Abstract
Gastrointestinal (GI) motility disorders are major contributing factors to functional GI diseases that account for >40% of patients seen in gastroenterology clinics and affect >20% of the general population. The autonomic and enteric nervous systems and the muscles within the luminal GI tract have key roles in motility. In health, this complex integrated system works seamlessly to transport liquid, solid, and gas through the GI tract. However, major and minor motility disorders occur when these systems fail. Common functional GI motility disorders include dysphagia, gastroesophageal reflux disease, functional dyspepsia, gastroparesis, chronic intestinal pseudo-obstruction, postoperative ileus, irritable bowel syndrome, functional diarrhea, functional constipation, and fecal incontinence. Although still in its infancy, bioelectronic therapy in the GI tract holds great promise through the targeted stimulation of nerves and muscles.
INTRODUCTION TO GI MOTILITY AND GI MOTILITY DISORDERS
Gastrointestinal (GI) motility disorders are major contributing factors of functional GI diseases that account for >40% of patients seen in gastroenterology clinics and affect >20% of the general population (Halder et al. 2007; Talley 2008; Chen et al. 2017). GI motility is the process by which luminal contents move through the GI tract. Motility encompasses many complex processes and is influenced by multiple internal and external factors. The enteric nervous system (ENS) has a key role in motility, and it is located within the wall of the luminal GI tract. The myenteric plexus and submucosal plexus are the major locations for ENS and are located between circular and longitudinal muscle layers and the submucosal space, respectively. The ENS can act autonomously without central control to propel contents through the GI tract. However, motility is also influenced by the central nervous system and the sympathetic (which is mostly inhibitory) and parasympathetic (which is mostly excitatory) nervous systems, by the hormonal milieu, by receptors throughout the GI tract, by luminal contents, and by the microbiome of the GI tract.
In health, this complex integrated system can sense luminal contents and works seamlessly to transport liquid, solid, and gas through the GI tract. Motility can be adjusted to transport accordingly by speeding, slowing, or stopping the flow of contents within the lumen and by increasing or decreasing pressure within the sphincters. The sphincters act as barriers or breaks to the flow and as control mechanisms for motility along the GI tract as well as protective mechanisms preventing reflux of luminal contents from one organ to another.
The GI tract, which is composed of lumens and sphincters, is a complex integrated system, which is subject to acute and chronic pathophysiologic changes that can lead to short- and/or long-term dysfunction. The lumen needs coordinated contractions to transport its contents. Weak contractions (hypomotility) lead to delayed transit or emptying. Impairment in propagation direction can slow or prevent luminal transit. The lumen also has to relax appropriately to accommodate luminal contents. Similarly, a weak sphincter can result in reflux of luminal content or incontinence, whereas a hypertensive sphincter may prevent passage of luminal contents (Chen et al. 2017).
PHYSIOLOGY OF GI MOTILITY—NERVES, MUSCLES, INTERSTITIAL CELLS OF CAJAL (ICCs), SPHINCTERS, AND THE ENTERIC NERVOUS SYSTEM
Different aspects of the physiology and pathophysiology of motility within the luminal GI tract can be evaluated using various techniques. At a molecular level, one can look at the receptors and the GI hormones on the muscles and nerves within the luminal GI tract. At a cellular level, one can look at the smooth and skeletal muscle cells and the neural cells and ganglia within the GI tract. At a histologic level, one can evaluate the muscle that makes up the circular, longitudinal smooth muscle and the muscularis propria within the wall of the GI tract. At an organ level, one can look at the coordination of the muscle layers as they contract to propel luminal contents through the GI tract and at the innervation of each of the organs by the sympathetic and parasympathetic nervous system. Finally, at a systems level, one can determine how each organ “talks” to the other organs within and outside of the GI tract, through hormones, nerves, and the passage of luminal contents. Common functional GI motility disorders include dysphagia, gastroesophageal reflux disease (GERD), functional dyspepsia, gastroparesis, chronic intestinal pseudo-obstruction (CIPO), postoperative ileus, irritable bowel syndrome, diarrhea, constipation, and fecal incontinence (Miller et al. 2018).
TARGETS OF ELECTRICAL ACTIVITY: NERVES, MUSCLE, SPINAL CORD, AND TRANSCUTANEOUS STIMULATION
Electrical stimulation has been used as a therapy for various functional GI disorders. Electrical therapy can be applied to various locations within and outside of the GI tract to modulate GI motility. The ultimate goal of electrical therapies is to improve GI motility, such as enhance contractions, normalize propagation, and improve the function of a sphincter or an accommodating organ (Chen et al. 2017). In addition, electrical therapies can be used to treat adverse symptoms such as nausea and vomiting. Electrical current can be applied directly to the muscle within the wall of the GI tract. An example of direct muscle stimulation is the pacing of the lower esophageal sphincter (LES) to increase LES pressure and treat GERD. Endostim is currently in clinical trial phase to obtain Food and Drug Administration (FDA) approvals for treating GERD. Electrical stimulation can also be applied directly to the wall of the GI tract to activate nerves. This type of electrical stimuli uses short pulse stimulation that mainly activates nerves. For example, intestinal electrical stimulation (IES) applied to the duodenum of rats has shown inhibitory effects on food intake and body weight (Chen et al. 2017). It was shown that the pulse width choice directly affects the modulation of the intestine (Chen et al. 2017). An example of electrical stimulation of nerves is use of a gastric pacemaker for treating nausea and vomiting (Yin et al. 2012). Direct nerve stimulation can also be used, for example, sacral nerve stimulation (SNS) has been successfully used for treating fecal incontinence. Most recently, spinal cord stimulation (SCS) has been proposed as a potential therapy for gastroparesis (Song et al. 2014) and functional dyspepsia (Song et al. 2017). In addition, the vagus nerve can be electrically stimulated directly. Transcutaneous electrical modulation methods have been explored as a potential therapy for functional constipation (Zhang et al. 2014).
METHODS AND PARAMETERS OF STIMULATION: LONG PULSE, SHORT PULSE, PULSE TRAIN, INFERENTIAL CURRENT STIMULATION
Electrical stimulation has been used as a therapy for GI motility disorders. Electrical stimulation can be adjusted based on the waveform of the stimulation, amplitude, pulse width, frequency, and duty cycle (burst patterns). In the following, we have discussed each stimulation parameter, its range, and potentially widely used combinations.
Waveform
Traditionally, square waves either in the form of monophasic or biphasic pulses have been used for stimulation. The biphasic pulses can be either charge balanced or imbalanced, meaning that the amount of charge delivered to the tissue can be identical or different in positive or negative phases of the stimulation. Compared with monophasic, biphasic pulses cause less damage to the electrodes or tissues. There have been studies that investigated other waveforms such as sinusoidal, triangle, rising exponential, rising ramp, and decaying exponential pulses (Bennie et al. 2002; Wongsarnpigoon et al. 2010; Nielsen et al. 2017; Yip et al. 2017); however, the number of these studies is limited compared with the studies that used square waves as the shape of stimulation pulses.
Amplitude/Intensity
The amplitude of the stimulation pulses often varies depending on the delivery target, that is, muscle or nerve. The amplitudes used to stimulate muscle are often in the range of a few milliamperes (1 mA–10 mA), whereas for nerve the amplitudes are often in the range of hundreds of microamperes. It is worth noting that some investigators have applied a few milliamperes intensity to larger nerve bundles such as vagus nerve in mammals.
Frequency
The frequency of the stimulation varies from very low frequencies closer to natural frequency of the motility in the GI tract to medium frequencies in the range of tens of hertz, to extremely high frequencies in the range of a few kilohertz. The frequency choice directly depends on the application. For example, high-frequency electrical stimulations in the range of a few kilohertz (5 kHz–10 kHz) applied to the nerves can block the conduction of neural activity through the nerve. Blockage applied to the vagus nerve is the method used in an FDA-approved therapy for obesity known as VBLOC.
Duty Cycle
Often, trains of stimulation pulses are interrupted with silent periods. Duty cycle, also called “pulse train” or “burst” stimulation, defines the on- and off-time periods of the stimulation pulses.
Pulse-Width
Short-pulse and long-pulse stimulation often referred to as low- and high-energy stimulation have been studied extensively in the GI field. Long pulses are often in the range of ten to hundreds of milliseconds, whereas the short pulses are in the range of hundreds of microseconds. The high-energy pulses applied directly to the muscle tissue has been shown to modulate the stomach (Lin et al. 1998, 2011; McCallum et al. 2010). The frequency of pulses is often close to the stomach’s natural frequency that is three cycles per minute. However, because of the shortage in appropriate devices (mainly, battery limitations), and electrode erosion high-energy stimulation has not been used in chronic clinical practices. Low-energy stimulation is typically delivered with a pulse-width on the order of a few hundred microseconds, at frequencies ranging from 5 Hz to 100 Hz, and may improve symptoms such as nausea, vomiting, and bloating (O’Grady et al. 2009; Deb et al. 2012). The best example of low-energy stimulation is the Enterra therapy that has been used to treat nausea and vomiting symptoms in gastroparesis patients. The pulse-width in this case is often set at 330 µs, and pulses are applied at 14 Hz, with the duty cycle of 0.1 sec “on” and 5 sec “off” intervals.
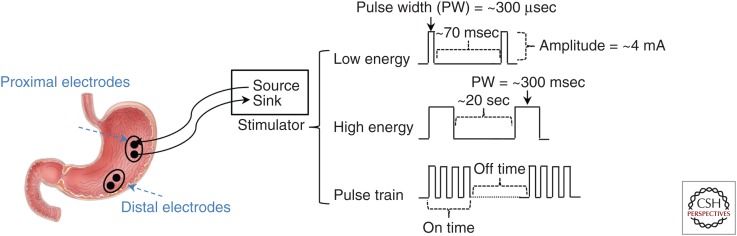
Using multiple stimulation electrodes or stimulation configuration, other stimulation methodologies have been derived from the aforementioned stimulation protocols. Figure 1 shows three of the most common types of stimulation configurations used to stimulate the stomach. Presentation of all stimulation configurations is not possible in this review; however, examples of some important ones follow.
Figure 1.
Placement of the stimulation electrodes on the proximal and distal locations on the greater curvature is shown. The electrodes are connected to a current stimulator device with source and sink. Three common stimulation types, including low-energy, high-energy, and pulse-train stimulations and related parameters for each stimulation, is shown.
Multichannel Stimulation
More than one channel is used to deliver electrical stimulation to multiple locations (Chen et al. 2005). For example, Song et al. (2005) and Mintchev et al. (1998) have used two-channel and four-channel stimulation configurations that were directly applied to the stomach in their studies.
The justification for multichannel stimulation of the stomach is to mimic normal functionality of the stomach motility that propagates circumferentially and distally. Motility in the distal region is important in emptying the stomach and pushing solids into the duodenum. Multichannel stimulation applied sequentially from the greater curvature (proximal stomach) to distal stomach can be effective in avoiding the retrograde pacing, which results in the solids to be pushed back in the wrong direction.
Dual-Pulse Stimulation
Combining high- and low-energy pulses, Qi et al. (2007) have shown that the dual-pulse modulation applied to the intestine is potentially effective in both normalizing dysrhythmia and improving nausea and vomiting symptoms. This study, which was performed on canines, benefits from the therapeutic effects of both long and short pulses.
Interferential Current Stimulation
In this method, two sites (or more) will be stimulated at different frequencies (sinusoidal waveform) in the range of a few kilohertz. The resulted stimulation (also called beat) at the cross-section of the two stimulation locations will have a lower frequency equal to the difference between the two stimulating frequencies. The interferential stimulation is often applied transcutaneously and allows the penetration of the beat frequency to deeper tissue, where direct application of low-frequency stimulation cannot penetrate. This method is used in treating constipation (Hutson et al. 2015; Iqbal et al. 2016b).
Synchronized Stimulation
Conventionally, stimulation has been applied without thorough consideration of the intrinsic frequency of slow waves. However, synchronizing the stimulation, especially high-energy stimulation, which results in motility, can potentially enhance contractions and result in accelerated transit in the stomach and intestine (Yin and Chen 2007). Synchronized stimulation is the basis for closed-loop and smart modulation of the GI tract (Wang et al. 2018).
TYPES OF ELECTRODES
Various types of electrodes have been developed for neuromuscular stimulation and recording. Generally speaking, these electrodes can be categorized based on their shapes, materials, charge transfer mechanisms, biocompatibility, and invasiveness. In this section, we intend to only review the common electrodes used to stimulate neuromuscular tissue in the GI field. Comprehensive review of each category is discussed elsewhere (Merrill et al. 2005; Navarro et al. 2005; Cogan 2008; Kim and Romero-Ortega 2012).
Surface electrodes are the only noninvasive electrodes that are placed over the skin of the subject. These electrodes made from Ag/AgCl have been used to deliver transcutaneous electrical stimulation (TES) (e.g., in interferential stimulation) or record electrogastrograms when placed on the abdominal area. Similar to surface electrodes, epimysial and epineurial electrodes have been used for invasive contact with muscle and nerve, respectively. The concept of surface electrodes has been used to develop electrodes with more but smaller contacts. These electrodes, which are embedded on a biocompatible flexible material, are often used for higher resolution purposes. For example, Du et al. (2009) developed an electrode with 32 contacts on flexible printed circuit board, and applied the electrode on the serosa of the stomach to map the gastric slow wave activity.
Other types of intramuscular electrodes have been used to either record or stimulate the GI tract. These electrodes, which are also used in the cardiology field, are either in the form of needle or have a helical tip (similar to screw) that can be affixed in the muscle tissue. Most of these electrodes are commercially available, and are used in clinical practices. Temporary gastric stimulation is a good example of the use of these electrodes that were implanted through an endoscopy procedure in patients with gastroparesis (Ayinala et al. 2005).
Electrodes interfacing peripheral nerves are generally divided into extraneural and intraneural categories. The extraneural electrodes are less invasive, and can be as simple as a pair of L-shaped or U-shaped Ag/AgCl wires that can be hooked to the nerve. Epineural electrodes can be also used to record and stimulate.
In recent decades, novel electrodes with the capability of delivering more precise stimulation with high resolution to the nerve has been developed using microelectromechanical systems (MEMS) fabrication technologies. Cuff and helicoidal electrodes are examples of extraneural electrodes that can be wrapped around the nerve, and often record compound action potentials or stimulate the nerve. These electrodes can have multiple stimulation/recording sites, and conform to the shape of the nerve. Another prominent example of extraneural electrodes with multiple stimulating sites is the ones used for SCS.
Intraneural electrodes are more invasive compared with extraneural; however, have higher specificity in recording and stimulation. Utah slanted array (USEA) is an example of such electrodes that have 48 to 96 silicon microelectrodes with varying lengths from 0.5 mm to 1.5 mm (Mathews et al. 2014; Wark et al. 2015). These microelectrodes penetrate the nerve and can be randomly interfaced with various types of fibers. Because USEA is not flexible, it does not conform to the shape of the nerve. Thus, to interface with neural ganglia, microelectrode arrays fabricated on flexible substrate has been developed (Zachariah et al. 2018).
SPECIFIC DISEASES TARGETED CLINICAL TRIALS AND EXPERIMENTAL TRIALS USING ELECTRICAL STIMULATION: GERD, GASTROPARESIS, CONSTIPATION, FECAL INCONTINENCE
Upper GI Neurostimulation (GERD and Gastroparesis)
Electrical Therapy for Gastroesophageal Reflux
GERD affects up to 40% of the population. Evidence is mounting that the prevalence is coincident with the increased incidence of obesity. GERD can cause esophagitis, esophageal erosions, ulcers, strictures, Barrett’s esophagus, adenocarcinoma, laryngitis, chronic bronchitis, chronic cough, asthma, and dental erosions. Although there are effective treatments to suppress stomach acid production (e.g., proton pump inhibitors [PPIs]), these drugs were associated with numerous adverse events including osteoporosis, hip fracture, aspiration pneumonia, and opportunistic infections. Surgical (fundoplication) and endoscopic (TIFF) interventions are also available. A number of motility abnormalities have been identified that contribute to the pathophysiologic mechanisms of reflux including reduction of tone and pressure within the muscles of the gastroesophageal junction high-pressure zone (GEJ HPZ) (Mittal et al. 1995; Meneghetti et al. 2005; Massey et al. 2006; Brasseur et al. 2007; Miller et al. 2007, 2009; Kwiatek et al. 2010; Vegesna et al. 2010, 2014). In addition, the GEJ HPZ pressure is known to drop transiently to baseline gastric pressure over extended periods not associated with swallowing, indicating transient loss of sphincter tone. This phenomenon is referred to as “transient lower esophageal sphincter relaxation” (TLESR) (Mittal et al. 1995).
Electrical stimulation of the LES is designed to alter the pressure of the LES. Short pulses at a frequency of 40 Hz have been shown to increase LES pressure mediated via a neural mechanism (Xing et al. 2005; Xing and Chen 2006). A clinical trial performed with electrical stimulation delivered to the LES has been shown to improve clinical outcomes (a reduction in acid reflux from 10% to 4% and normalization in 71% of the patients) in patients with GERD at 2 years (Rodríguez et al. 2015). In a follow-up study evaluating the safety and efficacy of LES stimulation, the same GERD patients with a partial response to PPI, with hiatal hernia <3 cm and with esophagitis grade C, were treated with LES stimulation in an open-label 2-year trial. At 3 years, there was a significant and sustained improvement in esophageal acid exposure and reduction in GERD symptoms and PPI use (Rodríguez et al. 2016).
Electrical Therapy for Gastroparesis
Gastroparesis is a major clinical problem in the United States resulting in substantial long-term disability and loss of time from work. It is caused by a delay in gastric emptying and is characterized by recurrent and often incapacitating nausea and vomiting. Often patients are disabled with a marked reduction in quality of life.
Under normal conditions, gastric emptying is a highly regulated process reflecting the integration of propulsive forces generated by proximal fundic tone and distal antral contractions with the resistance to flow occurring at the pyloric sphincter. Gastroparesis, or delayed gastric emptying, is a disorder from which patients suffer a variety of symptoms, including nausea, vomiting, abdominal pain, early satiety, and postprandial bloating (Achem et al. 1997). Antral hypomotility, as well as increased gastric outlet resistance because of pyloric dysfunction or pylorospasm appear to be important physiologic disturbances in gastroparesis (Klein et al. 1993; Paterson et al. 1995; Parkman et al. 1996). Gastroparesis is a common complication of patients with both type 1 and type 2 diabetes. The precise etiology is unclear but there appears to be degeneration of the ENS. It can also be idiopathic in patients without diabetes.
Current treatments for gastroparesis have suboptimal efficacy. Standard pharmacotherapy with prokinetic agents that increase gastric motility are not well tolerated, have many side effects, and are of limited utility. Intramuscular injection of botulinum toxin into the pyloric muscle is a therapeutic approach that has been effective in open-labeled studies. However, not all patients respond, and when they do the duration of therapeutic relief averages only about 7 months and the treatment needs to be repeated when symptoms return (Miller et al. 2002). Endoscopic pyloromyotomy (G-POEM) is a promising new technique.
Treatment of Gastroparesis by Altering Gastric Slow Waves
Long pulses delivered at frequencies slightly higher than the normal frequency of the gastric slow waves can be used to pace the stomach and entrain the gastric slow waves (Kelly and La Force 1972; Lin et al. 1998) with normalization of gastric dysrhythmia and improved gastric emptying in animal models (Chen et al. 2003; Xu et al. 2008). Gastric electrical stimulation (GES) with long pulses exerts direct effects on gastric slow waves and gastric emptying. However, there are few clinical trials using these techniques as a result of the unavailability of devices because of difficulty in manufacturing an implantable device. The long pulse makes it difficult to deliver and also difficult to balance electrical charges. Using an external device for a period of 4 weeks, GES was performed in nine patients with gastroparesis using repetitive long pulses (∼300 msec) (McCallum et al. 1998). Entrainment of gastric slow waves occurred in all patients and tachygastria observed in two patients was overridden by GES. Gastric emptying was improved significantly. Symptoms of gastroparesis were reduced at the end of the treatment that was performed 2 h daily after each meal. In a second study using GES with long pulses, the pulses were delivered at two locations in a sequential manner (Lin et al. 2011). Similar improvement was observed on gastric slow waves, gastric emptying, and symptoms of gastroparesis.
Treatment of Nausea and Vomiting in Gastroparesis
GES can be used for treating nausea and vomiting in gastroparesis using short pulses. It is thought that the main mechanism of action is not an alteration in the gastric motility or gastric slow waves (Yin et al. 2012) as in the use of long pulses. A number of studies show that possible mechanisms of action involve improved gastric accommodation (Chen et al. 2003; Liu et al. 2004; McCallum et al. 2010), enhanced vagal activity (Chen et al. 2003), or have a central nervous system effect (Zhang and Chen 2006; Frokjaer et al. 2008).
Dual-Pulse Method of the Stomach
A dual-pulse method of stimulation combines the features of long-pulse stimulation, which activates muscle and short-pulse stimulation, which activates nerves (Liu and Chen 2006). Synchronized electrical stimulation, delivers each stimulus (long pulse or pulse trains) in synchronization with the intrinsic slow wave. Thus, enhancing gastric or small intestinal contractions (Song and Chen 2007; Yin and Chen 2007; Zhu et al. 2007). Electrical stimulation can also be performed at different locations of the stomach or the small/large intestine in a sequential order. Multichannel stimulation has been shown to be more effective in enhancing gastric motility and gastric emptying than single-channel stimulation (Amaris et al. 2002; Chen et al. 2005).
Spinal Cord Stimulation for Gastroparesis
GI motility is regulated by the autonomic nervous system. Activation of the vagal nerve enhances GI motility, whereas excitation of sympathetic nerves inhibits GI motility (Ouyang et al. 2005). Suppression of sympathetic activity is expected to improve GI motility. Sympathetic activity and the sympathovagal ratio were reduced with SCS (Song et al. 2014). In a rodent model of postoperative ileus, SCS was also shown to improve gastric emptying (Maher et al. 2009). These findings suggest a therapeutic potential of SCS for gastroparesis.
Electrical Therapies for Functional Dyspepsia
Functional dyspepsia is common and affects ∼20% of the general population. Symptoms of functional dyspepsia are similar to those of gastroparesis but to a lesser degree. Electrical therapies for functional dyspepsia use noninvasive methods: weak electrical stimulation is delivered via needles or electrodes placed at acupuncture points. This method is called transcutaneous electroacupuncture (TEA). For the therapy to be effective, electrical stimulation must be performed on a daily basis because the effect of electrical stimulation does not last longer than 24 h (Yin and Chen 2010).
In one study, 27 patients with functional dyspepsia were treated with TEA or sham-TEA twice daily for 2 weeks using a transcutaneous electrical nerve stimulation (TENS) unit (Liu et al. 2008). There was a significantly greater decrease in symptoms in the TEA group than the sham-TEA group. Vagal activity was increased with both acute and chronic TEA and neuropeptide Y was increased with chronic TEA. In another crossover study (Ji et al. 2014), it was reported that TEA but not sham-TEA improved dyspeptic symptoms by 35%, and quality of life, gastric emptying, and gastric accommodation by various degrees.
Neurostimulation to Treat Obesity
Obesity, as defined by a body mass index (BMI) of 30 kg/m2 or more, is a rapidly growing problem, currently affecting >30% of adults in the United States (Flegal et al. 2002; Hedley et al. 2004). Morbid obesity is defined by a BMI of 40 kg/m2 or more or a BMI of 35 kg/m2 or more in the presence of comorbidities (Brolin 2002; Flegal et al. 2002). Obesity is commonly associated with many serious medical disorders, including heart disease, diabetes, hypertension, dyslipidemia, osteoarthritis, and sleep apnea. In addition, ∼300,000 adults in the United States die each year as a result of obesity-related causes (Allison et al. 1999).
The primary treatment objective for obese patients is weight reduction, which can improve comorbid conditions and also reduces risk factors for disease. Even moderate weight loss (5%–10% of initial weight) produces health benefits and has been associated with marked reductions in the risk for medical disorders (Goldstein 1992; de Leiva 1998; Maggard et al. 2005). Although nonoperative and pharmacologic weight loss therapies have met with only limited success, surgical intervention for morbid obesity, most frequently gastric bypass and gastric sleeve, are becoming increasingly common (Brolin 2002; Holzwarth et al. 2002; Livingston 2002; Buchwald et al. 2004). However, the decision to undergo surgery is a difficult one. Patients who choose to undergo gastric bypass are at risk for developing metabolic/nutritional complications, resulting from the long-term malabsorptive effects of gastric bypass and food intake restriction. Long-term complications of gastric bypass including anemia secondary to iron or B12 deficiency, mineral deficiencies (hypokalemia and hypomagnesia), and bone disease associated with secondary hyperparathyroidism are not uncommon (De Prisco and Levine 2005; Fujioka 2005).
The mechanism of action of GES for treatment of obesity appears most likely to induce satiety by modulation of the gut–brain neural axis, gut peptide hormone release, and gastric motor activity (Hasler 2009). There have been a number of devices and a number of trials used to treat obesity. A device called Transcend was used between 2002 and 2006. In open-labeled studies, this device appeared to work well (Champion et al. 2006). However, in a randomized controlled trial there was no difference in weight loss between the stimulation group and the sham treatment (Shikora et al. 2009). In trials using a device called Tantalus, there were variable outcome measures. Reports indicate ∼20% excess weight loss and improved glycemic control in diabetic patients (Bohdjalian et al. 2006, 2009a,b; Policker et al. 2009; Sanmiguel et al. 2009). Another commercially available technology—termed VBLOC—involves surgical placement of cuff-like electrodes around the anterior and posterior vagal trunks at the level of the esophageal hiatus in the abdomen. There is only one published study on VBLOC:EMPOWER, a randomized controlled trial, comprising 294 patients from 15 U.S. centers. There was no difference in weight loss with treatment versus sham at 12 months. However, there may have been confounding factors because of unplanned delivery of stimulus to the sham treatment group (Sarr et al. 2012).
IES may have an inhibitory effect on gastric motility suggesting a therapeutic potential for obesity. Gastric tone, antral contractions, and gastric emptying were reported to be inhibited with long pulse IES in dogs (Zhao et al. 2009). In rats, IES was reported to delay gastric emptying and reduce food intake and body weight (Yin et al. 2007). Similar inhibitory effects of IES on gastric motility were also noted in humans: IES with long pulses delivered via intraluminal ring electrodes attached to a feeding tube reduced gastric accommodation and delayed gastric emptying (Liu et al. 2005). IES-induced acceleration in small intestinal transit may result in reduced absorption (Sun and Chen 2004; Liu et al. 2011) and have a therapeutic potential for obesity (Yin and Chen 2010).
Electrical Therapies for Intestinal Motility Disorders
Chronic Intestinal Pseudo-Obstruction
CIPO is a severe digestive syndrome characterized by derangement of gut propulsive motility, which results in a clinical picture mimicking mechanical obstruction in the absence of any lesion occluding the gut. CIPO is one of the most important causes of chronic intestinal failure both in pediatrics (15%) and adults (20%), because affected individuals are often unable to maintain normal body weight and/or normal oral nutrition (Antonucci et al. 2008). This syndrome represents one of the main causes of intestinal failure and is characterized by high morbidity and mortality. The severity of the clinical picture, generally characterized by disabling digestive symptoms, contributes to deterioration of quality of life of the patients (De Giorgio et al. 2004).
Pseudo-obstructive syndromes may be either acute (i.e., the result of abdominal surgery, retroperitoneal hemorrhage, spinal or pelvic trauma, myocardial infarction, or hypokalemia) or, more commonly, chronic. CIPO can be idiopathic or secondary to a variety of diseases. CIPO can be classified into three major entities: neuropathies, mesenchymopathies, and myopathies, depending on the predominant involvement of enteric neurons (enteric glial cells), ICCs, or smooth muscle cells, respectively. Most commonly, CIPO is idiopathic, and occurs in isolation, affecting either neural or muscular elements of the intestinal wall (Antonucci et al. 2008).
Clinical manifestations of CIPO are characterized by recurrent episodes of abdominal pain, abdominal distension, and inability to defecate and can present with or without vomiting mimicking a mechanical subocclusion (Mann et al. 1997). Nausea, vomiting, and weight loss are predominant symptoms when the functional derangement primarily affects the upper GI tract while diffuse abdominal pain, abdominal distension, and constipation are suggestive of a more distal involvement of the gut (Amiot et al. 2009). CIPO is a difficult clinical problem because of the inadequacy of treatment (Antonucci et al. 2008).
Most of the studies on small intestinal electric stimulation have been performed in animal studies (Yin and Chen 2010). In dogs, IES with long pulses delivered at a frequency slightly higher than the frequency of the intrinsic small intestinal slow wave was reported to entrain intestinal slow waves and normalize dysrhythmia (Sun et al. 2009). Although the conventional method of long-pulse IES or intestinal pacing does not improve intestinal contractions, intestinal transit is accelerated (Yin and Chen 2010). Synchronized IES has shown an enhancing effect on intestinal contractions when each stimulus is delivered in synchronization with the intrinsic intestinal slow wave (Yin and Chen 2007). Acceleration of intestinal transit with IES in a diseased condition may have a therapeutic potential, such as for treating postoperative ileus (Wang et al. 2015).
Constipation
Using Rome IV criteria, functional constipation is diagnosed when there is the presence of two out of six symptoms, which may include straining at defecation, sensation of incomplete evacuation, lumpy or hard stools, sensation of anorectal obstruction or blockage, manual maneuvers to facilitate defecations for at least 25% of the time, and/or fewer than three defecations per week. Furthermore, there is an absence of loose stools without aid of laxatives. Constipation can be further subdivided into three groups: normal transit constipation, isolated slow transit constipation, and functional defecatory disorders. In defecatory disorders, colonic transit may be normal or slow (Rao et al. 1998; Ravi et al. 2010).
Slow transit constipation may include colonic inertia, or megacolon. Slow transit constipation is associated with colonic motor dysfunction. There is a marked decrease in the number of enteric cells or ICCs. It is also associated with increased nonpropagating or retrograde propagating pressure waves in the sigmoid colon or rectum.
Electrical Therapies for Slow Transit Constipation
A number of electrical therapies have been introduced for treating slow transit constipation. These include colonic electrical stimulation (CES), SNS, tibial nerve stimulation (TNS), TEA, and TES.
Colonic Electrical Stimulation for Constipation
Colon motility is composed of individually localized contractions and giant migrating contractions (Sarna 2010). The giant migrating contractions are regular and distinct but occur only once or twice daily and are often associated with mass movement along the colon, leading to defecation. Slow waves with different frequencies may appear sequentially or superimposed on each other. There are two CES methods. The first is pulse trains at a frequency of 20 Hz–50 Hz and the second is repetitive long pulses at a frequency of below 1 Hz. These methods both accelerate colonic transit (Bruninga et al. 1998; Liu et al. 2006). Pulse train CES induces colon contractions and facilitates mass movement (Sanmiguel et al. 2006) more effectively than long-pulse CES in a dog model (Sallam and Chen 2013). Preliminary clinical studies using long-pulse CES improved evacuation of the colon in patients with colonic inertia (Shafik et al. 2004).
Sacral Nerve Stimulation for Constipation
Conflicting results have been reported in clinical studies and most of the studies have not been controlled (Thomas et al. 2013). A controlled clinical study of 55 patients with slow transit constipation showed no improvement in stool frequency or constipation symptoms (Dinning et al. 2007).
Most of the physiological studies with SNS were performed in patients with fecal incontinence and not constipation (Carrington et al. 2014). The effects of SNS on colon motility/transit are inconclusive. Studies in patients with fecal incontinence suggest an inhibitory effect of SNS on colon motility and transit; whereas SNS using the same parameters showed an excitatory effect on colon motility and transit in constipation studies. In one SNS study, patients with slow transit constipation had an increased number of giant contractions and increased percentage of distally propagated colon contractions and improved clinical symptoms (Dinning et al. 2007).
Tibial Nerve Stimulation for Constipation
A pilot noncontrolled clinical study with percutaneous TNS (12 sessions of 30 min in a period of 4 to 12 weeks) in 18 patients with slow transit constipation showed a significant improvement in stool frequency and reduction in constipation symptom score and laxative use but no change in colonic transit time (Collins et al. 2012). Transcutaneous TNS has shown limited clinical efficacy (Iqbal et al. 2016a).
Electroacupuncture and Transcutaneous Electrical Acustimulation
A multicenter randomized, sham-controlled study involving 1075 patients with severe chronic functional constipation, showed significantly increased number of complete spontaneous bowel movement at 8 weeks with the increase maintained following 10 weeks after the completion of the treatment. Electroacupuncture (EA) or sham-EA was administrated in 28 sessions within a period of 8 weeks (Wang and Yin 2015).
Transcutaneous Electrical Stimulation
In a sham-controlled study of TES directed at the posterior tibial nerve and another acupressure site, the treatment was given twice daily in a blind manner. TES but not sham-TES significantly increased the frequency of spontaneous bowel movement, reduced constipation-related symptoms, and improved sensation to rectal distention (Zhang et al. 2014). In another study, transcutaneous SNS was performed 12 h/day for 4 weeks in 20 patients with chronic functional constipation (Iqbal et al. 2016b). No significant improvement was noted in spontaneous bowel movement- or constipation-related symptoms.
In one pediatric study, TES was performed 1 h daily for 3 to 6 months. Significant improvement was noted in weekly bowel movement (69% increase) in 13 patients with less than three bowel movements per week at enrollment and in soiling frequency in 16 patients with more than three bowel movements per week at enrollment. Improvement in colon transit was noted in ∼50% of these patients. However, the study was not placebo controlled (Yik et al. 2012). In a recent meta-analysis, the outcomes were uncertain (Ng et al. 2016).
THE FUTURE OF BIOELECTRONICS IN GASTROENTEROLOGY
Although still in its infancy, bioelectronic therapy in the GI tract is very promising. Currently, most bioelectronic stimulation in the GI tract is performed through stimulation of large portions of the muscle or through large truncal nerves. However, functional and anatomical mapping of the organs and of the peripheral innervation of the nerves to the organs will allow more defined and targeted stimulation. In addition to better stimulate targets (locations), better stimulation parameters need to be defined.
Closed-loop therapy, in which the physiologic effect of the stimulation is monitored and the stimulation parameters are automatically adjusted through a feedback signal, will allow fine tuning of the stimulation parameters and of the physiologic effect (Wang et al. 2017). Closed-loop therapy can also help in optimizing the battery life of the implanted stimulator by delivering the stimulation pulses whenever necessary (Farajidavar 2018).
Improvements in battery technology, energy harvesting, and wireless power transfer will also help in the development of novel electrical stimulators that can deliver more intense electrical stimulations (e.g., high-energy stimulation) for prolonged periods of therapy.
Footnotes
Editors: Valentin A. Pavlov and Kevin J. Tracey
Additional Perspectives on Bioelectronic Medicine available at www.perspectivesinmedicine.org
REFERENCES
- Achem SR, Kolts BE, Macmath T, Richter J, Mohr D, Burton L, Castell DO. 1997. Effects of omeprazole versus placebo in treatment of noncardiac chest pain and gastroesophageal reflux. Dig Dis Sci 42: 2138–2145. [DOI] [PubMed] [Google Scholar]
- Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB. 1999. Annual deaths attributable to obesity in the United States. JAMA 282: 1530–1538. [DOI] [PubMed] [Google Scholar]
- Amaris MA, Rashev PZ, Mintchev MP, Bowes KL. 2002. Microprocessor controlled movement of solid colonic content using sequential neural electrical stimulation. Gut 50: 475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiot A, Joly F, Alves A, Panis Y, Bouhnik Y, Messing B. 2009. Long-term outcome of chronic intestinal pseudo-obstruction adult patients requiring home parenteral nutrition. Am J Gastroenterol 104: 1262–1270. [DOI] [PubMed] [Google Scholar]
- Antonucci A, Fronzoni L, Cogliandro L, Cogliandro RF, Caputo C, Giorgio RD, Pallotti F, Barbara G, Corinaldesi R, Stanghellini V. 2008. Chronic intestinal pseudo-obstruction. World J Gastroenterol 14: 2953–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayinala S, Batista O, Goyal A, Al-Juburi A, Abidi N, Familoni B, Abell T. 2005. Temporary gastric electrical stimulation with orally or PEG-placed electrodes in patients with drug refractory gastroparesis. Gastrointest Endosc 61: 455–461. [DOI] [PubMed] [Google Scholar]
- Bennie SD, Petrofsky JS, Nisperos J, Tsurudome M, Laymon M. 2002. Toward the optimal waveform for electrical stimulation of human muscle. Eur J Appl Physiol 88: 13–19. [DOI] [PubMed] [Google Scholar]
- Bohdjalian A, Prager G, Aviv R, Policker S, Schindler K, Kretschmer S, Riener R, Zacherl J, Ludvik B. 2006. One-year experience with Tantalus: A new surgical approach to treat morbid obesity. Obes Surg 16: 627–634. [DOI] [PubMed] [Google Scholar]
- Bohdjalian A, Ludvik B, Guerci B, Bresler L, Renard E, Nocca D, Karnieli E, Assalia A, Prager R, Prager G. 2009a. Improvement in glycemic control by gastric electrical stimulation (TANTALUS) in overweight subjects with type 2 diabetes. Surg Endosc 23: 1955–1960. [DOI] [PubMed] [Google Scholar]
- Bohdjalian A, Prager G, Rosak C, Weiner R, Jung R, Schramm M, Aviv R, Schindler K, Haddad W, Rosenthal N, et al. 2009b. Improvement in glycemic control in morbidly obese type 2 diabetic subjects by gastric stimulation. Obes Surg 19: 1221–1227. [DOI] [PubMed] [Google Scholar]
- Brasseur JG, Ulerich R, Dai Q, Patel DK, Soliman AM, Miller LS. 2007. Pharmacological dissection of the human gastro-oesophageal segment into three sphincteric components. J Physiol 580: 961–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brolin RE. 2002. Bariatric surgery and long-term control of morbid obesity. JAMA 288: 2793–2796. [DOI] [PubMed] [Google Scholar]
- Bruninga K, Riedy L, Keshavarzian A, Walter J. 1998. The effect of electrical stimulation on colonic transit following spinal cord injury in cats. Spinal Cord 36: 847–853. [DOI] [PubMed] [Google Scholar]
- Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. 2004. Bariatric surgery: A systematic review and meta-analysis. JAMA 292: 1724–1737. [DOI] [PubMed] [Google Scholar]
- Carrington EV, Evers J, Grossi U, Dinning PG, Scott SM, O’Connell PR, Jones JF, Knowles CH. 2014. A systematic review of sacral nerve stimulation mechanisms in the treatment of fecal incontinence and constipation. Neurogastroenterol Motil 26: 1222–1237. [DOI] [PubMed] [Google Scholar]
- Champion JK, Williams M, Champion S, Gianos J, Carrasquilla C. 2006. Implantable gastric stimulation to achieve weight loss in patients with a low body mass index: Early clinical trial results. Surg Endosc 20: 444–447. [DOI] [PubMed] [Google Scholar]
- Chen JD, Qian L, Ouyang H, Yin J. 2003. Gastric electrical stimulation with short pulses reduces vomiting but not dysrhythmias in dogs. Gastroenterology 124: 401–409. [DOI] [PubMed] [Google Scholar]
- Chen JD, Xu X, Zhang J, Abo M, Lin X, McCallum RW, Ross B. 2005. Efficiency and efficacy of multi-channel gastric electrical stimulation. Neurogastroenterol Motil 17: 878–882. [DOI] [PubMed] [Google Scholar]
- Chen JD, Yin J, Wei W. 2017. Electrical therapies for gastrointestinal motility disorders. Expert Rev Gastroenterol Hepatol 11: 407–418. [DOI] [PubMed] [Google Scholar]
- Cogan SF. 2008. Neural stimulation and recording electrodes. Annu Rev Biomed Eng 10: 275–309. [DOI] [PubMed] [Google Scholar]
- Collins B, Norton C, Maeda Y. 2012. Percutaneous tibial nerve stimulation for slow transit constipation: A pilot study. Colorectal Dis 14: e165–e170. [DOI] [PubMed] [Google Scholar]
- Deb S, Tang SJ, Abell TL, Rao S, Huang WD, To SD, Lahr C, Chiao JC. 2012. An endoscopic wireless gastrostimulator (with video). Gastrointest Endosc 75: 411–415, 415.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Giorgio R, Sarnelli G, Corinaldesi R, Stanghellini V. 2004. Advances in our understanding of the pathology of chronic intestinal pseudo-obstruction. Gut 53: 1549–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leiva A. 1998. What are the benefits of moderate weight loss? Exp Clin Endocrinol Diabetes 106: 10–13. [DOI] [PubMed] [Google Scholar]
- De Prisco C, Levine SN. 2005. Metabolic bone disease after gastric bypass surgery for obesity. Am J Med Sci 329: 57–61. [DOI] [PubMed] [Google Scholar]
- Dinning PG, Fuentealba SE, Kennedy ML, Lubowski DZ, Cook IJ. 2007. Sacral nerve stimulation induces pan-colonic propagating pressure waves and increases defecation frequency in patients with slow-transit constipation. Colorectal Dis 9: 123–132. [DOI] [PubMed] [Google Scholar]
- Du P, O’Grady G, Egbuji JU, Lammers WJ, Budgett D, Nielsen P, Windsor JA, Pullan AJ, Cheng LK. 2009. High-resolution mapping of in vivo gastrointestinal slow wave activity using flexible printed circuit board electrodes: Methodology and validation. Ann Biomed Eng 37: 839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajidavar A. 2018. Bioelectronics for mapping gut activity. Brain Res 1693: 169–173. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Johnson CL. 2002. Prevalence and trends in obesity among US adults, 1999–2000. JAMA 288: 1723–1727. [DOI] [PubMed] [Google Scholar]
- Frokjaer JB, Ejskjaer N, Rask P, Andersen SD, Gregersen H, Drewes AM, Funch-Jensen P. 2008. Central neuronal mechanisms of gastric electrical stimulation in diabetic gastroparesis. Scand J Gastroenterol 43: 1066–1075. [DOI] [PubMed] [Google Scholar]
- Fujioka K. 2005. Follow-up of nutritional and metabolic problems after bariatric surgery. Diabetes Care 28: 481–484. [DOI] [PubMed] [Google Scholar]
- Goldstein DJ. 1992. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord 16: 397–415. [PubMed] [Google Scholar]
- Halder SL, Locke GR III, Schleck CD, Zinsmeister AR, Melton LJ III, Talley NJ. 2007. Natural history of functional gastrointestinal disorders: A 12-year longitudinal population-based study. Gastroenterology 133: 799–807. [DOI] [PubMed] [Google Scholar]
- Hasler WL. 2009. Methods of gastric electrical stimulation and pacing: A review of their benefits and mechanisms of action in gastroparesis and obesity. Neurogastroenterol Motil 21: 229–243. [DOI] [PubMed] [Google Scholar]
- Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. 2004. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA 291: 2847–2850. [DOI] [PubMed] [Google Scholar]
- Holzwarth R, Huber D, Majkrzak A, Tareen B. 2002. Outcome of gastric bypass patients. Obes Surg 12: 261–264. [DOI] [PubMed] [Google Scholar]
- Hutson JM, Dughetti L, Stathopoulos L, Southwell BR. 2015. Transabdominal electrical stimulation (TES) for the treatment of slow-transit constipation (STC). Pediatr Surg Int 31: 445–451. [DOI] [PubMed] [Google Scholar]
- Iqbal F, Collins B, Thomas GP, Askari A, Tan E, Nicholls RJ, Vaizey CJ. 2016a. Bilateral transcutaneous tibial nerve stimulation for chronic constipation. Colorectal Dis 18: 173–178. [DOI] [PubMed] [Google Scholar]
- Iqbal F, Thomas GP, Tan E, Askari A, Dastur JK, Nicholls J, Vaizey CJ. 2016b. Transcutaneous sacral electrical stimulation for chronic functional constipation. Dis Colon Rectum 59: 132–139. [DOI] [PubMed] [Google Scholar]
- Ji T, Li X, Lin L, Jiang L, Wang M, Zhou X, Zhang R, Chen J. 2014. An alternative to current therapies of functional dyspepsia: Self-administrated transcutaneous electroacupuncture improves dyspeptic symptoms. Evid Based Complement Alternat Med 2014: 832523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KA, La Force RC. 1972. Pacing the canine stomach with electric stimulation. Am J Physiol 222: 588–594. [DOI] [PubMed] [Google Scholar]
- Kim Yt, Romero-Ortega MI. 2012. Material considerations for peripheral nerve interfacing. MRS Bull 37: 573–580. [Google Scholar]
- Klein WA, Parkman HP, Dempsey DT, Fisher RS. 1993. Sphincterlike thoracoabdominal high pressure zone after esophagogastrectomy. Gastroenterology 105: 1362–1369. [DOI] [PubMed] [Google Scholar]
- Kwiatek MA, Kahrilas K, Soper NJ, Bulsiewicz WJ, McMahon BP, Gregersen H, Pandolfino JE. 2010. Esophagogastric junction distensibility after fundoplication assessed with a novel functional luminal imaging probe. J Gastrointest Surg 14: 268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin ZY, McCallum RW, Schirmer BD, Chen JD. 1998. Effects of pacing parameters on entrainment of gastric slow waves in patients with gastroparesis. Am J Physiol 274: G186–G191. [DOI] [PubMed] [Google Scholar]
- Lin Z, Sarosiek I, Forster J, Ross RA, Chen JD, McCallum RW. 2011. Two-channel gastric pacing in patients with diabetic gastroparesis. Neurogastroenterol Motil 23: 912–e396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Chen JD. 2006. Colonic electrical stimulation regulates colonic transit via the nitrergic pathway in rats. Dig Dis Sci 51: 502–505. [DOI] [PubMed] [Google Scholar]
- Liu J, Qiao X, Chen JD. 2004. Vagal afferent is involved in short-pulse gastric electrical stimulation in rats. Dig Dis Sci 49: 729–737. [DOI] [PubMed] [Google Scholar]
- Liu S, Hou X, Chen JD. 2005. Therapeutic potential of duodenal electrical stimulation for obesity: Acute effects on gastric emptying and water intake. Am J Gastroenterol 100: 792–796. [DOI] [PubMed] [Google Scholar]
- Liu J, Qiao X, Chen JDZ. 2006. Therapeutic potentials of a novel method of dual-pulse gastric electrical stimulation for gastric dysrhythmia and symptoms of nausea and vomiting. Am J Surg 191: 255–261. [DOI] [PubMed] [Google Scholar]
- Liu S, Peng S, Hou X, Ke M, Chen JD. 2008. Transcutaneous electroacupuncture improves dyspeptic symptoms and increases high frequency heart rate variability in patients with functional dyspepsia. Neurogastroenterol Motil 20: 1204–1211. [DOI] [PubMed] [Google Scholar]
- Liu J, Xiang Y, Qiao X, Dai Y, Chen JD. 2011. Hypoglycemic effects of intraluminal intestinal electrical stimulation in healthy volunteers. Obes Surg 21: 224–230. [DOI] [PubMed] [Google Scholar]
- Livingston EH. 2002. Obesity and its surgical management. Am J Surg 184: 103–113. [DOI] [PubMed] [Google Scholar]
- Maggard MA, Shugarman LR, Suttorp M, Maglione M, Sugerman HJ, Livingston EH, Nguyen NT, Li Z, Mojica WA, Hilton L, et al. 2005. Meta-analysis: Surgical treatment of obesity. Ann Intern Med 142: 547–559. [DOI] [PubMed] [Google Scholar]
- Maher J, Johnson AC, Newman R, Mendez S, Hoffmann TJ, Foreman R, Greenwood-Van Meerveld B. 2009. Effect of spinal cord stimulation in a rodent model of post-operative ileus. Neurogastroenterol Motil 21: 672–677, e33–e34. [DOI] [PubMed] [Google Scholar]
- Mann SD, Debinski HS, Kamm MA. 1997. Clinical characteristics of chronic idiopathic intestinal pseudo-obstruction in adults. Gut 41: 675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey BT, Simuncak C, LeCapitaine-Dana NJ, Pudur S. 2006. Transient lower esophageal sphincter relaxations do not result from passive opening of the cardia by gastric distention. Gastroenterology 130: 89–95. [DOI] [PubMed] [Google Scholar]
- Mathews KS, Wark HA, Normann RA. 2014. Assessment of rat sciatic nerve function following acute implantation of high density Utah slanted electrode array (25 electrodes/mm2) based on neural recordings and evoked muscle activity. Muscle Nerve 50: 417–424. [DOI] [PubMed] [Google Scholar]
- McCallum RW, Chen JD, Lin Z, Schirmer BD, Williams RD, Ross RA. 1998. Gastric pacing improves emptying and symptoms in patients with gastroparesis. Gastroenterology 114: 456–461. [DOI] [PubMed] [Google Scholar]
- McCallum RW, Dusing RW, Sarosiek I, Cocjin J, Forster J, Lin Z. 2010. Mechanisms of symptomatic improvement after gastric electrical stimulation in gastroparetic patients. Neurogastroenterol Motil 22: 161–167, e50–e51. [DOI] [PubMed] [Google Scholar]
- Meneghetti AT, Tedesco P, Damani T, Patti MG. 2005. Esophageal mucosal damage may promote dysmotility and worsen esophageal acid exposure. J Gastrointest Surg 9: 1313–1317. [DOI] [PubMed] [Google Scholar]
- Merrill DR, Bikson M, Jefferys JG. 2005. Electrical stimulation of excitable tissue: Design of efficacious and safe protocols. J Neurosci Methods 141: 171–198. [DOI] [PubMed] [Google Scholar]
- Miller LS, Szych GA, Kantor SB, Bromer MQ, Knight LC, Maurer AH, Fisher RS, Parkman HP. 2002. Treatment of idiopathic gastroparesis with injection of botulinum toxin into the pyloric sphincter muscle. Am J Gastroenterol 97: 1653–1660. [DOI] [PubMed] [Google Scholar]
- Miller L, Vegesna A, Kalra A, Besetty R, Dai Q, Korimilli A, Brasseur JG. 2007. New observations on the gastroesophageal antireflux barrier. Gastroenterol Clin North Am 36: 601–617. [DOI] [PubMed] [Google Scholar]
- Miller L, Dai Q, Vegesna A, Korimilli A, Ulerich R, Schiffner B, Brassuer J. 2009. A missing sphincteric component of the gastro-esophageal junction in patients with GERD. Neurogastroenterol Motil 21: 813–e52.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L, Roland BC, Whitson M, Passi M, Cheung M, Vegesna A. 2018. Clinical and translational aspects of normal and abnormal motility in the esophagus, small intestine and colon. In Physiology of the gastrointestinal tract, 6th ed (ed. Said HM), pp. 485–516. Academic, New York. [Google Scholar]
- Mintchev M, Sanmiguel C, Otto S, Bowes K. 1998. Microprocessor controlled movement of liquid gastric content using sequential neural electrical stimulation. Gut 43: 607–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal RK, Holloway RH, Penagini R, Blackshaw LA, Dent J. 1995. Transient lower esophageal sphincter relaxation. Gastroenterology 109: 601–610. [DOI] [PubMed] [Google Scholar]
- Navarro X, Krueger TB, Lago N, Micera S, Stieglitz T, Dario P. 2005. A critical review of interfaces with the peripheral nervous system for the control of neuroprostheses and hybrid bionic systems. J Peripher Nerv Syst 10: 229–258. [DOI] [PubMed] [Google Scholar]
- Ng RT, Lee WS, Ang HL, Teo KM, Yik YI, Lai NM. 2016. Transcutaneous electrical stimulation (TES) for treatment of constipation in children. Cochrane Database Syst Rev 7: Cd010873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen TN, Struijk JJ, Sevcencu C. 2017. Stimulation waveforms for the selective activation of baroreceptor nerve fibers in the cervical vagus nerve. In Converging clinical and engineering research on neurorehabilitation II. Biosystems & biorobotics (ed. Ibáñez J, González-Vargas J, Azorín J, Akay M, Pons J), pp. 995–999. Springer, Cham, Switzerland. [Google Scholar]
- O’Grady G, Egbuji JU, Du P, Cheng LK, Pullan AJ, Windsor JA. 2009. High-frequency gastric electrical stimulation for the treatment of gastroparesis: A meta-analysis. World J Surg 33: 1693–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang H, Xing J, Chen JD. 2005. Tachygastria induced by gastric electrical stimulation is mediated via α- and β-adrenergic pathway and inhibits antral motility in dogs. Neurogastroenterol Motil 17: 846–853. [DOI] [PubMed] [Google Scholar]
- Parkman HP, Maurer AH, Caroline DF, Miller DL, Krevsky B, Fisher RS. 1996. Optimal evaluation of patients with nonobstructive esophageal dysphagia. Dig Dis Sci 41: 1355–1368. [DOI] [PubMed] [Google Scholar]
- Paterson WG, Wang H, Vanner SJ. 1995. Increasing pain sensation to repeated esophageal balloon distension in patients with chest pain of undetermined etiology. Dig Dis Sci 40: 1325–1331. [DOI] [PubMed] [Google Scholar]
- Policker S, Haddad W, Yaniv I. 2009. Treatment of type 2 diabetes using meal-triggered gastric electrical stimulation. Isr Med Assoc J 11: 206–208. [PubMed] [Google Scholar]
- Qi H, Liu S, Chen JD. 2007. Dual pulse intestinal electrical stimulation normalizes intestinal dysrhythmia and improves symptoms induced by vasopressin in fed state in dogs. Neurogastroenterol Motil 19: 411–418. [DOI] [PubMed] [Google Scholar]
- Rao SS, Welcher KD, Leistikow JS. 1998. Obstructive defecation: A failure of rectoanal coordination. Am J Gastroenterol 93: 1042–1050. [DOI] [PubMed] [Google Scholar]
- Ravi K, Bharucha AE, Camilleri M, Rhoten D, Bakken T, Zinsmeister AR. 2010. Phenotypic variation of colonic motor functions in chronic constipation. Gastroenterology 138: 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez L, Rodriguez P, Gómez B, Ayala JC, Oxenberg D, Perez-Castilla A, Netto MG, Soffer E, Boscardin WJ, Crowell MD. 2015. Two-year results of intermittent electrical stimulation of the lower esophageal sphincter treatment of gastroesophageal reflux disease. Surgery 157: 556–567. [DOI] [PubMed] [Google Scholar]
- Rodríguez L, Rodriguez PA, Gómez B, Netto MG, Crowell MD, Soffer E. 2016. Electrical stimulation therapy of the lower esophageal sphincter is successful in treating GERD: Long-term 3-year results. Surg Endosc 30: 2666–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallam HS, Chen JD. 2013. Colonic electrical stimulation: Potential use for treatment of delayed colonic transit. Colorectal Dis 15: e244–e249. [DOI] [PubMed] [Google Scholar]
- Sanmiguel CP, Casillas S, Senagore A, Mintchev MP, Soffer EE. 2006. Neural gastrointestinal electrical stimulation enhances colonic motility in a chronic canine model of delayed colonic transit. Neurogastroenterol Motil 18: 647–653. [DOI] [PubMed] [Google Scholar]
- Sanmiguel CP, Conklin JL, Cunneen SA, Barnett P, Phillips EH, Kipnes M, Pilcher J, Soffer EE. 2009. Gastric electrical stimulation with the TANTALUS System in obese type 2 diabetes patients: Effect on weight and glycemic control. J Diabetes Sci Technol 3: 964–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarna SK. 2010. Integrated systems physiology: From molecule to function to disease. In Colonic motility: From bench side to bedside. Morgan & Claypool Life Sciences, San Rafael, CA. [PubMed] [Google Scholar]
- Sarr MG, Billington CJ, Brancatisano R, Brancatisano A, Toouli J, Kow L, Nguyen NT, Blackstone R, Maher JW, Shikora S, et al. 2012. The EMPOWER study: Randomized, prospective, double-blind, multicenter trial of vagal blockade to induce weight loss in morbid obesity. Obes Surg 22: 1771–1782. [DOI] [PubMed] [Google Scholar]
- Shafik A, Shafik AA, El-Sibai O, Ahmed I. 2004. Colonic pacing: A therapeutic option for the treatment of constipation due to total colonic inertia. Arch Surg 139: 775–779. [DOI] [PubMed] [Google Scholar]
- Shikora SA, Bergenstal R, Bessler M, Brody F, Foster G, Frank A, Gold M, Klein S, Kushner R, Sarwer DB. 2009. Implantable gastric stimulation for the treatment of clinically severe obesity: Results of the SHAPE trial. Surg Obes Relat Dis 5: 31–37. [DOI] [PubMed] [Google Scholar]
- Song GQ, Chen JD. 2007. Synchronized gastric electrical stimulation improves delayed gastric emptying in nonobese mice with diabetic gastroparesis. J Appl Physiol (1985) 103: 1560–1564. [DOI] [PubMed] [Google Scholar]
- Song G, Hou X, Yang B, Liu J, Qian W, Chen JD. 2005. Two-channel gastric electrical stimulation accelerates delayed gastric emptying induced by vasopressin. Dig Dis Sci 50: 662–668. [DOI] [PubMed] [Google Scholar]
- Song GQ, Sun Y, Foreman RD, Chen JD. 2014. Therapeutic potential of spinal cord stimulation for gastrointestinal motility disorders: A preliminary rodent study. Neurogastroenterol Motil 26: 377–384. [DOI] [PubMed] [Google Scholar]
- Song G, Sun Y, Chen J. 2017. Spinal cord electrical stimulation improves visceral hypersensitivity in a rodent model of functional dyspepsia. Gastroenterology 152: S933. [Google Scholar]
- Sun Y, Chen J. 2004. Intestinal electric stimulation decreases fat absorption in rats: Therapeutic potential for obesity. Obes Res 12: 1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Song GQ, Yin J, Lei Y, Chen JD. 2009. Effects and mechanisms of gastrointestinal electrical stimulation on slow waves: A systematic canine study. Am J Physiol Regul Integr Comp Physiol 297: R1392–R1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley NJ. 2008. Functional gastrointestinal disorders as a public health problem. Neurogastroenterol Motil 20: 121–129. [DOI] [PubMed] [Google Scholar]
- Thomas GP, Dudding TC, Rahbour G, Nicholls RJ, Vaizey CJ. 2013. Sacral nerve stimulation for constipation. Br J Surg 100: 174–181. [DOI] [PubMed] [Google Scholar]
- Vegesna A, Besetty R, Kalra A, Farooq U, Korimilli A, Chuang KY, Fisher R, Parkman H, Miller L. 2010. Induced opening of the gastroesophageal junction occurs at a lower gastric pressure in gerd patients and in hiatal hernia subjects than in normal control subjects. Gastroenterol Res Pract 2010: 857654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegesna AK, Patel H, Weissman S, Patel A, Kissel M, Indukuri S, Nimma A, Dai Q, Miller LS. 2014. Defective mucosal movement at the gastroesophageal junction in patients with gastroesophageal reflux disease. Dig Dis Sci 59: 1870–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Yin J. 2015. Complementary and alternative therapies for chronic constipation. Evid Based Complement Alternat Med 2015: 396396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WF, Yin JY, De Dz Chen J. 2015. Acceleration of small bowel transit in a canine hypermotility model with intestinal electrical stimulation. J Dig Dis 16: 135–142. [DOI] [PubMed] [Google Scholar]
- Wang R, Abukhalaf Z, Javan-Khoshkholgh A, Stocker A, Abell T, Farajidavar A. 2017. A novel system and methodology for continuous ambulatory monitoring of gastric slow waves. Gastroenterology 152: S516. [Google Scholar]
- Wang R, Abukhalaf Z, Javan-Khoshkholgh A, Wang THH, Sathar S, Du P, Angeli TR, Cheng LK, O’Grady G, Paskaranandavadivel N, et al. 2018. A miniature configurable wireless system for recording gastric electrophysiological activity and delivering high-energy electrical stimulation. IEEE J Emerg Sel Top Circuits Syst 8: 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wark HA, Black SR, Mathews KS, Cartwright PC, Gustafson KJ, Normann RA. 2015. Restoration from acute urinary dysfunction using Utah electrode arrays implanted into the feline pudendal nerve. Neuromodulation 18: 317–323. [DOI] [PubMed] [Google Scholar]
- Wongsarnpigoon A, Woock JP, Grill WM. 2010. Efficiency analysis of waveform shape for electrical excitation of nerve fibers. IEEE Trans Neural Syst Rehabil Eng 18: 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing JH, Chen JD. 2006. Gastric electrical stimulation with parameters for gastroparesis enhances gastric accommodation and alleviates distention-induced symptoms in dogs. Dig Dis Sci 51: 2160–2164. [DOI] [PubMed] [Google Scholar]
- Xing JH, Lei Y, Chen JD. 2005. Gastric electrical stimulation (GES) with parameters for morbid obesity elevates lower esophageal sphincter (LES) pressure in conscious dogs. Obes Surg 15: 1321–1327. [DOI] [PubMed] [Google Scholar]
- Xu J, Ross RA, McCallum RW, Chen JD. 2008. Two-channel gastric pacing with a novel implantable gastric pacemaker accelerates glucagon-induced delayed gastric emptying in dogs. Am J Surg 195: 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yik YI, Ismail KA, Hutson JM, Southwell BR. 2012. Home transcutaneous electrical stimulation to treat children with slow-transit constipation. J Pediatr Surg 47: 1285–1290. [DOI] [PubMed] [Google Scholar]
- Yin J, Chen J. 2007. Excitatory effects of synchronized intestinal electrical stimulation on small intestinal motility in dogs. Am J Physiol Gastrointest Liver Physiol 293: G1190–G1195. [DOI] [PubMed] [Google Scholar]
- Yin J, Chen JD. 2010. Mechanisms and potential applications of intestinal electrical stimulation. Dig Dis Sci 55: 1208–1220. [DOI] [PubMed] [Google Scholar]
- Yin J, Zhang J, Chen JD. 2007. Inhibitory effects of intestinal electrical stimulation on food intake, weight loss and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol 293: R78–R82. [DOI] [PubMed] [Google Scholar]
- Yin J, Abell TD, McCallum RW, Chen JD. 2012. Gastric neuromodulation with Enterra system for nausea and vomiting in patients with gastroparesis. Neuromodulation 15: 224–231; discussion 231. [DOI] [PubMed] [Google Scholar]
- Yip M, Bowers P, Noel V, Chandrakasan A, Stankovic KM. 2017. Energy-efficient waveform for electrical stimulation of the cochlear nerve. Sci Rep 7: 13582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariah JS, Kyounghwan N, Saman SP, Hillel JC, John S, Euisik Y, Tim MB. 2018. Flexible microelectrode array for interfacing with the surface of neural ganglia. J Neural Eng 15: 036027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Chen JD. 2006. Systematic review: Applications and future of gastric electrical stimulation. Aliment Pharmacol Ther 24: 991–1002. [DOI] [PubMed] [Google Scholar]
- Zhang N, Huang Z, Xu F, Xu Y, Chen J, Yin J, Lin L, Chen JDZ. 2014. Transcutaneous neuromodulation at posterior tibial nerve and ST36 for chronic constipation. Evid Based Complement Alternat Med 2014: 560802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Yin J, Chen J, Song G, Wang L, Zhu H, Brining D, Chen JD. 2009. Inhibitory effects and mechanisms of intestinal electrical stimulation on gastric tone, antral contractions, pyloric tone, and gastric emptying in dogs. Am J Physiol Regul Integr Comp Physiol 296: R36–R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Sallam H, Chen DD, Chen JD. 2007. Therapeutic potential of synchronized gastric electrical stimulation for gastroparesis: Enhanced gastric motility in dogs. Am J Physiol Regul Integr Comp Physiol 293: R1875–R1881. [DOI] [PubMed] [Google Scholar]