Abstract
Background:
Advances in diabetes technology have been exponential in the last few decades. With evolution in continuous glucose monitoring (CGM) systems and its progressive automation in control of insulin delivery, these advances have changed type 1 diabetes mellitus (T1DM) management. These novel technologies have the potential to improve glycated haemoglobin (HbA1c), reduce hypoglycaemic events, increase time spent in range and improve quality of life (QoL). Our aim was to evaluate the sustained effects in free-living unsupervised conditions of CGM systems (intermittently scanned and real time) and insulin delivery [from multiple daily injections, via sensor-augmented pump therapy and (predictive) low-glucose insulin suspension to hybrid closed-loop systems] on glucose control and QoL in adults and children with T1DM.
Methods:
We performed a systematic review of randomized controlled trials (RCTs), using PubMed and the Cochrane library up to 30 May 2019. Inclusion of RCTs was based on type of intervention (comparing glucose-monitoring devices and insulin-delivery devices), population (nonpregnant adults and children with T1DM), follow-up (outpatient setting for at least 8 weeks) and relevant outcomes [HbA1c, time in range (TIR), time in target, time in hypoglycaemia and QoL]. Exclusion of RCTs was based on intervention (exercise, only overnight use). The Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines were used to score the quality of the papers and for the final selection of the articles.
Results:
Our search resulted in 214 articles, of which 19 were eligible. Studies on advanced use in adults and children with T1DM reported increased TIR (all 9 studies); decreased time in hypoglycaemia (13 out of 15 studies); lowered HbA1c levels (5 out of 15 studies); improved QoL (10 of 16 studies) and treatment satisfaction (7 studies).
Conclusions:
Recent technologies have dramatically changed the course of T1DM. They are proving useful in controlling glycaemia in patients with T1DM, without increasing the treatment burden.
Keywords: HbA1c, hybrid closed-loop, hypoglycaemia, intermittently scanned (flash) continuous glucose monitoring, quality of life, (real-time) continuous glucose monitoring, sensor-augmented pump, time in range, type 1 diabetes
Introduction
For many patients with type 1 diabetes (T1DM), it is challenging to maintain near-normal blood glucose levels and to reduce the risk of both acute (hypoglycaemia, ketoacidosis) and chronic complications (retinopathy, nephropathy, neuropathy). In the era of self-monitoring of blood glucose (SMBG), a lower glycated haemoglobin (HbA1c) was associated with more hypoglycaemic events,1 thereby limiting the ability to reach tight glucose control. Advances in diabetes technology have been exponential in the last few decades. The evolution of continuous glucose-monitoring (CGM) systems and the progressive automation in control of insulin delivery up to the first hybrid closed-loop systems (HCLs) have changed the way T1DM is managed nowadays.
CGM, either intermittently scanned or real time, provides a comprehensive picture of glucose profiles, allowing patients and physicians to make therapeutic adjustments to improve metabolic control. They have the potential to improve HbA1c, reduce frequency and time spent in hypo- and hyperglycaemia, increase time spent in range, reduce glycaemic variability, and improve quality of life (QoL), especially if subjects wear the sensor for more than 70% of the time.2–7
In recent decades, both CGM and pump technology have advanced tremendously, with improved functional features and integration together with control algorithms to deliver insulin in a glucose-responsive manner, initially enabling automated low-glucose suspend (LGS), later predictive low-glucose suspend (PLGS) and now even the first HCL. Furthermore, a dual-hormone HCL can also deliver glucagon in addition to insulin, both in a glucose-responsive manner.8
Safety and efficacy of these systems was gradually evaluated in many trials, initially under supervised conditions, such as in-hospital, hotel or diabetes-camp settings and eventually, in outpatient free-living conditions. Many trials evaluated only overnight use of these systems. A meta-analysis of 40 trials concluded that artificial pancreas systems are efficacious and safe in outpatients with type 1 diabetes, but a short follow-up, a small sample size and inconsistency in reporting outcomes are the main limitations of current research evidence.9,10 In addition, guidance on the use of these systems is, however, scarce11–15 (see also part A of this review, ‘The road from intermittently scanned continuous glucose monitoring to hybrid closed loop systems. Part A: keys to success: patient profiles, choice of systems, education’).
In this manuscript, we systematically reviewed the evidence of randomized controlled trials (RCTs) of the last 5 years up to 30 May 2019, on CGM systems (intermittently scanned and real time) with its progressive automation in control of insulin delivery (from multiple daily injections to HCLs), in nonpregnant adults and children with T1DM on HbA1c, time in range (TIR), time in target (TIT), time in hypoglycaemia and QoL. We aimed to investigate these technologies in sustained unsupervised free-living conditions to establish a real-life evaluation of the different glucose-monitoring devices, therefore, only including studies with (approximately) 24 h per day use and a minimum follow-up duration of at least 8 weeks.
Methods
We performed this systematic review using the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines.16
Data sources and study selection
We searched the electronic databases Medline (‘PubMed’) and Cochrane library (‘Cochrane Central Register of Controlled Trials’) for studies published in the last 5 years up to 30 May 2019. In addition to the Medline search, the Cochrane library search revealed no additional fully published RCTs. Our search strategy was based on search terms describing the intervention [‘intermittently scanned (flash) continuous glucose monitoring’, ‘real-time continuous glucose monitoring’, ‘sensor-augmented pump therapy’, ‘low-glucose insulin suspension’, ‘predictive low-glucose suspension’, ‘hybrid closed-loop insulin delivery’ or ‘artificial pancreas’ in addition to a filter of RCT, population (T1DM) and publication date (last 5 years); Appendix 1: search strategy].
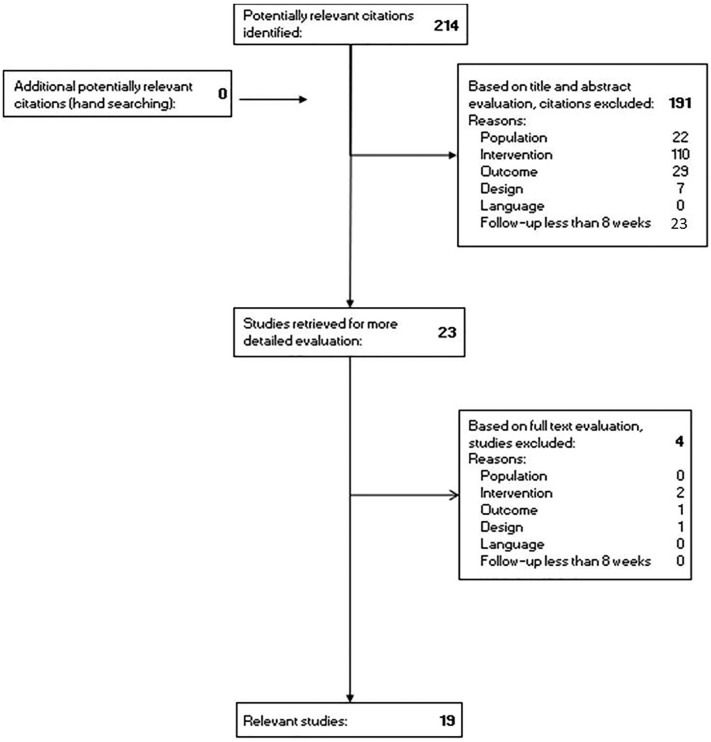
Study selection was based on population, intervention, outcome, design, follow-up and language (Figure 1). We included RCTs in adults or children with T1DM, comparing the above-mentioned new technologies with conventional therapy or a less advanced step in the treatment ladder, with 24 h/day use in normal living conditions for at least 8 weeks and evaluating one or more of the following outcome parameters: HbA1c, TIR, TIT, hypoglycaemia and QoL.
Figure 1.
Flowchart of search and study selection.
We excluded studies not meeting these criteria, or when the population included type 2 diabetes, pregnant women, virtual experiments, other diseases (e.g. depression, eating disorders); when the intervention was exercise; when evaluation of the outcome parameters was only performed during night time; when the study design was only a study protocol or a trial registration; and when the language was not English.
We aimed to investigate these recent technologies in sustained unsupervised free-living conditions to establish a real-life evaluation of the different glucose-monitoring devices. We used a cut-off of 8 weeks as a minimum follow-up because patients need time to learn how to (optimally) use the medical device (estimated time of 2–4 weeks) and need to have used the device long enough to show relevant results (estimated time of at least 4 weeks), especially as we evaluated HbA1c and QoL.
Data extraction and study quality assessment
Two independent researchers (FDR, MdB) screened and selected the articles. In case of disagreement, the third researcher (CDB) was consulted and a consensus was reached. To determine the risk of bias in each individual study, the checklist for RCTs of the Dutch Cochrane Centre was used17 and an extra relevant question;18 with methodological quality defined as high quality (score ⩾ 70%), moderate quality (score < 70% and ⩾ 50%) and low quality (score < 50%; Appendix 2: scoring the methodological quality of RCTs).
We labelled studies for strength of evidence according to the ‘Hierarchy of quality of individual studies and strength of evidence’ criteria.19,20 We stratified levels as: A1: systematic reviews, with at least some trials at quality level A2, and of which the results of each trial are consistent; A2: RCTs with a good quality and enough strength and consistency; B: RCTs with a moderate (weak) quality or insufficient strength, or other comparative trials (nonrandomized controlled studies); C: noncontrolled trials; D: expert opinion.
Finally, we summarized the results of the diabetes technologies on the different outcomes (HbA1c, TIR, TIT, hypoglycaemia, QoL) with the respective methodological quality of each study. Four levels of evidence were allocated to the conclusions. Level 1: conclusion based on one A1 systematic review or at least two independent studies at level A2; level 2: conclusion based on at least two independent studies of level B; level 3: conclusion based on one study of level A2 or B or C; level 4: conclusion based on solely expert opinion. By using these levels, we can formulate recommendations. Level 1: ‘Studies have shown that. . .’; level 2: ‘According to studies, it is likely that. . .’; level 3: ‘There are indications that. . .’; level 4: ‘The expert opinion is. . .’ (Appendix 3: quality of evidence).
Results
Study selection and characteristics
A total of 214 studies were identified, of which 195 were excluded, resulting in 19 relevant RCTs in patients with T1DM,4,6,21–37 involving 1450 participants: 1107 adult and 343 paediatric subjects ranging from 2 years to 76 years. The populations per study ranged from 20 to 241 patients. The male/female distribution was balanced and almost the same in every study. The follow-up period ranged from 8 weeks up to 24 months (median 6 months). Two studies compared intermittently scanned (flash) CGM (isCGM) with SMBG. Most studies compared real-time CGM (RT-CGM) with SMBG (n = 10) and some to isCGM (n = 2), which could be both in combination with continuous subcutaneous insulin infusion (CSII) or multiple daily injections (MDIs). Some studies compared HCL with predicted low-glucose suspension (n = 4) to sensor-augmented pump therapy (SAP). The effect of novel technology intervention was evaluated on HbA1c (n = 15), on TIR (n = 9), on hypoglycaemia (n = 15) and on QoL (n = 16) and is shown in Table 1.
Table 1.
Study characteristics and significant results, ordered by intervention and control devices.
| Author(s) and study name | Number of adults and children (therapy) |
Study design |
Follow-up (months) |
HbA1c (group difference) |
Time in range/time in target mean (±SD)/ median (IQR) mean/median group difference (95% CI) |
Time in hypoglycaemia mean (±SD)/median (IQR) mean/median group difference (95% CI) |
Quality of life (group difference) |
|
|---|---|---|---|---|---|---|---|---|
| Intervention (device) |
Control (device) |
|||||||
| Oskarsson et al.25
IMPACT Subgroup analysis |
167 adults (MDI) |
isCGM (FreeStyle Libre) (n = 75) |
SMBG (n = 69) |
6 | No difference |
70–180 mg/dl (3.9–10.0 mmol/l) Mean (baseline to follow-up) isCGM 15.0 (±2.6) to 15.7 (±2.8) h/day = 62.5 (±10.8) to 65.4 (±11.6)% SMBG 14.3 (±2.9) to 14.4 (±3.0) h/day = 59.6 (±12.1) to 59.6 (±12.5)% Difference in adjusted means 0.9 (0.2, 1.7) h/day** = 3.4 (0.08, 7.1)% Difference 6.5% |
<70 mg/dl (3.9 mmol/l)
Mean (baseline to follow-up) isCGM 3.44 (±2.10) to 1.86 (±1.36) h/day = 14.3 (±8.8) to 7.8 (±5.7)% SMBG 3.73 (±2.72) to 1.97 (±2.24) h/day = 15.5 (±11.3) to 15.3 (±11.6)% Difference in adjusted means −1.65 (−2.21, −1.09) h/day$$ = −6.9 (−9.2, −4.5)% Difference −46% <55 mg/dl (3.1 mmol/l) Mean (baseline to follow-up) isCGM 1.75 (±1.53) to 0.75 (±0.88) h/day = 7.3 (±6.4) to 3.1 (±3.7)% SMBG 1.99 (±1.97) to 1.97 (±2.24) h/day = 8.3 (±8.2) to 8.2 (±9.3)% Difference in adjusted means −1.10 (−1.55, −0.65) h/day$$ = −4.6 (−6.5, −2.7)% Difference −57.7% |
↑ Satisfaction$$
↓ Perceived frequency of hypoglycaemia** ↓ Perceived frequency of hyperglycaemia$$ |
| Bolinder et al.32
IMPACT |
241 adults (161 MDI; 78 CSII) |
isCGM (FreeStyle Libre) (n = 119) |
SMBG (n = 120) |
6 | No difference |
70–180 mg/dl (3.9–10.0 mmol/l) Mean (baseline to follow-up) isCGM 15.0 (±2.5) to 15.8 (±2.9) h/day = 62.5 (±10.4) to 65.8 (±12.1)% SMBG 14.8 (±2.8) to 14.6 (±2.9) h/day = 65.8 (±11.7) to 60.8 (±12.1)% Difference in adjusted means 1.0 (±0.3) h/day3$$ = 4.2 (±1.3)% |
<70 mg/dl (3.9 mmol/l)
Mean (baseline to follow-up) isCGM 3.38 (±2.31) to 2.03 (±1.93) h/day = 14.1 (±9.6) to 8.5 (±8.0)% SMBG 3.44 (±2.62) to 3.27 (±2.58) h/day = 14.3 (±10.9) to 13.6 (±10.8)% Difference in adjusted means −1.24 (±0.239) h/day$$ = −5.2 (±1.0)% Difference −38.0% <55 mg/dl (3.1 mmol/l) Mean (baseline to follow-up) isCGM 1.59 (±1.42) to 0.80 (±0.96) h/day = 6.6 (±5.9) to 3.3 (±4.0)% SMBG 1.77 (±1.86) to 1.65 (±1.97) h/day = 7.4 (±7.8) to 6.9 (±8.3)% Difference in adjusted means −0.82 (±0.175) h/day$$ = −3.4 (±0.7)% Difference −50.3% |
↑ Satisfaction$$
↓ Perceived frequency of hyperglycaemia$$ |
| Reddy et al.23
I HART extension |
40 adults (MDI) |
RT-CGM (Dexcom G5) (n = 16) |
isCGM switching to RT-CGM (FreeStyle Libre) (n = 20) |
2–4 | No difference |
70–180 mg/dl (3.9–10.0 mmol/l)
Median (8 weeks to 16 weeks) RT-CGM 65.9–64.9% isCGM to RT-CGM 60.0–67.4% Median change RT-CGM −1.0 (−4.4 to 4.1)% isCGM to RT-CGM 3.5 (−0.4 to 7.2)%** 70–140 mg/dl (3.9–7.8 mmol/l) Median (8 weeks to 16 weeks) RT-CGM 43.7–43.1% isCGM to RT-CGM 40.4–42.9% Median change RT-CGM 1.0 (−2.6 to 3.2)% isCGM to RT-CGM 2.2 (−5.2 to 4.7)% |
<70 mg/dl (3.9 mmol/l)
Median (8 weeks to 16 weeks) RT-CGM 6.2–5.4% isCGM to RT-CGM 11.0–3.9%$$ Median change RT-CGM continued 0.4 (−0.2 to 2.1)% isCGM to RT-CGM −6.6 (−9.4 to −3.7)%$$ <54 mg/dl (3.0 mmol/l) Median (8 weeks to 16 weeks) RT-CGM 1.3–1.3% isCGM to RT-CGM 5.0–0.8%$$ Median change RT-CGM continued 0.02 (–1.2–0.5)% isCGM to RT-CGM −4.0 (−4.8 to −2.9)%$$ <50 mg/dl (2.8 mmol/l) Median (8 weeks to 16 weeks) RT-CGM 0.8–0.9% isCGM to RT-CGM 3.8–0.5%$$ Median change RT-CGM 0.0 (−0.9 to 0.3)% Is CGM to RT-CGM −3.1 (−4.6 to −2.4)%$$ |
↓ Hypo fear** |
| Reddy et al.26
I HART |
40 adults (MDI) |
RT-CGM (Dexcom G5) (n = 20) |
isCGM (FreeStyle Libre) (n = 20) |
2 | No difference |
70–180 mg/dl (3.9–10.0 mmol/l)
Median (baseline to endpoint) RT-CGM 50.2–65.9% isCGM 54.1–60.0% Median change RT-CGM 12.7 (7.2–15.8)% isCGM 5.3 (1.1–11.7)%* Median between-group difference 7.4%* 70–140 mg/dl (3.9–7.8 mmol/l) Median (baseline to endpoint) RT-CGM 31.7–43.7% isCGM 34.8–40.4% Median change RT-CGM 10.6 (3.3–14.4)% isCGM 5.9 (−2.4 to 9.0)% Median between-group difference 4.7% |
<70 mg/dl (3.9 mmol/l)
Median (baseline to endpoint) RT-CGM 8.8–6.2% isCGM 11.9–11.0% Median change RT-CGM −2.7 (−6.1 to −0.1)% isCGM 0.6 (−2.1 to 5.4)%** Median between group difference −2.5% <50 mg/dl (2.8 mmol/l) Median (baseline to endpoint) RT-CGM 2.3–0.9% isCGM 4.1–3.8% Median change RT-CGM −1.2 (−4.3 to −0.5)% isCGM 1.3 (−1.0 to 2.4)%$ Median between group difference −4.3% |
↓ Hypo fear** |
| Olafsdottir et al.6
GOLD-3 |
161 adults (MDI) |
RT-CGM first (Dexcom G4) (n = 69) |
SMBG first (n = 73) | 16 (cross-over) |
NI | NI |
<70 mg/dl (3.9 mmol/l)
Calculated mean (day + night) RT-CGM 2.8% = 0.7 h/day SMBG 4.8% = 1.1 h/day$$ <54 mg/dl (3.0 mmol/l) Calculated mean (day + night) RT-CGM 0.8% = 0.2 h/day SMBG 1.9% = 0.4 h/day$$ |
↑ Hypo confidence in social situations** and in avoiding problems$ and in avoiding hypo$
↑ Free living$ |
| Lind et al.30
GOLD |
161 adults (MDI) |
RT-CGM (Dexcom G4 Platinum) (n = 142) |
SMBG (n = 142) |
6 (cross-over) |
Mean (baseline to follow-up)
RT-CGM 8.6–7.92% = 70–63 mmol/mol SMBG 8.6 8.35% = 70–68 mmol/mol Mean of differences −0.43 (−0.57 to −0.29)%$$ = −4.7 (−6.3 to −3.1) mmol/mol |
NI |
<70 mg/dl (3.9 mmol/l)
Mean (baseline to follow-up) RT-CGM 5.52 (±4.33) to 2.79 (±2.97)% SMBG 5.12 (±4.24) to 4.79 (±4.03)% <54 mg/dl (3.0 mmol/l) Mean (baseline to follow-up) RT-CGM 2.31 (±2.39) to 0.79 (±1.23)% SMBG 2.06 (±2.42) to 1.89 (±2.12)% |
↓ Hypo fear$$
↑ Satisfaction$$ |
| Heinemann et al.24
HypoDE |
149 adults (MDI) |
RT-CGM (Dexcom G5 Mobile) (n = 75) |
SMBG (n = 74) |
6 | No difference |
70–180 mg/dl (3.9–10.0 mmol/l)
Median (baseline to follow-up) RT-CGM 57.8–58.5% = 13.9–14.0 h/day SMBG 59.1–56.5% = 14.2–13.6 h/day Adjusted between-group difference 3.1 (0.0–6.2)% = 0.75 (0.0–1.5) h/day* |
<70 mg/dl (3.9 mmol/l)
Median (baseline to follow-up) RT-CGM 5.0–1.6% = 1.2–0.4 h/day SMBG 6.9–6.4% = 1.7–1.5 h/day$$ <54 mg/dl (3.0 mmol/l) Median (baseline to follow-up) RT-CGM 1.7–0.3% = 0.4–0.1 h/day SMBG 2.7–2.5% = 0.65 to 0.6 h/day$$ |
↓ Hypo distress ↑ Satisfaction |
| Little et al.37
Hypo-COMPaSS 2-year follow-up |
76 adults | RT-CGM (RT-CGM system, Medtronic) (n = 37) |
SMBG (n = 39) |
24 | No difference | NI | No difference | No difference (± ↓ Hypo fear) |
| Little et al.35
Hypo-COMPaSS |
96 adults (after randomization: 41 MDI; 42 CSII) |
RT-CGM (RT-CGM system, Medtronic) (n = 42) |
SMBG (n = 42) |
6 | No difference | NI | No difference | No difference |
| Polonsky et al.28
Further findings from DIAMOND |
158 adults (MDI) |
RT-CGM (Dexcom G4 Platinum) (n = 105) |
SMBG (Bayer Contour Next USB; n = 53) |
6 | NI | NI | NI | ↑ Hypo confidence**
↓ Diabetes distress** ↑ Satisfaction |
| Beck et al.31
DIAMOND |
158 adults (MDI) |
RT-CGM (Dexcom G4 Platinum) (n = 105) |
SMBG (Bayer Contour Next USB) (n = 53) |
6 |
Mean (baseline to follow-up)
RT-CGM 8.6 (±0.7) to 7.7 (±0.8)% 70 (±7) to 61 (±9) mmol/mol SMBG 8.6 (±0.6) to 8.2 (±0.8)% 70 (±7) to 66 (±9) mmol/mol Mean change RT-CGM –1.0 (±0.8)% –11 (±9) mmol/mol SMBG 0.4 (±0.7)% –4 (±7) mmol/mol Between-group difference −0.6 (−0.8 to −0.3)%$$ −6 (−8 to −3) mmol/mol |
70–180 mg/dl (3.9–10.0 mmol/l)
Mean (baseline to follow-up) RT-CGM 11 (±3) to 12.3 (±3.4) h/day SMBG 10.8 (±2.8) to 10.8 (±3.2) h/day Mean adjusted difference 1.3 (0.1, 2.5) h/day** = 5.4 (0.4, 10.4)% |
<70 mg/dl (3.9 mmol/l)
Mean (baseline to follow-up) RT-CGM 1.1 (0.6–1.7) to 0.7 (0.5–1.2) h/day = 4.6 (2.5–7.1) to 2.9 (2.1–5.0)% SMBG 1.2 (0.6–2.3) to 1.3 (0.6–1.9) h/day** = 5.0 (2.5–9.6) to 5.4 (2.5–7.9)% <50 mg/dl (2.8 mmol/l) Mean (baseline to follow-up) RT-CGM 0.2 (0.1–0.5) to 0.1 (0.03–0.2) h/day = 0.8 (0.4–2.1) to 0.4 (0.1–0.8)% SMBG 0.3 (0.1–0.7) to 0.3 (0.1–0.7) h/day** = 1.3 (0.4–2.9) to 1.3 (0.4–2.9)% |
NI |
| Van Beers et al.4
IN CONTROL |
52 adults (29 MDI; 23 CSII) |
RT-CGM (Enlite glucose sensor; Medtronic) (n = 26) |
SMBG (n = 26) |
2 × 4 (cross-over) |
No difference |
72–180 mg/dl (4.0–10.0 mmol/l)
Mean (CI) RT-CGM 65.0 (62.8–67.3)% = 15.6 (15.1–16.2) h/day SMBG 55.4 (53.1–57.7)% = 13.3 (12.7–13.8)$$ Mean difference (CI) 9.6 (8.0–11.2)% = 2.3 (1.9–2.7) h/day$$ |
<70 mg/dl (3.9 mmol/l)
Mean (CI) RT-CGM 6.8 (5.2–8.3)% = 1.6 (1.3–2.0) h/day SMBG 11.4 (9.9–13.0)% = 2.7 (2.4–3.1) h/day$$ Mean difference (CI) −4.7 (−5.9 to −3.4)% = −1.1 (−1.4 to −0.8) h/day$$ |
No difference |
| Hommel et al.34
SWITCH |
81 adults and 72 children (CSII) |
RT-CGM (MiniMed SofSensor; Medtronic) (n = 77) |
SMBG (n = 76) |
2 × 6 (cross-over) |
NI | NI | NI | No difference in children’s self-rating ↑ Parents’ proxy rating$ ↑ Satisfaction** ↑ Convenience** ↑ Flexibility** |
| Tumminia et al.36 | 20 adults (10 MDI; 10 CSII) |
RT-CGM first (Guardian REAL-Time Clinical; Medtronic) (n = 20) |
SMBG first (n = 20) |
2 × 6 (cross-over) |
Baseline to follow-up
MDI with RT-CGM 8.58 (±0.2) to 7.71 (±0.2)%** 70 (±3) to 61 (±3) mmol/mol** Calculated change −0. 87% (−9 mmol/mol) SAP 8.50 (±0.3) to 7.82 (±0.2)%** Calculated change −0.68% (−7 mmol/mol) |
NI |
<70 mg/dl (3.9 mmol/l)
Baseline to follow-up MDI + RT-CGM AUC 1.5 (±2.4) to 0.5 (±0.5)** MDI + SMBG AUC 1.5 (±0.5) to 1.8 (±1.0) SAP AUC from 1.7 (±0.3) to 1.5 (±0.3) CSII + SMBG AUC from 1.7 (±1.0) to 3 (±1.3) |
NI |
| Burckhardt et al.21 | 49 Children & their Parents (20 MDI; 29 CSII) |
RT-CGM (Dexcom G5 Mobile) (n = 48) |
SMBG (n = 48) |
2 × 3 (cross-over) |
No difference | NI | NI | ↓ Hypo fear$$
↓ Family impact$ ↓ Stress$ ↑ Improvement of psychosocial metrics$ ↑ Satisfaction** |
| Abraham et al.27 | 154 Children (CSII) |
HCL with PLGS (MiniMed 640G pump with Suspend before low, Medtronic; Enlite Sensor and Guardian 2 Link transmitter, Medtronic) (n = 80) |
SAP (same devices but without suspend on low and suspend before low) (n = 74) |
6 | No difference | NI |
<54 mg/dl (3.0 mmol/l)
Mean (baseline to follow-up) PLGS 1.3–0.6%$$ SAP 1.4–1.2%** Difference in LS means −0.44 (−0.64 to −0.24)%$$ |
No difference |
| Tauschmann et al.22 | 44 adults and 42 children (CSII) |
HCL with PLGS (640G, Medtronic; Enlite 3 glucose sensor, Medtronic; Contour Next Link 2.4 glucometer, Ascensia Diabetes Care) (n = 46) |
SAP (same devices but without suspend on low and suspend before low; n = 40) |
3 |
Mean (baseline to follow-up)
HCL 8.0–7.4% = 63–57 mmol/mol SAP 7.8–7.7% = 62–60 mmol/mol Difference (CI) −0.36 (−0.53 to −0.19)%$$ = −4 (−5.8 to −2.2) mmol/mol |
70–180 mg/dl (3.9–10.0 mmol/l)
Mean (baseline to follow-up) HCL 52 (±10) to 65 (±8)% SAP 52 (±9) to 54 (±9)% Difference (CI) 10.8 (8.2–13.5)%$$ |
<70 mg/dl (3.9 mmol/l)
Median (baseline to follow-up) HCL 3.5 (2.0–5.4) to 2.6 (1.9–3.6)% SAP 3.3 (1.2–5.5) to 3.9 (1.7–5.3)% Difference (CI) −0.83 (−1.40 to −0.16)%** <50 mg/dl (2.8 mmol/l) Median (baseline to follow-up) HCL 0.4 (0.1–1.0) to 0.3 (0.2–0.6)% SAP 0.5 (0.1–1.0) to 0.5 (0.2–0.9)% Difference (CI) −0.09 (−0.24 to 0.01)% (p = 0.11) |
No difference |
| Barnard et al.29 | 32 adults and 26 children (CSII) |
HCL with PLGS (Dana R pump, Diabecare; FreeStyle Navigator II, Abbott Diabetes Care) (n = 38) |
SAP (same devices but without Suspend on low and Suspend before low) (n = 30) |
2 × 3 (cross-over) |
NI | NI | NI | No difference (both groups ↑ satisfaction) |
| Thabit et al.33 | 33 adults (CSII) |
HCL with PLGS (Florence D2A or similar automated closed-loop glucose control system; FreeStyle Navigator II CGM System, Abbott Diabetes Care) (n = 32) |
SAP (CSII Dana R Diabecare; real-time FreeStyle Navi-gator CGM) (n = 33) |
2 × 3 (cross-over) |
Baseline to follow-up
HCL 7.6–7.3% = 60– 56 mmol/mol SAP 7.6–7.6% 60–60 mmol/mol Paired difference (CI) −0.3 (−0.5 to −0.1)%$ = −4 (−6 to −2) mmol/mol |
70–180 mg/dl (3.9–10.0 mmol/l)
Mean (at follow-up) HCL 67.7 (±10.6)% SAP 56.8 (±14.2)% Paired difference (CI) 11.0 (8.1–13.8)%$$ |
<70 mg/dl (3.9 mmol/l)
Median (at follow-up) HCL 2.9 (1.4–4.5)% SAP 3.0 (1.8–6.1)% Paired difference (CI) −0.81 (0.68–0.96)%** <50 mg/dl (2.8 mmol/l) Median (at follow-up) HCL 0.3 (0.1–0.7)% SAP 0.4 (0.1–0.9)% Paired difference (CI) −0.45 (0.31–0.65)%$$ |
NI |
To compare RCTs, time in range, time in target and time in hypoglycaemia were calculated to %/day if they reported hours or minutes/day.
p = 0.05.
p < 0.05.
p < 0.005.
p < 0.001.
AUC, area under curve; CI, confidence interval; CSII, continuous subcutaneous insulin infusion; isCGM, intermittently scanned (flash) continuous glucose monitoring; HCL, hybrid closed-loop system; IQR, interquartile range; MDI, multiple daily injection; NI, not investigated; PLGS, predictive low-glucose suspend; SAP, sensor-augmented pump therapy; SD, standard deviation; SMBG, self-monitoring of blood glucose; RT-CGM, real-time continuous glucose monitoring.
Risk of bias within studies
Appendix 2 shows the results of the individual risk of bias. All studies were of high (n = 8) or moderate (n = 11) methodological quality.
HbA1c
A total of 15 studies evaluated the effect of the new technologies on Hb1Ac: 11 in adults, 2 in children and 2 in both children and adults with T1DM (Table 1).
isCGM compared with SMBG or CGM. The IMPACT trial and prespecified subsequent subanalysis25,32 compared isCGM (FreeStyle Libre, Abbott Diabetes Care, Witney, Oxfordshire, UK) with SMBG in 241 well-regulated T1DM adult patients (Hb1Ac < 7.5% or <58 mmol/mol on inclusion) using MDI (n = 167; 67%) or CSII (n = 78; 33%). Hb1Ac did not significantly change over 6 months, but TIR increased significantly and time in hypoglycaemia decreased significantly (level 3).
The I HART CGM study26 evaluated randomization to isCGM (FreeStyle Libre) or RT-CGM (Dexcom G5, Dexcom, Inc., San Diego, CA, USA) in 40 hypo-unaware patients using MDI. The extension phase of this study evaluated the switch from isCGM to RT-CGM.23 In hypo-unaware patients, neither initiation of isCGM or RT-CGM, nor switching from isCGM to RT-CGM influenced the Hb1Ac levels (level 3), but patients randomized to RT-CGM spent significantly less time in hypoglycaemia and more TIR (cfr time in range in The I HART CGM study; level 3).
No recent RCTs on is CGM with a follow-up of at least 8 weeks were performed in children.
RT-CGM compared with SMBG. In studies on the use of RT-CGM (Dexcom G4, Guardian REAL-Time Clinical, Medtronic, Northridge, CA, USA) in poorly controlled diabetic patients [baseline HbA1c > 7.5% (or >58 mmol/mol), reported a decrease in HbA1c levels between 0.43% and 0.7% (4–7 mmol/mol], together with an increase in TIR and a decrease in time in hypoglycaemia (cfr infra) and less events of severe hypoglycaemia30,31,36 (level 2).
In most studies in hypo-prone patients, the use of RT-CGM (Dexcom G5, Guardian REAL-Time Clinical, Enlite glucose sensor), HbA1c levels did not significantly decrease4,24,35,37 (level 2).
In children aged 2–12 years, RT-CGM did not change Hb1Ac compared with SMBG,21 but the QoL of parents improved (cfr infra; level 2).
HCL with PLGS compared with SAP. In total, three studies evaluated the use of HCL with PLGS versus SAP (without insulin suspension) on Hb1Ac. In 154 children and adolescents with T1DM on CSII (Medtronic 640G with Guardian 2 Link, Medtronic, USA), the add-on of RT-CGM did not influence Hb1Ac but reduced time in hypoglycaemia27 (cfr infra). In two other studies on HCL with PLGS compared with SAP use, Hb1Ac levels decreased with 0.36% (4 mmol/mol) in 86 suboptimal controlled adults and children on Medtronic 640G with Enlite 3 (Medtronic, USA);22 and with 0.3% (3 mmol/mol) in 33 adults on Florence D2A (Sooil, Seoul, South Korea) with FreeStyle Navigator II (Abbott Diabetes Care, Witney, Oxfordshire, UK). In addition to Hb1Ac reduction, TIR increased and time in hypoglycaemia decreased (cfr infra; level 2).
Time in target and time in range
We focused on glycaemic levels between 70 and 180 mg/dl (3.9–10.0 mmol/l) for TIR and values between 70 and 140 mg/dl (3.9–7.8 mmol/l) for TIT. Nine studies evaluated the effect of the new technologies on TIR and TIT: eight in adults and one in both children and adults with T1DM (Table 1).
isCGM compared with SMBG or RT-CGM. In the IMPACT trial and subsequent substudy of 241 well-regulated T1DM adult patients, isCGM (FreeStyle Libre) compared with SMBG (with intermittent double-blinded sensor wear), the use of isCGM increased TIR significantly in patients on MDI with a group difference of 3.4% (0.9 h/day) and in all patients (MDI and CSII) with 4.2% (1 h/day), reaching a TIR over 65% (15.7 h/day;25,32 level 3).25,32
The I HART study evaluated isCGM (FreeStyle Libre) versus RT-CGM (Dexcom G5) in 40 hypo-unaware adults using multiple daily injection.26 TIR increased in RT-CGM from 50.2% to up to 65.9% (15.8 h/day) and in isCGM from 54% to up to 60% (14.4 h/day), with a group difference of 7.4% (1.8 h/day; p = 0.05) in favour of RT-CGM. In the extension phase of this study, patients were switched from isCGM to RT-CGM,23 resulting in an additional significant increase in TIR up to 67.4% (16.2 h/d; p = 0.04; level 3). TIT increased from 31.7 to 43.7% (10.5 h/day) in the RT-CGM group and from 34.8 to 40.4% (9.7 h/day) in the isCGM group, with a group difference of 5% (1.2 h/d; p = 0.15) in favour of RT-CGM. In the extension phase of this study where the patients were switched from isCGM to RT-CGM, there was a small additional increase in TIT to 42.9% (10.3 h/d; p = 0.68; level 3).
No RCTs on isCGM in children (that met the inclusion criteria) were performed.
RT-CGM compared with SMBG. RT-CGM (Dexcom G5) in hypo-prone T1DM patients on MDI or CSII,4,24,31 as well as RT-CGM (Dexcom G4) in poorly regulated T1DM patients on MDI,31 significantly increased TIR compared with SMBG with a group difference between 3.1 and 9.6% (0.7–2.3 h/day; level 2).
HCL with PLGS compared with SAP. Two studies evaluated the use of HCL (Medtronic 640G with Enlite 3, Florence D2A with FreeStyle Navigator II) versus SAP on TIR in adults and children.22,33 After 12 weeks, TIR increased significantly with 10.8–11% (2.6 h/day) reaching TIRs of 65.0–67.7% (15.6–16.2 h/day; level 2).
Time in hypoglycaemia
Fifteen studies evaluated the effect of the novel technologies on hypoglycaemia in adults (n = 13), in children (n = 1) or in both children and adults (n = 1). Different studies used different cut-off levels of hypoglycaemia. We focused on hypoglycaemia < 70 mg/dl (<3.9 mmol/l; level 1 hypoglycaemia), hypoglycaemia < 50, 54 or 55 mg/dl (<2.8, 3.0 or 3.1 mmol/l; level 2 hypoglycaemia), and severe hypoglycaemia, if not otherwise described (Table 1).
isCGM compared with SMBG or CGM. In the IMPACT trial and subsequent substudy, isCGM (FreeStyle Libre) decreased time spent in hypoglycaemia significantly compared with SMBG.25,32 Level 1 hypoglycaemias (<70 mg/dl or <3.9 mmol/l) decreased with a group difference of 1.2 h/day (5.2%)32 in patients on CSII or MDI and 1.65 h/day (6.8%)25 in patients on MDI. Level 2 hypoglycaemias (defined as <55 mg/dl or <3.1 mmol/l) decreased with a group difference of −0.82 h/day (−3.4%)32 in patients on CSII and MDI and −1.1 h/day (−4.6%) in patients on MDI (level 3).
The I HART study evaluated isCGM (FreeStyle Libre) versus RT-CGM (Dexcom G5) in 40 hypo-unaware adults using MDI,26 and found a significantly greater reduction in time spent in hypoglycaemia at all levels in RT-CGM compared with isCGM, with a group difference of RT-CGM over isCGM of 0.8 h/day (3.3%) at level 1 hypoglycaemia, and 0.6 h/day (2.5%) at level 2 hypoglycaemia. In the extension phase of this study, patients were switched from isCGM to RT-CGM,23 resulting in an additional significant decrease in time spent in clinically relevant hypoglycaemia (level 2 hypoglycaemia, <54 mg/dl or <3.0 mmol/l) from −1.2 h/day (−5.0%) to −0.2 h/day (−0.8%; level 3).
No RCTs on isCGM in children were performed under the desired circumstances for inclusion in this review.
RT-CGM compared with SMBG. The introduction of RT-CGM (Dexcom G4, G5, Gardian REAL-Time Clinical) compared with SMBG significantly reduced frequency of and time in hypoglycaemia significantly in T1DM patients, both in inadequately regulated patients6,30,31,36 as well as in hypo-unaware patients,4,24 independent of CSII or MDI use (level 2).
Little and colleagues,35,37 did not report time in hypoglycaemia between the intervention groups separately at 6 months or at 24 months; however, they showed a progressive decline in impaired hypo-awareness at 6 and 24 months (level 3).
In children, no RCTs comparing RT-CGM with SMBG were performed.
HCL with PLGS compared with SAP. One study evaluated HCL (Medtronic 640G) with or without PLGS in 154 children with T1DM, for 6 months. HCL with PLGS decreased hypoglycaemia in day and night time, compared with those with SAP without PLGS; time spent in clinically relevant hypoglycaemia (level 2 hypoglycaemia, <54 mg/dl or <3.0 mmol/l) decreased significantly with a mean difference of 0.44% in favour of PLGS, without negatively affecting Hb1Ac.27 Two other studies evaluated the use of HCL versus SAP on time in hypoglycaemia in adults and children over a period of 3 months22,33 (level 2). As mentioned above, in both studies, Hb1Ac levels decreased and TIR increased with HCL use compared with SAP. In addition, time in level 1 hypoglycaemia (<70 mg/dl or 3.9 mmol/l) reduced significantly with −0.8% (0.2 h/day; level 1).
Quality of life
Sixteen studies evaluated the effect of the new technologies on QoL: in adults (n = 11), children (n = 2) and in both adults and children (n = 3) (Table 1).
isCGM compared with SMBG or RT-CGM. Treatment satisfaction, hypo- and hyperperception improved significantly over 6 months with isCGM (FreeStyle Libre), in the above-mentioned IMPACT trials25,32 (level 3).
In the above-mentioned I HART CGM study26 in hypo-unaware patients, RT-CGM (Dexcom G5) decreased the Hypoglycaemia Fear Survey II (HFS-II) worry subscore in relation to the group difference of hypoglycaemia compared with isCGM (FreeStyle Libre). In the extension phase of this study, the HFS-II worry subscore also significantly improved when switching from isCGM to RT-CGM23 (level 3).
No RCTs with a sufficient follow-up on isCGM in children were performed.
RT-CGM compared with SMBG. In adults on MDI with suboptimal glycaemic control, RT-CGM (Dexcom G4) decreased fear of hypoglycaemia (GOLD study),30 improved hypoglycaemia-related confidence (GOLD 3 trial),6 especially in social situations, contributing to greater well-being and quality of life (GOLD study; GOLD 3 trial);6,30 and increased treatment satisfaction [HypoDE (Hypoglycemia in Deutschland) (Dexcom G5); GOLD study24,30 (level 1)].
In the SWITCH study of 153 adults on CSII with suboptimal T1DM control, RT-CGM (MiniMed SofSensor, Medtronic, USA) decreased hypo-fear and increased social flexibility and overall treatment satisfaction34 (level 1).
In adults with hypo-unawareness, regardless of MDI or CSII use, RT-CGM reduced hypoglycaemia fear and increased overall treatment satisfaction in some studies [HypoDE (Dexcom G5); IN CONTROL (Enlite Medtronic)],4,24 although these results were not seen in other studies [HypoCOMPaSS (Newcastle Upon Tyne Hospitals NHS Foundation Trust) (Medtronic);35,37 level 3].
In children, RT-CGM use did not significantly change children’s self-reports.21,34 In parents, however, RT-CGM with remote control increased parents’ proxy rates on children’s QoL, decreased familial distress and increased parental sleep without changes in children’s self-report on QoL21 (level 3).
HCL with PLGS compared with SAP. Three studies evaluated HCL (Metronic 640G, Dana R pump (Sooil, Seoul, South Korea) with FreeStyle Libre Navigator II (Abbott Diabetes Care, UK)) with or without PLGS in adults and children (and their parents) with T1DM22,27,29 and reported no change in QoL (level 1). One study reported increased treatment satisfaction in adults and children with both treatments after the follow-up period, with no favour for either of the treatments.29
Discussion
This systematic review shows promising results of the use of isCGM, RT-CGM, SAP and HCL with PLGS that influences the management of T1DM, particularly in preventing hypoglycaemia, decreasing hypoglycaemia fear and improving QoL, in combination with improving TIR and preserving or improving Hb1Ac levels. If implemented successfully in diabetes care, these medical devices could thereby prevent potential acute complications and possibly also chronic complications. In addition, in almost all RCTs, HCL with PLGS and SAP were more likely to have a more beneficial outcome compared with isCGM and RT-CGM in conventional therapies (CSII and MDI).
Patients with high HbA1c values at the introduction of RT-CGM and HCL with PLGS achieved the greatest reduction in HbA1c levels. It was not surprising that in patients who already managed their diabetes well, only a little additional improvement in HbA1c was possible. However, new technologies (isCGM, RT-CGM, SAP or HCL with PLGS) in those patients proved to be beneficial in increasing TIR and decreasing time in hypoglycaemia.
isCGM (FreeStyle Libre) increased TIR significantly compared with SMBG in well-controlled T1DM patients on MDI or CSII, reaching TIR > 65%.25,32 According to studies that randomized hypo-prone patients on MDI to either RT-CGM (Dexcom G5) or isCGM (FreeStyle Libre), TIR increased more in RT-CGM (>65%) than in isCGM (>60%)26 and TIR increased even more in those switching from isCGM to RT-CGM afterwards (>67%).23 Studies on hypo-prone patients on MDI or CSII reported TIRs of 58.5–65.0% with RT-CGM (Dexcom G5, Enlite glucose sensor, respectively). In addition, studies on poorly controlled T1DM patients reported increased TIR on RT-CGM (Dexcom G4) compared with SMBG, reaching 51.3% TIR on RT-CGM.31 RCTs on HCL systems with PLGS showed TIR of 65.0–67.7%.22,33
Compared with conventional SMBG, all systems (isCGM, RT-CGM, PLGS and HCL) decreased frequency and time in hypoglycaemia, and one study indicated improved hypo-awareness.35,37 isCGM (FreeStyle Libre) does not have alarms, but there are indications that isCGM decreases time in hypoglycaemia compared with SMBG in adults who were already well controlled (baseline HbA1c < 7.5% or 58 mmol/mol) and motivated to scan (flash) regularly. However, the I HART study indicates that switch to RT-CGM (Dexcom G5) further decreases time in hypoglycaemia.23 Indeed, the use of RT-CGM with (predictive) alarms when glucose levels (tend to) drop under a predefined threshold enabled adults and children (or their parents) to anticipate hypoglycaemia. Consequently, SAP therapy with alarms had an additional beneficial effect, lowering the time spend in hypoglycaemia without negatively affecting HbA1c. Studies both in adults and children showed that the use of HCLs with PLGS significantly decreased time in hypoglycaemia.
Studies in adults showed improved treatment satisfaction with all new technologies. The new technologies with alarms (RT-CGM, SAP and HCL) reduced fear, worry and distress of hypoglycaemia and improved QoL. Studies in children indicate that self-reports did not change in RT-CGM, but parents reported increased QoL, decreased familial distress and increased parental sleep, in case of RT-CGM.21 More studies on QoL should be done to investigate the best treatment for each individual patient with the lowest treatment burden.
The most frequent methodological difficulty was that patients and clinicians were not blinded to the treatment. However, it is not possible to blind patients for this kind of treatment and it was often unclear if the effect assessors (researchers) were blinded to the treatment.
Furthermore, up till now, the RCTs with a long follow-up on HCL systems using control algorithms to deliver insulin in a glucose-responsive manner, evaluated the predictive LGS function. Recently, these algorithms can also support increments of insulin dosing, and dual-hormone HCLs with glucagon dosing will also be administered. RCTs on these most recent adjustments included only a short follow-up and were therefore not assessed in this review.10
In addition, it is important to note that RCTs are subject to selection bias, and that real-world studies might show less impressive results. To evaluate the sustainable effect of HCLs, more RCTs with a longer follow-up are needed, as while most recent studies were indeed performed in real-life, they were undertaken in supervised situations such as camps, with only a very short follow-up of a few days.10
Finally, it is important to understand that these new technologies have a time lag compared with actual blood glucose levels, especially when those levels change rapidly, like during physical activity.38 For the future, there is a challenge in overcoming this time lag in HCL algorithms. Currently, this time lag is challenging for patients and physicians, as the success of implementation of these new technologies depends on effective guidance on use of these systems, which is, up till now, scarce11–15 (see also part A of this review).
Nevertheless, the results of RCTs are promising and prove the beneficial effects of novel technologies.
Conclusion
The introduction of isCGM and RT-CGM has transformed diabetes care. SAP and HCLs can make an additional difference in the daily life of our patients by reducing time in hypoglycaemia, increasing TIR and improving QoL. The success of these novel technologies, however, depends on the level to which people are educated, capable and motivated to use them. Successful implementation of these novel technologies might eventually reduce severe acute and chronic invalidating complications.
Supplemental Material
Supplemental material, Supplementary_material_pdf for The road from intermittently scanned continuous glucose monitoring to hybrid closed-loop systems. Part B: results from randomized controlled trials by Francesca De Ridder, Marieke den Brinker and Christophe De Block in Therapeutic Advances in Endocrinology and Metabolism
Footnotes
Authors’ note: CDB conceived the idea for the manuscript and decided the overall theme and content. MdB and FDR drafted the manuscript. All authors critically reviewed and approved the final submission.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: CDB is a consultant for Abbott, A. Menarini Diagnostics, Lilly, Medtronic, Novo Nordisk, and Roche Diagnostics.
ORCID iD: Christophe De Block  https://orcid.org/0000-0002-0679-3203
https://orcid.org/0000-0002-0679-3203
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Francesca De Ridder, Faculty of Medicine and Health Sciences, University of Antwerp, Antwerp, Belgium; Department of Endocrinology, Diabetology and Metabolism, Antwerp University Hospital, Antwerp, Belgium.
Marieke den Brinker, Faculty of Medicine and Health Sciences, University of Antwerp, Antwerp, Belgium; Department of Paediatrics, Antwerp University Hospital, Antwerp, Belgium.
Christophe De Block, Faculty of Medicine and Health Sciences, University of Antwerp, Antwerp, Belgium; Department of Endocrinology, Diabetology and Metabolism, Antwerp University Hospital, Antwerp, Belgium.
References
- 1. Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986. [DOI] [PubMed] [Google Scholar]
- 2. Bergenstal RM, Tamborlane WV, Ahmann A, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010; 363: 311–320. [DOI] [PubMed] [Google Scholar]
- 3. Norgaard K, Scaramuzza A, Bratina N, et al. Routine sensor-augmented pump therapy in type 1 diabetes: the INTERPRET study. Diabetes Technol Ther 2013; 15: 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Beers CA, DeVries JH, Kleijer SJ, et al. Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open-label, crossover trial. Lancet Diabetes Endocrinol. Epub ahead of print 15 September 2016. DOI: 10.1016/S2213-8587(16)30193-0. [DOI] [PubMed] [Google Scholar]
- 5. Klonoff DC, Ahn D, Drincic A. Continuous glucose monitoring: a review of the technology and clinical use. Diabetes Res Clin Pract 2017; 133: 178–192. [DOI] [PubMed] [Google Scholar]
- 6. Olafsdottir AF, Polonsky W, Bolinder J, et al. A randomized clinical trial of the effect of continuous glucose monitoring on nocturnal hypoglycemia, daytime hypoglycemia, glycemic variability, and hypoglycemia confidence in persons with type 1 diabetes treated with multiple daily insulin injections (GOLD-3). Diabetes Technol Ther 2018; 20: 274–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Charleer SMC, Nobels F, De Block C, et al. Improving glycaemic control, acute complication risk and quality of life through reimbursement of sensor-augmented pump therapy in patients with type 1 diabetes: a real-world nationwide prospective cohort study. J Clin Endocrinol Metab 2018; 103: 1224–1232.29342264 [Google Scholar]
- 8. Kowalski A. Pathway to artificial pancreas systems revisited: moving downstream. Diabetes Care 2015; 38: 1036–1043. [DOI] [PubMed] [Google Scholar]
- 9. Bekiari E, Karagiannis T, Haidich AB, et al. Meta-analysis of artificial pancreas trials: methodological considerations. Lancet Diabetes Endocrinol 2017; 5: 685. [DOI] [PubMed] [Google Scholar]
- 10. Bekiari E, Kitsios K, Thabit H, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ 2018; 361: k1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peters AL, Ahmann AJ, Battelino T, et al. Diabetes technology-continuous subcutaneous insulin infusion therapy and continuous glucose monitoring in adults: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2016; 101: 3922–3937. [DOI] [PubMed] [Google Scholar]
- 12. Aleppo G, Laffel LM, Ahmann AJ, et al. A Practical approach to using trend arrows on the dexcom G5 CGM system for the management of adults with diabetes. J Endocr Soc 2017; 1: 1445–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kudva YC, Ahmann AJ, Bergenstal RM, et al. Approach to using trend arrows in the FreeStyle Libre flash glucose monitoring systems in adults. J Endocr Soc 2018; 2: 1320–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grunberger G, Handelsman Y, Bloomgarden ZT, et al. American Association of Clinical Endocrinologists and American College of Endocrinology 2018 position statement on integration of insulin pumps and continuous glucose monitoring in patients with diabetes mellitus. Endocr Pract 2018; 24: 302–308. [DOI] [PubMed] [Google Scholar]
- 15. Kruger DF, Edelman SV, Hinnen DA, et al. Reference guide for integrating continuous glucose monitoring into clinical practice. Diabetes Educ 2019; 45(Suppl. 1): 3S–20S. [DOI] [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009; 62: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 17. Scholten RJPM, Offringa M, Assendelft WJJ. (Red.). Inleiding in evidence-based medicine, 10.1007/978-90-368-1978-7_4 [DOI]
- 18. Van der Wees PJ, Lenssen AF, Hendriks EJ, et al. Effectiveness of exercise therapy and manual mobilisation in ankle sprain and functional instability: a systematic review. Aust J Physiother 2006; 52: 27–37. [DOI] [PubMed] [Google Scholar]
- 19. Petrisor B, Bhandari M. The hierarchy of evidence: levels and grades of recommendation. Indian J Orthop 2007; 41: 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg 2011; 128: 305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burckhardt MA, Roberts A, Smith GJ, et al. The use of continuous glucose monitoring with remote monitoring improves psychosocial measures in parents of children with type 1 diabetes: a randomized crossover trial. Diabetes Care 2018; 41: 2641–2643. [DOI] [PubMed] [Google Scholar]
- 22. Tauschmann M, Thabit H, Bally L, et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet 2018; 392: 1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reddy M, Jugnee N, Anantharaja S, et al. Switching from flash glucose monitoring to continuous glucose monitoring on hypoglycemia in adults with type 1 diabetes at high hypoglycemia risk: the extension phase of the I HART CGM study. Diabetes Technol Ther 2018; 20: 751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heinemann L, Freckmann G, Ehrmann D, et al. Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet 2018; 391: 1367–1377. [DOI] [PubMed] [Google Scholar]
- 25. Oskarsson P, Antuna R, Geelhoed-Duijvestijn P, et al. Impact of flash glucose monitoring on hypoglycaemia in adults with type 1 diabetes managed with multiple daily injection therapy: a pre-specified subgroup analysis of the IMPACT randomised controlled trial. Diabetologia 2018; 61: 539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reddy M, Jugnee N, El Laboudi A, et al. A randomized controlled pilot study of continuous glucose monitoring and flash glucose monitoring in people with type 1 diabetes and impaired awareness of hypoglycaemia. Diabet Med 2018; 35: 483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abraham MB, Nicholas JA, Smith GJ, et al. Reduction in hypoglycemia with the predictive low-glucose management system: a long-term randomized controlled trial in adolescents with type 1 diabetes. Diabetes Care 2018; 41: 303–310. [DOI] [PubMed] [Google Scholar]
- 28. Polonsky WH, Hessler D, Ruedy KJ, et al. The impact of continuous glucose monitoring on markers of quality of life in adults with type 1 diabetes: further findings from the DIAMOND randomized clinical trial. Diabetes Care 2017; 40: 736–741. [DOI] [PubMed] [Google Scholar]
- 29. Barnard KD, Wysocki T, Ully V, et al. Closing the loop in adults, children and adolescents with suboptimally controlled type 1 diabetes under free living conditions: a psychosocial substudy. J Diabetes Sci Technol 2017; 11: 1080–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lind M, Polonsky W, Hirsch IB, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA 2017; 317: 379–387. [DOI] [PubMed] [Google Scholar]
- 31. Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA 2017; 317: 371–378. [DOI] [PubMed] [Google Scholar]
- 32. Bolinder J, Antuna R, Geelhoed-Duijvestijn P, et al. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet 2016; 388: 2254–2263. [DOI] [PubMed] [Google Scholar]
- 33. Thabit H, Tauschmann M, Allen JM, et al. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med 2015; 373: 2129–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hommel E, Olsen B, Battelino T, et al. Impact of continuous glucose monitoring on quality of life, treatment satisfaction, and use of medical care resources: analyses from the SWITCH study. Acta Diabetol 2014; 51: 845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Little SA, Leelarathna L, Walkinshaw E, et al. Recovery of hypoglycemia awareness in long-standing type 1 diabetes: a multicenter 2 x 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care 2014; 37: 2114–2122. [DOI] [PubMed] [Google Scholar]
- 36. Tumminia A, Crimi S, Sciacca L, et al. Efficacy of real-time continuous glucose monitoring on glycaemic control and glucose variability in type 1 diabetic patients treated with either insulin pumps or multiple insulin injection therapy: a randomized controlled crossover trial. Diabetes Metab Res Rev 2015; 31: 61–68. [DOI] [PubMed] [Google Scholar]
- 37. Little SA, Speight J, Leelarathna L, et al. Sustained reduction in severe hypoglycemia in adults with type 1 diabetes complicated by impaired awareness of hypoglycemia: two-year follow-up in the HypoCOMPaSS randomized clinical trial. Diabetes Care 2018; 41: 1600–1607. [DOI] [PubMed] [Google Scholar]
- 38. Moser O, Eckstein ML, Mueller A, et al. Impact of physical exercise on sensor performance of the FreeStyle Libre intermittently viewed continuous glucose monitoring system in people with type 1 diabetes: a randomized crossover trial. Diabet Med 2019; 36: 606–611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_material_pdf for The road from intermittently scanned continuous glucose monitoring to hybrid closed-loop systems. Part B: results from randomized controlled trials by Francesca De Ridder, Marieke den Brinker and Christophe De Block in Therapeutic Advances in Endocrinology and Metabolism