Abstract
We have previously reported human fetal cartilage progenitor cells (hFCPCs) as a novel source of therapeutic cells showing high proliferation and stem cell properties superior to those of adult mesenchymal stem cells (MSCs). In this study, we investigated the immunophenotype and immune-modulatory activities of hFCPCs. With institutional review board approval, hFCPCs were isolated from fetuses at 11–13 weeks of gestation. hFCPCs showed strong expression of HLA class I molecules but low or no expression of HLA class II and co-stimulatory molecules, which was not changed significantly after 4 days of IFN-γ treatment. In a mixed lymphocyte reaction (MLR), hFCPCs showed no allogeneic immune response to peripheral blood lymphocytes (PBLs) and suppressed concanavalin A (Con A)-mediated proliferation of PBLs in a dose-dependent manner. In addition, hFCPCs inhibited Con A-induced secretion of pro-inflammatory cytokines TNF-α and IFN-γ from PBLs but showed no significant decrease of secretion of IL-10, anti-inflammatory cytokine. Co-culture of hFCPCs with stimulated PBLs for 4 days resulted in a significant increase in CD4+CD25+FoxP3+ T regulatory cells (Tregs). hFCPCs expressed LIF, TGF-β1, TSG-6, and sHLA-G5 but did not express IDO and HGF. Stimulation of hFCPCs with TNF-α for 12 h showed slight induction in the expression of LIF, TSG-6, IDO, and HGF, whereas stimulation with IFN-γ did not affect expression of any of these factors. These results suggest that hFCPCs have low allogeneic immunogenicity and immune-modulatory activity in vitro, comparable to those of MSCs. However, compared with MSCs, hFCPCs were less responsive to TNF-α and IFN-γ, and the mechanisms underlying responses to these two cell types appeared distinct.
Keywords: human fetal cartilage-derived progenitor cells, immunogenicity, immunomodulation, cell therapy
Introduction
Mesenchymal stem cells (MSCs) are an attractive source of cells for therapeutic use, because they are easy to obtain from several tissues in adults, have low immunogenicity and chemotactic activity, and can differentiate into a series of mesengenic cell lineages. The ability of MSCs to modulate immune responses and inflammation in vitro and in vivo raised considerable interest because of their potential for use in treating many immune-related diseases1. However, MSCs have an insufficient differentiation ability, limiting their potential to meet clinical needs for tissue regeneration, and they show phenotypic drift during long-term expansion, hindering their mass production. Studies are currently underway to overcome these practical limitations of MSCs, but there is also a keen demand to find a novel source of cells. Embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) are good sources of therapeutic cells, but there are high safety concerns and technical challenges associated with their use, and these cells do not have immune-privilege and immune-modulatory functions2,3.
In contrast, stem or progenitor cells from fetal tissues may complement, or be a substitute for, MSCs. They can be isolated from a variety of different fetal tissues, including bone marrow, liver, blood4, lung5, brain6, cartilage7, heart8, umbilical cord blood9, Wharton’s jelly10, and placenta11. Fetal stem/progenitor cells have a greater proliferative capacity and differentiation potential than MSCs12. In addition, they have the advantages of low tumorigenicity and immunogenicity13,14,15. Several studies have shown that fetal stem/progenitor cells have an immune-modulatory activity similar to those of MSCs14,15. However, most of the studies have been done using post-natal placenta or umbilical cord blood-derived MSCs and immune-modulatory activity of MSCs from pre-natal fetus is limited. In addition, it is not clear what the differences are between the immune-modulatory activity of selected subpopulation of MSCs and total fetal progenitor cells. Therefore, it is imperative to understand the immune characteristics and immune-modulatory functions of cells from many different fetal tissues for their clinical adoption.
Many previous studies have established the mechanism of immune-privileged and immune-modulatory abilities of MSCs. MSCs express MHC class I molecules but do not express HLA class II molecules and co-stimulatory factors such as CD80, CD86, and CD4016. Functional assays show that MSCs inhibit proliferation of T and B lymphocytes17, reduce cytotoxicity of T lymphocytes18,19 and natural killer cells18, suppress differentiation and maturation of monocytes into dendritic cells20, and stimulate production of T regulatory cells (Tregs) from immature T cells21. Many cytokines and ligands secreted by MSCs are known to modulate these processes, including interleukin 10 (IL-10)22, leukemia inhibitory factor (LIF)19, indoleamine 2,3-dioxygenase (IDO)18,23, prostaglandin E2 (PGE2)18, hepatocyte growth factor (HGF)24, transforming growth factor (TGF)-β124, soluble human leukocyte antigen-G5 (sHLA-G5)25, and TNF-α stimulated gene 6 (TSG-6)26.
Fetal tissues are immune tolerant to limit their reactions to the mother27. They show low level expression of HLA class I and co-stimulatory molecules, and produce immune modulatory molecules such as TGF-β13. The mechanisms of immune tolerance involve stimulation of CD4+CD25+FoxP3+ Tregs and auto-reactive T cell clones from the thymus28. MSCs are also found in some fetal tissues, such as those of the liver14 and bone marrow29, and they show low immunogenicity, immune-modulatory activity, and inflammatory cytokine secretion, similar to adult MSCs. Fetal neural progenitor cells (NPCs)30 and fetal bone cells13 are also reported to have similar immuno-phenotypes, but not much information is available on other fetal tissues. Interestingly, these two fetal tissue-derived cells exhibit differential expression patterns of HLA class I and II molecules in an unstimulated state and upon stimulation with TNF-α and IFN-γ. Fetal NPCs express higher levels of HLA class I molecules than class II molecules, and neither of these was induced by treatment with TNF-α or INF-γ. In contrast, fetal bone cells show very low expression of both HLA class I and II molecules, but their expression increases significantly in response to TNF-α or INF-γ. These results suggest that immune-related activities of fetal stem/progenitor cells might vary with tissue type, and these differences need to be clarified for therapeutic development of each cell type.
Previously, we isolated human fetal cartilage progenitor cells (hFCPCs) from a fetus at 12 weeks of gestation and identified their stem cell properties of high colony formation and proliferation, and multi-potent differentiation ability into chondrogenic, osteogenic, and adipogenic lineages7. hFCPCs express most MSC markers and show a clearer stem cell phenotype than that of MSCs. There are other reports describing similar stem or progenitor cell properties of hFCPCs at 6–20 weeks of gestation but their immune-phenotypic characteristics are not provided in these studeis31,32. Human chondrocytes from neocartilage were previously shown to have immune-privileged and immune-modulatory activities. However, chondrocytes are different from our fetal progenitor cells with stem cell properties, and information on Tregs and cytokine profiles was not provided33. We have previously shown that hFCPCs do not induce severe immune rejection when injected into the synovial cavity of rats34. In the present study, we characterized the immunological properties of hFCPCs from fetuses at 11–13 weeks of gestation and investigated their immune-modulatory activity on allogeneic lymphocyte proliferation. We found that hFCPCs share many characteristics with other fetal progenitor cells, as well as MSCs, but that they also display features unique to this cell type.
Materials and Methods
Culture of hFCPCs and MSCs
The use of human fetal tissues and bone marrow aspirate was approved by the institutional review board (IRB) of Ajou University Medical Center (AJIRB-MED-SMP-11-205). Cartilages from three fetuses between 11 and 13 weeks of gestation were obtained from donors undergoing elective termination with written and informed consent. Human fetal cartilage tissue has a large number of progenitor cells evenly distributed throughout the tissue7. The fetal cartilage tissue was carefully separated from femoral head with a scalpel blade, chopped into small pieces, and treated with 0.2% collagenase type II (Worthington Biochemical, Lakewood, NJ, USA) in serum-free Dulbecco’s modified Eagle medium (DMEM-LG; Hyclone, Logan, UT, USA) for 3 h at 37°C. Dissociated cells were collected by centrifugation at 1700 rpm for 10 min. After several washes with DMEM, cells were plated in a 150-mm culture plate at 8000 cells/cm2 and cultivated in DMEM-LG supplemented with 10% fetal bovine serum (FBS), 10,000 U/ml penicillin, and 0.1 mg/ml streptomycin (all from Hyclone). The medium was changed twice a week. Cells were passaged at 80% confluence using trypsin/EDTA (Invitrogen, Carlsbad, CA, USA). MSCs were isolated from the bone marrow of fractured femurs from patients 11–25 years old undergoing orthopedic surgery. Briefly, mononuclear cells were collected by Ficoll-Paque PLUS (GE Healthcare Bio-Sciences AB, Sweden) density gradient centrifugation, suspended in α-modified Eagle’s medium (α-MEM) supplemented with 10% FBS, 10,000 U/ml penicillin, and 0.1 mg/ml streptomycin (all from Hyclone), and plated at a density of 80,000 cells/cm2. After 6 days, non-adherent cells were removed, and the adherent MSCs were supplied with fresh medium. MSCs were passaged at 80% confluence using trypsin/EDTA, and plated at a density of 8,000 cells/cm2.
Reverse Transcriptase-Polymerase Chain Reaction
Total RNA was isolated from hFCPCs using Tri-reagent (Invitrogen) according to the manufacturer’s instructions. cDNA synthesis was performed using a First Strand cDNA Synthesis Kit (Roche Diagnostics, Rotkreuz, Switzerland), and PCR was conducted using 1 μg cDNA, the primer pairs listed in Table 1, and HiPi PCR Premix (ELPis Biotech, Daejeon, Korea). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control.
Table 1.
Primers Used in the Present Study.
| Gene | Primer Sequence (5′ – 3′) | Annealing temperature | Size | Accession Number |
|---|---|---|---|---|
| HLA-ABC | GTATTTCTTCACATCCTGGTCCCG GTCCGCCGCGGTCCAAGAGCGCAG |
70 | 394 | NM_002164.5 |
| HLA-DR | CTGATGAGCGCTCAGGAATCATTG TGCATTGGCCAACATAGCTG |
60 | 220 | NM_001242758.1 |
| HLA-DM | CCAGCCCAATGGAGACTG CAGCCCAGGTGTCCAGTC |
57 | 136 | NM002118.4 |
| HLA-G | CTGACCCTGACCGAGACCTGG GTCGCAGCCAATCATCCACTGGAG |
65 | 331 | NM_002127.5 |
| Beta2M | GTGGAGCATTCAGACTTGTC AACAAGCTTTGAGTGCAAGAG |
57 | 479 | NM_004048.2 |
| CD80 | ACTCGCATCTACTGGCAAAAGGA ATGGGAGCAGGTTATCAGGAAAA |
59 | 553 | NM_005191.3 |
| CD86 | GTATTTTGGCAGGACCAGGA GCCGCTTCTTCTTCTTCCAT |
57 | 664 | NM_006889.4 |
| CD40 | AGAAGGCTGGCACTGTACGA CAGTGTTGGAGCCAGGAAGA |
59 | 363 | NM_152854.2 |
| TAP1 | TCTCCTCTCTTGGGGAGATG GAGACATGATGTTACCTGTCTG |
58 | 273 | NM000593.5 |
| TAP2 | GTCGTGTCATTGACATCCTG TCAGCTCCCCTGTCTTAGTC |
57 | 228 | NM_000544.3 |
| IDO | CGCTGTTGGAAATAGCTTC CAGGACGTCAAAGCACTGAA |
53 | 234 | NM_002164.5 |
| LIF | GGCCCGGACACCCATAGACG CCACGCGCCATCCAGGTAAA |
53 | 455 | NM_002309.4 |
| TGF-β | ACCGGCCTTTCCTGCTTCTCA CGCCCGGGTTATGCTGGTTGT |
63 | 288 | NM_000660.4 |
| TSG-6 | GGTGTGTACCACAGAGAAGCA GGGTTGTAGCAATAGGCATCC |
63 | 284 | NM_007115.3 |
| sHLA-G | CCACCACCCTGTCTTTGACT TGGCACGTGTATCTCTGCTC |
63 | 210 | NM_002127.5 |
| HGF | ATGCATCCAAGGTCAAGGAG TTCCATGTTCTTTTGTCCCACA |
55 | 249 | NM_001010932 |
| GAPDH | GGTCATGAGTCCTTCCACGAT GGTGAAGGTCGGAGTCAACGG | 58 | 520 | NM_002046.3 |
Flow Cytometry for Surface Marker Analysis
For the surface marker analysis, hFCPCs at passage four were untreated or stimulated with 200 U/ml of recombinant human IFN-γ (BD Biosciences, San Jose, CA, USA) for 4 days, and then incubated with fluorescence-conjugated primary antibodies against HLA-ABC, HLA-DR, CD80, CD86, CD40, CD40L, and CD11c (all from BD Biosciences) for 30 min at 4°C. After washing, the stained cells were analyzed on a BD FACSCanto II flow cytometer using Cell Quest software (BD Biosciences).
Mixed Lymphocyte Reaction
Peripheral blood was obtained from five healthy volunteers (median age 39.3 years, range 31–46 years) with informed consent and IRB approval from Ajou University Medical Center (AJIRB-MED-SMP-11-205). Lymphocytes were isolated by Ficoll-Paque PLUS (1.077 g/ml; GE Healthcare Bio-Sciences AB, Sweden) density gradient centrifugation according to the manufacturer’s instructions. Peripheral blood lymphocytes (PBLs) at a concentration of 105 cells in 0.5 ml medium were untreated or stimulated with 10 μg/ml concanavalin A (Con A; Sigma-Aldrich, St. Louis, MO, USA) for use in the MLR assay. hFCPCs or hMSCs were irradiated with 3000 rads Cs137 as previously described35 and plated at decreasing amounts of 105, 104, 103, and 102 cells in 96-well plates and mixed with 105 Con A-stimulated or -unstimulated PBLs. After 4 days, 0.1 ml from each sample was transferred to a new 96-well plate, and 20 µl of BrdU labeling solution (BrdU ELISA kit, Roche Diagnostics, Mannheim, Germany) was added to each well. After 6 hours at 37°C, absorbance were measured at 492 nm using a microplate reader (Infinite m200; TECAN, Männedorf, Switzerland).
Enzyme-Linked Immunosorbent Assays for Cytokines Analysis
PBLs (106 cells) were stimulated with 10 μg/ml Con A and incubated with 105 or 106 Cs137-inactivated hFCPCs in 12-well plates. After 4 days, the culture medium was collected and the amount of IFN-γ, TNF-α, and IL-10 in samples was measured using enzyme-linked immunosorbent assay (ELISA) kits for each cytokine (eBioscience, San Diego, CA, USA) according to the manufacturer’s instructions. Absorbance at 450 nm was measured using a microplate reader (Infinite m200; TECAN).
Analysis of Tregs
PBLs (106 cells) were stimulated with 10 μg/ml Con A and incubated with Cs137-inactivated hFCPCs at a 1:1 ratio, as above, in a 60-mm dish for 4 days. PBLs were harvested after 4 days and immuno-stained simultaneously with fluorescence-conjugated anti-CD4-FITC, anti-CD25-APC, and anti-FoxP3-PE antibodies using a FoxP3 staining Kit (BD Biosciences) according to the manufacturer’s protocol. Cells were analyzed on a BD FACSCanto II flow cytometer using Cell Quest software (BD Biosciences).
Statistical Analysis
Data were expressed as the mean ± standard deviation (SD) from multiple independent experiments, as indicated for each assay. Statistical significance was determined by one-way analysis of variance (ANOVA) with Tukey’s post hoc test using GraphPad Prism version 6.0 software (GraphPad Software, Inc., La Jolla, CA, USA). Values of P < 0.05 were regarded as statistically significant. Statistical significance was assigned as *P < 0.05, **P < 0.01, or ***P < 0.001.
Results
Immuno-Phenotypic Characterization of hFCPCs
Expression of HLA class I and II and complement molecules in hFCPCs was examined by reverse transcriptase-polymerase chain reaction (RT-PCR) and flow cytometry. In the RT-PCR analysis, selected genes exhibited three different expression patterns at passages 2 and 10 of hFCPCs (Fig. 1A). HLA-ABC was expressed at high levels at passage 2, and expression decreased at passage 10. Beta-2 microglobulin (β2 M) and transporter associated with antigen processing molecules 1 (TAP1) and 2 (TAP2) were expressed at a consistent level at passages 2 and 10. HLA-DM, HLA-DR, CD80, CD86, and CD40 were not expressed at either passage. Flow cytometry was performed to examine the levels of selected immune-related antigens at passage 4 in the absence or presence of IFN-γ (Fig. 1B). HLA-ABC was present in 99.2 ± 0.6% of FCPCs, and CD86 was present only in 11.2 ± 0.4% of cells, while other cell surface markers, i.e. HLA-DR, CD80, CD40, CD11c, and CD40L were detected in less than 1% of cells. IFN-γ treatment is known to increase expression of HLA and complement molecules in MSCs36, but treatment with this cytokine but did not affect their expression in FCPCs. In the case of CD86, expression was decreased slightly to 7.0 ± 3.9% following IFN-γ treatment.
Fig 1.
Immuno-phenotypic profiles of hFCPCs. (A) The expression of human immune-related genes was examined by RT-PCR analysis in hFCPCs at passages 2 (P2) and 10 (P10). The level of GAPDH mRNA were used as an internal control. (B) The expression of immune-related surface markers was examined in hFCPCs at passage 4 by flow cytometry. Cells were treated or untreated with IFN-γ for 4 days before analysis. In each panel, percentages of immune-positive cells are indicated by mean values with standard deviations (SD) from three independent experiments.
hFCPCs Show Immune-Modulatory Activity in an MLR
MLR was performed using allogenic PLBs to understand the immune-stimulatory or -modulatory function of FCPCs. T cell proliferation by mitogens such as Con A has been regarded as mimicking T cell activation, and, therefore, has been used to establish positive controls. When hFCPCs were growth arrested and mixed with 105 PBLs for 4 days at decreasing amounts of 105, 104, 103, and 102 cells, they did not stimulate T cell proliferation at all concentrations (Fig. 2A). Treatment of PBLs with 10 μg/ml Con A showed T cell proliferation of approximately 2.3 ± 0.5-fold (***P < 0.001). MSCs are known to suppress the immune response of PBLs stimulated with Con A17. We investigated whether hFCPCs show similar effects. When growth-arrested hFCPCs were mixed with Con A-stimulated PBLs under the same conditions, the hFCPCs inhibited T cell proliferation by Con A in a dose-dependent manner (Fig. 2B). When compared with Con A-stimulated PBLs alone, the effects of 105 and 104 hFCPCs were statistically significant (**P < 001 and *P < 0.05, respectively). In particular, 105 hFCPCs mixed with PBLs at a 1:1 ratio almost completely blocked T cell proliferation in response to Con A. In this set of experiments, Con A induced relatively high levels of T cell proliferation with large variation (9.6 ± 7.9 fold) and showed a statistically significant difference from levels of proliferation of untreated PBLs (***P < 0.001). For comparison, hMSCs from bone marrow also inhibited Con A-induced T cell proliferation, but this effect was not greater than that of hFCPCs. The effect was statistically significant only at 105 hFCPCs (***P < 0.001), with high variation observed with less hFCPCs. In a separate experiment, we investigated the immune-modulatory activity of human young (1 year old) and adult chondrocytes (56 years old) and found they did not efficiently suppress PBL activation by Con A (Supplementary Fig 1). Interestingly, we found that adult chondrocytes further increased proliferation of PBLs.
Fig 2.
Effects of hFCPCs on the proliferation of allogeneic PBLs. (A) hFCPCs were irradiated with 3000 rads of Cs137 to abolish cell proliferation. Human PBLs (105 cells) were cultured in 96-well plates in the absence or presence of inactivated hFCPCs at ratios of 1:1 and 1:1000 (105 to 102 cells) for 4 days. Human PBLs treated with 10 μg/ml Con A was used as a positive control. (B) Human PBLs (105 cells) stimulated with Con A were co-cultured with inactivated hFCPCs or BM-MSCs at ratios of 1:1 and 1:1000 (105 to 102 cells) for 4 days. The proliferation of PBLs was measured by BrdU labeling and subsequent ELISA at 492 nm using a BrdU ELISA kit (Roche Diagnostics). Fold inductions from the values of untreated PBLs are shown in the histograms by mean values with SD from independent experiments (A, n = 3; B, n = 8 for hFCPCs, and n = 3 for MSCs). *P < 0.05, **P < 0.01, and ***P < 0.001.
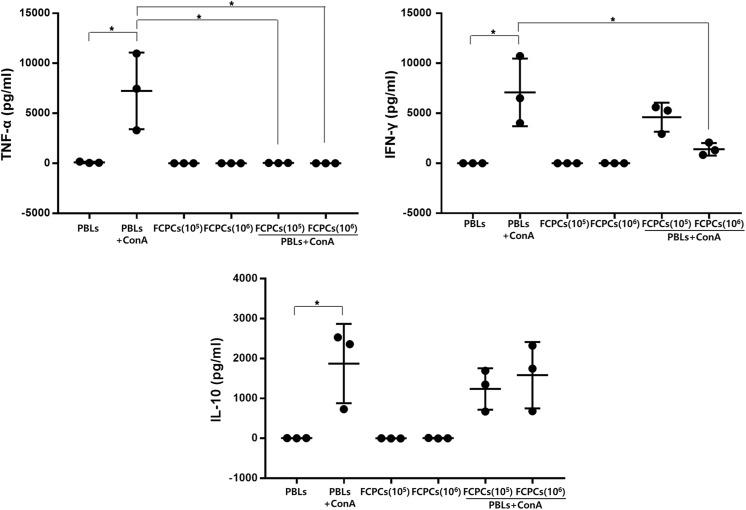
hFCPCs Modulate Cytokine Production from PBLs
To determine whether hFCPCs affect the cytokine profiles of activated PBLs, 105 or 106 hFCPCs were co-cultured with 106 Con A-treated PBLs for 4 days, and expression of TNF-1α, IFN-γ, and IL-10 was examined (Fig. 3). TNF-1α and IFN-γ are known to be pro-inflammatory cytokines, whereas IL-10 is an anti-inflammatory cytokine37. The Con A-treated samples showed substantial variation, and the data from all three samples were presented together with mean values ±SD. In spite of individual differences, Con A significantly increased secretion of TNF-α from 85.4 ± 71.4 to 7242.8 ± 3838.8 pg/ml, IFN-γ from 5.4 ± 9.3 to 7087.0 ± 3385.7 pg/ml, and IL-10 from 4.4 ± 2.4 to 1874.6 ± 993.0 pg/ml. Co-culture of 105 or 106 hFCPCs with 106 Con A-treated PBLs almost completely abolished TNF-α secretion (36.6 ± 9.1 and 3.4 ± 1.4 pg/ml, respectively) and significantly reduced IFN-γ secretion to 4606 ± 1448.5 and 1404.8 ± 623.4 pg/ml, respectively. In contrast, co-culture with these cells did not hamper Con A-induced IL-10 secretion significantly, with levels of IL-10 secretion of 1238.3 ± 518.6 and 1584.5 ± 831.7 pg/ml, respectively.
Fig 3.
Effect of hFCPCs on the expression of inflammation-related cytokines in PBLs stimulated with Con A. PBLs (106 cells) were stimulated with 10 μg/ml Con A in the absence or presence of inactivated hFCPCs (105 or 106 cells) for 4 days. Untreated PBLs and inactivated hFCPCs were used as controls. The culture media were collected, and TNF-α, IFN-γ, and IL-10 levels were determined by ELISA (eBioscience). The graphs shows individual values and means with SD from three independent experiments. The statistical significance of differences between the PBL + Con A and PBL + Con A groups and the hFCPCs groups was assessed using a paired t test. *P < 0.05, **P < 0.01, and ***P < 0.001.
hFCPCs Increase the Number of Tregs in PBLs
CD4+CD25+ Treg cells are capable of modulating tolerance to immune responses38, and Foxp3, another Treg cell marker, can control Treg development39. Growth-arrested hFCPCs were co-cultured with unstimulated PBLs at a 1:1 ratio for 4 days, and the number of CD4+CD25+Foxp3+ T cells was determined by flow cytometry (Fig. 4). Combining two markers, co-culture with hFCPCs increased the amount of CD4+Foxp3+ cells from 1.6 ± 0.3% to 4.2 ± 0.2%, that of CD4+CD25+ cells from 0.6 ± 0.4% to 2.0 ± 1.3%, and that of FoxP3+CD25+ cells was from 0.5 ± 0.4% to 2.0 ± 1.1%). In addition, the percentage of CD4+CD25+ Foxp3+ T cells of total PBLs was 0.5 ± 0.4%, but this percentage was increased after co-culture with hFCPCs (2.0 ± 1.1%).
Fig 4.
Effects of hFCPCs on the activation of CD4+CD25+FoxP3+ cells in PBLs. Human PBLs were cultured with inactivated hFCPCs for 4 days and subjected to triple staining with anti-human CD4-FITC, CD25-APC, and FoxP3-PE using a FoxP3 staining kit (BD Bioscience). The graphs show representative flow cytometry data that indicate the subpopulations of CD4+CD25+, CD4+FoxP3+, and CD4+/CD25+/FoxP3+ cells. Percentages of double- or triple-positive cells were calculated from four independent experiments and are presented in the upper right quadrant (Q2) as the means with SD (n = 4).
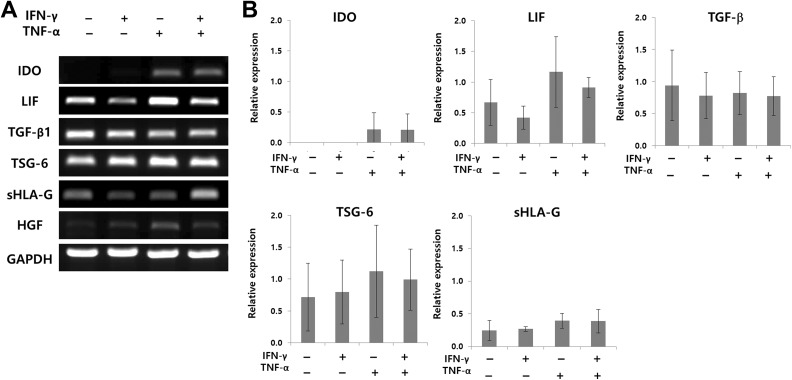
hFCPCs Express Many Immune-Modulatory Factors
The effect of MSCs on T cells is modulated mainly through cell contact-independent processes, indicating the importance of soluble factors such as IDO, LIF, TGF-β1, sHLA-G5, and HGF15. Expression of these factors in MSCs is induced by IFN-γ and TNF-α40. hFCPCs were untreated or treated with IFN-γ and/or TNF-α for 12 h, and the expression levels of these factors were measured by RT-PCR (Fig. 5A). Untreated hFCPCs showed high levels of LIF, TGF-β, and TSG-6 expression, low levels of sHLA-G5 expression, and no evident IDO and HGF expression. Treatment with INF-γ did not increase expression of these factors and only slightly decreased expression of LIF and HLA-G. Treatment with TNF-α clearly increased IDO, TSG-6, and HGF expression. Co-treatment with INF-γ and TNF-α showed mixed results, with only IDO and sHLA-G5 expression increased when compared with that of the untreated control. Quantitative data of the RT-PCR results showed similar patterns to the representative image but the statistical significance was not observed due to the large variation among three different donors (Fig. 5B). The graph on HGF is not included because its band intensity was too weak to obtain meaningful quantification.
Fig 5.
Expression of selected cytokines in hFCPCs stimulated with IFN-γ and/or TNF-α. hFCPCs from three donors were treated with 500 U/ml of TNF-α, 200 U/ml of IFN-γ, or their combination for 12 h. (A) A representative data on the mRNA levels of IDO, LIF, TGF-β, TSG-6, HLA-G, and HGF in hFCPCs examined by RT-PCR. GAPDH was used as an internal control. (B) RT-PCR data from three different donors were quantified using Image J program. Relative expression of each cytokine normalized to that of GAPDH are presented by mean values with SD (n = 3).
Discussion
This study is the first to define the immune-privileged and immune-modulatory characteristics of hFCPCs in vitro. We found that hFCPCs express HLA class I and associated molecules but do not express HLA class II molecules or co-stimulatory factors such as CD80 (B7 -1), CD86 (B7-2), and CD40. HLA class I molecules are expressed in almost all mammalian cells, whereas class II molecules are mainly found in antigen presenting cells (APCs) such as dendritic cells (DCs). HLA molecules and co-stimulatory factors are mainly responsible for antigen presentation and immune responses36. Cells that express HLA molecules stimulate T cells directly if co-stimulatory factors such as CD80 or CD86 are also present; otherwise, they activate T cells indirectly by cross presentation of their peptides on APCs such as DCs36. Therefore, cells without HLA molecules might have a low risk of alloimmunity. hFCPCs are expected not to express co-stimulatory molecules, and, therefore, may not directly activate T cells. Because direct activation of T cells is known to be about 10-fold stronger than activation through the indirect APC-dependent mechanism41, the allogenic immune response of hFCPCs themselves might not be a significant problem, as confirmed by our MLR assay. Although there should be a mechanistic difference between the allogeneic and xenogeneic immune rejection, these findings might be in connection with our previous report that hFCPCs did not cause immune rejection in rat synovial cavity34. Previously, adult or fetal MSCs were shown to express HLA class I molecules without expressing HLA class II or co-stimulatory molecules14,16. However, the expression of both HLA class I and II molecules on cell surfaces increased when cells were exposed to INF-γ for 7 days. Similarly, expression of HLA molecules increased on the surfaces of fetal and adult MSCs in response to IFN-γ14. In contrast to fetal or adult MSCs, treatment with IFN-γ for 4 days did not increase HLA molecules of either type on the surfaces of hFCPCs, as demonstrated by flow cytometry. A previous report has shown that chondrocytes isolated from human iPSC-derived cartilages express low levels of both HLA types I and II, and treatment of IFN- γ induces expression of HLA type I only42. Taken together with our findings using hFCPCS, it could be speculated that chondrocytes have an innate property of low expression of HLA type II and no significant induction of its expression upon IFN-γ stimulation.
MSCs from the bone marrow can suppress alloreactive T cells43. They also significantly inhibit proliferation of T cells stimulated by potent mitogens such as Con A and phytohemagglutinin (PHA). This effect of mitogens is regarded as similar to that of T cell activation by APCs44. We found in this study that the immunosuppressive effect of hFCPCs is stronger than that of adult MSCs. hFCPCs almost completely blocked Con A-induced proliferation of allogeneic PBLs when they were mixed at a 1:1 ratio. Our preliminary in vitro data also shows that TNF-α but not IFN-γ induced the expression of anti-inflammatory factors, including IDO, LIF, TSG-6, and HGF, in hFCPCs. Interestingly, we found that both young (1 year) and aged (56 years) chondrocytes showed no clear immunosuppressive activity in MLR (supplementary Fig 1), while human iPSCs-derived chondrocytes were reported to have an immunosuppressive activity42. These findings suggest that the immunosuppressive activity is specific to pre-natal chondrocytes and the iPSCs-derived chondrocytes might share their characteristics.
To better understand the immunosuppressive effects of hFCPCs, we measured the secretion of INF-γ, TNF-α, and IL-10 in Con A-activated PBLs in the presence of hFCPCs. IFN-γ and TNF-α are key players in allogeneic immune responses and T cell proliferation. IFN-γ induces expression of HLA class I and II molecules on cell surfaces and reinforces T cell activity45. TNF-α is produced by monocytes or macrophages and enhances proliferation of mature T cells46. Our results indicate that hFCPCs can suppress expression of these two inflammatory cytokines in Con A-activated PBLs. This result agrees with previous reports on different types of MSCs from bone marrow, Wharton’s jelly, or umbilical cord blood47,48. IL-10 is a potent immunosuppressive cytokine in vitro and in vivo that downregulates production of pro-inflammatory cytokines and chemokines by immune cells49. Previously, bone marrow MSCs were shown to induce IL-10 expression in MLR assays, particularly when primed with IFN-γ or TNF-α before co-culture with PBLs47. Interestingly, in our study, hFCPCs did not increase IL-10 expression in either resting or Con A-activated PBLs in the MLR assay. Similar results have been reported for MSCs from bone marrow, which did not increase IL-10 expression in either suppressed and unsuppressed conditions in an MLR assay36. Taken together, these results suggest that MSCs and hFCPCs shows various effects on cytokine section from PBLs, depending on their specific cell type and source, which might affect their immune-modulatory activities.
Numerous reports have shown the importance of CD4+CD25+Foxp3+ Tregs in immune regulation. Tregs have pleiotropic suppressive effects on immune responses to allo-antigens and infectious agents50 and play a key role in immune tolerance of a fetus to its mother28. MSCs modulate immune responses by de novo induction and expansion of CD4+CD25+Foxp3+ and CD8+ Tregs, which can promote immune tolerance in certain circumstances51. Our results showed a 2.63-fold increase in the number of CD4+Foxp3+ Tregs in PBLs co-cultured with hFCPCs, which is significant when compared with previous data for MSCs. Induction of Tregs by MSCs is mediated by direct contact and indirectly through CD4+ T cells via TGF-β, IDO, HLA-G5, LIF, and PGE2 secreted by MSCs25,52. We found that hFCPCs also express TGF-β, LIF, and HLA-G5, which suggests that expression of these cytokines from hFCPCs may play a role in expanding the Treg population.
The immunosuppressive effect of MSCs can be mediated in a cell-cell contact-independent manner. Soluble factors such as TGF-β1, HO-1, PGE2, IDO, HLA-G5, LIF, TSG-6, and HGF are known to participate in this process15. hFCPCs in this study expressed LIF, TGF-β1, and TSG-6 at high levels and HLA-G at moderate levels, whereas they did not express IDO and HGF. Treatment of hFCPCs with TNF-α induced expression of IDO, LIF, TSG-6, and HGF slightly, but treatment with IFN-γ showed almost no effect on expression of these genes. IDO is an enzyme that catabolizes tryptophan into kynurenine, which regulates T cell proliferation53. IDO is expressed in MSCs, and its expression has been shown to increase significantly in response to IFN-γ23, which is different from what was observed with hFCPCs. Expression of LIF increases in response to various inflammatory insults, such as exposure to lipopolysaccharide, IL-6, IL-1β, or G-CSF54. MSCs also express LIF, and expression of this factor is further induced by interactions between MSCs and PBLs, which play a role in the proliferation of Foxp3+ Tregs19. HLA-G is an important factor that mediates the immunosuppressive function of MSCs; in particular, it inhibits the innate immune responses of natural killer (NK) cells and secreted IFN-γ25. TGF-β1 also contributes to the immunosuppressive function of MSCs and is probably induced by direct contact between MSCs and monocytes55. Specifically, TGF-β1 secreted by MSCs induces Tregs and suppresses T cell responses. Overall, the secretion of these immune- and inflammation-modulating factors by hFCPCs is similar to that of MSCs, but there are clear differences as well particularly in that hFCPCs did not respond to IFN-γ. Further studies are needed to clarify the secretome profile and mechanisms underlying the immune-modulatory functions of hFCPCs.
The results of this study strongly suggest the therapeutic potential of hFCPCs to treat immune problems or inflammatory diseases. We have previously tried to inject hFCPCs into the synovial cavity of rats with Complete Freund’s Adjuvant-induced knee arthritis and compare its therapeutic effect with that of triamcinolone (TRA), a representative anti-arthritis drug34. We found in the study that hFCPCs was not highly efficient but reduced the knee circumference at a delayed time point 7 days after injection with no immune rejection or serological toxicity. It is not clear if this result has implications for the therapeutically effective dose of hFCPCs needed in physiological or other disease environments in vivo. However, hFCPCs appear not to be inferior to MSCs in their immunosuppressive activity, detailed in many previous reports47,48,52. Therefore, we speculate that the previous result suggests both clinical possibility of hFCPCs and needs for further optimization in the target indications and therapeutic protocol. The behavior and mechanism of hFCPCs in an inflammatory environment in vivo, including whether these cells would have a significant therapeutic benefit, should be explored in a future study.
Stem or progenitor cells of fetal origin have been regarded as a good source of therapeutic transplantation56. Fetal cells are know to have high proliferation ability and high differentiation ability to the committed lineages. We have also revealed that hFCPCs have proliferation ability and produce high quality cartilage tissue both in vitro and in vivo7. Although the availability of donor tissue is limited, a fetal cartilage yields more than 20-fold the number of cells than the same amount of adult cartilage. In addition, we have also shown that hFCPCs can be expanded for more than 30 passages without losing their proliferation ability in a xeno-free medium, which resulted in approximately 1030 cells from 5 × 105 cells of initial culture57. Therefore, we believe hFCPCs have a competitive commercial value, but understand that actual commercialization requires further investigation on their characteristics and behavior during long-term expansion and development of means for mass production and quality control as well. Lastly, there are ethical concerns with using cells of fetal origin, although it is not absolutely illegal and there are many clinical trials underway using fetal stem/progenitors57. We think the balance between the ethical concerns and patient benefits should be considered as well.
In conclusion, the results of this study revealed that hFCPCs have immune-privileged and immune-modulatory characteristics similar to those of MSCs. The mechanisms underlying these hFCPCs functions also appear to be similar to those of MSCs, but the secretion of immune-modulatory factors and the response to inflammatory cytokines of TNF-α and IFN-γ are distinct between these two cell types. Together with the advantages of their high proliferation ability, stem cell properties, and low safety concerns, the immune-privileged and immune-modulatory functions of hFCPCs suggest their utility in diverse applications and high therapeutic value in regenerative medicine.
Supplemental Material
Supplementary_Figure_1 for Immunophenotype and Immune-Modulatory Activities of Human Fetal Cartilage-Derived Progenitor Cells by Su Jeong Lee, Jiyoung Kim, Woo Hee Choi, So Ra Park, Byung Hyune Choi and Byoung-Hyun Min in Cell Transplantation
Acknowledgments
The authors thank Prof. Young Jick Kim, Ajou University Hospital for technical help and assistance.
Footnotes
Ethical Approval: This study was approved by the institutional review board (IRB) of Ajou University Medical Center (AJIRB-MED-SMP-11-205).
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the institutional review board (IRB) of Ajou University Medical Center (AJIRB-MED-SMP-11-205). This article does not contain any studies with animal subjects.
Statement of Informed Consent: Written informed consent was obtained from the donors for their anonymized information to e published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI17C2191).
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Barry FP, Murphy JM, English K, Mahon BP. Immunogenicity of adult mesenchymal stem cells: lessons from the fetal allograft. Stem Cells Dev. 2005;14(3):252–265. [DOI] [PubMed] [Google Scholar]
- 2. Perez-Cunningham J, Ames E, Smith RC, Peter AK, Naidu R, Nolta JA, Murphy WJ. Natural killer cell subsets differentially reject embryonic stem cells based on licensing. Transplantation. 2014;97(10):992–998. [DOI] [PubMed] [Google Scholar]
- 3. Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474(7350):212–215. [DOI] [PubMed] [Google Scholar]
- 4. Campagnoli C. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98(8):2396–2402. [DOI] [PubMed] [Google Scholar]
- 5. Fan CG, Tang FW, Zhang QJ, Lu SH, Liu HY, Zhao ZM, Liu B, Han ZB, Han ZC. Characterization and neural differentiation of fetal lung mesenchymal stem cells. Cell Transplant. 2005;14(5):311–321. [DOI] [PubMed] [Google Scholar]
- 6. Tamaki S, Eckert K, He D, Sutton R, Doshe M, Jain G, Tushinski R, Reitsma M, Harris B, Tsukamoto A, Gage F, Weissman I, Uchida N. Engraftment of sorted/expanded human central nervous system stem cells from fetal brain. J Neurosci Res. 2002;69(6):976–986. [DOI] [PubMed] [Google Scholar]
- 7. Choi WH, Kim HR, Lee SJ, Jeong N, Park SR, Choi BH, Min BH. Fetal cartilage-derived cells have stem cell properties and are a highly potent cell source for cartilage regeneration. Cell Transplant. 2016;25(3):449–461. [DOI] [PubMed] [Google Scholar]
- 8. Thanh TN, Shin HC, Kim HR, Park SR, Kim J, Choi BH. Characteristics and cardiomyogenic potential of rat fetal cardiac progenitor cells at different developmental stage. Tissue Eng Regen Med. 2017;14(3):253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109(1):235–242. [DOI] [PubMed] [Google Scholar]
- 10. Lim J, Razi ZRM, Law JX, Nawi AM, Idrus RBH, Chin TG, Mustangin M, Ng MH. Mesenchymal stromal cells from the maternal segment of human umbilical cord is ideal for bone regeneration in allogenic setting. Tissue Eng Regen Med. 2018;15(1):75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. In ‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, Kanhai HH. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22(7):1338–1345. [DOI] [PubMed] [Google Scholar]
- 12. Guillot PV, Gotherstrom C, Chan J, Kurata H, Fisk NM. Human first-trimester fetal MSCs express pluripotency markers and grow faster and have longer telomeres than adult msc. Stem Cells. 2007;25(3):646–654. [DOI] [PubMed] [Google Scholar]
- 13. Montjovent MO, Bocelli-Tyndall C, Scaletta C, Scherberich A, Mark S, Martin I, Applegate LA, Pioletti DP. In vitro characterization of immune-related properties of human fetal bone cells for potential tissue engineering applications. Tissue Eng Part A. 2009;15(7):1523–1532. [DOI] [PubMed] [Google Scholar]
- 14. Gotherstrom C, Ringden O, Tammik C, Zetterberg E, Westgren M, Le Blanc K. Immunologic properties of human fetal mesenchymal stem cells. Am J Obstet Gynecol. 2004;190(1):239–245. [DOI] [PubMed] [Google Scholar]
- 15. Chen PM, Yen ML, Liu KJ, Sytwu HK, Yen BL. Immunomodulatory properties of human adult and fetal multipotent mesenchymal stem cells. J Biomed Sci. 2011;18:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31(10):890–896. [DOI] [PubMed] [Google Scholar]
- 17. Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30(1):42–48. [DOI] [PubMed] [Google Scholar]
- 18. Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, Romagnani P, Maggi E, Romagnani S, Annunziato F. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24(2):386–398. [DOI] [PubMed] [Google Scholar]
- 19. Nasef A, Mazurier C, Bouchet S, Francois S, Chapel A, Thierry D, Gorin NC, Fouillard L. Leukemia inhibitory factor: role in human mesenchymal stem cells mediated immunosuppression. Cell Immunol. 2008;253(1–2):16–22. [DOI] [PubMed] [Google Scholar]
- 20. Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105(10):4120–4126. [DOI] [PubMed] [Google Scholar]
- 21. Sioud M. New insights into mesenchymal stromal cell-mediated T-cell suppression through galectins. Scand J Immunol. 2011;73(2):79–84. [DOI] [PubMed] [Google Scholar]
- 22. Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. [DOI] [PubMed] [Google Scholar]
- 23. Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103(12):4619–4621. [DOI] [PubMed] [Google Scholar]
- 24. Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–3843. [DOI] [PubMed] [Google Scholar]
- 25. Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, Borg C, Saas P, Tiberghien P, Rouas-Freiss N, Carosella ED, Deschaseaux F. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26(1):212–222. [DOI] [PubMed] [Google Scholar]
- 26. Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (mscs): role as guardians of inflammation. Mol Ther. 2012;20(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gaunt G, Ramin K. Immunological tolerance of the human fetus. Am J Perinatol. 2001;18(6):299–312. [DOI] [PubMed] [Google Scholar]
- 28. Burt TD. Fetal regulatory T cells and peripheral immune tolerance in utero: implications for development and disease. Am J Reprod Immunol. 2013;69(4):346–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Q, Yang Q, Wang Z, Tong H, Ma L, Zhang Y, Shan F, Meng Y, Yuan Z. Comparative analysis of human mesenchymal stem cells from fetal-bone marrow, adipose tissue, and Warton’s jelly as sources of cell immunomodulatory therapy. Hum Vaccin Immunother. 2016;12(1):85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu J, Gotherstrom C, Forsberg M, Samuelsson EB, Wu J, Calzarossa C, Hovatta O, Sundstrom E, Akesson E. Human neural stem/progenitor cells derived from embryonic stem cells and fetal nervous system present differences in immunogenicity and immunomodulatory potentials in vitro. Stem Cell Res. 2013;10(3):325–337. [DOI] [PubMed] [Google Scholar]
- 31. Quintin A, Schizas C, Scaletta C, Jaccoud S, Applegate LA, Pioletti DP. Plasticity of fetal cartilaginous cells. Cell Transplant. 2010;19(10):1349–1357. [DOI] [PubMed] [Google Scholar]
- 32. Mirmalek-Sani SH, Tare RS, Morgan SM, Roach HI, Wilson DI, Hanley NA, Oreffo RO. Characterization and multipotentiality of human fetal femur-derived cells: implications for skeletal tissue regeneration. Stem Cells. 2006;24(4):1042–1053. [DOI] [PubMed] [Google Scholar]
- 33. Adkisson HD, Milliman C, Zhang X, Mauch K, Maziarz RT, Streeter PR. Immune evasion by neocartilage-derived chondrocytes: implications for biologic repair of joint articular cartilage. Stem Cell Res. 2010;4(1):57–68. [DOI] [PubMed] [Google Scholar]
- 34. Lee SJ, Oh HJ, Trung MD, Lee KB, Kim JY, Kim YJ, Park SR, Min BH. Therapeutic possibility of human fetal cartilage-derived progenitor cells in rat arthritis model. Tissue Eng Regen Med. 2015;12(2):147–154. [Google Scholar]
- 35. Bocelli-Tyndall C, Bracci L, Spagnoli G, Braccini A, Bouchenaki M, Ceredig R, Pistoia V, Martin I, Tyndall A. Bone marrow mesenchymal stromal cells (BM-MSCs) from healthy donors and auto-immune disease patients reduce the proliferation of autologous- and allogeneic-stimulated lymphocytes in vitro. Rheumatology. 2007;46(3):403–408. [DOI] [PubMed] [Google Scholar]
- 36. Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW, Deans RJ, McIntosh KR. T cell responses to allogeneic human mesenchymal stem cells: Immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12(1):47–57. [DOI] [PubMed] [Google Scholar]
- 37. Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. [DOI] [PubMed] [Google Scholar]
- 39. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. [DOI] [PubMed] [Google Scholar]
- 40. De Miguel MP, Fuentes-Julian S, Blazquez-Martinez A, Pascual CY, Aller MA, Arias J, Arnalich-Montiel F. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med. 2012;12(5):574–591. [DOI] [PubMed] [Google Scholar]
- 41. Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J Immunol. 1999;162(1):352–358. [PubMed] [Google Scholar]
- 42. Kimura T, Yamashita A, Ozono K, Tsumaki N. Limited immunogenicity of human induced pluripotent stem cell-derived cartilages. Tissue Eng. 2016;22(23–24):1367–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005;305(1):33–41. [DOI] [PubMed] [Google Scholar]
- 44. Perrin PJ, Davis TA, Smoot DS, Abe R, June CH, Lee KP. Mitogenic stimulation of T cells reveals differing contributions for B7 -1 (CD80) and B7-2 (CD86) costimulation. Immunology. 1997;90(4):534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Robb RJ, Smith KA. Heterogeneity of human t-cell growth factor(s) due to variable glycosylation. Mol Immunol. 1981;18(12):1087–1094. [DOI] [PubMed] [Google Scholar]
- 46. Debets JM, van der Linden CJ, Spronken IE, Buurman WA. T cell-mediated production of tumour necrosis factor-alpha by monocytes. Scand J Immunol. 1988;27(5):601–608. [DOI] [PubMed] [Google Scholar]
- 47. Prasanna SJ, Gopalakrishnan D, Shankar SR, Vasandan AB. Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PloS One. 2010;5(2): e9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Oh W, Kim DS, Yang YS, Lee JK. Immunological properties of umbilical cord blood-derived mesenchymal stromal cells. Cell Immunol. 2008;251(2):116–123. [DOI] [PubMed] [Google Scholar]
- 49. de Vries JE. Immunosuppressive and anti-inflammatory properties of interleukin 10. Ann Med. 1995;27(5):537–541. [DOI] [PubMed] [Google Scholar]
- 50. Shevach EM, DiPaolo RA, Andersson J, Zhao DM, Stephens GL, Thornton AM. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol Rev. 2006;212:60–73. [DOI] [PubMed] [Google Scholar]
- 51. Gebler A, Zabel O, Seliger B. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol Med. 2012;18(2):128–134. [DOI] [PubMed] [Google Scholar]
- 52. Shi M, Liu ZW, Wang FS. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin Exp Immunol. 2011;164(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mellor AL, Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol Today. 1999;20(10):469–473. [DOI] [PubMed] [Google Scholar]
- 54. Wetzler M, Talpaz M, Lowe DG, Baiocchi G, Gutterman JU, Kurzrock R. Constitutive expression of leukemia inhibitory factor RNA by human bone marrow stromal cells and modulation by IL-1, TNF-alpha, and TGF-beta. Exp Hematol. 1991;19(5):347–351. [PubMed] [Google Scholar]
- 55. Nasef A, Chapel A, Mazurier C, Bouchet S, Lopez M, Mathieu N, Sensebe L, Zhang Y, Gorin NC, Thierry D, Fouillard L. Identification of IL-10 and TGF-beta transcripts involved in the inhibition of T-lymphocyte proliferation during cell contact with human mesenchymal stem cells. Gene Expr. 2007;13(4–5):217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ishii T, Eto K. Fetal stem cell transplantation: past, present, and future. World J Stem Cells. 2014;6(4):404–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kim HR, Kim J, Park SR, Min BH, Choi BH. Characterization of human fetal cartilage progenitor cells during long-term expansion in a xeno-free medium. Tissue Eng Regn Med. 2018;15(5):649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_Figure_1 for Immunophenotype and Immune-Modulatory Activities of Human Fetal Cartilage-Derived Progenitor Cells by Su Jeong Lee, Jiyoung Kim, Woo Hee Choi, So Ra Park, Byung Hyune Choi and Byoung-Hyun Min in Cell Transplantation