5613-1582
References
- 1. Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260-272. [DOI] [PubMed] [Google Scholar]
- 2. Klarenbach SW, Tonelli M, Chui B, et al. Economic evaluation of dialysis therapies. Nature Rev Nephrol. 2014;10(11):644-652. [DOI] [PubMed] [Google Scholar]
- 3. Pereira B, Fernandes N, de Melo NP, Abrita R, Grincenkov FRDS, Fernandes NMDS. Beyond quality of life: a cross sectional study on the mental health of patients with chronic kidney disease undergoing dialysis and their caregivers. Health Qual Life Outcomes. 2017;15(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Global Regional National Age-Sex-Specific Mortality for 282. 1980-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1736-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arora P, Vasa P, Brenner D, et al. Prevalence estimates of chronic kidney disease in Canada: results of a nationally representative survey. CMAJ. 2013;185(9):E417-E423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Terner M, Redding N, Wu J. Increasing rates of kidney failure care in Canada strains demand for kidney donors. Healthc Q. 2016;19(3):10-12. [DOI] [PubMed] [Google Scholar]
- 7. Hajian-Tilaki K, Heidari B, Hajian-Tilaki A. A comparison of health-related quality of life in patients with renal failure under hemodialysis and healthy participants. Saudi J Kidney Dis Transpl. 2017;28(1):133-140. [DOI] [PubMed] [Google Scholar]
- 8. Levin A, Hemmelgarn B, Culleton B, et al. Guidelines for the management of chronic kidney disease. Can Med Assoc J. 2008;179(11):1154-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Manns L, Scott-Douglas N, Tonelli M, et al. A population-based analysis of quality indicators in CKD. Clin J Am Soc Nephrol. 2017;12(5):727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hsu CY. Linking the population epidemiology of acute renal failure, chronic kidney disease and end-stage renal disease. Curr Opin Nephrol Hypertens. 2007;16(3):221-226. [DOI] [PubMed] [Google Scholar]
- 11. Mehta RL, Burdmann EA, Cerda J, et al. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: a multinational cross-sectional study. Lancet. 2016;387(10032):2017-2025. [DOI] [PubMed] [Google Scholar]
- 12. Olowu WA, Niang A, Osafo C, et al. Outcomes of acute kidney injury in children and adults in Sub-Saharan Africa: a systematic review. Lancet Glob Health. 2016;4(4):e242-e250. [DOI] [PubMed] [Google Scholar]
- 13. Collister DPN, Pannu N, Ye F, et al. Health care costs associated with AKI. Clin J Am Soc Nephrol. 2017;12(11):1733-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heung M, Steffick DE, Zivin K, et al. Acute kidney injury recovery pattern and subsequent risk of CKD: an analysis of veterans health administration data. Am J Kidney Dis. 2016;67(5):742-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Canadian Society of Nephrology. Multinational inventory on chronic kidney disease is released. https://www.csnscn.ca/news-events/press-releases/145-multinational-inventory-on-chronic-kidney-disease-is-released. Accessed May 27, 2018.
- 16. Canadian Institute for Health Information. CORR Annual Statistics, 2007 to 2016. https://www.cihi.ca/en/corr-annual-statistics-2007-to-2016. Accessed May 27, 2018.
- 17. Central Intelligence Agency. The World Factbook. https://www.cia.gov/library/Publications/the-world-factbook/geos/ca.html. Accessed May 27, 2018.
- 18. Bello AK, Levin A, Lunney M, et al. Global Kidney Health Atlas: A Report by the International Society of Nephrology on the Global Burden of End-Stage Kidney Disease and Capacity for Kidney Replacement Therapy and Conservative Care Across World Countries and Regions. Brussels: International Society of Nephrology; 2019. [Google Scholar]
- 19. World Health Organization. Monitoring the Building Blocks of Health Systems: A Handbook of Indicators and Their Measurement Strategies. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 20. Organisation for Economic Co-operation and Development. List of OECD member countries. http://www.oecd.org/about/membersandpartners/list-oecd-member-countries.htm. Published 2018. Accessed August 12, 2019. [PubMed]
- 21. Government of Canada. Canada’s Health Care System. https://www.canada.ca/en/health-canada/services/health-care-system/reports-publications/health-care-system/canada.html. Published 2012. Accessed October 31, 2017.
- 22. Bello AK, Johnson D, Feehally J, et al. Global kidney health atlas: design and methods. Kidney Int Suppl. 2017;7(2):145-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bello AK, Levin A, Tonelli M, et al. Assessment of global kidney health care status. JAMA. 2017;317(18):1864-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leowski J, Krishnan A. Capacity to control noncommunicable diseases in the countries of South-East Asia. Health Policy. 2009;92(1):43-48. [DOI] [PubMed] [Google Scholar]
- 25. Can-SOLVE CKD. https://cansolveckd.ca. Accessed January 19, 2018.
- 26. Daw JR, Morgan SG, Thomson PA, Law MR. Here today, gone tomorrow: the issue attention cycle and national print media coverage of prescription drug financing in Canada. Health Policy. 2013;110(1):67-75. [DOI] [PubMed] [Google Scholar]
- 27. Law MR, Cheng L, Dhalla IA, Heard D, Morgan SG. The effect of cost on adherence to prescription medications in Canada. CMAJ. 2012;184(3):297-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Campbell DJ, King-Shier K, Hemmelgarn BR, et al. Self-reported financial barriers to care among patients with cardiovascular-related chronic conditions. Health Rep. 2014;25(5):3-12. [PubMed] [Google Scholar]
- 29. Gagnon MA. The role and impact of cost-sharing mechanisms for prescription drug coverage. CMAJ. 2017;189(19):E680-E681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morgan SG, Leopold C, Wagner AK. Drivers of expenditure on primary care prescription drugs in 10 high-income countries with universal health coverage. CMAJ. 2017;189(23):E794-E799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sharif MU, Elsayed ME, Stack AG. The global nephrology workforce: emerging threats and potential solutions! Clin Kidney J. 2016;9(1):11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ku E, Johansen KL, Portale AA, Grimes B, Hsu CY. State level variations in nephrology workforce and timing and incidence of dialysis in the United States among children and adults: a retrospective cohort study. BMC Nephrol. 2015;16:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Levin A, Steven S, Selina A, Flora A, Sarah G, Braden M. Canadian chronic kidney disease clinics: a national survey of structure, function and models of care. Can J Kidney Health Dis. 2014;1:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kierkegaard P. Mapping telemedicine efforts: surveying regional initiatives in Denmark. Telemed J E Health. 2015;21(5):427-435. [DOI] [PubMed] [Google Scholar]
- 35. Tuot DS, Boulware LE. Telehealth applications to enhance CKD knowledge and awareness among patients and providers. Adv Chronic Kidney Dis. 2017;24(1):39-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Keely E, Li J, Magner P, Afkham A, Liddy C. Nephrology eConsults for primary care providers: original investigation. Can J Kidney Health Dis. 2018;5:PMC5788130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Venuthurupalli SK, Hoy WE, Healy HG, Cameron A, Fassett RG. CKD.QLD: establishment of a chronic kidney disease [CKD] registry in Queensland, Australia. BMC Nephrol. 2017;18(1):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Levey AS, Stevens LA, Coresh J. Conceptual model of CKD: applications and implications. Am J Kidney Dis. 2009;53(3, suppl 3):S4-S16. [DOI] [PubMed] [Google Scholar]
- 39. Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825-830. [DOI] [PubMed] [Google Scholar]