Abstract
Background
Adequate endometrial growth is principal for implantation and pregnancy. Thin endometrium is associated with lower pregnancy rate in assisted reproductive technology. Some frozen-thawed embryo transfer cycles are cancelled due to inadequate endometrial growth.
Objective
To assess the effectiveness of autologous platelet-rich plasma (PRP) intrauterine infusion for the treatment of thin endometrium.
Materials and Methods
A total of 72 patients who had a history of cancelled frozen-thawed embryo transfer cycle due to the thin endometrium ( 7mm) were assessed for the eligibility to enter the study between 2016 and 2017. Twelve patients were excluded for different reasons, and 60 included patients were randomly assigned to PRP or sham-catheter groups in a double-blind manner. Hormone replacement therapy was administered for endometrial preparation in all participants. PRP intrauterine infusion or shamcatheter was performed on day 11-12 due to the thin endometrium and it was repeated after 48 hr if necessary.
Results
Endometrial thickness increased at 48 hr after the first intervention in both groups. All participants needed second intervention due to an inadequate endometrial expansion. After second intervention, endometrial thickness was 7.21 0.18 and 5.76 0.97 mm in the PRP group and sham-catheter group, respectively. There was a significant difference between the two groups. (p 0.001). Embryo transfer was done for all patients in PRP group and just in six cases in the sham-catheter group. Chemical pregnancy was reported in twelve cases in the PRP group and two cases in the sham-catheter group.
Conclusion
According to this trial, PRP was effective in endometrial expansion in patients with refractory thin endometrium.
Keywords: Platelet-rich plasma, Endometrium, Embryo transfer, Randomized controlled trial.
1. Introduction
Implantation and pregnancy are influenced by two main factors, embryo and endometrium. A healthy embryo and receptive endometrium are necessary for implantation. Several treatments are used for endometrial preparation in frozen-thawed embryo transfer (FET) cycles (1). Endometrial pattern and thickness are evaluated by ultrasound examination. There is no agreement about the most recipient endometrium, however, most clinicians prefer endometrial thickness more than 7 mm for embryo transfer (2-5). Some FET cycles are canceled due to inadequate endometrial growth despite several treatments. Different protocols such as extended estrogen treatment, Pentoxifylline, low-dose Aspirin, Tamoxifen, vaginal Sildenafil, intrauterine perfusion with granulocyte-colony stimulating factor, and platelet-rich plasma (PRP) have been performed for the management of thin endometrium, but there is little consensus on the most effective one (6-14). PRP intrauterine infusion is a new approach for the treatment of refractory thin endometrium, which can stimulate proliferation and angiogenesis with a large number of growth factors and cytokines (7-11).PRP is achieved easily from an autologous blood sample that eliminates the risk of immunological reactions and transmission infections with low cost.
The aim of this double-blind randomized sham-controlled trial was to evaluate the effectiveness of PRP intrauterine infusion for the treatment of thin endometrium.
2. Materials and Methods
Study population
A total of 72 woman who had a history of cancelled FET cycle due to inadequate endometrial growth ( 7 mm) despite standard treatments were assessed for the eligibility to enter the study between 2016 and 2017. The inclusion criteria were age 38 yr and body mass index 30 kg/m. The exclusion criteria were uterine abnormalities, hormonal disorders, and hematological disorders. All participants were subjected to the laboratory evaluation of hormonal and hematological disorders. The hysteroscopic examination was performed before the cycle if it was not previously done. Twelve women were excluded for different reasons; sixty were included in the study (Figure 1).
Study design
This was a single-center, double-blind randomized sham-controlled trial (with balanced randomization), which was conducted in the IVF Center, Taleghani Educational Hospital, Tehran, Iran. Women were randomly selected to one of the two different groups according to the method of treatment: PRP or sham_catheter. Patients, outcome assessor, and statistician were blinded as to randomization. Randomization was carried out using computer-generated simple random tables in a 1:1 ratio. According to our pilot study the sample size was calculated after the consideration of type 1 statistical error 5%; and type 2 statistical error 20% based on the expected difference of endometrial thickness between the two groups. Hormone replacement therapy was administered for endometrial preparation in all patients: estradiol valerate (Aburaihan Co., Tehran, Iran) 6 mg/d was started on the 2nd or 3rd day of the menstrual cycle and that was increased to 8 mg/d on day 9-10 due to inadequate endometrial growth ( 7 mm). Intrauterine infusion of PRP or sham_catheter was performed on day 11-12 in intervention or control group due to the thin endometrium (thickness less than 7 mm), and it was repeated after 48 hr if required. PRP infusion or sham_catheter was performed under an ultrasound guidance. During this cycle, whenever endometrial thickness increased more than 7 mm, suppository progesterone (Cyclogest; Actavis, the UK limited, England) 400 mg twice a day was started, and ET was carried out on cleavage stage (on embryonic day 3). Estradiol valerate and progesterone supplementation were continued for next 2 weeks after ET and until 12 weeks of gestation in pregnancy. Transvaginal ultrasound was carried out by an expert gynecologist with a fellowship in infertility by one machine. Endometrial thickness was measured at the thickest part in the longitudinal axis of the uterus. PRP was prepared from autologous blood using a two-step centrifuge process; as the same protocol as reported in our pilot study (8). Then, 0.5 ml of PRP was infused into the uterine cavity with the IUI catheter (Takwin, Iran) under ultrasound guidance. The dosage and timing of PRP infusion were determined according to the pilot studies that were published in reproductive medicine.
Outcome assessment
Transvaginal ultrasound examination was done 48 hr after PRP infusion or sham_catheter to evaluate the endometrial thickness.
Figure 1.
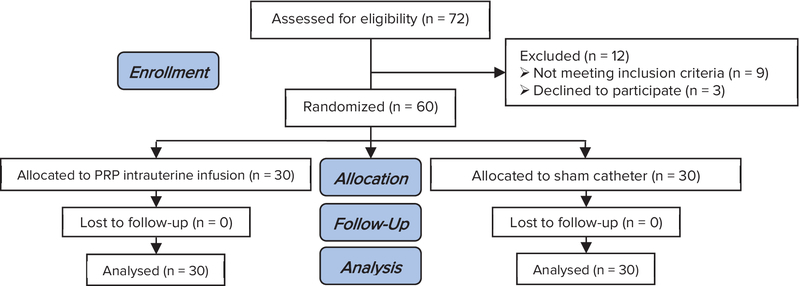
Consort flow diagram.
Ethical consideration
All patients signed an informed written consent. The study was approved by the ethical committee of Shahid Beheshti University of medical sciences, Tehran, Iran (IR.SBMU.MSP.REC.1394.92).
Statistical analysis
Results were given as mean SD. Statistical analysis was performed using the SPSS 21.0 software package (Statistical Package for the social sciences, SPSS Inc, Chicago, IL, USA). According to the distribution of data, t-test and chi-square test were performed. A p 0.05 was considered significant. The primary outcome was endometrial expansion and secondary outcomes were the chemical and clinical pregnancy that was determined by a positive serum βHCG and the presence of fetal heart beat in transvaginal ultrasound, 2 wk and 5 wk after ET, respectively.
3. Results
Baseline characteristics
Sixty consenting participants were enrolled in this study who fulfilled the entry criteria and abled to complete the study and their data were analyzed. Table I shows a summary of the baseline characteristics of both groups. In term of age, BMI, and etiology of infertility there were no significant differences between the groups.
Outcomes
Table II summarized the endometrial thickness before and after PRP or sham-catheter intervention. All participants needed PRP or sham-catheter intervention in the treatment cycles due to inadequate endometrium growth on day 11-12. As the time of first intervention, the endometrial thickness was 4.92 0.67 and 5.06 0.82 mm in the PRP group and sham-catheter group, respectively. There was no significant difference between the two groups (p = 0.613). After the first intervention, endometrial thickness was increased in both group; 5.99 0.7 and 5.45 0.82 mm in the PRP group and sham-catheter group, respectively. There was no significant difference between the two groups (p = 0.63). All participants needed second intervention 48 hr after the first one due to the inadequate endometrial thickness. After second intervention, endometrial thickness was 7.21 0.18 and 5.76 0.97 mm in the PRP group and sham-catheter group, respectively. There was a significant difference between the two groups (p 0.001). FET cycle was cancelled in 24 patients in the sham-catheter group due to the persistent thin endometrium (endometrial thickness less than 7 mm) 48 hr after second intervention. Embryo transfer was done for all patients in the PRP group and just in six cases in the sham-catheter group. Chemical pregnancy was reported in twelve cases in the PRP group and two cases in the sham-catheter group (p = 0.031). Clinical pregnancy was reported in ten cases in the PRP group and one case in the sham-catheter group (p = 0.048).
Table 1.
Baseline characteristics
|
| |||
| PRP (n = 30) | Sham (n = 30) | p-value | |
| Age (yr)* | 33.93 2.76 | 32.33 4.79 | 0.274 |
| BMI (kg)* | 24.3 2.24 | 25.46 2.68 | 0.262 |
| Etiology of Infertility** | |||
| Male Factor | 6 (20) | 8 (26.6) | |
| DOR | 4 (13.3) | 2 (6.6) | |
| Tubal Factor | 2 (6.6) | 2 (6.6) | |
| Anovulation | 6 (20) | 4 (13.3) | |
| Mixed | 12 (40) | 14 (46.6) | 0.499 |
| Note: *Data presented as mean SD; **data presented as n (%) | |||
| No staticalsignificancewas observed between the two groups: | |||
| BMI: Body mass index DOR: Diminished ovarian reserve | |||
Table 2.
Outcomes including endometrial thickness and pregnancy rate
|
| |||
| PRP (n = 30) | Sham (n = 30) | p-value | |
| Endometrial thickness (mm)* | |||
| before intervention | 4.92 0.671 | 5.06 0.821 | 0.613 |
| after 1 intervention | 5.993 0.701 | 5.453 0.823 | 0.63 |
| after 2 intervention | 7.213 0.188 | 5.767 0.973 | 0.001$ |
| Chemical pregnancy** | 12 (40) | 2 (6.7) | 0.031$ |
| Clinical pregnancy** | 10 (33.3) | 1 (3.3) | 0.048$ |
| Note: *data presented as mean SD; **Data presented as n (%); $: Statically significant; SD: Standard deviation; PRP: Platelet-rich plasma | |||
4. Discussion
Adequate endometrial growth is principal for embryo implantation and pregnancy. According to the literature, the incidence of the thin endometrium is about 2.5% in in-vitro fertilization (IVF) cycles which can lead to cycle cancellation. Also, an endometrial thickness 7 mm is associated with lower pregnancy rate in IVF cycles. Besides the important role of endometrial thickness for implantation, there is some evidence about its effects on neonatal birth weight in assisted reproductive technology (15-17). Intrauterine infusion of PRP to increase of endometrial thickness was first proposed by Change and colleagues in 2015 (7). Successful endometrial growth was reported in all of five patients with a history of refractory thin endometrium. Recent reports from our center have recommended that intrauterine infusion of PRP may be effective in the improvement of endometrial growth, expansion and implantation. In our pilot trial, 10 patients who had a history of refractory thin endometrium were recruited in the study and PRP intrauterine infusion was administered. Endometrial thickness increased during 48 hours after first PRP and reached 7 mm after second PRP. Embryo transfer was carried out for all patients. Five chemical pregnancies and four live births were reported (7, 8, 18). Another pilot study revealed the role of PRP in the improvement of endometrial thickness and vascularity in women with suboptimal endometrial thickness and reduced the incidence of cycle cancellation (9). Two recent studies suggested PRP intrauterine infusion for two novel approaches in assisted reproductive technology. These papers revealed the effective role of PRP in regeneration in Asherman's syndrome and the positive effects of PRP in the improvement of pregnancy outcome in repeated implantation failure (11, 18). PRP is an autologous fraction of the blood that contains a large amount of platelet, growth factors and cytokines such as vascular endothelial growth factor, platelet-derived growth factor, epidermal growth factor, fibroblast growth factor, insulin-like growth factor I, II, transforming growth factor, interleukin 8, hepatocyte growth factor, and connective tissue growth factor. Recently, the stimulating, proliferating, and tissue regenerative effects of PRP have been used in several medical conditions in orthopedics, ophthalmology, dental surgery, and wound healing. According to our pilot trials, there isn't any report of maternal, fetal, and neonatal adverse effects (8, 18-23). The result of this trial revealed the efficacy of PRP intrauterine infusion on endometrial expansion and proliferation in women with idiopathic thin endometrium. As we know, this is the first double-blind randomized sham-controlled trial that evaluated the efficacy of PRP in endometrial growth. We performed two-dimensional transvaginal ultrasound examination for measure endometrial thickness. According to the role of endometrial vascularity on implantation and pregnancy and the angiogenetic effects of PRP, it is suggested to design further studies and apply color Doppler and three-dimensional ultrasound examination for the detection of endometrial and sub-endometrial vasculature pattern and cavity volume before and after PRP infusion (24).
5. Conclusion
According to this trial, PRP was effective in endometrial expansion in patients with refractory thin endometrium.
Conflict of Interests
No conflict of interests has been declared.
Acknowledgments
The present study was financially supported by the Research Department of the School of Medicine, Shahid Beheshti University of Medical Sciences (Grant no. 6776).
References
- 1.Acosta-Olivo C, Garza-Borjon A, Simental-Mendia M, Vilchez-Cavazos F, Tamez-Mata Y, Pena-Martinez V. Delayed union of humeral shaft fractures: comparison of autograft with and without platelet-rich plasma treatment: a randomized, single blinded clinical trial. Arch Orthop Trauma Surg 2017; 137: 1247–1252. [DOI] [PubMed]
- 2.Gleicher N, Kim A, Michaeli T, Lee HJ, Shohat-Tal A, Lazzaroni E, et al A pilot cohort study of granulocyte colony-stimulating factor in the treatment of unresponsive thin endometrium resistant to standard therapies. Hum Reprod 2013; 28: 172–177. [DOI] [PubMed]
- 3.Garcia-Velasco JA, Acevedo B, Alvarez C, Alvarez M, Bellver J, Fontes J, et al Strategies to manage refractory endometrium: state of the art in 2016. Reprod Biomed Online 2016; 32: 474–489. [DOI] [PubMed]
- 4.Groenewoud ER, Cantineau AE, Kollen BJ, Macklon NS, Cohlen BJ. What is the optimal means of preparing the endometrium in frozen-thawed embryo transfer cycles? A systematic review and meta-analysis. Hum Reprod Update 2013; 19: 458–470. [DOI] [PubMed]
- 5.El-Toukhy T, Coomarasamy A, Khairy M, Sunkara K, Seed P, Khalaf Y, et al The relationship between endometrial thickness and outcome of medicated frozen embryo replacement cycles. Fertil Steril 2008; 89: 832–839. [DOI] [PubMed]
- 6.Barad DH, Yu Y, Kushnir VA, Shohat-Tal A, Lazzaroni E, Lee HJ, et al A randomized clinical trial of endometrial perfusion with granulocyte colony-stimulating factor in in vitro fertilization cycles: impact on endometrial thickness and clinical pregnancy rates. Fertil Steril 2014; 101: 710–715. [DOI] [PubMed]
- 7.Chang Y, Li J, Chen Y, Wei L, Yang X, Shi Y, et al Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int J Clin Exp Med 2015; 8: 1286–1290. [PMC free article] [PubMed]
- 8.Zadehmodarres S, Salehpour S, Saharkhiz N, Nazari L. Treatment of thin endometrium with autologous platelet-rich plasma: a pilot study. JBRA Assist Reprod 2017; 21: 54–56. [DOI] [PMC free article] [PubMed]
- 9.Tandulwadkar SR, Naralkar MV, Surana AD, Selvakarthick M, Kharat AH. Autologous intrauterine platelet-rich plasma instillation for suboptimal endometrium in frozen embryo transfer cycles: a pilot study. J Hum Repord Sci 2017; 10: 208–212. [DOI] [PMC free article] [PubMed]
- 10.Colombo GVL, Fanton V, Sosa D, Criado Scholz E, Lotti J, Aragona SE, et al Use of platelete rich plasma in human infertility. J Biol Regul Homeost Agents 2017; 31: 179–182. [PubMed]
- 11.Aghajanova L, Houshdaran S, Balayan S, Irwin J, Huddlestom H, Giudice L. Platelets for endometrial regeneration: a novel approach. Fertil Steril 2016; 106: e82
- 12.Xie Y, Zhang T, Tian Z, Zhang J, Wang W, Zhang H, et al Efficacy of intrauterine perfusion of granulocyte colony-stimulating factor (G-CSF) for Infertile women with thin endometrium: A systematic review and meta-analysis. Am J Reprod Immunol 2017; 78: doi: 10.1111/aji.12701. [DOI] [PubMed]
- 13.Ke H, Jiang J, Xia M, Tang R, Qin Y, Chen ZJ. The effect of tamoxifen on thin endometrium in patients undergoing frozen-thawed embryo transfer. Reprod Sci 2018; 25: 861–866. [DOI] [PubMed]
- 14.Li J, Mo S, Chen Y. The effect of G-CSF on infertile women undergoing IVF treatment: A meta-analysis. Syst Biol Reprod Med 2017; 63: 239–247. [DOI] [PubMed]
- 15.Lee D, Jo JD, Kim SK, Jee BC, Kim SH. The efficacy of intrauterine instillation of granulocyte colony-stimulating factor in infertile women with a thin endometrium: A pilot study. Clin Exp Reprod Med 2016; 43: 240–246. [DOI] [PMC free article] [PubMed]
- 16.Mouhayar Y, Sharara FI. G-CSF and stem cell therapy for the treatment of refractory thin lining in assisted reproductive technology. J Assist Reprod Genet 2017; 34: 831–837. [DOI] [PMC free article] [PubMed]
- 17.Moffat R, Beutler S, Schotzau A, De Geyter M, De Geyter C. Endometrial thickness influences neonatal birth weight in pregnancies with obstetric complications achieved after fresh IVF-ICSI cycles. Arch Gynecol Obstet 2017; 296: 115–122. [DOI] [PubMed]
- 18.Nazari L, Salehpour S, Hoseini S, Zadehmodarres S, Ajori L. Effects of autologous platelet-rich plasma on implantation and pregnancy in repeated implantation failure: A pilot study. Int J Reprod Biomed 2016; 14: 625–628. [PMC free article] [PubMed]
- 19.Lee JH, Kim MJ, Ha SW, Kim HK. Autologous platelet-rich plasma eye drops in the treatment of recurrent corneal erosions. Korean J Ophthalmol 2016; 30: 101–107. [DOI] [PMC free article] [PubMed]
- 20.Rossi LA, Molina Romoli AR, Bertona Altieri BA, Burgos Flor JA, Scordo WE, Elizondo CM. Does platelet-rich plasma decrease time to return to sports in acute muscle tear? A randomized controlled trial. Knee Surg Sports Traumatol Arthrosc 2017; 25: 3319–3325. [DOI] [PubMed]
- 21.Mohammadi S, Nasiri S, Mohammadi MH, Malek Mohammadi A, Nikbakht M, Zahed Panah M, et al Evaluation of platelet-rich plasma gel potential in acceleration of wound healing duration in patients underwent pilonidal sinus surgery: A randomized controlled parallel clinical trial. Transfus Apher Sci 2017; 56: 226–232. [DOI] [PubMed]
- 22.Marini MG, Perrini C, Esposti P, Corradetti B, Bizzaro D, Riccaboni P, et al Effects of platelet-rich plasma in a model of bovine endometrial inflammation in vitro. Reprod Biol Endocrinol 2016; 14: 58–74. [DOI] [PMC free article] [PubMed]
- 23.Picard F, Hersant B, Niddam J, Meningaud JP. Injections of Platelet-Rich Plasma for androgenic alopecia: A systematic review. J Stomatol Oral Max Surg 2017; 118: 291-297. [DOI] [PubMed]
- 24.Kim A, Jung H, Choi WJ, Hong SN, Kim HY. Detection of endometrial and subendometrial vasculature on the day of embryo transfer and prediction of pregnancy during fresh in vitro fertilization cycles. Taiwan J Obstet Gynecol 2014; 53: 360–365. [DOI] [PubMed]


