Abstract
Human CD133+ stem cells were injected into the bone marrow cavity of NOG (NOD Shi-SCID IL2Rγcnull) mice with or without preconditioning of busulfan in order to assess the efficiency of human CD133+ cells engraftment. Peripheral blood from CD133+-engrafted NOG mice was analyzed by flow cytometry. The results showed that human CD19+ B lymphocytes could be detected at 4 weeks post-transplantation, and human CD4+, CD8+ subsets of T lymphocytes, CD19– CD14– HLA-DR+ DCs and CD19– CD14+ monocytes could be detected at 16 weeks post-transplantation. The survival rate of mice in busulfan-untreated group (100%) was slightly higher than that in the busulfan-pretreated group (83%) (P > 0.05). However, the differentiation efficiency of CD133+ stem cells in busulfan-pretreated group was significantly higher than that in the untreated group (P < 0.05). This data imply that CD133+ cells could be a good resource for a humanized mouse model, and the preconditioning of busulfan could be more conducive to accelerating the differentiation of human CD133+ cells in NOG mice by intra-bone marrow injection.
Keywords: CD133+ stem cells, hematopoietic differentiation, busulfan, humanized mouse model
Introduction
Humanized mouse models with human hematopoietic and lymphoid systems are regarded as novel approaches to study the basic immunological mechanisms underlying clinical diseases, such as infectious diseases, autoimmune diseases, and cancer1–4. Such models can be established by transplanting human hematopoietic stem/progenitor cells (HSCs) into immunodeficient mice. However, the efficacy of HSCs engraftment can be affected by various factors, such as immunodeficient mouse strains, route of HSCs injection, HSCs, and irradiation sources.
A popular method for the establishment of a humanized mouse model is to inject human cord blood-derived CD34+ cells intravenously into newborn immunodeficient mice with preconditioning radiation5–8. However, it was reported that CD133+ HSCs separated from cord blood performed better than CD34+ in quantity and quality of differentiation after engraftment. Moreover, it was also suggested that intra-bone marrow injection (IBMI) provides a more efficient engraftment of HSCs9–11. As for mouse strains, NOG (NOD Shi-SCID IL2Rγcnull) is accepted worldwide to be the best recipient for HSCs, showing a higher ratio of human cell differentiation after transplantation1,12. However, radiosensitive NOG mice and the requirement for special equipment and professionals for irradiation increase the costs of successfully achieving humanized mice. Therefore, in present study, busulfan was used to replace the preconditioning irradiation in NOG mice.
Busulfan is commonly used as a preconditioning drug in clinical transplantation of HSCs, and is also a traditional therapeutic drug for chronic myeloid leukemia. The side effects of busulfan included bone marrow suppression, pulmonary fibrosis, and skin pigmentation. Research has shown that preconditioning using busulfan is more effective than irradiation in the case of human B cell differentiation13,14. In this study, we evaluated the effects on the establishment of humanized mouse model of intra-bone marrow injection of human CD133+ stem cells into NOG mice after preconditioning with busulfan.
Materials and Methods
Experimental Animals
Female NOG mice (4–5 weeks old) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd, (Beijing, China). All mice were placed in a sterile isolator for a week to acclimate, and all experiments were conducted under aseptic conditions. The animal experiments were performed in accordance with Chinese Regulations for the administration of experimental animals, and were approved by the local authorities and Ethics Committee of Xinxiang Medical University.
Reagents and Antibodies
Busulfan (B2635, Sigma-Aldrich, St. Louis, MO, USA) was dissolved in dimethyl sulfoxide (DMSO), stored at a concentration of 30g/L, and diluted to a working solution using 0.9% physiological saline just before use. A CD133+ magnetic beads isolation kit, anti-human CD133-FITC antibody, and MACS sorting system were provided by Miltenyi Biotec (Bergisch Gladbach, Germany). Ficoll lymphocyte separation solution was from Tian Jin Hao Yang Biological Manufacture (Tianjin, China). Anti-human CD45-FITC, CD19-PE-Cyanine5.5, CD8-APC-eFluor®780, anti-mouse CD45.1-Biotin antibody, and Red cell lysis buffer were from Thermo Fisher Scientific (Waltham, MA, USA). Anti-human CD3-BV421 antibody was from Biolegend (San Diego, CA, USA). Anti-human CD4-PE-Cy7, CD56-PE, CD11c-PE-Cy7, CD16-APC, CD3-APC-H7, CD19-APC-H7, CD14-V450, HLA-DR-V500, and Streptavidin-BV605 antibody were from BD Biosciences (San Jose, CA, USA).
Purification of CD133+ HSCs
Mononuclear cells were first isolated from umbilical cord blood by ficoll-density gradient centrifugation after written informed consent was obtained from healthy women donors according to the declaration of Helsinki. CD133+ hematopoietic stem cells were isolated by using the MACS magnetic separation column according to the Manufacturer’s manual with the CD133+ MicroBead Kit. The purity of CD133+ hematopoietic stem cells was detected by BD FACS Caliber flow cytometry after staining the cells with FITC labelled anti-human CD133 antibody. All procedures involving human samples were approved by the Ethics Committee.
Intra Bone Marrow Injection of CD133+ HSCs
Busulfan was administered to 6- to 7-week-old NOG mice intraperitoneally at dosage of 30mg/kg, 24 h before intra-bone marrow injection. After anaesthetizing mice with 0.2% pentobarbital sodium, the upper limb of each mouse was fixed in supine position, and the area of the knee joint was disinfected by iodophor. After pulling the proximal tibia to the front position with a 90° bend of the knee, a 26-gauge needle was pierced into the bone marrow cavity from the articular surface of the tibia. A 20 µl suspension of CD133+HSCs containing 4 × 104 cells was injected slowly into bone marrow cavity.
Flow Cytometry Analysis
Venous blood was drawn from the orbit of CD133+-engrafted NOG mice every other 4 weeks; 30 µl anticoagulant peripheral blood was added with antibody mixture, which included human anti-CD45, -CD19, -CD8, -CD3, -CD4, -CD11c, -CD56, -CD14, -CD16, -HLA-DR to label human various leukocytes and mouse anti-CD45.1.antibody to label mouse leukocytes. After incubating with antibody mixture on ice for 30 min, 500 μl red cell-lysing buffer was added to the peripheral blood and incubated at room temperature for 5 min. Cell sediments were collected by centrifugation, washed three times with FACS buffer (1×PBS, 2% FBS, 2 mM EDTA) and then suspended in the same buffer. The samples were detected by FACS Canto flow cytometer and analyzed by Flowjo software.
Measurement of Survival Curves in Engrafted-NOG Mice
Physical and behavioral changes, including rough pelage, poor ability to ambulate, mental fatigue, increasing intensity of palpation of the whole body, and the number of deaths in both groups (n = 6) were monitored every 2 weeks after engraftment. Individual lifespan data were used to calculate the Kaplan-Meier estimator, and survival curves were compared using a Log rank test.
Statistical Analysis
Data were analyzed by GraphPad Prism 5 and expressed as mean ± SEM. Two groups were compared using two-tailed paired t-test. Differences between multiple groups were analyzed by one-way ANOVA and Tukey’s post hoc test. P < 0.05 was considered statistically significant.
Results
Construction of Humanized NOG Mice by Intra-Bone Marrow Engraftment of CD133+ Stem Cells
First, CD133+ stem cells were purified from human umbilical cord blood by immune-magnetic bead sorting technology. Mononuclear cells [(3.6 ± 1.2) × 107] were isolated from 60–80 ml fresh umbilical cord blood by density gradient centrifugation. And [1.5 ± 0.43) × 106] of CD133+ HSCs were obtained from mononuclear cells by immunomagnetic separation with a proportion of (0.42 ± 0.15)%. The purity of CD133+ cells was (93.5 ± 2.12)% as determined by flow cytometry (Fig. S1). Then, 4 × 104 of CD133+ cells were injected into the bone marrow cavity of tibia (Fig. 1A). The condition of the mice was monitored every 2 weeks after engraftment. In busulfan-preconditioned group (N = 6), one mouse with ruffled fur and reduced activity was observed gradually after engraftment. At 2 weeks post-transplantation (wpt), hunched posture and increasing intensity of palpation of the whole body occurred. During the observation period, one mouse in the busulfan-treated group died at 4 wpt. Excluding pathogen infection and graft rejection, we considered that lethal busulfan sensitivity may have been the reason. No deaths were observed in the busulfan-untreated group (n = 6). The survival curves of the two groups were not statistically significantly different (P = 0.3) (Fig. 1B).
Fig 1.
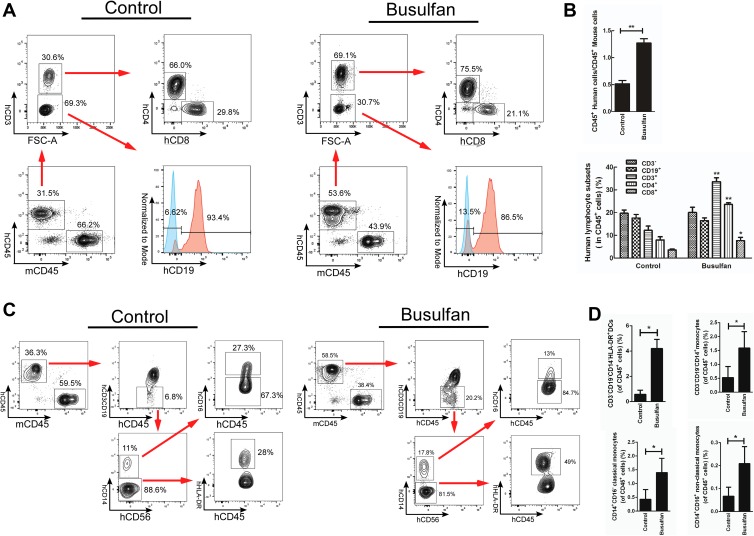
Generation and assessment of humanized mice. (A) Operation of intra-bone marrow injection. (B) Assessment of percent survival of humanized mice. Six humanized mice in each group were monitored weekly. (C) Differentiation of human CD45+ and CD19+ cells in each groups at 4 weeks post-transplantation (wpt). D. the significant difference between control and busulfan groups in the ratio of hCD45 to mCD45 and percentage of human lymphocytes subsets at 4 wpt. **P < 0.01, Data are mean ± SEMs in humanized mice (n = 6, each group).
Differentiation of Human Immune Cells in CD133+-Engrafted Humanized NOG Mice
The efficiency of human immune cells differentiated in humanized NOG mice was estimated by analysis of cell surface biomarkers. At 4 wpt, the relative percentage of human lymphocytes in all CD45+ leukocytes of the busulfan-pretreated group was 7.49±0.4% of CD19+ cells, 7.87±0.38% of CD3– cells, respectively. However, in the busulfan-untreated group, the corresponding percentages were 0.72±0.13% and 0.77±0.13%, respectively. Human CD3+ cells were not detected in either group (Fig. 1C,D; Table 1). At 16 wpt, the relative percentage of human lymphocytes in all CD45+ leukocytes of busulfan-pretreated group was 20±2.3% of CD3– cells, 16.48±1.2% of CD19+ cells, 33.54±1.8% of CD3+ cells, 23.66±0.6% of CD4+ cells, 7.66±1.5% of CD8+ cells, respectively. The ratio of CD4+ to CD8+ cells was 3.52±0.4%. And the corresponding percentage in busulfan-untreated group was 19.7±1.45%, 17.66±1.5%, 12.2±1.88%, 7.97±1.4%, 3.58±0.46%, respectively. The ratio of CD4+ to CD8+ cells was 2.2±0.15% (Fig. 2A,B; Table 1).
Table 1.
Relative Percentage of Mouse and Human Leucocyte in Humanized Mice.
| Groups Cells |
Control | Busulfan | ||
|---|---|---|---|---|
| 4 weeksa | 16 weeksb | 4 weeksa | 16 weeksb | |
| mCD45+ | 95.7 ± 0.15 | 64.6 ± 2.4 | 88.5±0.76 | 40.3±1.6 |
| hCD45+ | 0.8 ± 0.14 | 32.2 ± 2.6 | 7.9±0.4** | 57.5±1.2## |
| hCD3– CD19+ | 0.7 ± 0.13 | 17.7 ± 1.5 | 7.5±0.4** | 16.5±1.2 |
| hCD3+ CD4+ | — | 7.97 ± 1.4 | — | 23.7±0.6## |
| hCD3+ CD8+ | — | 3.6 ± 0.5 | — | 7.7±1.5# |
| hCD3– CD19– CD14– HLA-DR+ DCs | — | 0.5 ± 0.2 | — | 6.6±2.4# |
| hCD3– CD19– CD14+ monocytes | — | 0.6 ± 0.3 | — | 1.8±0.21# |
aBlood detected from humanized NOG mice after 4 weeks post-transplantation.
bBlood detected from mice after 16 weeks post-transplantation.
**P < 0.01, *P < 0.05 vs control of 4 weeks; ##P < 0.01, #P < 0.05 vs control of 16 weeks.
Fig 2.
Human lymphocytes, DCs and monocytes detection in peripheral blood of CD133+-transplanted NOG mice at 16 wpt. (A) the differentiation of human CD4+, CD8+ and CD19+ cells in each groups. (B) Significant difference between control and busulfan groups in the ratio of hCD45 to mCD45 and percentage of human lymphocytes subsets. *P < 0.05, **P < 0.01, Data are mean ± SEMs in humanized mice (n = 6, each group). (C) Differentiation of human CD14–HLA-DR+, CD14+CD16– and CD14+CD16+ cells in each group. (D) Significant difference between control and busulfan groups in percentage of human DCs and monocytes. *P < 0.05. Data are mean ± SEMs in humanized mice (n = 6, each group).
Compared with the untreated group, the ratio of human CD45+ to mouse CD45+ in the busulfan-pretreated group was significantly higher (P < 0.01). And the efficiency of human CD133+ cells differentiated into CD45+ leukocytes, CD19+, CD4+, CD8+ lymphocytes and the ratio of CD4+ to CD8+ cells was significantly higher than in the untreated group (P < 0.01) (Figs 1D, 2B, S2, Table 1). But there was no significant difference in the differentiation efficiency of CD19+ lymphocytes at 16 wpt (Fig. 2B, Table 1).
In addition, human CD3– CD19– CD14– HLA-DR+ dendritic cells (DCs) and CD3– CD19– CD14+ monocytes were also detected at 16 wpt. Compared with the untreated group, there was a significant improvement in DC and monocyte differentiation in busulfan-pretreated group, exhibiting increases of seven-fold and three-fold, respectively (Fig. 2C, D, Table 1).
Discussion
NOG (NOD Shi-SCID IL2Rγcnull) mice with the characteristics of defective T, B, and NK cells were established by Mamoru Ito by crossing NOD/scid strain mice with a strain of mice in which the gamma chain of the IL-2 receptor (IL-2 R) was knocked out12. Due to their greater efficiency in HSC differentiation7,15, NOG mice are used widely as a humanized mice model in by CD34+ stem cell engraftment. Although an important biomarker for HSCs, CD34 was found not to be expressed on any of the resulting HSCs, and CD34– HSCs could differentiate into CD34+ HSCs16–18. As another important biomarker of HSCs, CD133 was expressed on both of CD34+ and CD34– stem cells. CD34 was also expressed on 98% of CD133+ stem cells. Regarding potential for differentiation and proliferation in the absence of serum and mesenchymal cells, it was found that CD133+ cells in G0 phase had a higher cellular events in long-term culture and colony formation9–11. The results of our study show that CD133+ cells could be a better resource for humanized mice, as we found that the humanized mice model could be established successfully even using only 2 × 104 CD133+ cells for engraftment (data not shown). Furthermore, the route of engraftment can also contribute to the efficiency of establishment of humanized mice. Using intra-bone marrow injection, HSCs can directly enter the bone marrow microenvironment, therefore providing a better environment for proliferation and differentiation than when entering peripheral blood via a tail vein19.
According to cell-surface markers in standardized flow cytometry assays20, our study demonstrated that differentiated human CD3– CD19+ B lymphocytes could be detected in all NOG mice in busulfan-treated or untreated groups at 4 wpt. And differentiation of CD3+CD4+ and CD3+CD8+ T lymphocytes could be detected at 16 wpt, accompanied by a relatively stable proportion of CD19+ lymphocytes. More importantly, human CD3– CD19– CD14– HLA-DR+ DCs and CD3– CD19– CD14+ monocytes, which are pivotal participants in phagocytosis, antigen presentation and cytokine production, could also be detected at 16 wpt. These data suggest that the humanized mice established by intra-bone marrow injection of human CD133+ HSCs could possess human innate and adaptive immune cells, as did CD34+-engrafted humanized mice21,22. With busulfan preconditioning, the proportion of human CD45+ leukocytes, various human lymphocytes subsets, DCs and the ratio of CD4+ to CD8+ cells in peripheral blood of NOG mice were significantly higher than those of the untreated group (P < 0.05). Moreover, two subsets of monocytes, classic (CD14+ CD16–) and non-classic (CD14+ CD16+), were also differentiated and showed a significant difference between the two groups (P < 0.05). Although it was reported that busulfan at 30 mg/kg was harmless to NOG mice21, a mouse death in the busulfan-treated group after transplantation was observed in our study. But there was no statistically significant difference in survival rate of the two groups. Excluding pathogen infection and immune rejection, it should be considered that the death may have been due to the existence of individual differences in busulfan sensitivity. This remains to be verified by further research with large samples.
In conclusion, our study suggested that 1) CD133+ HSCs could be a good resource for the establishment of humanized mouse models. 2) Preconditioning with busulfan and intra-bone marrow injection could be more conducive to accelerating differentiation of human CD133+ HSCs in NOG mice. This may be an effective and practical method of creating humanized mice models.
Supplemental Material
Supplemental_file for The Preconditioning of Busulfan Promotes Efficiency of Human CD133+ Cells Engraftment in NOD Shi-SCID IL2Rγcnull (NOG) Mice via Intra-Bone Marrow Injection by Xiaofang Guo, Xiaoxiao Yin, Wenjuan Zhu, Ying Pan, Hui Wang, Yinming Liang and Xiaofei Zhu in Cell Transplantation
Acknowledgments
The authors are grateful to Dr. Mamoru Ito, Central Institute for Experimental Animals, Kawasaki, Japan for technical assistance, and to the Chinese mothers who voluntarily donated cord blood to this research.
Footnotes
Ethical Approval: This study was approved by Ethics Committee of Xinxiang Medical University, Henan province, China.
Statement of Human and Animal Rights: All experimental procedures involving animals were conducted in accordance with Chinese Regulations for the administration of experimental animals and the rules of clinical practice involving blood samples were followed to respect the human rights of trial participants according to the Helsinki Declaration
Statement of Informed Consent: Written informed consent was obtained from donors of cord blood.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Natural Science Foundation of China (No. 81373135, No.81771690), Innovative Talents in Science and Technology of fund program of universities of Henan (No. 15HASTIT040) and the Graduate Innovative Practice Base for clinical medicine of Xinxiang Medical University.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Ito R, Takahashi T, Katano I, Ito M. Current advances in humanized mouse models. Cell Mol Immunol. 2012;9(3):208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brehm MA, Cuthbert A, Yang C, Miller DM, DiIorio P, Laning J, Burzenski L, Gott B, Foreman O, Kavirayani A, Herlihy M, Rossini AA, Shultz LD, Greiner DL. Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rgamma(null) mutation. Clin Immunol. 2010;135(1):84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Custer RP, Bosma GC, Bosma MJ. Severe combined immunodeficiency (SCID) in the mouse. Pathology, reconstitution, neoplasms. Am J Pathol. 1985;120(3):464–477. [PMC free article] [PubMed] [Google Scholar]
- 4. Van Duyne R, Pedati C, Guendel I, Carpio L, Kehn-Hall K, Saifuddin M, Kashanchi F. The utilization of humanized mouse models for the study of human retroviral infections. Retrovirology. 2009;6(76):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, Manz MG. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304(5667):104–107. [DOI] [PubMed] [Google Scholar]
- 6. Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108(2):487–492. [DOI] [PubMed] [Google Scholar]
- 7. Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, Watanabe T, Akashi K, Shultz LD, Harada M. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor c chainnull mice. Blood. 2005;106(5):1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, Greiner DL, Handgretinger R. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2 R gamma nullmice engraftedwith mobilized human hemopoietic stem cells. J Immunol. 2005;174(10):6477–6489. [DOI] [PubMed] [Google Scholar]
- 9. Summers YJ, Heyworth CM, de Wynter EA, Hart CA, Chang J, Testa NG. AC133+ G0 cells from cord blood show a high incidence of long-term culture-initiating cells and a capacity for more than 100 million-fold ampli fication of colony-forming cells in vitro. Stem Cells. 2004;22(5):704–715. [DOI] [PubMed] [Google Scholar]
- 10. Takahashi M, Matsuoka Y, Sumide K, Nakatsuka R, Fujioka T, Kohno H, Sasaki Y, Matsui K, Asano H, Kaneko K, Sonoda Y. CD133 is a positive marker for a distinct class of primitive human cord blood-derived CD34-negative hematopoietic stem cells. Leukemia. 2014;28(6):1308–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Handgretinger R, Kuçi S. CD133-positive hematopoietic stem cells: from biology to medicine. Adv Exp Med Biol. 2013;777:99–111. [DOI] [PubMed] [Google Scholar]
- 12. Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, Heike T, Nakahata T. NOD/SCID/γcNull mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100(9):3175–3182. [DOI] [PubMed] [Google Scholar]
- 13. Krivoy N, Hoffer E, Lurie Y, Bentur Y, Rowe JM. Busulfan use in hematopoietic stem cell transplantation: pharmacology, dose adjustment, safety and efficacy in adults and children. Curr Drug Saf. 2008;3(1):60–66. [DOI] [PubMed] [Google Scholar]
- 14. Hayakawa J, Hsieh MM, Uchida N, Phang O, Tisdale JF. Busulfan produces efficient human cell engraftment in NOD/LtSz-Scid IL2Rcnull mice. Stem Cells. 2009;27(1):175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bosma MJ. B and T cell leakiness in the scid mouse mutant. Immunodefic Rev. 1992;3(4):261–276. [PubMed] [Google Scholar]
- 16. Hogan CJ, Shpall EJ, Keller G. Differential long-term and multilineage engraftment potential from subfractions of human CD34+cord blood cells transplanted into NOD/SCID mice. Proc Natl Acad Sci USA. 2002;99(1):413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I, Dick JE. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333(6039):218–221. [DOI] [PubMed] [Google Scholar]
- 18. Zanjani ED, Almeida-Porada G, Livingston AG, Flake AW, Ogawa M. Human bone marrow CD34- cells engraft in vivo and undergo multilineage expression that includes giving rise to CD34+cells. Exp Hematol. 1998;26(4):353–360. [PubMed] [Google Scholar]
- 19. Kushida T, Inaba M, Hisha H, Ichioka N, Esumi T, Ogawa R, Iida H, Ikehara S. Intra-bone marrow injection of allogeneic bone marrow cells: a powerful new strategy for treatment of intractable autoimmune diseases in MRL/lpr mice. Blood. 2001;97(10):3292–3299. [DOI] [PubMed] [Google Scholar]
- 20. Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nat Rev Immunol. 2012;12(3):191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Choi B, Chun E, Kim M, Kim ST, Yoon K, Lee KY, Kim SJ. Human B cell development and antibody production in humanized NOD/SCID/IL-2Rγ(null) (NSG) mice conditioned by busulfan. J Clin Immunol. 2011;31(2):253–264. [DOI] [PubMed] [Google Scholar]
- 22. Nie C, Sato K, Misawa N, Kitayama H, Fujino H, Hiramatsu H, Heike T, Nakahata T, Tanaka Y, Ito M, Koyanagi Y. Selective infection of CD4+effector memory T lymphocytes leads to preferential depletion of memory T lymphocytes in R5 HIV-1-infected humanized NOD/SCID/IL-2Rγnull mice. Virology. 2009;394(1):64–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental_file for The Preconditioning of Busulfan Promotes Efficiency of Human CD133+ Cells Engraftment in NOD Shi-SCID IL2Rγcnull (NOG) Mice via Intra-Bone Marrow Injection by Xiaofang Guo, Xiaoxiao Yin, Wenjuan Zhu, Ying Pan, Hui Wang, Yinming Liang and Xiaofei Zhu in Cell Transplantation