Abstract
MiR-128, one of the most enriched miRNAs in the human brain, has been reported to protect MCAO mice via inhibiting P38α MAPK. Whether it is involved in pathogenesis in acute ischemic stroke patients remains to be determined. The present study focused on the clinical importance of miR-128 and its underlying mechanisms. We detected miR-128 levels in the circulating lymphocytes, neutrophils, and plasma of acute ischemic stroke patients by using RT-PCR. miR-128 levels were significantly elevated in circulating lymphocytes, neutrophils, and plasma of patients with acute ischemic stroke. In addition, miR-128 levels in circulating lymphocytes correlated positively with the infarction volume, NIHSS scores at 7 days and mRS at 90 days after ischemic stroke onset. Subsequent KEGG pathway analysis showed that the MAPK signaling pathway and cell cycle are among the pathways targeted by miR-128. Although no correlation was found between miR-128 in plasma and peripheral inflammatory cell numbers, miR-128 decreased in the penumbra and increased in the infarction core of ipsilateral brain tissues in MCAO mice. Moreover, an in vitro study demonstrated that miR-128 antagomir aggravated primary neuronal damage and exacerbated cell cycle reactivation induced by OGD/R stimulation; the underlying mechanism involved increasing cyclin A2, PTEN, and ERK expression and promoting phosphorylation of PTEN and ERK. From the above results, we concluded that the upregulation of miR-128 in circulating lymphocytes of acute ischemic stroke patients was correlated with stroke severity and miR-128 antagomir exacerbated ischemia-reperfusion induced neuronal injury via promoting neuronal cell cycle reentry.
Keywords: ischemic stroke, microRNA, ischemia-reperfusion, cell cycle
Introduction
MicroRNAs (miRNAs) are a class of endogenously expressed small noncoding RNAs, which are usually 18–24 nucleotides long and play an important role in various pathophysiological processes via regulating gene expression post-transcriptionally1. Because of their master role in gene control and their ease of isolation, dozens of miRNAs isolated from plasma/serum, cerebrospinal fluid, or circulating blood cells of ischemic stroke patients have been identified as potential biomarker candidates2–7.
MiR-128 is one of the most enriched miRNAs in the adult mouse and human brain8,9. It is encoded by two separate genes, miR-128 -1 and miR-128-2, on mouse chromosomes 1 and 9, or human chromosomes 2 and 3. In mice, germline miR-128-2 deficiency results in an 80% decrease in miR-128 expression in the forebrain, whereas ablation of the miR-128 -1 gene eliminates only 20% of miR-128 expression10. During postnatal development, the expression of miR-128 in the mouse brain increases gradually and peaks in adulthood11,12.
The diverse expression of miR-128 throughout regions of the brain suggests its important roles in processes common to various cell-types. Franzoni et al have reported that miR-128 promotes functional neuronal maturation during cortical development via its involvement in neuronal migration, outgrowth, and intrinsic excitability13. Chan et al have found that miR-128 governs motor activity by suppressing the expression of various ion channels and signaling components of the extracellular signal-regulated kinase ERK2 network that regulates neuronal excitability10. Moreover, miR-128 has also been shown to regulate the proliferation and differentiation of neural progenitor cells, cell-cycle progression in glioblastoma multiforme, and myocardial ischemia/reperfusion injury-induced cardiomyocyte apoptosis through targeting pericentriolar material 1 (PCM1), the transcription factor E2F-3a, or peroxisome proliferator-activated receptor gamma (PPARG)14–16. In addition, Mao et al have demonstrated that miR-128 protects against cerebral ischemic injury through decreasing p38α mitogen activated protein kinase activity in an MCAO mouse model17. In the present study, we aimed to further verify the specific effect of miR-128 on acute ischemic stroke patients and to explore its potential regulatory role in neuronal ischemia-reperfusion injury and cell cycle activation following cerebral ischemia.
Materials and Methods
Blood Samples of Patients with Acute Ischemic Stroke
The use of the human blood samples for research purposes was approved by the Ethics Committee of Capital Medical University. Written informed consent was obtained from all participants. We enrolled 40 patients with acute ischemic stroke who were treated in the Department of Neurology, Xuanwu Hospital of Capital Medical University. The inclusion criteria were as follows: (1) diagnosis of first ischemic stroke on the basis of clinical information and MRI images; (2) patients aged 55–65 years old; (3) presentation of subjects within 72 h of the event; (4) National Institutes of Health Stroke Scale (NIHSS) score of 4–15; and (5) Trial of ORG 10172 in Acute Stroke Treatment stroke (TOAST) subtype of large-artery atherosclerosis. Equivalent numbers of age-matched patients were enrolled as controls. Blood was collected from each patient/volunteer for analysis.
Neurological Deficit Scores and Magnetic Resonance Imaging
All patients underwent standard neurological and general medical evaluation and assessment on the basis of the NIHSS score at 7 days and the modified Rankin scale (mRS) at 90 days after stroke onset.
Magnetic resonance imaging (MRI images were acquired within 72 h of stroke onset on a 3.0 T Magnetom Verio Syngo instrument (Siemens, Munich, Germany). The radiologist used diagnostic workstations with a unisight system for interpretation and measurement of the infarct volume. The analysis was performed blind to the clinical history, physical findings, patient identity, and final diagnostic results. The total infarct volume was calculated on the basis of the infarct size on the diffusion-weighted imaging sequence and then multiplied by the thickness. All image data were standardized according to intracranial cross-sectional area18. The formula of the volume standardization of cerebral infarction was as follows: infarct volume × mean of intracranial cross-sectional area/intracranial cross-sectional area of patient.
Separation of Neutrophils, Lymphocytes, and Plasma
Blood samples (2 × 4 ml) were collected into EDTA-anticoagulant tubes and processed according to the following procedures: first, each blood sample was immediately centrifuged at 200 g for 10 min at 4°C, and plasma was obtained. Second, the blood cells were diluted with 8 ml normal saline and slowly added to the surface of the lymphocyte separation medium (Tian Jin Hao Yang Biological Manufacture Co., Beijing, China) in two 15 ml centrifuge tubes. After the tubes were centrifuged at 400 g for 20 min at 20°C, the lymphocytes were separated and saved. Finally, erythrocytes were dissociated with erythrocyte lysis solution three times and discarded, and the remaining neutrophils were saved. Neutrophils, lymphocytes, and plasma were stored at –80°C in RNase/DNase-free tubes for further laboratory testing.
MiR Extraction and Real-Time Reverse Transcription Polymerase Chain Reaction
Total RNA in neutrophils, lymphocytes, and plasma was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and processed as previously described19. The PCR primers for miR-128 were 5′GCGGAGCCGTAGCACTGT3′ and 5′GTGCGTGTCG -TGGAGTCG3′. The PCR primers for U6 were 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and 5′-CGCTTCACGAATTTGCGTGTCAT-3′. Relative gene expression was calculated via the 2–△△CT method and expressed as fold change relative to U6 expression.
Prediction of miR-128 Related Inflammation Pathways and a miRNA-Pathway Network
Targetscan (http://www.targetscan.org), miRanda (http://www.microRNA.org), and miRBase target prediction database (http://www.ebi.ac.uk/enrightsrv/microcosm) were used to determine the predicted target genes of miR-128 as previously described20. The overlapping targets predicted by the above three databases were subjected to Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis; the selection criterion for significant KEGG pathway terms was a P value <0.01.
Mouse Model of Focal Cerebral Ischemia
Male C57Bl/6 mice weighing 20–25 g were purchased from Vital River Laboratory Animal Technology Co. (Beijing, China). All animal experiments in this study were approved by the Institutional Animal Care and Use Committee of Capital Medical University. Mice models of transient focal cerebral ischemia were induced as described previously21. To ensure the occurrence of ischemia by MCAO, regional cerebral blood flow was monitored using laser Doppler flowmetry (PeriFlux System 5000, Perimed, Sweden) at a location 0.5 mm anterior and 5.0 mm lateral from the bregma. The rectal temperature was maintained at 37.0°C during and after surgery with a temperature-controlled heating pad (CMA 150; Carnegie Medicin, Sweden).
Primary Neuronal Culture and Treatment
The primary neurons were dissociated carefully from the cortex of P16-18 C57BL/6 mice. They were seeded at a density of 5×105 cells/cm2 and grown in neurobasal medium with 2% B27 supplement (Gibco, Carlsbad, CA, USA), which were replaced half of fresh medium every 3 days. After being cultured for 7 days, the primary neurons were transfected with miR-128 antagomir using MessengerMAXTM Reagent (Lipofectamine MessengerMAX™, Invitrogen) for 48 h as pre the manufacturer’s protocol. Sense of miR-128 antagomir were synthesized by GenePharma and the sequences were as follows: 5′AAAGAGACCGGUUCACUGUGA3′.
In order to establish the oxygen and glucose deprivation /reoxygenation (OGD/R) cell model, after 48 h transduction, primary neurons were subjected to 2.5 h OGD/24 h R. For OGD treatment, the cells were cultured in glucose-free DMEM and kept in a hypoxic incubator chamber (Billups- Rothenberg, San Diego, CA, USA) filled with 95% N2/5% CO2 at 37°C. After OGD for 2.5 h, the cells were transferred to normal medium under normoxic conditions (5% CO2/21%O2/74% N2) for 24 h.
Quantification of Lactate Dehydrogenase Activity
Lactate dehydrogenase (LDH) activity was determined as the manufacturer’s protocol. Simply speaking, 10 μl medium cultured with primary neuron and 190 μl working reagent were transferred into 96-well plate and OD565 were read 0 min (ODS0) and 25 min (ODS25) after they were mixed briefly; 200 μl H2O (ODH2O) and 200 μl Calibrator (ODCAL) solution were also transferred into 96-well plate. LDH activity were quantified as the following calculation: LDH activity = 43.68×ODS25–ODS0/ODCAL–ODH2O×10 (IU/L).
Cell-Cycle Analysis by Flow Cytometry
At 24 h after reoxygenation, primary neurons were washed with PBS, detached with 0.25% trypsin, and fixed with 70% ethanol overnight. The samples were then resuspended in 0.5 mL PBS, treated with RNase to remove RNA and stained with propidium iodide (Sigma-Aldrich, St. Louis, MO, USA) in the dark for 30 min. The DNA content was measured by fluorescence-activated cell sorting on a FACSCanto flow cytometer with Cellfit software (Becton-Dickinson, San Jose, CA, USA).
Western Blotting
The ipsilateral penumbra and ischemic core were collected 1 h after ischemia and 1 h, 4 h, and 24 h after reperfusion, then processed for western blotting as previously described22. The antibodies (1:1000) specific to cyclin A2, PTEN/P-PTEN, and ERK/P-ERK (Cell Signaling Technology, Boston, MA, USA), and β-actin (Bioworld, Nanjing, China) were used. Blots were detected with horseradish peroxidase-conjugated secondary antibody (1:2000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and an enhanced luminescence kit.
Statistical Analysis
Statistical analyses were carried out using SPSS Version 17.0 software (SPSS Inc., Chicago, IL, USA). Data are expressed as the mean ± SEM. Statistical significance between groups was determined with unpaired t-tests. For correlation analyses, we used the Pearson correlation test. P < 0.05 was considered statistically significant.
Results
MiR-128 is Upregulated in the Peripheral Blood (Lymphocytes, Neutrophils, and Plasma) of Acute Ischemic Stroke Patients
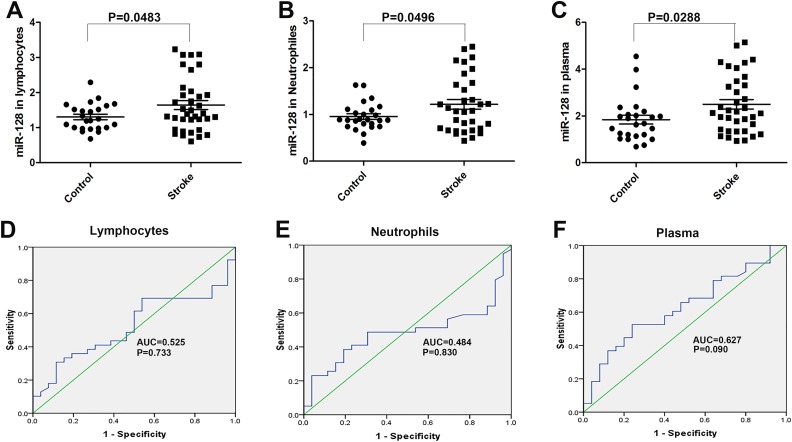
A total of 40 acute ischemic stroke patients and 25 healthy controls were enrolled in this study. There were no marked differences between the two groups in age, sex, or risk factors, including histories of diabetes, hypertension, hypercholesterolemia, or smoking. We collected blood samples from all patients and controls, then analyzed miR-128 levels in the circulating lymphocytes, neutrophils, and plasma. RT-PCR analysis revealed that the miR-128 levels in the circulating lymphocytes, neutrophils, and plasma were significantly higher in acute ischemic stroke patients than healthy controls (Fig. 1A-C, P < 0.05). These results suggested that miR-128 might be involved in the pathophysiological progression of acute ischemic stroke, although the underlying mechanisms require further study.
Fig 1.
Expression and diagnostic value of miR-128 in the peripheral blood (lymphocytes, neutrophils, and plasma) of acute ischemic stroke patients. (A–C) The miR-128 levels in the circulating lymphocytes (n = 24 in the control group, n = 36 in the stroke group), neutrophils (n = 25 in the control group, n = 33 in the stroke group), and plasma (n = 25 in the control group, n = 36 in the stroke group) in acute ischemic stroke (AIS) patients and healthy controls, as determined by semi-quantitative RT-PCR. U6 was used to normalize the expression levels of target miRNAs in different samples. (D–F) ROC analysis and AUC were used for AIS patients.
To investigate the potential for miR-128 to be used as a diagnostic biomarker of acute ischemic stroke, receiver operating characteristic (ROC) analysis was performed, and the area under the curve (AUC) was calculated. Generally, AUC > 0.5 was considered diagnostic, AUC < 0.7 indicated a lower diagnostic value, 0.7 < AUC < 0.9 indicated a moderate diagnostic value, and AUC > 0.9 indicated a high diagnostic value. The AUC of miR-128 in lymphocytes was 0.525, and that in plasma was 0.627; both were indicated as weakly diagnostic for acute ischemic stroke (Fig. 1D–F). This result suggested that the upregulation of miR-128 in the peripheral plasma may potentially be used as a biomarker for rapid diagnosis of acute ischemic stroke, but this possibility remains to be further confirmed in more acute ischemic patients.
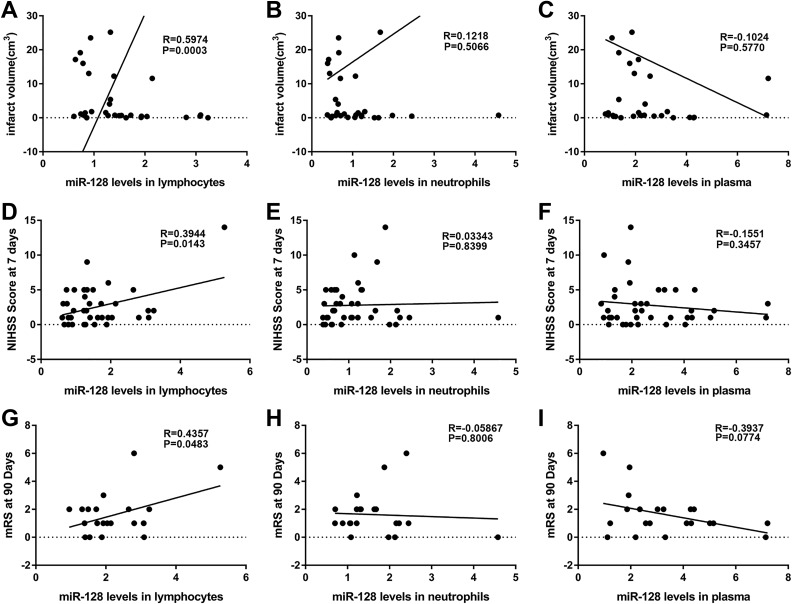
MiR-128 levels in the circulating lymphocytes were positively correlated with the infarction volume and neurologic scores of acute ischemic stroke patients
To further explore whether miR-128 is involved in acute ischemic stroke progression, we analyzed the correlation of the miR-128 levels in the circulating lymphocytes, neutrophils, and plasma with the infarction volume and neurologic scores of acute ischemic stroke patients. The miR-128 levels in circulating lymphocytes were positively correlated with the infarction volume, NIHSS scores at 7 days, and mRS at 90 days after ischemic stroke onset (Fig. 2A, D and G, P < 0.05). These results suggested a bidirectionally regulatory role of miR-128 in acute ischemic stroke. However, the miR-128 levels in the circulating neutrophils and plasma were not correlated with the infarction volume and neurologic score in acute ischemic stroke.
Fig 2.
Correlations of miR-128 levels in the circulating lymphocytes, neutrophils, and plasma with the infarction volume and neurological scores of acute ischemic stroke. (A–C) Correlations of miR-128 levels in lymphocytes, neutrophils, and plasma with cerebral infarct volume in 32 AIS patients. (D–F) Correlations of miR-128 levels in lymphocytes (n = 38), neutrophils (n = 39), and plasma (n = 39) with NIHSS score at 7 days after AIS onset in AIS patients. (G–I) Correlations of miR-128 levels in lymphocytes, neutrophils, and plasma with mRS scores at 90 days after AIS onset in 21 AIS patients.
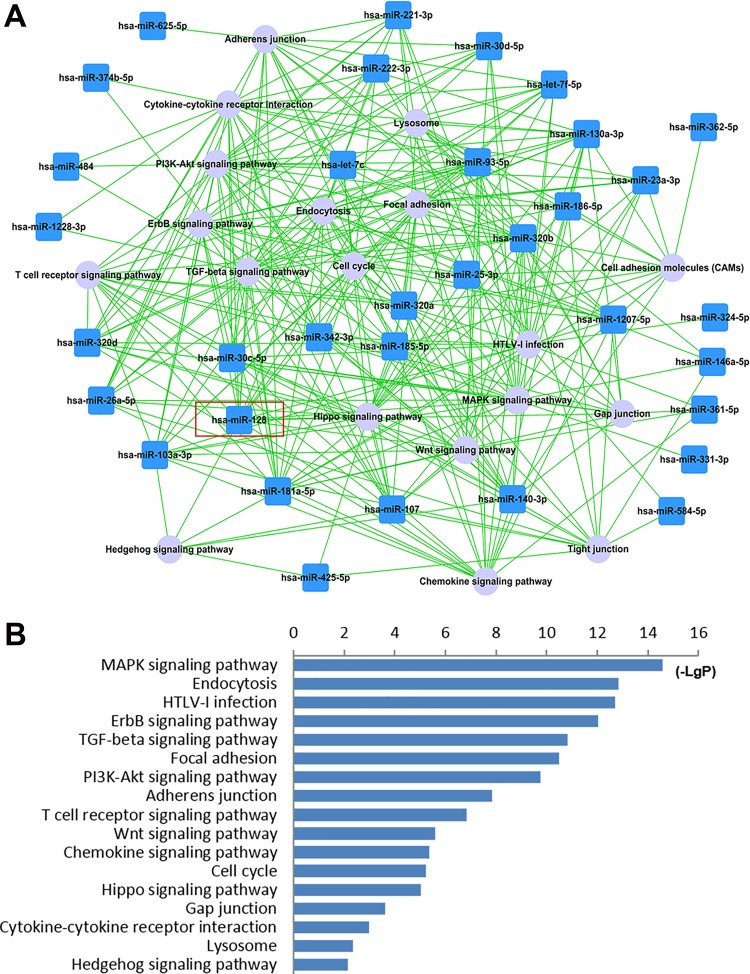
Bioinformatics Analysis Showed that miR-128 is Involved in the MAPK Pathway and Cell Cycle Progression
On the basis of the miRNA microarray analysis (data not shown), we further predicted the inflammation related pathways targeted by deregulated miRNAs in circulating lymphocytes of acute ischemic stroke patients by using KEGG pathway analysis. The results demonstrated that miR-128 played a central role within the miRNA-pathway network (Fig. 3A). Moreover, we determined the miR-128 targeted inflammation related pathways and produced a graphical heat map, which showed that the MAPK signaling pathway and cell cycle related pathways were among those significantly targeted by miR-128 (Fig. 3B).
Fig 3.
Bioinformatic analysis of miR-128 targeted pathways related to the inflammatory response. (A) Dysregulated miRNA-inflammation related pathway network of circulating lymphocytes of AIS patients, based on microarray and KEGG pathway analysis. The blue box nodes represent dysregulated miRNAs, and gray cycle nodes represent KEGG pathways. Edges show the effects of miRNAs on KEGG pathways. The red box marks the inflammation related pathways targeted by miR-128. (B) Inflammation related pathways significantly targeted by miR-128. The vertical axis is the pathway category, the horizontal axis is the significance of pathways represented by –log(P value) (LgP), and P < 0.01 is considered significant.
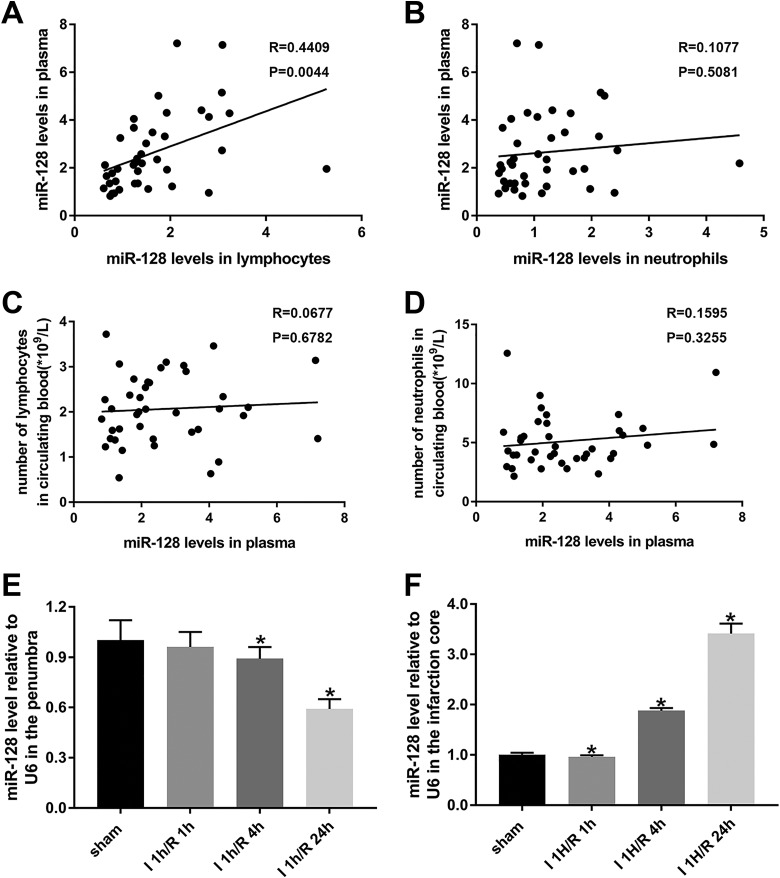
Plasma miR-128 levels were positively correlated with those in circulating lymphocytes; although they were not correlated with the number of circulating lymphocytes or neutrophils, they were decreased in the ipsilateral penumbra and increased in the ischemic core of MCAO mice.
To determine the origin of the miR-128 plasma expression, we analyzed the correlation between miR-128 expression in plasma and that in circulating lymphocytes and neutrophils. The miR-128 levels in plasma were positively correlated with the miR-128 levels in lymphocytes (Fig. 4A, P < 0.05), thus suggesting that miR-128 in plasma partially originated from circulating lymphocytes.
Fig 4.
Correlations between plasma miR-128 levels and lymphocytes or neutrophil miR-128 levels and the circulating blood number of AIS patients; time-dependent changes in miR-128 expression in the MCAO mouse model. (A and B) Correlations between plasma miR-128 levels and lymphocyte or neutrophil miR-128 levels in AIS patients. (C and D) Correlations between plasma miR-128 levels and the numbers of lymphocytes or neutrophils in the circulating blood of AIS patients. N = 40. (E and F) Real-time PCR analysis of miR-128 expression changes in the penumbra and ischemic core in an MCAO mouse model after ischemia for 1 h with different reperfusion durations of 1 h, 4 h, and 24 h and in the sham-operated group. U6 was used to normalize expression levels of target miRNAs in different samples. *P < 0.05 versus sham; n = 3 per group.
We next sought to determine whether miR-128 upregulation in the peripheral blood influenced the inflammatory cell number through acting on cell cycle progression. To answer this, we analyzed the correlation between miR-128 levels in plasma and the numbers of lymphocytes and neutrophils. Surprisingly, the correlations were not significant (Fig. 4C and D). Given that the miR-128 levels in plasma did not influence the peripheral inflammatory cell numbers, whether miR-128 might enter the brain through a damaged blood brain barrier and might be involved in neuronal injury after cerebral ischemia deserves further study.
To explore the role of miR-128 in neuronal injuries following cerebral ischemia, we first detected its expression in the penumbra and ischemic core in an MCAO mouse model by using real-time reverse transcription PCR (RT-PCR). The miR-128 levels in the penumbra of ipsilateral brain tissues decreased progressively after ischemia for 1 h and reperfusion for 1 h, 4 h, and 24 h, as compared with those observed after sham operation (Fig. 4E, P < 0.05). However, after a transient decrease, miR-128 expression in the ischemic core increased progressively and reached 3.5 times that in the sham group after reperfusion for 24 h (Fig. 4F, P < 0.05), a result consistent with the upregulation of miR-128 levels in the circulating lymphocytes, neutrophils, and plasma of acute ischemic stroke patients. These results further demonstrate that miR-128 might have bidirectionally regulatory effects on ischemia-reperfusion cerebral injury and thus may be a potential therapeutic target for acute ischemic stroke.
MiR-128 Antagomir Exacerbates OGD/R Induced Primary Neuronal Injury Partially Through Promoting Cell Cycle Reentry
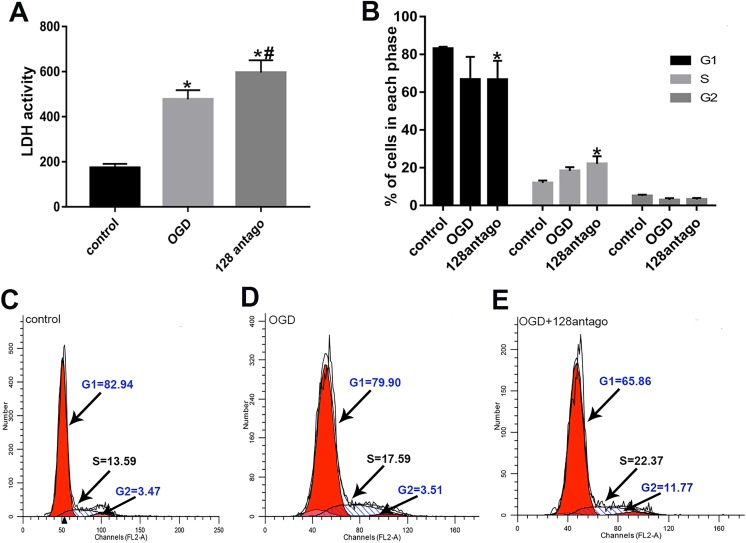
LDH is an endocellular enzyme and can be released outside the cells upon cell damage, so it is most often measured to evaluate the presence of tissue or cell damage23. To investigate the potential mechanisms of miR-128, we assessed the LDH activity in primary neurons after miR-128 antagomir and OGD/R treatment using an LDH kit. The results demonstrated that LDH activity was significantly upregulated after only OGD/R stimulation, whereas miR-128 antagomir plus OGD/R treatment induced a marked increase in LDH activity (Fig. 5A, P < 0.05). These results demonstrated that miR-128 antagomir aggravated primary neuronal injury induced by OGD/R stimulation.
Fig 5.
Effects of miR-128 antagomir on primary neuronal injury and cell cycle after OGD/R treatment. (A) Quantification and analysis of LDH activity in primary neurons under OGD/R and miR-128 antagomir treatment. LDH, lactate dehydrogenase; antago, antagomir; OGD, OGD/R. N = 6. (B) Flow cytometry analysis and semi-quantification of the effects of miR-128 on the cell cycle of OGD/R induced primary neurons; *P < 0.05 vs. control group; n = 4. (C–E) Representative flow cytometry of cell cycle in control, OGD/R and OGD/R plus miR-128 antagomir groups.
Neuronal cell cycle reentry is required for the neuronal injury evoked by cerebral ischemia24. In addition, we demonstrated that cell cycle progression was among the inflammation related pathways targeted by miR-128 using KEGG pathway analysis. To verify whether miR-128 acts on cell cycle progression, we performed flow cytometry assays to explore the cell cycle changes in primary neurons after OGD/R stimulation and miR-128 antagomir transfection. We found a tendency of proportionately fewer primary neurons in G1 phase but more cells in the S phases after OGD/R stimulation in the treated cells compared with the controls (Fig. 6B). These results confirmed cell cycle reentry induced by OGD/R. However, miR-128 antagomir plus OGD/R treatment exacerbated this cell cycle activation, i.e., markedly fewer cells were found in G1 phase and significantly more primary neurons were in S phase than were observed in control groups (Fig. 6B, P < 0.05). These results suggested that miR-128 antagomir may aggravate neuronal injury via promoting ischemia-reperfusion induced cell cycle reentry.
Fig 6.
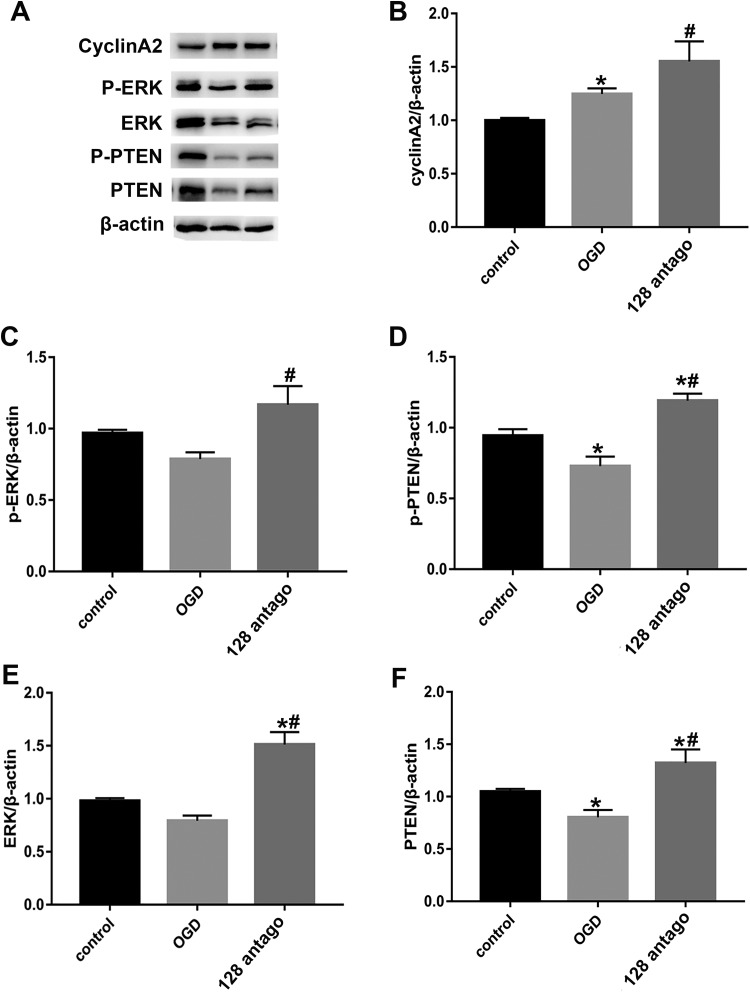
Effects of miR-128 antagomir on expression and phosphorylation of ERK, PTEN, and cyclinA2 in primary neurons after OGD/R treatment. (A–F) Western blot analysis and semi-quantitation of the effects of miR-128 on the expression of p-PTEN, p-ERK, and cyclin A2, p-ERK, p-PTEN, ERK, and PTEN in OGD/R induced primary neurons. Values represent the mean ± SEM. *P < 0.05 vs. control group; #P<0.05 vs. control+OGD/R group, n = 5.
To further explore how miR-128 acts on cell cycle progression, we used western blotting to detect the expression of cell cycle-related signaling molecules including PTEN and ERK and the cell cycle regulatory protein cyclin A2 in primary neurons after miR-128 antagomir and OGD/R treatment. The results demonstrated that compared with control groups, the cyclin A2 levels was upregulated markedly while protein expression and phosphorylation of PTEN decreased significantly under OGD/R stimulation (Fig. 6B, D and F, P < 0.05). Moreover, cyclin A2 expression were further increased after miR-128 antagomir plus OGD/R treatment and both the protein expressions and phosphorylation of ERK and PTEN were upregulated as well (Fig. 6B–F, P < 0.05).
These results suggested that miR-128 antagomir exacerbated ischemia-reperfusion induced cell cycle reentry via upregulating protein expressions of both PTEN and ERK, promoting their phosphorylation and increasing cyclin A2 expression, thereby potentially aggravating neuronal injury after cerebral ischemia.
Discussion
Although sustained efforts have been made to find the proper target for its prevention and therapy, ischemic stroke remains a major public health problem, owing to its high morbidity and narrow therapeutic window25,26. MiRNA-128, a small brain-enriched RNA, has been shown to be involved in neurogenesis, neuronal differentiation, apoptosis, and brain development in humans27–29. In addition, miR-128 levels have been reported to be deregulated in the brain in many diseases such as prion-induced neurodegeneration, Huntington’s Disease, Parkinson’s Disease and Alzheimer’s Disease, thus suggesting a possibility of exploiting miR-128 for diagnosis, prognosis, and development of novel therapies30–32. Moreover, miRNA-128 has been reported to be increased in the brain tissue in MCAO mice compared with sham-operated mice, and it attenuates ischemic injury through decreasing protein expression of P38α MAPK17. However, its role in ischemic stroke patients remains to be determined.
In the present study, we investigated the expression of miR-128 in acute ischemic stroke patients and explored its potential role in primary neuronal injury induced by OGD/R and cell cycle reentry. The results showed that the miR-128 levels increased in the peripheral blood of acute ischemic stroke patients and were positively correlated with infarction volume, NIHSS score at 7 days, and mRS at 90 days after stroke onset. Together, these results suggested that miR-128 might be involved in the progression of cerebral ischemic injury. Further bioinformatics analysis and in vitro studies confirmed that miR-128 antagomir exerted its neurotoxic effect partially by aggravating OGD/R induced neuronal injury and promoting neuronal cell cycle activation.
Quantitative RT-PCR showed that miR-128 levels increased in the circulating lymphocytes, neutrophils, and plasma of patients with acute ischemic stroke. Because obtaining a blood sample for measurement of miRNA is more convenient and less invasive than other auxiliary examinations such as computed tomography (CT) or MRI, plasma miR-128 levels may serve as a diagnostic biomarker for acute ischemic stroke. Moreover, we found significantly positive correlations between miR-128 levels in the circulating lymphocytes and the infarction volume, the NIHSS score at 7 days, and the mRS at 90 days after stroke onset. From KEGG pathway analysis, we predicted and found that the MAPK signaling pathway and cell cycle related pathways were among the inflammation related pathways targeted by miR-128. These findings provided clues as to the underlying mechanisms of miR-128 potentially acted following cerebral ischemia. The subsequent correlation analysis suggested that miR-128 in plasma might originate partially from circulating lymphocytes. However, plasma miR-128 levels did not influence the number of peripheral inflammatory cells.
To answer whether miR-128 in the plasma enters the brain and is involved in neuronal injury after cerebral ischemia, we detected its expression in the brain tissues of MCAO mice and explored its underlying mechanisms in vitro. In accordance with the clinical data, after a transient decrease, the miR-128 levels were markedly upregulated in the infarction core in MCAO mice, in agreement with the changes in the ipsilateral brain tissues in an MCAO mouse model reported by Mao et al17. The results from both the patients and animal models suggested a critical role of miR-128 in the pathogenesis of ischemic stroke.
OGD/R is commonly used to mimic cerebral ischemia and reperfusion in vitro. To address the involvement of miR-128 in neuronal injury, miR-128 antagomir was transfected into primary neurons and was found to significantly exacerbate OGD/R induced neuronal injury. In agreement with our results, Mao et al have reported that miR-128 may protect neurons against cell apoptosis by targeting p38α in an early stage of cerebral ischemia17. Yolanda et al also has shown that miRNA-128 overexpression downregulates genes that induce apoptosis and upregulates genes implicated in cell survival33. Contrary to our findings, Zeng et al have reported that miR-128 leads to cardiomyocyte apoptosis during myocardial ischemia-reperfusion, through activating PPARG16. In addition, several independent studies have illustrated the pro-apoptotic role of miRNA-128 in various cell types34,35. From the results of the present study, we concluded that miRNA-128 antagomir has neurotoxic functions opon OGD/R induced primary neuron. However, depending on the cell types on which miR-128 acts, further investigation is warranted to develop it as a therapeutic target for ischemic brain injury.
Inappropriate activation of cell cycle proteins is implicated in neuronal death induced by various pathologic stresses, including ischemia and oxidative stress36. Inhibiting the reactivation of cell cycle regulatory proteins such as cyclin D/Cdk4 and transcription factor E2F4 have been identified as a therapeutic target for ischemic injury24,37,38. Multiple articles have reported a role of miR-128 in cell cycle progression39,40. In the present study, we found that OGD/R stimulation induced a tendency of less neuron in G1 phase and more cells in S phase, a result consistent with cell cycle reactivation following ischemia. However, miR-128 antagomir aggravated OGD/R induced cell cycle reentry significantly via upregulating protein expression of cyclinA2, ERK and PTEN, as well as increasing phosphorylation of both ERK and PTEN. Thereby this demonstrated that miR-128 antagomir potentially exerted neurotoxic effects after cerebral ischemia through reactivating cell cycle entry and thus exacerbating neuronal injury.
Conclusions
In summary, the present study demonstrated that miR-128 was involved in the pathogenesis of acute ischemic stroke and miR-128 antagomir exerted neurotoxic effects against ischemia-reperfusion injury through activating cell cycle reentry by promoting ERK and PTEN phosphorylation and increasing ERK, PTEN and cyclinA2 expression. These results suggest that miR-128 holds promise as a miRNA-based therapeutic target that may be exploited for treating ischemic stroke.
Footnotes
Ethical Approval: The study was approved by the Ethics Committee of Capital Medical University.
Statement of Human and Animal Rights: All animal care complied with the guide for the Care and Use of Laboratory Animals. Human blood samples were obtained using protocols approved by the Ethics Committee of Xuanwu Hospital, Capital Medical University.
Statement of Informed Consent: We confirm that guidelines on patients consent have all been met and any details of informed consent obtained are indicated within the text of the submitted manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by the Natural Science Foundation in China (81601157, 81771413, 81571280).
References
- 1. Bartel DP. Micrornas: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. [DOI] [PubMed] [Google Scholar]
- 2. Li P, Teng F, Gao F, Zhang M, Wu J, Zhang C. Identification of circulating micrornas as potential biomarkers for detecting acute ischemic stroke. Cell Mol Neurobiol. 2015;35(3):433–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang W, Sun G, Zhang L, Shi L, Zeng Y. Circulating micrornas as novel potential biomarkers for early diagnosis of acute stroke in humans. J Stroke Cerebrovasc Dis. 2014;23(10):2607–2613. [DOI] [PubMed] [Google Scholar]
- 4. Sorensen SS, Nygaard AB, Nielsen MY, Jensen K, Christensen T. Mirna expression profiles in cerebrospinal fluid and blood of patients with acute ischemic stroke. Transl Stroke Res. 2014;5(6):711–718. [DOI] [PubMed] [Google Scholar]
- 5. Zhou J, Zhang J. Identification of mirna-21 and mirna-24 in plasma as potential early stage markers of acute cerebral infarction. Mol Med Rep. 2014;10(2):971–976. [DOI] [PubMed] [Google Scholar]
- 6. Dewdney B, Trollope A, Moxon J, Thomas Manapurathe D, Biros E, Golledge J. Circulating micrornas as biomarkers for acute ischemic stroke: a systematic review. J Stroke Cerebrovasc Dis. 2018;27(3):522–530. [DOI] [PubMed] [Google Scholar]
- 7. Bam M, Yang X, Sen S, Zumbrun EE, Dennis L, Zhang J, Nagarkatti PS, Nagarkatti M. Characterization of dysregulated mirna in peripheral blood mononuclear cells from ischemic stroke patients. Mol Neurobiol. 2018;55(2):1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. He M, Liu Y, Wang X, Zhang MQ, Hannon GJ, Huang ZJ. Cell-type-based analysis of microrna profiles in the mouse brain. Neuron. 2012;73(1):35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shao NY, Hu HY, Yan Z, Xu Y, Hu H, Menzel C, Li N, Chen W, Khaitovich P. Comprehensive survey of human brain microrna by deep sequencing. BMC Genomics. 2010;11:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tan CL, Plotkin JL, Venø MT, von Schimmelmann M, Feinberg P, Mann S, Handler A, Kjems J, Surmeier DJ, O’Carroll D, Greengard P, Schaefer A. Microrna-128 governs neuronal excitability and motor behavior in mice. Science. 2013;342(6163):1254–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miska EA, Alvarez-Saavedra E, Townsend M, Yoshii A, Sestan N, Rakic P, Constantine-Paton M, Horvitz HR. Microarray analysis of microrna expression in the developing mammalian brain. Genome Biol. 2004;5(9):R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS. A microrna array reveals extensive regulation of micrornas during brain development. RNA. 2003;9(10):1274–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Franzoni E, Booker SA, Parthasarathy S, Rehfeld F, Grosser S, Srivatsa S, Fuchs HR, Tarabykin V, Vida I, Wulczyn FG. Mir-128 regulates neuronal migration, outgrowth and intrinsic excitability via the intellectual disability gene phf6. ELife. 2015;4:e04263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang W, Kim PJ, Chen Z, Lokman H, Qiu L, Zhang K, Rozen SG, Tan EK, Je HS, Zeng L. Mirna-128 regulates the proliferation and neurogenesis of neural precursors by targeting pcm1 in the developing cortex. ELife. 2016;5:e11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cui JG, Zhao Y, Sethi P, Li YY, Mahta A, Culicchia F, Lukiw WJ. Micro-rna-128 (mirna-128) down-regulation in glioblastoma targets arp5 (angptl6), bmi-1 and e2f-3a, key regulators of brain cell proliferation. J Neurooncol. 2010;98(3):297–304. [DOI] [PubMed] [Google Scholar]
- 16. Zeng XC, Li L, Wen H, Bi Q. Microrna-128 inhibition attenuates myocardial ischemia/reperfusion injury-induced cardiomyocyte apoptosis by the targeted activation of peroxisome proliferator-activated receptor gamma. Mol Med Rep. 2016;14(1):129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mao G, Ren P, Wang G, Yan F, Zhang Y. Microrna-128-3p protects mouse against cerebral ischemia through reducing p38alpha mitogen-activated protein kinase activity. J Mol Neurosci. 2017;61(2):152–158. [DOI] [PubMed] [Google Scholar]
- 18. Ferguson KJ, Wardlaw JM, Edmond CL, Deary IJ, Maclullich AM. Intracranial area: a validated method for estimating intracranial volume. J Neuroimaging. 2005;15(1):76–78. [DOI] [PubMed] [Google Scholar]
- 19. Ma Q, Zhao H, Tao Z, Wang R, Liu P, Han Z, Ma S, Luo Y, Jia J. Microrna-181c exacerbates brain injury in acute ischemic stroke. Aging Dis. 2016;7(6):705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao HP, Liu P, Xu CM, Li GW, Gao L, Luo YM. Unique micrornas signature of lymphocyte of yang and yin syndromes in acute ischemic stroke patients. Chin J Integr Med. 2019;25(8):590–597. [DOI] [PubMed] [Google Scholar]
- 21. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. [DOI] [PubMed] [Google Scholar]
- 22. Yin XM, Luo Y, Cao G, Bai L, Pei W, Kuharsky DK, Chen J. Bid-mediated mitochondrial pathway is critical to ischemic neuronal apoptosis and focal cerebral ischemia. J Biol Chem. 2002;277(44):42074–42081. [DOI] [PubMed] [Google Scholar]
- 23. Liu Q, Hu Y, Zhang M, Yan Y, Yu H, Ge L. Microrna-451 protects neurons against ischemia/reperfusion injury-induced cell death by targeting celf2. Neuropsychiatr Dis Treat. 2018;14:2773–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Osuga H, Osuga S, Wang F, Fetni R, Hogan MJ, Slack RS, Hakim AM, Ikeda JE, Park DS. Cyclin-dependent kinases as a therapeutic target for stroke. Proc Natl Acad Sci U S A. 2000;97(18):10254–10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Global BMI Mortality Collaboration; Di Angelantonio E, Bhupathiraju ShN, Wormser D, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smadja D. Pharmacological revascularization of acute ischaemic stroke: Focus on challenges and novel strategies. CNS Drugs. 2012;26(4):309–318. [DOI] [PubMed] [Google Scholar]
- 27. Adlakha YK, Saini N. Brain micrornas and insights into biological functions and therapeutic potential of brain enriched mirna-128. Mol Cancer. 2014;13:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sempere LF, Freemantle S, Pitha-Rowe I, Moss E, Dmitrovsky E, Ambros V. Expression profiling of mammalian micrornas uncovers a subset of brain-expressed micrornas with possible roles in murine and human neuronal differentiation. Genome Biol. 2004;5(3):R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cernilogar FM, Di Giaimo R, Rehfeld F, Cappello S, Lie DC. RNA interference machinery-mediated gene regulation in mouse adult neural stem cells. BMC Neurosci. 2015;16:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee ST, Chu K, Im WS, Yoon HJ, Im JY, Park JE, Park KH, Jung KH, Lee SK, Kim M, Roh JK. Altered microRNA regulation in Huntington’s Disease models. Exp Neurol. 2011;227(1):172–179. [DOI] [PubMed] [Google Scholar]
- 31. Saba R, Goodman CD, Huzarewich RL, Robertson C, Booth SA. A miRNA signature of prion induced neurodegeneration. Plos One. 2008;3(11):e3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lukiw WJ. Micro-RNA speciation in fetal, adult and Alzheimer’s Disease hippocampus. Neuroreport. 2007;18(3):297–300. [DOI] [PubMed] [Google Scholar]
- 33. Guidi M, Muinos-Gimeno M, Kagerbauer B, Marti E, Estivill X, Espinosa- Parrilla Y. Overexpression of mir-128 specifically inhibits the truncated isoform of ntrk3 and upregulates bcl2 in sh-sy5y neuroblastoma cells. BMC Mol Biol. 2010;11:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Adlakha YK, Saini N. Microrna-128 downregulates bax and induces apoptosis in human embryonic kidney cells. Cell Mol Life Sci. 2011;68(8):1415–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Adlakha YK, Saini N. Mir-128 exerts pro-apoptotic effect in a p53 transcription-dependent and -independent manner via puma-bak axis. Cell Death Dis. 2013;4:e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iyirhiaro GO Zhang Y Estey C O’Hare MJ Safarpour F Parsanejad M Wang S Abdel-Messih E Callaghan SM During MJ Slack RS, Park DS. Regulation of ischemic neuronal death by e2f4-p130 protein complexes. J Biol Chem. 2014;289(26):18202–18213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rashidian J, Iyirhiaro G, Aleyasin H, Rios M, Vincent I, Callaghan S, Bland RJ, Slack RS, During MJ, Park DS. Multiple cyclin-dependent kinases signals are critical mediators of ischemia/hypoxic neuronal death in vitro and in vivo. Proc Natl Acad Sci U S A. 2005;102(39):14080–14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang F, Corbett D, Osuga H, Osuga S, Ikeda JE, Slack RS, Hogan MJ, Hakim AM, Park DS. Inhibition of cyclin-dependent kinases improves ca1 neuronal survival and behavioral performance after global ischemia in the rat. J Cereb Blood Flow Metab. 2002;22(2):171–182. [DOI] [PubMed] [Google Scholar]
- 39. Shan ZN, Tian R, Zhang M, Gui ZH, Wu J, Ding M, Zhou XF, He J. Mir128-1 inhibits the growth of glioblastoma multiforme and glioma stem-like cells via targeting bmi1 and e2f3. Oncotarget. 2016;7(48):78813–78826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dong Q, Cai N, Tao T, Zhang R, Yan W, Li R, Zhang J, Luo H, Shi Y, Luan W, Zhang Y, et al. An axis involving snai1, microrna-128 and sp1 modulates glioma progression. Plos One. 2014;9(6):e98651. [DOI] [PMC free article] [PubMed] [Google Scholar]