Abstract
Traumatic brain injury is one of the leading causes of mortality and morbidity worldwide. At present there is no effective treatment. Previous studies have demonstrated that topical application of adipose tissue-derived mesenchymal stem cells can improve functional recovery in experimental traumatic brain injury. In this study, we evaluated whether hypoxic preconditioned mesenchymal stem cells could enhance the recovery from traumatic brain injury. Traumatic brain injury was induced with an electromagnetically controlled cortical impact device. Two million mesenchymal stem cells derived from the adipose tissue of transgenic green fluorescent protein Sprague-Dawley rats were cultured under either hypoxic (2.5% O2 for 18 hours) (N = 30) or normoxic (18% O2) (N = 30) conditions, then topically applied to the exposed cerebral cortex within 1 hour after traumatic brain injury. A thin layer of fibrin was used to fix the cells in position. No treatment was given to the animals with traumatic brain injury (N = 30). Animals that underwent craniectomy without traumatic brain injury were treated as the sham group (N = 15). Neurological functions were evaluated with water maze, Roto-rod and gait analysis. Animals were sacrificed at days 3, 7, and 14 for microscopic examinations and real-time polymerase chain reaction analysis. The rats treated with hypoxic mesenchymal stem cells showed the greatest improvement in neurological function recovery. More green fluorescent protein-positive cells were found in the injured brain parenchyma treated with hypoxic mesenchymal stem cells that co-expressed glial fibrillary acidic protein, Nestin, and NeuN. Moreover, there was early astrocytosis triggered by the infiltration of more glial fibrillary acidic protein-positive cells and microgliosis was suppressed with fewer ionized calcium binding adapter molecule 1-positive cells in the penumbra region of hypoxic mesenchymal stem cells group at day 3. Compared with normoxic mesenchymal stem cells and traumatic brain injury only groups, there was significantly (p < 0.05) less neuronal death in both the hippocampus and penumbral regions in sections treated with hypoxic mesenchymal stem cells as determined by Cresyl violet and terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling staining respectively. The expression of pro-inflammatory genes (interleukin 6, interleukin 1a, interleukin 1b, tumor necrosis factor α) was upregulated and apoptotic gene (Caspase-3) expression was suppressed at day 3. Anti-inflammatory (interleukin 10) and anti-apoptotic (BCL2 associated agonist of cell death) gene expression was upregulated at days 7 and 14. Our study showed that a hypoxic precondition enhanced the beneficial effects of mesenchymal stem cells on neurological recovery after traumatic brain injury.
Keywords: Traumatic brain injury, mesenchymal stem cells, hypoxia, topical application
Introduction
Traumatic brain injury (TBI) is a major health and socioeconomic problem throughout the world. It affects people of all ages. Most people suffer from behavioral deficits or TBI-related disability for a long period of time after TBI1,2.
TBI is characterized by both neuronal and white-matter loss in the primary injury and brain atrophy in the secondary injury with functional neurological impairment (secondary injury)3. Direct mechanical impact causes the primary insult. The secondary injury involves a series of complex pathophysiological changes such as excitotoxicity, apoptosis, mitochondrial injury, cerebral edema, and inflammation. Current therapies are limited to supportive management including monitoring intracerebral pressure, maintaining cerebral perfusion, optimizing cerebral oxygenation, and fluid support, which focus on reducing the extent of secondary injury4.
Mesenchymal stem cells (MSCs) are multipotent, self-renewing cells that can differentiate into osteoblasts, adipocytes, and chondroblasts. It is likely that MSCs secrete a variety of paracrine factors, including interleukins, colony-stimulating factors, prostaglandins, and growth factors, which regulate interactions with the environment. In contrast to pharmacologic agents, which target only a single pathway, MSCs have the potential to accelerate tissue repair by multiple mechanisms. MSCs derived from adipose tissue have been shown to improve outcomes in the rat model of TBI5. However, poor survival rate, short survival time, and migration ability limit therapeutic efficacy after cell transplantation6. Different strategies including preconditioning of the cell product by heat shock, oxidative stress, or hypoxia have been investigated to solve the problem. Among them, a hypoxic precondition can enhance major MSC features including genetic stability, proliferation capacity, differentiation, and migration ability6,7. Our previous study has shown that topically applied MSCs can migrate from the surface of the cerebral cortex and home in on the injured parenchyma8.
In this study, we aimed to investigate whether a hypoxic precondition could enhance the beneficial effects of topical MSCs against experimental TBI.
Materials and Methods
Adipose-derived MSCs
Adipose-derived MSCs (ADMSCs) were harvested from the subcutaneous fat of male transgenic Sprague-Dawley (SD) rats (300–350 g) that expressed green fluorescent protein (GFP) (SDTg(CAG-EGFP) CZ-0040sb; SLC Inc, Shizuoka, Japan). The subcutaneous adipose tissue was washed extensively with sterile phosphate buffered saline (PBS) and then treated with 0.1% collagenase (type I; Sigma-Aldrich, HK) in PBS for 30 minutes at 37°C with gentle agitation. After passing through a 100-μm mesh filter to remove debris, the filtrate was washed three times and suspended in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mM L-glutamine. The cultures were maintained in an incubator with a humidified atmosphere of 5% CO2 8.
Hypoxic preconditioning
Before transplantation, ADMSCs were exposed to an atmosphere of 2.5% O2 and 5% CO2, achieved by replacing O2 with N2 in an O2- and CO2-controlled multi gas incubator while kept in full medium for 18 hours.
Cell phenotyping
ADMSCs at three to five passages were characterized by flow cytometry with a fluorescent-activated cell sorting argon laser (BD Biosciences, San Jose, CA, USA). Cells were washed, then centrifuged for 10 minutes at low speed, before 1x106 cells were fixed in 3% paraformaldehyde for 30 minutes at 4°C. The cells were labelled with phycoerythrin-conjugated antibodies against CD29, CD45, and CD90 (Abcam Inc., Cambridge, UK). Isotype-matched negative controls were used to assess background fluorescence. The data were analyzed by Cell-Quest software (Becton Dickson, HK)8.
Adipogenic, chondrogenic, and osteogenic differentiation potential
ADMSCs were seeded at 5000 cells/cm2 and cultured in adipogenic, chondrogenic, and osteogenic differentiation culture media according to the manufacturer’s protocols (Invitrogen, LifeTechnologiesTM, HK). The differentiated adipocytes were stained with Oil Red O, chondrocytes with Alcian Blue, and osteocytes with Alizarin Red S stain to identify intracytoplasmic lipid, extracellular glycosaminoglycans, and calcium deposits, respectively. All chemicals were purchased from Sigma-Aldrich, Shanghai, China8.
Animal experiment
The animals were randomly assigned to one of four experimental groups: sham, N = 15; TBI only, N = 30; normoxic MSCs, N = 30; or hypoxic MSCs, N = 30.
Experimental TBI
Adult female SD rats weighing 200–250 g were used for this experiment. All animals were anesthetized through intra-peritoneal injection with a solution of ketamine (50 mg/kg) and xylazine (10 mg/kg). During surgery, the body temperature was maintained at 37oC with a warm pad. A piece of skull bone (7 mm x 7 mm, 1.6 mm posterior to the lambda and 2.0 mm right to the midline) was removed with a dental microdrill. TBI was induced by driving the 3 mm diameter tip of an electromagnetically controlled cortical impact (CCI) device at a rate of 3.5 m/s with 2.5 mm of compression. Within 1 hour, 2 million hypoxic (N = 30) or normoxic (N = 30) MSCs were topically applied to the exposed cerebral cortex and a thin layer of fibrin glue (TISSEEL Baxter, Round Lake, IL, USA) was applied to keep the cells in place and the skin was closed with 3-0 silk sutures. The same procedures were performed in the control rats, but they did not receive MSC transplantation or fibrin (N = 30). In total, 15 animals were used for sham surgery and underwent craniectomy without TBI.
Functional evaluation
In all animals, neurological functions were tested within 14 days after TBI by a Morris water maze (Stoelting Co., Wood Dale, IL, USA), Roto-rod training assessment, and catwalk gait analysis (CatWalkTM XT from Noldus, Beijing, China). For each test, there were 10 animals in each of the TBI, normoxic MSC, and hypoxic MSC groups; five animals were assigned to the sham group.
The Morris water maze evaluates the spatial learning and memory. The circular pool was divided into four quarters: NE, SE, SW, and NW. An invisible platform hidden underwater (1.5–2 cm) was placed in NW. The swimming pool was filled the night before the test, so the water was at room temperature. Then 1 day after TBI, rats were placed in a large circular pool and allowed to find the location of an invisible platform using external cues starting from NE, SE, SW, and NW respectively. On the 11th day, the escape platform was removed, and probe test was performed. The activities of the rats were recorded by camera. Parameters including the average distance to platform, average travelled distance, and time used to reach platform were analyzed by SPSS. Animals were sacrificed at day 14 after TBI and the brain was harvested for further investigations.
Roto-rod training assessment was used to evaluate the balance ability of the rats. Then 3 days before TBI, rats were trained on an accelerating (10 to 30 rpm) Roto-rod with three trials each day. After TBI, assessment of balance ability was conducted at day 3, 7, and 14. Each assessment included five trials. Between each trial, the rats were allowed to rest for 30 minutes. With the highest and lowest values excluded, the latency to fall value (in seconds) was measured.
Catwalk gait analysis is an automated quantitative gait analysis system to detect speed-controlled gait deficits. Animals were placed in the runway and three runs with about four step cycles were analyzed by catwalk software. Foot intensity was recorded as parameter.
Microscopic examinations
Animals were sacrificed on day 3, day 7, and day 14 after surgery. Five high-magnification (400x) photos at the penumbral and hippocampus regions of each slide were taken for examination.
Cresyl violet stain was used to identify cell death. Hydrated sections were placed in Cresyl violet solution for 60 seconds. Subsequently, they were washed in tap water and checked under a microscope.
To localize GFP-MSCs, standard immunohistochemical staining was performed using anti-GFP antibody (Abcam, Cambridge, MA, USA) on hydrated paraffin sections. The co-expressions of glial fibrillary acidic protein (GFAP) (Abcam, Cambridge, UK), Nestin (Santa Cruz, Dallas, TX, USA), and NeuN (Millipore, HK) were detected by immunofluorescence staining.
Mouse monoclonal antibody against GFAP (Abcam, Cambridge, UK,) and ionized calcium binding adaptor molecule 1 (Iba1) (WAKO, Osaka, Japan) were used in histochemistry staining to evaluate neuroinflammation.
Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling assay
To analyze apoptosis, slides were stained by terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling using a detection kit (Roche, Basel, Switzerland) according to the manufacturer’s instructions. Positive cells were counted.
Real-time polymer chain reaction
Total RNA was isolated using TRIzol* Reagent (Invitrogen, LifeTechnologiesTM, HK). The polymer chain reaction (PCR) cycle was performed under specific conditions, 95°C for 10 minutes followed by 40 cycles of 95°C for 15 seconds and 60°C for 60 seconds. The threshold cycle (Ct) of each target gene was automatically defined and normalized to the control glyceraldehyde 3-phosphate dehydrogenase (ΔCt value). The relative differences in the expression levels of each mRNA in ΔΔCt were calculated and presented as fold induction (2−ΔΔ Ct). Real-time PCR primers are listed in Table 1.
Table 1.
List of primers used in real-time PCR.
| Gene | |
|---|---|
| GAPDH | forward AGA CAG CCG CAT CTT CTT GT |
| reverse CTT GCC GTG GGT AGA GTC AT | |
| IL-6 | forward TCT CTC CGC AAG AGA CTT CCA |
| reverse ATA CTG GTC TGT TGT GGG TGG | |
| IL-1a | forward AAG ACA AGC CTG TGT TGC TGA AGG |
| reverse TCC CAG AAG AAA ATG AGG TCG GTC | |
| TNF-α | forward ACC ACG CTC TTC TGT CTA CTG |
| reverse CTT GGT GGT TTG CTA CGA C | |
| IL-1b | forward GCA ATG GTC GGG ACA TAG TT |
| reverse AGA CCT GAC TTG GCA GAG GA | |
| IL-10 | forward TGC CTT CAG TCA AGT GAA GAC |
| reverse AAA CTC ATT CAT GGC CTT GTA | |
| Capase-3 | forward TTC ATT ATT CAG GCC TGC CGA GG |
| reverse TTC TGA CAG GCC ATG TCA TCC TCA | |
| BAD | forward GGA GCA TCG TTC AGC AGC AG |
| reverse CCA TCC CTT CAT CTT CCT CAG TC |
BAD: BCL2 associated agonist of cell death; IL: interleukin; GADPH: glyceraldehyde 3-phosphate dehydrogenase; PCR: polymer chain reaction; TNF: tumor necrosis factor.
Statistical analysis
All data are expressed in mean ± standard error of mean. Statistical analysis was performed by IBM (Armonk, NY, USA) SPSS 21.0 software and underwent either one- or two-way analysis of variance (ANOVA). A p value less than 0.05 was reported as significant. Tukey’s honestly significant difference (HSD) test was applied as post hoc comparison.
Results
Isolation and characterization of MSCs
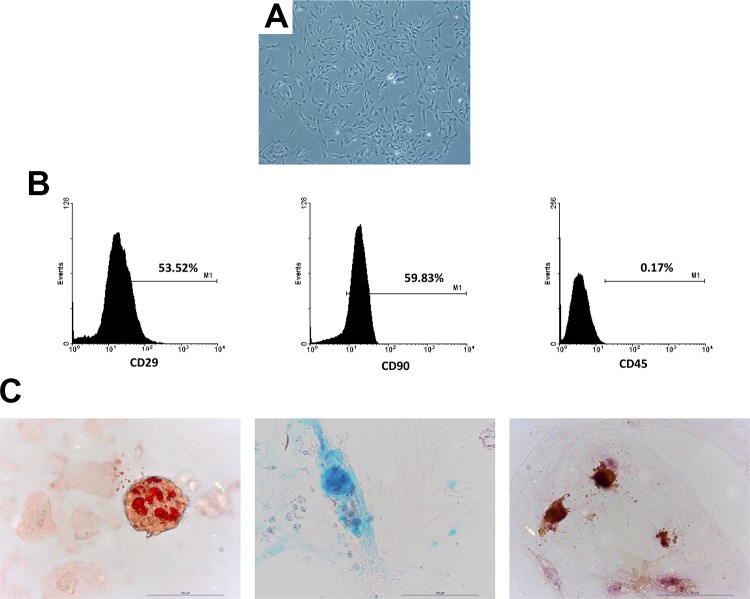
MSCs showed spindle-shaped morphology in the culture dishes (Figure 1(a)). The flow cytometry analysis indicated MSCs expressed CD29 and CD90 but not CD45 (Figure 1(b)). Under specific conditions, they differentiated into adipocytes, chondroblasts, and osteoblasts (Figure 1(c)).
Figure 1.
Mesenchymal stem cell (MSC) morphology in culture and characterization. Picture of green fluorescent protein (GFP) MSCs showed a spindle shape in culture (a). Flow cytometry analysis using anti-CD29, anti-CD45, anti-CD90 antibody for both normoxic and hypoxic MSCs (b). Potential of differentiation into adipocytes, chondroblasts, and osteoblasts was confirmed by culture under specific media (c).
Behavioral tests
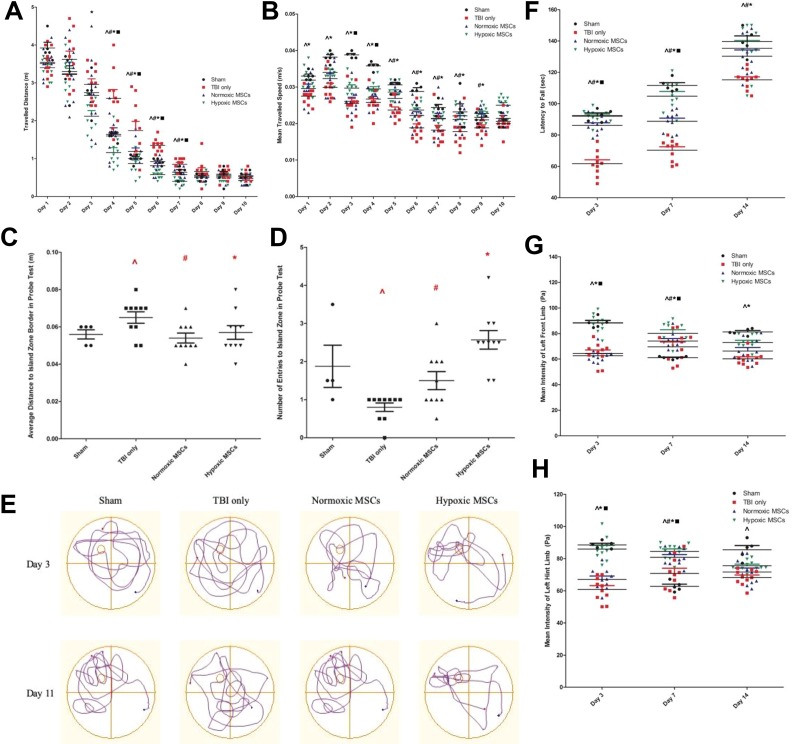
In the Morris water maze, both normoxic and hypoxic MSCs-treated rats showed a better functional outcome. They took a shorter time to find the location of platform (2 days for normoxic and hypoxic MSCs treated rats, 3 days for control rats). From day 3 to day 7, animals treated with hypoxic MSCs swam for the shortest distance to reach the platform (p < 0.05). The therapeutic effects of normoxic MSCs were shown from day 4 (p < 0.05). Compared with normoxic MSC treatment, hypoxic MSC administration further shortened the swimming distance (p < 0.05). After 7 days, there was no remarkable difference among the four groups (Figure 2(a)). The swimming speed was apparently affected from the first day after TBI (p < 0.05). Within 9 days, rats treated with hypoxic MSCs swam at the highest speed among the four groups (p < 0.05). From day 5 to day 9, the improvement in speed was shown in the normoxic MSC group (p < 0.05) (Figure 2(b)). In the probe test at day 11, both normoxic and hypoxic MSC-treated rats stayed closer to the island zone (Figure 2(c)), and they entered the island zone more frequently (Figure 2(d)). Track plots showed they stayed close in the island zone (Figure 2(e)).
Figure 2.
Results of behavioral tests. For each test, there were 10 animals in traumatic brain injury (TBI) only group, normoxic mesenchymal stem cell (MSC) group and hypoxic MSC group respectively; five animals were assigned to the sham group. (a)-(e) Results of water maze. (a) Distance travelled for rats before they reached the platform. From day 3 to day 7, rats treated with hypoxic MSCs swam for the shortest distance before they reached the platform (day 3, *p = 0.005 vs TBI only; day 4, *p < 0.001 vs TBI only, ▪p = 0.050 vs normoxic MSCs; day 5, *p = 0.0003 vs TBI only, ▪p = 0.045 vs normoxic MSCs; day 6, *p < 0.001 vs TBI only, ▪p = 0.050 vs normoxic MSCs; day 7, *p < 0.001 vs TBI only, ▪p = 0.039 vs normoxic MSCs). From day 4 to day 7, rats treated with normoxic MSCs showed improvement compared with control animals (day 4, #p = 0.012 vs TBI only; day 5, #p = 0.010 vs TBI only; day 6, #p = 0.001 vs TBI only; day 7, #p = 0.016 vs TBI only). There was significant memory deficit after surgery in the control group from day 4 to day 7 (day 4, ^ p = 0.008 vs sham group; day 5, ^p = 0.00 vs sham group; day 6, ^p = 0.050 vs sham group; day 7, ^p = 0.039 vs sham group). Difference was not remarkable after day 8. (b) Mean swimming speed. From day 1 to day 8, rats in the control group was significantly affected in swimming speed (day 1, ^p = 0.002 vs sham group; day 2, ^p < 0.001 vs sham group; day 3, ^p < 0.001 vs sham group; day 4, ^p = 0.0001 vs sham group; day 5, ^p = 0.008 vs sham group; day 6, ^p < 0.001 vs sham group; day 7, ^p = 0.003 vs sham group; day 8, ^p = 0.017 vs sham group). Rats in the hypoxic MSC group swam faster from day 1 to day 9 and they were shown to swim at the highest speed at day 3 and day 4 (day 1, *p = 0.006 vs TBI only; day 2, *p = 0.040 vs TBI only; day 3, *p = 0.023 vs TBI only, ▪p = 0.033 vs normoxic MSCs; day 4, *p = 0.027 vs TBI only, ▪p = 0.050 vs normoxic MSCs; day 5, *p = 0.002 vs TBI only; day 6, *p = 0.011 vs TBI only; day 7, *p = 0.039 vs TBI only; day 8, *p = 0.038 vs TBI only; day 9, *p = 0.020 vs TBI only). The swimming speed of rats in the normoxic MSC group showed improvement from day 5 to day 9 (day 5, # p = 0.040 vs TBI only; day 6, #p = 0.050 vs TBI only; day 7, #p = 0.037 vs TBI only; day 8, #p = 0.042 vs TBI only; day 9, #p = 0.046 vs TBI only). (c) Average distance to island zone in probe test. The memory of rats in control group was impaired (^p = 0.049 vs sham group). Both normoxic (#p = 0.041 vs TBI only) and hypoxic MSC (*p = 0.032 vs TBI only) treated rats stayed closer to the island zone. No difference was shown between two treatments. (d) Number of entries to the island zone in the probe test. Rats in control group entered the island zone fewer times than the sham group (^p = 0.05). Both normoxic (#p = 0.050 vs TBI only) and hypoxic MSC (#p = 0.003 vs TBI only) treated rats went through the island zone more frequently. No remarkable difference was shown between two treatments. (e) Schematic pictures of learning phase (day 3) and probe test showed animals in the hypoxic group had the greatest memory improvement. (f) Balance ability tested by Roto-rod training assessment. After the surgery, the balance ability of control animals was significantly affected (day 3, ^p < 0.001 vs sham group; day 7, ^p < 0.001 vs sham group; day 14, ^p = 0.001 vs sham group). Both normoxic and hypoxic MSC treatments improved balance ability (day 3, #p < 0.001 vs TBI only, *p < 0.001 vs TBI only; day 7, #p < 0.001 vs TBI only, *p < 0.001 vs TBI only; day 14, #p = 0.019 vs TBI only, *p = 0.011 vs TBI only). At day 3 and day 7, rats with hypoxic MSC treatment stayed on the rod for the longest time (day 3, ▪p = 0.042 vs normoxic MSCs; day 7, ▪p = 0.005 vs normoxic MSCs). (g) and (h) Results of catwalk gait analysis. (g) Foot intensity of left front limb. The power of the left front limb was significantly impaired after TBI (day 3, ^p < 0.001 vs sham group; day 7, ^p = 0.050 vs sham group; day 14, ^p < 0.001 vs sham group). Hypoxic MSC-treated rats indicated higher foot intensity (day 3, *p < 0.001 vs TBI only, ▪p < 0.001 vs normoxic MSCs; day 7, *p = 0.002 vs TBI only, ▪p = 0.042 vs normoxic MSCs; day 14, *p = 0.015 vs TBI only), rats in the normoxic MSC group showed improvement at day 7 only (#p = 0.043 vs TBI only). (h) Foot intensity of left hind limb. The hind limb power was affected remarkably after TBI (day 3, ^p < 0.001 vs sham group; day 7, ^p = 0.049 vs sham group; day 14, ^p < 0.001 vs sham group). Rats that received hypoxic MSCs showed the greatest foot intensity at day 3 and day 7 (day 3, *p < 0.001 vs TBI only, ▪p < 0.001 vs normoxic MSCs; day 7, *p < 0.001 vs TBI only, ▪p = 0.030 vs normoxic MSCs). Rats in the normoxic MSC group showed improvement at day 7 only (#p = 0.026 vs TBI only).
In the Roto-rod assessment, rats in both the normoxic and hypoxic MSC groups stayed on the rod for a longer time (p < 0.05). At day 3 and day 7, rats that received hypoxic MSCs retained the best balance ability among the four groups (p < 0.05). The difference between the normoxic and hypoxic MSC groups was not significant at day 14 (Figure 2(f)).
In gait analysis, the foot intensity of the left front limb was significantly greater in the hypoxic MSC group than the normoxic MSC group and the control group when running across the walkway (p < 0.05). There was no significant difference between normoxic MSCs and control group at day 14 (Figure 2(g)). The intensity of the left hind limb was highest in rats from the hypoxic MSC group at day 3 and day 7 (p < 0.05). There was no remarkable difference between the treatment and control groups at day 14 (Figure 2(h)).
Microscopic examination
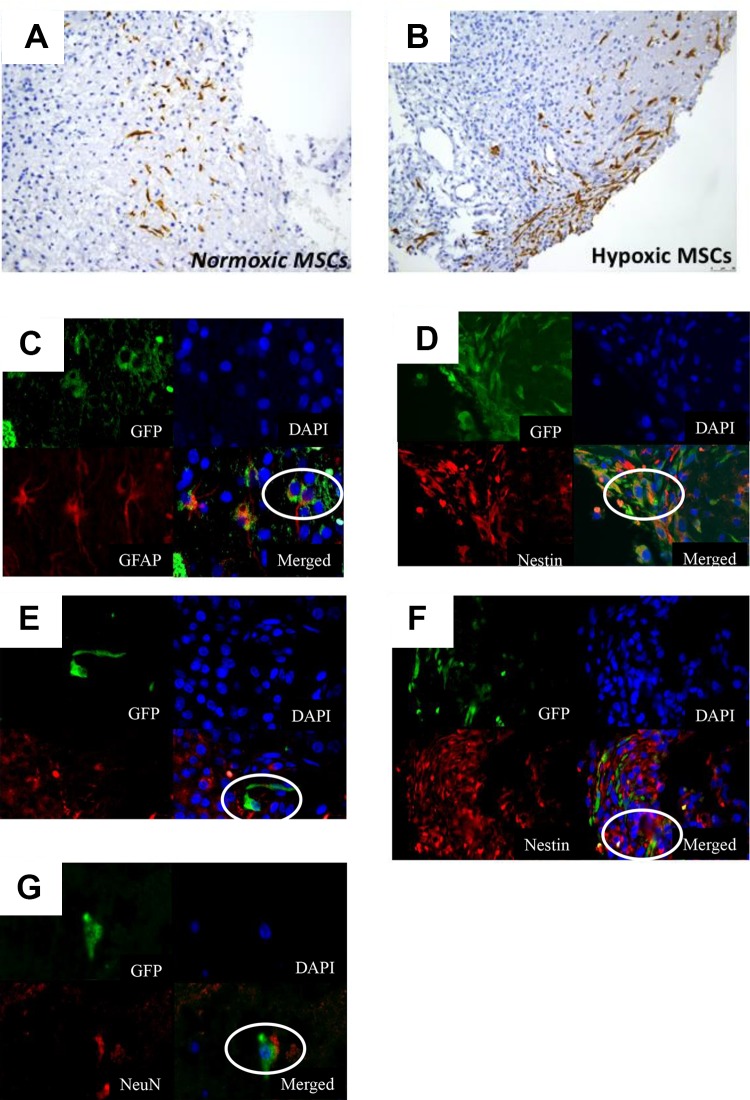
At day 7, more GFP+ve cells were found in the penumbral region of the injury in Group A (Figure 3(a)). The normoxic MSCs co-expressed GFAP (a marker of astrocytes) and Nestin (marker of neural progenitor cells) (Figure 3(b) and 3(c)), hypoxic MSCs co-expressed GFAP, Nestin, and NeuN (a marker of neurons) in immunofluorescent staining (Figure 3(d), 3(e), 3(f)). No GFP-positive cells were detected after day 14.
Figure 3.
Normoxic/hypoxic mesenchymal stem cell (MSC) homing and in vivo differentiation ability. More green fluorescent protein (GFP)+ve cells were found in the penumbral region of traumatic brain injury (TBI) treated with hypoxic cells 7 days after transplantation (a). The homed MSCs co-expressed markers of astrocytes and neurons (b)-(d).
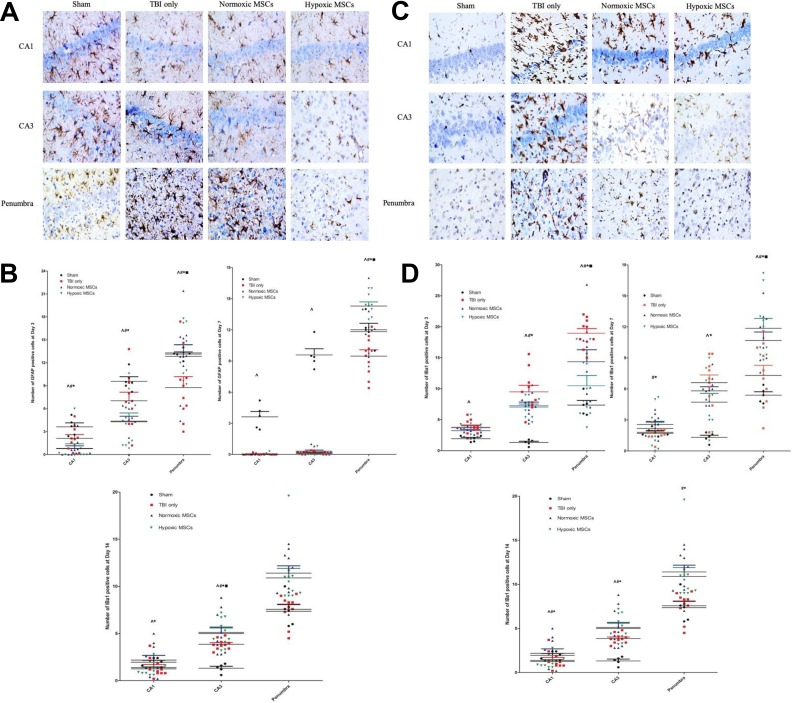
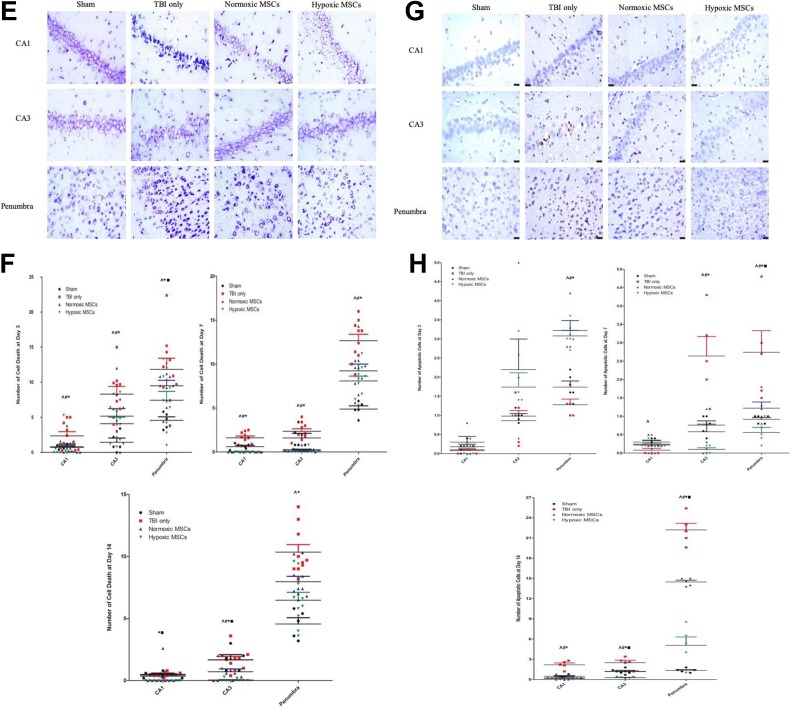
Early reactive astrocytosis was triggered in both the penumbra and hippocampus with more GFAP-positive cells (Figure 4(a) and 4(b)). Microgliosis was suppressed. There were fewer Iba1-positive cells (Figure 4(c),4(d)) in the hypoxic MSC group at day 3. At days 3, 7, and 14, Cresyl violet staining (Figure 4(e) and 4(f)) showed remarkably less neuron death in both the hippocampus and penumbra in the hypoxic MSC group. Fewer apoptotic cells were found at day 7 and day 14 in both the hippocampus and penumbral region in the hypoxic MSC-treated group (Figure 4(g) and 4(h)).
Figure 4.
Photos of histochemical staining and quantity results at different time points after treatment in the hippocampus and penumbral region. (a) and (b) At day 3, more glial fibrillary acidic protein (GFAP)+ve cells were observed in both the hippocampus and penumbral region in the hypoxic mesenchymal stem cell (MSC) group (CA1: *p = 0.036 vs traumatic brain injury (TBI) only, #p = 0.018 vs TBI only, ^p = 0.028 vs sham group; CA3: *p = 0.050 vs TBI only, #p = 0.047 vs TBI only, ^p = 0.049 vs sham group; penumbra: *p = 0.008 vs TBI only, #p = 0.050 vs TBI only, ▪p = 0.050 vs normoxic MSCs, ^p = 0.031 vs sham group). At day 7, the increased number of GFAP-positive cells was only found in penumbra (*p < 0.001 vs TBI only, #p = 0.033 vs TBI only, ▪p = 0.045 vs normoxic MSC, ^p = 0.037 vs sham). More GFAP+ve cells were found in the sham group in hippocampus (CA1: ^p < 0.001; CA3: ^p < 0.001). At day 14, less astrocytes infiltration was found in the hippocampus in hypoxic MSCs group (CA1: *p = 0.038 vs TBI only; CA3: *p < 0.001 vs TBI only, ▪p < 0.001 vs normoxic MSCs, #p = 0.041 vs TBI only). (c)-(d) At day 3, more microglia were activated after TBI in both hippocampus and penumbra (CA1: ^p < 0.001 vs sham group; CA3: ^p < 0.001 vs sham group; penumbra, ^p < 0.001 vs sham group). Fewer ionized calcium binding adapter molecule (Iba)+ve cells were found in the hypoxic MSC group in CA3 and penumbra (CA3: *p = 0.035 vs TBI only; penumbra: *p < 0.001 vs TBI only, ▪p = 0.002 vs normoxic MSCs). Normoxic MSC treatment also reduced the number of Iba+ve cells at CA3 and penumbra (CA3: #p = 0.034; penumbra, #p = 0.036). At day 7, fewer microglia were found in the hypoxic MSC group in both the hippocampus and penumbra (CA1: *p = 0.036 vs TBI only; CA3: *p = 0.040 vs TBI only; penumbra: *p < 0.001 vs TBI only, ▪p = 0.035 vs normoxic MSCs). Normoxic MSC treatment reduced microglia activation at CA1 and penumbra significantly (CA1: #p = 0.011; penumbra: #p = 0.037). Both normoxic and hypoxic MSCs reduced the number of microglia at day 14, no significant difference was found between the two treatments (CA1: *p = 0.043, #p = 0.010; CA3: *p = 0.029, #p = 0.043; penumbra: *p < 0.001, #p < 0.001). (e)-(f) Number of cell death increased significantly after TBI from day 3 to day 14 in both the hippocampus and penumbra (^p < 0.05 vs sham). At day 3, cell death was reduced remarkably in the hypoxic MSC group in both the hippocampus and penumbra (CA1: *p = 0.014 vs TBI only; CA3: *p = 0.005 vs TBI only; penumbra: *p = 0.013 vs TBI only, ▪p = 0.022 vs normoxic MSCs). Normoxic MSCs reduced cell death at the hippocampus only (CA1: #p = 0.024; CA3: #p = 0.038). At day 7, both normoxic
Real-time PCR
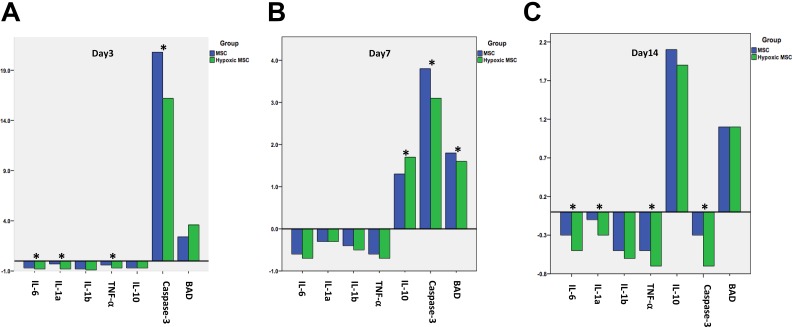
The expression of genes associated with inflammation and apoptosis was analyzed at days 3, 7, and 14. The expression of anti-inflammatory (interleukin (IL)-10) and pro-inflammatory (IL-6, IL-1a, IL-1b, tumor necrosis factor (TNF)-α) genes was significantly downregulated at day 3 in the hypoxic MSC group (p < 0.05 vs normoxic MSCs) (Figure 5(a)). At day 7, the expression of pro-apoptotic gene (Caspase-3) was downregulated and anti-inflammatory gene (IL-10) was upregulated remarkably in the hypoxic MSC group (p < 0.05 vs the normoxic MSCs group) (Figure 5(b)). At day 14, the expression of Caspase-3 was downregulated with upregulation of anti-apoptotic gene (BCL2 associated agonist of cell death (BAD)) in the hypoxic MSC group (p < 0.05 vs normoxic MSC group) (Figure 5(c)).
Figure 4.
(Continued). and hypoxic MSCs reduced cell death, no significant difference was observed between the two treatments (CA1: *p < 0.001, #p < 0.001; CA3: *p < 0.001, #p < 0.001; penumbra: *p = 0.002, #p = 0.017). At day 14, the hypoxic MSC group showed less cell death in both the hippocampus and penumbra (CA1: *p = 0.004 vs TBI only, ▪p = 0.038 vs normoxic MSCs; CA3: *p < 0.001 vs TBI only, ▪p < 0.001 vs normoxic MSCs; penumbra: *p = 0.036 vs TBI only). (g)-(h) At day 7 and day 14, normoxic and hypoxic MSCs reduced the number of apoptotic cells significantly in the hippocampus and penumbra (day 7: CA3, *p < 0.001, #p = 0.003, penumbra, *p = 0.005, #p = 0.034; day 14: CA1, *p < 0.001, #p = 0.001, CA3, *p < 0.001, #p = 0.023 penumbra, *p < 0.001, #p = 0.009). Hypoxic MSCs further reduced number of apoptotic cells at day 7 and 14 in the penumbra (day 7: ▪p = 0.042 vs normoxic MSCs; day 14: ▪p < 0.001 vs normoxic MSCs).
Figure 5.
Inflammatory and apoptotic gene expression changes after transplantation. Then 3 days after transplantation, both anti-(interleukin (IL)-1, IL-6, tumor necrosis factor (TNF)-a) and pro-inflammatory (IL-10) gene expression were downregulated (*p < 0.05 vs normoxic), the pro-apoptotic gene (Caspase-3 was upregulated remarkably, but the upregulation in hypoxic group was less (*p < 0.05 vs normoxic). From day 7 to day 14, the expression of anti-inflammatory (IL-10) and anti-apoptotic genes increased significantly, with downregulation of the pro-apoptotic gene (Capase-3) (*p < 0.05 vs normoxic).
Discussion
The US Centers for Disease Control defines TBI as an alteration in brain function because of a bump, blow, jolt to the head or penetrating head injury8. At present there is no effective treatment regime and survivors are left with debilitating long-term motor, cognitive, and behavioral deficits. TBI has become a major public health concern. The CCI model of brain injury is the most commonly used model of TBI in neuro-trauma research. It causes both cortical and hippocampal damage resulting in motor and cognitive deficits in rodents8.
MSCs have real appeal for tissue engineering and therapeutic applications due to their multipotent differentiation ability, trophic activity, and immunomodulation. There are different routes to delivering MSCs, including intravenous/intra-arterial infusion or local injection. Previous studies have shown that intravenous MSC therapy can improve functional recovery after TBI9. Meanwhile, intravenous infusion is adopted more in clinical trials transplanting a large number of MSCs. However, the majority of cells are trapped in the pulmonary microvasculature, which may cause an embolism. Due to the blood-brain barrier, less than 0.001% of infused cells reach the brain tissue after transplantation10. The risk of arterial embolism and occlusion limits intra-arterial infusion of MSCs. Local injection of MSCs using a Hamilton syringe needle may cause severe bleeding. Compared with systemic infusion, topical application can provide efficient delivery of MSCs to the injured brain. The beneficial effects of topical MSCs on functional recovery post TBI has been demonstrated in our previous study.11
In this study, hypoxic MSCs were found to improve the motor function and hippocampus-mediated visual-spatial memory after TBI. In a Morris water maze, animals showed a shorter learning time and stronger ability to position an invisible target. The lack of significance in the probe test between hypoxic and normoxic MSCs might be due to recovery from TBI in both groups after 14 days.
After MSCs were cultured in a hypoxic environment, the capacity of self-renewal was enhanced with a higher proliferative rate, whereas multilineage differentiation potential was maintained. Hypoxia induces different responses in MSCs by transcription factor hypoxia-inducible factor-1 activation12. The activation of this molecule plays an important role in the growth, multiplication, differentiation, and gene expression profile of MSCs in their niche by a complex of signals. Hypoxia cultivation provides a better microenvironment that is similar to that of bone marrow for MSC proliferation and differentiation. Homing to a target organ is a key factor in MSC therapy. The migration ability was shown to be stronger after exposure to a hypoxic condition due to the upregulation of chemokine receptors C-X-C chemokine receptor type 4 (CXCR4), CXCR7, and CX3C chemokine receptor 113. They play an important role in damaged-tissue-specific trafficking and homing of MSCs.
Previous studies have shown that MSCs modulate the inflammatory response including reactive astrocytosis and migration of microglia14. After injury, astrocytes are activated. They home to the damaged area and protect healthy brain tissue from inflammation. In acute phase after TBI, topical MSC transplantation induced more reactive astrocytes in both the hippocampus and penumbra. However, these reactive astrocytes were properly managed as the number of astrocytes decreased in the subacute/late stage. After TBI, fewer microglia migrated to CA1/CA3 in animals treated with topical MSCs. In the penumbral region, more microglia were detected in the subacute/late stage due to different phenotypes of microglia. M1 and M2 are two subtypes of microglia and have opposite effects on inflammation. The increase in cell numbers could be an anti-inflammatory response mediated by M2 phenotype microglia15.
The inflammatory response was suppressed by downregulation of pro-inflammatory cytokines and upregulation of anti-inflammatory cytokines. Pro-inflammatory cytokines such as IL-1, IL-6, and TNF-α were downregulated after topical MSC. TNF-α is one of the key activators of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) signaling pathway, which regulates a large number of genes associated with inflammation and apoptosis16. Its downregulation suppresses the NF-kB pathway after TBI. The anti-inflammatory cytokine IL-10 increased in the subacute/late stage because it is mainly produced by M2 phenotype microglia. Less neuron death and apoptosis were found after topical MSC treatment. The expression of pro-apoptosis gene Caspase-3 decreased in the late stage, whereas anti-apoptosis gene BAD increased in the early phase. However, the reason why more apoptotic cells appeared in the acute phase remained unclear.
The underlying mechanisms of MSC therapy remain to be investigated. There are two main principles by which cells facilitate therapeutic potential: cell differentiation and paracrine mechanism17,18. Because limited evidence for cell differentiation has been observed, the paracrine mechanism seems ultimately responsible for the repair of injured tissues. Therefore, the therapeutic potential of the conditioned medium (cell supernatant) should be investigated in our future research. The literature has pointed out that conditioned medium is able to captivate the benefits of stem-cell therapy. For example, the conditioned medium, particularly from genetically modified MSCs overexpressing Akt-1, demonstrated the ability of cardiomyocyte protection19. The angiogenic and antiangiogenic factors in the conditioned medium was effective in treating cardiovascular disease20.
However, there are some limitations in the study. Firstly, it was unable to track the cells’ migration routine and destination. Double florescent staining only showed the cell location at the studying time point. Secondly, different doses of transplanted cells were not tested. We selected the dose according to our previous study. Thirdly, multiple transplantation was not studied because the severity of the CCI model was limited and one-time transplantation was enough for functional recovery. Fourthly, we did not include the topically applied fibrin glue group because no functional improvement was found in our preliminary study. Fibrin was used as an adhesive agent to keep the cells in position.
Conclusion
Topical application is safe and efficient for delivering a large number of MSCs. Hypoxic preconditions can enhance the potential of MSCs in neuroprotection through suppressing inflammation and reducing neuron death.
Footnotes
Author Contributions: HM performed the experiments and wrote the manuscript. CSWT and KKYL helped with the immunohistochemistry staining. PKL, GW and WSP supervised the study and provided language help and writing assistance. All authors reviewed and commented on the manuscript. All authors approved the final version of the manuscript.
Ethical Approval: All procedures were conducted in accordance with the Animals (Control of Experiments) Ordinance Chapter 340, Department of Health, Hong Kong. The study was approved by the Animal Experimentation Ethnics Committee of the Chinese University of Hong Kong.
Statement of Human and Animal Rights: All procedures were conducted in accordance with the Animal Experimentation Ethnics Committee of the Chinese University of Hong Kong approved protocols.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Roozenbeek B, Maas AI, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol. 2013;9(4):231–236. [DOI] [PubMed] [Google Scholar]
- 2. Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic brain injury–related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Surveill Summ. 2017;66(9):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Greve MW, Zink BJ. Pathophysiology of traumatic brain injury. Mt Sinai J Med. 2009;76(2):97–104. [DOI] [PubMed] [Google Scholar]
- 4. Frattalone AR, Ling GS. Moderate and severe traumatic brain injury: pathophysiology and management. Neurosurg Clin N Am. 2013;24(3):309–319. [DOI] [PubMed] [Google Scholar]
- 5. Mastro-Martínez I, Pérez-Suárez E, Melen G, González-Murillo Á, Casco F, Lozano-Carbonero N, Gutiérrez-Fernández M, Díez-Tejedor E, Casado-Flores J, Ramírez-Orellana M, Serrano-González A. Effects of local administration of allogenic adipose tissue-derived mesenchymal stem cells on functional recovery in experimental traumatic brain injury. Brain Inj. 2015;29(12):1497–1510. [DOI] [PubMed] [Google Scholar]
- 6. Paquet J, Deschepper M, Moya A, Logeart-Avramoglou D, Boisson-Vidal C, Petite H. Oxygen tension regulates human mesenchymal stem cell paracrine functions. Stem Cells Transl Med. 2015;4(7):809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fotia C, Massa A, Boriani F, Baldini N, Granchi D. Hypoxia enhances proliferation and stemness of human adipose-derived mesenchymal stem cells. Cytotechnology. 2015;67(6):1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Revi Neurosci. 2013;14(2):128–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mahmood A, Lu D, Chopp M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J Neurotrauma. 2004;21(1):33–39. [DOI] [PubMed] [Google Scholar]
- 10. Harting MT, Jimenez F, Xue H. Intravenous mesenchymal stem cell therapy for traumatic brain injury. J Neurosurg. 2009;110(6):1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lam PK, Lo AWI, Wang KKW, Lau HCH, Leung KKC, Li KTC, Lai PBS, Poon WS. Transplantation of mesenchymal stem cells to the brain by topical application in an experimental traumatic brain injury model. J Clin Neurosci. 2013;20(2):306–309. [DOI] [PubMed] [Google Scholar]
- 12. Ejtehadifar M, Shamsasenjan K, Movassaghpour A. The effect of hypoxia on mesenchymal stem cell biology. Adv Pharm Bull. 2015;5(2):141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rasmussen JG, Frøbert O, Pilgaard L. Prolonged hypoxic culture and trypsinization increase the pro-angiogenic potential of human adipose tissue-derived stem cells. Cytotherapy. 2011;13(3):318–328. [DOI] [PubMed] [Google Scholar]
- 14. Zhang R, Liu Y, Yan K, Chen L, Chen XR, Li P, Chen FF, Jiang XD. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J Neuroinflammation. 2013;10:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cherry JD, Olschowka JA, O’Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation. 2014;11:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O’Neill LAJ, Kaltschmidt C. NF-kB: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997;20(6):252–258. [DOI] [PubMed] [Google Scholar]
- 17. Kawada H, Fujita J, Kinjo K, Matsuzaki Y, Tsuma M, Miyatake H, Muguruma Y, Tsuboi K, Itabashi Y, Ikeda Y, Ogawa S. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood. 2004;104(12):3581–3587. [DOI] [PubMed] [Google Scholar]
- 18. Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N, Yarmush ML. Mesenchymal stem cells: mechanisms of immunomodulation and homing. Cell Transplant. 2010;19(6–1):667–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103(11):1204–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109(10):1292–1298. [DOI] [PubMed] [Google Scholar]