Abstract
More than half of the patients with advanced hepatocellular carcinoma (HCC) do not respond to primary treatment with sorafenib. Currently, there are no universally accepted methods for further treatment. This pilot study was performed to assess the safety and effectiveness of apatinib as an optional treatment for patients with sorafenib-refractory HCC. Between January 2015 and May 2017, 43 consecutive patients with sorafenib-refractory advanced HCC who received apatinib were reviewed. The objective response rate (ORR) and disease control rate (DCR) were assessed using modified response evaluation criteria in solid tumors. The time to progression (TTP) and overall survival (OS) were determined using the Kaplan-Meier method. Toxicities associated with apatinib were assessed. All patients had hepatitis B virus (HBV) related HCC. The mean follow-up time was 11 months (range: 3-37) and the mean duration of apatinib was 7.6 months (range: 1-32). After treatment, 11 patients had partial response (PR), 18 had stable disease (SD), and 14 had progressive disease (PD); accordingly, the ORR and DCR were 25.6% and 67.4%, respectively. The median TTP and OS were 3 months (95% confidence interval [CI]: 1.9-4.1) and 8 months (95% CI: 6.9-9.0), respectively. The median OS times for PR, SD, and PD were 19 months (95% CI: 15.8-22.2), 8 months (95% CI: 7.3-8.7), and 4 months (95% CI: 3.1-4.9), respectively (P < .001). The median TTP for PR, SD, and PD was 14 months (95% CI: 11.9-16.1), 3 months (95% CI: 2.3-3.7) and 1 month, respectively (P < .001). No patients experienced toxicity-related death. The most common toxicities were weight loss, hand–foot skin reaction, and hypertension. Twelve adverse events of grade 3 or higher were observed. Based on our findings, apatinib is a promising treatment for patients with sorafenib-refractory advanced HBV-related HCC.
Keywords: hepatocellular carcinoma, refractory, apatinib, sorafenib, survival
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy and the third leading cause of cancer-related death worldwide.1 Approximately 40% of all cases with HCC are diagnosed at an advanced stage (cancer with portal invasion or extrahepatic metastasis according to the Barcelona Clinic Liver Cancer [BCLC] system), and thus, patients must rely on palliative therapy to prolong their survival time.2–4 Since the Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP) trial and the Asia-Pacific trial showed that sorafenib significantly improved survival benefits, sorafenib was recommend as the first-line systemic therapy for advanced HCC.5–7 However, the objective response rate (ORR) was rarely achieved, and the disease control rate (DCR) was also limited (reported at 35.3%-43%).5,6 In other words, more than half of the patients with advanced HCC did not respond to primary treatment with sorafenib. What can we do for those patients with sorafenib-refractory HCC?
Recently, regorafenib was recommended as a second-line systemic targeted therapy for patients with HCC who progressed on sorafenib, since Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE) trial showed that regorafenib compared with placebo significantly improved the overall survival (OS) of patients with HCC who progressed on sorafenib.8,9 However, we found the majority of enrolled patients had acquired resistance to sorafenib.10 Therefore, whether regorafenib benefits patients with primary resistance to sorafenib is unclear. Apatinib is a novel receptor tyrosine kinase inhibitor that selectively targets vascular endothelial growth factor receptor (VEGFR) 2, with a binding affinity 10 times that of sorafenib.11,12 Apatinib significantly reduced tumor growth in several established human tumor xenograft models by inhibiting tumor-induced angiogenesis.11,12 Recently, apatinib has been widely used for treatment of HCC, and studies have indicated that apatinib is a safe and effective option for unresectable HCC.13–19 Furthermore, the low price of apatinib facilitates its application in China.
Currently, there are no universally accepted treatments for patients with sorafenib-refractory HCC. Although some treatments have been reported for patients with sorafenib-refractory HCC, some of the adverse events were severe and offset the moderate beneficial effects.20–22 Recently, 2 case reports showed that apatinib demonstrated an excellent antitumor effect in patients with sorafenib-refractory HCC.14,16 Therefore, we conducted a pilot study to investigate apatinib use for patients with sorafenib-refractory advanced HCC (sorafenib-refractory HCC was defined as a target lesion with 2 successive instances of progression, determined using modified response evaluation criteria in solid tumors [mRECIST]23 every month after initiation of sorafenib treatment) to evaluate the safety and effectiveness of apatinib in these patients.
Materials and Methods
Patient Selection
Between January 2015 and May 2017, consecutive patients with HCC treated with sorafenib were reviewed at our center. The inclusion criteria were as follows: (1) all patients had pathologically or radiologically (contrast-enhanced computed tomography) confirmed advanced HCC based on the European Association for the Study of the Liver diagnostic criteria3; (2) all patients had sorafenib-refractory HCC and must have tolerated sorafenib (at least ≥ 400 mg daily). Sorafenib-refractory HCC was defined as 2 successive instances of radiological progression to confirm real disease progression and eliminate the possibility that sorafenib did not work due to insufficient treatment time (previous studies have reported that the onset of reaction to sorafenib occurred within the first month of sorafenib initiation in most patients)24,25; (3) all patients were transferred to apatinib treatment within 4 weeks after confirmation of sorafenib resistance; and (4) patients must have Child-Pugh score of less than 7 and Eastern Cooperative Oncology Group (ECOG) score of 0 or 1. Patients were excluded if they had received any other previous systemic treatment for HCC, if they discontinued sorafenib due to toxicity, the duration of apatinib administration was less than 4 weeks, or patients data were missing.
All patients were informed that apatinib was an optional treatment because the first-line sorafenib treatment was ineffective. In addition, patients were informed of the anticipated outcomes, economic cost, and toxicity. Final treatment decisions were generally made by the patients. All patients provided written informed consent, and the protocol was approved by the ethics committees of the institution.
Treatment Protocol
The sorafenib treatment protocol was based on that described in a previous study.26 The initial dose of oral sorafenib was 400 mg twice daily. Doses were modified depending on the intolerable toxicity. If a patient experienced progression 2 consecutive times after initiation of sorafenib treatment, apatinib treatment was advised (250 mg per tablet; Jiangsu Hengrui Medicine, Lianyungang, China). The initial dose of apatinib was 500 mg every day, reduced to 250 mg in the event of intolerable toxicity. If further dose reduction was required, treatment was discontinued. Treatment continued until disease progression (defined by mRECIST), clinical progression (defined as an ECOG score ≥3 or symptomatic deterioration, including liver function tests), death, unacceptable toxicity, or withdrawal of consent by the patient.17,18
Assessment and Follow-Up
Contrast-enhanced computed tomography scans of the chest and upper abdomen, liver function tests, and alpha-fetoprotein level assessments were performed 1 month after treatment to evaluate the treatment effect. The ORR was defined as the sum of the complete response (CR) and partial response (PR), while the DCR was defined as the sum of the CR, PR, and stable disease (SD). Apatinib treatment could be continued beyond progression if the physician judged the patient would benefit from the continuation. Overall survival was defined as the time from the start of sorafenib treatment until death or the last follow-up. Time to progression (TTP) was defined as the time from initiation of apatinib treatment to radiologic or clinical progression. Disease progression was measured using mRECIST.23 The grade of toxicity was recorded according to version 3.0 of the National Cancer Institute’s Common Terminology Criteria for Adverse Events.27
Statistical Analysis
All statistical analyses were performed using SPSS software (SPSS version 16.0; SPSS, Chicago, Illinois). For baseline characteristics, continuous variables are described as the mean ± standard deviation, and categorical variables are expressed as frequencies and percentages. The Kaplan-Meier method was used to calculate the TTP and OS. Univariate analyses were performed using a log-rank test. Variables with a P < .1 in univariate analysis were entered into a multivariate analysis. The multivariate Cox model was used to identify risk factors that affected OS. All statistical tests were 2-sided, and P < .05 was considered statistically significant.
Results
Study Population
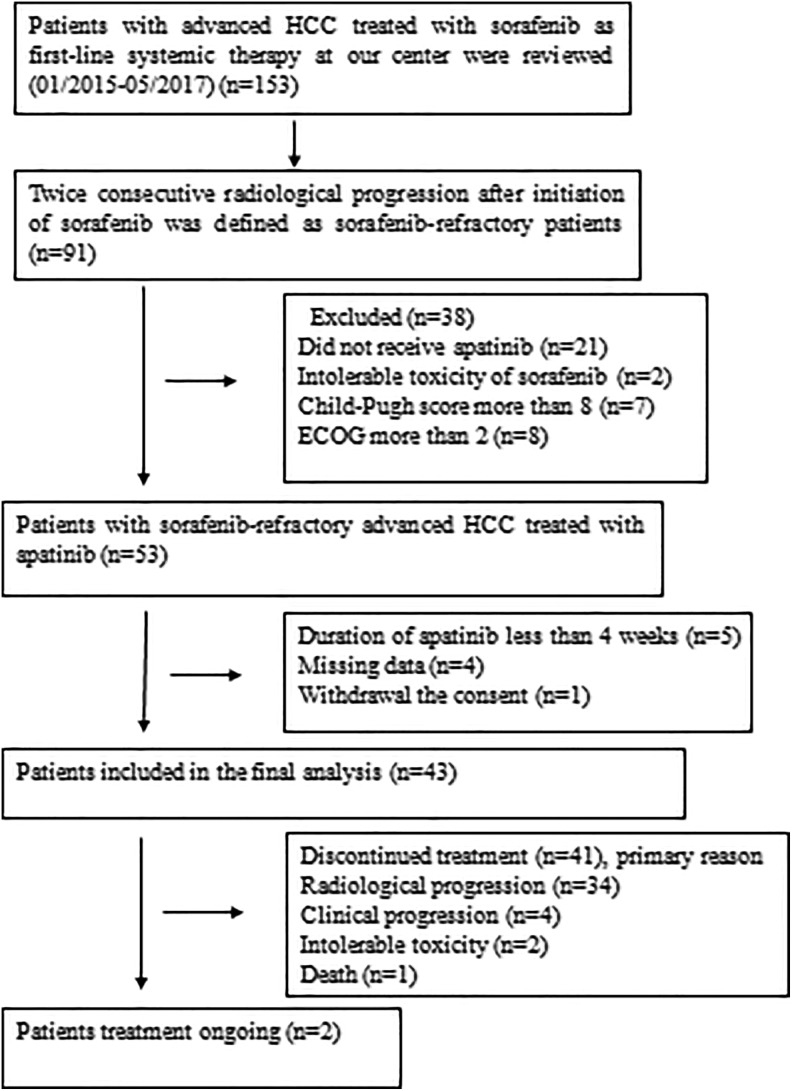
In total, between January 2015 and May 2017, 153 consecutive patients with advanced HCC treated with sorafenib were reviewed, and 91 patients were confirmed to have sorafenib-refractory HCC. A total of 38 patients were excluded from the analysis because they did not satisfy the inclusion criteria. As a result, 53 patients with sorafenib-refractory HCC received apatinib treatment. Additionally, 10 patients were excluded. Finally, 43 patients with sorafenib-refractory advanced HCC were enrolled in this analysis (Figure 1). The baseline characteristics of all patients are shown in Table 1. The majority of the patients were male (90.7%), and hepatitis B (100%) and cirrhosis (88.4%) were the most common underlying disease. Among the patients, 41 (95.3%) of 43 had BCLC stage C HCC, and the remaining 2 had multiple bilateral liver tumors (BCLC stage B) and did not respond to chemoembolization. Moreover, 34 (79.1%) of 43 patients had a portal vein thrombosis (PVTT).
Figure 1.
Flow diagram showing patient selection. ECOG indicates Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma.
Table 1.
Baseline Characteristics of Patients With Sorafenib-Refractory Advanced HCC.
| Characteristics | N = 43 |
|---|---|
| Age, years, mean ± SD | 50.1 ± 9.2 |
| Sex (male/female) | 39/4 |
| Etiology (HBV/HCV/other) | 43/0/0 |
| Cirrhosis (present/absent) | 38/5 |
| No. of tumors (1-3/>3) | 9/34 |
| Liver tumor burden (<50%/≥50%) | 17/26 |
| ECOG (0/1) | 13/30 |
| Child-Pugh score 5/6/7 | 30/9/4 |
| BCLC B/C | 2/41 |
| PVTT (branch/main/no) | 18/16/9 |
| Extrahepatic spread (lung/enterocoelia/no) | 6/2/35 |
| AFP (≤400/>400), ng/mL | 11/32 |
| Ascites (present/absent) | 6/37 |
| ALT, U/L | 50.4 ± 29.6 |
| GGT, U/L | 173.7 ± 101.6 |
| Albumin, g/L | 37.9 ± 4.1 |
| Bilirubin, µmol/L | 19.5 ± 6.4 |
Abbreviations: AFP, alpha-fetoprotein; ALT, alanine aminotransferase; BCLC, Barcelona Clinic Liver Cancer; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; GGT, γ-glutamyl transpeptidase; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; PVTT, portal vein tumor thrombus; SD, standard deviation.
Treatment Outcome
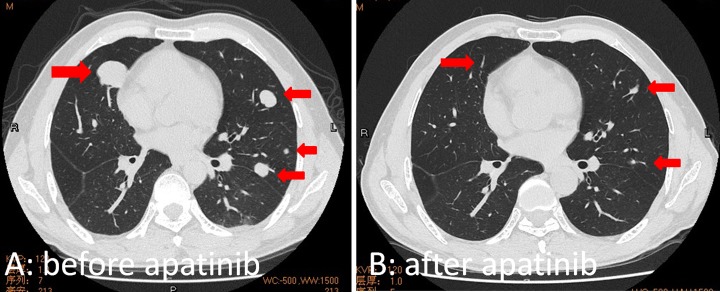
The mean duration of sorafenib treatment was 2.1 months (range: 1.5-2.5). The mean duration of the apatinib administration was 7.6 months (range: 1-32). At month 2 after apatinib treatment, 11 (25.6%) patients had PR, 18 (41.9%) had SD, and 14 (32.6%) had progressive disease (PD). Accordingly, the ORR was 25.6% and the DCR was 67.4%. The treatment outcome details are shown in Table 2. A 51-year-old man with HCC and multiple pulmonary metastasis experienced a disappearance of all lung lesions after 6 months of apatinib treatment, at the time of this writing, the patient has survived for 37 months and is fully ambulatory (Figure 2).
Table 2.
Tumor Response Assessed by mRECIST at Month 2 After Apatinib Treatment.
| Tumor Response | n (%) |
|---|---|
| Complete response | 0 |
| Partial response | 11 (25.6) |
| Stable disease | 18 (41.9) |
| Progressive disease | 14 (32.6) |
| Objective response rate | 11 (25.6) |
| Disease control rate | 29 (67.4) |
Abbreviation: mRECIST, modified response evaluation criteria in solid tumors.
Figure 2.
Images of a 51-year-old man with biopsy-confirmed HCC and multiple pulmonary metastases, who received apatinib treatment due to primary resistance to sorafenib; the patient experienced disappearance of all lung lesions after 6 months of apatinib treatment. At the time of this writing, the patient has survived for 37 months and is fully ambulatory. A, Chest computed tomography image showing multiple lesions in both lungs before apatinib treatment. B, At 6 months after apatinib treatment, the chest computed tomography image showed disappearance of all lesions and lung fibrosis. HCC indicates hepatocellular carcinoma.
Survival
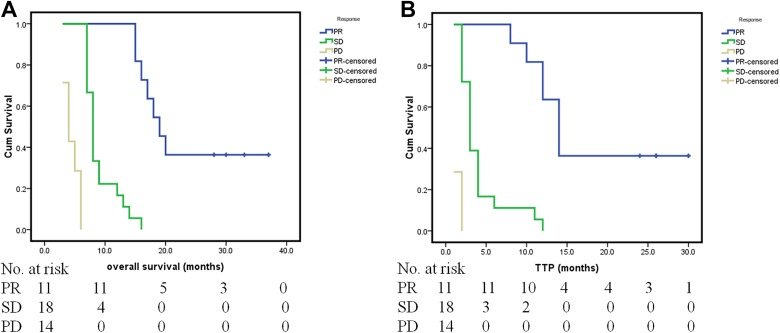
At the end of follow-up (May 2018), 39 (90.7%) of 43 patients had died. The mean follow-up for these patients was 11 months (range: 3-37). The median total patients OS was 8 months (95% confidence interval [CI]: 6.9-9.0). The 6-month, 1-year, and 2-year survival rates were 76.7%, 34.9%, and 9.3%, respectively. The median OS was 19 months (95% CI: 15.8-22.2), 8 months (95% CI: 7.3-8.7), and 4 months (95% CI: 3.1-4.9) for PR, SD, and PD, respectively (P < .001; Figure 3A).The median total patient TTP after apatinib treatment was 3 months (95% CI: 1.9-4.1). The median and mean TTP after sorafenib treatment was 1 month and 1.1 months, respectively. The median TTP was 14 months (95% CI: 11.9-16.1), 3 months (95% CI: 2.3-3.7), and 1 month for PR, SD, and PD, respectively (P < .001; Figure 3B).
Figure 3.
Kaplan-Meier curves showing overall survival (OS) and time to progression (TTP) for partial response (PR), stable disease (SD), and progressive disease (PD) in patients with sorafenib-refractory advanced HCC treated with apatinib. A, The median OS times were 19 months (95% CI: 15.8-22.2), 8 months (95% CI: 7.3, 8.7), and 4 months (95% CI: 3.1-4.9) for PR, SD, and PD, respectively (P < .001). B, The median TTP was 14 months (95% CI: 11.9-16.1), 3 months (95% CI: 2.3-3.7), and 1 month for PR, SD, and PD, respectively (P < .001). CI indicates confidence interval; HCC, hepatocellular carcinoma.
In univariate analysis, cirrhosis, liver tumor burden, extrahepatic metastases, and PVTT were significantly associated with OS. In multivariate Cox analysis, liver tumor burden (≥50% vs <50%; hazard ratio [HR] = 1.543; 95% CI: 1.067-2.598; P = .001) and extrahepatic metastases (present vs absent; HR = 0.545; 95% CI: 0.333-0.895; P = .013) were demonstrated as the independent prognostic factors for OS. The outcome details are shown in Table 3. The median OS was 16 months (95% CI: 13.3-18.7) for patients with liver tumor burden <50% and 6 months (95% CI: 4.8-7.2) for patients with liver tumor burden ≥50% (P < .001). The median OS was 18 months (95% CI: 14.6-20.2) for patients with extrahepatic metastases and 7 months (95% CI: 5.8-8.2) for patients without extrahepatic metastasis (P < .001).
Table 3.
Univariate and Multivariate Analyses of Prognostic Factors for Overall Survival of Patients With Sorafenib-Refractory Advanced HCC.
| Factors | Overall Survival | |||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| P Value | HR | 95% CI | P Value | |
| Gender (M/F) | .557 | |||
| Age, years | .283 | |||
| Cirrhosis (present/absent) | .026 | 0.354 | 0.103-1.222 | .101 |
| ALT (≤40/>40) | .744 | |||
| Albumin (<35/≥35) | .050 | 0.396 | 0.152-1.030 | .058 |
| Bilirubin (≤20/>20) | .211 | |||
| GGT (≤100/>100) | .068 | 1.395 | 0.613-3.178 | .428 |
| Ascites (present/absent) | .126 | |||
| Child-Pugh (A/B) | .059 | 2.172 | 0.538-8.766 | .276 |
| ECOG (0/1) | .729 | |||
| AFP (≤400/>400) | .912 | |||
| Liver tumor burden (≥50%/<50%) | <.001 | 1.543 | 1.067-2.598 | .001 |
| No. of tumors (1-3/>3) | .129 | |||
| PVTT (present/absent) | .001 | 2.695 | 1.783-3.457 | .104 |
| Extrahepatic metastases (present/absent) | <.001 | 0.235 | 0.106-0.795 | .013 |
Abbreviations: AFP, alpha-fetoprotein; ALT, alanine aminotransferase; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; F, female; GGT, γ-glutamyl transpeptidase; HCC, hepatocellular carcinoma; HR, hazard ratio; M, male; PVTT, portal vein tumor thrombosis.
Safety and Toxicity
No patients experienced the toxicity-related death. Ten temporary reductions in apatinib dose were necessary because of its toxicity. Two patients discontinued the apatinib treatment due to dizziness and dysphagia. The most common toxicities associated with apatinib included weight loss, hand–foot skin reaction (HFSR), hypertension, fatigue, alopecia, and diarrhea. Twelve grade 3 or higher adverse events were observed. The most common grade 3 or higher adverse events were HFSR and diarrhea. Toxicities related to apatinib are detailed in Table 4.
Table 4.
Adverse Events Associated With Apatinib in Patients With Sorafenib-Refractory Advanced HCC.
| Adverse Event | All Grades, n (%) | Grades 1-2, n (%) | Grades 3-4, n (%) |
|---|---|---|---|
| Weight loss | 34 (79.1) | 34 (79.1) | 0 |
| HFSR | 32 (74.4) | 30 (69.8) | 2 (4.7) |
| Hypertension | 26 (60.5) | 25 (58.1) | 1 (2.3) |
| Fatigue | 23 (53.5) | 23 (53.5) | 0 |
| Alopecia | 21 (48.8) | 21 (48.8) | 0 |
| Diarrhea | 20 (46.5) | 18 (41.9) | 2 (4.7) |
| Anorexia | 16 (37.2) | 15 (37.2) | 1 (2.3) |
| Proteinuria | 12 (27.9) | 11 (25.6) | 1 (2.3) |
| Pharyngolaryngeal pain | 5 (11.6) | 4 (9.3) | 1 (2.3) |
| Hoarseness | 5 (11.6) | 5 (11.6) | 0 |
| Oral mucositis | 4 (9.3) | 3 (7.0) | 1 (2.3) |
| Headache/dizziness | 3 (7.0) | 2 (4.7) | 1 (2.3) |
| Stomachache | 3 (7.0) | 2 (4.7) | 1 (2.3) |
| Vomiting | 3 (7.0) | 3 (7.0) | 0 |
| Dysphagia | 1 (2.3) | 0 | 1 (2.3) |
Abbreviations: HCC, hepatocellular carcinoma; HFSR, hand–foot skin reaction.
Discussion
The most important finding in our study is that we provide an optional treatment for the majority of patients with advanced HCC. The RESORCE study demonstrated that regorafenib can be treated as a second-line systemic targeted therapy in patients with HCC who progressed on sorafenib.8 However, we found that the median TTP after sorafenib treatment was 7.2 months,10 while the median TTP after sorafenib treatment in our study was only 1 month. In other words, most of the patients enrolled in the RESORCE study acquired resistance to sorafenib. From the 2 large randomized control trials (RCTs), we found that more than half of the patients with advanced HCC did not respond to sorafenib,5,6 specifically, more than half of the patients with advanced HCC were resistant to sorafenib. Therefore, the management of patients with sorafenib-refractory advanced HCC is urgent because those patients bear a very poor prognosis with a median survival of less than 3 months.28
In the present study, the median TTP and OS in our population were 3 months and 8 months, respectively. The 1-year and 2-year survival rates were 34.9% and 9.3%, respectively. These results are exciting and promising. The subsequent apatinib treatment dramatically prolonged the survival rate of patients with an initially poor status. The median OS of 8 months in the present study is longer than that in Goldstein’s (median OS: 6.3 months) and Ogasawara’s (median OS: 7.2 months) reported for patients with sorafenib-refractory advanced HCC.21,22 That is also longer than that of Asia-Pacific trial (median OS: 6.5 months).6 Especially for the patients with lower liver tumor burden, extrahepatic metastasis, and had a PR to apatinib, the median OS reached to 16 months, 18 months, and 19 months, respectively, which is significantly longer than that of other studies.5,6,15,17,19 The most likely reason is that apatinib is a receptor tyrosine kinase inhibitor that selectively targets VEGFR-2, with a binding affinity 10 times higher than that of sorafenib.12 Therefore, we propose a hypothesis that the VEGFR-2 signaling pathway may play a leading role in hepatocarcinogenesis in patients with sorafenib-refractory HCC. Sorafenib is a small-molecule agent that inhibits the activities of the serine-threonine kinases Raf-1 (c-Raf) and B-Raf; the receptor tyrosine kinases VEGFR-1, -2, and -3; and platelet-derived growth factor receptor α (PDGFR-α) and PDGFR-β.29 Therefore, sorafenib with one-tenth the anti-VEGFR-2 efficacy is insufficient for antitumor angiogenesis. Of course, the mechanism of antitumor angiogenesis is complex, and this hypothesis requires further confirmation in experimental studies.
In this study, which focused on apatinib treatment for patients with sorafenib-refractory advanced HCC, liver tumor burden and extrahepatic metastasis were identified as the independent prognostic factors for survival. In the present study, liver tumor burden was the most significant risk factor for patients with advanced HCC treated with apatinib. There are reports showing that most patients with HCC die due to the progression of interhepatic tumors, resulting in liver function failure.30,31 Other studies indicate that tumor burden is a significant risk factor in patients with HCC, which was confirmed by our results.31,32 Although extrahepatic metastasis is a known risk factor for patients with HCC, it was found to be a favorable factor in survival in our study. In fact, this does not contradict to previous reports. It is known that advanced HCC includes cancer with either PVTT or extrahepatic metastasis. In other words, PVTT was a worse factor compared with extrahepatic metastasis for patients with advanced HCC treated with apatinib. This is accordant with the fact that PVTT is one of the most robust risk factors for patients with HCC.31,33 Recently, an ePoster in the Annual of International Liver Cancer Association showed that apatinib treatment had a significant benefit for patients having HCC with lung metastasis was confirmed by our results.34
The toxicity of apatinib is has also been a concern. Apatinib induces many potential adverse events, similar to other molecular targeted drugs, although no serious adverse events were observed in the current study. The most frequent adverse events were weight loss, HFSR, hypertension, fatigue, alopecia, and diarrhea, which are easily managed and can be alleviated through dose reduction or suspension of apatinib treatment. Although some cytotoxic chemotherapies and targeted therapies, such as capecitabine, Leucovocin and oxaliplatin (FOLFOX), epirubicin, cisplatin, and cetuximab, are also used in the treatment of sorafenib-refractory advanced HCC, associated morbidities, such as neutropenia, thrombocytopenia, and gastrointestinal toxicity, frequently occur.20–22 More serious toxicities, including cytotoxicity-related neutropenic sepsis and liver injury, have also been reported after treatment.21 However, in the present study, patients treated with apatinib for sorafenib-refractory HCC did not experience treatment-related death. Although a few grade 3 or more toxicities related to apatinib treatment occurred, they were alleviated when the apatinib dose was reduced or treatment was suspended.
This study has some limitations. First, it is a retrospective pilot study, and the data were obtained from a single center. The center had the largest population of liver cancer patients and had considerable experience with treatment of HCC in South China.29 Second, the enrolled patients were all diagnosed with hepatitis B virus related HCC, the other causes of HCC, including hepatitis C virus and alcohol abuse, should be studied further. Third, it was a single-arm study with a small sample size. Thus, large randomized controlled trials are needed to examine the utility of apatinib in this setting.
In conclusion, the results suggest that apatinib treatment prolonged the TTP and OS of patients with sorafenib-refractory advanced HBV-related HCC. The results provided a promising option for the majority of patients with advanced HCC. This finding is important and a prospective RCT is ongoing to confirm these results.
Acknowledgments
The authors would like to thank Shiting Feng (Department of Radiology, the First Affiliated Hospital, Sun Yat-sen University) for imaging assistance, and thank Yuantao Hao (School of Public Health, Sun Yat-sen University) for statistical assistance in the manuscript.
Authors’ Note: Yingqiang Zhang and Wenzhe Fan would like to share co-first authorship. The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The study protocol was approved by the Ethics Committees of the Seventh Affiliated Hospital, Sun Yat-sen University (No. 2015SYSUSH-002). Written informed consent was obtained from each participant in accordance with the Declaration of Helsinki.
Author Contributions: Yingqiang Zhang and Jiaping Li contributed conception/design; Yingqiang Zhang, Wenzhe Fan, and Yu Wang contributed in provision of the study material; Yingqiang, Wenzhe Fan, and Guihua Huang contributed in collection of assembly of data; Yingqiang Zhang and Wenzhe Fan performed data analysis and interpretation; All authors involved in manuscript writing and final approval of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Grant support was provided by the Natural Science Foundation of China (No. 81371653).
ORCID iD: Yingqiang Zhang  https://orcid.org/0000-0003-0945-7405
https://orcid.org/0000-0003-0945-7405
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. [DOI] [PubMed] [Google Scholar]
- 2. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. European Association For The Study Of The Liver1; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943. [DOI] [PubMed] [Google Scholar]
- 4. Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30(1):61–74. [DOI] [PubMed] [Google Scholar]
- 5. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. [DOI] [PubMed] [Google Scholar]
- 6. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. [DOI] [PubMed] [Google Scholar]
- 7. Kane RC, Farrell AT, Madabushi R, et al. Sorafenib for the treatment of unresectable hepatocellular carcinoma. Oncologist. 2009;14(1):95–100. [DOI] [PubMed] [Google Scholar]
- 8. Bruix J, Qin S, Merle P, Granito A, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. [DOI] [PubMed] [Google Scholar]
- 9. Pelosof L, Lemery S, Casak S, et al. Benefit-risk summary of regorafenib for the treatment of patients with advanced hepatocellular carcinoma that has progressed on sorafenib. Oncologist. 2018; 23(4):496–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Finn RS, Merle P, Granito A, et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: additional analyses from the phase III RESORCE trial. J Hepatol. 2018;69(2):353–358. [DOI] [PubMed] [Google Scholar]
- 11. Yang C, Qin S. Apatinib targets both tumor and endothelial cells in hepatocellular carcinoma. Cancer Med. 2018;7(9):4570–4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011;102(7):1374–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yu WC, Zhang KZ, Chen SG, Liu WF. Efficacy and safety of apatinib in patients with intermediate/advanced hepatocellular carcinoma: a prospective observation study. Medicine. 2018;97(3):e9704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu H, Ma X, Zhao Y, Duo J. The excellent antitumor effect of apatinib alone as second-line therapy in a patient with sorafenib-refractory hepatocellular carcinoma: a case report. Medicine. 2018;97(25):e11214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu Z, Chen J, Fang Y, Han X, Pan H, Han W. The efficacy and safety of apatinib treatment for patients with unresectable or relapsed liver cancer: a retrospective study. J Cancer. 2018;9(16):2773–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhu H, Zhao Y, Wang X. The radiosensitive effect of apatinib for hepatocellualr carcinoma patient with big paraspinal metastasis: a case report. Medicine. 2018;97(2):e9598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu C, Xing W, Si T, Yu H, Guo Z: Efficacy and safety of apatinib combined with transarterial chemoembolization for hepatocellular carcinoma with portal venous tumor thrombus: a retrospective study. Oncotarget. 2017;8(59):100734–100745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lu W, Jin XL, Yang C, et al. Comparison of efficacy between TACE combined with apatinib and TACE alone in the treatment of intermediate and advanced hepatocellular carcinoma: a single-center randomized controlled trial. Cancer Biol Ther. 2017;18(6):433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Y, Guo Q, Xu R, Chen X, Zhou Z. Efficacy and safety of sorafenib versus apatinib in the treatment of intermediate and advanced hepatocellular carcinoma: a comparative retrospective study. Onco Targets Ther. 2018;11:3407–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Poggi G, Montagna B, Melchiorre F, et al. Hepatic intra-arterial cetuximab in combination with 5-fluorouracil and cisplatin as salvage treatment for sorafenib-refractory hepatocellular carcinoma. Anticancer Res. 2011;31(11):3927–3933. [PubMed] [Google Scholar]
- 21. Goldstein R, Yu D, Gillmore R, et al. Oxaliplatin/5-fluorouracil in advanced hepatocellular carcinoma: case report and single-center retrospective review. Future Oncol. 2014;10(13):2007–2014. [DOI] [PubMed] [Google Scholar]
- 22. Ogasawara S, Chiba T, Ooka Y, et al. A phase I/II trial of capecitabine combined with peginterferon α-2a in patients with sorafenib-refractory advanced hepatocellular carcinoma. Invest New Drugs. 2014;32(4):762–768. [DOI] [PubMed] [Google Scholar]
- 23. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhao Y, Li H, Bai W, et al. Early sorafenib-related adverse events predict therapy response of TACE plus sorafenib: a multicenter clinical study of 606 HCC patients. Int J Cancer. 2016;139(4):928–937. [DOI] [PubMed] [Google Scholar]
- 25. Zhong BY, Ni CF, Chen L, Zhu HD, Teng GJ. Early sorafenib-related biomarkers for combination treatment with transarterial chemoembolization and sorafenib in patients with hepatocellular carcinoma. Radiology. 2017;284(2):583–592. [DOI] [PubMed] [Google Scholar]
- 26. Zhang Y, Fan W, Wang Y, et al. Sorafenib with and without transarterial chemoembolization for advanced hepatocellular carcinoma with main portal vein tumor thrombosis: a retrospective analysis. Oncologist. 2015;20(12):1417–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. National Cancer Institute. Common terminology criteria for adverse events, version 3.0. Available at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed Aug 17, 2019.
- 28. Cho JY, Paik YH, Lim HY, et al. Clinical parameters predictive of outcomes in sorafenib-treated patients with advanced hepatocellular carcinoma. Liver Int. 2013;33(6):950–957. [DOI] [PubMed] [Google Scholar]
- 29. Wilhelm SM, Carter C, Tang L, et al. BAY 43-9306 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64(19):7099–7109. [DOI] [PubMed] [Google Scholar]
- 30. Yoo DJ, Kim KM, Jin YJ, et al. Clinical outcome of 251 patients with extrahepatic metastasis at initial diagnosis of hepatocellular carcinoma: does transarterial chemoembolization improve survival in these patients? J Gastroenterol Hepatol. 2011;26(1):145–154. [DOI] [PubMed] [Google Scholar]
- 31. Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies. Liver Int. 2009;29(4):502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Spreafico C, Sposito C, Vaiani M, et al. Development of a prognostic score to predict response to Yttrium-90 radioembolization for hepatocellular carcinoma with portal vein invasion. J Hepatol. 2018;68(4):724–732. [DOI] [PubMed] [Google Scholar]
- 33. Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Liovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J Hepatol. 2017;67(5):999–1008. [DOI] [PubMed] [Google Scholar]
- 34. Du X, Chen D, Lin Z, Dong Z, Liu L. Efficacy of apatinib for advanced hepatocellular carcinoma with lung metastasis: a retrospective, multicenter study. Annual ILCA. 2018. ePoster. [PubMed] [Google Scholar]