Abstract
Purpose:
Distinguishing aggressive prostate cancer (PrCa) from indolent disease improves personalized treatment. Although, only few genetic variants are known to predispose to aggressive PrCa, synergistic interactions of HOXB13 G84E high-risk PrCa susceptibility mutation with other genetic loci remains unknown. The purpose of this study was to examine the interplay of HOXB13 rs138213197 (G84E) and CIP2A rs2278911 (R229Q) germline variants on PrCa risk.
Experimental Design:
Genotyping was done in Finnish discovery cohort (n=2738), validated in Swedish (n=3132) and independent Finnish (n=1155) PrCa cohorts. Expression pattern analysis was followed by functional studies in PrCa cell models.
Results:
Interplay of HOXB13 (G84E) and CIP2A (R229Q) variants results in highest observed inherited PrCa risk (OR 21.1; p=0.000024). In addition, this synergism indicates a significant association of HOXB13 T and CIP2A T dual carriers with elevated risk for high Gleason score (OR 2.3; p=0.025) and worse PrCa specific life expectancy (HR 3.9; p=0.048), and it is linked with high PSA at diagnosis (OR 3.30; p=0.028). Furthermore, combined high expression of HOXB13-CIP2A correlates with earlier biochemical recurrence. Finally, functional experiments showed that ectopic expression of variants stimulates PrCa cell growth and migration. In addition, we observed strong chromatin binding of HOXB13 at CIP2A locus, and revealed that HOXB13 functionally promotes CIP2A transcription. The study is limited to retrospective Nordic cohorts.
Conclusions:
Simultaneous presence of HOXB13 T and CIP2A T alleles confers for high PrCa risk and aggressiveness disease, earlier biochemical relapse, lower disease-specific life expectancy. HOXB13 protein binds to CIP2A gene and functionally promotes CIP2A transcription.
Keywords: Prostate cancer, Aggressive, Synergistic genetic risk, HOXB13, CIP2A
INTRODUCTION
Prostate cancer (PrCa) is the second most common cancer in men, with an estimated 1.1 million men diagnosed worldwide, accounting for 15% of the cancers diagnosed in men. PrCa represents the fifth leading cause of male cancer-related death with over 300.000 annual deaths (http://globocan.iarc.fr, GLOBOCAN 2012). The Nordic Twin Study of Cancer, the world’s largest population-based twin cohort, recently estimated genetic factors to account for 57% of the PrCa risk (95% CI 51%–63%)(1). The twin-based findings collaborate results of genome wide association studies (GWAS) that have discovered about 105 susceptibility loci, each low penetrance common variant individually modestly increasing the risk (average per allele odds ratios (OR) 1.1- 1.3)(2). These single nucleotide polymorphisms (SNPs) are estimated to explain about 30% of the inherited risk of PrCa(3,4). Despite these findings, the polygenic and very heterogeneous nature of PrCa has not yet been dissected.
One of the strongest PrCa risk predictors is the rare recurrent and highly penetrant missense mutation rs138213197/G84E in HOXB13, which codes a homeobox transcription factor that is important in prostate development(5). There are few additional HOXB13 variants (e.g. Y88D, L144P, G216C, R229G), identified in US Caucasians(5). However, the G84E is a Finnish founder mutation and was recently found to be linked to androgen receptor (AR) cistrome reprogramming through cooperating with the pioneer factor FOXA1 in human prostate tumorigenesis(6). Sharing common haplotype in mutation carriers suggested a founder effect, which was estimated to occur in 1792 in Finland(7). It is present in 8.4% of Finnish familial PrCa cases (OR 8.8) and represents significantly increased PrCa risk also in unselected cases (OR 3.6)(8). In unselected PrCa cases, the G84E variant was associated also with a considerable risk of PrCa in Swedish and UK populations (OR 3.5 and OR 2.93, respectively)(9),(10). G84E mutation has been connected with early-onset, familial cases worldwide (OR 7.9)(11) and explains partly the hereditary component of PrCa(5). Although overexpression of HOXB13 has been linked to several prognostic predictors of PrCa(12) and prostate tumourigenesis in men, the associations of G84E with PrCa clinical outcomes are not fully known(8).
Protein Phosphatase 2A (PP2A) has recently emerged as a potential PrCa tumour suppressor(13). Tumour suppressor activity of PP2A is inhibited in many cancers by overexpression of endogenous inhibitor proteins, such as SET and CIP2A(14). Importantly, expression of SET and CIP2A is associated with high Gleason scores and the presence of metastatic disease(15). CIP2A protein expression is increased in a variety of cancers, including PrCa(16), where it has been associated with castration-resistant prostate cancer (CRPC)(16,17). Recently, depletion of CIP2A in CRPC cell lines was shown to sensitize the cells to therapeutic agents(18). However, even though CIP2A can be considered functionally as a cancer driver protein, genetic evidence for CIP2A mutations in human cancers is very rare. The only genetic study to date of CIP2A in human cancer found that it exerted a synergetic effect with the exonic missense mutation (rs2278911, C/T, exon 6, p. R229Q) on the risk of hepatocellular carcinoma (HCC) in hepatitis B and C virus infection in a Han Chinese population(19). The high prevalence of C carriers (50%), together with observation that the allele is not associated with HCC risk, but only has a role in the context of hepatitis infection, suggests that CIP2A rs2278911 variant might constitute a co-operating oncogene. However, neither the prevalence of rs2278911 in other populations or disease groups, or the interaction with other genes, nor the potential functional role of this mutation, has been studied thus far.
Exact molecular mechanisms underlying the initiation and progression of PrCa still remain largely unknown. One potential reason for this is that the interaction of genetic variants using large population based cohort has not been widely studied. In British men no evidence of interaction between the HOXB13 G84E variant and polygenic risk score was found(10). The Finnish cohort is particularly suited for thorough further search for the interactive genetic variants in clinically differentiated PrCa cases. Therefore, considering the overexpression of both HOXB13 and CIP2A in PrCa, our aim was to elucidate the role of CIP2A in PrCa genetic risk and investigate the possible interaction of HOXB13 rs138213197 and CIP2A rs2278911 in PrCa susceptibility, and in relation to several disease progression and clinical outcome parameters.
PATIENTS AND METHODS
In the discovery study, the genotyped cancer patients and controls were of Finnish origin. The study was conducted in accordance with the ethical guidelines of Helsinki Declaration (1975). Written informed consent was obtained from each study subject. The human investigations were performed after approval of the study protocol by the research ethics committee at Pirkanmaa Hospital District (Tampere, Finland) and by the National Supervisory Authority for Welfare and Health (VALVIRA). For HOXB13 mutation 2669 and for CIP2A variant 2738 unselected non-familial eligible PrCa cases were analysed, respectively. The genotyping was partly carried out by the PRACTICAL (Prostate Cancer Association group to Investigate Cancer Associated Alterations in the Genome) consortium. Of cases, 2281 were clinically diagnosed cases from the Pirkanmaa Hospital District, confirmed from medical records. Another set of subjects consisted of 457 Finnish screen detected cancer cases recruited by the Finnish arm of The European Randomized Study of Screening for Prostate Cancer (ERSPC)(20). Clinical characteristics of genotyped Finnish PrCa patients are summarized in Supplementary Table 1. PrCa control subjects (HOXB13 G84E, n=2423; CIP2A R229Q, n=2427) belonging to the screening trial control group were derived from the Finnish arm of the ERSPC(20). Control subjects were population-matched healthy individuals of ages between 70 and 86 years who had undergone PSA screening. Their disease status is annually evaluated from the records of the Finnish Cancer Registry.
In the validation phase of the study, the Swedish Stockholm2 (STHM2) cohort (PrCa patients n=3132, controls n=1429) and the TAMPERE2 cohort (PrCa n=1155, controls n=1184) were studied. High PSA (>20 ng/mL) was present in 8.9% (n=278) of STHM2 and in 18.3% (n=211) of TAMPERE2 patients. Similarly, a high Gleason score ≥ 8 was observed for 10.5% (n=329) of STHM2 patients and for 5.1% of TAMPERE2 patients. Written informed consent was obtained from each study subject.
Clinical and pathological sub-classification of PrCa patients can be found in Supplementary material including the combined modality staging according to ERSPC classification (Supplementary Table 2.).
SNP genotyping and sequencing details are given in Supplementary file.
In statistical analyses the Hardy-Weinberg equilibrium equation was used to determine whether the proportion of each genotype obtained was in agreement with the expected values as calculated from the allele frequencies.
Statistical analyses were performed with IBM SPSS version 22 (SPSS Inc., Chicago, USA) unless otherwise specified. As the number of parameters was relatively small compared to the number of subjects, unconditional logistic regression analyses was used to measure the association between HOXB13 and CIP2A variants and the risk of PrCa by estimation of odds ratio (OR) and its 95% confidence interval (CI). P-values were 2-sided and p<0.05 was considered to indicate a statistically significant result.
To evaluate the relative effects of HOXB13 and CIP2A variants on PrCa development, binary stepwise logistic-regression with backward elimination method was used as described by (21). By fitting statistical models with main effects, we employed a test with few degrees of freedom that is likely to be powerful for detecting primary etiological determinants. This approach is applicable to case-control study modelled via unconditional logistic regression. By testing two polymorphisms, small number of degrees of freedom will be present. In this procedure we started with all candidate variables, and tested if the deletion of the variable improves the model the most by being deleted, and repeating this process until no further improvement is possible.
For the time-to-event analyses, Cox regression method was used.
We conducted binary logistic regression analyses to evaluate the impact of the mutations on tumour phenotype and the following selected clinical features: age at onset, PSA at diagnosis, Gleason-score, tumour stage (T), the presence of nodal (N) or distant metastases (M), general progression, PSA progression, local and distant progression, age at progression, T2:ERG fusion status (transmembrane protease, serine 2 (TMPRSS2):v-ets erythroblastosis virus E26 oncogene homolog (avian) (ERG)), other cancer, clinical and screen detection, CRPC (Castration Resistant Prostate Cancer) development, BPH status and PrCa development, general and disease specific death.
In analyses of the association between HOXB13 and CIP2A variants and the combined modality stage (ERSPC classification) of PrCa patients, we applied case-case multinomial logistic regression analyses.
In order to explore the role of HOXB13 and CIP2A in overall and PrCa specific survival, we applied Kaplan-Meier survival analyses. Survival time (years) was compared between carriers and non-carriers. Follow-up characteristics, defined follow-up periods (birth-death, diagnosis-progression, progression-death, diagnosis-death) and follow-up periods used in survival analyses of Finnish PrCa patients are summarized in Supplementary Table 3.
The pathogenicity prediction of G84E and R229Q variants was investigated by using in silico tool for missense variants, see Supplementary material.
Assessment of the effect of combined HOXB13-CIP2A expression on prediction of PrCa biochemical recurrence was based on TCGA dataset (22) containing 333 patients with primary PrCa were obtained from cBioPortal for Cancer Genomics (23,24). The mRNA expression levels of HOXB13 and CIP2A (KIAA1524) were clustered by the k-means method from the “amap” package in R (25). Parameters were set as “n=2, nstart=25, method= pearson”, which aims to partition the patients into 2 groups whose members share some measure of similarity in their expression pattern according to the expression levels of their genes. The Kaplan-Meier analysis from the R “Survival” package was used to estimate the biochemical recurrence free survival of patients after which were partitioned into 2 groups across the TCGA data sets.
Methodological details of functional studies are detailed in Supplementary file, including plasmids and site-directed mutagenesis, cell culture protocol and Western blot analyses, the cell viability, proliferation and wound healing assays, chromatin immunoprecipitation followed by quantitative PCR, lentivirus production and infection, and shRNA-, and siRNA-mediated knockdown of HOXB13, RNA extraction and quantitative RT-PCR (Supplementary Table 4)., RNA-seq and data analysis.
RESULTS
Germline risk of PrCa defined by dual T alleles of HOXB13 G84E and CIP2A R229Q
In the Finnish discovery cohort the overall minor T allele frequency of HOXB13 G84E variant was 1.8% and for the CIP2A R229Q 13.8%. The G84E mutation was more frequently observed in patients (n=167, carrier frequency 6.24%) than in healthy controls (n=20, carrier frequency 0.80%). The carrier frequency of R229Q variant was similar in cases (24.7%, n=675) and in controls (26.5%, n=642) (Table 1). HOXB13 G84E T allele carriers had an 8.0-fold increased risk of PrCa (95% CI 5.0-12.8; p=7.69E-25) in unselected non-familial cases in the analysed Finnish samples. Similarly, CT heterozygotes showed also significantly elevated risk for PrCa (OR 7.9; 95% CI 4.9-12.7; p=1.22E-24). CIP2A T allele had no significant effect on PrCa risk. However, most importantly HOXB13 T and CIP2A T dual carriers showed striking 21.1 fold increased odds for PrCa (95% CI 5.2-87.5; p=0.000024).
Table 1.
Association between the HOXB13 rs138213197 and CIP2A rs2278911 variants and the risk of prostate cancer
| Locus genotype | Case, n (%) | Control, n (%) |
OR (95% CI) | P value |
|---|---|---|---|---|
| DISCOVERY COHORT (TAMPERE) | ||||
| HOXB13 rs138213197 | ||||
| CC | 2502 (93.7) | 2403 (99.2) | 1.0 | |
| CT | 166 (6.20) | 20 (0.80) | 7.9 (4.9-12.7) | 1.22E-24 |
| TT1 | 1 (0.10) | 0 (0.00) | - | - |
| T carriers | 167 (6.24) | 20 (0.80) | 8.0 (5.0-12.8) | 7.69E-25 |
| CIP2A rs2278911 | ||||
| CC | 2063 (75.3) | 1785 (73.5) | 1.0 | |
| CT | 625 (22.8) | 588 (24.2) | 0.9 (0.8-1.1) | 0.236 |
| TT | 50 (1.80) | 54 (2.20) | 0.8 (0.6-1.2) | 0.308 |
| T carriers | 675 (24.7) | 642 (26.5) | 0.9 (0.8-1.0) | 0.139 |
| Dual carriers of HOXB13 and CIP2A | ||||
| HOXB13 C&CIP2A C | 2620 (98.2) | 2369 (97.8) | 1.0 | |
| HOXB13 T&CIP2A T | 46 (1.70) | 2 (0.10) | 21.2 (5.2 - 87.5) | 0.000024 |
| VALIDATION COHORT (STOCKHOLM) | ||||
| HOXB13 rs138213197 | ||||
| CC | 3022 (96.5) | 1418 (99.2) | 1.0 | |
| CT | 108 (3.44) | 11 (0.77) | 4.6 (2.5-8.6) | 1.57E-6 |
| TT1 | 2 (0.06) | 0 (0.00) | - | - |
| T carriers | 110 (3.51) | 11 (0.77) | 4.7 (2.5-8.7) | 1.15E-6 |
| CIP2A rs2278911 | ||||
| CC | 2455 (78.4) | 1103 (77.2) | 1.0 | |
| CT | 626 (20.0) | 303 (21.2) | 0.9 (0.8-1.1) | 0.345 |
| TT | 51 (1.63) | 23 (1.61) | 1.0 (0.6-1.6) | 0.988 |
| T carriers | 677 (21.6) | 326 (22.8) | 0.9 (0.8-1.1) | 0.365 |
| Dual carriers of HOXB13 and CIP2A | ||||
| HOXB13 C&CIP2A C | 3104 (99.1) | 1427 (99.9) | 1.0 | |
| HOXB13 T&CIP2A T | 28 (0.89) | 2 (0.14) | 6.4 (1.5 – 27.0) | 0.011 |
| VALIDATION COHORT (TAMPERE2) | ||||
| HOXB13 rs138213197 | ||||
| CC | 1067 (92.4) | 1178 (99.5) | 1.0 | |
| CT | 88 (7.6) | 6 (0.50) | 16.2 (7.1-37.2) | 5.15E-11 |
| TT1 | 0 (0.00) | 0 (0.00) | - | - |
| T carriers | 88 (7.6) | 6 (0.77) | 16.2 (7.1-37.2) | 5.15E-11 |
| CIP2A rs2278911 | ||||
| CC | 857 (74.2) | 871 (73.6) | 1.0 | |
| CT | 272 (23.5) | 295 (24.9) | 0.9 (0.8-1.1) | 0.441 |
| TT | 26 (2.3) | 18 (1.5) | 1.5 (0.8-2.7) | 0.196 |
| T carriers | 298 (25.8) | 313 (26.4) | 0.9 (0.8-1.2) | 0.727 |
| Dual carriers of HOXB13 and CIP2A | ||||
| HOXB13 C&CIP2A C | 1129 (97.7) | 1166 (98.5) | 1.0 | |
| HOXB13 T&CIP2A T | 23 (2.0) | 1 (0.10) | 24.0 (3.2 – 178.3) | 0.002 |
Case-control logistic regression analyses; OR, odds ratio, CI, confidence interval;
OR couldn't be calculated because of the limitations of log regression method; Results are in bold & grey highlighted, if the 95% CI excluded 1 and the association significant at p<0.05 vs. controls;
In the validation phase of this study, we examined the possible risk of PrCa as defined by studied polymorphisms in a Swedish (PrCa patients n=3132, controls n=1429) and in an independent Finnish (PrCa n=1155, Controls n=1184) cohort (Table 1). In the Swedish validation cohort, the same trends of associations were observed, supporting the original findings. HOXB13 G84E T allele carriers showed a 4.7-fold increased risk of PrCa (95% CI 2.5-8.7; p=1.15E-6). Although the frequency of dual T allele carriers was lower in the Swedish cohort (0.89% in PrCa patients vs 0.14% in controls) than in the Finnish cohort, we observed a significant 6.4-fold increased risk of PrCa in these Swedish carriers (95% CI 1.5-27.0; p=0.011). In addition, the results of the Finnish validation study fully support the synergistic effect of studied combination variants on PrCa risk (OR 24.0; 95% CI 3.2-178.3; p<0.003). The significantly high PrCa risk (OR 16.2; 95% CI 7.1-37.2; p=5.15E-11) caused by the HOXB13 G84E T allele was replicated also successfully. Important to state, that the Finnish validation cohort study resulted in similar carrier frequencies of the studied variants in general as it was observed in discovery cohort of Finland.
The increases in PrCa risk observed for carriers of only one of the HOXB13 T and CIP2A T alleles were statistically less augmented than the increase observed for dual T-carriers (Supplementary Table 5), suggesting that the simultaneous presence of variant T alleles at both loci might play an explicit biological role in PrCa oncogenesis. Statistical dissection of the effect of HOXB13 G84E and CIP2A R229Q variants can be find in Supplementary result.
Clinical features of PrCa associated with dual carriers of HOXB13 G84E and CIP2A R229Q
Statistically significant associations of HOXB13 G84E carriers and dual carriers of HOXB13 G84E and CIP2A R229Q (vs non-carriers) with clinical characteristics are presented in Table 2. Similar to the findings regarding disease risk, no evidence of an association between CIP2A R229Q T allele carrier status and any clinical feature of PrCa was found in any of the studied cohorts (data not shown). Significant association was observed between the HOXB13 mutation status and high PSA at diagnosis (PSA>20 ng/mL; OR 1.5; 95% CI 1.1-2.3; p=0.012). No significant association was observed between HOXB13 mutation status and Gleason score (Gleason score ≤ 6 vs ≥ 8, p=0.093). Importantly, dual HOXB13 T and CIP2A T carriers showed a significant association with Gleason score (Gleason ≥ 8, OR 2.3; 95% CI 1.1-4.8; p=0.025). This score is a commonly used clinical parameter to define aggressive PrCa and poor prognosis when predicting disease. We also examined the effect of HOXB13 and CIP2A mutation status on age at diagnosis, age at progression, PSA progression, local or distant progression, TNM stage, T2:ERG fusion status, but no significant associations were found (data not shown). Uniquely, in the Swedish PrCa validation cohort, dual T carrier status was associated with high PSA at diagnosis (OR 3.30; 95% CI 1.1-9.6; p=0.028) but not with elevated Gleason score. In the Finnish validation cohort we successfully replicated the significant association between the HOXB13 T allele and high PSA at diagnosis (OR 1.86; 95% CI 1.1-3.1; p=0.01). However, possibly due to different selection criteria of patients for the validation cohorts, such as clinical vs. PSA screening cases, and smaller sample size, statistically significant association between dual T carriers and high Gleason score of PrCa biopsy was not seen neither in Finnish nor in Swedish validation cohorts.
Table 2.
Association between HOXB13 and CIP2A carrier status and selected clinical features of prostate cancer1
| Clinical parameter |
HOXB13 rs138213197 | HOXB13 rs138213197 and CIP2A rs2278911 | ||||||
|---|---|---|---|---|---|---|---|---|
| T carrier % (n) |
T non-carrier % (n) |
OR (95% CI) | P value | Dual T carrier % (n) |
Dual T non-carrier % (n) |
OR (95% CI) | P value | |
| DISCOVERY COHORT (TAMPERE) | ||||||||
| PSA at diagnosis | ||||||||
| ≤ 20 ng/mL | 5.7 (117) | 94.3 (1924) | 1.0 | 1.7 (35) | 98.3 (2006) | 1.0 | ||
| >20 ng/mL | 8.9 (42) | 91.1 (432) | 1.50 (1.11-2.31) | 0.012 | 2.1 (10) | 97.9 (464) | 1.24 (0.61-2.51) | 0.560 |
| Gleason score | ||||||||
| ≤ 6 | 5.9 (76) | 94.7 (1207) | 1.0 | 1.5 (19) | 98.5 (1264) | 1.0 | ||
| ≥ 8 | 8.4 (30) | 91.6 (327) | 1.46 (0.94-2.26) | 0.093 | 3.4 (12) | 96.6 (345) | 2.31 (1.11-4.81) | 0.025 |
| VALIDATION COHORT (STOCKHOLM) | ||||||||
| PSA at diagnosis | ||||||||
| ≤ 20 ng/mL | 3.2 (64) | 96.8 (1930) | 1.0 | 0.6 (11) | 99.4 (1983) | 1.0 | ||
| >20 ng/mL | 3.6 (10) | 96.4 (268) | 1.21 (0.64-2.28) | 0.556 | 1.8 (5) | 98.2 (273) | 3.30 (1.14-9.57) | 0.028 |
| Gleason score | ||||||||
| ≤ 6 | 4.1 (65) | 95.9 (1515) | 1.0 | 1.1 (17) | 98.9 (1562) | 1.0 | ||
| ≥ 8 | 3.0 (10) | 97.0 (319) | 0.72 (0.37-1.41) | 0.346 | 1.2 (4) | 98.8 (325) | 1.13 (0.38-2.28) | 0.825 |
| VALIDATION COHORT (TAMPERE2) | ||||||||
| PSA at diagnosis | ||||||||
| ≤ 20 ng/mL | 6.7 (58) | 93.3 (803) | 1.0 | 1.7 (15) | 98.3 (846) | 1.0 | ||
| >20 ng/mL | 11.8 (25) | 88.2 (186) | 1.86 (1.13-3.05) | 0.01 | 3.8 (8) | 96.2 (203) | 2.22 (0.93-5.31) | 0.07 |
| Gleason score | ||||||||
| ≤ 6 | 6.3 (26) | 93.7 (384) | 1.0 | 0.7 (3) | 99.3 (407) | 1.0 | ||
| ≥ 8 | 7.5 (12) | 92.5 (149) | 1.19 (0.59-2.42) | 0.63 | 1.9 (3) | 98.1 (158) | 2.58 (0.51-12.90) | 0.25 |
Case-case logistic regression analyses;
summary of statistically significant associations; OR, odds ratio; CI, confidence interval; Results are in bold, if the 95% CI excluded 1 and the association significant at p<0.05 vs. non-carriers (bold);
Additional associations with clinical features of PrCa are detailed in Supplementary results. In short, we show that HOXB13 and CIP2A dual T carriers develop PrCa 7.2 months earlier (HR 2.1; p=4.52E-7) (Supplementary Table 6). Dual T carriers have remarkably high risk to be detected both through clinical symptoms (OR, 23.4; p=0.000013) and through screening (OR 10.7; p=0.006) compared to controls (Supplementary Table 7). This study also shows that G84E variant confers for a 12.6-fold risk of developing PrCa in benign prostatic hyperplasia (BPH) cases compared to BPH controls (95% CI 2.8-56.8; p=0.001) (Supplementary Table 7). In comprehensive evaluation of the clinical factors, using the combined modality stage based on the European Randomized study of Screening for Prostate Cancer (ERSPC) classification, we could not reveal reasonable association with dual T carriers (Supplementary Table 8).
Life-span survival of PrCa patients is shaped by dual T alleles of HOXB13 and CIP2A
Kaplan–Meier survival analyses was applied to assess the overall survival and PrCa specific survival differences between HOXB13 and CIP2A carriers and PrCa patients without the mutations. We sub-divided the follow-up period between birth and death into periods of diagnosis-death, diagnoses-progression and progression-death.
Based on the data, our survival analyses suggested no worse PrCa specific prognosis for HOXB13 T carriers after the disease progressed (case-only, Breslow test, HR 1.4; 95% CI 15.6-16.9; p=0.240). No association was found between CIP2A T carriers and the survival of PrCa patients. Exploring the longest follow up time (birth-death), dual T carriers of HOXB13 and CIP2A have significantly worse PrCa related life expectancy and die earlier of PrCa (Breslow test, HR 3.9; 95% CI 91.6-94.5; p=0.048) vs non-dual T carriers.
HOXB13 G84E and CIP2A R229Q variants are predicted to be pathogenic (in silico)
We applied CADD framework for functional effect prediction analysis(26). HOXB13 G84E was predicted to be deleterious with a scaled C-score of 29.6, which indicates it to be a potentially causative mutation. CIP2A R229Q variant has a scaled C-score of 23.9, denoting similarly deleterious variant as well. In addition, we applied M-CAP classifier and identified the pathogenicity score of 0.110 (T allele, possibly pathogenic) with high clinical sensitivity for HOXB13 G84E rare missense variant (recommended threshold >0.025)(27).
High expression pattern of HOXB13-CIP2A associates with earlier biochemical recurrence
We next assessed the effect of combined HOXB13-CIP2A expression pattern on clinical features of PrCa patients (see Methods). We observed a significant association in a TCGA cohort of PrCa (22) (Supplementary Figure 1). The time to PSA relapse was marginally significantly shorter in the patient group with higher CIP2A expression (HR, 1.718; 95% CI, 0.993-2.975; p=0.0505), but not with HOXB13. Remarkably, the combination of HOXB13-CIP2A expression data is somewhat better than CIP2A alone in prediction of the risk of biochemical recurrence of these PrCa patients (HR, 1.86; 95% CI, 1.09-3.20; p=0.0219), suggesting an obvious additive value of two genes in disease prognosis.
Gene level allele-specific expression (ASE) of CIP2A R229Q
Gene-level ASE analyses using RNA sequencing (RNA-Seq) gene-expression data and genome-wide high-density genotypes from 471 samples of benign primary prostate tissue(28) was conducted to evaluate the effect of studied variant alleles on gene expression. This analysis identified a significant association of CIP2A R229Q T allele with CIP2A upregulation (p=0.0187), suggesting that the CIP2A R229Q T allele is associated with increased expression of CIP2A. Due to the rare frequency of HOXB13 G84E mutation analyses of ASE was not feasible.
Ectopic expression of HOXB13 G84E and CIP2A R229Q variants stimulates prostate cell growth and migration
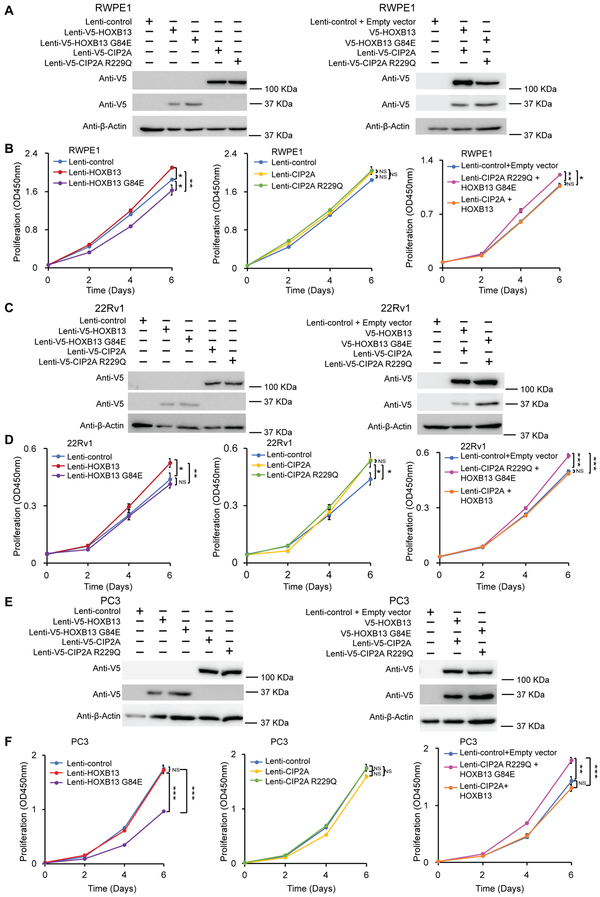
We next sought to examine the functional impact of HOXB13 G84E and CIP2A R229Q variants on cellular behaviour of prostate normal and cancer cells. Thus, we overexpressed wild-type alleles and their variants in multiple human prostate cell models to monitor cell growth using cell proliferation assays (Figure 1). We firstly confirmedthe overexpression of indicated V5-tagged genes in the tested immortalized benign prostate cell line RWPE1, and PrCa cell models, 22Rv1and PC3 (Figure 1A, 1C, 1E). Although the HOXB13 G84E variant was reported to be associated with an increased risk of PrCa in previous work (5), we found that overexpression of HOXB13 other than HOXB13 G84E promoted cell growth in RWPE1 (Figure 1B) and 22Rv1 (Figure 1D). Overexpression of HOXB13 G84E somehow reduced cell growth of RWPE1 and PC3 (Figure 1B and 1F) RWPE1 and PC3 cells overexpressing CIP2A or CIP2A R229Q showed no difference in proliferation from control cells, while overexpression of CIP2A or CIP2A R229Q in 22Rv1 cells slightly promoted cell growth (Supplementary Table 9). In contrast, all the tested cell lines with co-overexpression of HOXB13 G84E and CIP2A R229Q displayed elevated proliferation in comparison with the cells co-expressing both wild type HOXB13 and CIP2A, consistent with our genetic finding of a synergistic interaction between the two variants.
Figure 1. Overexpression of HOXB13 G84E and CIP2A R229Q variants promotes cell proliferation.
A, C, E. The ectopic expression of V5-tagged wild type HOXB13, HOXB13 G84E, CIP2A or CIP2A R229Q was assessed using western blotting with anti-V5 antibody. Parallel blots of β-actin were applied to probe for equal protein loading of RWPE1, 22Rv1 or PC3 cell lysates.
B, D, F. Cell proliferation was measured at the indicated time points by XTT colorimetric assay (absorbance at 450 nm) in PrCa RWPE1(B), 22Rv1(D) and PC3(F) cells overexpressing HOXB13, HOXB13 G84E, CIP2A, CIP2A R229Q, HOXB13 with CIP2A or HOXB13 G84E with CIP2A R229Q, respectively. Error bars, s.d. from three technical replicates. *P < 0.05, **P < 0.01, ***P < 0.01 assessed by two-tailed t-tests. NS, not significant.
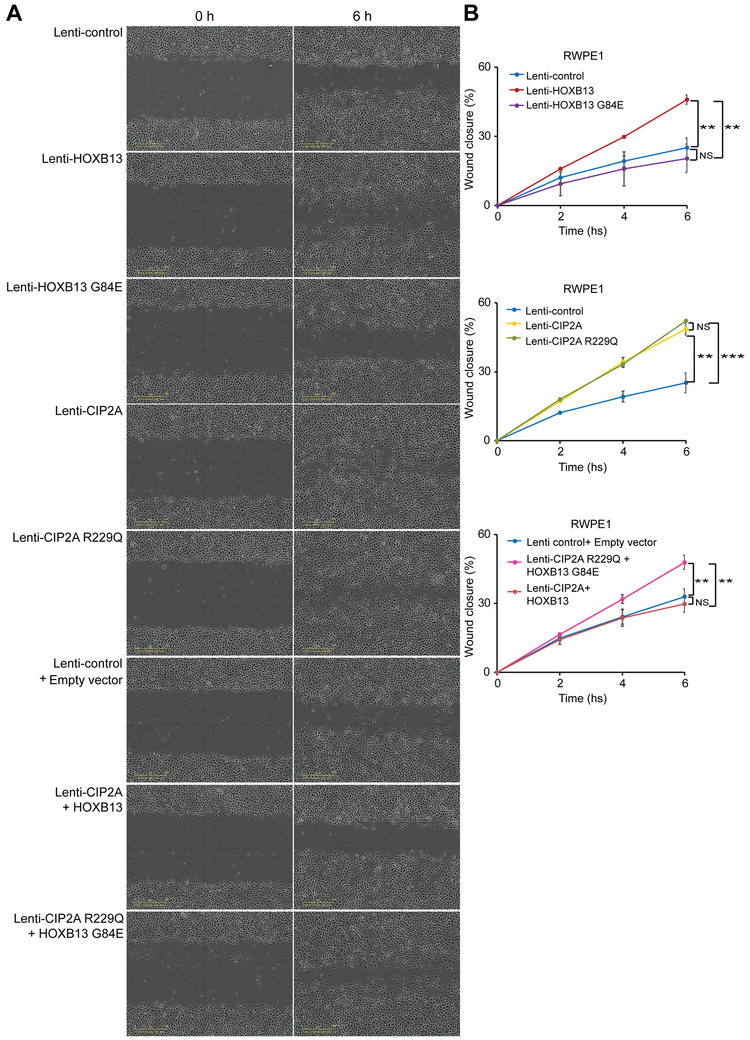
In agreement with these results, our wound healing assays showed that the co-overexpression of HOXB13 G84E and CIP2A R229Q increased the wound closure rate in a time-dependent manner in RWPE1 and 22Rv1 cells (Figure 2, Supplementary Figure 2, Supplementary Table 9), suggesting synergistic roles of these variants in cell migration. Moreover, similar to the results of cell proliferation assays, overexpression of HOXB13, HOXB13 G84E, CIP2A or CIP2A R229Q in different cell line has different impact on cell migration, which might be due to the different genetic background of these prostate cell lines or their representation of different stage of PrCa.
Figure 2. Effects of HOXB13 G84E and CIP2A R229Q variants on prostate cell migration.
A. Representative images of wound healing assays for RWPE1 cells overexpressing HOXB13, HOXB13 G84E, CIP2A, CIP2A R229Q, HOXB13 with CIP2A or HOXB13 G84E with CIP2A R229Q, respectively.
B. Quantification of the fraction of the closure rate (percentage) of original wound area in triplicate wells.
Error bars, s.d. from three biological replicates. *P < 0.05, **P < 0.01, ***P < 0.01 from two-tailed t-tests. NS, not significant. Note that ectopic expression of both HOXB13 G84E and CIP2A R229Q variants obviously increases wound closure rate compared with that of wild type HOXB13 and CIP2A expressing RWPE1 cells.
To further investigate the molecular alterations in PrCa cells co-expressing HOXB13 G84E and CIP2A R229Q, we sought to identify their potential downstream target genes. We thus performed RNA-Seq analysis of 22Rv1 cells co-overexpressing HOXB13 G84E and CIP2A R229Q and of 22Rv1 cells co-expressing HOXB13 and CIP2A. We found that overexpression of HOXB13 G84E and CIP2A R229Q upregulated known oncogenic drivers of PrCa, such as NAMPT(29) and MALAT-1(30), and downregulated tumour suppressors, including AHNAK(31) and HUWE1(32) (Supplementary Table 9), thereby potentially promoting PrCa cell proliferation and migration. Together, these functional results further support the synergistic roles of these variants in PrCa predisposition.
HOXB13 transcriptionally regulates CIP2A expression
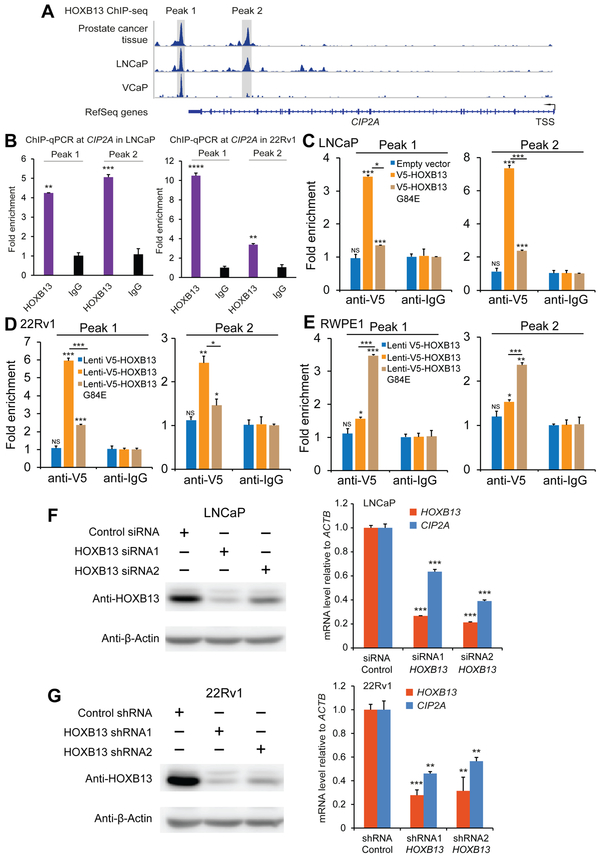
HOXB13 is a prostate-lineage-specific transcription factor and known to be important for prostate normal development and tumorigenesis. CIP2A is often highly expressed in PrCa samples and cell lines. We thus sought to examine whether HOXB13 potentially regulates the transcriptional expression of CIP2A. We have previously mapped genome-wide binding sites for HOXB13 using chromatin immunoprecipitation coupled with massively parallel sequencing (ChIP-seq) (33). Interestingly, query from this HOXB13 ChIP-seq data set, we observed a strong chromatin binding of HOXB13 at CIP2A locus in thePrCa cell line VCaP (Figure 3A). Consistently, HOXB13 binding at CIP2A was also observed using additional ChIP-seq data derived from both LNCaP cells and PrCa tissue samples (Figure 3A). We next performed HOXB13 ChIP-qPCR assays and confirmed HOXB13 chromatin binding at CIP2A regulatory regions in both the LNCaP and 22Rv1 PrCa cell lines (Figure 3B and for VCaP cell line in Supplementary Figure 3). To assess whether the HOXB13 variant influences the transcriptional regulation of CIP2A, we performed ectopic expression of V5-tagged HOXB13 or HOXB13 G84E in 22Rv1, RWPE1 and LNCaP cell lines (Figure 1A, 1C and Supplementary Figure 4) followed by ChIP-qPCR with V5 antibody. Intriguingly, HOXB13 indicated stronger binding at CIP2A regulatory regions than HOXB13 G84E in both LNCaP and 22Rv1 PrCa cell lines (Figure 3C and 3D). In contrast, the binding affinity of HOXB13 G84E at CIP2A regulatory regions is higher than that of HOXB13 in the human immortalized prostatic epithelial RWPE1 cells (Figure 3E), suggesting different roles of HOXB13 and HOXB13 G84E in gene regulation at different stages of PrCa tumorigenesis. We next examined if HOXB13 functionally regulates the expression of CIP2A, and thus performed RNA interference (RNAi)-mediated knockdown of HOXB13 in PrCa LNCaP and 22Rv1 cell lines, respectively. Knockdown efficiency was confirmed at both mRNA and protein level (Figure 3F and 3G). The results showed that depletion of HOXB13 led to greatly diminished mRNA levels of CIP2A (Figure 3F and 3G). Taken together, these results demonstrate that CIP2A is a direct regulatory target of HOXB13.
Figure 3. CIP2A is directly regulated by HOXB13 at transcriptional level.
A. ChIP-seq assays indicate strong binding of HOXB13 at CIP2A gene in prostate cancer cells and tissue (data sets from refs. (48) and (6)).
B. ChIP-qPCR of HOXB13 at CIP2A in the PrCa LNCaP and 22Rv1 cell lines. ** p < 0.01; *** p < 0.001, ****p<0.0001
C-E. ChIP-qPCR at CIP2A in the PrCa LNCaP (C), and 22Rv1 cells (D) and immortalized benign prostate RWPE1 (E) cell line overexpressing V5 tagged HOXB13 or G84E. *p<0.05, ** p < 0.01; *** p < 0.001, from two-tailed t-tests. NS, not significant.
F-G. Real time qPCR analyses reveal markedly reduced mRNA levels of CIP2A (KIAA1524) upon knockdown of HOXB13 both in the LNCaP (siRNA) and 22Rv1 (shRNA) prostate cancer cell lines. Western blot results indicate depletion of HOXB13 in the PrCa LNCaP (siRNA) and 22Rv1 (shRNA) cell lines. Error bars, s.e.m. ** p < 0.01, *** p < 0.001, two-tailed student t-test.
DISCUSSION
Shown evidence of HOXB13 G84E in PrCa susceptibility(33) is validated here through the significantly larger risk effect compared to common SNPs identified by GWAS with ORs ranging between 1.04-2.90(3,34,35). Present study justifies the earlier association (OR 3.6) in unselected non-familial Finnish PrCa cases(8) with 8-time higher odds in carriers. Our results are consistent with Laitinen and colleagues, that in the presence of HOXB13 G84E mutation the risk to have more likely >20 ng/mL PSA at diagnosis is significantly higher(8), providing strengthened clinical evidence for combined application of HOXB13 G84E variant and PSA as biomarkers in population screening trials(36). Similar to the findings of other studies, no association was observed between G84E and Gleason score, which is commonly considered as a marker of aggressive disease(8,10). However, the speculated carcinogenic potential of HOXB13 G84E carriers in the shift between BPH and PrCa was successfully revealed in this study, supporting earlier observations(8,29,37).
Here, in addition to risk evaluation, we assessed the prognostic impact of HOXB13 G84E mutation on survival. This is the first study to report on longer than 10 years follow up of PrCa patients. Earlier studies analysed the whole survival time after diagnoses until death(8), or 5 and 10 years follow-up(10), and no association with HOXB13 G84E mutation was found. Through the applied thorough follow-up approach we were able to show a suggestive association that patients possessing the HOXB13 G84E had higher chance of dying from PrCa when PSA progresses, or when any of local or distant progression is present; though, the difference was not statistically significant.
This study represents the first comprehensive case-control study assessing the prevalence of the CIP2A (rs2278911, R229Q) variant in PrCa. Prior to this analysis, the role of CIP2A R229Q genetic variation has been investigated only in Asian hepatocellular carcinoma patients(19). Contrary to the results from Asians, in our analysis the ancestral C allele is the major and the variant T is the minor allele with a 25.6% carrier frequency in entire sample set vs the 82.3% reported in Asians. As rs2278911 variant did not show risk for PrCa, we next focused on potential clinico-pathological, predictive and prognostic roles of CIP2A variant in unselected PrCa samples in Finland. We were able to show no clinical role of the CIP2A alone. This is consistent with findings in PP2A, which showed that it may enhance tumorigenic potential only in combination with other cancer drivers(38).
The biggest tribute of this study was the interactive modelling of HOXB13 G84E and CIP2A R229Q variants on PrCa outcome. Exceeding all previous expectations, dual carriers of the HOXB13 T and CIP2A T alleles were at considerably high risk of PrCa. The odds for PrCa development were 21.1 times higher in dual carriers vs non-dual T carriers in the Finnish discovery cohort. Further to this end, synergistic effect of HOXB13 and CIP2A variants enables us to detect the risk of PrCa with three-fold higher odds than the HOXB13 T allele alone. Additionally, the earlier time-to-event (7.2 months) reflects the clinical importance of synergistic risk effect of HOXB13 and CIP2A in dual T carriers. A high PrCa risk conferred by the combination of HOXB13 T and CIP2A T alleles was verified both in independent Finnish (OR 24.0) and in Swedish (OR 6.4) validation cohorts.
To date, only few variant and no synergistic effect have been shown to be associated with clinical features and aggressive disease. In a meta-analysis, the rs11672691 SNP on chromosome 19 showed association with aggressive PrCa (Gleason score ≥ 8; OR 1.12)(39). The rs1571801 (9q33) in the DAB2IP gene was associated with aggressive PrCa in European Americans, as measured by a complex set of clinical variables (OR 1.32)(40). In a Finnish study, a rare SNP (rs200331695) within the EMSY intron in 11q13.5 region was associated with aggressive PrCa (PSA ≥ 20 μg/L or Gleason grade ≥ 7) in unselected cases compared to controls (OR = 6.0)(41). Here, we showed for the first time a synergistic effect of two variants on aggressive PrCa, obtaining a high odds ratio of 2.3. Significant association between the synergistic variants and high Gleason score, i.e. aggressive disease, was not seen in the Swedish population possibly because of the underlying genetic heterogeneity between the two populations. In addition, no association with aggressive disease in the validation cohorts might be due, at least in part, to patient selection criteria and clinical differences between the discovery and validation cohorts. Higher percentage of non-clinically detected PrCa cases were included in the validation cohort (TAMPERE2 48%) compared to the discovery cohort (17%). This is reflected also in clinical criteria: in the discovery cohort the percentage of high Gleason score patients is higher (21.8%) than in the STHLM2 (17.2%) or in the TAMPERE2 (18.7%).
In addition, dual T carrier status was significantly associated with high PSA at diagnosis, with an observed effect size of OR 3.30 in Swedish cohort, which might be population specific. In addition, other interacting genes may differ in Swedish population compared to the Finnish and this may modify the effect on PSA.
To the best of our knowledge, this is the first study on synergistic interaction of genetic biomarkers in PrCa to report association with already existing biomarker and high-grade, aggressive disease. To take advantage of these findings, employment of both variants in PrCa screening would enable the identification of clinically relevant cases already at screening, and at the same time enhance the efficacy of it. Overall, CIP2A T allele seemingly increased the effect of HOXB13 T allele in more than one aspect of PrCa clinical characteristics.
Here, our functional experiments provide evidence of a synergistic role of HOXB13 G84E and CIP2A R229Q variants in promoting PrCa cell proliferation and migration.
It has been demonstrated that both HOXB13(42) and CIP2A(15) are overexpressed in CRPCs. Here we add, that combined high expression of HOXB13-CIP2A outperforms each gene alone in prediction of the time to biochemical relapse, which underpins the prognostic potential of HOXB13-CIP2A in PrCa. Notably, synergism at the expression level and its association with the clinical features of PrCa has not been described to date.
The suggested dual role of HOXB13 gene, namely to act both as a tumour suppressor gene and as an oncogene, has been described previously in the literature(5). Recently, Pomerantz et al reported the first demonstration of an oncogenic effect of HOXB13 in combination with pioneer transcription factor FOXA1 involving the reprogramming of genome-wide AR binding sites (cistrome) during human prostate epithelial transformation(6). Nevertheless, the molecular explanation for oncogenic role of HOXB13 G48E remains largely unknown(37) and therefore requires further investigation. Novel molecular pathways driving PrCa in HOXB13 G84E carriers have been suggested. Smith et al identified aberrant molecular features in HOXB13 G84E carriers. These patients had low prevalence of ERG-fusion positive cancers, and increased prevalence of SPINK1 overexpression(37). Here, we revealed the molecular basis of synergistic activities of HOXB13 and CIP2A mutants in clinical PrCa progression and the promotion of PrCa cell proliferation and migration. Our results also demonstrate that HOXB13 functionally regulates CIP2A transcriptional expression. We provided strong evidence supporting CIP2A as a direct target of HOXB13.
Furthermore, if we take into account cancer pathways as a whole it is easy to see that CIP2A-mediated PP2A inhibition may promote activities of various PrCa pathways(43,44). For example, HOXB13 has been shown to promote PrCa metastasis by stimulation of NF-κB signaling(45) and NF-κB is a target for PP2A tumour suppressor activity in PrCa cells(46). Importantly, we also identify here HOXB13 as a positive regulator of CIP2A transcription in PrCa cell lines. This result, together with the results of a previous study demonstrating the role of AR in positively regulating CIP2A expression (47), provides a well-defined example of the lineage-specific roles of HOXB13 in reprogramming the AR cistrome and driving gene expression in human prostate tumorigenesis (29). Obviously, we cannot rule out the possibility that HOXB13 might regulate many important genes other than CIP2A in driving prostate carcinogenesis. Collectively, HOXB13 regulation of CIP2A expression and other findings of this study demonstrate the co-operation of HOXB13 and CIP2A at multiple levels in PrCa.
In conclusion, the combination of HOXB13 T and CIP2A T alleles appears to have a definite effect on PrCa risk, the time to develop the disease, disease aggressiveness, the detection of clinically relevant PrCa disease-specific life expectancy and PrCa cell proliferation and migration. Genetic synergism was confirmed through the synergistic findings of higher HOXB13-CIP2A mRNA expression, predicting earlier biochemical relapse of PrCa patients. Furthermore, we describe here the molecular basis of the synergism, namely that HOXB13 protein binds to CIP2A gene and functionally promotes CIP2A transcription. Synergistic effects need to be confirmed in other ethnic groups and populations, and in familial background of PrCa. Further molecular studies needed as well.
Supplementary Material
Translational Relevance.
Synergistic genetic interaction of HOXB13 rs138213197 (G84E) germline mutation with a common variant CIP2A rs2278911 (R229Q), first demonstrated here, confers risk for aggressive PrCa. Our unique findings suggest exceptional clinical potential of HOXB13-CIP2A as novel synergistic genetic markers in aggressive PrCa. Combined high expression of HOXB13-CIP2A outperforms each gene alone in prediction of the time to biochemical relapse, showing also prognostic potential. Genetic biomarkers such HOXB13/CIP2A and their germline genetic testing may bring new opportunities for precision oncology in PrCa. Understanding of the clinical relevance of the molecular sub-classification may have a critical role in the developments of patient selection strategies and new therapeutic approaches.
Acknowledgments
The authors thank the patients who participated in this study. Liisa Määttänen is thanked for assistance related to FinRSPC screening trial samples, Riina Kylätie, Elina Kaikkonen and Jukka Karhu for help in laboratory work, and Katri Pylkäs and Meeri Otsukka in helping next-generation sequencing. Tero Vahlberg’s help is acknowledged for his advice in biostatistics. This work was financially supported by the Academy of Finland grants (#251074 JS) and (#284618 and #279760 G-HW), The Finnish Cancer Organisations, and the Sigrid Juselius Foundation.
We thank for the members from the Prostate Cancer Association Group to Investigate Cancer Associated Alterations in the Genome (PRACTICAL) consortium who are provided in the Supplement/foot notes. Information of the consortium can be found at http://practical.icr.ac.uk/.
Genotyping of the OncoArray was funded by the US National Institutes of Health (NIH) [U19 CA 148537 for ELucidating Loci Involved in Prostate cancer SuscEptibility (ELLIPSE) project and X01HG007492 to the Center for Inherited Disease Research (CIDR) under contract number HHSN268201200008I]. Additional analytic support was provided by NIH NCI U01 CA188392 (PI: Schumacher).
The PRACTICAL consortium was supported by Cancer Research UK Grants C5047/A7357, C1287/A10118, C1287/A16563, C5047/A3354, C5047/A10692, C16913/A6135, European Commission's Seventh Framework Programme grant agreement n° 223175 (HEALTH-F2-2009-223175), and The National Institute of Health (NIH) Cancer Post-Cancer GWAS initiative grant: No. 1 U19 CA 148537-01 (the GAME-ON initiative).
We would also like to thank the following for funding support: The Institute of Cancer Research and The Everyman Campaign, The Prostate Cancer Research Foundation, Prostate Research Campaign UK (now Prostate Action), The Orchid Cancer Appeal, The National Cancer Research Network UK, The National Cancer Research Institute (NCRI) UK. We are grateful for support of NIHR funding to the NIHR Biomedical Research Centre at The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust.
Financial support:
Johanna Schleutker Academy of Finland grant (#251074)
Gong-Hong Wei Academy of Finland grant (#284618 and #279760)
Footnotes
Disclosure of potential conflicts of interest:
The authors declare no potential conflicts of interest.
REFERENCES
- 1.Mucci LA, Hjelmborg JB, Harris JR, Czene K, Havelick DJ, Scheike T, et al. Familial Risk and Heritability of Cancer Among Twins in Nordic Countries. JAMA 2016;315(1):68–76 doi 10.1001/jama.2015.17703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eeles R, Goh C, Castro E, Bancroft E, Guy M, Al Olama AA, et al. The genetic epidemiology of prostate cancer and its clinical implications. Nat Rev Urol 2014;11(1):18–31 doi 10.1038/nrurol.2013.266. [DOI] [PubMed] [Google Scholar]
- 3.Eeles RA, Olama AA, Benlloch S, Saunders EJ, Leongamornlert DA, Tymrakiewicz M, et al. Identification of 23 new prostate cancer susceptibility loci using the iCOGS custom genotyping array. Nat Genet 2013;45(4):385–91, 91e1-2 doi 10.1038/ng.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amin Al Olama A, Benlloch S, Antoniou AC, Giles GG, Severi G, Neal DE, et al. Risk Analysis of Prostate Cancer in PRACTICAL, a Multinational Consortium, Using 25 Known Prostate Cancer Susceptibility Loci. Cancer Epidemiol Biomarkers Prev 2015;24(7):1121–9 doi 10.1158/1055-9965.EPI-14-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ewing CM, Ray AM, Lange EM, Zuhlke KA, Robbins CM, Tembe WD, et al. Germline mutations in HOXB13 and prostate-cancer risk. N Engl J Med 2012;366(2):141–9 doi 10.1056/NEJMoa1110000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pomerantz MM, Li F, Takeda DY, Lenci R, Chonkar A, Chabot M, et al. The androgen receptor cistrome is extensively reprogrammed in human prostate tumorigenesis. Nat Genet 2015;47(11):1346–51 doi 10.1038/ng.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Z, Greenwood C, Isaacs WB, Foulkes WD, Sun J, Zheng SL, et al. The G84E mutation of HOXB13 is associated with increased risk for prostate cancer: results from the REDUCE trial. Carcinogenesis 2013;34(6):1260–4 doi 10.1093/carcin/bgt055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laitinen VH, Wahlfors T, Saaristo L, Rantapero T, Pelttari LM, Kilpivaara O, et al. HOXB13 G84E mutation in Finland: population-based analysis of prostate, breast, and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev 2013;22(3):452–60 doi 10.1158/1055-9965.EPI-12-1000-T. [DOI] [PubMed] [Google Scholar]
- 9.Karlsson R, Aly M, Clements M, Zheng L, Adolfsson J, Xu J, et al. A population-based assessment of germline HOXB13 G84E mutation and prostate cancer risk. Eur Urol 2014;65(1):169–76 doi 10.1016/j.eururo.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 10.Kote-Jarai Z, Mikropoulos C, Leongamornlert DA, Dadaev T, Tymrakiewicz M, Saunders EJ, et al. Prevalence of the HOXB13 G84E germline mutation in British men and correlation with prostate cancer risk, tumour characteristics and clinical outcomes. Ann Oncol 2015;26(4):756–61 doi 10.1093/annonc/mdv004. [DOI] [PubMed] [Google Scholar]
- 11.Breyer JP, Avritt TG, McReynolds KM, Dupont WD, Smith JR. Confirmation of the HOXB13 G84E germline mutation in familial prostate cancer. Cancer Epidemiol Biomarkers Prev 2012;21(8):1348–53 doi 10.1158/1055-9965.EPI-12-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zabalza CV, Adam M, Burdelski C, Wilczak W, Wittmer C, Kraft S, et al. HOXB13 overexpression is an independent predictor of early PSA recurrence in prostate cancer treated by radical prostatectomy. Oncotarget 2015;6(14):12822–34 doi 10.18632/oncotarget.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel R, Gao M, Ahmad I, Fleming J, Singh LB, Rai TS, et al. Sprouty2, PTEN, and PP2A interact to regulate prostate cancer progression. J Clin Invest 2013;123(3):1157–75 doi 10.1172/JCI63672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perrotti D, Neviani P. Protein phosphatase 2A: a target for anticancer therapy. Lancet Oncol 2013;14(6):e229–38 doi 10.1016/S1470-2045(12)70558-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khanna A, Rane JK, Kivinummi KK, Urbanucci A, Helenius MA, Tolonen TT, et al. CIP2A is a candidate therapeutic target in clinically challenging prostate cancer cell populations. Oncotarget 2015;6(23):19661–70 doi 10.18632/oncotarget.3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaarala MH, Väisänen MR, Ristimäki A. CIP2A expression is increased in prostate cancer. J Exp Clin Cancer Res 2010;29:136 doi 10.1186/1756-9966-29-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khanna A, Pimanda JE. Clinical significance of Cancerous Inhibitor of Protein Phosphatase 2A (CIP2A) in human cancers. Int J Cancer 2015. doi 10.1002/ijc.29431. [DOI] [PubMed] [Google Scholar]
- 18.Huang J, Jia J, Tong Q, Liu J, Qiu J, Sun R, et al. Knockdown of cancerous inhibitor of protein phosphatase 2A may sensitize metastatic castration-resistant prostate cancer cells to cabazitaxel chemotherapy. Tumour Biol 2014. doi 10.1007/s13277-014-2748-5. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Wang K, Dai L, Wang P, Song C, Shi J, et al. HapMap-based study of CIP2A gene polymorphisms and HCC susceptibility. Oncol Lett 2012;4(2):358–64 doi 10.3892/ol.2012.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009;360(13):1320–8 doi 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 21.Cordell HJ, Clayton DG. A unified stepwise regression procedure for evaluating the relative effects of polymorphisms within a gene using case/control or family data: application to HLA in type 1 diabetes. Am J Hum Genet 2002;70(1):124–41 doi 10.1086/338007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Network CGAR. The Molecular Taxonomy of Primary Prostate Cancer. Cell 2015;163(4):1011–25 doi 10.1016/j.cell.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012;2(5):401–4 doi 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Souto MC, Costa IG, de Araujo DS, Ludermir TB, Schliep A. Clustering cancer gene expression data: a comparative study. BMC Bioinformatics 2008;9:497 doi 10.1186/1471-2105-9-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tavazoie S, Hughes JD, Campbell MJ, Cho RJ, Church GM. Systematic determination of genetic network architecture. Nat Genet 1999;22(3):281–5 doi 10.1038/10343. [DOI] [PubMed] [Google Scholar]
- 26.Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet 2014;46(3):310–5 doi 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jagadeesh KA, Wenger AM, Berger MJ, Guturu H, Stenson PD, Cooper DN, et al. M-CAP eliminates a majority of variants of uncertain significance in clinical exomes at high sensitivity. Nat Genet 2016. doi 10.1038/ng.3703. [DOI] [PubMed] [Google Scholar]
- 28.Larson NB, McDonnell S, French AJ, Fogarty Z, Cheville J, Middha S, et al. Comprehensively evaluating cis-regulatory variation in the human prostate transcriptome by using gene-level allele-specific expression. Am J Hum Genet 2015;96(6):869–82 doi 10.1016/j.ajhg.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albitar F, Diep K, Ma W, Albitar M. Synonymous Polymorphisms in HOXB13 as a Protective Factor for Prostate Cancer. J Cancer 2015;6(5):409–11 doi 10.7150/jca.11413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren S, Liu Y, Xu W, Sun Y, Lu J, Wang F, et al. Long noncoding RNA MALAT-1 is a new potential therapeutic target for castration resistant prostate cancer. J Urol 2013;190(6):2278–87 doi 10.1016/j.juro.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Lee IH, Sohn M, Lim HJ, Yoon S, Oh H, Shin S, et al. Ahnak functions as a tumor suppressor via modulation of TGFβ/Smad signaling pathway. Oncogene 2014;33(38):4675–84 doi 10.1038/onc.2014.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan L, Peng G, Sahgal N, Fazli L, Gleave M, Zhang Y, et al. Regulation of c-Myc expression by the histone demethylase JMJD1A is essential for prostate cancer cell growth and survival. Oncogene 2016;35(19):2441–52 doi 10.1038/onc.2015.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang H, Cai B. G84E mutation in HOXB13 is firmly associated with prostate cancer risk: a meta-analysis. Tumour Biol 2014;35(2):1177–82 doi 10.1007/s13277-013-1157-5. [DOI] [PubMed] [Google Scholar]
- 34.Eeles RA, Kote-Jarai Z, Al Olama AA, Giles GG, Guy M, Severi G, et al. Identification of seven new prostate cancer susceptibility loci through a genome-wide association study. Nat Genet 2009;41(10):1116–21 doi 10.1038/ng.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kote-Jarai Z, Olama AA, Giles GG, Severi G, Schleutker J, Weischer M, et al. Seven prostate cancer susceptibility loci identified by a multi-stage genome-wide association study. Nat Genet 2011;43(8):785–91 doi 10.1038/ng.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Zappa M, Nelen V, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014;384(9959):2027–35 doi 10.1016/S0140-6736(14)60525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith SC, Palanisamy N, Zuhlke KA, Johnson AM, Siddiqui J, Chinnaiyan AM, et al. HOXB13 G84E-related familial prostate cancers: a clinical, histologic, and molecular survey. Am J Surg Pathol 2014;38(5):615–26 doi 10.1097/PAS.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu X, Garcia C, Fazli L, Gleave M, Vitek MP, Jansen M, et al. Inhibition of Pten deficient Castration Resistant Prostate Cancer by Targeting of the SET - PP2A Signaling axis. Sci Rep 2015;5:15182 doi 10.1038/srep15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amin Al Olama A, Kote-Jarai Z, Schumacher FR, Wiklund F, Berndt SI, Benlloch S, et al. A meta-analysis of genome-wide association studies to identify prostate cancer susceptibility loci associated with aggressive and non-aggressive disease. Hum Mol Genet 2013;22(2):408–15 doi 10.1093/hmg/dds425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duggan D, Zheng SL, Knowlton M, Benitez D, Dimitrov L, Wiklund F, et al. Two genome-wide association studies of aggressive prostate cancer implicate putative prostate tumor suppressor gene DAB2IP. J Natl Cancer Inst 2007;99(24):1836–44 doi 10.1093/jnci/djm250. [DOI] [PubMed] [Google Scholar]
- 41.Nurminen R, Wahlfors T, Tammela TL, Schleutker J. Identification of an aggressive prostate cancer predisposing variant at 11q13. Int J Cancer 2011;129(3):599–606 doi 10.1002/ijc.25754. [DOI] [PubMed] [Google Scholar]
- 42.Kim YR, Kang TW, To PK, Xuan Nguyen NT, Cho YS, Jung C, et al. HOXB13-mediated suppression of p21WAF1/CIP1 regulates JNK/c-Jun signaling in prostate cancer cells. Oncol Rep 2016;35(4):2011–6 doi 10.3892/or.2016.4563. [DOI] [PubMed] [Google Scholar]
- 43.Westermarck J, Hahn WC. Multiple pathways regulated by the tumor suppressor PP2A in transformation. Trends Mol Med 2008;14(4):152–60 doi 10.1016/j.molmed.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Khanna A, Pimanda JE, Westermarck J. Cancerous inhibitor of protein phosphatase 2A, an emerging human oncoprotein and a potential cancer therapy target. Cancer Res 2013;73(22):6548–53 doi 10.1158/0008-5472.CAN-13-1994. [DOI] [PubMed] [Google Scholar]
- 45.Kim YR, Kim IJ, Kang TW, Choi C, Kim KK, Kim MS, et al. HOXB13 downregulates intracellular zinc and increases NF-κB signaling to promote prostate cancer metastasis. Oncogene 2014;33(37):4558–67 doi 10.1038/onc.2013.404. [DOI] [PubMed] [Google Scholar]
- 46.Kar S, Palit S, Ball WB, Das PK. Carnosic acid modulates Akt/IKK/NF-κB signaling by PP2A and induces intrinsic and extrinsic pathway mediated apoptosis in human prostate carcinoma PC-3 cells. Apoptosis 2012;17(7):735–47 doi 10.1007/s10495-012-0715-4. [DOI] [PubMed] [Google Scholar]
- 47.Cristóbal I, González-Alonso P, Daoud L, Solano E, Torrejón B, Manso R, et al. Activation of the Tumor Suppressor PP2A Emerges as a Potential Therapeutic Strategy for Treating Prostate Cancer. Mar Drugs 2015;13(6):3276–86 doi 10.3390/md13063276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang Q, Whitington T, Gao P, Lindberg JF, Yang Y, Sun J, et al. A prostate cancer susceptibility allele at 6q22 increases RFX6 expression by modulating HOXB13 chromatin binding. Nat Genet 2014;46(2):126–35 doi 10.1038/ng.2862. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.