Abstract
PD-1 inhibitors are approved for treating advanced melanoma, but resistance has been observed. This phase Ib trial evaluated intratumoral SD-101, a synthetic CpG oligonucleotide that stimulates Toll-like receptor 9 (TLR9), in combination with pembrolizumab in patients with unresectable or metastatic malignant melanoma. The most common adverse events related to SD-101 were injection-site reactions and transient, mild-to-moderate “flu-like” symptoms. Among the 9 patients naïve to anti-PD-1 therapy, the overall response rate (ORR) was 78%. The estimated 12-month progression-free survival rate was 88%, and the overall survival rate was 89%. Among 13 patients having prior anti-PD-1 therapy, the ORR was 15%. RNA profiling of tumor biopsies demonstrated increased CD8+ T cells, natural killer cells, cytotoxic cells, dendritic cells, and B cells. The combination of intratumoral SD-101 and pembrolizumab was well tolerated and induced broad immune activation in the tumor microenvironment with durable tumor responses in both peripheral and visceral lesions.
Trial Registration clinicaltrials.gov Identifier:
INTRODUCTION
Two monoclonal antibodies to PD-1, pembrolizumab and nivolumab, have been approved by the FDA for treatment of metastatic melanoma; each has achieved an overall response rate (ORR) of approximately 35% to 40% (1, 2). Responses to single-agent anti-PD-1 therapy are dependent primarily on a preexisting T-cell infiltrate that is inhibited by PD-1-PD-L1 interactions (3). Higher ORRs have been reported when combined with additional immune modulation with potential to recruit new antigen-specific T cells into tumors. The PD-1-blocking antibody nivolumab in combination with the anti-CTLA4-blocking antibody ipilimumab demonstrated an ORR of 58% among 314 patients treated in a phase III clinical trial (4). Intratumoral injection of the oncolytic virus talimogene laherparepvec in combination with pembrolizumab in 21 patients with peripherally injectable lesions had an ORR of 62% (5). Despite the improvement in response rates with combination immunotherapy, a large unmet need remains.
SD-101 is a synthetic oligonucleotide with cytidine-phosphoguanosine (CpG) motifs that stimulates plasmacytoid dendritic cells (pDC) through engagement of TLR9. This stimulation causes pDCs to release IFNα and mature into efficient antigen-presenting cells, strengthening both innate and adaptive immune responses. Preclinical studies in mice demonstrated that the combination of intratumorally injected SD-101 and systemic anti-PD-1 led to a complete, durable rejection of essentially all injected tumors and a majority of uninjected, distant-site tumors (6). Clinically, in 27 patients with low-grade non-Hodgkin lymphoma, direct injection of SD-101 into tumors in combination with low-dose radiation not only activated local immune responses, but also induced a systemic (abscopal) effect (7).
We hypothesized that injection of SD-101 into peripheral metastatic lesions would change the tumor microenvironment at that site, resulting in the local production of type I IFNs and subsequent stimulation of a cytotoxic antitumor T-cell immune response. By concomitantly releasing PD-1-mediated inhibition with pembrolizumab, this antitumor immune response would be amplified sufficiently to be active in distant lesions. This combination therapy would be anticipated to work in patients whose baseline tumors have or do not have a preexisting immune response and may reverse primary resistance in some patients who did not respond to single-agent anti-PD-1. Here, we provide results from the dose-escalation phase of an ongoing clinical study that is assessing the safety, efficacy, and pharmacodynamic effect of the combination of SD-101 and pembrolizumab in patients with advanced melanoma.
RESULTS
Patients and Disease Characteristics
Twenty-two patients were enrolled in this phase Ib study; 9 patients were naïve to anti-PD-1/PD-L1 therapy at base-line and 13 had received prior anti-PD-1/PD-L1 therapy. All patients who were naïve to anti-PD-1 therapy had stage IV disease; 3 had BRAFV600E mutations; and 5 had received prior therapy for their melanoma including 4 who had received anti-CTLA4 therapy (Table 1). Seven patients received 1 or 2 mg of intralesional SD-101 (Supplementary Table S1). Ten patients who had previously received anti-PD-1/PD-L1 therapy had stage IV disease; 3 had BRAFV600E mutations; and 12 had two or more lines of previous therapy including anti-CTLA4 therapy (Table 1). Nine of 13 patients in this group received 4 or 8 mg of intralesional SD-101 (Supplementary Table S1).
Table 1.
Demographic and baseline characteristics of patients
| Characteristic | Naïve to prior anti-PD-1/ PD-L1 therapy (N = 9) |
Received prior anti-PD-1/ PD-L1 therapy (N = 13) |
Total (N = 22) |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 6 (67) | 9 (69) | 15 (68) |
| Female | 3 (33) | 4 (31) | 7 (32) |
| Age (years) | |||
| Median | 67 | 64 | 64 |
| Min, max | 46, 78 | 34, 77 | 34, 78 |
| ECOG PS, n (%) | |||
| 0 | 7 (78) | 9 (69) | 16 (73) |
| 1 | 2 (22) | 4 (31) | 6 (27) |
| Stage at screening, n (%) | |||
| IIIC | 0 | 3 (23) | 3 (14) |
| IV | 9 (100) | 10 (77) | 19 (86) |
| Ma | 4 (44) | 3 (23) | 4 (18) |
| Mb | 2 (22) | 4 (31) | 6 (27) |
| Mc | 3 (33) | 6 (46) | 9 (41) |
| Baseline LDH (U/L), mean (SD) | 397 (533) | 292 (198) | 335 (365) |
| ≤ULN, n (%) | 8 (89) | 8 (62) | 16 (73) |
| >1ULN to ≤2 ULN, n (%) | 0 | 4 (31) | 4 (18) |
| >2ULN, n (%) | 1 (11) | 1 (8) | 2 (9) |
| BRAFV600E mutation, n (%) | |||
| Wild-type | 6 (67) | 6 (46) | 12 (55) |
| Mutated | 3 (33) | 3 (23) | 6 (27) |
| Not tested | 0 | 4 (31) | 4 (18) |
| 0/1/2/≥3 prior lines of therapy, n (%) | 4/4/1/0 | 0/1/4/8 | 4/5/5/8 |
| Anti-CTLA4 | 4 (44) | 12 (92) | 16 (73) |
| Interferon | 2 (22) | 3 (23) | 5 (23) |
| IL2 | 0 | 3 (23) | 3 (14) |
| IL 10 | 0 | 1 (8) | 1 (5) |
| Chemotherapy | 0 | 5 (39) | 5 (23) |
| BRAF or MEK inhibitor | 0 | 2 (15) | 2 (9) |
| Responded to prior anti-PD-1/PD-L1, n (%) | NA | 3 (24) | NA |
| Tissue involvement, n (%)a | |||
| Liver | 2 (22) | 6 (46) | 8 (36) |
| Lung | 5 (56) | 5 (39) | 10 (46) |
| Bone | 2 (22) | 0 | 2 (9) |
| Skin/subcutaneous tissue | 5(56) | 10 (77) | 15 (68) |
| Lymph nodes | 6 (67) | 6 (46) | 12 (55) |
| Other organs | 4 (44) | 5 (39) | 9 (41) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; NA, not applicable; SD, standard deviation; ULN, upper limit of normal.
Patients may have >1 site of tissue involvement.
Safety
All 22 patients had at least one treatment-emergent adverse event (TEAE; Supplementary Table S2). Most adverse events (AE) were grades 1 to 2 in severity, and there was no clear relationship of AEs to the SD-101 dose level (Supplementary Table S3). Transient flu-like illness occurred more frequently at the higher doses of SD-101, but patients at every dose level had some symptoms consistent with a flu-like illness. The most common grade 3 to 4 TEAEs related to SD-101 were chills, myalgia, and injection-site pain (each 14%; Supplementary Table S3). Most occurred the night of an injection of SD-101 and were managed with over-the-counter medications such as ibuprofen or acetaminophen. Many patients had redness at the injection site and approximately a third of patients had injection-site pain. One patient had an AE in the injected limb that led to discontinuation of SD-101 alone.
Three patients reported new-onset immune-related AEs (irAE) comprising 1 patient in the 1-mg dose cohort with grade 2 pneumonitis (day 22; resulted in withdrawal of pembrolizumab and SD-101), 1 patient in the 1-mg dose cohort with grade 3 polymyalgia rheumatica (at 5 months), and 1 patient in the 2-mg cohort with grade 1 hypothyroidism (at 8 months) and grade 3 hypophysitis that occurred 3 months after the last pembrolizumab dose (at 10.5 months).
Treatment Response
Patients Naïve to Prior Anti-PD-1/PD-L1 Therapy
Of the 9 patients naïve to anti-PD-1/PD-L1 therapy, 7 had at least one evaluation of their tumors (Table 2). One 70-year-old patient did not have a scan because of rapidly progressive disease (PD) and was discontinued from the study at day 23. A 67-year-old man who did not have a scan was discontinued from the study at day 35 because of an irAE of pneumonitis. By investigator-assessed RECIST version 1.1 (8), all 7 patients with scans had a confirmed objective response, including two complete responses (CR). Tumor shrinkage was observed in all target lesions (Fig. 1A), injected target lesions (Supplementary Fig. S1A), noninjected target lesions (Supplementary Fig. S1B), subcutaneous tissue target lesions (Supplementary Fig. S1C), and visceral lesions (Supplementary Fig. S1D) including complete resolution of lesions in the lung (an example is shown in Fig. 1C). Responses were seen at each dose level with CRs in the 2- and 4-mg cohorts. One patient with a BRAFV600E mutation had a CR and 1 patient had a partial response (PR). Responses were unrelated to baseline PD-L1 expression (Fig. 1D). The median time to response was 18 weeks (range, 8–44 weeks) with tumor shrinkage continuing to occur more than 8 months after first dose. One patient developed a CR more than 10 months after study onset. One patient with an objective response developed PD 15 months after enrollment. The median progression-free survival (PFS), duration of response, and overall survival (OS) have not been reached. The estimated 12-month PFS rate was 88% and OS rate was 89%. After a median of 18 months of follow-up, 86% of responses were ongoing.
Table 2.
Best overall treatment response (intention-to-treat population) as determined by the investigator using RECIST v1.1
| Naïve to prior anti-PD-1/PD-L1 therapy (N = 9) |
Received prior anti-PD-1/PD-L1 therapy (N = 13) |
|
|---|---|---|
| BOR by investigator | n (%) | n (%) |
| ORR | 7 (78) | 2 (15) |
| CR | 2 (22) | 0 |
| PR | 5 (56) | 2 (15) |
| SD | 0 | 5 (38) |
| PD | 1 (11) | 5 (38) |
| Disease control rate | 7 (78) | 7 (54) |
| Unevaluable | 1 (11) | 1 (8) |
Abbreviations: BOR, best overall response; ORR, overall response rate; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Figure 1.
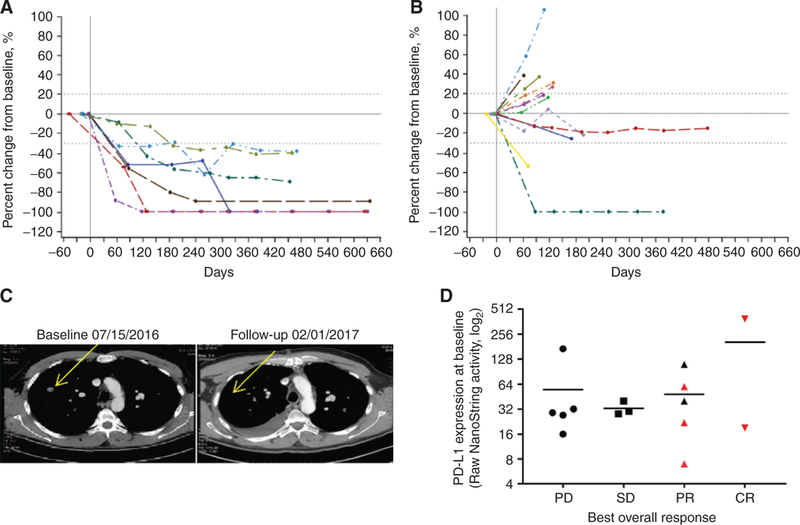
Responses to the combination of SD-101 and pembrolizumab. Percent change from baseline over time in tumor burden in all target lesions in patients naïve to anti-PD-1/PD-L1 therapy (A) and in patients with prior anti-PD-1/PD-L1 therapy (B). Dashed lines denote cutoffs for PD (+20%) or response (−30%) according to RECIST v1.1. C, Response to therapy in a noninjected lesion is shown in a CT scan from a 61-year-old male patient with stage IV disease at baseline. The patient had not received anti-PD-1/PD-L1 therapy prior to enrollment and had a PR to SD-101/pembrolizumab therapy. D, PD-L1 RNA expression profiling using the NanoString PanCancer Immune Profiling Panel at baseline by best overall response. Red symbols indicate patients who did not have prior anti-PD-1 therapy.
Patients with Prior Anti-PD-1/PD-L1 Experience
Of the 13 patients who had received prior anti-PD-1/PD-L1 therapy, 12 had at least one evaluation of their tumors. One patient withdrew consent prior to receiving a scan and later had PD. By investigator-assessed RECIST v1.1, 2 patients with scans had a confirmed PR (Table 2). PRs occurred in 1 patient who received 1 mg of SD-101 and an unconfirmed PR occurred in a patient who received 8 mg of SD-101. One patient with a BRAFV600E mutation had a confirmed PR. Reduction of target lesions occurred in patients including 2 in the 1-mg SD-101 cohort and 2 in the 8-mg SD-101 cohort (Fig. 1B). One patient who received 1 mg of SD-101 and was refractory to prior anti-PD-1 therapy had a PR ongoing at 10.5 months of follow-up, and another patient who received 1 mg of SD-101 who had responded to prior anti-PD-1 therapy had stable disease ongoing at 10.5 months of follow-up. The other 10 patients developed PD ranging from 1.5 to 8 months after enrollment. The median time on study was 2.8 months.
Pharmacodynamics
IFN-Responsive Gene Signature in Blood Cells
The pharmacodynamic response to SD-101 was assessed by the induction of IFN-responsive genes 24 hours after the second SD-101 injection. Induction of IFNα-responsive genes in circulating leukocytes was used as a surrogate of the production of IFNα by pDCs in the tumor. All patients demonstrated induction of IFN-responsive genes and a clear dose-response relationship was observed over the range of 1 to 4 mg, with less differentiation between the 4- and 8-mg doses (Supplementary Fig. S2).
Immune Expression Profiling in Tumors
Postdose biopsies from injected lesions showed consistent increases in the expression of genes representing a variety of immune cell types, including CD8+ T cells, in patients who were naïve to prior anti-PD-1/PD-L1 therapy when compared with baseline samples (Supplementary Fig. S3). The changes in patients who had received prior anti-PD-1 therapy were not as pronounced; 5 of 11 patients had greater than 2-fold increases in genes representing CD8+, natural killer, cytotoxic, and T cells while receiving the combination therapy (Supplementary Fig. S3). There were also increases in the expression of genes representing Th1 cells and a concomitant decrease in Th2 cells consistent with the induction of a type I IFN response in the tumor microenvironment (Supplementary Fig. S3). These immune responses correlated with changes in tumor response (Fig. 2A). Analysis of changes in gene expression of the entire NanoString PanCancer panel (Supplementary Table S4) by distances between samples showed unsupervised clustering by responder status, consistent with a broad mechanistic basis for the response to this combination therapy (Fig. 2B).
Figure 2.
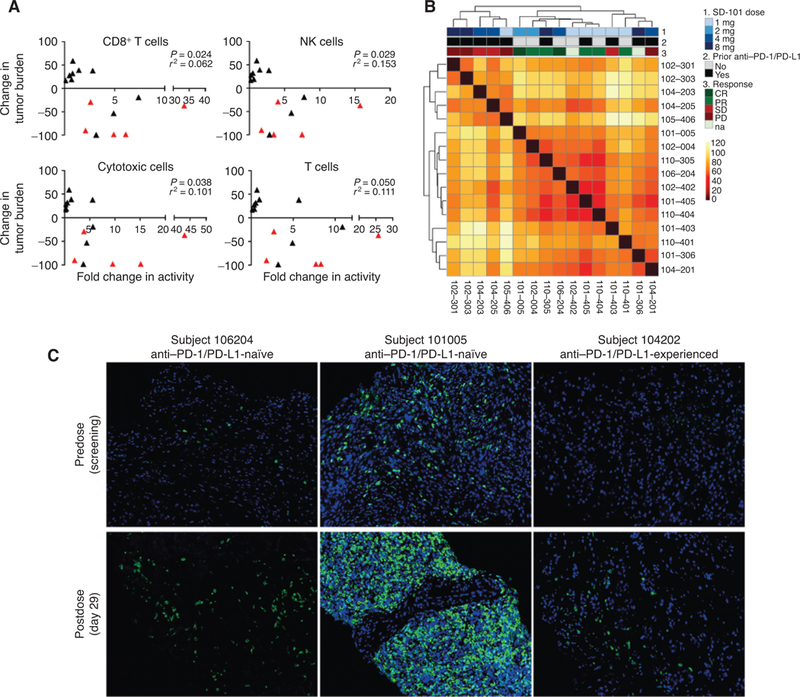
Pharmacodynamic changes in the tumor microenvironment. A, Changes in tumor-infiltrating lymphocytes significantly correlate with changes in size of all target lesions. Correlation plots between the fold change in activity (day 29 relative to day 1) defining a specific cell type by NanoString and change in size of all target lesions from baseline as assessed at a patient’s last scan. Red triangles indicate patients who did not have prior anti-PD-1 therapy. B, Heat map of Euclidean distances between samples, based on log2-transformed fold changes (day 29 vs. day 1) across genes from the NanoString data set. The dendogram shows the unbiased hierarchical clustering of samples. Samples are annotated by response (CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; na, not available), prior treatment with anti-PD1/PD-L1 and SD-101 dose level. C, Fluorescent IHC results of biopsies comparing pretreatment (predose) samples collected during screening with on-treatment (postdose) samples collected at day 29.
IHC for Infiltrating Lymphocytes
Increases in tumor-infiltrating lymphocytes were observed in patients who were anti-PD-1/PD-L1 naïve as well as those who previously received such therapy (Fig. 2C; Supplementary Fig. S4). There was a good correlation between T-cell changes measured by gene expression and IHC in the subset of samples for which data were available (Supplementary Fig. S5).
DISCUSSION
This is the first report of the combination of an intratu- moral CpG oligonucleotide and checkpoint inhibitor with assessment of the tumor microenvironment in advanced melanoma. Previously, a B-class, CpG oligonucleotide PF-3512676 was administered subcutaneously and in combination with dacarbazine in patients with advanced melanoma and failed to show efficacy over dacarbazine alone (9). This early-stage, first-in-human trial of an intratumoral TLR9 agonist with an anti-PD-1 checkpoint inhibitor demonstrated the combination was well tolerated with a high response rate in a small number of patients who were naïve to anti-PD-1 therapy at baseline. Tumor biopsies demonstrated that the drug combination resulted in an increased number of infiltrating lymphocytes in the tumor microenvironment, including CD8+ T cells.
The combination of SD-101 and pembrolizumab did not induce dose-limiting toxicity at any dose level of SD-101. Most AEs related to SD-101 treatment were transient, mild to moderate flu-like symptoms (fatigue, malaise, chills, headache, and myalgia) and injection-site reactions that responded to over-the-counter medications. There was no increase in the frequency of immune-related AEs over individual monotherapies reported in previous studies (2, 10), nor was there evidence of a unique safety signal for the combination. Increased rates of febrile neutropenia observed in previous studies of combinations of CpG oligonucleotides and chemotherapy (11, 12) were not observed in this study.
Intratumoral injection of SD-101 in combination with pembrolizumab produced antitumor responses in patients with stage IIIC/IV melanoma, both those naïve to and those who had received prior anti-PD-1/PD-L1 therapy. Responses were observed not only in the injected lesion, but also in distant lesions, including visceral metastases in the lung. Responses appeared to be independent of baseline PD-L1 expression. Of note, 2 of the 12 evaluable patients who had PD on prior anti-PD-1/PD-L1 therapy had a PR or stable disease for 10.5 months. These early results suggest that SD-101 in combination with pembrolizumab may have the potential for greater efficacy than in prior trials of pembrolizumab monotherapy.
The clinical responses were supported by mechanistic data consistent with the anticipated mode of action of SD-101 (9). SD-101 engaged its target, TLR9, independent of prior anti-PD-1/PD-L1 therapy in a dose-related manner up to a dose of 4 mg. Further, in patients who were naïve and those who had received prior anti-PD-1/PD-L1 therapy, treatment with SD-101 and pembrolizumab was associated with broad immune activation within the tumor microenvironment including infiltration of CD8+ T cells. This increased immune activity was variable, but generally correlated with increased clinical response.
The combination of SD-101 and pembrolizumab demonstrated active immune stimulation (i.e., increased PD-L1 expression) in both patient groups. Markers of antitumor response appeared to be more robust in patients who were naïve to anti-PD-1/PD-L1 therapy because more of these patients showed changes of greater magnitude. However, the fact that similar changes were seen in patients who had previously received anti-PD-1 therapy suggests that SD-101 may enhance the induction of the immune responses even in these previously treated patients. The correlation of immune activity with response suggests that, independent of prior anti-PD-1/PD-L1 therapy, the addition of SD-101 may have the potential to reverse the resistance to anti-PD-1/PD-L1 monotherapy.
In conclusion, intratumoral injection of SD-101 induces favorable changes in the tumor microenvironment with increased type I IFN and CD8+ T-cell infiltration. These changes may result in a high response rate in combination with pembrolizumab, especially in patients who have not previously received anti-PD-1 therapy, and with minimal additional toxicity.
METHODS
Study Design
This phase Ib (dose-escalation) study used a modified 3 + 3 dose-escalation design, evaluating escalating dose levels of SD-101 given with a fixed dose of pembrolizumab in patients with unresectable (stage IIIC) or metastatic (stage IV) melanoma.
Cohorts of 3 to 6 patients were enrolled at each dose level. Patients at each dose level were treated and observed for dose-limiting toxicities (DLT) for 28 days. Dose escalation was to proceed if ≤1 DLT was observed in 6 patients. If ≥2 of 6 patients experienced a DLT in any cohort, dose escalation was to cease and the previous lower dose of SD-101 would be designated as the maximum tolerated dose.
The planned dose cohorts for escalation of SD-101 were 2, 4, and 8. A 1-mg cohort was added after the 8-mg cohort to assess efficacy and not for safety reasons.
The study was approved by institutional review boards at each participating center. The study was conducted according to the Declaration of Helsinki and Good Clinical Practices. Written informed consent was obtained prior to enrollment.
Objectives
The primary objectives were to assess the safety and tolerability of the combination, evaluate the expression of IFN-inducible genes in whole blood 24 hours after intratumoral injection of SD-101 as a pharmacodynamic marker of SD-101 activity, and determine a recommended phase II dose of SD-101 in combination with pembrolizumab. Exploratory objectives were to assess the preliminary response of the injected and noninjected lesion(s) and to assess changes in tumor biomarkers.
Patient Population
Patients were 18 years of age and older with histologically or cyto-logically confirmed unresectable (stage IIIC) or metastatic (stage IV) melanoma. Patients were required to have at least 1 site of disease that qualified as a measurable (target) lesion by RECIST v1.1 (8) and was accessible for intratumoral injection. Eligible patients had an Eastern Cooperative Oncology Group Performance Status of 0 or 1 and adequate bone marrow, liver, and renal function. Patients could be anti-PD-1/PD-L1 treatment-naïve or have PD on prior anti-PD-1/PD-L1 therapy. Patients could not have a diagnosis of immunodeficiency. Patients could not have active hepatitis B, hepatitis C, or human immunodeficiency virus infection, or a history of or current uveal or ocular melanoma or active central nervous system metastases or carcinomatous meningitis. Patients could not be pregnant or breastfeeding, or expecting to conceive or father children within the projected duration of the trial through 120 days after the last dose of study treatment. Patients could not have active autoimmune disease requiring systemic treatment in the previous 2 years or a disease that required immunosuppressive medication, ongoing pneumonitis or history of (noninfectious) pneumonitis that required steroids, or an immune-related AE from a previous immunotherapeutic agent that had not resolved to grade 1 or less prior to study enrollment.
Treatment
SD-101 (1, 2, 4, or 8 mg) was administered intratumorally on days 1, 8, 15, 22, and then every 3 weeks (Q3W) for 7 more doses (days 43. 64, 85, 106, 127, 148, and 169). Pembrolizumab 200 mg was administered intravenously on day 1 and then Q3W until confirmed disease progression, unacceptable adverse reaction, or up to 2 years. SD-101 was administered before pembrolizumab on days when both drugs were scheduled to be administered. Intrapatient dose escalation or reduction was not permitted.
The protocol allowed patients who were clinically stable and had unconfirmed PD to continue on pembrolizumab until confirmed PD per investigator decision and Dynavax Medical Monitor approval. Patients had to discontinue all study treatment for confirmed PD unless discussed with and approved by the medical monitor. Patients who discontinued pembrolizumab also had to discontinue SD-101. Pembrolizumab could be continued as a single agent if SD-101 was discontinued due to AEs per investigator decision.
Outcome Measures
The safety of SD-101 in combination with pembrolizumab was assessed based on AE reporting, vital signs, and physical examination, electrocardiograms, and laboratory tests. The severity of injection-related AEs was graded using the FDA’s Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials (https://www.fda.gov/downloads/BiologicsBloodVaccines/ucm091977). All other AEs were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03 (https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010–06-14_QuickReference_5×7.pdf). DLTs were assessed for 28 days after the first dose of study drug and were defined as follows: grade ≥3 nonhematologic AE related to SD-101 (e.g., postinjection reaction or influenza-like illness) that did not resolve to grade ≤1 with standard treatment by the time of the next treatment, with the exclusion of fatigue; grade 3 nausea, vomiting, and diarrhea lasting >3 days despite optimal supportive care; clinically significant grade ≥3 nonhematologic laboratory AE; grade ≥4 hematologic AE; grade 3 hematologic laboratory AE which lasted >7 days, with the exception of lymphopenia; grade ≥3 febrile neutropenia; and prolonged delay (>3 weeks) of SD-101 or pembrolizumab dosing due to treatment-related toxicity.
Response to treatment was determined by investigators using RECIST v1.1. Initial PD per RECIST v1.1 required confirmation per irRECIST (13), with a follow-up scan obtained at least 4 weeks later that also met the criteria of PD per RECIST v1.1. All other treatment responses were confirmed by follow-up scan.
Pharmacodynamic Assessments
Target Engagement of SD-101.
Whole blood was collected on day 1 prior to injection of SD-101, and on day 9, 24 hours after the injection of SD-101. cDNA was prepared from the RNA isolated from the samples and subjected to quantitative PCR examining the expression of IFNα induced genes (Gbpl, Ifit2, Ccl2, and Mx2). Ct values relative to ubiquitin were then calculated for the IFN-responsive genes using the following formula: Relative Ct = 100,000 × 1.8[Ct(ubiquitin) - Ct(gene)]. A composite score averaging the Ct values for the four genes was generated to determine engagement of TLR9.
RNA Expression Profiling of Tumor Biopsies
Tissue from the intratumorally injected lesion was collected via punch or core-needle biopsy during screening and on day 29, and placed into RNA stabilizing agent (RNAlater, Qiagen) and frozen. RNA was isolated and analyzed with the nCounter PanCancer Immune Profiling Panel (NanoString Technologies, Inc.) to evaluate the immunophenotype of the tumor microenvironment. NanoString data were analyzed using the nSolver Analysis Software. Additional analyses were performed in R, version 3.5.0. Log2-transformed fold changes between day 29 and 1 samples were used to generate sample-to-sample distances, and heat maps were generated using the pheatmap package.
Fluorescent IHC
Formaldehyde-fixed, paraffin-embedded tumor biopsies were sectioned and stained using serial application of Opal Polymer anti-Mouse/Rabbit antibodies, HRP secondary antibodies, and fluorochrome-conjugated tyramide signal amplification reagents for detection (PerkinElmer). Microwave stripping was applied between antibodies. After the final antibody detection, the slides were stained with DAPI. Slides were imaged with a Vectra Automated Quantitative Pathology Imaging System and analyzed using inForm Software (PerkinElmer).
Statistical Methods
This phase Ib trial was designed to allow assessments of safety based on a 3 + 3 dose-escalation design, biological activity, and preliminary efficacy in approximately 24 patients. No prespecified hypothesis testing was performed. All analyses of demographics, safety, biological activity, and efficacy were descriptive.
Supplementary Material
SIGNIFICANCE:
These early data demonstrate that the combination of pembrolizumab with intratumoral SD-101 is well tolerated and can induce immune activation at the tumor site. Combining an intratumoral TLR9 innate immune stimulant with PD-1 blockade can potentially increase clinical efficacy with minimal additional toxicity relative to PD-1 blockade alone.
Acknowledgments
This study was funded by Dynavax Technologies Corporation, which provided SD-101. Merck & Co. provided pembrolizumab. A. Ribas is funded by the Parker Institute for Cancer Immunotherapy and NIH grant R35 CA197633. We thank the patients and their families and caregivers for participating in the study; the participating study teams including Sanjiv Agarwala and his team; Rene Gonzalez for comments on the manuscript; Elliot Chartash for input into study design (Merck & Co.); and Biao Xing, Brit Harvey, and Tripta Dahiya for contributions to the analysis of the data (Dynavax Technologies Corporation).
A. Ribas has received honoraria from consulting with Dynavax and Merck. A. Amin has received honoraria from the speakers bureaus of BMS, Pfizer, and Exelixis and is a consultant/advisory board member for Merck. G.A. Daniels is a consultant/advisory board member for Merck. A. Candia has ownership interest (including stock, patents, etc.) in Dynavax. R.L. Coffman has ownership interest (including stock, patents, etc.) in Dynavax. R Janssen has ownership interest (including stock, patents, etc.) in Dynavax Technologies Corporation and Merck & Co.
Footnotes
Supplementary data for this article are available at Cancer Discovery Online (http://cancerdiscovery.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed by the other authors.
REFERENCES
- 1.Ribas A, Hamid O, Daud A, Hodi FS, Wolchok JD, Kefford R, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 2016;315:1600–9. [DOI] [PubMed] [Google Scholar]
- 2.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–32. [DOI] [PubMed] [Google Scholar]
- 3.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined Nivolumab and Ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell 2017; 170:1109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang S, Campos J, Gallotta M, Gong M, Crain C, Naik E, et al. Intra-tumoral injection of a CpG oligonucleotide reverts resistance to PD-1 blockade by expanding multifunctional CD8+ T cells. Proc Natl Acad Sci U S A 2016;113:E7240–E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frank MJ, Reagan PM, Bartlett NL, Gordon LI, Friedberg JW, Czerwinski DK, et al. In situ vaccination with a TLR9 agonist and local low-dose radiation induces systemic responses in untreated indolent lymphoma. Cancer Discov 2018;8:1258–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 9.Weber JS, Zarour H, Redman B, Trefzer U, O’Day S, van den Eertwegh AJ, et al. Randomized phase 2/3 trial of CpG oligodeoxynucleotide PF-3512676 alone or with dacarbazine for patients with unresectable stage III and IV melanoma. Cancer 2009;115:3944–54. [DOI] [PubMed] [Google Scholar]
- 10.Specenier P Pembrolizumab use for the treatment of advanced melanoma. Expert Opin Biol Ther 2017;17:765–80. [DOI] [PubMed] [Google Scholar]
- 11.Manegold C, van Zandwijk N, Szczesna A, Zatloukal P, Au JS, Blasinska-Morawiec M, et al. A phase III randomized study of gemcitabine and cisplatin with or without PF-3512676 (TLR9 agonist) as first-line treatment of advanced non-small-cell lung cancer. Ann Oncol 2012;23:72–7. [DOI] [PubMed] [Google Scholar]
- 12.Hirsh V, Paz-Ares L, Boyer M, Rosell R, Middleton G, Eberhardt WE, et al. Randomized phase III trial of paclitaxel/carboplatin with or without PF-3512676 (Toll-like receptor 9 agonist) as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol 2011;29: 2667–74. [DOI] [PubMed] [Google Scholar]
- 13.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009; 15:7412–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


