Abstract
Ventricular arrhythmias (VA) are of major concern in the field of cell therapy, potentially limiting its safety and efficacy. We sought to investigate the effects of CD34+ cell therapy on VA burden in patients with chronic heart failure (CHF). We performed registry data analysis of patients with CHF and implanted ICD/CRT devices treated with transendocardial CD 34+ cell therapy. Demographic, echocardiographic, and biochemical parameters were analyzed. Device records were reviewed and the number and type of VA 1 year prior to and 1 year after cell therapy were analyzed. All patients underwent electroanatomical mapping, and myocardial scar was defined as unipolar voltage (UV) <8.3 mV and linear local shortening (LLS) <6%. Of 209 patients screened, 48 met inclusion criteria. The mean age of the patients was 52 years and 88% were male. Nonischemic and ischemic cardiomyopathy were present in 55% and 45% of patients. The average serum creatinine was 91±26 µmol/L, serum bilirubin 18±9 µmol/L, NT-proBNP 1767 (468, 2446) pg/mL, LVEF 27±9% and 6’ walk test 442±123 m. The average scar burden in patients with nonischemic and ischemic DCM was 58±15% and 51±25% (P=0.48). No significant difference in VA burden was observed before and after cell therapy (48% vs. 44%; P=0.68). ICD activation occurred in 19% and 27% of patients before and after cell therapy (P=0.33). According to our results, transendocardial CD34+ cell therapy does not appear to increase the risk of VA in chronic heart failure patients.
Keywords: stem cell therapy, ventricular arrhythmias, heart failure
Introduction
Although a significant body of published data suggests3 that stem cell therapy is feasible and safe in the chronic heart failure patient population, several preclinical and clinical studies did raise some concern that stem cell therapy may be associated with ventricular arrhythmias, as potentially life-threatening ventricular tachycardia (VT) and/or ventricular fibrillation (VF) have been reported after stem cell therapy1–4. The occurrence of ventricular arrhythmias has not been consistently observed across the stem cell clinical trials, which is very likely due the variations in patient population, stem cell type, delivery methods, and the timing of stem cell therapy that were used in different studies4. Interestingly, most of the reported ventricular arrhythmias in stem cell studies were related to the use of skeletal myoblasts, with higher-than-expected rate of ventricular arrhythmias, including several deaths5,6.
In comparison to skeletal myoblasts, bone marrow stem cells (unfractionated or subpopulations) or mesenchymal stem cells (MSCs) appear to have much better safety profile. Although bone marrow stem cells have been associated with potentially proarrhythmic electrophysiological changes in preclinical models, stem cell therapy has not been shown to translate to clinically relevant ventricular arrhythmias in patients with acute coronary syndrome or chronic ischemic heart disease7. However, the potential proarrhythmic effects of intramyocardial stem cells transplantation in patients with chronic heart failure have not been explored and currently remain undefined. In the present study we sought to investigate the effects of transendocardial CD34+ cell therapy on the burden of ventricular arrhythmias in patients with chronic heart failure and reduced left ventricular ejection fraction (LVEF).
Materials and Methods
Patient Population
We performed a post-hoc analysis of the patients enrolled in two prospective open-label trials investigating the clinical effects of stem cell therapy in patients with ischemic cardiomyopathy (ICM, NCT01350310) and nonischemic dilated cardiomyopathy (DCM), and patients enrolled in a Registry of Cell Therapy in Nonischemic Dilated Cardiomyopathy (RECORD, NCT02445534). Patients with either nonischemic DCM or ICM who underwent transendocardial cell therapy at Advanced Heart Failure and Transplantation Program, University Medical Center Ljubljana, Slovenia from January 1st, 2006 and December 31st 2016 were screened for the study. Inclusion criteria were as follows: age 18 to 65 years, presence of ICD/CRT device ≥12 months prior to stem cell therapy, diagnosis of DCM according to European Society of Cardiology position statement8 or diagnosis of ICM without any option for further percutaneous or surgical myocardial revascularization9, and stem cell therapy ≥12 months prior enrollment. Patients without ICD/CRT device, with stem cell therapy <12 months prior to enrollment, with acute coronary syndrome or hospitalization for worsening heart failure requiring inotropic support within 12 months before and after stem cell therapy, and patients who underwent any medical or invasive arrhythmia treatment within 12 months before or after stem cell therapy were excluded from the study. Written informed consent was obtained in all patients before participation in the study, and the study protocol was approved by the National Ethics Committee of the Republic of Slovenia.
Study Design
In patients who met the inclusion criteria, baseline demographic, echocardiographic, and biochemical parameters were analyzed at the time of stem cell transplantation. In addition, an ICD/CRT event log was reviewed in the time interval of 12 months before and after stem cell therapy, and the number and type of ventricular arrhythmias and the number and type of device activations were reviewed. The data from all device interrogations that occurred within 12 months before cell transplantation and within 12 months after transplantation were included in the analysis. In all patients a device interrogation was also done on the day of cell transplantation and at 12 month follow-up as a part of our standard management protocol. All ventricular arrhythmic events that were recorded by the device (regardless of whether they triggered device activation in the form of shock or antitachycardia pacing (ATP)) were considered for the analysis. Patients were stratified based on the presence of ventricular arrhythmias after stem cell therapy.
Peripheral Blood Stem Cell Mobilization and Collection
All patients underwent stem cell mobilization and collection as previously described10. In short, bone marrow cells were mobilized into peripheral blood by daily subcutaneous injections of G-CSF (5 µg/kg BID). On the fifth day bone marrow-derived cells were collected by cytapheresis using Miltenyi cell separator (Miltenyi Biotec, Teterow, Germany). Subsequently immunoselection of obtained cells was done using Isolex 300i (Nexell Therapeutics Inc, Irvine, CA, USA) and the selected CD34+ cells were stored in the cell collection bag. The obtained cell product was further concentrated to a final volume of 6 mL.
Electroanatomical Mapping and Transendocardial Cell Delivery
Using electroanatomical mapping (Biosense-Webster, Diamond Bar, CA, USA), maps of color-coded myocardial viability (unipolar voltage; UV) and regional myocardial contraction (linear local shortening; LLS) and their corresponding bull’s-eye maps, consisting of ≥150 sampling points, were generated for each patient prior to stem cell transplantation. Hibernating myocardium was defined as areas with UV ≥8.3 mV and LLS <6%; using electroanatomical data, myocardial scar was defined as segments with UV <8.3 mV and LLS <6%11. Transendocardial delivery of cell suspension was performed with MyoStar (Biosense-Webster) injection catheter. Each patient received 20 injections (0.3 mL each) of stem cell suspension; all injections were performed targeting the areas of myocardial hibernation (UV ≥8.3 mV and LLS <6%).
Electrogram Analysis
We analyzed the electrograms of the stem cell injection regions. Electrograms (Fig. 1) were recorded with the NOGAStar mapping catheter and displayed with fixed gain of 1 mV/cm at 200 mm/s. Areas of slow conduction were defined as areas with fragmented QRS signal12. QRS fragmentation was defined as a multicomponent QRS signal and the cut-off value for normal QRS fragmentation was ≤4 positive peak deflections7.
Fig. 1.
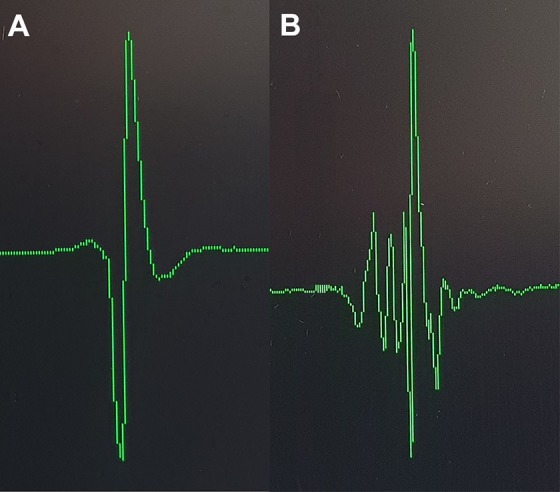
Examples of electrogram recordings. Electrogram recordings of normal (panel A) and fragmented (panel B) QRS, recorded at a gain of 1 mV/cm at 200 mm/sec.
Echocardiography, 6-Minute Walk Test, and NT-proBNP Measurements
The echocardiography data were recorded and analyzed at the end of the study by an independent echocardiographer who was blinded to the patient’s treatment status and the timing of the recordings. Left ventricular end-systolic volume and end-diastolic volume and LVEF were estimated using the Simpson’s biplane method, and left ventricular end-systolic dimension and end-diastolic dimension were measured in the parasternal long axis view. All echocardiographic measurements were averaged for five cycles. In all patients, 6-minute walk test was performed by a blinded observer according to the standard protocol13. All NT-proBNP assays were performed at a central independent laboratory, blinded to the patient’s clinical data using a commercially available kit (Roche Diagnostics, Mannheim, Germany).
Ventricular Arrhythmia Assessment
Device interrogations were performed in the outpatient clinic as a part of routine follow-up screening of the patients with ICD/CRT device, and the number and type of ventricular arrhythmias and the number and type of device activations were recorded. VT was defined as five consecutive ventricular ectopic beats. All ventricular arrhythmias that occurred in the time interval of 12 months before and after stem cell transplantation were considered for the analysis. Ventricular tachyarrhythmia episodes and appropriateness of device activation (ATP or shock) were adjudicated by two independent electrophysiologists blinded to the patient status. Recordings that were falsely labeled as ventricular arrhythmias and inappropriate device therapy were excluded from our analysis.
Study End Points
The primary end point was the change in the number of episodes of ventricular arrhythmias 1 year before and 1 year after stem cell transplantation. Secondary end points included changes in the number of episodes of device activation (shock or ATP) and changes in the mode of device activation before and after stem cell therapy.
Statistical Methods and Analysis
Continuous variables are presented as mean (±SD) or median (interquartile range) where appropriate and categorical data are given as count (percent). Categorical variables were compared with Chi-squared test (or Fischer exact nonparametric test). All continuous variables were compared using Student’s t-test and ANOVA (or Mann–Whitney nonparametric test). Cluster analysis of the points, obtained with electromechanical mapping, was performed using a two-step cluster analysis approach where clusters are automatically determined using Schwarz Bayesian criterion. Statistical significance was assumed for P-values of <0.05. All statistical analyses were performed with SPSS software version 20.0 (IBM SPSS Statistics, Armonk, NY, USA).
Results
Patient Characteristics
Baseline patient characteristics are shown in Table 1. The majority of the patients were male with nonischemic DCM, with a myocardial scar burden, as evaluated by electroanatomical mapping, of around 50%, in NYHA functional class III or higher and with a LVEF of around 30%. End-organ function was not significantly affected in any of the patients and no significant electrolyte disturbances were registered. All of the patients received optimal heart failure therapy that was not altered during the evaluation period. With regards to the antiarrhythmic management, two-thirds of patients had received an ICD and one-third of patients had received a CRT-D. Seven (15%) of the patients received an additional antiarrhythmic medical therapy (amiodarone) per discretion of the treating arrhythmologist that was stable throughout the evaluation period.
Table 1.
Baseline Patient Characteristics.
| All (N=48) |
|
|---|---|
| Age, y | 52±9 |
| Male gender, (%) | 43 (88) |
| Nonischemic CMP, (%) | 27 (55) |
| NYHA | 2.9±0.4 |
| LVEF, % | 27±9 |
| 6MWT, m | 442±123 |
| NT-proBNP, pg/mL (IQR) | 1767 (468, 2446) |
| Creatinine, mmol/L | 91±26 |
| Bilirubin, μmol/L | 18±9 |
| Potassium, mmol/L | 4.4±0.4 |
| Sodium, mmol/L | 140±2 |
| AST, μmol/L | 0.5±0.2 |
| AF, μmol/L | 1.4±0.8 |
| gGT, μmol/L | 1.3±1.8 |
| Leukocytes, ×109 | 7.0±1.7 |
| Hemoglobin, g/L | 146±12 |
| Medical management | |
| ACEI/ARB | 48 (100) |
| Beta blockers | 48 (100) |
| MRA | 48 (100) |
| Diuretic | 32 (67) |
| Aspirin | 25 (52) |
| Antiarrhythmic therapy | |
| CRT-D, (%) | 14 (29) |
| ICD, (%) | 34 (71) |
| Medication*, (%) | 7 (15) |
| Scar burden, % | 54±20 |
Baseline Patient Characteristics. Values are presented as mean±SD, number of patients (percent) or median (IQR) for NT-proBNP. CMP: cardiomyopathy; NYHA: New York Heart Association; LVEF: left ventricular ejection fraction; 6MWT: 6-minute walk test; AST: aspartate transaminase; AF: alkaline phosphatase; gGT: γ-glutamyltranspeptidase; ACEI: angiotensin convertase inhibitor; ARB: angiotensin receptor blocker; MRA: mineralocorticoid receptor antagonist; CRT-D: cardiac resynchronization therapy; ICD: implantable cardioverter defibrillator; *-antiarrhythmic medication other than beta blockers.
Effects of CD34+ Cell Therapy on Ventricular Arrhythmias
Overall, we found no significant differences in the burden of ventricular arrhythmias in the evaluation period of 12 months before and after stem cell therapy (Fig. 2). VT occurred in 23 (48%) and 21 (44%) patients before and after stem cell, respectively (P=0.68). In one (2%) patient an episode of VF was registered before stem cell therapy as opposed to two (4%) patients experiencing VF after stem cell therapy. At least one ICD activation occurred in nine (19%) patients before and in 13 (27%) patients after stem cell therapy, which was not significantly different (P=0.33).
Fig. 2.
Patients experiencing ventricular arrhythmias before and after stem cell transplantation. The total number of patients experiencing at least one ventricular arrhythmia before (dotted boxes) or after (meshed boxes) stem cell transplantation did not differ significantly. Also, the total number of patients with at least one ICD activation before and after stem cell transplantation was not significantly different.
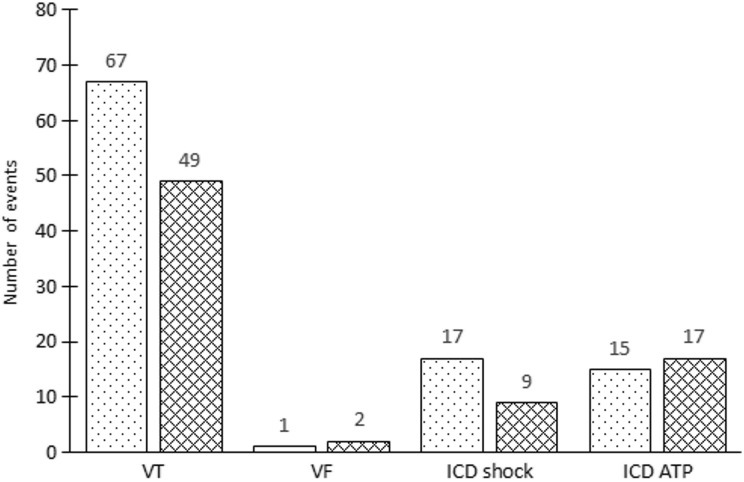
A total ventricular arrhythmia burden is outlined in Fig. 3. The VT burden before and after stem cell transplantation was similar, as a total of 67 VT episodes were registered before and 49 VT episodes were registered after stem cell therapy (P=0.46). One episode of VF was registered before and two episodes of VF were registered after stem cell therapy, which occurred >30 days after the procedure and were adjudicated not to be directly associated with stem cell therapy. In addition, the cumulative device activity in the analyzed patient cohort did not differ before and after stem cell therapy: 17 ICD shocks and nine ICD shocks were delivered before and after stem cell therapy, respectively, (P=0.38) and ICD ATP occurred 15 times before and 17 times after stem cell therapy (P=0.80).
Fig. 3.
Ventricular arrhythmia burden before and after stem cell therapy. The total number of ventricular tachycardia and ventricular fibrillation before (dotted boxes) or after (meshed boxes) stem cell transplantation did not differ significantly. Also, the total number ICD activations before and after stem cell transplantation was not significantly different.
Differential Arrhythmic Response to CD34+ Cell Therapy
We compared patients conditional to ventricular arrhythmia events after stem cell therapy (Table 2). We found no differences between Group A, that did not experience any arrhythmias (N=27) and Group B, that experienced at least one episode of VT/VF (N=21), considering gender, age, underlying heart failure etiology, exercise capacity, end-organ function, medical therapy, or antiarrhythmic management. In addition, the two groups did not differ with respect to the myocardial scar burden. However, significantly fewer patients in Group A experienced ventricular arrhythmias before stem cell therapy in comparison to Group B (22% vs. 81%, P<0.05). Not surprisingly, significantly fewer patients in Group A experienced device activation before stem cell therapy in comparison to Group B (7% vs. 33%, P=0.02).
Table 2.
Comparison of Patients With and Without Ventricular Arrhythmias After Transendocardial Stem Cell Transplantation.
| Group A (N=27) |
Group B (N=21) |
P | |
|---|---|---|---|
| Age, y | 52±11 | 52±8 | 0.89 |
| Male gender, (%) | 23 (85) | 19 (91) | 0.83 |
| NYHA | 2.8±0.7 | 2.9±0.8 | 0.61 |
| Nonischemic CMP | 14 (52) | 9 (43) | 0.72 |
| LVEF, % | 27±9 | 28±8 | 0.84 |
| 6MWT, m | 461±67 | 460±100 | 0.95 |
| NT-proBNP, pg/mL (IQR) | 1378 (417, 2747) | 1385 (482, 2181) | 0.34 |
| Creatinine, mmol/L | 95±29 | 86±21 | 0.24 |
| Bilirubin, μmol/L | 16±7 | 20±11 | 0.21 |
| Potassium, mmol/L | 4.3±0.3 | 4.5±0.4 | 0.44 |
| Sodium, mmol/L | 141±2 | 140±2 | 0.10 |
| AST, μmol/L | 0.4±0.1 | 0.5±0.1 | 0.64 |
| AF, μmol/L | 1.3±0.7 | 1.5±0.8 | 0.27 |
| gGT, μmol/L | 0.8±0.8 | 1.1±1.7 | 0.33 |
| Leukocytes, ×109 | 7.2±1.8 | 6.7±1.4 | 0.31 |
| Hemoglobin, g/L | 144±11 | 148±12 | 0.28 |
| Medical management | |||
| ACEI/ARB | 27 (100) | 48 (100) | / |
| Beta blockers | 27 (100) | 48 (100) | / |
| MRA | 27 (100) | 48 (100) | / |
| Diuretic | 18 (67) | 14 (67) | 0.86 |
| Aspirin | 13 (48) | 12 (57) | 0.27 |
| Antiarrhythmic therapy | |||
| CRT-D, (%) | 13 (48) | 7 (33) | 0.50 |
| ICD, (%) | 14 (52) | 14 (67) | 0.50 |
| Medication*, (%) | 5 (18) | 2 (10) | 0.39 |
| Scar burden, % | 54±19 | 53±21 | 0.86 |
Comparison of patients with and without ventricular arrhythmias after transendocardial stem cell transplantation. Values are presented as mean±SD, number of patients (percent) or median (IQR) for NT-proBNP. CMP: cardiomyopathy; NYHA: New York Heart Association; LVEF: left ventricular ejection fraction; 6MWT: 6-minute walk test; AST: aspartate transaminase; AF: alkaline phosphatase; gGT: γ-glutamyltranspeptidase; ACEI: angiotensin convertase inhibitor; ARB: angiotensin receptor blocker; MRA: mineralocorticoid receptor antagonist; CRT-D: cardiac resynchronization therapy; ICD: implantable cardioverter defibrillator; *-antiarrhythmic medication other than beta blockers.
Electrophysiological Properties of the Myocardium and Arrhythmic Response to CD34+ Cell Therapy
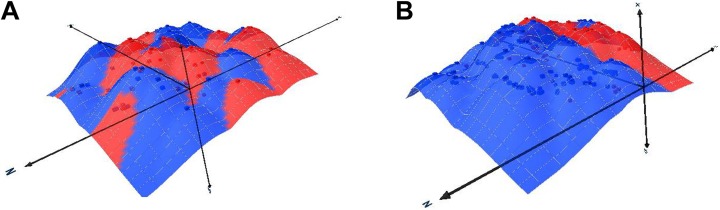
In Group A, six patients displayed ventricular arrhythmias (a total of nine VT events) before stem cell therapy but did not display any ventricular arrhythmias after the procedure. Conversely, in Group B, four patients did not display any ventricular arrhythmias before stem cell therapy but had arrhythmic events (a total of eight VT events) after stem cell therapy. Although a low patient number precludes any statistical comparison between the two groups, the initial observation of the patient data failed to identify any clinically relevant differences between the two subgroups. Interestingly, in six patients with the decrease in arrhythmic events the analysis of the electromechanical data showed a trend toward increased clustering (greater dispersion) of the myocardial scar (Fig. 4A). In patients in whom ventricular arrhythmic events increased after stem cell therapy, the scar was localized to a single cluster (Fig. 4B). In the remaining 17 patients the occurrence of ventricular arrhythmias did not change with the stem cell therapy.
Fig. 4.
Graphic presentation of ventricular scar dispersion as evaluated by electroanatomical data. Examples of 3-dimensional scatterplot charts are shown for patients in whom ventricular arrhythmia burden regressed (panel A) or increased after stem cell therapy (panel B). The red areas mark the myocardial scar and the blue areas mark the viable myocardium.
Heart Failure Etiology and Ventricular Arrhythmias after CD34+ Cell Therapy
When stratifying the patients according to the underlying cause of heart failure, patients with nonischemic dilated cardiomyopathy (DCM Group, N=26) did not differ from patients with ischemic heart failure (ICM, N=22) regarding age, gender, LVEF, NYHA functional class, serum levels of NT-proBNP, functional capacity, and end-organ function. We also found no differences in medical management, device therapy, or scar burden between the two groups. Importantly, there were no differences between the two groups with respect to the burden of ventricular arrhythmias or ICD activation before and after stem cell therapy.
Discussion
This is the first study to evaluate the effects of CD34+ cell therapy on ventricular arrhythmias in patients with ischemic and nonischemic chronic heart failure. Our results suggest that transendocardial CD34+ cell therapy does not increase the burden of ventricular arrhythmias in this patient cohort, and that it is not associated with increased rate or changed mode of ICD activation. Although not reaching significance in terms of antiarrhythmic capacity of cell therapy, our data do generate some new evidence to support further studies into the antiarrhythmic effects of this treatment modality.
Ever since the initial data on skeletal myoblasts and their proarrhythmic effects were published, a concern of potential association between stem cell therapy and ventricular arrhythmias has persisted in the stem cell community. Building on the data from skeletal myoblasts, three main mechanisms of ventricular arrhythmias after stem cell therapy were proposed. It was shown that transplantation of skeletal myoblasts into the diseased myocardium increases spatial dispersion of action potential duration in the target myocardium. This, in combination with decreased expression of connexin 43 of the maturing skeletal myoblasts, results in the lack of electrical coupling of the myoblast-derived myotubes in the host myocardium, increasing the risk of reentry3. Furthermore, transplanted skeletal myoblasts display enhanced automaticity. Even though differentiated myotubes are not connected to the neighboring cardiomyocytes by gap junctions, their intrinsic contractile activity could increase cardiac excitability through electrotonic interactions3. Also, the spontaneous electrical activity of engrafted skeletal myoblasts may provoke after-depolarizations and thus induce ventricular arrhythmias by triggered activity. In the clinical setting these interactions of skeletal myoblasts with the surrounding myocardium translated to potentially life-threatening ventricular arrhythmias1–4, and this was largely responsible for the shift in the stem cell field from skeletal myoblasts to safer bone marrow mononuclear cell populations.
In vitro studies of bone marrow stem cells have suggested that bone marrow stem cell transplantation generates an increased electrophysiological heterogeneity that could increase the likelihood of ventricular arrhythmias7. It was shown that, despite the presence of functional connexin-43 gap junctions between injected bone marrow stem cells and adjacent cardiomyocytes, the conduction velocity across bone marrow stem cells was 11-fold slower than across cardiomyocytes14. In addition, the impulse transmission across the bone marrow stem cells was characterized by reduced depolarization and low-amplitude electrical activity7,14. Thus, sustained reentry arrhythmia (an in vitro equivalent of sustained monomorphic VT) could repeatedly be induced by rapid pacing in co-cultures of cardiomyocytes and bone marrow stem cells. Interestingly, triggered activity and increased automacity, seen with skeletal myoblasts, were not observed with bone marrow stem cells, suggesting that potential proarrhythmic effects of one stem cell type cannot be generalized to the entire field of stem cell therapy. In accordance with the in vitro data, results from animal heart failure models showed that in the failing myocardium, bone marrow stem cells prolonged local activation time, increased the activation time dispersion, and shortened the effective refractory periods15,16. Furthermore, there was an association between the number of stem cells and the activation time dispersion. Importantly, despite these electrophysiological changes in the target myocardium, ventricular arrhythmias could not be induced by rapid ventricular pacing, questioning the clinical relevance of these findings15.
In the clinical setting, the potential proarrhythmic effects of stem cells were mainly explored in patients with chronic myocardial ischemia. Based on the Holter data and programmed ventricular stimulation, these studies showed that bone marrow stem cell therapy is not associated with increased incidence of ventricular arrhythmias17,18. Furthermore, detailed electrophysiological evaluation of the myocardium with the NOGA system at the time of intramyocardial bone marrow stem cell transplantation and at 6 month follow-up showed that intramyocardial stem cell injections are not associated with any alterations in electrogram amplitude, fragmentation, or duration. Taken together, these data suggest that in patients with chronic myocardial ischemia, intramyocardial bone marrow stem cells transplantation does not generate clinically detectable electrophysiological tissue heterogeneity, thus making ventricular arrhythmias after stem cell therapy highly unlikely. In accordance with these results, the findings of the present trial confirm that also in patients with chronic heart failure transendocardial stem cell therapy does not appear to generate unfavorable myocardial electrophysiological changes that would translate to clinically detectable ventricular arrhythmias.
Recently the proarrhythmic effects of stem cell therapy were evaluated in patients with chronic ischemic heart failure. A post-hoc analysis of the pooled POSEIDON and TAC-HFT data showed that, in this patient cohort, transendocardial injections of MSCs or bone marrow stem cells were not associated with short-term or long-term ventricular arrhythmias19. In addition, the authors observed that stem cell therapy may be associated with a potential antiarrhythmic effect as they demonstrated a numerical, albeit not statistically significant, increase in heart rate variability and a decrease in ventricular ectopy in patients receiving MSCs19. These results are supported by the data from the MSC-HF trial that showed similar rates of ventricular arrhythmias in MSC and placebo-injected patients20. As we did not observe any increase in the burden of ventricular arrhythmias or ICD activation after stem cell therapy, our results confirm the low arrhythmogenic potential of transendocardial stem cell therapy and are in line with previously published clinical data. Our data also suggest that transendocardial stem cell therapy is equally safe in patients with ischemic and nonischemic heart failure, which is a novel observation and has until now never been systematically addressed.
The results of our study additionally support the observation of Ramireddy et al.19 and Henry et al.21 that proposed potential antiarrhythmic effects of cell therapy. Although this issue needs to be addressed in a dedicated prospective study, our data suggest that myocardial scar distribution and the location of stem cell injections may play a role in defining the antiarrhythmic potential of stem cell therapy, as injecting stem cells in the areas of slow conduction in patients with multiple scar clusters resulted in decreased burden of ventricular arrhythmias. This could potentially be explained by the structural and functional actions of the stem cells on the failing myocardium, as several recent clinical trials have proposed that stem cell therapy may improve a segmental perfusion of the failing myocardium and might even beneficially affect myocardial scar burden22–29. These favorable changes may in turn lead to a decreased anisotropic conduction in the targeted region of the failing myocardium, decreasing a possibility of reentry and ultimately lowering the burden of ventricular arrhythmias.
This study has several limitations that need to be acknowledged. We have performed a post-hoc analysis of the two randomized clinical trials and a clinical registry. However, all patients received the same CD34+ cell type and were treated using the same cell mobilization protocols and transendocardial cell injection procedure using the NOGA system. Also, our analysis was performed on a limited number of patients, which may mask potential antiarrhythmic benefits of stem cell therapy in our patient cohort.
Conclusions
In patients with chronic heart failure of ischemic and non-ischemic etiology, transendocardial CD34+ cell transplantation was not associated with an increase in the burden of ventricular arrhythmias or ICD activation. In addition, it appears that CD34+ cell therapy may be associated with antiarrhythmic effects in a specific subgroup of chronic heart failure patients with multiple scar clusters. As these observations are based on post-hoc analysis, prospective randomized studies need to verify our data and specifically address the issue of the antiarrhythmic potential of stem cell therapy.
Footnotes
Ethical Approval: The study protocol was approved by the National Medical Ethics Committee of Republic of Slovenia (No. 136/03/11).
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the National Medical Ethics Committee of Republic of Slovenia approved protocols.
Statement of Informed Consent: Written informed consent was obtained from the patient for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Slovenian Research Agency grant # J3-7312-0381. The work was also supported through collaboration with Stanford Cardiovascular Institute. Support form the grant ERA-CVD (ERA-CVD) 2016 (project ID ANR-16-ECVD-0001), cofunded by Ministry of education, science and sport of Republic of Slovenia was also received for this study.
References
- 1. Higuchi T, Miyagawa S, Pearson JT, Fukushima S, Saito A, Tsuchimochi H, Sonobe T, Fujii Y, Yagi N, Astolfo A, Shirai M, Sawa Y. Functional and electrical integration of induced pluripotent stem cell-derived cardiomyocytes in a myocardial infarction rat heart. Cell Transplant. 2015;24(12):2479–2489. [DOI] [PubMed] [Google Scholar]
- 2. Menasché P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, Vilquin JT, Marolleau JP, Seymour B, Larghero J, Lake S, Chatellier G, Solomon S, Desnos M, Hagège AA. The myoblast autologous grafting in ischemic cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117(9):1189–1200. [DOI] [PubMed] [Google Scholar]
- 3. Menasché P. Stem cell therapy for heart failure. Are arrhythmias a real safety concern? Circulation. 2009;119(20):2735–2740. [DOI] [PubMed] [Google Scholar]
- 4. Liu YW, Su CT, Yen CY, Lin LJ, Hsieh PC. Arrhythmogenesis: a roadblock to cardiac stem cell therapy. Curr Treat Options Cardiovasc Med. 2016;18(10):61. [DOI] [PubMed] [Google Scholar]
- 5. Hagège AA, Marolleau JP, Vilquin JT, Alhéritière A, Peyrard S, Duboc D, Abergel E, Messas E, Mousseaux E, Schwartz K, Desnos M, Menasché P. Skeletal myoblast transplantation in ischemic heart failure: long-term follow-up of the first phase I cohort of patients. Circulation. 2006;114(suppl 1):I-108–I-113. [DOI] [PubMed] [Google Scholar]
- 6. Veltman CE, Soliman OI, Geleijnse ML, Vletter WB, Smits PC, ten Cate FJ, Jordaens LJ, Balk AH, Serruys PW, Boersma E, van Domburg RT, van der Giessen WJ. Four-year follow-up of treatment with intramyocardial skeletal myoblasts injection in patients with ischaemic cardiomyopathy. Eur Heart J. 2008;29(11):1386–1396. [DOI] [PubMed] [Google Scholar]
- 7. Beeres SL, Zeppenfeld K, Bax JJ, Dibbets-Schneider P, Stokkel MP, Fibbe WE, van der Wall EE, Atsma DE, Schalij MJ. Electrophysiological and arrhythmogenic effects of intramyocardial bone marrow cell injection in patients with chronic ischemic heart disease. Heart Rhythm. 2007;4(3):257–265. [DOI] [PubMed] [Google Scholar]
- 8. Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kühl U, Maisch B, McKenna WJ, Monserrat L, Pankuweit S, Rapezzi C, Seferovic P, Tavazzi L, Keren A. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology working group on myocardial and pericardial diseases. Eur Heart J. 2008;29(2):270–276. [DOI] [PubMed] [Google Scholar]
- 9. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members; Document Reviewers. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the heart failure association (HFA) of the ESC. Eur J Heart Fail. 2016;18(8):891–975. [DOI] [PubMed] [Google Scholar]
- 10. Dreger P, Grelle K, Eckstein V, Suttorp M, Müller-Ruchholtz W, Löffler H, Schmitz N. Granulocyte-colony-stimulating factor induces increased serum levels of soluble interleukin 2 receptors preceding engraftment in autologous bone marrow transplantation. Br J Haematol. 1993;83(1):7–13. [DOI] [PubMed] [Google Scholar]
- 11. Campos B, Jauregui ME, Park KM, Mountantonakis SE, Gerstenfeld EP, Haqqani H, Garcia FC, Hutchinson MD, Callans DJ, Dixit S, Lin D, Riley MP, Tzou W, Cooper JM, Bala R, Zado ES, Marchlinski FE. New unipolar electrogram criteria to identify irreversibility of nonischemic left ventricular cardiomyopathy. J Am Coll Cardiol. 2012;60(21):2194–2204. [DOI] [PubMed] [Google Scholar]
- 12. de Bakker JM, van Capelle FJ, Janse MJ, Tasseron S, Vermeulen JT, de Jonge N, Lahpor JR. Fractionated electrograms in dilated cardiomyopathy: origin and relation to abnormal conduction. J Am Coll Cardiol. 1996;27(5):1071–1078. [DOI] [PubMed] [Google Scholar]
- 13. Olsson LG, Swedberg K, Clark AL, Witte KK, Cleland JG. Six minute corridor walk test as an outcome measure for the assessment of treatment in randomized, blinded intervention trials of chronic heart failure: a systematic review. Eur Heart J. 2005;26(8):778–793. [DOI] [PubMed] [Google Scholar]
- 14. Chang MG, Tung L, Sekar RB, Chang CY, Cysyk J, Dong P, Marbán E, Abraham MR. Proarrhythmic potential of mesenchymal stem cell transplantation revealed in an in vitro coculture model. Circulation. 2006;113(15):1832–1841. [DOI] [PubMed] [Google Scholar]
- 15. Chen M, Fan ZC, Liu XJ, Deng JL, Zhang L, Rao L, Yang Q, Huang DJ. Effects of autologous stem cell transplantation on ventricular electrophysiology in doxorubicin-induced heart failure. Cell Biol Int. 2006;30(7):576–582. [DOI] [PubMed] [Google Scholar]
- 16. Price MJ, Chou CC, Frantzen M, Miyamoto T, Kar S, Lee S, Shah PK, Martin BJ, Lill M, Forrester JS, Chen PS, Makkar RR. Intravenous mesenchymal stem cell therapy early after reperfused acute myocardial infarction improves left ventricular function and alters electrophysiologic properties. Int J Cardiol. 2006;111(2):231–239. [DOI] [PubMed] [Google Scholar]
- 17. Beeres SLMA, Bax JJ, Dibbets P, Stokkel MPM, Zeppenfeld K, Fibbe WE, van der Wall EE, Schalij MJ, Atsma DE. Effect of intramyocardial injection of autologous bone marrow-derived mononuclear cells on perfusion, function, and viability in patients with drug-refractory chronic ischemia. J Nucl Med. 2006;47(4):574–580. [PubMed] [Google Scholar]
- 18. Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364(9429):141–148. [DOI] [PubMed] [Google Scholar]
- 19. Ramireddy A, Brodt CR, Mendizabal AM, DiFede DL, Healy C, Goyal V, Alansari Y, Coffey JO, Viles-Gonzalez JF, Heldman AW, Goldberger JJ, Myerburg RJ, Hare JM, Mitrani RD. Effects of Transendocardial stem cell injection on ventricular proarrhythmia in patients with ischemic cardiomyopathy: results from the POSEIDON and TAC-HFT Trials. Stem Cells Transl Med. 2017;6(5):1366–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mathiasen AB, Qayyum AA, Jorgensen E, Helqvist S, Fischer-Nielsen A, Kofoed KF, Haack-Sørensen M, Ekblond A, Kastrup J. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial (MSC-HF trial). Eur Heart J. 2015;36(27):1744–1753. [DOI] [PubMed] [Google Scholar]
- 21. Henry T, Patel A, Schaer G, DeMaria A, Remmers A, Recker DP. Reduction in ventricular arrhythmias with Ixmyelocel-T: results from the IxCell-DCM Trial. J Am Coll Cardiol. 2016;67(13):1447. [Google Scholar]
- 22. Lezaic L, Socan A, Poglajen G, Peitl PK, Sever M, Cukjati M, Cernelc P, Wu JC, Haddad F, Vrtovec B. Intracoronary transplantation of CD34(+) cells is associated with improved myocardial perfusion in patients with nonischemic dilated cardiomyopathy. J Card Fail. 2015;21(2):145–152. [DOI] [PubMed] [Google Scholar]
- 23. Poglajen G, Sever M, Cukjati M, Cernelc P, Knezevic I, Zemljic G, Haddad F, Wu JC, Vrtovec B. Effects of transendocardial CD34+ cell transplantation in patients with ischemic cardiomyopathy. Circ Cardiovasc Interv. 2014;7(4):552–559. [DOI] [PubMed] [Google Scholar]
- 24. Vrtovec B, Poglajen G, Lezaic L, Sever M, Socan A, Domanovic D, Cernelc P, Torre-Amione G, Haddad F, Wu JC. Comparison of transendocardial and intracoronary CD34+ cell transplantation in patients with nonischemic dilated cardiomyopathy. Circulation. 2013;128(11 suppl 1):S42–S49. [DOI] [PubMed] [Google Scholar]
- 25. Vrtovec B, Poglajen G, Lezaic L, Sever M, Domanovic D, Cernelc P, Socan A, Schrepfer S, Torre-Amione G, Haddad F, Wu JC. Effects of intracoronary CD34+ stem cell transplantation in nonischemic dilated cardiomyopathy patients: 5-year follow-up. Circ Res. 2013;112(1):165–173. [DOI] [PubMed] [Google Scholar]
- 26. Malliaras K, Makkar RR, Smith RR, Cheng K, Wu E, Bonow RO, Marbán L, Mendizabal A, Cingolani E, Johnston PV, Gerstenblith G, Schuleri KH, Lardo AC, Marbán E. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction). J Am Coll Cardiol. 2014;63(2):110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hare JM, DiFede DL, Rieger AC, Florea V, Landin AM, El-Khorazaty J, Khan A, Mushtaq M, Lowery MH, Byrnes JJ, Hendel RC, et al. Randomized comparison of allogeneic versus autologous mesenchymal stem cells for nonischemic dilated cardiomyopathy: POSEIDON-DCM Trial. J Am Coll Cardiol. 2017;69(5):526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karantalis V, DiFede DL, Gerstenblith G, Pham S, Symes J, Zambrano JP, Fishman J, Pattany P, McNiece I, Conte J, Schulman S, et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ Res. 2014;114(8):1302–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cerar A, Zemljic G, Frljak S, Jaklic M, Poglajen G, Sever M, Cukjati M, Vrtovec B. Transendocardial CD34+ cell transplantation in noncompaction cardiomyopathy: first-in-man case study. Cell Transplant. 2018;27(7):1027–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]