Abstract
Objectives
Preclinical data showed poly(methyl methacrylate) (PMMA) loaded with microsilver to be effective against a variety of bacteria. The purpose of this study was to assess patient safety of PMMA spacers with microsilver in prosthetic hip infections in a prospective cohort study.
Methods
A total of 12 patients with prosthetic hip infections were included for a three-stage revision procedure. All patients received either a gentamicin-PMMA spacer (80 g to 160 g PMMA depending on hip joint dimension) with additional loading of 1% (w/w) of microsilver (0.8 g to 1.6 g per spacer) at surgery 1 followed by a gentamicin-PMMA spacer without microsilver at surgery 2 or vice versa. Implantation of the revision prosthesis was carried out at surgery 3.
Results
In total, 11 of the 12 patients completed the study. No argyria or considerable differences in laboratory parameters were detected. Silver blood concentrations were below or around the detection limit of 1 ppb in ten of the 11 patients. A maximum of 5.6 ppb at 48 hours after implantation of the silver spacer, which is below the recommended maximum level of 10 ppb, was found in one patient. No silver was detected in the urine. Drainage fluids showed concentrations between 16.1 ppb and 23.3 ppb at 12 hours after implantation of the silver spacers, and between 16.8 ppb to 25.1 ppb at 48 hours after implantation. Pathohistological assessment of the periprosthetic membrane did not reveal any differences between the two groups.
Conclusion
Microsilver-loaded gentamicin-PMMA spacers showed good biocompatibility and the broad antimicrobial activity warrants further clinical research to assess its effectivity in reducing infection rates in prosthetic joint infection.
Cite this article: V. Alt, M. Rupp, K. Lemberger, T. Bechert, T. Konradt, P. Steinrücke, R. Schnettler, S. Söder, R. Ascherl. Safety assessment of microsilver-loaded poly(methyl methacrylate) (PMMA) cement spacers in patients with prosthetic hip infections: Results of a prospective cohort study. Bone Joint Res 2019;8:387–396. DOI: 10.1302/2046-3758.88.BJR-2018-0270.R1.
Keywords: Silver, Spacer, Prosthetic infection, Poly(methyl methacrylate), Hip
Article focus
This prospective cohort study assesses patient safety in relation to use of poly(methyl methacrylate) (PMMA) spacers with microsilver in prosthetic hip infections.
The study’s hypothesis is that the use of microsilver-loaded PMMA spacers is safe.
The study includes: an analysis of clinical and laboratory parameters, measurement of silver concentrations in blood, urine, and drainage fluids; and a histopathological evaluation of the periprosthetic membrane around the spacers.
Key messages
Microsilver-loaded gentamicin-PMMA spacers showed good biocompatibility, with very low blood and urine silver concentrations.
Drainage fluids showed concentrations between 16.1 ppb and 23.3 ppb at 12 hours after implantation of the silver spacers, and between 16.8 ppb and 25.1 ppb at 48 hours after implantation, which is in line with the concept of local application of antimicrobial agents.
Strengths and limitations
This study includes prospectively collected data in the target population of patients with a prosthetic hip infection.
Limitations of this study included low patient numbers and a limited follow-up period.
Introduction
Prosthetic joint infections (PJIs) remain a challenge and have a tremendous negative impact both on the quality of life of patients and on healthcare systems.1 Surgical treatment for PJIs can be divided into implant-retaining strategies, such as irrigation and debridement, and surgical options with implant removal.2 The latter includes one-stage or two-stage revision procedures, which differ in the timepoint of implantation of the revision implant. For one-stage revision procedures, implantation of the revision prosthesis is performed during the same surgery as the removal of the infected prosthesis. The two-stage concept consists of two interventions, starting with the removal of the prosthesis at surgery 1, followed by insertion of a new prosthesis during the second procedure (surgery 2), which is usually performed a few weeks later. Antibiotic-loaded spacers are frequently inserted during surgery 1, acting both as a drug delivery system and void filler to facilitate implantation of the revision prosthesis.3 However, after an initial burst from the poly(methyl methacrylate) (PMMA), subinhibitory levels of the released antibiotics have been described, with the risk of bacterial recolonization of the PMMA surface with antibiotic-resistant bacterial strains.4-6
While an increasing prevalence of infections in revision arthroplasty with resistant microbes has been reported, sobering clinical results were noted by Gomez et al7 for two-stage PJI revisions, the ‘gold standard’ of revision arthroplasty in knee and hip PJIs.8 In 504 cases, the following were reported: an interstage mortality of 7.5%, an inability to reimplant the prosthesis in 20% of cases; and a failure rate of nearly 20% in revision prosthesis implantation.7 In this context, the need to improve treatment of PJI is obvious.
Silver has been known and used as an antimicrobial substance in medicine for many decades, and has recently gained interest for the coating of tumour endoprostheses in orthopaedic oncology with convincing clinical results.9,10 The major benefit of silver is its antimicrobial activity, with preclinical data suggesting that PMMA bone cement loaded with microsilver particles is highly effective against a variety of bacteria, including methicillin-resistant Staphylococcus aureus (MRSA).11 Furthermore, it is uncommon for bacteria to develop resistance against silver, most likely due to the multiple mechanisms of antimicrobial action exploited by silver ions.12 The addition of silver to antibiotic-loaded PMMA spacers therefore offers the potential benefit of broadening and prolonging the antimicrobial protection of the spacer surface due to its wide-ranging antimicrobial properties and favourable release kinetics from PMMA.
However, adverse events due to silver-coated orthopaedic prostheses have also been reported, such as argyria with grey discolouration of the skin next to the implantation site or elevation of serum silver levels,13,14 emphasizing the need for cautious clinical evaluation of silver-loaded orthopaedic implants. Interestingly, argyria was not found to be associated with elevated serum silver concentrations or significant changes in laboratory parameters in these cases versus in patients without the development of argyria who were treated with the same type of silver-coated megaprostheses.13,14
We hypothesized that the application of microsilver-loaded PMMA spacers in PJI after total hip arthroplasty (THA) is safe. Therefore, the purpose of the current prospective case series was to assess patient safety after silver-loaded PMMA spacer implantation in patients undergoing infection revision procedures for infected THA. The study included: clinical observation of the patient; silver concentration analysis of the serum, urine, and drainage fluids; and histological analysis of the peri-implant membrane.
Materials and Methods
Study design and patients
The study was officially approved by the Ethical Committee of the University Erlangen-Nürnberg, Erlangen, Germany, and all patients gave informed consent for inclusion into the study. For inclusion in the study, patients with prosthetic total hip infections had to be aged 18 years or older and had to be able to give informed consent before surgery. Prosthetic hip infection was diagnosed by positive culture growth of puncture of the hip joint before surgery. Exclusion criteria were allergies to silver or bone cement, severe liver or renal insufficiency, or the participation in any other ongoing clinical trial.
Overall, 12 patients with prosthetic total hip infection treated with silver-loaded gentamicin-PMMA spacers were included in a prospective cohort study. The surgical protocol for the treatment of the infected THA consisted of a modified two-stage procedure with removal of the infected hip prosthesis and implantation of a PMMA spacer during the first revision procedure. During this surgery 1 intervention, the infected hip prosthesis was removed and a thorough debridement was performed, followed by the implantation of either a silver-loaded gentamicin-PMMA or silver-free gentamicin spacer. In contrast to a ‘classical’ two-stage procedure, in which a new prosthesis is implanted during surgery 2, the spacers were exchanged 14 days after the index procedure. Patients who had received a silver-loaded gentamicin-PMMA spacer were further treated with a silver-free gentamicin spacer, and vice versa. This second procedure was then followed by a third surgery with removal of the spacer and implantation of the revision prosthesis. Thus, this protocol can be seen as a modification of the ‘classical’ two-stage procedure to a three-stage procedure with exchange of the spacer in the prosthesis-free interim interval. This is the preferred protocol of the senior author (RA) of this study, with the intention of allowing for a four-week prosthesis-free interval. The aim of this protocol, for use in complex prosthetic total hip infections, is to improve local antibiotic therapy through enhanced release of antibiotics by the exchange of the gentamicin spacers at day 14. The protocol is not specifically designed for the current study with the use of silver-loaded spacers.
All of the patients were operated on by the senior author (RA). This enabled a standardized surgical treatment and PMMA spacer handling. The decision about the sequence of spacer implantation was randomized based on a randomization table generated by drawing lots before the start of the study.
For each intervention, clinical observation of adverse events and silver concentration analysis of blood, urine, and wound drainage samples were performed, as well as histological assessment of the perispacer membrane.
Silver
The microsilver used in this study (MicroSilver BG; Bio-Gate AG, Nuremberg, Germany) consisted of primary silver particles of a nanoparticulate size of 5 nm to 50 nm.11 The silver nanoparticles form aggregates of 2 µm to 5 µm, which were introduced as silver microparticles into the PMMA cement for the spacers.
Spacers
For silver-free spacers, commercially available gentamicin-loaded PMMA spacers with 0.5 g of gentamicin per 40 g of PMMA (Palacos R+G; Heraeus Medical GmbH, Wehrheim, Germany) were used. The amount of PMMA and the size of the used spacer moulds (StageOne; Biomet, Berlin, Germany) were adapted to the size of the hip joint. The following quantities of PMMA were used: 80 g in two cases; 120 g in nine cases; and 160 g in one case (Table I). In terms of silver spacers, 1% w/w of microsilver (Bio-Gate) was added to the PMMA (0.8 g in one case, 1.2 g in nine cases, and 1.6 g in one case; Table I). The silver was added to the PMMA powder and stirred with a spatula to achieve homogeneous distribution within the powder before the liquid was added. All other steps for the production of the spacers were identical to those for the silver-free spacers. In all cases, vacuum-assisted mixing (Optivac; Biomet, Berlin, Germany) was used.
Table I.
Patient demographics, microbiological findings, comorbidities, poly(methyl methacrylate) (PMMA) bone cement, and microsilver details
| Patient | Sex | Age, yrs | Identified bacteria | Relevant comorbidities | Weight of PMMA powder, g | Weight of microsilver added to PMMA powder, g | Surgery 1 |
Surgery 2 |
Surgery 3 |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Spacer type | Days between surgeries 1 and 2 | Spacer type | Days between surgeries 2 and 3 | Implantation of revision THA | |||||||
| 1 | Male | 61 | Staphylococcus aureus | Diabetes mellitus type II, arterial hypertension, spondylodiscitis | 120 | 1.2 | Silver | 21 | Non-silver | 21 | Yes |
| 2 | Male | 45 | Staphylococcus capitis | None | 120 | 1.2 | Non-silver | 21 | Silver | 21 | Yes |
| 3 | Male | 76 | Pseudomonas aeruginosa, MRSA | AV block grade 1 | 120 | 1.2 | Silver | 16 | Non-silver | 16 | Yes |
| 4 | Female | 75 | Culture-negative | Sacral ulcer, diarrhoea, highly elevated CRP | 120 | 1.2 | Silver | 15 | Non-silver | 13 | Yes |
| 5 | Female | 80 | Staph. aureus | Depression, weakness, osteoporosis, low oxygen saturation | 120 | 1.2 | Silver | 15 | Non-silver | N/A | No* |
| 6 | Male | 72 | Staphylococcus epidermidis | Arterial hypertension, CHF, moderate renal insufficiency, diabetes mellitus type II | 80 | 0.8 | Non-silver | 17 | Silver | 15 | Yes |
| 7 | Male | 80 | MRSE | Pacemaker, diabetes mellitus type II, hypertension | 160 | 1.6 | Silver | N/A† | N/A† | N/A† | N/A† |
| 8 | Female | 75 | Enterococcus faecalis | Depression, diabetes mellitus type II, arterial hypertension | 80 | 0.8 | Non-silver | 15 | Silver | 16 | Yes |
| 9 | Male | 31 | E. faecalis | None | 120 | 1.2 | Silver | 15 | Non-silver | 14 | Yes |
| 10 | Male | 64 | Staphylococcus lugdunensis | Arterial hypertension | 120 | 1.2 | Non-silver | 15 | Silver | 14 | Yes |
| 11 | Female | 77 | MRSA | CHF, arterial hypertension, anaemia, atrial fibrillation | 120 | 1.2 | Silver | 14 | Non-silver | 14 | Yes |
| 12 | Female | 64 | E. faecalis | COPD, CHF (NYHA class IV), allergies to chrome and nickel, hypercholesterolemia | 120 | 1.2 | Non-silver | 15 | Silver | 14 | Yes |
No reimplantation possible due to poor general health status
Patient chose to withdraw from the study after surgery 1
THA, total hip arthroplasty; MRSA, methicillin-resistant Staphylococcus aureus; AV, atrioventricular; CRP, C-reactive protein; CHF, congestive heart failure; MRSE, methicillin-resistant Staphylococcus epidermidis; N/A, not applicable; COPD, chronic obstructive pulmonary disease; NYHA, New York Heart Association
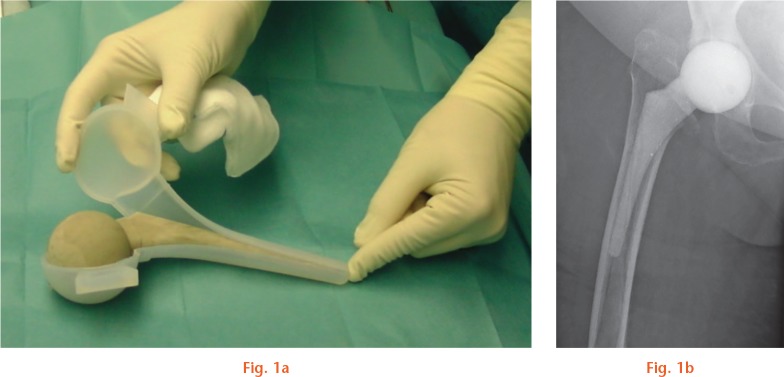
Preformed hip spacer moulds (StageOne) were then filled with bone cement using a cement gun to create articulating spacers (Fig. 1). After the PMMA spacer hardened, the moulds were cut, the spacers were inserted into the proximal femur, and the hip joint was reduced.
Fig. 1.
a) Microsilver-loaded poly(methyl methacrylate) (PMMA) spacer after hardening in a preformed hip spacer mould (StageOne; Biomet). b) Postoperative radiograph control in anteroposterior view with correct placement of the microsilver-loaded PMMA spacer.
Clinical and laboratory assessment
All side effects of the entire observation period were prospectively assessed, including clinical signs and symptoms, as well as laboratory parameters. Vital signs were measured three times a day and the patient’s temperature was measured twice a day. Wounds were checked daily for effusion, swelling, redness, or discolouration. Laboratory parameters tested from collected blood samples were as follows: erythrocyte count, leucocyte count, thrombocyte count, creatinine, urea, glutamate oxaloacetate transaminase (GOT), glutamate pyruvate transaminase (GPT), γ-glutamyl transferase (γ-GT), C-reactive protein (CRP), interleukin (IL)-6, sodium, potassium, and calcium. Laboratory tests were carried out after 12, 48, and 72 hours, as well as on postoperative days 7 and 14.
Silver analysis of blood, urine, and drainage fluids
For silver analysis, 10 ml of blood was collected 12 hours and 48 hours after surgery 1, and at the same timepoints after surgery 2. Urine was collected and pooled for the first 12 hours after surgery. Urine was further collected and pooled between 12 hours and 48 hours after surgery. Urine aliquots of 10 ml from the pooled material were sampled to measure the total silver concentration. Drainage was left in place for 48 hours and drainage fluid was collected for the first 12 hours after surgery, and from 12 hours to 48 hours after surgery as described already for urine, of which 10 ml were used for the analysis.
Urine, blood, drainage fluid, and tissue samples were then analyzed for their total silver concentration. In total, 1 ml of blood, urine, and drainage fluid was suspended in 2 ml nitric acid (65%, Merck Suprapur; Merck, Darmstadt, Germany) and wet-digested in an ultraviolet (UV)-reaction chamber. Inductively coupled plasma mass spectrometry (ICP-MS) was applied according to EN ISO 17294-2 (International Organization for Standardization (ISO), Geneva, Switzerland). A model ELAN 6100 instrument with autosampler AS90 (PerkinElmer, Waltham, Massachusetts) was used for the measurements. Its calibration was carried out with ICP standard solutions (Merck Certipur; Merck). For quality control, the certified reference material, Trace Metals in Drinking Water (TMDW), High-Purity Standards, was analyzed during all measurements. All measurements were performed in triplicate.
Pathohistological assessment of the periprosthetic membrane
For histological evaluation, tissue samples were collected during each surgical procedure. About 5 g of soft tissue was removed from the immediate vicinity of the cement spacers in order to allow for comparable assessment of the periprosthetic membrane. The blinded samples were then fixed in formalin and embedded in paraffin, and 4 µm thick longitudinal sections were stained with haematoxylin and eosin (H&E). All samples were evaluated by a senior pathologist experienced in orthopaedic pathology (SS) and were categorized according to the classification for periprosthetic membranes suggested by Morawietz et al.15 Samples with a mean of more than ten neutrophil granulocytes per ten high-power fields (HPFs) were considered to have a high-grade infection. In samples with a mean of between one and ten neutrophil granulocytes, a low-grade infection was diagnosed.16 Polarized light microscopy was used to detect polyethylene wear particles.
Results
Patients and microbiological findings
A total of 12 patients were included in the study after its official approval from the local ethics committee, and after informed consent was obtained from each patient. Ten of these 12 patients completed the observation period up until reimplantation of the revision prosthesis by surgery 3, according to the study protocol. No adverse events attributable to the silver spacer or other serious adverse events were observed. In one case, reimplantation of a revision prosthesis after surgery 2 was not possible in an 80-year-old female patient (patient 5) due to poor general health including physical weakness, depression, intermittent low oxygen saturation, and severe osteoporosis. One patient asked to be removed from the study for personal reasons without any adverse events detected.
There were six male and five female patients with diagnosed infected THA, with a mean age of 66.7 years (31 to 80) (Table I). Relevant comorbidities included atrial fibrillation, arterial hypertension, congestive heart failure, renal insufficiency, diabetes mellitus type II, depression, and sacral ulcers. All bacteria detected during the first surgery are listed in Table II. During the second intervention, five patients were diagnosed with positive culture growth. Four of them had previously been treated with a silver-free spacer and one of them with a silver-loaded spacer during surgery. In the last intervention (surgery 3), two patients were diagnosed with positive culture growth; one had previously been treated with a non-silver spacer and the other had previously been treated with a silver spacer during surgery 1 (Table II).
Table II.
Microbiological findings
| Patient | Sex | Age, yrs | Microbiological findings in surgery 1 | Type of spacer surgery 1 | Microbiological findings in surgery 2 | Type of spacer surgery 2 | Microbiological findings in surgery 3 |
|---|---|---|---|---|---|---|---|
| 1 | Male | 61 | Staphylococcus aureus | Silver | No growth | Non-silver | No growth |
| 2 | Male | 45 | Staphylococcus capitis | Non-silver | Staphylococcus capitis | Silver | No growth |
| 3 | Male | 76 | Pseudomonas aeruginosa, MRSA | Silver | Enterobacter spp. | Non-silver | MRSA |
| 4 | Female | 75 | Culture-negative | Silver | No growth | Non-silver | No growth |
| 5 | Female | 80 | Staph. aureus | Silver | No growth | Non-silver | N/A* |
| 6 | Male | 72 | Staphylococcus epidermidis | Non-silver | Pseudomonas aeruginosa | Silver | No growth |
| 7 | Male | 80 | MRSE | Silver | No growth | N/A† | N/A† |
| 8 | Female | 75 | Enterococcus faecalis | Non-silver | N/A‡ | Silver | N/A‡ |
| 9 | Male | 31 | E. faecalis | Silver | No growth | Non-silver | No growth |
| 10 | Male | 64 | Staphylococcus lugdunensis | Non-silver | Corynebacterium | Silver | No growth |
| 11 | Female | 77 | MRSA | Silver | No growth | Non-silver | No growth |
| 12 | Female | 64 | E. faecalis | Non-silver | Enterococcus faecalis | Silver | Citrobacter |
No reimplantation possible due to poor general health
Patient chose to withdraw from the study after surgery 1
Data not collected
MRSA, methicillin-resistant Staphylococcus aureus; N/A, not applicable; MRSE, methicillin-resistant Staphylococcus epidermidis
Clinical observation
At a mean timepoint of day 16 (14 to 21) after surgery 1, all of the 11 patients received their second spacer according to the study protocol. After another mean of 16 days (14 to 21), a revision total hip prosthesis could be implanted in ten of the 11 patients. Six patients initially received the microsilver-loaded spacer, followed by a silver-free spacer at surgery 2 after two weeks, while five patients were first implanted with a silver-free spacer, which was then exchanged for a silver-loaded spacer at surgery 2.
Adverse events included nausea, vomiting, low oxygen saturation, diarrhoea, hypo- and hypertension, renal function disturbances, leg oedema, disorientation, and hallucinations (Table III). Laboratory parameter changes included elevated leucocytes, elevated or decreased thrombocytes, elevated creatinine, or alterations of potassium. All of these changes were deemed to be mild to moderate and none of them was considered to be associated with the silver spacer. No silver-specific adverse events, such as argyria or impairment of the femoral or sciatic nerve, were observed.
Table III.
Adverse events
| Patient | Sex | Age, yrs | Type of spacer surgery 1 | Adverse events after surgery 1 | Type of spacer surgery 2 | Adverse events after surgery 2 |
|---|---|---|---|---|---|---|
| 1 | Male | 61 | Silver | Redness and itching of the back at day 1, elevated thrombocytes | Non-silver | Elevated leucocytes |
| 2 | Male | 45 | Non-silver | Low oxygen saturation for four days; mild: mouth dryness | Silver | None |
| 3 | Male | 76 | Silver | Leg oedema, nausea, elevated LDH, elevated urea | Non-silver | None |
| 4 | Female | 75 | Silver | Leg oedema, nausea | Non-silver | Nausea, leg oedema |
| 5 | Female | 80 | Silver | Low oxygen saturation for ten days | Non-silver | Diarrhoea |
| 6 | Male | 72 | Non-silver | Nausea, diarrhoea, hypertension, renal insufficiency, disorientation | Silver | Disorientation, diarrhoea |
| 7 | Male | 80 | Silver | Mycosis inguinal area, low potassium, elevated creatinine | N/A* | N/A* |
| 8 | Female | 75 | Non-silver | Low potassium, disorientation, bleeding of haemorrhoids, hypotension, vomiting, urinal mycosis, diarrhoea, high potassium | Silver | Hypotension, vomiting; moderate: urinal tract infection, hyper potassium |
| 9 | Male | 31 | Silver | None | Non-silver | None |
| 10 | Male | 64 | Non-silver | None | Silver | None |
| 11 | Female | 77 | Silver | Hallucinations, bradyarrhythmia; mild: limited renal function | Non-silver | Arterial hypertension, redness neck region, strong sweating, depression; mild: low potassium, limited renal function |
| 12 | Female | 64 | Non-silver | None | Silver | None |
Patient chose to withdraw from the study after surgery 1
LDH, lactate dehydrogenase; N/A, not applicable
Blood analysis
In all but one patient, the detected silver amount was close to or below the detection limit of 1 ppb of blood in all blood samples and thus several times below the reported values of up to 10 ppb in normal subjects.17 One patient (patient 4) had a silver concentration of 3.0 ppb and 2.8 ppb (3.0 µg and 2.8 µg silver per kilogram of blood) at 12 hours and 48 hours, respectively, after the implantation of the silver spacer during surgery 1 was detected. After exchange of the silver spacer to a silver-free spacer, the silver concentration was 4.1 ppb and 5.3 ppb at 12 hours and 48 hours, respectively, after the intervention.
Urine analysis
The overall urine silver burden in all urine samples was negligible and found to be below the detection limit of 1 ppb.
Drainage fluids
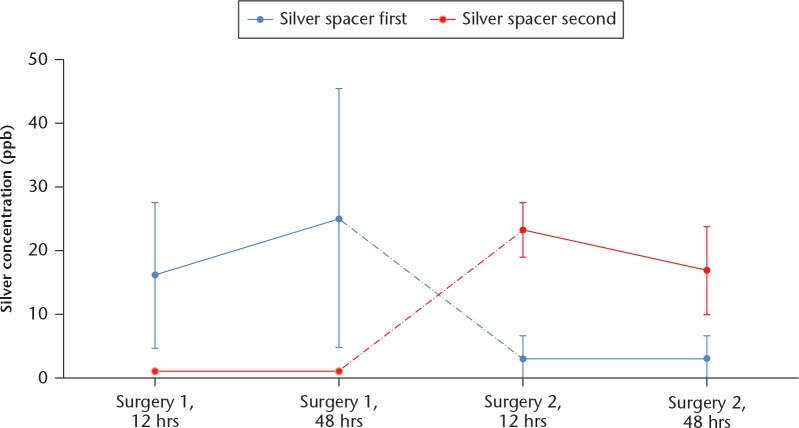
Drainage fluids showed silver concentrations from 6.0 ppb to 38.0 ppb and 5.6 ppb to 68.0 ppb after 12 hours and 48 hours, respectively, following surgical implantation of the silver spacer. In patients who received the silver-loaded spacer first, the mean silver concentration was 16.1 ppb (sd 11.5) at 12 hours after surgery and 25.1 ppb (sd 20.4) at 48 hours after surgery (Fig. 2). The mean silver concentration decreased to 3.1 ppb (sd 3.5) after surgery 2 with the silver-free spacer. The mean silver concentration in the drainage fluid was 1 ppb in all patients at 12 hours and 48 hours after surgery 1 with a silver-free spacer. Following implantation of the silver spacer in these patients, silver concentrations of 23.2 ppb (sd 4.4) at 12 hours after surgery and 16.8 ppb (sd 6.9) at 48 hours after surgery were observed.
Fig. 2.
Chart showing the silver concentration in the drainage fluid.
In six of the 11 cases, the silver concentrations between 12 hours and 48 hours only changed moderately, within a range of 5 ppb. The maximum concentration of 68.0 ppb was seen in one patient, who had already shown the highest concentration of 38.1 ppb after 12 hours.
Histology
In general, no considerable systematic differences between microsilver-loaded and silver-free PMMA spacers were detected and the histomorphological features were similar compared with regular diagnostic samples tested in the same period.
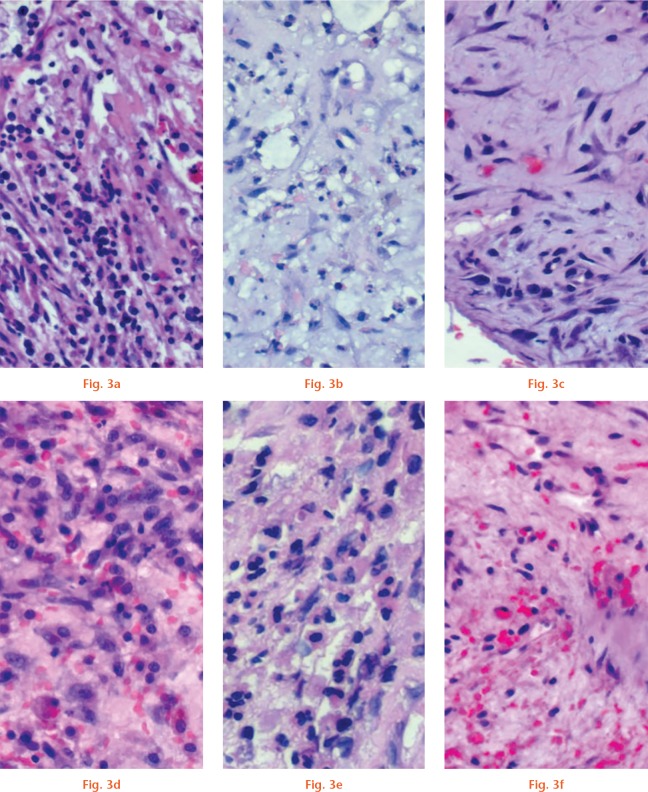
In both cases, the highest inflammatory activity (with a mean of more than ten neutrophil granulocytes per HPF) could be observed in the samples of surgery 1 with explantation of the infected prosthesis (Figs 3a and 3d). Samples taken from surgeries 2 and 3 mostly showed low-grade infection (Fig. 3b) or only a chronic inflammation (Fig. 3c), with a mean of neutrophil granulocytes below the diagnostic threshold of one per HPF (Figs 3b, 3c, 3e, and 3f). No silver particles or indications of toxic effects such as necrosis were found in any of the samples.
Fig. 3.
Haematoxylin and eosin (H&E) staining and pathohistological assessment of periprosthetic/perispacer membrane in two cases. a) to c) Microsilver poly(methyl methacrylate) (PMMA) spacer first followed by silver-free PMMA spacer. d) to f) Silver-free PMMA spacer first followed by microsilver PMMA spacer. Magnification: 400×.
Polyethylene wear particles were detected in three of the initial samples and two of the later samples. Since neither of the spacer variants contained polyethylene, these wear particles must have derived from the prosthesis that had already been removed. Light microscopy showed that in seven of the initial samples and five of the later samples, there was fine (< 10 µm) brown (e.g. hemosiderin or iron rich wear particles) or black (e.g. titanium wear particles) pigmented material. There was no association between the pigmented material and the type of spacer used.
Discussion
To the best of our knowledge, the present study examined for the first time the use of silver-loaded gentamicin-PMMA spacers in a clinical trial. Our group previously showed that PMMA bone cement with 1% w/w of microsilver exhibits promising in vitro effects, with high antibacterial effectiveness against multidrug-resistant bacteria.11 At the same time, no toxic effects on larger eukaryotic cells were seen.11 Combined, the biocompatibility with eukaryotic cells and cytotoxic effects against multidrug-resistant prokaryotic cells provide a ‘therapeutic window’ for clinical application.11 In the present study, 0.8 g microsilver was used in the PMMA spacers in most cases; in one patient, a total amount of 1.6 g microsilver was used. No silver-specific adverse events, such as argyria or impairment of the femoral or sciatic nerve, were observed. Any clinical adverse events detected, such as nausea, vomiting, low oxygen saturation, or laboratory changes (decreased thrombocytes, elevated creatinine, or alterations of potassium) were related to the procedure and to existing comorbidities of the patient, and not to the additional silver loading of the spacer. Serum and urine analysis revealed concentrations around or below the detection limit of 1 ppb in all but one patient. This patient exhibited silver concentrations of 3.0 ppb at 12 hours after the implantation of the silver spacer during surgery 1, and 2.8 ppb after 48 hours. Interestingly, these concentrations even increased up to 5.3 ppb at 48 hours after surgery 2, with the change of spacer from silver-loaded to silver-free PMMA. This patient suffered from multiple comorbidities, such as sacral ulcers, diarrhoea, and highly elevated CRP during admission, whereas creatinine levels were found to be low (0.6 mg/dl at surgery 1 and 0.58 mg/dl at surgery 2). No clear explanation for the marked difference in silver serum levels between this patient and the others could be found. Data from the literature suggest that silver serum levels in silver industry workers are between 0.1 ppb and 23.0 ppb,18 and around 11.0 ppb19 without adverse clinical effects, and, therefore, this maximum value of 5.3 ppb blood can be deemed safe for the patient. A limitation of the study is that no preoperative serum silver analysis was performed, which could have helped to explain why the silver levels reached 5.3 ppb blood in this patient.
Drainage fluid levels were between 6.0 ppb and 38.1 ppb after surgery 1 and between 5.6 ppb and 68.0 ppb after surgery 2, which is considerably higher than the measured silver levels in urine and in blood. This is fully in line with the concept of local application of antimicrobial agents, such as in antibiotic-loaded PMMA spacers or beads, to achieve high local concentration and minimize systemic concentrations of the antimicrobial agents at the same time, based on the initial ideas of Buchholz and Engelbrecht20 and Klemm.21 Our results confirm a considerable release of silver from the PMMA spacer, which is a prerequisite to fulfil its antimicrobial effects against bacteria in the wound. Due to the fact that no silver-related adverse events were detected, the observed local concentrations of up to 68.0 ppb in the drainage fluid can be considered safe for patients.
With regard to the clinical application of silver in other musculoskeletal contexts, silver poisoning was described following the use of nanoparticulate silver for topical wound dressing (Acticoat; Smith & Nephew, London, United Kingdom).22 In this case, clinical presentation showed greyish discoloration, fatigue, and lack of appetite.22 The silver concentration in the blood was determined as 107 ppb.22 A case series describing silver uptake in patients treated with the same wound dressing reported median maximum silver levels of 56.8 ppb with no haematological or biochemical indicators of toxicity.23 The maximum level of 5.3 ppb in our study was more than ten times lower than the median maximum levels found in the silver wound dressing group of this case series.23
Hardes et al13 reported on the biocompatibility of silver-coated megaendoprostheses (MUTARS; Implantcast GmbH, Buxtehude, Germany) in a clinical case series of 20 patients with bone tumours. A maximum concentration of 56.4 ppb was found in one patient’s serum 15 months after implantation of the silver-coated megaendoprosthesis.13 Laboratory tests excluded the kidney, liver, and haematological impairment within a follow-up period of 24 months.13 Again, the maximum measured concentration of 5.3 ppb blood in our study is more than ten times less than the concentration found in the study by Hardes et al.13 However, different coating techniques and different types of silver (metallic silver, microsilver, silver salts, other silver complexes) result in a significant variation in the release of free silver ions (Ag+), which are thought to be relevant for silver toxicity.24 Hence, comparison of toxicological studies should be critically evaluated.
Based on the findings of animal studies, silver and its ionic forms seem to be distributed to all organs.25 In argyria, the most prominent sign is discolouration of the skin. Local argyria around the microsilver-loaded cement spacers was not detected in any of our patients. The histological findings of tissue samples taken in the immediate vicinity of the spacers showed no difference between groups.
Silver excretion in general is described as urinary and faecal.24 A urinary-faecal excretion ratio in monkeys is reported as 0.019% to 0.026%.26 Furthermore, urinary excretion of orally applied silver nanoparticles was found to be between 0.005% and 0.057%.25 Hence, it is not surprising that no quantifiable excretion of silver was detectable in the urine of patients in our study.
Accumulation of silver in patients’ organs could not be determined directly. However, laboratory tests did not indicate any functional limitation of internal organs due to the deposition of silver. In a rabbit model, Gosheger et al27 found that no toxicological effects were associated with a silver-coated MUTARS tumour endoprosthesis within a 90-day follow-up period. Accumulation of silver was observed in the liver and spleen with mean values of 90.4 ppb and 27.9 ppb, whereas blood concentrations had a mean of 1.88 ppb.27
Our paper has several limitations, including a low number of patients. The case series was planned to test biocompatibility in temporary spacer implantation only. The long-term antimicrobial performance of silver-loaded PMMA cement in reducing reinfection rates after reimplantation of the prosthesis was therefore not evaluated. This will be of the utmost importance for further clinical studies with microsilver-loaded PMMA spacers. An interesting microbiological finding of the current study was the lack of bacterial growth after the use of silver spacers in six of seven cases after surgery 1, compared with proof of bacterial growth in all four cases in which non-silver spacers were used during surgery 1 (Table II). The small sample size and the primary safety study design characteristics do not allow for adequate statistical evaluation in this context.
Furthermore, the short observation period of 28 to 42 days from initial surgery until reimplantation of the revision prosthesis also limits the quality of the study. However, these spacers only serve as temporary implants, and an observation period limited to the duration of the in situ stage is acceptable. In the study conducted by Glehr et al,14 in which silver-coated megaendoprostheses were implanted during bone tumour surgery, patients developed argyria after a median exposure time of 25.7 months. This is substantially longer than the relatively short in situ period of silver spacers of only 14 to 21 days.
Bacterial resistance to silver nanoparticles and strategies to overcome this, as recently reported by Panáček et al,28 have to be addressed in future studies, especially given the large numbers of multidrug-resistant bacteria.29
In conclusion, this prospective cohort study on microsilver-loaded gentamicin-PMMA spacers was intended to evaluate the safety of gentamicin-loaded PMMA spacers with the additional loading of microsilver in prosthetic hip infections in a prospective cohort study. The microsilver spacers did not show any adverse clinical events related to silver, such as argyria of the skin. Blood and urine analyses were far below the published silver concentrations of other orthopaedic implants or wound dressings and confirmed the good biocompatibility. Detailed histology of the peri-implant membrane did not reveal any differences between microsilver and silver-free spacers. Therefore, the microsilver PMMA spacers used can be deemed safe, and their broad antimicrobial activity warrants further clinical research to assess their effectivity in reducing infection rates in prosthetic joint infections.
Footnotes
Author contributions: V. Alt: Analyzed the data, Wrote the manuscript.
M. Rupp: Analyzed the data, Wrote the manuscript.
K. Lemberger: Collected and analyzed the data.
T. Bechert: Conceptualized the study, Collected and analyzed the data.
T. Konradt: Conceptualized the study, Collected and analyzed the data.
P. Steinrücke: Conceptualized the study, Collected and analyzed the data.
R. Schnettler: Analyzed the data.
S. Söder: Analyzed the histopathological samples.
R. Ascherl: Performed the surgeries, Collected and analyzed the data.
V. Alt and M. Rupp are joint first authors.
Conflict of interest statement: V. Alt reports consultancy fees from Bio-Gate AG related to this study. T. Konradt and P. Steinrücke report employment from Bio-Gate AG, who acted as a sponsor and provided the MicroSilver bone cement.
Ethical review statement: The study was officially approved by the Ethical Committee of the University Erlangen-Nürnberg, Germany, with the reference #4100 (21.10.2009).
Follow us @BoneJointRes
Funding statement
The author or one or more of the authors have received or will receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this article. In addition, benefits have been or will be directed to a research fund, foundation, educational institution, or other non- profit organization with which one or more of the authors are associated.
References
- 1. Helwig P, Morlock J, Oberst M, et al. Periprosthetic joint infection–effect on quality of life. Int Orthop 2014;38:1077-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parvizi J, Zmistowski B, Adeli B. Periprosthetic joint infection: treatment options. Orthopedics 2010;33:659. [DOI] [PubMed] [Google Scholar]
- 3. Citak M, Argenson JN, Masri B, et al. Spacers. J Arthroplasty 2014;29(2 Suppl):93-99. [DOI] [PubMed] [Google Scholar]
- 4. Neut D, van de, Belt H, Stokroos I, et al. Biomaterial-associated infection of gentamicin-loaded PMMA beads in orthopaedic revision surgery. J Antimicrob Chemother 2001;47:885-891. [DOI] [PubMed] [Google Scholar]
- 5. Thomes B, Murray P, Bouchier-Hayes D. Development of resistant strains of Staphylococcus epidermidis on gentamicin-loaded bone cement in vivo. J Bone Joint Surg [Br] 2002;84-B:758-760. [DOI] [PubMed] [Google Scholar]
- 6. Yuenyongviwat V, Ingviya N, Pathaburee P, Tangtrakulwanich B. Inhibitory effects of vancomycin and fosfomycin on methicillin-resistant Staphylococcus aureus from antibiotic-impregnated articulating cement spacers. Bone Joint Res 2017;6:132-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gomez MM, Tan TL, Manrique J, Deirmengian GK, Parvizi J. The fate of spacers in the treatment of periprosthetic joint infection. J Bone Joint Surg [Am] 2015;97-A:1495-1502. [DOI] [PubMed] [Google Scholar]
- 8. Cooper HJ, Della Valle CJ. The two-stage standard in revision total hip replacement. Bone Joint J 2013;95-B(11 Suppl A):84-87. [DOI] [PubMed] [Google Scholar]
- 9. Wafa H, Grimer RJ, Reddy K, et al. Retrospective evaluation of the incidence of early periprosthetic infection with silver-treated endoprostheses in high-risk patients: case-control study. Bone Joint J 2015;97-B:252-257. [DOI] [PubMed] [Google Scholar]
- 10. Hardes J, von Eiff C, Streitbuerger A, et al. Reduction of periprosthetic infection with silver-coated megaprostheses in patients with bone sarcoma. J Surg Oncol 2010;101:389-395. [DOI] [PubMed] [Google Scholar]
- 11. Alt V, Bechert T, Steinrücke P, et al. An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials 2004;25:4383-4391. [DOI] [PubMed] [Google Scholar]
- 12. Marx DE, Barillo DJ. Silver in medicine: the basic science. Burns 2014;40(Suppl 1):S9-S18. [DOI] [PubMed] [Google Scholar]
- 13. Hardes J, Ahrens H, Gebert C, et al. Lack of toxicological side-effects in silver-coated megaprostheses in humans. Biomaterials 2007;28:2869-2875. [DOI] [PubMed] [Google Scholar]
- 14. Glehr M, Leithner A, Friesenbichler J, et al. Argyria following the use of silver-coated megaprostheses: no association between the development of local argyria and elevated silver levels. Bone Joint J 2013;95-B:988-992. [DOI] [PubMed] [Google Scholar]
- 15. Morawietz L, Classen RA, Schröder JH, et al. Proposal for a histopathological consensus classification of the periprosthetic interface membrane. J Clin Pathol 2006;59:591-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morawietz L, Tiddens O, Mueller M, et al. Twenty-three neutrophil granulocytes in 10 high-power fields is the best histopathological threshold to differentiate between aseptic and septic endoprosthesis loosening. Histopathology 2009;54:847-853. [DOI] [PubMed] [Google Scholar]
- 17. Perrelli G, Piolatto G. Tentative reference values for gold, silver and platinum: literature data analysis. Sci Total Environ 1992;120:93-96. [DOI] [PubMed] [Google Scholar]
- 18. Armitage SA, White MA, Wilson HK. The determination of silver in whole blood and its application to biological monitoring of occupationally exposed groups. Ann Occup Hyg 1996;40:331-338. [DOI] [PubMed] [Google Scholar]
- 19. DiVincenzo GD, Giordano CJ, Schriever LS. Biologic monitoring of workers exposed to silver. Int Arch Occup Environ Health 1985;56:207-215. [DOI] [PubMed] [Google Scholar]
- 20. Buchholz HW, Engelbrecht H. Depot effects of various antibiotics mixed with Palacos resins. Chirurg 1970;41:511-515. (Article in German) [PubMed] [Google Scholar]
- 21. Klemm K. Treatment of chronic bone infection with gentamicin-PMMA chains and beads. Accident Surg 1976;16:5-7. [Google Scholar]
- 22. Trop M, Novak M, Rodl S, et al. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma 2006;60:648-652. [DOI] [PubMed] [Google Scholar]
- 23. Vlachou E, Chipp E, Shale E, et al. The safety of nanocrystalline silver dressings on burns: a study of systemic silver absorption. Burns 2007;33:979-985. [DOI] [PubMed] [Google Scholar]
- 24. Wijnhoven SW, Peijnenburg WJ, Herberts CA, et al. Nano-silver–a review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology 2009;3:109-138. [Google Scholar]
- 25. Loeschner K, Hadrup N, Qvortrup K, et al. Distribution of silver in rats following 28 days of repeated oral exposure to silver nanoparticles or silver acetate. Part Fibre Toxicol 2011;8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Furchner JE, Richmond CR, Drake GA. Comparative metabolism of radionuclides in mammals-IV. Retention of silver-110m in the mouse, rat, monkey, and dog. Health Phys 1968;15:505-514. [DOI] [PubMed] [Google Scholar]
- 27. Gosheger G, Hardes J, Ahrens H, et al. Silver-coated megaendoprostheses in a rabbit model–an analysis of the infection rate and toxicological side effects. Biomaterials 2004;25:5547-5556. [DOI] [PubMed] [Google Scholar]
- 28. Panácˇek A, Kvítek L, Smékalová M, et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat Nanotechnol 2018;13:65-71. [DOI] [PubMed] [Google Scholar]
- 29. Barros CHN, Fulaz S, Stanisic D, Tasic L. Biogenic nanosilver against multidrug-resistant bacteria (MDRB). Antibiotics (Basel) 2018;7:69. [DOI] [PMC free article] [PubMed] [Google Scholar]