Abstract
Mucopolysaccharidosis IVA (OMIM 253000; also known as Morquio A syndrome) is associated with skeletal, airway, and hearing abnormalities. Cochlear implantation is an effective intervention for patients with severe-to-profound hearing loss. Patients can gain substantial improvement in auditory performance, speech perception, and their quality of life from cochlear implantation. Although severe progressive sensorineural hearing loss is a common feature of mucopolysaccharidosis IVA, no detailed description of cochlear implantation for mucopolysaccharidosis IVA has been reported. To review the effectiveness and special considerations associated with cochlear implantation in patients with mucopolysaccharidosis IVA, we here report the case of cochlear implantation in mucopolysaccharidosis IVA by a multidisciplinary team. A retrospective chart review was conducted on a 34-year-old female with mucopolysaccharidosis IVA, who received a cochlear implant. Audiometric thresholds, speech perception scores, and cochlear implant processor mapping information were reviewed during the first 12 months following cochlear implantation. The results of audiological tests indicate improved hearing thresholds as well as remarkable enhancement of speech perception skills over 12 months of cochlear implant use. Cochlear implantation improved auditory performance in a mucopolysaccharidosis IVA patient with postlingually severe-to-profound sensorineural hearing loss. The benefits of cochlear implantation could be meaningful for other Morquio patients with progressive hearing loss, although the risks of surgery and anesthesia should be carefully considered by a multidisciplinary team of experts during the cochlear implant candidacy process.
Keywords: Mucopolysaccharidosis, cochlear implantation, hearing loss, speech perception
Introduction
Mucopolysaccharidosis IVA (MPS IVA; OMIM 253000, also known as Morquio A syndrome) is a rare metabolic disorder caused by a deficiency of N-acetylgalactosamine-6-sulfate sulfatase enzyme to degrade glycosaminoglycans (GAGs) such as keratan sulfate and chondroitin-6-sulfate. The accumulated GAGs in the tissue cause cellular dysfunction, which affects various tissues and organs. The common symptoms include marked disproportionate short stature, short neck, skeletal abnormalities, upper and lower airway issues, hypermobile joints, upper respiratory tract infections, progressive upper airway obstructions, hearing loss, otitis media with effusion (OME), mildly coarse facial features, cardiac valve issues, and vision and dental issues.1,2 Unlike other MPS types, MPS IVA does not affect intelligence.1,3
Hearing loss is one of the most common health problems among patients with MPS IVA2 and can include both conductive and sensorineural types of hearing loss. Most patients with MPS IVA experience persistent OME throughout their life, and in some, sensorineural hearing loss appears during childhood and progressively worsens as the disease progresses.4,5 We have found that height correlates negatively with severity of hearing loss.5 The life expectancy of MPS IVA depends on the severity of symptoms. Patients with severe symptoms may not survive until young adulthood, but patients with mild MPS IVA may survive beyond young adulthood. Furthermore, life expectancy is increasing in MPS patients due to recent medical and surgical advancement.6 Consequently, there are now more cases of patients with MPS IVA with severe-to-profound hearing loss with age.
Successful cochlear implantation (CI) was reported in a child with MPS I and a child with MPS II, recently.7 The child with MPS I had moderate-to-profound hearing loss from infancy that progressed over his first several years of life. He underwent CI at 12 years of age. Before CI, he could respond to speech or recognize environmental sounds, but was not able to discriminate speech sounds. At 1 year after CI, the patient showed significant improvement of his hearing thresholds and was able to understand conversation without lip-reading with a familiar talker. The child with MPS II had congenital bilateral sensorineural hearing loss and received CI at 4 years of age. He substantially improved their hearing thresholds after CI and showed great speech discrimination abilities in both quiet and noisy environments. Until now, there has been no detailed report of CI outcomes in patients with MPS IVA. This report describes the CI outcomes in one adult patient with MPS IVA, as well as perioperative considerations important to successful CI in this patient group.
Case report
A retrospective review of the clinical chart of a female patient diagnosed with MPS IVA was performed to retrieve the pertinent information regarding her CI. To examine the outcome of CI, we used the hearing thresholds, speech reception thresholds, and speech perception test results at pre-implantation and 1, 3, 9, and 12 months post-activation. Speech perception tests include closed-set word recognition, open-set word recognition, and the Hearing In Noise Test (HINT) sentence recognition tests. All the speech perception tests were conducted in a quiet condition.
Clinical course
This female patient was diagnosed with MPS IVA at 5 years of age. Her first language is Cantonese, but the patient started to learn English at 3 years of age. The patient has a history of recurring ear infections. At 15 years of age, she reported that she failed a hearing screening at school. She started to use hearing aids regularly at 18 years of age. Her hearing loss worsened around the age of 26 years and continued to progress thereafter. She had started enzyme replacement therapy at 28 years of age. At the age of 34 years, she reported that hearing aids did not provide adequate benefit to support communication. Risks and benefits of CI were discussed by an interdisciplinary team, which included otolaryngologists (one expert for adult population and one expert on pediatric population), audiologists, anesthesiologists, radiologists, orthopedic physicians, and specialists with extensive experience with MPS patients. At the time of CI candidacy review, she was diagnosed with bilateral severe-to-profound sensorineural hearing loss. Her unaided pure-tone thresholds are shown in Table 1. Her unaided speech reception thresholds were 75 and 100 dBHL in the left and right ears, respectively. Her aided speech reception thresholds were 40 dBHL in the left ear and 70 dBHL in the right ear. The audiological evaluation before implantation suggested that hearing on the right side was no longer aidable, and her speech perception scores were very poor even with binaural amplification. A computed tomography (CT) scan was performed to examine the temporal bones and surrounding auditory areas with pediatric dose reduction strategies. Her right mastoid was found to be sclerotic, but inner ear anatomy was normal. Non-sedated magnetic resonance imaging (MRI) was conducted to examine the cervical, thoracic, and lumbar spine on a 1.5 T magnet, and no acute concerns were raised. The airway was evaluated according to our Morquio protocol using a CT angiogram which revealed a triangular-shaped trachea with moderate stenosis at the level of thoracic inlet as shown in Figure 1. The anesthetic plan was discussed extensively with the patient and parents. Due to the past history of difficult awake trans-nasal fiberoptic intubation, a sedated intubation using a video laryngoscope with or without a rigid bronchoscopic assist was planned. After careful review by the interdisciplinary team, a recommendation was made to move forward with right CI using a Med-El Synchrony device, which was selected for MRI compatibility.
Table 1.
Hearing thresholds in the unaided right and left ears at pre-implantation, and hearing thresholds of the (implanted) right ear at 1, 3, 9, and 12 months post-activation.
| Time | Ear | 0.25 kHz | 0.5 kHz | 1 kHz | 2 kHz | 3 kHz | 4 kHz | 6 kHz | 8 kHz |
|---|---|---|---|---|---|---|---|---|---|
| Pre-implant | R | 70 | 85 | NR | NR | NA | NR | NA | NR |
| Pre-implant | L | 60 | 75 | 80 | 80 | NA | 70 | NA | 80 |
| 1 month | R | 40 | 45 | 40 | 45 | 45 | 40 | 40 | 35 |
| 3 months | R | 40 | 35 | 35 | 25 | 35 | 35 | 35 | NA |
| 9 months | R | 35 | 30 | 30 | 30 | 30 | 35 | 25 | 25 |
| 12 months | R | 30 | 30 | 30 | 30 | NA | 25 | 25 | NA |
R: right; L: left; NR: no response; NA: not available.
All thresholds are reported in decibels hearing level (dBHL). The maximum levels tested were 115 dBHL at 1, 2, and 4 kHz and 90 dBHL at 8 kHz.
Figure 1.
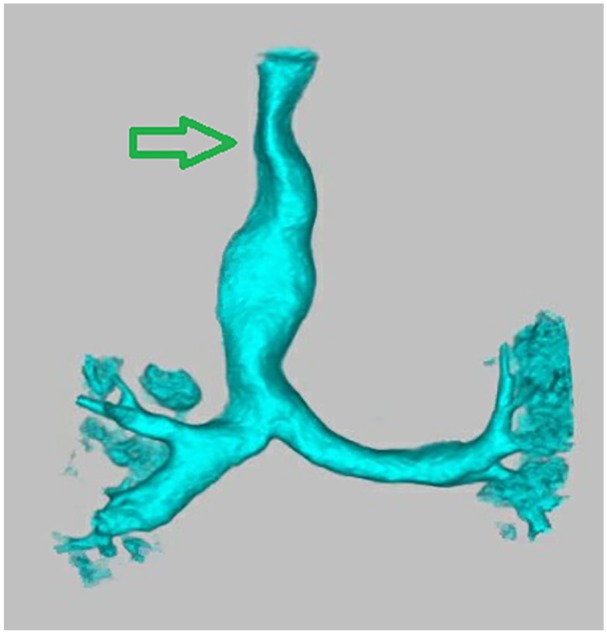
Three-dimensional reconstruction of the CT angiogram of chest with special attention to major airways. Tracheal narrowing is evident at the thoracic inlet. The arrow marks the narrowest part of the trachea at the level of the thoracic inlet with cross-sectional area measuring 64 mm2.
CI
At the time of surgery, the patient was 34 years old, 100 cm tall, and weighed 23.4 kg. Comprehensive neuromonitoring was carried out by a technician throughout both intubation and surgery. During induction of her anesthesia, she was adequately ventilated even though she had a mild-to-moderate degree of upper airway obstruction which was relieved using a jaw thrust. However, her larynx was not accessible with either the video laryngoscope or rigid bronchoscope. In the end, a trans-oral fiberoptic intubation was easily accomplished. While retracting her tongue anteriorly during the intubation, her lingual frenulum was torn and subsequently repaired with suture. Surgery was conducted by two experienced implant surgeons (an adult neurotologist and a pediatric otologist). Surgical positioning was challenging because of the inability to turn the head or extend the neck. Ultimate positioning resulted in an unusual orientation of the facial recess, elevated nearly 60° from the usual horizontal plane. The round window membrane could not be visualized, necessitating an anteroinferior cochleostomy into the scala tympani of the basal turn. The Med-El Flex 28 array was inserted without resistance through electrode 10, but began to recoil at electrode 11, so electrodes 11 and 12 were left extracochlear to avoid tip roll-over. Intraoperative neurotelemetry demonstrated normal im-pedance across the array (except two extracochlear electrodes 11 and 12) and acceptable neural responses. Surgery was completed without complications in 150 min. The patient spent one uneventful night on the inpatient floor and was discharged the following morning.
Post-CI
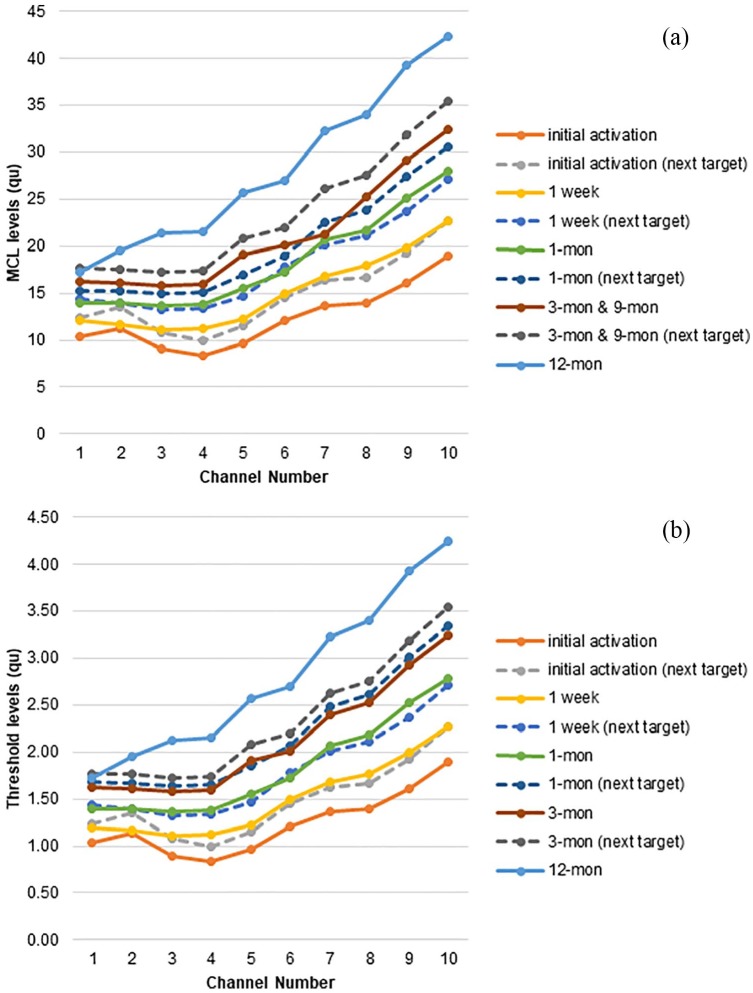
Her initial stimulation was done 19 days post-surgery. After activation of the cochlear implant, each electrode was re-programmed at each CI mapping session. Threshold (THR) level (the softest sound levels that the patient can detect through the implant) and most comfortable loudness (MCL) level were set by audiologists to obtain optimal performance of her CI. Loudness balancing was used to ensure that approximately equal loudness was perceived across all 10 electrodes. Electrodes 11 and 12 were turned off and set as extracochlear electrodes. At each CI mapping session, the levels of current were gradually increased. The patient uses two styles of Med-El audio processors (Sonnet and Rondo). Rondo is an all-in-one unit design and uses disposable batteries to last up to 75 h. The patient usually uses Rondo when she travels because of its long battery life. She uses a behind-the-ear design Sonnet more regularly than Rondo because batteries are rechargeable (despite shorter battery life of 6 or 10 h) and it is difficult to lose. Mapping results in this study were based on the programming of the Sonnet processor because her audiology evaluations were done with the Sonnet. Figure 2 shows the THR and MCL levels at initial mapping, 1-, 3-, 9-, and 12-month post-activation mapping sessions. In each session, additional CI mapping programs were loaded into the speech processor to progressively increase loudness. The patient was instructed to switch to a louder program until the next mapping appointment. As seen in Figure 2, both THR and MCL levels steadily increased after CI activation.
Figure 2.
(a) Most comfortable loudness (MCL) and (b) threshold (THR) levels (top and bottom panels) at active electrodes over 12 months.
Speech perception
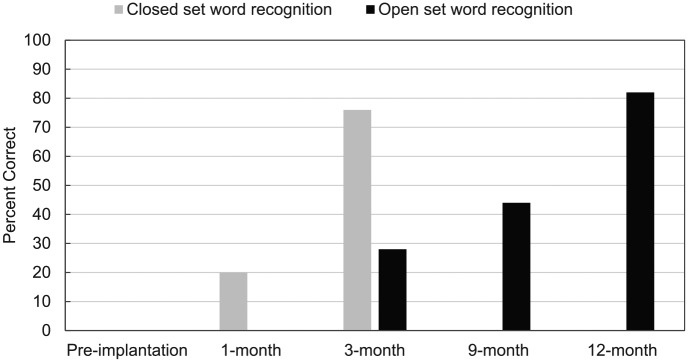
Table 1 shows the hearing thresholds results of her implanted right ear after CI activation. Her hearing thresholds and speech reception threshold in the right ear indicated mild hearing loss at 1 month post-activation. The speech perception results showed significant improvement in 12 months after CI activation. Figure 3 shows the word recognition test results. Her closed-set word recognition score increased to 76% at 3-month follow-up, which was a marked improvement from her 1 month post-activation test results. At the same time, her open-set word recognition was emerging and continuously improved through 12 months. Performance on the HINT sentence recognition test in quiet was 77% at 9 months and reached 86% at 12 months. This is a considerably improved performance compared to her score (0–5 words correct) at pre-implantation. All the tests were conducted in English, but the patient reported that she has a similar benefit when she communicates in Chinese.
Figure 3.
Speech perception scores before and after implantation.
Discussion
This is the first successful case report which underwent CI in MPS IVA patients. The patient was 100 cm tall, diagnosed as severe phenotype, and predicted severe progressive hearing loss.5
During the first couple of weeks of CI use, the patient only wore her device for approximately 1 h per day. She was advised to wear her CI device all waking hours and to practice using her implant with auditory training exercises available online or listening to audiobooks. She reported that it sounded like Donald Duck when she was listening to other people talk. Then, after 1 month of CI use, she reported that she was doing much better with her cochlear implant. She continued to report the sound quality like Donald Duck, but that strange voice quality seemed to be decreasing over time. Three months post-activation, she stated that she could hear clearly. After about 5 months of CI use, she reported that she was able to hear natural songs sung by birds and insects. The patient reported that the surgery was the easiest one she ever had and she is extremely happy with the outcomes of her CI.
Overall, these test results indicate that the patient receives a great amount of benefit with the cochlear implant. The patient is still adapting and learning how to use a CI. Although we did not have a measure of the quality of life before and after CI, the patient’s reports indicate that CI provides her better quality of life.
Because CI can provide positive outcomes to these patients, it might be beneficial for patients to consider the feasibility of CI earlier. The greatest risk of the procedure is related to the risk of general anesthesia.8 Patients with MPS IVA have particularly high anesthetic risks due to their progressive narrowing of the airway passage.9
Conclusion
We expect to see an increasing number of patients with MPS IVA with severe-to-profound hearing loss, and cochlear implants are a potential option for such patients. CI can improve speech perception and communication abilities in patients with MPS IVA. Special perioperative attention to musculoskeletal and airway abnormalities in these patients is necessary to ensure safe implantation. The implanting surgeon should also be aware of positioning limitations that can complicate what is usually a routine operation. It is important to carefully review the specific patient for CI candidacy by a multidisciplinary team and to make sure the benefits of the surgery outweigh the risks of surgery and anesthesia.
Acknowledgments
The authors thank the patient for providing us her insights as a cochlear implantation (CI) user. The authors thank all the clinical staff at Nemours/Alfred I. duPont Hospital for Children for their contributions to this study. The authors also thank Dr Meghan Lockard at Hackensack Meridian Health for extracting some of the audiological data in this report.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: ST was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of National Institutes of Health (NIH) under grant number P30GM114736.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iD: Kyoko Nagao  https://orcid.org/0000-0003-0917-6603
https://orcid.org/0000-0003-0917-6603
References
- 1. Hendriksz CJ, Harmatz P, Beck M, et al. Review of clinical presentation and diagnosis of mucopolysaccharidosis IVA. Mol Genet Metab 2013; 110(1–2): 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Montaño AM, Tomatsu S, Gottesman GS, et al. International Morquio A Registry: clinical manifestation and natural course of Morquio A disease. J Inherit Metab Dis 2007; 30(2): 165–174. [DOI] [PubMed] [Google Scholar]
- 3. Barone R, Pellico A, Pittalà A, et al. Neurobehavioral phenotypes of neuronopathic mucopolysaccharidoses. Ital J Pediatr 2018; 44(Suppl. 2): 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Riedner ED, Levin LS. Hearing patterns in Morquio’s syndrome (mucopolysaccharidosis IV). Arch Otolaryngol 1977; 103(9): 518–520. [DOI] [PubMed] [Google Scholar]
- 5. Nagao K, Morlet T, Haley E, et al. Neurophysiology of hearing in patients with mucopolysaccharidosis type IV. Mol Genet Metab 2018; 123(4): 472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lavery C, Hendriksz C. Mortality in patients with Morquio syndrome A. JIMD Rep 2015; 15: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saeed H, Nichani J, Melling C, et al. Feasibility of cochlear implantation in Mucopolysaccharidosis. Int J Pediatr Otorhinolaryngol 2013; 77(8): 1255–1258. [DOI] [PubMed] [Google Scholar]
- 8. Theroux MC, Nerker T, Ditro C, et al. Anesthetic care and perioperative complications of children with Morquio syndrome. Paediatr Anaesth 2012; 22(9): 901–907. [DOI] [PubMed] [Google Scholar]
- 9. Tomatsu S, Montaño AM, Oikawa H, et al. Mucopol-ysaccharidosis type IVA (Morquio A disease): clinical review and current treatment: a special review. Curr Pharm Biotechnol 2011; 12(6): 931–945. [DOI] [PubMed] [Google Scholar]