ABSTRACT
The childhood immunization schedule is well known and generally well implemented in developed countries. For various reasons, the same is not true of vaccines aimed at preventing infections in adults, in which vaccination coverage is incomplete and generally very deficient.
In order to assess the situation of adult vaccination in Spain, the Fundación de Ciencias de la Salud has brought together a series of experts in different fields, including doctors, nurses, representatives of patient associations, health managers and economists, health authorities and journalists to deal with this issue. The format was that of a round table in which a series of questions previously formulated by the coordinators were to be answered and debated. The document presented is not an exhaustive review of the topic, nor is it intended to make recommendations, but only to give a multidisciplinary opinion on topics that could be particularly debatable or controversial.
The paper reviews the main vaccine-preventable adult diseases, their clinical and economic impact, the possibilities of reducing them with vaccination programmes and the difficulties in carrying them out. The role of nursing, pharmacy services, patient associations and the health administration itself in changing the current situation was discussed. Prospects for new vaccines were discussed and we speculated on the future in this field. Finally, particularly relevant ethical aspects in decision-making regarding vaccination were discussed, which must be faced by both individuals and states.
We have tried to summarize, at the end of the presentation of each question, the environment of opinion that was agreed with all the members of the table.
Key words: adult vaccines, vaccination, Influenza, Hepatitis B, Hepatitis A, Human Papillomavirus, Pneumococcus, Streptococcus pneumoniae, Haemophilus influenzae, Meningococcus, Ethics
RESUMEN
El calendario de vacunación infantil es bien conocido y generalmente bien implementado en los países desarrollados. Por varias razones,no ocurre lo mismo en el caso de las vacunas destinadas a prevenir las infecciones en adultos, en los que la cobertura vacunal es incompleta y generalmente muy deficiente.
Con el fin de evaluar la situación de la vacunación de adultos en España, la Fundación de Ciencias de la Salud ha reunido a una serie de expertos en diferentes campos, incluyendo médicos, enfermeras, representantes de asociaciones de pacientes, gestores sanitarios, economistas, autoridades sanitarias y periodistas para discutir este asunto. El formato fue el de una mesa redonda en la que una serie de preguntas, formuladas previamente por los coordinadores, debían ser contestadas y debatidas. El documento presentado no es una revisión exhaustiva del tema, ni tiene por objeto hacer recomendaciones, simplemente pretende dar una opinión multidisciplinar sobre aspectos que pueden ser debatibles o controvertidos. El documento revisa las principales enfermedades de los adultos que pueden prevenirse con vacunas, su impacto clínico y económico, las posibilidades de reducirlos con los programas de vacunación y las dificultades para llevarlos a cabo. Se discutió el papel de la enfermería, la farmacia, los servicios de salud, las asociaciones de pacientes y la propia administración sanitaria para cambiar la situación actual. Se evaluaron las perspectivas para nuevas vacunas y se especuló sobre el futuro en este campo. Por último, se discutieron los aspectos éticos especialmente relevantes en la toma de decisiones con respecto a la vacunación, que deben ser afrontados tanto por los individuos como por los estados.
Hemos intentado resumir, al final de la presentación de cada pregunta, la opinión que representaba el consenso de todos los miembros de la mesa.
Palabras clave: Vacunas adultos, Vacunación, Gripe, Hepatitis B, Hepatitis A, Papilomavirus, Neumococo, Streptococcus pneumoniae, Haemophilus influenzae, Meningococo, Etica
INTRODUCTION
The benefits that a child vaccination calendar has had in reducing Infectious Diseases during paediatric age and for the rest of life do not need to be highlighted. However, despite the large number of infectious diseases with significant morbidity and mortality that can affect adults, and the availability of vaccines for many of them, adult vaccination is often neglected. This negligence may be attributable to the patients themselves, as well as to health-care professionals, and the administration.
In order to examine the situation of this problem in Spain, the Fundación de Ciencias de la Salud has brought together, at a round table, both experts in different aspects of the subject as well as representatives of affected communities and the media. All participants were asked a series of previously agreed questions to review the state of the art of each subject, with particular emphasis on the situation in Spain and searching for opportunities for improvement. The opinions expressed by each of the speakers are their own and do not necessarily represent those of the Institution or Institutions to which they belong. This document is not intended to provide recommendations or guidelines, but simply to collect opinions.
The meeting was held in Madrid on April the 18th, 2018 and this document reflects the main questions, answers and conclusions of the meeting updated by the literature available up to May 2018.
METHODS
Before the meeting, the different participants were sent some questions related to the situation of vaccines for adults in Spain, in general terms or in relation to some vaccines in particular. Some questions especially pulsed the vision of these problems on the part of particular groups as the nurses, the associations of transplanted patients, the health economists or the position and attitudes of the press. Each accepted question was introduced and presented by one of the panel members and then discussed by all the participants trying to reach a common opinion or consensus.
The original document, conveniently edited and referenced, was sent to all panel members for correction and final approval.
QUESTION 1.- What are the most prevalent vaccine-preventable diseases in adults?
Exposure:
The World Health Organization (W.H.O.) has listed the most important diseases preventable by vaccines in adults (table 1) [1]. Among them are several that do not currently represent a problem in the developed world, precisely because of the widespread use of vaccines among the population, so we will not address them in this text. Examples are measles, rubella, mumps, tetanus, diphtheria, polio or rabies. It is important to note that, as recent experiences have shown [2], if a population’s vaccination coverage is reduced, there is a clear risk of outbreaks of these diseases. WHO does not include in its list other diseases, also preventable by vaccines, but which either no longer represent a threat (smallpox), or because a fully established and accepted vaccination system (Bacillus anthracis, plague, Q fever) is not yet available.
Table 1.
Most prevalent diseases in adults preventable with vaccines
| Measles Rubella Mumps Poliomyelitis Hepatitis A Hepatitis B Influenza Varicella Herpes zoster Rotavirus |
Human Papillomavirus Rabies Tick-mediated encephalitis Japanese encephalitis Hepatitis E Pneumococcus Haemophilus influenzae type b Meningococcus Diphtheria |
Whooping cough Cholera Typhoid fever Tetanus Tuberculosis Malaria Dengue |
We will mention here, by way of example, seven infections that are vaccine-preventable but remain a challenge for adults in the developed world. The selection is based on the recent recommendations of the North American Advisory Committee on Immunization Practices (ACIP). These infections are, following the same order of the mentioned recommendations, those caused by the Influenza virus, Varicella Zoster, Human Papillomavirus, pneumococcus (Streptococcus pneumoniae), hepatitis B virus (HBV), meningococcus (Neisseria meningitidis) and Haemophilus influenzae type b [3].
Table 2 shows their incidence, some risk factors and the morbidity and mortality associated with them. Whenever possible, we have used figures provided by the CDC in order to harmonize them and recognizing that incidence and mortality figures are estimates [4-16].
Table 2.
Incidence, risk factors, morbidity and mortality of some infections preventable with vaccines.
| Estimation of cases per year | Estimation of deaths per year | Main Risk factors | Morbidity | Sequelae | Source | |
|---|---|---|---|---|---|---|
| Influenza | 3-5,000,000 severe cases | 650.000 | Age, Chronic diseases, Pregnancy Health-Care-Worker |
Fever, malaise, pneumonia | Infrequent | [4] |
| Herpes Zoster | 972,580 in USA (30% life risk) | 60/100,000 in adults >65 years | Age, trauma, immunosuppression, neoplasia, chronic medical conditions | Rash, pain | Neuralgia, meningoencephalitis, myelitis, vasculopathy, retinal necrosis | [5] |
| HPV | 529,000 cervix neoplasia 10-22% with normal Pap-smear |
274,000 | Sexual intercourse, immune compromised | Asymptomatic Genital and non-genital warts |
Cervix carcinoma, vagina, vulva, penis, anus and oropharyngeal recurrent papillomatosis | [6, 7] |
| Hepatitis B | 257-350 million persons with chronic infection | 887,000 (2015) | Mother-to-child transmission, Drug users, health-care workers, sexual transmission | Hepatitis, Fulminant hepatitis | Chronic hepatitis, cirrhosis, hepatoma, extra hepatic manifestations | [8, 9] |
| S. pneumoniae | ≥65: 36.4/ 100.000 <1 year: 34.2 /100,000 hematol. malignancies 186/100,000 HIV 173/ 100,000 |
500,000 children < 5 years | Age, chronic heart and lung disease, smoking and asplenia | Pneumonia, otitis, meningitis, sepsis, endocarditis, other infections | Severe disabilities after meningitis and endocarditis | [10-12] |
| Meningococcus | 1.2 million | 135,000 | Age, closed communities, certain medical conditions (asplenia, deficiency of complement components, HIV infection) and travel | Meningitis, sepsis, pneumonia, and other localized infections | Cognitive impairment, deafness, motor impairment, seizures, visual impairment, hydrocephalus, and limb loss | [13, 14] |
| Haemophilus influenzae type b | 8.13 million severe diseases | 371,000 (2000) 199,000(2008) |
Age, immunosuppression (complement deficit, hypogammaglobulinemia, sickle cell anemia, asplenia, malignancy, HIV), COPD, smoking, alcoholism. | Meningitis, epiglottitis, pneumonia, empyema, pericarditis, bacteraemia, septic arthritis, and other infections | Cognitive deficits and other serious sequelae in cases of meningitis. | [15, 16] |
We must also mention the immense health expenditure involved in these preventable diseases. A few examples are worth mentioning. It is estimated that “the flu” in the United States represents an annual expenditure of 10,400 million US dollars only as direct costs for hospitalization and medical visits of adults [17]. European sources refer to an expenditure attributable to influenza of 56.7 million euros per million inhabitants [18].
In the case of pneumococcus, the cost of an episode of pneumonia, meningitis and bacteraemia has been estimated at 6,283, 3,886 and 4,768 US dollars respectively [19]. Globally, pneumococcal pneumonia costs the United States US$ 4.9 billion annually, which increases by an additional US$ 324 million in the case of antimicrobial resistance [20]. Finally, the cost of bacterial meningitis in the event of sequelae has been estimated at £160,000-£200,000 in the first year after the episode and £590,000 - £1,090,000 per person for the rest of his life, assuming that the patient survives to the age of 70 years [21].
Conclusion:
At present, in the developed world, the 7 adult infections that could benefit most from a strict vaccination schedule are those caused by: Influenza virus, Herpes Zoster, Human Papillomavirus, Hepatitis B Virus, Pneumococcus, Meningococcus and Haemophilus influenzae type b.
QUESTION 2.- What proportion of vaccine-preventable diseases in adults could be reduced with currently available vaccines?
Exposure:
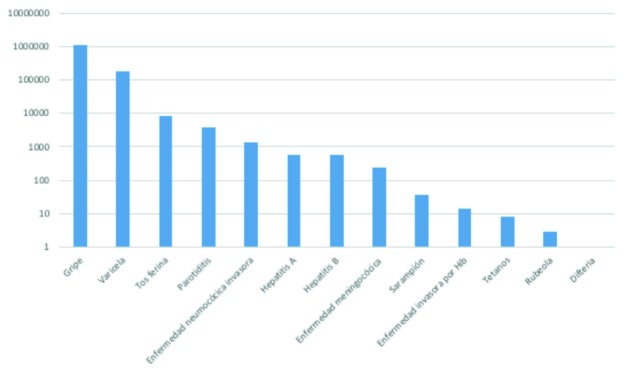
Figure 1 shows the incidence of vaccine-preventable diseases in Spain published in 2015 [22]. Episodes of influenza, followed by chickenpox, whooping cough, mumps, invasive pneumococcal disease, hepatitis A and B, meningococcal disease and others, stand out for their magnitude. The number of deaths attributable to notifiable infectious diseases in Spain in 2015 is estimated to be close to 28,000.
Figure 1.
Incidence of vaccine-preventable diseases in Spain (2015)
The recommendations of vaccines for adults are provided by the Health Department of the United States of America [3].
Despite evidence of a drastic reduction in the incidence, morbidity and mortality of vaccine-preventable diseases since the late 19th century [23-25], American adults, as an example of a developed nation, remain inadequately vaccinated [25-27].
The reasons include poor information, fear of undesirable effects, reluctance to use vaccines, low priority on the list of individual concerns, cost, problems of access to vaccines and other [26-28].
It is estimated that since 1924, more than 100 million cases of smallpox, measles, polio, mumps, rubella, hepatitis A, diphtheria and pertussis have been prevented.
Considering the period from 1980 to the present day, the reductions in incidence and mortality caused by some of the aforementioned diseases exceed 90% and in some cases 99%. and vaccination is considered an efficient and cost-effective procedure as a public health strategy [27, 28].
Following the line of argument of the previous question, the potential for substantial reductions that could be achieved in the different diseases could be summarised as follows:
Influenza
A study of 18 elderly cohorts in the United States of America that collected 713,872 observational persons/-seasons estimated the effectiveness of Influenza vaccines as follows: There was a 27% reduction in the risk of hospitalization for pneumonia or Influenza and a 48% [29] reduction in the risk of death for those vaccinated. These figures were maintained for different age groups and risk subgroups.
A historical cohort of England and Wales in people over 64 years of age compares the rates of acute respiratory infection admissions and death from acute respiratory infections in people vaccinated against Influenza (692,819 person-years) and in unvaccinated people (1,534,280 person/ years). The reduction in hospitalization was 21% and the death rate was reduced by 12% [30].
Gross et al [31], in a meta-analysis of 20 cohort studies, estimate the effectiveness of influenza vaccination at 56% in preventing respiratory infections, 53% in preventing pneumonia, 50% in preventing hospitalizations, and 68% in preventing deaths. In case-control studies, the prevention of hospitalization for pneumonia ranged from 32% to 45%; between 31% and 65% in preventing hospital deaths caused by pneumonia or Influenza, between 43% and 50% in preventing deaths from any respiratory cause and between 27% and 30% in preventing deaths from any cause.
Herpes Zoster
In Western countries, the incidence of shingles is approximately 11 cases per 1,000 inhabitants over 80 years of age/ year, compared to 1 to 3 episodes in people under 50 years of age [27]. A study carried out in the United States shows that approximately 1 million cases of Zoster episodes occur in adults per year and that a high proportion of them developed post-herpetic neuralgia [5].
Lal and colleagues [32] conducted a clinical trial in 18 nations on patients ≥ 50 years with two doses of VZV vaccine two months apart, the results of which were stratified by decades of age (50 to 59, 60 to 69, and ≥70 years). Of a total of 15,411 participants, 7,698 and 7,713 participants received the vaccine or placebo, respectively. During a follow-up time of 3.2 years, herpes zoster was confirmed in 6 and 210 participants in the respective groups (incidence, 0.3 vs. 9.1 per 1,000 people/year). Overall, vaccine efficacy was 97.2%. Adverse effects were minimal and there were no differences between the two groups. In another prospective and comparative study, that enrolled 13,900 evaluable participants (mean age, 75.6 years), observed over an average period of 3.7 years, the efficacy of the vaccine in protecting from episodes of zoster in patients over 70 years was 91.3% and against postherpetic neuralgia, 88.8% [33]. This data offers enormous possibilities for controlling the problem.
Human Papillomavirus
No example more clearly demonstrates the paradigm of infection as a cause of cancer as in the case of the Human Papillomavirus (HPV). Infectious agents are estimated to cause 17.8% of all cancers in the world and their main agents are Helicobacter pylori (5.5% of the total), Hepatitis B and C viruses (4.9%), EBV (1%), HIV along with Herpes viruses (0.9%) and HPV (5.2%) [6].
The last decade of the last century and the first decade of the present one have served to demonstrate the relationship between HPV and cervical cancer in women, a cancer that constitutes the second cause of death by neoplasia for women in the world [6, 34-38].
In a paper published in 2015, looking for HPV on tissues archived with different forms of human cancer, HPV was present in 91% of cervical cancers, in 69% of vulva cancers, 75% of vaginal cancers, 63% of penile cancers, 89% and 93% of anal cancers in men and women respectively and 72 and 63%, respectively, of oropharyngeal cancers in men and women [39].
After 4 multicentric and multinational, similar design, clinical trials, a tetravalent vaccine against HPV was introduced. After a follow-up of 40 months, it showed a protection of almost 100% against genital warts, cervical cancer and the persistence of HPV types contained in the vaccine that were 6, 11, 16 and 18 [40-43]. These results were maintained after 5 years of follow-up [44].
A vaccination with tetravalent vaccine, recommended then only for girls between 11 and 12 years of age, with a rescue for women between 13 and 26 years of age, was introduced in 2006 in the United States [45].
Other recommendations have been added to this, that take into account that there is an older population that can benefit from this vaccine and that men are not excluded from these benefits. However, information is being collected suggesting that the immunogenic capacity of these vaccines decreases when applied to populations over 19 years of age. To the 2 and 4 serotypes vaccines, a 9-serotype vaccine has been added more recently.
For all the above reasons, the current recommendations for vaccination against HPV in the US ideally indicate it between 11 and 12 years old, in both boys and girls, with a potential rescue until the age of 26 for those who did not receive it previously and particularly for groups at risk such as men who have sex with men or immunocompromised men [45]. In Spain, the current recommendations of vaccination against HPV indicate 12 years old girls (vaccinate only girls, with 2 doses) and some risk groups adult women (HIV, ICs (non-HIV) & conizated), in 13 autonomous regions [46].
The CDC of the United States of America estimates, with data from 2008-2012, that about 30,700 episodes of cancer per year, 19,100 in women and 11,600 in men, can be attributed to HPV and that a correct vaccination could prevent 24,600 cancers in the U.S. population each year whether vaccinated with bivalent or tetravalent vaccine, to which an additional 3,800 cases could be added if vaccinated with new serotypes, which would add up to a potential prevention of 28,500 tumours if HPV vaccination is properly implemented, only in the United States of America [47]. Population studies in Denmark and Australia seem to confirm these assumptions [48-52].
Hepatitis B virus
In most European nations, the prevalence of chronic HBV is estimated at 0.5-0.7% of the general population. It is estimated that cirrhosis will develop in 20-30% of those infected with HBV, with another 25% developing hepatocarcinoma [8, 9, 53].
Hepatitis B vaccine is not strictly considered an adult vaccine since it must be administered at paediatric age. It is recommended in adults only for those not previously vaccinated in which there is a medical, occupational or behavioural risk factor or in non-immunized adults who lack these conditions and wish to be protected. The incidence of Hepatitis B in developed societies is already very low since the beginning of childhood vaccination in 1991. It is estimated that the decrease in incidence has been 82%. Despite this, in 2015, the incidence of acute hepatitis B was 2.6 cases per 100,000 people aged 30-39 in the USA [54, 55].
Medical indications for vaccination against HBV in the adult, not previously vaccinated, are primarily chronic renal failure (including haemodialysis), patients with chronic liver diseases, diabetes mellitus and HIV infection. Professional indications focus on health-care workers and security forces who may be exposed to blood or body fluids and people with risky behaviours such as parenteral drug users, those who have had more than one different sexual contact in the last 6 months, men who have sex with men and those who have had a recent Sexually Transmitted Infection (STI).
The WHO aims to eliminate Hepatitis B by 2030, reducing chronic Hepatitis B infections by 90% and associated mortality by 65% [56].
Streptococcus pneumoniae
The importance of Invasive Pneumococcal Disease (IPD) does not need to be highlighted and constitutes a very important cause of morbidity and mortality, mainly in the populations of children and adults over 50 years of age.
The impact that conjugate pneumococcal vaccines have had on the evolution of IPD in children is well known, with clear decreases in the overall incidence of episodes and particularly those caused by serotypes included in them [11].
The impact that has been achieved in the reduction of IPDs in the adult population is not so well known. On this aspect, a recent systematic review assesses the evolution of IPD between 2000 and 2016 using only articles written in English and collected in PubMed, finding 49 valid papers that met the selection criteria. Most of them came from Canada, the United Kingdom or the United States of America and showed statistically significant decreases in episodes of IPD after the introduction of childhood vaccination. This indirect effect on older populations was associated with coverage rates that had been achieved in different situations and particularly benefited those over 65 years of age [12].
IPD incidence reductions ranged from 61% as a combined effect of PCV7, PCV10 and PCV13 use in people over 65 years of age in Canada [12], with up to 21% reduction as an effect of the use of PCV7 and PCV13 in Israel [57].
An Alaskan study reported a significant reduction in IPD following the introduction of PCV13 [58] but reduction did not reach statistical significance in other studies [59], one of them from Barcelona (Spain) [60]. In the latter case, mortality from IPD in people over 65 years of age did not change significantly (24 vs. 22%); but mortality dependent on specific serotypes included in PCV7 did, which in three successive periods were 4.94 vs. 3.58 vs. 2.45 deaths/100,000 population/year.
Neisseria meningitidis
N. meningitidis (meningococcus) are Gram-negative, encapsulated bacteria that are grouped into pairs that cause invasive meningococcal disease (IMI), characterized primarily by meningitis but also by other extrameningeal manifestations such as disseminated meningococcaemia. Mortality, in one form or another, can vary between 10 and 40% of the episodes of infection. Of the 12 existing capsular groups, A, B, C, W, X and Y are the cause of most IMI episodes. IMI episodes are usually grouped into three life stages: childhood, adolescence, and a third group in people over the age of 65. The classic quadrivalent vaccines include polysaccharide antigens from serogroups A, C, W and Y and induce specific antibody responses in more than 90% of receptors [14].
None of these vaccines, however, offers protection against infection by N. meningitidis serogroup B, which is nevertheless the cause of more than 50% of meningococcal infections in different parts of the world, today. There are two vaccines against N. meningitidis type B on the market that are recommended not only for children but also for adults with anatomic or functional asplenia, for those who have deficiencies of complement components, people being treated with eculizumab, microbiologists, and people exposed to epidemic infection situations caused by this bacterium [61]. They contain protein antigens from the external membrane that have been incorporated with different techniques [62].
Given its recent introduction, the long-term impact experience of this vaccine is still scarce. In outbreak situations there has been a 42% reduction in expected cases. In the UK, the efficacy of 4CMenB has been estimated at 83% after the administration of the two doses [63, 64].
In a recent systematic review, the proportion of children and adolescents in whom seroconversion occurs at 30 days versus the original 4 strains was, respectively: 92% for strain 44/76-SL, 91% for 5/99n, 84% for NZ98-254 and 87% for M10713. The incidence of serious adverse events in patients receiving the 4CMenB vaccine was low (5.4 per 1,000 individuals), although higher than other routine vaccines (1.2 per 1,000 individuals)[65].
Haemophilus influenzae type b
H. influenzae type b is a well-known cause of meningitis and other invasive infections, usually accompanied by bacteraemia. Most of them occur in children in whom the vaccine is recommended. In 2012, the rate of invasive Hib disease in Europe in children under 5 years of age was 0.19/100,000 children. In the United States, after the introduction of the vaccine, the incidence of the disease has been reduced by 99% [15, 66] and remains below 0.27 cases/100,000 in children under 5 years estimated by the Healthy People project for 2020 [67, 68]. This has diverted the current focus of incidence to older adults [69-71].
In adults, the H. influenzae type b vaccine is recommended only in immunosuppressed patients at high risk of acquiring this infection, including those with anatomic or functional asplenia or who are scheduled for splenectomy, as well as patients with bone marrow transplants, including those previously vaccinated, beginning 6-12 months after transplantation. This vaccine is not recommended for HIV-positive patients at this time.
In conclusion, with the data summarized above, it is possible to imagine the added protection that would result from adequate immunization coverage. American adults have particularly poor immunization coverage against Influenza, hepatitis B, tetanus, and diphtheria/pertussis, which means that millions of infections in the U.S. [26, 29] could be avoided with the corresponding vaccines. One of the greatest risks is the association between influenza and pneumonia [28], for which vaccination coverage rates among adults did not reach 50%.
The consequences of all this is that some 50,000 Americans die annually from diseases that could have been prevented by vaccination and 99% of the deaths are in adults [27, 28].
In 2008, an estimated 4,500 people died in the U.S. from invasive pneumococcal disease, the vast majority of whom were adults over 35 years of age [72]
In terms of the reasons for this low coverage, in a recent survey, vaccines are perceived as a low health priority for both doctors and patients and to be vaccinated is not required for the vast majority of employment situations. Many adults are not even aware that they need vaccines or the benefits of vaccines, nor do they understand that booster doses of vaccines they have received in the past may be necessary. In general, adults are aware that there are vaccines for influenza or tetanus, however, only 36% of those vaccinated for tetanus received a booster dose every 10 years. In the same survey, 56% of patients who knew there was a pneumococcal vaccine had not had it because “the doctor did not recommend it”. Added to this is the fear of vaccines, punctures and their effects, and in some cases the high cost of vaccines not covered by public services or health insurers.
Conclusion:
The possibilities of reducing the problem with adequate vaccination in adults are always estimated to be above 50% and often more than 90%. The savings in morbidity, mortality and money would be immense if the vaccines were applied in all their indications and with an adequate vaccination calendar in adults.
QUESTION 3.- What data is available on vaccine tolerance in adults?
Exposure:
Local reactions at the injection site of parenterally administered vaccines are common and may include pain, swelling, and erythema, usually of a moderate nature and of short duration. Systemic manifestations such as fever, irritability, or rash may also occur but are also rare and unimportant [73].
Some vaccines contain traces of antibiotics such as neomycin or gelatin as in the case of the MMR vaccine, or egg proteins and can produce an allergic reaction in people with hypersensitivity to these substances. Anaphylactic reactions are estimated to occur in one out of every million doses administered [74].
Thimerosal is a mercuric compound used to prevent bacterial and viral contamination of vaccines, used since the 1930s. No serious effect associated with it has been demonstrated but a hypothetical relationship between this product and autism or other neuropsychiatric diseases has caused a great damage to confidence in vaccines. Such an effect, we insist, was never demonstrated and the work in question was withdrawn for fraud [75-79].
Other risks such as febrile seizures or immune thrombocytopenia are known but extremely rare. The FDA and the CDC maintain a Vaccine Adverse Event Reporting System (VAERS) in the United States of America where manufacturers and physicians report about 30,000 adverse effects annually [80-82].
In this section we will try to respond specifically to the question posed in the adult population and in the vaccines that we have selected as the most relevant and most discussed at the present time.
Influenza vaccine
Influenza vaccination in adults, particularly in people over 65 years, has a somewhat higher incidence of local manifestations (30%) than in the younger population. There is no evidence that the presence of systemic manifestations after influenza vaccination is greater than in a population receiving placebo. A special mention is deserved for the risk of developing Guillain-Barré syndrome, whose incidence in the general population is about 10-20 cases per million inhabitants. With some contradictory data, it is not clear that this rate is increased in the influenza vaccinated population nor that there is a causal relationship between these two problems [83].
A recent systematic review compares the results of influenza vaccination carried out with normal doses in young people or with high doses in the elderly. Although the volume of information is scarce, high-dose vaccine would reduce the risk of influenza by 24%, without clearly being associated with a risk of higher adverse effects [84].
Older patients receiving tetravalent influenza vaccines had neither significant serious adverse effects nor a higher incidence of common adverse effects than trivalent vaccine recipients [85].
Human Papillomavirus vaccines
Serious adverse effects of HPV vaccines are minimal and refer, in the vast majority of cases, to local manifestations of pain or erythema. Occasionally, febrile episodes may occur that rarely exceed a temperature of 39°C. In a safety study, 6 girls had potentially immunomediated reactions (0.8%) such as reactive arthritis, idiopathic juvenile arthritis, erythema nodosum, alopecia areata, ulcerative colitis and celiac disease, of which only one was possibly considered as related to the vaccine [86].
This safety profile is maintained in women who are vaccinated between the ages of 15 and 55 years in which no serious adverse events attributable to the vaccine were detected within an observation period of 10 years [87].
Serious adverse effects were also not detected in other groups of adults who received the vaccine because they belonged to high-risk groups [88, 89] or during pregnancy [90]. There is no evidence of increased risk of Guillain-Barré syndrome in the HPV vaccinated population [91].
Nonavalent vaccines are as harmless as tetravalent vaccines and there is no difference between them in the incidence of headache, dizziness or tiredness [92].
Zoster vaccine
There are two vaccines available for the prevention of Zoster in adults over 50 years of age: an older live attenuated virus (ZVL) vaccine on the market, and a recombinant vaccine, produced primarily with more recently introduced glycoprotein E (RVZ) [93-99]. Although the two vaccines have not been compared face-to-face in clinical trials, the efficacy of RZV seen in two clinical trials appears superior to that of ZVL. The protection of ZVL Zoster is estimated at 70% [100], whereas in the case of RVZ the protection was 90 to 97% in two randomized clinical trials [33, 101].
Vaccines are preferably indicated for non-immunosuppressed individuals over the age of 50 and data on immunosuppressed individuals is limited. Safety data does not allow these vaccines to be indicated in individuals with multiple sclerosis, rheumatoid arthritis and other autoimmune diseases because of the risk of the vaccines inducing flare-ups. RZV is preferred for vaccinating people who have immunosuppressed home contacts. There are no contraindications to RZV vaccination for people who have had a previous Zoster more than three years ago or who have previously received ZVL.
The incidence of local reactions is higher with RZV and consists primarily of local pain at the injection site that only limits routine activities in 9% of recipients [33, 101]. The most common systemic reactions to RZV are myalgia, tiredness, headaches, chills and fever that only limit daily activities in 10.8% of cases. The duration of these side effects is usually less than 3 days and do not prevent the vast majority of recipients from receiving the second dose.
The ZVL vaccine is administered in single doses and its local and systemic effects are qualitatively similar to those of RVZ. However, 6 cases of acute retinal necrosis, uveitis or keratitis with ZVL have been reported between 6 days and 2 months after vaccination. Contraindications to VZL include allergy to gelatin or neomycin, immunosuppression that may facilitate dissemination of the vaccine strain, and pregnancy [102, 103].
Hepatitis B vaccine
There are several recombinant hepatitis B vaccines currently available on the market and all of them are considered extraordinarily safe although the protection rate drops substantially as administration takes place later in life.
The most important adverse effect with classic vaccines is pain at the injection site that occurs in less than 25% of vaccines. In much lower percentages there may be fever, malaise, headaches, arthralgias and myalgias, generally mild and of short duration.
Suspicions of a link between vaccination against hepatitis B and multiple sclerosis, raised in France, have not been confirmed in studies carried out in the United States of America [104-109].
In the case of the recombinant HBV vaccine using a new adjuvant (HepB-CpG), the adverse effects are similar to those for the other vaccines [110], but suspicion has recently been raised that it may be associated with a higher incidence of myocardial infarction in one of the three major clinical trials, as well as new-onset autoimmune diseases [111].
Pneumococcal vaccines
In many developed countries, vaccination with the 23-valent pneumococcal vaccine is recommended to prevent IPD in adults over 50 years of age or with underlying diseases that justify the fact that it has been available for decades. Immunity declines with age and the revaccination recommendation is under discussion. In a meta-analysis that includes 14 studies in vaccinated and revaccinated patients [112], most of them have significant biases, but local and general adverse effects during vaccination and revaccination were few and limited in time, although they were more frequent during the second vaccination than during the first.
For conjugate vaccines, tolerance is also very good and serious adverse effects are minimal [113]. Most studies have found no adverse reactions of particular interest, with the doubt of an increase in asthmatic reactions in some of the studies. The application of these vaccines to patients who have previously received unconjugated vaccines does not increase their intolerance [114, 115].
Meningococcal vaccines
Vaccines to prevent invasive meningococcal disease are usually given before adulthood and are only given in adults if there is a particular risk of contracting this disease. This risk is particularly important in travellers to hyperendemic areas of meningococcal disease, in military personnel working in these areas, and for people frequently in contact with Neisseria meningitidis, such as microbiologists. They are also indicated in individuals with functional or anatomical asplenia, patients with complement deficiency, patients treated with eculizumab, men that have sex with men and patients in some areas where there is an epidemic outbreak of this disease in this population group.
In addition to the classic quadrivalent vaccines, there are two vaccines against Neisseria meningitidis serotype B (Trumemba® and Bexsero®) that can be used in adults with risk factors such as those mentioned above.
The most common adverse effects with tetravalent vaccines include local pain and erythema, along with fever and headache as systemic effects. Although occasional cases of Guillain-Barré syndrome have been reported following meningococcal vaccination, a clear causal association between these vaccines and this syndrome has not been demonstrated [116, 117].
In the case of N. meningitis serotype B vaccine, administration to adults (microbiologists with occupational risk of invasive meningococcal disease) showed local discomfort was frequent but there were no serious adverse effects [118].
Haemophilus influenzae type b vaccine
The vaccine for the prevention of invasive disease caused by H. influenzae type b is rarely administered in adulthood. The most frequent reasons for this are the existence of anatomic or functional asplenia, HIV infection, humoral immunodeficiency, defects of the complement chain, bone marrow transplant recipients and some patients with chemo or radiotherapy [119, 120]. Adverse effects of this vaccine in adults are very uncommon [121].
Conclusion:
Apart from local effects such as pain or systemic effects such as general malaise or fever, of little significance and short duration, adult vaccines have shown a very high degree of safety and a very low number of serious adverse effects. The very few hypersensitivity reactions described are generally related to substances added to preserve them, such as gelatin or neomycin.
QUESTION 4.- What is the situation of whooping cough in adults and the elderly? How are things in Spain?
Exposure:
Whooping cough or Pertussis is a disease caused by the bacterium Bordetella pertussis that causes a respiratory infection in childhood, characterized by violent attacks of spasmodic cough that can last for weeks and are usually followed, in children, by post-episodium emesis. The only reservoir is human and it is a highly contagious disease that can be fatal. Transmission in the close circle is very frequent but does not always translate into a symptomatic clinical picture.
The introduction of a full-cell vaccine at the end of the 1940s, for use in children, dropped the incidence in the United States of America from about 250,000 cases per year in 1935 to about 1,000 cases per year in 1976 [122].
This vaccine was replaced by an acellular vaccine in 1997, better tolerated than the previous one, but against which the immune response decreases at 5-10 years, resulting in a higher risk of late infection in adolescents and adults [123-126].
Therefore, we are witnessing a resurgence of this disease and in these circumstances; there are currently some 30 to 50 million cases of whooping cough in developing countries, of which some 300,000 cause death [127]. Some important outbreaks have occurred in the United States that have reached 25,827 episodes in 2004, 25,616 in 2005, 27,500 in 2010 and 48,277 cases in 2012 [128] [129]. In the United Kingdom there has also been a significant rebound in recent cases, which reached 12 episodes per 100,000 people aged 15 and over in 2012 [130]. There have also been major outbreaks of the disease in South America, Asia, Africa, Australia and New Zealand with thousands of episodes published since 2008 and finally a severe epidemic that continues since 2008 in West Darfur [131-136].
In Spain, vaccination with whole cell vaccine began in 1975 and acellular vaccine was introduced in 2005. From 1998 to 2009, the numbers of whooping cough cases remained below 1.5 episodes/100,000 population. But, those numbers have risen dramatically in recent years and across all age groups [137]. The evolution of whooping cough in Spain between 1982 and 2016 shows a recent upturn in the number of cases reaching 17.9 episodes / 100,000 inhabitants in 2016 and growing since 2010. The upturn affects all age groups.
Fernandez-Cano et al. analized the hospitalizated cases in Spain by whooping cough between 1997 and 2011, which amounts to 8,331, of which 92% were children under 1 year [138]. The overall mortality was 0.56%, the vast majority of which occurs in infants who acquire the disease transmitted from their parents or siblings. The causes for this resurgence are the loss of natural and vaccinal immunity over time, the lower antigenic potency of acellular vaccines (DTaP), the scarcity of mucosal immunity induction, greater clinical suspicion, improvements in the use and precision of the techniques and the genetic changes of Bordetella pertussis that facilitate the escape from vaccine protection, along with the existence of strains with higher toxin production. Whole cell vaccines differ from acellular vaccines in different aspects. They have a protective efficacy ranging from 38-92% [139], prevent the transmission of disease and infection, interrupt the carrier state, confer a certain group immunity, induce a potent mucosal immunity and an immune response Th17. The acellular ones have an efficacy calculated between 71-85% [140], protect from disease but not from infection, do not prevent carrier status and allow transmission (experimental studies). They do not confer group immunity, do not induce mucosal immunity and produce a Th2-type immune response.
Conclusion:
There has been a clear increase in cases of whooping cough over the last decade, affecting all population groups, including adults. The problem has multiple causes, one of which is the change to acellular vaccines, better tolerated but with less permanence of immunogenic capacity. Spain is no exception to the problem and has multiplied its incidence of pertussis more than 10 times in the last decade.
QUESTION 5. - What has been the reality of the recent flu vaccination campaign in Spain?
Exposure:
The reality of the 2017-2018 flu campaign in Spain is that it has been a real “perfect storm” with declining vaccination figures, a multiple circulation of different types and subtypes of virus A, coupled with a predominance of type B commanding the seasonal epidemic, aggravated by the almost absolute discordance between the B virus lineage that has circulated in the last seasonal epidemic 2017-2018 (Yamagata lineage) and the content of the trivalent vaccines (Victoria lineage) administered this season.
Spanish flu vaccination figures are known almost every year at the beginning of the following year’s campaign, when the different Autonomous Communities provided their data to the Ministry of Health. For the umpteenth consecutive year since the 2009 pandemic, Spain shows a consecutive decrease in these figures. The only official global record available to Spain is for people over 65 years of age and indicates that the Spanish average for this population group stands at 55.5% coverage far from the 65.7% of the 2009-2010 flu season; maximum reached in the Spanish time series and twenty points away from the WHO set at 75% for ≥ 65 years. Only two Spanish communities, Castilla y León and La Rioja, have exceeded 60% of vaccination of their elderly. (Ministry of Health, Social Services and Equality, data from 2017).
In this regard, WHO has expressed concern that half of European countries vaccinate less than 1 in three older people [141]. In this sense, the recommendations of many of the more and more extensive, detailed and individual European countries recommendations reach lower real vaccination figures, which shows that extending vaccination indications to particular populations does not necessarily guarantee an increase in coverage [142].
Spain, like most European countries, has included health care workers in its guidelines for influenza vaccination for years, but coverage in this strategic group is less than 40% and even lower. In general, there tends to be a certain parallelism between vaccinated health-care workers and coverage in a given community. Some Spanish publications have reliably demonstrated an association between this fact and also the reasons associated with a higher frequency of vaccination in Health-care workers [143].
Much more worrying is this vaccination in pregnant women; a priority population group for WHO, recommended in more than 90% of countries surveyed, barely reaches 10% coverage in more than half of European countries despite the demonstrated risk of severe influenza in pregnant women and the additional protection of the new-born by vaccinated mothers [142].
This year’s seasonal influenza epidemic (2017-2018) has also had some different peculiarities with respect to others, such as the slightly earlier onset than other times, the prominence of the B virus over the A viruses and the presence of a lineage (Yamagata) different from the content of the seasonal vaccine (Victoria lineage). A virus has also circulated, mostly of subtype H3, strain A/Singapore/16-0019/2016 different from that contained in the vaccine (A/Hong Kong/4801). This vaccine H3 virus has accounted for only a third of the infections by Influenza A virus. (Spanish Influenza Surveillance System, April 2018). Despite this, the effectiveness of the vaccine has been reasonable with a certain cross-response.
In Spain, inactivated vaccines have been available in their different forms. Vaccines of fractionated viruses, subunits and adjuvant vaccines mainly and to a lesser extent, modern tetravalent with two B virus lineages, in addition to subtypes H1 and H3 of type A influenza virus. The viruses recommended by the WHO in the 2017-18 vaccine have been: A/Michigan/45/2015 (H1N1) pdm09-like virus; A/Hong Kong/4801/2014 (H3N2)-like virus, B/Brisbane/60/2008-like virus (Victoria lineage). With the recommendation that quadrivalent vaccines containing two B viruses in addition to the three previous viruses include a B/ Phuket/3073/2013-like virus strain (of the Yamagata lineage).
Almost all the Autonomous Communities have vaccinated with trivalent inactivated vaccines in any of the existing modalities. This has left approximately 60% of the main viruses without specific homologous coverage, although, as explained above, there has been some heterologous cross-protection.
The explanation for the use of trivalent vaccines instead of quadrivalent ones lies fundamentally in the price differences between them. Spain, like other countries, has a very conservative stance in this regard. The WHO has noted that in the 2017/2018 season there were many hospitalizations among elderly people caused by the influenza B virus of the lineage that was not included in the classic trivalent vaccines. Although price can be a barrier to implementation in countries with limited resources, due to the higher price of quadrivalents, WHO considers that given the total costs to the health sector, quadrivalent vaccines can prove to be cost effective [144].
As far as its general explanation is concerned, the healthcare world tends to have a personal and simplistic knowledge about Influenza, which, together with a lack of trust in a vaccine that is not absolutely effective, means that it is not linked, as in other countries, to criteria of healthcare quality and efficiency and does not appear constantly in the lifestyle and clinical protocols of many common chronic diseases.
As for the challenges and possible future solutions, the first challenge in Spain lies in agreeing on a universal vaccination indication or one almost similar to that of the USA, Canada or the UK. Only this indication has been shown to increase coverage and therefore reduce risks and healthcare costs [145]. The current WHO coverage percentage targets (>75% in >64 years) do not achieve group protection (herd immunity) that would be achieved with the US coverage targets (>80% in healthy people) [146].
On the other hand, Spain, like many European countries, is far from the coverage targets and does not include among its indications that of children between 2 and 5 years that exist, for example, in Finland and the UK. In this sense, it is surprising that countries with very low coverage of influenza vaccination in classic population groups (chronically ill, elderly, etc.) recommend vaccination in children as a more gestural measure than fulfilled, since in many of them the influenza vaccine is not free or reimbursed [142].
The next challenge is the development of vaccines with elongated immunity in order to increase the immunizing potency and its spectrum of effectiveness against different viruses, thus avoiding the problem of flu variation or lengthening the period of influenza revaccinations. These vaccines have been denominated by the WHO as NGIV (Next Generation Influenza Vaccines) that has elaborated and published some objectives to 5 and 10 years. Some of them are easier to achieve and reach; others may require more time [147]. Among these future vaccines are the popularly-called “universal flu vaccines” claimed by different authors [148, 149].
The approaches to these vaccines are multiple and not all have the same degree of experimental development. The viral targets against which they are directed include, in order of development, the M2 protein, the chimera haemagglutinins, the inclusion of neuraminidase and nucleoprotein (NP), the antibodies against the stem of the haemagglutinin in serial administration, etc. [150, 151].
Until these challenges are met, the low coverage of influenza in many European countries, especially those in the East, must be addressed by clarifying misunderstandings among the population, doctors and health-care administrators homogeneously throughout the Union [152]. This is the only way to increase the coverage in the elderly and people with chronic diseases and add other population groups with scarce or testimonial coverage (pregnant women and children) reaching at least the 75% targets set by the WHO [153]. Until we reach the Holy Grail of an almost universal flu vaccine, there are quite a few preliminary goals to be met [154].
Conclusion:
The proportion of people over the age of 65 vaccinated against influenza in Spain continues to decline and is far from a coverage of more than 75% of the population WHO objectives. The situation in populations such as health care workers, pregnant women and children is regrettable and does not reach significant figures. There are very important challenges in the flu vaccination until the Holy Grail of an almost universal vaccine against this virus is reached. Vaccination of children is effective not only in the prevention of hospitalizations but also in indirect herd immunity in older people before much greater coverage is achieved.
QUESTION 6.- What is the situation of pneumococcal vaccination in Spain?
Exposure:
S. pneumoniae infection is a major cause of morbidity and mortality worldwide and the pneumococcal disease is potentially preventable by vaccination in the world. According to WHO estimates, S. pneumoniae causes 1.6 million deaths annually; the disease preventable by vaccines that causes the most mortality, with the youngest children and older adults being the most affected. Probably, routine childhood vaccination could prevent morbidity and mortality associated with pneumococcal infection in adults (indirect protection). However, until systematic vaccination of the child population is maintained for several years, the use of PCV13 seems to be justified in adults at higher risk, as the prevention of pneumococcal disease is based exclusively on the use of vaccines [155].
In Spain, S. pneumoniae is the most frequently identified pathogen in community-acquired pneumonia (CAP), causing up to 63.7% of cases in some series. During the period 2003- 2007, a total of 75,932 deaths due to CAP were registered in adults aged 50 years or over and the incidence of CAP in our country in people over 65 is estimated at 14 cases per 1,000 person-years (IC95% 12.7-15.3) and increases with age (29.4 cases per 1,000 person-years in people over 85 years). In addition, it carries an important burden, as up to 75% of cases require hospital admission [155].
We have the pneumococcal polysaccharide 23 valent vaccine (PPV23), indicated for active immunization for the prevention of S. pneumoniae disease in people older than 2 years. In Spain, there are two authorised vaccines: Pneumo23 (pre-filled syringe) and Pneumovax23 (vial). In addition, conjugate pneumococcal vaccines are available, 7, 10 and 13 valents indicated for active immunization for the prevention of Invasive Pneumococcal Disease (IPD), pneumonia and Acute Otitis Media (AOM) caused by S. pneumoniae in children and adolescents aged 6 weeks to 17 years and for the prevention of IPD in adults ≥ 18 years and older. The conjugate vaccines authorised in Spain are Synflorix® and Prevenar® [156].
In Spain it is estimated that approximately 50% of the population over 50 years of age has risk factors for pneumococcal disease and would be candidates for vaccination [157]. The impact of polysaccharide vaccines have shown only a modest reduction in hospitalizations, ICU admissions, and death in elderly patients diagnosed with CAP [158]. On the contrary, the impact of the use of conjugate vaccines in children on the incidence of disease by vaccine serotypes in adults has been demonstrated by Cámara et al [159]. The PPV23 has shown a good safety profile both as primary doses and after the administration of booster doses, but does not generate immune memory, with antibody levels decreasing over time, causing a phenomenon of immune tolerance, and also does not act on nasopharyngeal colonization. However, the conjugate vaccines (PCV13) generate a more potent immune response and a greater impact by acting on nasopharyngeal colonization.
Prior to the introduction of PCV7 in children, a study in 10 European countries, including Spain, evaluated the cost-effectiveness of PPV23 in preventing IPD in adults, which was found acceptable in all countries. For Spain, the cost-effectiveness rate per QALY (Quality of Life Adjusted Life-Year Earned) among adults aged 65 and over was estimated at 9,187 euros.
Using the CAPiTA study efficacy data, the CAPA study serotype coverage data and the CMBD 2010-13 incidence of pneumococcal disease, it determined that the use of PCV13 in 5 years would hope to avoid in a cohort between 65 and 69 years of age 10,360 cases of IPD, 699 deaths, 14,736 years of life gained that only in direct costs would represent an accumulated net saving of 3.8 million euros at constant prices (4.9 at current prices), being efficient for the National Health System of Spain.
PPV23 is financed by all the Autonomous Communities in Spain, in risk groups and systematically for people over 60 years in each Autonomous Community. Only 5 Autonomous Communities (Castilla León, Madrid, Galicia, Asturias and La Rioja) finance in their calendar the vaccination of adults with a valent conjugate vaccine from 60-65 years of age. The fact that it is not financed does not mean that it cannot be recommended. In fact, the Ministry of Health, Social Services and Equality itself, in the review document published by the Working Group on Vaccination against pneumococcus in risk groups of the Presentation of Programmes and Registration of Vaccinations and approved by the Public Health Commission in June 2015, urges physicians that “it is necessary to adequately inform the elderly and/or those belonging to at-risk groups of the possibilities of vaccination against pneumococcus (...)”. In those cases in which the vaccine recommended by the health authorities is the PPV23, it is necessary not only to inform that the choice of the vaccine obeys public health criteria, but that the PCV13 also exists and is marketed, which, although it is not financed in all cases, is not contraindicated [156].
Conclusion:
Invasive pneumococcal infection in Spain is a very important cause of morbidity and mortality in adults and the elderly. Although the polyvalent polysaccharide vaccine has shown only a modest impact in reducing hospital admissions and deaths, conjugate vaccines applied to children have a greater impact on the adult population. The financing of these vaccines does not follow a homogeneous pattern in the different Autonomous Communities of Spain.
QUESTION 7. - What is the future of Vaccine Clinical Research?
Exposure:
According to a report issued by a prestigious consortium of manufacturers and researchers, almost three hundred vaccines are in the development phase, half of them aimed at infectious diseases [160]. The dynamism of this field of knowledge is illustrated by the fact that access to PubMed through the terms “vaccines research” currently offers one hundred and thirteen thousand references [161]. To offer a structured view of the topic, we will try to answer four questions.
What vaccines are in Phase 3 clinical trials at the present time?
The field of infectious diseases includes vaccines against bacteria, viruses, fungi and parasites, which employ various production strategies and techniques. Those aimed at identifying new protective antigens include inverse vaccinology, structural vaccinology and immunomics; those aimed at acquiring or enhancing immunogenicity include vaccinomics, systems vaccinology, use of new adjuvants and delivery modalities, heterologous vaccination, polysaccharide to protein conjugation and adversomics. Among the innovative routes of administration: edible, mucous, and transcutaneous. And as new types of vaccines: recombinant (with or without vectors), nucleic acid, peptide, attenuated and molecularly inactivated, reordered virus (reassorted) and adapted to the cold [162].
The antiviral vaccines that are at a more advanced level of research development include those aimed at the prevention of infection by Cytomegalovirus (CMV) in stem cell transplantation, recurrent infection by Herpes Simplex (HSV) and Varicella-Zoster Virus (VZV). At the same level of development are framed different flu vaccines, against Respiratory Syncytial Virus (RSV), new modalities of triple virus (Measles-Rubella-Parotiditis) and those aimed at the prevention of HPV and HIV, whose search is a relevant challenge, with high budgets and great media attention [163]. Of the “emerging” agents it seems appropriate to cite Dengue, Ebola and Zika. Dengue is based on another flavivirus (yellow fever), which is attenuated and recombined with genes from the premembrane and the envelopes of wild strains of the different serotypes [164].
The Ebola outbreak in 2014 has accelerated the development of vaccines, being an adenovirus derived from chimpanzee (ChiAd3) that encodes the glycoprotein of the species Zaire (GP EBOV), which has become the vector of the same at an advanced stage Zika is working on vaccines that can activate the response B and T together and also include Dengue [165, 166].
Among the antibacterial vaccines, those against staphylococcal infection (due to S aureus which includes several antigens, given its host adaptation systems that allow it to colonize numerous niches and elude the immune system) stand out [167], as well as those against pneumococcal infection (recombinant vaccines), conjugate anti-meningococcal vaccines, and against Streptococcus agalactiae, H. influenzae, and Clostridium difficile. [168].
Of the parasitic diseases that present a strong research investment, Malaria, Chagas Disease and Leishmaniasis stand out, the latter being autochthonous in our country. Vaccines are developed with recombinant antigens, by vectors (adenovirus or vaccinia), DNA vaccines and a heterologous vaccination strategy through induction by plasmidic DNA and a later reinforcement with a viral vector (adenovirus) or with recombinant proteins adjuvated with IL-2 and cytokines [169].
Which of the research vaccines will be most useful in Spain?
The conventional meaning of “usefulness” refers to the capacity of a measure (in this case a vaccine) to serve or to be used for a specific purpose. The criteria that must prevail in order to implement “useful” vaccination strategies in our environment must assess the economic and social impact of prevention programs. To this end, at least two entities are involved that combine healthcare and preventive activity in each Regional Management of the health system: the “Direcciones Generales de Asistencia y Salud Pública” of the different Autonomous Communities. Among others, we could consider from the assistance both the assessment of the real burden of each disease, identified by the Basic Minimum Data Set to hospital discharge and scientific literature; and have a system of access to “big-data” that allows a quantification of the most prevalent infectious diseases. Public Health and Preventive Medicine should define vaccination priorities by age segments and patient groups. From the managerial point of view, it is pertinent to implement economic evaluation studies with robust and consolidated models that allow to endorse the decisions adopted and render accounts with transparency.
In our country, in addition to those commented by the previous speakers, priority would be given to those against RSV, CMV and other Herpesviruses. Among the bacterial ones, it would be desirable to promote those directed against Staphylococcus aureus and C. difficile.
What problems are foreseen for its future implementation?
The definition of health-care priorities represents a challenge that presupposes equity, access to the system and budgetary availability. Among the actors that will have a joint impact on its application and, consequently, on the reduction of the problems for its application, it is worth mentioning:
Firstly, the Pharmaceutical Companies which, through their R+D+I strategies, develop and manufacture effective and safe vaccines and contribute to their post-marketing implantation/surveillance. Secondly, basic researchers, contributing new concepts and technologies and connecting with groups that apply their findings. Thirdly, health-care professionals who advise the population on their benefits and develop vaccination programs. Investment in continuing education will never be weighed sufficiently. In Spain, Primary Care exhibits exemplary behaviour in achieving recommendations and coverage that place us in paediatrics among the most advanced countries [170], a fact that should be taken advantage of in the vaccinology of adults and patients with special indications. Fourthly, the necessary involvement of the media in the dissemination of truthful and responsible information in support of vaccination campaigns should be highlighted. Finally, it is opportune to point out the role of the Health Authorities, who define the conditions of use and ensure access to vaccines and their implementation, provide budget, support and promote vaccination policies. Likewise, they must preserve the protagonism and independence of the Regulatory Agencies, which evaluate and control their effectiveness, safety and quality.
What impact will they have on the problems they aim to reduce?
It is clear that the purpose of any vaccination strategy is to measure its capacity to reduce the burden of disease to be prevented, to reduce its morbidity and to avoid its potential mortality.
It is possible to introduce new vaccines from the modalities of economic evaluation in the field of health. These can be summarised in two types of techniques: analysis where the measurement of the effect is collected in monetary units (Cost-Benefit Analysis) and analysis where the measurement of the effect is collected in non-monetary units, where the Cost-Utility Analysis (CUA) is inscribed. Specifically, in a CUA (to which the second question referred) we compare two or more alternatives in relation to its costs and results, expressed in terms of utility units or quality of life, according to the user’s perception. The unit of measurement can be the QALY (Quality Adjusted Life Year) or AVAC (Quality Adjusted Life Years); this measure relates the years of life that the individual would enjoy (thanks to a health intervention) with the quality of life of that extra period [171].
A particularly attractive field will be to apply these evaluation models from vaccinomics, studying individual phenotypes and genotypes, correlating genetic polymorphisms with a certain predisposition to suffer the infection, a singular immune response, an adjusted vaccine dosage, an adequate administration route or quantifying the probability of suffering an adverse effect [162]. This will lead to the possibility of designing vaccines for each individual or group that are safer, cheaper and easier to conserve/administer, against prevalent and emerging pathogens such as those mentioned above.
Conclusion:
An enormous number of Phase 3 clinical trials are currently studying the effectiveness of new vaccines, approximately half of which are aimed at controlling infectious diseases. These include vaccines for viral, bacterial and parasitic processes, and their future application will depend on very diverse factors that must consider the size of the problem, the effectiveness of the vaccine, its tolerance, and economic aspects of unquestionable importance.
QUESTION 8.- What is the administration’s vision of vaccines in Spain?
Exposure:
Following the transfer of public health competencies from the State to the Autonomous Communities (AA.CC.), between 1979 and 1985, and through the General Health Law 14/1986, the “Interterritorial Council of the National Health System, ICNHS (Consejo Interterritorial del Sistema Nacional de Salud)” was created as a permanent body for coordination, cooperation and communication between the State and the AA.CC. In this way, the Ministry of Health, Social Services and Equality coordinates and harmonizes health strategies in order to maintain equity and cohesion in access to health services. [172].
The Committee on Vaccination Programme and Registries, created in 1991, advises the Public Health Commission of the ICNHS from a scientific and technical point of view in making decisions on vaccination programmes in Spain [173]. Vaccination in risk groups and healthy adults is currently being reviewed within the ICNHS. These recommendations, which are still in the consultation phase, are expected to be agreed in 2018.
Vaccines for adults authorised in Spain.
Vaccines are authorised through national or centralised procedures, the latter coordinated at European Union (EU) level and the most widely used at present. The Spanish Agency for Medicines and Health Products (AEMPS), existing under the Ministry of Health, Social Services and Equality, is the regulatory body that participates together with the other EU countries in the evaluation of medicines dossiers in the European Medicines Agency (EMA) [174-176].
Most of the vaccines authorized in Spain are for use in a wide range of ages including adults, with the exception of combined vaccines that contain high loads of diphtheria toxoid and components against whooping cough (D and Pa), rotavirus vaccines (up to 24 or 32 weeks depending on the product), attenuated influenza (2 to 18 years), shingles (from 50 years of age and older), ten serotypes pneumococcal conjugate vaccine (6 weeks to 5 years of age) and H. influenzae type b (2 to 5 years of age) [177].
It is important to distinguish between the authorization and the recommendation of vaccines. In the evaluation for vaccine authorization, it is considered that the benefit/risk ratio is favourable. To establish vaccination recommendations, it is necessary to consider other additional criteria, such as the epidemiological characteristics of the disease to be prevented, the pattern and target group to obtain the expected benefits in the population, indirect adverse effects of its use, implementation aspects and economic aspects.
The recommendations for vaccination in adults approved from the ICNHS currently include:
- Systematic vaccination in ≥ 65 years against tetanus and diphtheria (Td), influenza and pneumococcus (VPP23).
Between the ages of 15 and 64, any contact with the health system should be used to review vaccination and update it in case of susceptibility, especially Td, MMR (measles, mumps and rubella) and varicella; and in young adults, hepatitis B, meningococcus C and HPV.
- In addition, people of any age with risk conditions, the relevance of recommending DTap, hepatitis A, hepatitis B, conjugated meningococcal, conjugated pneumococcal and influenza vaccines should be taken into account.
How are vaccines financed in Spain?
According to current legislation, referring to the portfolio of common services in the NHS, “vaccinations are covered in all age groups and, where appropriate, risk groups, according to the current vaccination schedule approved by the ICNHS and the competent health administrations, as well as those that may be indicated, in general population or risk groups, for situations that epidemiologically advise it”.
Should there be some others?
At the moment, vaccination recommendations for different risk groups and healthy adults are in the final phase of the evaluation process at the ICNHS. The evaluation of vaccination recommendations against shingles in healthy adults and meningococcal disease will begin with 2018.
What are the major differences between Autonomous Communities?
In recent years, the ICNHS has worked to reach a broad consensus on vaccination recommendations aimed at the child population, reflected in the common childhood vaccination schedule. Although there has also been joint work on the recommendations for certain vaccines in risk groups, some AA.CC have extended the age of vaccination and the use of certain vaccines to certain population groups. The main differences relate to the age of influenza vaccination, type of vaccination used for routine pneumococcal and risk group vaccination, and human papillomavirus (HPV) vaccination in certain risk groups.
What can be improved and what is needed to do so?
Some of the aspects to be improved in terms of vaccination policy in general and in terms of vaccination in adults, in particular, would include the following:
- Recommendations on vaccination programs are agreed by consensus in the ICNHS. However, sometimes these recommendations may not be followed by all AA.CCs. Unilateral decisions different than those agreed in the ICNHS may cause confusion in the population and the healthcare workers. Political commitment and institutional loyalty are required to maintain agreements adopted within an institution, the ICNHS, of which all the Autonomous Communities are a part.
- In order to improve confidence in the decisions adopted by the ICNHS, it would be necessary to find mechanisms for participation in the proposal of recommendations from the different stakeholders involved in vaccination, as well as greater transparency and communication between them.
- There is a need for greater awareness of the benefits that vaccination programs bring to the health of the population, establishing communication strategies aimed at health professionals and the general population.
Conclusion:
The authorisation of vaccines in Spain is mainly carried out at European Union level. Most of the vaccines authorised in Spain are for use in a wide range of ages including adults, with some exceptions authorised only for children or for the erderly. They are financed in any age group, as long as they are included into the current vaccination schedule, approved by the Interterritorial Council of the National Health System or the Autonomous Communities, with few differences between them. Throughout 2018, the evaluation of vaccination recommendations against herpes zoster in healthy adults and invasive meningococcal disease will begin.
QUESTION 9.- What is the vision on vaccination of a group of affected people such as patients with Solid Organ Transplants?
Exposure:
First of all, it would be appropriate to highlight, as an idea for discussion, the potential role of vaccines as a mechanism to avoid solid organ transplants (SOT). We do not know a precise answer to this question, but it is enough to recall, as an example, that a substantial proportion of liver transplants are a consequence of the evolution of hepatitis B and therefore potentially avoidable in almost 100% of cases.
Preventing infection is a key element in SOT patients, since it is clear that infections contribute to the morbidity and mortality of these patients and often to graft loss. Prevention is also necessary because many avoidable infections either have no medical treatment or patients respond poorly to it. Immunization in these patients, with frank immunosuppression, also has its particularities since, generally, vaccines made with live attenuated agents cannot be administered, in addition to the ability to mount an adequate immune response being limited in some situations [178].
The International and National Societies have issued Guidelines with recommendations for Immunization in this population both in the paediatric age and for adults that include their health and personal contacts. [120, 179, 180]. Ideally, vaccines should be given before transplantation to achieve the greatest possible immune response. During this period, the patient may receive vaccines with live attenuated agents (measles, mumps, rubella, chickenpox, etc.) that they will not be able to receive if administration is made after transplantation.
In the post-transplant period, vaccinations are generally avoided in the first two to six months after transplantation, during the period of maximum immunosuppression. An exception to this rule is the case of Influenza, in which vaccination is justified after the first month post-transplant with inactivated influenza virus vaccines [181].
It is known that influenza is more severe in the population with SOT, occurs more frequently with pneumonia, causes more intensive care admissions and more deaths than in the non-transplanted population [182]. Vaccine protection is lower than in the immunocompetent population and administration of higher antigenic doses in this population is associated with a better immune response [183, 184]. A Spanish group has demonstrated the best efficacy of a second dose (booster) of inactivated flu vaccine, 5 weeks after the first, in the transplanted population [185].
For other inactivated vaccines, a summary of the situation would be as follows:
Vaccination guidelines for diphtheria and tetanus should be the same as in the normal population, and vaccines are considered safe, although diphtheria-toxin antibody levels may fall more rapidly than in the normal population. Booster doses with tetanus diphtheria toxoid should be given at least every 10 years [186, 187]. The ACIP recommends that booster doses be made with a triple vaccine including tetanus toxoid, diphtheria toxoid and acellular pertussis (Tdap) type Boostrix® or Adacel® for all adults older than 19 years in which a decrease in immunity is suspected.
In relation to polio, given the situation close to the eradication of poliomyelitis, only transplant recipients who could be exposed due to travel or risk would require prevention with inactivated vaccine, in case of doubt of previous vaccination, and only a booster dose is recommended if the risk of exposure continues, once in a lifetime [188-190].
Solid organ transplant recipients should receive pneumococcal polysaccharide vaccine 23 valent, and conjugate vaccine 10 or 13 valent, but it is interesting that the recommendations depend on the vaccines previously received and the order of the vaccinations. For those who have not previously received either of the two, we recommend first the conjugate followed by the 23 valent, at least 8 weeks apart [191]. For those who have previously received one or more doses of 23 valent vaccine, a single dose of separate conjugate vaccine a minimum of one year after the 23 valent vaccine is recommended. Finally, for those who have received previous conjugate vaccine and require other doses of 23 valent vaccine, a delay of at least 8 weeks from the administration of the conjugate, and not less than 5 years from the last dose of 23 valent, is desirable.
The relatively low incidence of H. influenzae type b pneumonia in adult transplant recipients and the poor immunogenic response that occurs with the vaccine do not make this one an essential vaccine for this population group [192, 193]. The same occurs with the meningococcal vaccine in this population. Among adults, there is a low incidence of meningococcal infection in SOT patients and the response to it is also poorly known [194]. The vaccine is therefore reserved for those with particular risk factors for contracting the disease. When indicated, it seems reasonable to opt for a conjugate vaccine [179].
All SOT that are Anti-HBs negative should be vaccinated against HBV. The population with chronic HBV liver disease in the post-transplant period has a high rate of post-transplant complications and a high rate of related mortality [195-197]. Therefore, if after the usual three doses a rate of antibodies > 10mIU/ml is not reached in this population, a second cycle should be repeated. The response to HBV vaccine is quite variable when done in post-transplantation and also in cirrhotic patients, vaccinated at any time, which makes it necessary to periodically re-check the level of protective antibodies [198-203].
With regard to Hepatitis A (HAV), vaccination is mandatory for all unvaccinated transplant recipients, whether children or adults, since there is an increased risk of fulminant liver failure when contracting hepatitis A in a SOT recipient. The antibody response is also more limited in time than in the normal population and vaccination should therefore be attempted prior to transplantation, whenever possible [204-207].
With regard to the HPV vaccine, it is a known fact that infection with this virus is associated with a risk of up to 100 times greater incidence of cervical neoplasms in transplanted women and anogenital cancers in men. Therefore, those who have vaccination criteria, regardless of whether or not they have been transplanted, should receive the HPV vaccine. If they have already been transplanted, it is advisable to wait 3-6 months after the transplant. The immunogenicity of the HPV vaccine in this population is not well known but the risks-benefits incline to the recommendation. In the future, indications may be extended to groups of transplanted adults who are not in the age ranges in which the vaccine is now indicated [179, 208-210].
RZV was immunogenic in patients with solid tumors receiving immunosuppressive chemotherapies. Humoral and cell-mediated immune responses persisted 1 year after vaccination and no safety concerns were identified [211].
At the time of writing this document, there is no recommendation for vaccination against Zoster with the recombinant vaccine in the population with SOT, but at least two clinical trials are underway in this population group that will shortly clarify its indications that seem favourable [212]. In patients with hematopoietic transplantation, the result of a clinical trial has just been published that proves its efficacy and good tolerance [213].
To conclude, we should remember that the responsibility for implementing the vaccine schedule in transplant patients is often diluted between the transplant team of the patient, the family and community medicine teams that also follow the cases and transplant patient organizations. International data show that there are clear opportunities to improve the implementation of the vaccine schedule in this population [214, 215]. Our opinion is that this dilution of responsibilities is also a frequent reason for omissions or forgetfulness in the vaccination calendar of patients with SOT in our environment.
Conclusion:
Adult transplanted patients constitute a very particular group in relation to the prevention of diseases through vaccines, for several reasons. Firstly, because they cannot receive vaccines produced with attenuated micro-organisms in the post-transplant period. Secondly, because the immune response is not the same as in the non-immunosuppressed population. Finally, some vaccines should be administered in this population with higher doses and at different rates. Despite the high level of the transplant system in Spain, there is an opportunity to improve coordination when implementing a rigorous vaccine schedule in solid organ transplant recipients.
QUESTION 10.- What is the role and work of professional and scientific associations / societies specifically dedicated to vaccines?
Exposure:
The Scientific Societies must provide evidence to the Health Authorities, so that they issue recommendations on vaccination in adults belonging to risk groups, and that these are as homogeneous as possible, and duly supported by scientific evidence. They must collaborate with the health-care authorities to ensure that the health professional is the first defender of vaccines and to provide agile mechanisms for consultation with those professionals who are better prepared in the field of prevention. In Spain there are several scientific societies that have specific sections or working groups dedicated to vaccines groups. The Spanish Vaccinology Association (AEV) works specifically and monographically on the topic.
It is a non-profit Medical-Scientific Association, constituted under Law 191/1964, of 24 December. Its general objective is to protect health by means of primary and, where appropriate, secondary prevention actions against immunopreventable infectious diseases, with biological preparations for immunisation practices, thereby contributing to better life expectancy and quality of life for citizens, with special reference to the child population and risk groups by age, immunocompromised people, people with occupational risks, international travel and basic diseases, increasing the quality of life of the population.
The aims of the Association include:
a) To disseminate scientific advances in the area of “Artificial, active and passive acquired immunity” and to promote the development of knowledge of immunobiological vaccines and preparations for infectious diseases.
b) To permanently review medical, clinical, epidemiological, immunobiological research and cost-benefit analysis criteria in order to make judgments that may be useful for a rational use and in accordance with the socio-sanitary development in the aforementioned preparations, in the practice of Health Sciences professionals, both private practice and at the service of the Administrations.
c) To expand the Vaccination Programs recommended by the Health-Care Authorities to support the coverages, as well as to foment the evaluation of the same ones and to stimulate the Pharmacovigilance in the use of the preparations.
d) To organize, sponsor and promote conferences, courses, congresses and meetings in order to disseminate and update the knowledge that is being incorporated into Vaccinology, with expression of technological development in this field of Health Sciences.
e) To promote research in Vaccinology, cooperating where necessary, in the Projects at the Design and Planning level, stimulating the streamlining at the level of the Clinical Research Ethics Committee of the Welfare Network.
f) To raise awareness on the importance of the correct use of immunization practices to social agents (politicians, media, general population) bearing in mind the competences of the different Public Administrations including Foreign Health.
g) To establish relations with those National, International and International Scientific Societies with thematic affinity, as well as with the Health Administrations with competences in this professional or Regulatory praxis, creating meeting spaces between professionals of different levels and disciplines, for what is related to this scientific field.
h) To cooperate in those Programmes of Health Dissemination and Information and Education for Health (EPS) in which topics on vaccines and other immunobiological preparations of social, scientific or journalistic interest can be submitted for debate.
i) To carry out Publications (printed, digital, Web), to summon scholarships or aids for national and foreign research studies, to organize Prizes, Courses or Seminars, or any other action conducive to metializing the previous points.
The activity of the Spanish Vaccinology Association is not restricted exclusively to promoting the scientific technical knowledge of its members but is open to any other possible beneficiary who meets the conditions and characters required by the nature of its own purposes.
The activity of the Association may also consist of the collection and management of funds and patronage for the granting of Scholarships or Grants for studies and research, the organisation of Awards, Courses and Seminars, grants for all kinds of Institutions and other activities that the Governing Body considers appropriate for the strict fulfilment of its aims.
Conclusion:
There are several Scientific Societies that have specific sections or working groups dedicated to vaccines in Spain. The Spanish Vaccinology Association (AEV) works specifically in the field of vaccines and its objectives are aimed at promoting knowledge, research and the appropriate use of vaccines as a means of immunoprevention.
QUESTION 11.- What is the role of nursing in promoting health with vaccines in adults?
Exposure:
Health promotion, as part of the comprehensive care process, is the essence of nursing. This process includes, in addition to promotion, assistance (primary and specialized), prevention (primary, secondary and tertiary) and social adaptation (rehabilitation and integration). At any of these levels, health education is a key instrument [216, 217]. The concept of nursing care has also evolved from a disease-oriented care system to a preventive and health promotion system [218]. According to the World Health Organization at the 9th World Health Promotion Conference, “getting vaccinated” is one of the 12 tips for good health. It therefore integrates vaccines into healthy lifestyles [219, 220].
The role of nursing, specifically in the field of promotion and administration of vaccines, is very broad and varies from recruitment of vaccination subsidiaries to conviction campaigns, continuing education and follow-up. As in other groups, the knowledge and skills of nurses, both graduated and school nurses, in the problem of vaccination, still offer opportunities for improvement in both groups today and in highly developed countries such as Finland [221, 222].
In a study conducted in Israel, the role of the flu vaccine nurse in gaining acceptance of the flu vaccine among those recommended for vaccination is demonstrated [223]. The reminder role of certain programmes on the at-risk population, carried out by doctors and nurses, has proved effective [224]. In the case of the pneumococcal vaccine, a study carried out in Hong Kong, showed that a brief process of health education, lasting only 3 minutes, carried out by nurses, increased acceptance and coverage with pneumococcal vaccine from 48 to 57% [225].
Nursing can also play a relevant role in detecting cases eligible for pneumococcal vaccination during hospital admission for any reason, and so a CDC-sponsored study increased the vaccination rate from 19% to 74% after implementing a screening program and vaccination offer [226].
Another example of the potential nursing work in the acceptance by school girls and their families, through a simple reminder call of the convenience of getting vaccinated against HPV [227]. This work may be particularly necessary when it is carried out in particularly defenceless groups or in social exclusion. This is the case of vaccination against hepatitis B, where the role of nursing has also been shown to be fundamental, particularly by ensuring, through a follow-up programme, that patients complete their third dose of vaccine [228].
The literature collects a miscellany of situations in which the role of nursing is key in the global vaccination process, both in poor and developed countries and with vaccines of a different nature, including polio [229-231].
It seems, therefore, that this would be a very appropriate area, due to the characteristics we have mentioned, for the creation of consultations, or vaccine promotion groups, particularly coordinated by nurses, although we have not been able to find concrete examples in the professional literature studying (with the appropriate methodology) the clinical, economic and social impact of their introduction.
Conclusion:
Vaccination, and particularly adult vaccination, is one of the paradigms of the work and competence of nursing. Many studies demonstrate the effectiveness of nursing intervention in different groups, with different vaccines and with different impacts. Nursing has to promote and manage all adult vaccines and their complete vaccination schedule, and in our opinion, this work is a very clear area for nursing consultations or working groups managed by nurses.
QUESTION 12.- What is the role of Pharmacy Services in the vaccination of adults?
Exposure:
The Community Pharmacist plays, or must play, an essential role in adult vaccination. Not only in aspects such as the correct conservation and storage of vaccines, but also in all aspects related to tolerance and safety of vaccination. There is the possibility of making a clear contribution to health education from the Community Pharmacy. This must be done not only in relation to the vaccines of regular use, but also in those that are needed occasionally as it is the case of some vaccines for travellers. If this is not the case, it is due, in our opinion, to the lack of necessary training and the necessary coordination with other structures. The attitude of the Community Pharmacist as a health-care agent is changing significantly for the benefit of the patient, not only in Spain, but also in numerous countries of the European Union and beyond.
The wide network of pharmacies distributed uniformly throughout Spain can undoubtedly help to increase the vaccination coverage of the adult population along with other health-care centres. We must not forget that an average Spaniard goes to the pharmacy 7 times more frequently than to any other health centre or medical consult.
At the Community Pharmacy, it is possible to identify and guide risk patients who may benefit from vaccination, strengthen the recruitment of people included in these risk groups by collaborating with other health professionals and involve the pharmacy in health education, transmitting truthful and clear information on the importance of vaccination to prevent different diseases. In addition, it is easy to carry out pharmacovigilance work from the Community Pharmacy. Another important objective is to fight against the “anti-vaccine” philosophy from which some elements of the supposedly better educated classes are not free.
The other area that we must discuss is the role of the Hospital Pharmacy Department in the immunization policy of the population. The hospital pharmacy is currently one of the points with the highest volume of data on patients in the hospital, not only from their own information but for being the coordinating node of many other databases to create campaigns and strategies based on combination and confrontation of the data. Alert campaigns to doctors and nurses responsible for certain patients with risk factors of certain diseases can very well be done with a warning from the pharmacy services. Taking advantage of admission to the hospital to facilitate such vaccination is a perfectly feasible contribution. In the United States of America, the “pharmacy-delivered immunization services” or groups that promote vaccination based on the pharmacy department are well known. One recent study estimates that they administer an additional 6.2 million doses of flu vaccine and 3.5 million additional pneumococcal vaccines each year [232]. Something similar happens with Community Pharmacies. A recent evaluation estimates that almost 80% of them in the U.S. offer and promote the use of at least one vaccine that can be administered at the pharmacy itself [233- 235].
There are data on the effectiveness of such vaccination programs implemented from pharmacy, community or hospital services, which have demonstrated effectiveness in influenza, pneumococcal infection and HPV fundamentally [236-243]. A good example in the case of influenza and invasive pneumococcal infection is a working group created by a pharmacy technician and a nurse that increased influenza vaccination from 72 to 93% of the candidates in one institution [244].
Similar experiences have been carried out in countries other than the United States [245-252], but we have not been able to find information on the activity and impact of promoting vaccination in Spanish pharmacy services and offices.
Conclusion:
Both hospital pharmacy departments and Community Pharmacy offices can do a great deal in infection prevention and health education from their respective departments, working to promote the proper use of vaccines in adults. In the United States, a high percentage of these services have such programs, and the data in the literature show a clear impact on vaccination rates and educational capacity. We have not been able to find data on the quantitative and qualitative importance of this activity in pharmacy departments and pharmacy offices in Spain.
QUESTION 13.- What is the economic value of vaccines, as seen by a health economist?
Exposure:
The Choiseul Institute has recently produced two publications proposing “A Vaccine Strategy for Spain” [253], and assessing “The economic impact of vaccines” [254], of which we summarise some aspects in the following lines.
Health expenditure in Spain has been reduced to below 6% of Gross Domestic Product (GDP) in 2018 according to the General State Budget (not yet approved). This means that Spain is not among the leading European countries in “per capita” health-care spending but is in a second place that it shares with Italy. In a way, this situation is aggravated by the dispersion that exists in the different Autonomous Communities (AA.CC.). The fact that health policies have been transferred to AA.CC. adds to the inequality between the different regions of Spain.
On the other hand, it is interesting to note one of the problems that are not normally analysed, such as the effect on the economy of absenteeism due to illness. Although there is no very recent data, that provided by Eurostat in 2012 of the 13,000 million euros representing temporary incapacity says enough. An influenza episode, for example, results in a worker, on average, to take sick leave for five days. The cost of this incapacity represents more than 20% of health expenditure, part of which could be covered by prevention policies. This is where vaccines come in; an area which, like many others in health-care policy, has suffered significant cuts. Suffice it to say that spending on vaccines is around 1.8% of total pharmaceutical spending in Spain. This quantity is clearly insufficient if the benefits of vaccination are considered.
The vaccination policy in young people is well known. In this chapter, Spain is among the most advanced countries in the world with coverage rates above 95%. However, vaccination must be considered as a policy during all ages of life. And here, the situation is frankly improvable. It is enough to look at vaccination data for those over 18 years of age, or older. Coverage in adolescents is estimated at 79% and in adulthood at 57% [254]. Not to mention the laxity that health professionals themselves have when it comes to getting vaccinated, an aspect that requires special attention.
The results of phase I of the DOVE study published in 2011 in the journal of Health Affairs conclude that with a package of only 6 vaccines, the death of 6.4 million children could be avoided over the following 10 years, to which should be added the disappearance of 426 million episodes of disease. Economically, the potential savings for the 72 poorest countries, if such a vaccination program were to be implemented, would mean saving 151,000 million dollars, the result of less spending on diseases and greater productivity [255].
It has been proven that vaccination, a healthy life, and one would also say, life expectancy itself, are related aspects that translate into well-being for individuals while providing very positive effects on the economy of any country. People with deteriorated health produce less, consume less, increase public spending, affect the public deficit and have effects on foreign trade, because buying less weakens exports, and producing less affects, in some way, on exports; in short, harm the production of goods and services of the country and, therefore, deteriorate global wealth. The importance of vaccination in a country’s wealth is therefore evident. A European calculation estimates that for every euro invested in health-care, a return of 4 euros is obtained and that 5 years of increase in life expectancy has an impact on the GDP of an annual 0.5% increase in developed countries. In Spain, it is estimated that for every euro invested in vaccines, €22 is saved in direct and indirect costs.
Vaccinating a person throughout his or her life is calculated at a variable cost of between €443 and €3,953 per person at a cost of €44 to €226 per “protected” pathogen [255].
There are also other no less important considerations, such as new vaccines that address new health problems. At this point, it is important to highlight the fact that the pharmaceutical industry is one of the most R+D-intensive, this being necessary for the production of new solutions to specific health-care problems. One of them has to do, for example, with changes in the patterns of sexual relations, due to relationships occurring at increasingly younger ages and without the proper precautions, to which has been added the new problem that comes from the famous “morning-after pill”, which is proven to be used without any medical control and which, in the long run, according to many experts, could have incidence in breast cancers. However, going back to vaccines, it is important to point out the HPV vaccine, which is now on the vaccination calendar for young women, but not for men. Given the promiscuity in young people, the difference in criteria from one European country to another, the “Erasmus effect”, and other considerations, this is a problem that, without being apparently serious, also affects boys in a multitude of health problems that affect the physical and, above all, the psychological area. This is an aspect of vaccination policy that calls for review. This aspect, moreover, without being deadly, has enormous economic effects because it affects work performance and other aspects that are undoubtedly very relevant. In all this, public authorities must be sensitive to the times.
Conclusion:
The money spent on vaccines should not be seen as an expense but as an investment. In strictly economic terms, it is estimated that every euro spent on vaccines has a return of 4 euros to a country’s economy. Each pathogen against which a vaccine protects cost an estimated €44 to €226. The implementation of a programme of only 6 vaccines in the 72 poorest countries of the world would have the effect of preventing the death of 6.4 million children and 426 million episodes of illness in the next 10 years and would mean a saving of 151,000 million dollars, as a result of less spending on diseases and greater productivity.
QUESTION 14.- What is the role of the press in the promotion of Health through vaccines?
Exposure:
The role of the press in the vaccination of adults and children should focus on conveying to the population the importance of this fact and its repercussions as a major public health issue. The press should demand from the health authorities, beyond information, a commitment to adopt measures to guarantee access for adults, on equal terms, to all the necessary vaccines. Furthermore, it should call on the responsibility of scientific societies to promote the implementation of a homogeneous adult vaccination schedule for the whole country.
In addition to these general principles, it is also pertinent to comment on the news that leads to the dissemination of vaccine hoaxes, particularly via the Internet. In a study conducted between July 2014 and September 2017, researchers followed thousands of tweets, proving that many of them had origins similar to those that tried to influence the electoral process in 2016 in the United States of America. In general, they try to project the image of a public opinion much more divided than it actually is about the safety or insecurity of available vaccines. The study shows that the vast majority of American society believes that vaccines are safe and effective.
The reluctance of some parents to allow their children to be vaccinated is another issue in which the press can play a very important role, just providing proper information. In general, parents who do so tend to make three kinds of arguments: some believe that their children are at little risk of diseases such as polio, measles or tetanus because others are vaccinated already. Others believe that many of the diseases that vaccines prevent are not really too serious, such as chickenpox or measles itself. Finally, there is a group of parents whose primary concern is the incidence of adverse effects such as autism. The press, offering truthful and rigorous information and fleeing sensationalism, can do an extraordinary job in this sense as well [256-266].
In conclusion, we would like to convey our opinion to point out that the press is an excellent vehicle for transmitting public demands to the political class, which must legislate on the financing of vaccines so that their application is feasible for all those who need them.
Conclusion:
The role of the press in the subject of vaccination is potentially multiple. It must contribute to disseminating truthful and rigorous information to the population, promoting the acceptance of essential Public Health measures. The media must contribute to the elimination of hoaxes and misinformation and create social pressure in favour of making legislative decisions that, as in the case of vaccines, have a great impact on individual and collective health.
QUESTION 15.- What ethical aspects deserve to be particularly highlighted in the policy of using vaccines for the prevention of infections in adults?
Exposure:
Vaccination has raised ethical issues from the very beginning. But this does not mean that these problems have always been the same. Quite the opposite is true, that each era has posed its own problems.
When Jenner fine-tuned the antivariolic vaccination procedure and wanted to spread it and generalize it to the whole population, the problem arose as to whether it was correct to inoculate, in healthy people, a very serious disease and from which more than 30% of those affected died. This was the great debate in the final years of the 18th century and the first decades of the 19th century.
In the middle of that century another problem arose. Faced with the cholera epidemics that filled the entire century, Jaime Ferrán fine-tuned his controversial vaccine. Here the debate was mainly scientific, and the question was whether this vaccine was effective and safe enough to be extended to the whole population.
Today there is no question about the effectiveness and safety of vaccines. But it happens that, precisely because they are effective, they have side effects, which in some cases can be serious. And the problem that arises is whether the State can make a vaccine obligatory, which, although there is no doubt that it has a clear collective benefit, can nevertheless be harmful at an individual level.
For obvious reasons, in the following lines we are going to focus on the analysis of this last problem, that of the obligatory nature of vaccines.
Until very modern times it was never doubted that the State had the power to oblige people to provide services, not only financially or in terms of goods, but also personally or in terms of their own lives, in certain cases in which the good of the community was at serious risk. Such has always been the case of the obligatory nature of military service and of actively intervening in actions of war, even at the risk of one’s own life.
In fact, ethics has never questioned the legality of this type of service. The problems have started very recently. In the case of armies, one of the reasons has been the progressive complication and technification of military tasks, which can no longer be covered by non-specialized personnel, which has forced the professionalization not only of military commanders but of the militia in general.
But in addition to these reasons that we can call technical or professional, there are others that depend on culture. We live in a liberal culture, where perhaps the most appreciated value is freedom. It is, of course, about individual freedom, in such a way that all the orders coming from any instance outside the person are questioned by principle.
On the other hand, Western culture generally puts freedom at the service of maximum utility or maximum personal interest. This means that the idea of the collective interest has lost much of its previous strength, and that today it clearly conflicts with the individual interest inasmuch as the search for that collective interest may result in some damage to the individual himself.
Thus, the great moral conflict of compulsory vaccination is the conflict between the collective or common good and the individual good. As long as the protection of the former can put the latter at risk, there are criticisms that individual rights are being violated which are inviolable.
The two values at stake or, in these cases, in conflict, are collective benefit vs. individual benefit. In all classical ethics it was assumed that the first of these values had priority over the second. Aristotle says it at the beginning of his Politics, and it became an undisputed principle, which was self-evident. It was a clear “deontological principle”, which was soon justified by criteria of natural law and even divine law.
But as of the 18th century, a new approach was developed, not deontological but “teleological”; that of utilitarianism. The only moral obligation is to maximize utilities, and therefore the question is whether the utilities of the individual benefit are superior to those of the public benefit or not. Act Utilitarianism calculates utility “act by act”. This means that the usefulness of an individual’s vaccination must be calculated both for the community and for the individual himself. If the vaccine entails serious risks, even if its probability is very low, it is normal that the usefulness of vaccinating a single individual is socially or collectively very small, and that the usefulness of avoiding the individual risk of secondary effects may be greater. Knowingly or not, this is how most objectors to social vaccination reason.
There is another utilitarianism called Rule Utilitarianism. It seeks to optimize the usefulness not of each act, but of each norm or rule. In the case of compulsory vaccination, the rule says that collective vaccination has a great social benefit, even though it may entail some individual risks, which, in some very extraordinary cases, may become serious. But Rule Utilitarianism does not easily eliminate Act Utilitarianism. The result is that the rational utilitarianist will look for others to act according to Rule Utilitarianism whilst he decides according to Act Utilitarianism. This is the case of the “freeloader”, who takes advantage of the altruism of others to increase the usefulness of his own selfishness. For example, if everyone is vaccinated, my child will not be infected with the disease, and therefore I do not need to vaccinate him, thus avoiding discomfort and possible side effects. It is the same as when it is forbidden to fill swimming pools with water during a very dry summer, but a someone decides to fill his because this consumption is almost imperceptible, as long as the others respect the rule. I take advantage of the respect of the rule of others, at the same time that I decide not to respect it.
What has been said about compulsory vaccination changes when vaccination is free, in such a way that it is advised but not obligatory. In those cases, which are the most frequent, the conflicting values are, in general, the balance between benefit and risk, that is to say, between the prevention of a potential serious disease on the one hand and the risk of secondary effects of the vaccine on the other. These are obviously two distinct manifestations of non-maleficence: preventing something that is in our hands and that is maleficent on the one hand and, on the other hand, avoiding or not carrying out something that can be, though certainly with a low probability.
This conflict is more apparent than real. This is due to the fact that clinical research into vaccines allows us to have scientific evidence that the benefit of the disease it prevents, or the harm it avoids, is far greater than the harm from the side effects of the vaccine itself, and this in the individual himself. Therefore, now we are not talking about collective benefit versus individual benefit, but we are comparing the individual benefit of getting vaccinated and avoiding a serious illness versus the benefit of not getting vaccinated and avoiding the side effects of the vaccine, whilst accepting the risk that the person may suffer a serious illness. This is therefore a utilitarian calculation, and not a “Rule” but an “Act”. And it is clear that in vaccines, utilitarian calculus is always on the side of vaccinating, because its benefits are much greater than the risks assumed.
So far, we have appealed to the language of values and conflicts of values. We have approached analysis as a choice between different values. This is what is usually done in ethics. However, it is not the ideal procedure. The optimal solution is never in the extreme courses but in the intermediate ones.
It is sometimes said that between vaccinating and not vaccinating, there are no intermediate courses. But that is not the case. This is a typical bias in the decision-making process that we human beings commit with unusual frequency.
The optimal intermediate course is citizen education and the promotion of responsibility. Most of the objections to vaccination are due to false prejudices, which an adequate deliberation allows, in most cases, to overcome.
One of the most common prejudices is that of “naturalism”, i.e. natural is good and artificial is bad. This, however paradoxical it may seem, is in the collective unconscious of our culture. Ethics was born as a scientific discipline in Greece from this principle, which later reinforced Stoic philosophy and Abrahamic root religions. This is the basis of Western naturalism, which although it has been very positive in many aspects of our culture, also has highly problematic and negative consequences, such as the conversion of the order of nature into a criterion of morality. At present, this moral prejudice is at the basis of many of the “ecological” movements.
But as pernicious to ethics as deontological naturalism has been teleological utilitarianism, of which we have already spoken, the moral obligations of human beings are not aimed solely at optimizing individual benefits. We are social beings, we benefit from social life which, obviously, has the right to demand certain benefits by reciprocity. These must be as harmless as possible to individual goods, and that is why vaccines should be made compulsory only in exceptional cases. But we must all assume our duty to contribute to the common or collective good, even assuming, in exceptional cases, vital risks. Whoever does not act in this way must be seen for what he is, a “non-solidarity” subject, a “profiteer” or, simply, a “freeloader”. In 1714, a British physician and philosopher, Bernard Mandeville, published a famous book with the following title: The Fable of Bees, or How Private Vices Make Prosperity Public. It is a classic topic in liberal culture thereafter. Some time later, in 1759, Adam Smith brought to light his great book of ethics, the Theory of Moral Sentiments. In the part entitled “From the Effect of Utility on the Sentiment of Approval,” he wrote: “The rich consume barely more than the poor, and despite their natural selfishness and greed, even though they seek only their own convenience, even though the only aim they set themselves is the satisfaction of their own vain and insatiable desires, they divide with the poor the fruit of all their properties. An invisible hand leads them to realize almost the same distribution of things necessary for life that would have taken place if the earth had been divided into equal portions among all its inhabitants, and so without pretending it, without knowing it, they promote the interest of society”. Hence the importance that this invisible hand ended up having in the other great work written by Adam Smith, this one on political economy, An investigation on the nature and cause of the wealth of nations, published in 1776. Does such an invisible hand exist? Does it fix everything? Does it make private vices contribute to public prosperity? Sometimes yes, but not in all cases. Both situations occur in the world of vaccines. In some of them, the pure individual interest in tackling the ills of the disease protects even those not directly vaccinated and thus contributes to the collective benefit. This is the case of the Sabin vaccine against the polio virus. By seeking individual protection, the immunization of other individuals is indirectly achieved (“herd effect”). These are win-win situations, so studied in game theory. It is unreasonable to collaborate on a collective good if one does not take personal advantage of it. The problem is that this is not always achieved, because there are times when it is necessary to lose something individually in order to maximize the collective benefit. The analysis of these situations has been worrying theoreticians for a long time, from Pareto to Nash. Mancur Olson’s study of this type of social behaviour in his book The Logic of Collective Action: Public Goods and the Theory of Groups (1965) is a classic. The study of the case of the “sponger”, “stowaway”, “parasite” or free-rider, the one who tries to go unnoticed to take advantage of the collective benefit, without contributing to it in its aliquot part, also comes from the theory of games: “If everyone gets vaccinated, then I don’t have to, because the germ won’t be able to live and spread.”
The problem increases by degree when the individual loss does not consist so much in the discomfort of the very fact of vaccination, but as the possibility of some more serious and persistent effect on the life of the person being vaccinated. There is also a paradigmatic example of this: the human smallpox vaccine, which managed to eradicate this very serious disease from the face of the earth. In addition to the discomfort inherent in its application, there were extremely rare encephalitis, but with permanent and often serious sequelae. It should be remembered that antivariolic vaccination was compulsory until the eradication of the disease in 1980. The argument for requiring vaccination, even knowing that a small group of people would be harmed by it, was one of “public health”. The risk had to be taken that individual health might be affected for public health reasons. It was a social benefit, a contribution to the collective good like others, including the obligation of military service or the defence of the country in the event of war.
Today, in Spain, there is no vaccine that is compulsory. There is a very positive reason for doing so. The use of persuasive methods is always preferable to coercive measures, especially in our liberal societies, where it is getting worse and worse that someone has to suffer harm for reasons of public benefit. We all understand well and are willing to collaborate in win-win situations, but we resist by all means at our disposal those in which the collective good requires a sacrifice, sometimes serious, very serious, of certain individuals. These are the lose-win situations. Someone has to lose, and we try by all means not to be us, although we are delighted to receive the positive social consequences generated by the sacrifice of others. The paradigmatic case is that of wars. With which we become, velis nolis, freeloaders.
The ethical consequences of this type of behaviour are evident. We receive many benefits from the community, and that is why we are also obliged to contribute to the collective good with different types of sacrifices. It is necessary to do everything humanly possible so that these are the least possible, not only in number but also in gravity. But it is no longer so clear that the solution, when it is necessary to distribute risks, consists in trusting everything to the voluntary will of those who, for whatever reasons, are willing to assume them freely and voluntarily. Because this, in a collateral way, encourages the proliferation of freeloaders. Public burdens must be distributed equitably, otherwise they cannot be considered fair. When persuasive measures do not fully cover health objectives, as in the case of several vaccines, the only right thing to do is to make them obligatory. The rest is a serious dereliction of duty. This seems to have been understood by other countries, such as France, where there are currently eleven obligatory vaccines in children, or Italy, where the presentation of the health booklet attesting to the application of the twelve types of vaccines required by law is required upon entry into kindergarten or school. The argument put forward by the Spanish scientific societies is that, with the current procedure, very high percentages of vaccination are achieved, even higher than that achieved in some other country through compulsory vaccination. This is a markedly consequentialist criterion. And while the consequences are an important factor in moral reasoning, they are not the only one. There are also the principles; in this case, that of justice, the equitable distribution of burdens. We must not endorse voluntarism and altruism, which is basically a pure duty of justice. The invisible hand, as Adam Smith rightly said, gets “almost” the same distribution as justice does. But only “almost”.
Conclusion:
We receive many benefits from the community, and that is why we are also obliged to contribute to the collective good with different types of benefits. It is necessary to do everything humanly possible so that these are the least possible, not only in number but also in severity. But when it is necessary to distribute risks, it is not possible to trust everything to the voluntary will of those who, for whatever reasons, are willing to assume them freely and voluntarily. Because this, in a collateral way, encourages the proliferation of freeloaders. Public burdens must be distributed equitably, otherwise they cannot be considered fair. When persuasive measures do not fully cover health objectives, as in the case of several vaccines, the only right thing to do is to make them obligatory. The rest is a serious dereliction of duty.
TRANSPARENCY DECLARATIONS/POTENTIAL CONFLICTS OF INTEREST
The authors declare no conflicts of interest. This publication was financed by GSK and the authors have not received any fee for their collaboration.
References
- 1.World Health Organization Global Vaccine Plan 2010-2020. Geneva, 2012. 2017
- 2.World Health Organization 16th Meeting of the SAGE Polio Working Group. http://www.who.int/immunization//sage/meetings/2017/april/1_Final_report_Clarke_april3.pdf
- 3.Kim DK, Riley LE, Harriman KH, Hunter P, Bridges CB. Recommended Immunization Schedule for Adults Aged 19 Years or Older, United States, 2017. Ann Intern Med. 2017;166(3):209-19. DOI: 10.7326/m16-2936 [DOI] [PubMed] [Google Scholar]
- 4.Uhart M, Bricout H, Clay E, Largeron N. Public health and eco-nomic impact of seasonal influenza vaccination with quadriva-lent influenza vaccines compared to trivalent influenza vaccines in Europe. Hum Vaccin Immunother. 2016;12(9):2259-68. DOI: 10.1080/21645515.2016.1180490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le P, Rothberg MB. Cost-Effectiveness of Herpes Zoster Vaccine for Persons Aged 50 Years. Ann Intern Med. 2015;163(7):489-97. DOI: 10.7326/m15-0093 [DOI] [PubMed] [Google Scholar]
- 6.Parkin DM. The global health burden of infection-associated can-cers in the year 2002. Int J Cancer. 2006;118(12):3030-44. DOI: 10.1002/ijc.21731 [DOI] [PubMed] [Google Scholar]
- 7.No authors listed. Human papillomavirus and cervical cancer. Lan-cet. 1988;1(8588):756-8. [PubMed] [Google Scholar]
- 8.Schweitze A, Horn J, Mikolajczyk R, Krause G, Ott J. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a sys-tematic review of data published between 1965 and 2013. Lancet. 2015;386:1546-55. [DOI] [PubMed] [Google Scholar]
- 9.Blachier M, Leleu H, Peck-Radosavljevic M, Valla D, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epide-miological data. J Hepatol 2013;58:593-608. [DOI] [PubMed] [Google Scholar]
- 10.Thorrington D, Andrews N, Stowe J, Miller E, van Hoek AJ. Elucidat-ing the impact of the pneumococcal conjugate vaccine programme on pneumonia, sepsis and otitis media hospital admissions in Eng-land using a composite control. BMC Med. 2018;16(1):13. DOI: 10.1186/s12916-018-1004-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsaban G, Ben-Shimol S. Indirect (herd) protection, following pneu-mococcal conjugated vaccines introduction: A systematic review of the literature. Vaccine. 2017;35(22):2882-91. DOI: 10.1016/j.vac-cine.2017.04.032 [DOI] [PubMed] [Google Scholar]
- 12.Rudnick W, Liu Z, Shigayeva A, Low DE, Green K, Plevneshi A, et al. . Pneumococcal vaccination programs and the burden of invasive pneumococcal disease in Ontario, Canada, 1995-2011. Vaccine. 2013;31(49):5863-71. DOI: 10.1016/j.vaccine.2013.09.049 [DOI] [PubMed] [Google Scholar]
- 13.Reisinger KS, Baxter R, Block SL, Shah J, Bedell L, Dull PM. Quad-rivalent meningococcal vaccination of adults: phase III compari-son of an investigational conjugate vaccine, MenACWY-CRM, with the licensed vaccine, Menactra. Clin Vaccine Immunol. 2009;16(12):1810-5. DOI: 10.1128/cvi.00207-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jafri RZ, Ali A, Messonnier NE, Tevi-Benissan C, Durrheim D, Esko-la J, et al. . Global epidemiology of meningococcal disease. Popul Health Metr. 2013;11(1):17. doi: 10.1186/1478-7954-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams AI, Barron AO. Pertussis in Sydney: an indicator of the prob-lem facing health educators. Med J Aust. 1966;1(2):41-5. [PubMed] [Google Scholar]
- 16.Adams WG, Deaver KA, Cochi SL, Plikaytis BD, Zell ER, Broome CV, et al. . Decline of childhood Haemophilus influenzae type b (Hib) dis-ease in the Hib vaccine era. Jama. 1993;269(2):221-6. [PubMed] [Google Scholar]
- 17.Putri WCWS, Muscatello DJ, Stockwell MS, Newall AT. Econom-ic burden of seasonal influenza in the United States. Vaccine. 2018;36(27):3960-3966. doi: 10.1016/j.vaccine.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 18.European CDC. Factsheet about seasonal influenza.. https://ecdec.europa.eu/en/seasonal-influenza8facts/factsheet. 2017.
- 19.Calderon C, Dennis R. Economic cost of community-acquired pneumonia, meningitis and bacteremia in an adult population that required hospitalization in Bogota, Colombia. Biomedica. 2014;34(1):92-101. DOI: 10.1590/s0120-41572014000100012 [DOI] [PubMed] [Google Scholar]
- 20.Tong S, Amand C, Kieffer A, Moe H. Trends in healthcare utilization and costs associated with pneumonia in the United States during 2008–2014. BMC Health Serv Res. 2018;18(1):715. doi: 10.1186/s12913-018-3529-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright C, Wordsworth R, Glennie L. Counting the cost of meningo-coccal disease : scenarios of severe meningitis and septicemia. Pae-diatr Drugs. 2013;15(1):49-58. DOI: 10.1007/s40272-012-0006-0 [DOI] [PubMed] [Google Scholar]
- 22.Centro Nacional de Epidemiología CIBER Epidemiología y Salud Pública (CIBERESP). Instituto de Salud Carlos III. Resultados de la Vigilancia Epidemiológica de las enfermedades transmisibles. Informe anual 2015. Madrid, 2017.
- 23.Williams WW, Hickson MA, Kane MA, Kendal AP, Spika JS, Hinman AR. Immunization policies and vaccine coverage among adults. The risk for missed opportunities. Ann Intern Med. 1988;108(4):616-25. [DOI] [PubMed] [Google Scholar]
- 24.Williams WW, Lu PJ, O’Halloran A, Kim DK, Grohskopf LA, Pilishvili T, et al. . Surveillance of Vaccination Coverage among Adult Popula-tions-United States, 2015. MMWR Surveill Summ. 2017;66(11):1-28. DOI: 10.15585/mmwr.ss6611a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ventola CL. Immunization in the United States: Recommendations, Barriers, and Measures to Improve Compliance: Part 2: Adult Vacci-nations. P t. 2016;41(8):492-506. [PMC free article] [PubMed] [Google Scholar]
- 26.Esposito S, Durando P, Bosis S, Ansaldi F, Tagliabue C, Icardi G. Vac-cine-preventable diseases: from paediatric to adult targets. Eur J Intern Med. 2014;25(3):203-12. DOI: 10.1016/j.ejim.2013.12.004 [DOI] [PubMed] [Google Scholar]
- 27.Poland GA, Schaffner W, Hopkins RH Jr, Health USDo, Human S. Immunization guidelines in the United States: new vaccines and new recommendations for children, adolescents, and adults. Vac-cine. 2013;31(42):4689-93. DOI: 10.1016/j.vaccine.2013.03.031 [DOI] [PubMed] [Google Scholar]
- 28.Anderson EL. Recommended solutions to the barriers to immuniza-tion in children and adults. Mo Med. 2014;111(4):344-8. [PMC free article] [PubMed] [Google Scholar]
- 29.Nichol KL, Nordin J D, Nelson D B, Mullooly J P,Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med. 2007;357(14):1373-81. doi: 10.1056/NEJMoa070844 [DOI] [PubMed] [Google Scholar]
- 30.Mangtani P, Cumberland P, Hodgson CR, Roberts JA, Cutts FT, Hall AJ. A cohort study of the effectiveness of influenza vaccine in older people, performed using the United Kingdom general practice research database. J Infect Dis. 2004;190(1):1-10. DOI: 10.1086/421274 [DOI] [PubMed] [Google Scholar]
- 31.Gross PA, Hermogenes AW, Sacks HS, Lau J, Levandowski RA. The efficacy of influenza vaccine in elderly persons. A meta-analysis and review of the literature. Ann Intern Med. 1995;123(7):518-27. [DOI] [PubMed] [Google Scholar]
- 32.Lal H, Cunningham AL, Heineman TC. Adjuvanted Herpes Zoster Subunit Vaccine in Older Adults. N Engl J Med. 2015;373(16):1576-7. DOI: 10.1056/NEJMc1508392 [DOI] [PubMed] [Google Scholar]
- 33.Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang SJ, Diez-Domin-go J, et al. . Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. N Engl J Med. 2016;375(11):1019-32. DOI: 10.1056/NEJMoa1603800 [DOI] [PubMed] [Google Scholar]
- 34.Tilston P. Anal human papillomavirus and anal cancer. J Clin Pathol. 1997;50(8):625-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal re-lation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55(4):244-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dawar M, Deeks S, Dobson S. Human papillomavirus vaccines launch a new era in cervical cancer prevention. Cmaj. 2007;177(5):456-61. DOI: 10.1503/cmaj.070771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wachold-er S. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890-907. DOI: 10.1016/s0140-6736(07)61416-0 [DOI] [PubMed] [Google Scholar]
- 38.Palma DA, Nichols AC. Human papillomavirus in head and neck cancer. Cmaj. 2014;186(5):370 DOI: 10.1503/cmaj.130849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saraiya M, Unger ER, Thompson TD, Lynch CF, Hernandez BY, Lyu CW, et al. . US assessment of HPV types in cancers: implica-tions for current and 9-valent HPV vaccines. J Natl Cancer Inst. 2015;107(6):djv086 DOI: 10.1093/jnci/djv086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mao C, Koutsky LA, Ault KA, Wheeler CM, Brown DR, Wiley DJ, et al. . Efficacy of human papillomavirus-16 vaccine to prevent cervical in-traepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2006;107(1):18-27. DOI: 10.1097/01.AOG.0000192397.41191.fb [DOI] [PubMed] [Google Scholar]
- 41.Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR, et al. . Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a ran-domised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6(5):271-8. DOI: 10.1016/s1470-2045(05)70101-7 [DOI] [PubMed] [Google Scholar]
- 42.Sattler C, editor Efficacy of a prophylactic quadrivalent human papillomavirus (HPV) (types 6,11,16,18) L1 virus-like particle (VLP) vaccine for prevention of cervical dysplasia and external genital lesions (EGL) . 45th Interscience Conference on Antimicrobial Agents and Chemnotherapy 2005; Washington DC. [Google Scholar]
- 43.Skjelkdestad F, editor Prophylactic quadrivalent human papillomavirus (HPV) (Types 6,11, 16, 18) L1 virus-like particle (VPL) vaccine (GardasilTM) reduces cervical intraepithelial neoplasia (CIN) 2/3 risk Abstract LB-8a . 43rd Infectious Disease S ociety of America; 2005; San Francisco. [Google Scholar]
- 44.Villa LL, Costa RL, Petta CA, Andrade RP, Paavonen J, Iversen OE, et al. . High sustained efficacy of a prophylactic quadrivalent hu-man papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer. 2006;95(11):1459-66. DOI: 10.1038/sj.bjc.6603469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent Human Papillomavirus Vaccine: Recommenda-tions of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56(Rr-2):1-24. [PubMed] [Google Scholar]
- 46.Calendario de Vacunación del Consejo Interterritorial. https://www.mscbs.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/Calendario2018.htm
- 47.Viens LJ, Henley SJ, Watson M, Markowitz LE, Thomas CC, Thompson TD, et al. . Human Papillomavirus-Associated Cancers-United States, 2008-2012. MMWR Morb Mortal Wkly Rep. 2016;65(26):661-6. DOI: 10.15585/mmwr.mm6526a1 [DOI] [PubMed] [Google Scholar]
- 48.Baldur-Felskov B, Dehlendorff C, Munk C, Kjaer SK. Early impact of human papillomavirus vaccination on cervical neoplasia--na-tionwide follow-up of young Danish women. J Natl Cancer Inst. 2014;106(3):djt460 DOI: 10.1093/jnci/djt460 [DOI] [PubMed] [Google Scholar]
- 49.Baldur-Felskov B, Dehlendorff C, Junge J, Munk C, Kjaer SK. Incidence of cervical lesions in Danish women before and after imple-mentation of a national HPV vaccination program. Cancer Causes Control. 2014;25(7):915-22. DOI: 10.1007/s10552-014-0392-4 [DOI] [PubMed] [Google Scholar]
- 50.Baldur-Felskov B, Munk C, Nielsen TS, Dehlendorff C, Kirschner B, Junge J, et al. . Trends in the incidence of cervical cancer and severe precancerous lesions in Denmark, 1997-2012. Cancer Causes Control. 2015;26(8):1105-16. DOI: 10.1007/s10552-015-0603-7 [DOI] [PubMed] [Google Scholar]
- 51.Gertig DM, Brotherton JM, Saville M. Measuring human papilloma-virus (HPV) vaccination coverage and the role of the National HPV Vaccination Program Register, Australia. Sex Health. 2011;8(2):171-8. DOI: 10.1071/sh10001 [DOI] [PubMed] [Google Scholar]
- 52.Gertig DM, Brotherton JM, Budd AC, Drennan K, Chappell G, Saville AM. Impact of a population-based HPV vaccination pro-gram on cervical abnormalities: a data linkage study. BMC Med. 2013;11:227 DOI: 10.1186/1741-7015-11-227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prevention CfDCa Yellow Book. Hepatitis B. 2016. https://www.nccdcgov/travel/yellowbook/2016/infectious-diseases-related-totravel/hepatitis-b#46212
- 54.Daniels D, Grytdal S, Wasley A. Surveillance for acute viral hepatitis-United States, 2007. MMWR Surveill Summ. 2009;58(3):1-27. [PubMed] [Google Scholar]
- 55.Iqbal K, Klevens RM, Kainer MA, Baumgartner J, Gerard K, Pois-sant T, et al. . Epidemiology of Acute Hepatitis B in the United States From Population-Based Surveillance, 2006-2011. Clin Infect Dis. 2015;61(4):584-92. DOI: 10.1093/cid/civ332 [DOI] [PubMed] [Google Scholar]
- 56.World Health Organization Combating hepatitis B and C to reach elimination by 2030
- 57.Regev-Yochay G, Paran Y, Bishara J, Oren I, Chowers M, Tziba Y, et al. . Early impact of PCV7/PCV13 sequential introduction to the national pediatric immunization plan, on adult invasive pneu-mococcal disease: A nationwide surveillance study. Vaccine. 2015;33(9):1135-42. DOI: 10.1016/j.vaccine.2015.01.030 [DOI] [PubMed] [Google Scholar]
- 58.Bruce MG, Singleton R, Bulkow L, Rudolph K, Zulz T, Gounder P, et al. . Impact of the 13-valent pneumococcal conjugate vaccine (pcv13) on invasive pneumococcal disease and carriage in Alaska. Vaccine. 2015;33(38):4813-9. DOI: 10.1016/j.vaccine.2015.07.080 [DOI] [PubMed] [Google Scholar]
- 59.Demczuk WH, Martin I, Griffith A, Lefebvre B, McGeer A, Lovgren M, et al. . Serotype distribution of invasive in Canada after the introduc-tion of the 13-valent pneumococcal conjugate vaccine, 2010-2012. Can J Microbiol. 2013;59(12):778-88. DOI: 10.1139/cjm-2013-0614 [DOI] [PubMed] [Google Scholar]
- 60.Grau I, Ardanuy C, Cubero M, Benitez MA, Linares J, Pallares R. Declining mortality from adult pneumococcal infections linked to children’s vaccination. J Infect. 2016;72(4):439-49. DOI: 10.1016/j.jinf.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 61.No author listed. Adult immunization. Med Lett Drugs Ther. 2018;60(1546):73-82. [PubMed] [Google Scholar]
- 62.Wang NY, Pollard AJ. The next chapter for group B meningo-coccal vaccines. Crit Rev Microbiol. 2018;44(1):95-111. DOI: 10.1080/1040841x.2017.1329276 [DOI] [PubMed] [Google Scholar]
- 63.Parikh SR, Campbell H, Beebeejaun K, Ribeiro S, Gray SJ, Borrow R, et al. . Meningococcal Group W Disease in Infants and Potential Pre-vention by Vaccination. Emerg Infect Dis. 2016;22(8):1505-7. DOI: 10.3201/eid2208.160128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parikh SR, Newbold L, Slater S, Stella M, Moschioni M, Lucidarme J, et al. . Meningococcal serogroup B strain coverage of the mul-ticomponent 4CMenB vaccine with corresponding regional distri-bution and clinical characteristics in England, Wales, and North-ern Ireland, 2007-08 and 2014-15: a qualitative and quantitative assessment. Lancet Infect Dis. 2017;17(7):754-62. DOI: 10.1016/s1473-3099(17)30170-6 [DOI] [PubMed] [Google Scholar]
- 65.Flacco ME, Manzoli L, Rosso A, Marzuillo C, Bergamini M, Stefa-nati A, et al. . Immunogenicity and safety of the multicomponent meningococcal B vaccine (4CMenB) in children and adoles-cents: a systematic review and meta-analysis. Lancet Infect Dis. 2018;18(4):461-72. DOI: 10.1016/s1473-3099(18)30048-3 [DOI] [PubMed] [Google Scholar]
- 66.Bisgard KM, Kao A, Leake J, Strebel PM, Perkins BA, Wharton M. Haemophilus influenzae invasive disease in the United States, 1994-1995: near disappearance of a vaccine-preventable child-hood disease. Emerg Infect Dis. 1998;4(2):229-37. DOI: 10.3201/eid0402.980210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.No author listed From the Centers for Disease Control and Prevention. Progress toward elimination of Haemophilus influenzae type b invasive disease among infants and children--United States, 1998-2000. Jama. 2002;287(17):2206-7. [PubMed] [Google Scholar]
- 68.No author listed. Progress toward elimination of Haemophi-lus influenzae type b invasive disease among infants and chil-dren--United States, 1998-2000. MMWR Morb Mortal Wkly Rep. 2002;51(11):234-7. [PubMed] [Google Scholar]
- 69.MacNeil JR, Cohn AC, Farley M, Mair R, Baumbach J, Bennett N, et al. . Current epidemiology and trends in invasive Haemophilus influenzae disease--United States, 1989-2008. Clin Infect Dis. 2011;53(12):1230-6. DOI: 10.1093/cid/cir735 [DOI] [PubMed] [Google Scholar]
- 70.Blain A, MacNeil J, Wang X, Bennett N, Farley MM, Harrison LH, et al. . Invasive Haemophilus influenzae Disease in Adults >/=65 Years, United States, 2011. Open Forum Infect Dis. 2014;1(2):ofu044 DOI: 10.1093/ofid/ofu044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Livorsi DJ, Macneil JR, Cohn AC, Bareta J, Zansky S, Petit S, et al. . Invasive Haemophilus influenzae in the United States, 1999-2008: epidemiology and outcomes. J Infect. 2012;65(6):496-504. DOI: 10.1016/j.jinf.2012.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maine LL, Knapp KK, Scheckelhoff DJ. Pharmacists and techni-cians can enhance patient care even more once national pol-icies, practices, and priorities are aligned. Health Aff (Millwood). 2013;32(11):1956-62. DOI: 10.1377/hlthaff.2013.0529 [DOI] [PubMed] [Google Scholar]
- 73.Spencer JP, Trondsen Pawlowski RH, Thomas S. Vaccine Ad-verse Events: Separating Myth from Reality. Am Fam Physician. 2017;95(12):786-94. [PubMed] [Google Scholar]
- 74.Kelso JM, Greenhawt MJ, Li JT, Nicklas RA, Bernstein DI, Bless-ing-Moore J, et al. . Adverse reactions to vaccines practice parame-ter 2012 update. J Allergy Clin Immunol. 2012;130(1):25-43. DOI: 10.1016/j.jaci.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 75.Ball LK, Ball R, Pratt RD. An assessment of thimerosal use in child-hood vaccines. Pediatrics. 2001;107(5):1147-54. [DOI] [PubMed] [Google Scholar]
- 76.Thompson WW, Price C, Goodson B, Shay DK, Benson P, Hinrichsen VL, et al. . Early thimerosal exposure and neuropsychological out-comes at 7 to 10 years. N Engl J Med. 2007;357(13):1281-92. DOI: 10.1056/NEJMoa071434 [DOI] [PubMed] [Google Scholar]
- 77.Godlee F, Smith J, Marcovitch H. Wakefield’s article linking MMR vaccine and autism was fraudulent. Bmj. 2011;342:c7452 DOI: 10.1136/bmj.c7452 [DOI] [PubMed] [Google Scholar]
- 78.Wakefield AJ. Measles, mumps, and rubella vaccination and autism. N Engl J Med. 2003;348(10):951-4; author reply-4. [PubMed] [Google Scholar]
- 79.Wakefield AJ, Harvey P, Linnell J. MMR--responding to retraction. Lancet. 2004;363(9417):1327-8; discussion 8. DOI: 10.1016/s0140-6736(04)16017-0 [DOI] [PubMed] [Google Scholar]
- 80.Hambidge SJ, Ross C, Shoup JA, Wain K, Narwaney K, Breslin K, et al. . Integration of data from a safety net health care system into the Vaccine Safety Datalink. Vaccine. 2017;35(9):1329-34. DOI: 10.1016/j.vaccine.2017.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Izurieta HS, Moro PL, Chen RT. Hospital-based collaboration for ep-idemiological investigation of vaccine safety: A potential solution for low and middle-income countries ? Vaccine. 2018;36(3):345-6. DOI: 10.1016/j.vaccine.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lei J, Balakrishnan MR, Gidudu JF, Zuber PLF. Use of a new glob-al indicator for vaccine safety surveillance and trends in adverse events following immunization reporting 2000-2015. Vaccine. 2018;36(12):1577-82. DOI: 10.1016/j.vaccine.2018.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rowhani-Rahbar A, Klein NP, Baxter R. Assessing the safety of in-fluenza vaccination in specific populations: children and the elder-ly. Expert Rev Vaccines. 2012;11(8):973-84. DOI: 10.1586/erv.12.66 [DOI] [PubMed] [Google Scholar]
- 84.Wilkinson K, Wei Y, Szwajcer A, Rabbani R, Zarychanski R, Abou-Set-ta AM, et al. . Efficacy and safety of high-dose influenza vaccine in elderly adults: A systematic review and meta-analysis. Vaccine. 2017;35(21):2775-80. DOI: 10.1016/j.vaccine.2017.03.092 [DOI] [PubMed] [Google Scholar]
- 85.Moa AM, Chughtai AA, Muscatello DJ, Turner RM, MacIntyre CR. Immunogenicity and safety of inactivated quadrivalent influenza vaccine in adults: A systematic review and meta-analysis of ran-domised controlled trials. Vaccine. 2016;34(35):4092-102. DOI: 10.1016/j.vaccine.2016.06.064 [DOI] [PubMed] [Google Scholar]
- 86.Leung TF, Liu AP, Lim FS, Thollot F, Oh HM, Lee BW, et al. . Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine adminis-tered according to 2-and 3-dose schedules in girls aged 9-14 years: Results to month 12 from a randomized trial. Hum Vaccin Immuno-ther. 2015;11(7):1689-702. DOI: 10.1080/21645515.2015.1050570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schwarz TF, Galaj A, Spaczynski M, Wysocki J, Kaufmann AM, Poncelet S, et al. . Ten-year immune persistence and safety of the HPV-16/18 AS04-adjuvanted vaccine in females vaccinated at 15-55 years of age. Cancer Med. 2017;6(11):2723-31. DOI: 10.1002/cam4.1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hidalgo-Tenorio C, Ramirez-Taboada J, Gil-Anguita C, Esquivias J, Omar-Mohamed-Balgahata M, SamPedro A, et al. . Safety and im-munogenicity of the quadrivalent human papillomavirus (qHPV) vaccine in HIV-positive Spanish men who have sex with men (MSM). AIDS Res Ther. 2017;14:34 DOI: 10.1186/s12981-017-0160-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wheeler CM, Skinner SR, Del Rosario-Raymundo MR, Garland SM, Chatterjee A, Lazcano-Ponce E, et al. . Efficacy, safety, and immuno-genicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 7-year follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet Infect Dis. 2016;16(10):1154-68. DOI: 10.1016/s1473-3099(16)30120-7 [DOI] [PubMed] [Google Scholar]
- 90.Moro PL, Zheteyeva Y, Lewis P, Shi J, Yue X, Museru OI, et al. . Safety of quadrivalent human papillomavirus vaccine (Gardasil) in preg-nancy: adverse events among non-manufacturer reports in the Vaccine Adverse Event Reporting System, 2006-2013. Vaccine. 2015;33(4):519-22. DOI: 10.1016/j.vaccine.2014.11.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gee J, Sukumaran L, Weintraub E. Risk of Guillain-Barre Syndrome following quadrivalent human papillomavirus vaccine in the Vac-cine Safety Datalink. Vaccine. 2017;35(43):5756-8. DOI: 10.1016/j.vaccine.2017.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Costa APF, Cobucci RNO, da Silva JM, da Costa Lima PH, Giral-do PC, Goncalves AK. Safety of Human Papillomavirus 9-Valent Vaccine: A Meta-Analysis of Randomized Trials. J Immunol Res. 2017;2017:3736201 DOI: 10.1155/2017/3736201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sacks HS. In adults ≥70 years of age, an adjuvanted herpes zoster subunit vaccine reduced herpes zoster at a mean 3.7 years. Ann Intern Med. 2017;166(2):Jc5 DOI: 10.7326/acpjc-2017-166-2-005 [DOI] [PubMed] [Google Scholar]
- 94.Parrino J, McNeil SA, Lawrence SJ, Kimby E, Pagnoni MF, Stek JE, et al. . Safety and immunogenicity of inactivated varicella-zoster virus vaccine in adults with hematologic malignancies receiv-ing treatment with anti-CD20 monoclonal antibodies. Vaccine. 2017;35(14):1764-9. DOI: 10.1016/j.vaccine.2016.10.055 [DOI] [PubMed] [Google Scholar]
- 95.Oxman MN, Levin MJ. Vaccination against Herpes Zoster and Pos-therpetic Neuralgia. J Infect Dis. 2008;197 Suppl 2:S228-36. DOI: 10.1086/522159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Herpes zoster vaccine (Zostavax). Med Lett Drugs Ther. 2006;48(1243):73-4. [PubMed] [Google Scholar]
- 97.Vazquez M, Shapiro ED. Varicella vaccine and infection with vari-cella-zoster virus. N Engl J Med. 2005;352(5):439-40. DOI: 10.1056/NEJMp048320 [DOI] [PubMed] [Google Scholar]
- 98.Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, et al. . A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271-84. DOI: 10.1056/NEJMoa051016 [DOI] [PubMed] [Google Scholar]
- 99.Kessler KM. A vaccine to prevent herpes zoster. N Engl J Med. 2005;353(13):1414-5; author reply-5. DOI: 10.1056/NEJMc051795 [DOI] [PubMed] [Google Scholar]
- 100.Schmader KE, Levin MJ, Gnann JW Jr., McNeil SA, Vesikari T, Betts RF, et al. . Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years. Clin Infect Dis. 2012;54(7):922-8. DOI: 10.1093/cid/cir970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, et al. . Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087-96. DOI: 10.1056/NEJMoa1501184 [DOI] [PubMed] [Google Scholar]
- 102.Winthrop KL, Wouters AG, Choy EH, Soma K, Hodge JA, Nduaka CI, et al. . The Safety and Immunogenicity of Live Zoster Vaccination in Patients With Rheumatoid Arthritis Before Starting Tofacitinib: A Randomized Phase II Trial. Arthritis Rheumatol. 2017;69(10):1969-77. DOI: 10.1002/art.40187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Willis ED, Woodward M, Brown E, Popmihajlov Z, Saddier P, An-nunziato PW, et al. . Herpes zoster vaccine live: A 10year review of post-marketing safety experience. Vaccine. 2017;35(52):7231-9. DOI: 10.1016/j.vaccine.2017.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shaw FE Jr., Graham DJ, Guess HA, Milstien JB, Johnson JM, Schatz GC, et al. . Postmarketing surveillance for neurologic adverse events reported after hepatitis B vaccination. Experience of the first three years. Am J Epidemiol. 1988;127(2):337-52. [DOI] [PubMed] [Google Scholar]
- 105.McMahon BJ, Helminiak C, Wainwright RB, Bulkow L, Trimble BA, Wainwright K. Frequency of adverse reactions to hepatitis B vac-cine in 43,618 persons. Am J Med. 1992;92(3):254-6. [DOI] [PubMed] [Google Scholar]
- 106.Niu MT, Davis DM, Ellenberg S. Recombinant hepatitis B vacci-nation of neonates and infants: emerging safety data from the Vaccine Adverse Event Reporting System. Pediatr Infect Dis J. 1996;15(9):771-6. [DOI] [PubMed] [Google Scholar]
- 107.Ascherio A, Zhang SM, Hernan MA, Olek MJ, Coplan PM, Bro-dovicz K, et al. . Hepatitis B vaccination and the risk of multi-ple sclerosis. N Engl J Med. 2001;344(5):327-32. DOI: 10.1056/nejm200102013440502 [DOI] [PubMed] [Google Scholar]
- 108.Hall A, Kane M, Roure C, Meheus A. Multiple sclerosis and hepatitis B vaccine ? Vaccine. 1999;17(20-21):2473-5. [DOI] [PubMed] [Google Scholar]
- 109.Confavreux C, Suissa S, Saddier P, Bourdes V, Vukusic S. Vaccina-tions and the risk of relapse in multiple sclerosis. Vaccines in Multiple Sclerosis Study Group. N Engl J Med. 2001;344(5):319-26. DOI: 10.1056/nejm200102013440501 [DOI] [PubMed] [Google Scholar]
- 110.Schillie S, Harris A, Link-Gelles R, Romero J, Ward J, Nelson N. Recommendations of the Advisory Committee on Immunization Practices for Use of a Hepatitis B Vaccine with a Novel Adjuvant. MMWR Morb Mortal Wkly Rep. 2018;67(15):455-8. DOI: 10.15585/mmwr.mm6715a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.BLA Clinical Review Memorandum, 2017. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM590189.pdf (Accessed on May 10, 2018). [Internet]. 2018.
- 112.Remschmidt C, Harder T, Wichmann O, Bogdan C, Falkenhorst G. Effectiveness, immunogenicity and safety of 23-valent pneu-mococcal polysaccharide vaccine revaccinations in the elderly: a systematic review. BMC Infect Dis. 2016;16(1):711 DOI: 10.1186/s12879-016-2040-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Solanki BB, Juergens C, Chopada MB, Supe P, Sundaraiyer V, Le Dren-Narayanin N, et al. . Safety and immunogenicity of a 13-va-lent pneumococcal conjugate vaccine in adults 50 to 65 years of age in India: An open-label trial. Hum Vaccin Immunother. 2017;13(9):2065-71. DOI: 10.1080/21645515.2017.1331796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Destefano F, Pfeifer D, Nohynek H. Safety profile of pneumococcal conjugate vaccines: systematic review of pre-and post-licensure data. Bull World Health Organ. 2008;86(5):373-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Urbancikova I, Prymula R, Goldblatt D, Roalfe L, Prymulova K, Ko-sina P. Immunogenicity and safety of a booster dose of the 13-va-lent pneumococcal conjugate vaccine in children primed with the 10-valent or 13-valent pneumococcal conjugate vaccine in the Czech Republic and Slovakia. Vaccine. 2017;35(38):5186-93. DOI: 10.1016/j.vaccine.2017.07.103 [DOI] [PubMed] [Google Scholar]
- 116.Centers for Disease Control and Prevention (CDC) Notice to read-ers: Recommendation from the Advisory Committee on Immuniza-tion Practices (ACIP) for use of quadrivalent meningococcal conjugate vaccine (MCV4) in children aged 2--10 years at increased risk for invasive meningococcal disease. MMWR Morb Mortal Wkly Rep 2007;56:1265. [Google Scholar]
- 117.Centers for Disease Control and Prevention (CDC) Updated recom-mendations for use of meningococcal conjugate vaccines---Ad-visory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2011;60(3):72-6. [PubMed] [Google Scholar]
- 118.Hong E, Terrade A, Taha MK. Immunogenicity and safe-ty among laboratory workers vaccinated with Bexsero(R) vaccine. Hum Vaccin Immunother. 2017;13(3):645-8. DOI: 10.1080/21645515.2016.1241358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Briere EC, Rubin L, Moro PL, Cohn A, Clark T, Messonnier N. Preven-tion and control of haemophilus influenzae type b disease: recom-mendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep. 2014;63(Rr-01):1-14. [PubMed] [Google Scholar]
- 120.Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, et al. . 2013. IDSA clinical practice guideline for vaccination of the im-munocompromised host. Clin Infect Dis. 2014;58(3):e44-100. DOI: 10.1093/cid/cit684 [DOI] [PubMed] [Google Scholar]
- 121.Lottenbach KR, Granoff DM, Barenkamp SJ, Powers DC, Kennedy D, Irby-Moore S, et al. . Safety and immunogenicity of haemophilus influenzae type B polysaccharide or conjugate vaccines in an el-derly adult population. J Am Geriatr Soc. 2004;52(11):1883-7. DOI: 10.1111/j.1532-5415.2004.52511.x [DOI] [PubMed] [Google Scholar]
- 122.(CDC) CfDCaP. Pertussis--United States, 1997-2000. MMWR Morb Mortal Wkly Rep. 2002;51(4):73-6. [PubMed] [Google Scholar]
- 123.Lambert HJ. Epidemiology of a small Pertussis outbreak in Kent county, Michigan. Public Health Rep. 1965;80:365-9. [PMC free article] [PubMed] [Google Scholar]
- 124.Jenkinson D. Duration of effectiveness of pertussis vaccine: evi-dence from a 10 year community study. Br Med J (Clin Res Ed). 1988;296(6622):612-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cody CL, Baraff LJ, Cherry JD, Marcy SM, Manclark CR. Nature and rates of adverse reactions associated with DTP and DT immuniza-tions in infants and children. Pediatrics. 1981;68(5):650-60. [PubMed] [Google Scholar]
- 126.Zhang L, Prietsch SO, Axelsson I, Halperin SA. Acellular vaccines for preventing whooping cough in children. Cochrane Database Syst Rev. 2014(9):Cd001478 DOI: 10.1002/14651858.CD001478.pub6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Prevention Pertussis (Whooping Cough). Pertussis in Other Countries. http://www.cdc.gov/pertussis/countries.html.
- 128.Liang JL, Tiwari T, Moro P, Messonnier NE, Reingold A, Sawyer M, et al. . Prevention of Pertussis, Tetanus, and Diphtheria with Vac-cines in the United States: Recommendations of the Advisory Com-mittee on Immunization Practices (ACIP). MMWR Recomm Rep. 2018;67(2):1-44. DOI: 10.15585/mmwr.rr6702a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.MMMWR Notifiable Diseases and Mortality Tables. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6115md.htm?s_cid=mm6115md_w 2012. [PubMed]
- 130.Amirthalingam G, Gupta S, Campbell H. Pertussis immunisation and control in England and Wales, 1957 to 2012: a historical review. Euro Surveill. 2013;18(38). [DOI] [PubMed] [Google Scholar]
- 131.Fernandes EG, Sartori AMC, de Soarez PC, Carvalhanas T, Rodrigues M, Novaes HMD. Challenges of interpreting epidemiologic surveillance pertussis data with changing diagnostic and immunization practices: the case of the state of Sao Paulo, Brazil. BMC Infect Dis. 2018;18(1):126 DOI: 10.1186/s12879-018-3004-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Alamaw SD, Kassa AW, Gelaw YA. Pertussis outbreak investigation of Mekdela district, South Wollo zone, Amhara region, North-West Ethiopia. BMC Res Notes. 2017;10(1):420. DOI: 10.1186/s13104-17-2735-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Saul N, Gilmour R, Spokes P. Annual vaccine-preventable disease report for New South Wales, Australia, 2014. Western Pac Surveill Response J. 2017;8(2):5-11. DOI: 10.5365/wpsar.2016.7.3.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bailon H, Leon-Janampa N, Padilla C, Hozbor D. Increase in pertus-sis cases along with high prevalence of two emerging genotypes of Bordetella pertussis in Peru, 2012. BMC Infect Dis. 2016;16:422 DOI: 10.1186/s12879-016-1700-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Aslanabadi A, Ghabili K, Shad K, Khalili M, Sajadi MM. Emergence of whooping cough: notes from three early epidemics in Persia. Lancet Infect Dis. 2015;15(12):1480-4. DOI: 10.1016/s1473-3099(15)00292-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Javed S, Said F, Eqani SA, Bokhari H. Bordetella parapertussis out-break in Bisham, Pakistan in 2009-2010: fallout of the 9/11 syn-drome. Epidemiol Infect. 2015;143(12):2619-23. DOI: 10.1017/s0950268814003732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sizaire V, Garrido-Estepa M, Masa-Calles J, Martinez de Aragon MV. Increase of pertussis incidence in 2010 to 2012 after 12 years of low circulation in Spain. Euro Surveill. 2014;19(32). [DOI] [PubMed] [Google Scholar]
- 138.Fernandez-Cano MI, Armadans-Gil L, Alvarez-Bartolome M, Rodri-go-Pendas JA, Campins-Marti M. [Hospitalization due to whooping cough in Spain (1997-2011)]. Enferm Infecc Microbiol Clin. 2014;32(10):638-42. DOI: 10.1016/j.eimc.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 139.Heininger U. Pertussis: what the pediatric infectious disease specialist should know. Pediatr Infect Dis J. 2012;31(1):78-9. DOI: 10.1097/INF.0b013e31823b034e [DOI] [PubMed] [Google Scholar]
- 140.Zhang L, Prietsch SO, Axelsson I, Halperin SA. Acellular vaccines for preventing whooping cough in children. Cochrane Database Syst Rev. 2012(3):Cd001478 DOI: 10.1002/14651858.CD001478.pub5 [DOI] [PubMed] [Google Scholar]
- 141.WHO Europe/ECDC joint statement Low uptake of seasonal influenza vaccination in Europe may jeopardize capacity to protect people in next pandemic. http://www.eurowhoint/en/media-centre/sections/press-releases/2018/who-europeecdc-joint-statement. 2018.
- 142.Jorgensen P, Mereckiene J, Cotter S, Johansen K, Tsolova S, Brown C. How close are countries of the WHO European Region to achieving the goal of vaccinating 75% of key risk groups against influenza ? Results from national surveys on seasonal influenza vaccination programmes, 2008/2009 to 2014/2015. Vaccine. 2018;36(4):442-52. DOI: 10.1016/j.vaccine.2017.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Godoy P, Castilla J, Mayoral JM, Martin V, Astray J, Torner N, et al. . Influenza vaccination of primary healthcare physicians may be associated with vaccination in their patients: a vaccination cover-age study. BMC Fam Pract. 2015;16:44 DOI: 10.1186/s12875-015-0259-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Word Health Organization http://www.euro.who.int/en/health-topics/communicable-diseases/influenza/news/news/2018/6/is-european-region-ready-to-respond-to-next-in-fluenza-pandemic accessed 28 June 2018.
- 145.Ortiz de Lejarazu R, Tamames S. [Influenza vaccination. Effective-ness of current vaccines and future challenges]. Enferm Infecc Mi-crobiol Clin. 2015;33(7):480-90. DOI: 10.1016/j.eimc.2015.06.011 [DOI] [PubMed] [Google Scholar]
- 146.Plans-Rubio P. The vaccination coverage required to establish herd immunity against influenza viruses. Prev Med. 2012;55(1):72-7. DOI: 10.1016/j.ypmed.2012.02.015 [DOI] [PubMed] [Google Scholar]
- 147.Ortiz JR, Hickling J, Jones R, Donabedian A, Engelhardt OG, Katz JM, et al. . Report on eighth WHO meeting on development of in-fluenza vaccines that induce broadly protective and long-lasting immune responses: Chicago, USA, 23-24 August 2016. Vaccine. 2018;36(7):932-8. DOI: 10.1016/j.vaccine.2017.11.061 [DOI] [PubMed] [Google Scholar]
- 148.Paules CI, Sullivan SG, Subbarao K, Fauci AS. Chasing Seasonal In-fluenza-The Need for a Universal Influenza Vaccine. N Engl J Med. 2018;378(1):7-9. DOI: 10.1056/NEJMp1714916 [DOI] [PubMed] [Google Scholar]
- 149.Osterhaus A, Fouchier R, Rimmelzwaan G. Towards univer-sal influenza vaccines ? Philos Trans R Soc Lond B Biol Sci. 2011;366(1579):2766-73. DOI: 10.1098/rstb.2011.0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Scorza FB, Pardi N. New Kids on the Block: RNA-Based Influen-za Virus Vaccines. Vaccines (Basel). 2018;6(2). DOI: 10.3390/vac-cines6020020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Krammer F, Palese P. Influenza virus hemagglutinin stalk-based antibodies and vaccines. Curr Opin Virol. 2013;3(5):521-30. DOI: 10.1016/j.coviro.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kassianos G, Blank P, Falup-Pecurariu O, Kuchar E, Kyncl J, De Lejarazu RO, et al. . Influenza vaccination: key facts for general practitioners in Europe-a synthesis by European experts based on national guidelines and best practices in the United Kingdom and the Netherlands. Drugs Context. 2016;5:212293 DOI: 10.7573/dic.212293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Blank PR, van Essen GA, Ortiz de Lejarazu R, Kyncl J, Nitsch-Osuch A, Kuchar EP, et al. . Impact of European vaccination policies on seasonal influenza vaccination coverage rates: An update seven years later. Hum Vaccin Immunother: 2018:1-9. DOI: 10.1080/21645515.2018.1489948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rudolph W, Ben Yedidia T. A universal influenza vaccine: where are we in the pursuit of this. “Holy Grail” ? Hum Vaccin. 2011;7(1):10-1. [DOI] [PubMed] [Google Scholar]
- 155.Gonzalez-Romo F, Picazo JJ, Garcia Rojas A, Labrador Horrillo M, Barrios V, Magro MC, et al. . Consenso sobre la vacunación anti-neu-mocócica en el adulto por riesgo de edad y patología de base. Ac-tualización 2017. Rev Esp Quimioter. 2017;30(2):142-68. PMid: [PubMed] [Google Scholar]
- 156.Ministerio de Sanidad SSeI Grupo de trabajo vacunación frente a neumococo en grupos de riesgo 2015 de la Ponencia de Programas y Registro de Vacunaciones. Utilización de la vacuna frente a neumococo en grupos de riesgo. Comisión de Salud Pública del Consejo Interterritorial del Sistema Nacional de Salud. 2015.
- 157.Ochoa-Gondar O, Hospital I, Vila-Corcoles A, Aragon M, Jariod M, de Diego C, et al. . Prevalence of high, medium and low-risk med-ical conditions for pneumococcal vaccination in Catalonian mid-dle-aged and older adults: a population-based study. BMC Public Health. 2017;17(1):610 DOI: 10.1186/s12889-017-4529-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dominguez A, Soldevila N, Toledo D, Torner N, Force L, Perez MJ, et al. . Effectiveness of 23-valent pneumococcal polysaccharide vaccination in preventing community-acquired pneumonia hospi-talization and severe outcomes in the elderly in Spain. PLoS One. 2017;12(2):e0171943 DOI: 10.1371/journal.pone.0171943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Camara J, Marimon JM, Cercenado E, Larrosa N, Quesada MD, Fon-tanals D, et al. . Decrease of invasive pneumococcal disease (IPD) in adults after introduction of pneumococcal 13-valent conjugate vaccine in Spain. PLoS One. 2017;12(4):e0175224 DOI: 10.1371/journal.pone.0175224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pharmaceutical Research and Manufacturers of America. VACCINES: HARNESSING SCIENCE TO DRIVE INNOVATION FOR PATIENTS. https://wwwphrmaorg/report/2017-state-of-vaccines. 2017;Assessed march 24, 2018. [Google Scholar]
- 161.PubMed. https://www.ncbi.nlm.nih.gov/pubmed/?term=vaccines+research [assessed April 1st 2018]. 2018. [Google Scholar]
- 162.González-Romo. F, Picazo. FJ. El desarrollo de nuevas vacunas. En-ferm Infecc Microbiol Clin. 2015;33:557-68. [DOI] [PubMed] [Google Scholar]
- 163.Gao Y, McKay PF, Mann JFS. Advances in HIV-1 Vaccine Develop-ment. Viruses. 2018;10(4). DOI: 10.3390/v10040167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tian YS, Zhou Y, Takagi T, Kameoka M, Kawashita N. Dengue Vi-rus and Its Inhibitors: A Brief Review. Chem Pharm Bull (Tokyo). 2018;66(3):191-206. DOI: 10.1248/cpb.c17-00794 [DOI] [PubMed] [Google Scholar]
- 165.Ewer K, Sebastian S, Spencer AJ, Gilbert S, Hill AVS, Lambe T. Chimpanzee adenoviral vectors as vaccines for outbreak path-ogens. Hum Vaccin Immunother. 2017;13(12):3020-32. DOI: 10.1080/21645515.2017.1383575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Roth C, Delgado FG, Simon-Loriere E, Sakuntabhai A. Immune Re-sponses to Dengue and Zika Viruses-Guidance for T Cell Vaccine Development. Int J Environ Res Public Health. 2018;15(2). DOI: 10.3390/ijerph15020385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Goldmann O, Medina E. strategies to evade the host acquired im-mune response. Int J Med Microbiol. 2018;308(6):625-30. DOI: 10.1016/j.ijmm.2017.09.013 [DOI] [PubMed] [Google Scholar]
- 168.Bruxelle JF, Pechine S, Collignon A. Immunization Strategies Against Clostridium difficile. Adv Exp Med Biol. 2018;1050:197-225. DOI: 10.1007/978-3-319-72799-8_12 [DOI] [PubMed] [Google Scholar]
- 169.Zucca M, Scutera S, Savoia D. New chemotherapeutic strategies against malaria, leishmaniasis and trypanosomiases. Curr Med Chem. 2013;20(4):502-26. [DOI] [PubMed] [Google Scholar]
- 170.Moreno-Perez D, Alvarez Garcia FJ, Alvarez Aldean J, Cilleruelo Ortega MJ, Garces Sanchez M, Garcia Sanchez N, et al. . [Immuni-sation schedule of the Spanish Association of Paediatrics: 2018 recommendations]. An Pediatr (Barc). 2018;88(1):53.e1-.e9. DOI: 10.1016/j.anpedi.2017.10.001 [DOI] [PubMed] [Google Scholar]
- 171.Cabezas Pascual CF, Pérez Rubio A, Eiros Bouza JM, Cortés Lorenzo I. Cien cuestiones básicas de Economía de la Salud y Evaluaciones Económicas. In: (ed). EA, editor. I. Valladolid: Iglesias Comunicación; 2017. [Google Scholar]
- 172.Ministerio de Sanidad SSeI Consejo Interterritorial. Introducción y marco legal. Ley 14/1986, de 25 de abril, General de Sanidad (LGS) y disposiciones de desarrollo. https://www.msssigobes/organizacion/consejoInterterri/introduccionhtm [assessed April 5th, 2018]. 1986.
- 173.España. Gd.Real Decreto 1030/2006, de 15 de septiembre, por el que se establece la cartera de servicios comunes del Sistema Nacional de Salud y el procedimiento para su actualización.,
- 174.Agencia Española de Medicamentos y Productos Sanitarios Vacunas de uso humano autorizadas en España. . https://www.aempsgobes/medicamentosUsoHumano/vacunas/autorizadasEspana/home.htm [consultado el 5 de abril de 2018].
- 175.European Medicines Agency Authorisation of medicines. Available at http://www.emaeuropaeu/ema/indexjsp?curl=pag-es/about_us/general/general_content_000109jsp&mid=W-C0b01ac0580028a47 [consulted April 5, 2018].
- 176.European Medicines Agency Marketing authorisation. Available at: http://www.emaeuropaeu/ema/indexjsp?curl=pages/regulation/general/general_content_001595jsp&mid=W-C0b01ac0580b18a3d [Colsulted April 5 2018]
- 177.Ministerio de Sanidad SSeI Vacunas y programas de vacunación. Available at: http://www.msssigobes/profesionales/saludPublica/prevPromocion/vacunaciones/home.htm [assessed April 5th, 2018]
- 178.Chong PP, Avery RK. A Comprehensive Review of Immuniza-tion Practices in Solid Organ Transplant and Hematopoietic Stem Cell Transplant Recipients. Clin Ther. 2017;39(8):1581-98. DOI: 10.1016/j.clinthera.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 179.Danziger-Isakov L, Kumar D. Vaccination in solid organ transplan-tation. Am J Transplant. 2013;13 Suppl 4:311-7. DOI: 10.1111/ajt.12122 [DOI] [PubMed] [Google Scholar]
- 180.Danziger-Isakov L, Posfay-Barbe KM. Optimal approach to immuni-zation in pediatric solid organ transplantation. Pediatr Transplant. 2012;16(7):680-3. DOI: 10.1111/j.1399-3046.2012.01689.x [DOI] [PubMed] [Google Scholar]
- 181.Hirzel C, Kumar D. Influenza vaccine strategies for solid organ transplant recipients. Curr Opin Infect Dis. 2018;31(4):309-15. DOI: 10.1097/qco.0000000000000461 [DOI] [PubMed] [Google Scholar]
- 182.Kumar D, Ferreira VH, Blumberg E, Silveira F, Cordero E, Perez-Romero P, et al. . A Five-year Prospective Multi-center Evaluation of Influenza Infection in Transplant Recipients. Clin Infect Dis. 2018. DOI: 10.1093/cid/ciy294 [DOI] [PubMed] [Google Scholar]
- 183.Natori Y, Shiotsuka M, Slomovic J, Hoschler K, Ferreira V, Ashton P, et al. . A Double-Blind, Randomized Trial of High-Dose vs Stand-ard-Dose Influenza Vaccine in Adult Solid-Organ Transplant Re-cipients. Clin Infect Dis. 2018;66(11):1698-704. DOI: 10.1093/cid/cix1082 [DOI] [PubMed] [Google Scholar]
- 184.Natori Y, Shiotsuka M, Slomovic J, Hoschler K, Ferreira V, Ashton P, et al. . A Double Blind Randomized Trial of High Dose vs. Standard Dose Influenza Vaccine in Adult Solid Organ Transplant Recipients. Clin Infect Dis. 2017. DOI: 10.1093/cid/cix1082 [DOI] [PubMed] [Google Scholar]
- 185.Cordero E, Roca-Oporto C, Bulnes-Ramos A, Aydillo T, Gavalda J, Moreno A, et al. . Two Doses of Inactivated Influenza Vaccine Im-prove Immune Response in Solid Organ Transplant Recipients: Re-sults of TRANSGRIPE 1-2, a Randomized Controlled Clinical Trial. Clin Infect Dis. 2017;64(7):829-38. DOI: 10.1093/cid/ciw855 [DOI] [PubMed] [Google Scholar]
- 186.Rohde KA, Cunningham KC, Henriquez KM, Nielsen AR, Worzella SL, Hayney MS. A cross-sectional study of tetanus and diphtheria antibody concentrations post vaccination among lung transplant patients compared with healthy individuals. Transpl Infect Dis. 2014;16(6):871-7. DOI: 10.1111/tid.12288 [DOI] [PubMed] [Google Scholar]
- 187.Broeders EN, Wissing KM, Ghisdal L, Lemy A, Hoang AD, Vereer-straeten P, et al. . Large decrease of anti-tetanus anatoxin and an-ti-pneumococcal antibodies at one year after renal transplantation. Clin Nephrol. 2013;79(4):313-7. DOI: 10.5414/cn107779 [DOI] [PubMed] [Google Scholar]
- 188.Prevots DR, Burr RK, Sutter RW, Murphy TV. Poliomyelitis preven-tion in the United States. Updated recommendations of the Adviso-ry Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000;49(Rr-5):1-22; quiz CE1-7. [PubMed] [Google Scholar]
- 189.Brandao LGP, Santoro-Lopes G, Oliveira SS, da Silva EE, do Brasil P. Seroprevalence of antibodies against the three serotypes of po-liovirus and IPV vaccine response in adult solid organ transplant candidates. Vaccine. 2018;36(31):4681-6. DOI: 10.1016/j.vac-cine.2018.06.031 [DOI] [PubMed] [Google Scholar]
- 190.Ljungman P, Aschan J, Gustafsson B, Lewensohn-Fuchs I, Winiarski J, Ringden O. Long-term immunity to poliovirus after vaccination of allogeneic stem cell transplant recipients. Bone Marrow Trans-plant. 2004;34(12):1067-9. DOI: 10.1038/sj.bmt.1704678 [DOI] [PubMed] [Google Scholar]
- 191.No author listed. Use of 13-valent pneumococcal conjugate vac-cine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61(40):816-9. [PubMed] [Google Scholar]
- 192.Sever MS, Yildiz A, Eraksoy H, Badur S, Yuksel-Onel D, Gorcin B, et al. . Immune response to Haemophilus influenzae type B vaccina-tion in renal transplant recipients with well-functioning allografts. Nephron. 1999;81(1):55-9. DOI: 10.1159/000045246 [DOI] [PubMed] [Google Scholar]
- 193.Pao M, Papadopoulos EB, Chou J, Glenn H, Castro-Malaspina H, Jakubowski AA, et al. . Response to pneumococcal (PNCRM7) and haemophilus influenzae conjugate vaccines (HIB) in pediatric and adult recipients of an allogeneic hematopoietic cell transplantation (alloHCT). Biol Blood Marrow Transplant. 2008;14(9):1022-30. DOI: 10.1016/j.bbmt.2008.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wyplosz B, Derradji O, Hong E, Francois H, Durrbach A, Duclos-Vallee JC, et al. . Low immunogenicity of quadrivalent meningococ-cal vaccines in solid organ transplant recipients. Transpl Infect Dis. 2015;17(2):322-7. DOI: 10.1111/tid.12359 [DOI] [PubMed] [Google Scholar]
- 195.Wedemeyer H, Pethig K, Wagner D, Flemming P, Oppelt P, Petzold DR, et al. . Long-term outcome of chronic hepatitis B in heart trans-plant recipients. Transplantation. 1998;66(10):1347-53. [DOI] [PubMed] [Google Scholar]
- 196.Pessoa MG, Terrault NA, Ferrell LD, Detmer J, Kolberg J, Collins ML, et al. . Hepatitis after liver transplantation: the role of the known and unknown viruses. Liver Transpl Surg. 1998;4(6):461-8. [DOI] [PubMed] [Google Scholar]
- 197.Kliem V, Ringe B, Holhorst K, Frei U. Kidney transplantation in hep-atitis B surface antigen carriers. Clin Investig. 1994;72(12):1000-6. [DOI] [PubMed] [Google Scholar]
- 198.Chung RT, Feng S, Delmonico FL. Approach to the management of allograft recipients following the detection of hepatitis B virus in the prospective organ donor. Am J Transplant. 2001;1(2):185-91. [PubMed] [Google Scholar]
- 199.Villeneuve E, Vincelette J, Villeneuve JP. Ineffectiveness of hepatitis B vaccination in cirrhotic patients waiting for liver transplantation. Can J Gastroenterol. 2000;14 Suppl B:59b-62b. [DOI] [PubMed] [Google Scholar]
- 200.Dominguez M, Barcena R, Garcia M, Lopez-Sanroman A, Nuno J. Vaccination against hepatitis B virus in cirrhotic patients on liver transplant waiting list. Liver Transpl. 2000;6(4):440-2. DOI: 10.1053/jlts.2000.8313 [DOI] [PubMed] [Google Scholar]
- 201.Barcena R, Fernandez-Braso M, Urman J, Lopez-San Roman A, del Campo S, Moreno N, et al. . Response to hepatitis B virus vaccine in patients transplanted for HBV-related liver disease under specific gammaglobulin prophylaxis. Transplant Proc. 1999;31(6):2459-60. [DOI] [PubMed] [Google Scholar]
- 202.Loinaz C, de Juanes JR, Gonzalez EM, Lopez A, Lumbreras C, Gomez R, et al. . Hepatitis B vaccination results in 140 liver transplant recip-ients. Hepatogastroenterology. 1997;44(13):235-8. [PubMed] [Google Scholar]
- 203.Serrano B, Bayas JM, Bruni L, Diez C. Solid organ transplantation and response to vaccination. Vaccine. 2007;25(42):7331-8. DOI: 10.1016/j.vaccine.2007.08.031 [DOI] [PubMed] [Google Scholar]
- 204.No author listed. Prevention of hepatitis A through active or passive im-munization: Recommendations of the Advisory Committee on Immuni-zation Practices (ACIP). MMWR Recomm Rep. 1999;48(Rr-12):1-37. [PubMed] [Google Scholar]
- 205.Vento S, Garofano T, Renzini C, Cainelli F, Casali F, Ghironzi G, et al. . Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med. 1998;338(5):286-90. DOI: 10.1056/nejm199801293380503 [DOI] [PubMed] [Google Scholar]
- 206.Willner IR, Uhl MD, Howard SC, Williams EQ, Riely CA, Waters B. Serious hepatitis A: an analysis of patients hospitalized dur-ing an urban epidemic in the United States. Ann Intern Med. 1998;128(2):111-4. [DOI] [PubMed] [Google Scholar]
- 207.Arslan M, Wiesner RH, Poterucha JJ, Gross JB Jr., Zein NN. Hepa-titis A antibodies in liver transplant recipients: evidence for loss of immunity posttransplantation. Liver Transpl. 2000;6(2):191-5. DOI: 10.1002/lt.500060216 [DOI] [PubMed] [Google Scholar]
- 208.Chin-Hong PV, Kwak EJ. Human papillomavirus in solid organ transplantation. Am J Transplant. 2013;13 Suppl 4:189-200. DOI: 10.1111/ajt.12142 [DOI] [PubMed] [Google Scholar]
- 209.Kumar D, Unger ER, Panicker G, Medvedev P, Wilson L, Humar A. Immunogenicity of quadrivalent human papillomavirus vaccine in organ transplant recipients. Am J Transplant. 2013;13(9):2411-7. DOI: 10.1111/ajt.12329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kwak EJ, Julian K. Human papillomavirus infection in solid organ transplant recipients. Am J Transplant. 2009;9 Suppl 4:S151-60. DOI: 10.1111/j.1600-6143.2009.02906.x [DOI] [PubMed] [Google Scholar]
- 211.Vink P, Delgado Mingorance I, Maximiano Alonso C, Rubio-Viqueira B, Jung KH, Rodriguez Moreno JF, et al. . Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in patients with solid tumors, vaccinated before or during chemotherapy: A randomized trial. Cancer. 2019. DOI: 10.1002/cncr.31909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wang L, Verschuuren EAM, van Leer-Buter CC, Bakker SJL, de Joode AAE, Westra J, et al. . Herpes Zoster and Immunogenicity and Safe-ty of Zoster Vaccines in Transplant Patients: A Narrative Review of the Literature. Front Immunol. 2018;9:1632 DOI: 10.3389/fim-mu.2018.01632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Winston DJ, Mullane KM, Cornely OA, Boeckh MJ, Brown JW, Pergam SA, et al. . Inactivated varicella zoster vaccine in autologous hae-mopoietic stem-cell transplant recipients: an international, multi-centre, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391(10135):2116-27. DOI: 10.1016/s0140-6736(18)30631-7 [DOI] [PubMed] [Google Scholar]
- 214.Kumar D. Immunizations following solid-organ transplantation. Curr Opin Infect Dis. 2014;27(4):329-35. DOI: 10.1097/qco.0000000000000078 [DOI] [PubMed] [Google Scholar]
- 215.Berben L, Denhaerynck K, Schaub S, De Geest S. Prevalence and cor-relates of influenza vaccination among kidney transplant patients. Prog Transplant. 2009;19(4):312-7. [DOI] [PubMed] [Google Scholar]
- 216.Formación en Promoción y Educación para la Salud Informe del Grupo de Trabajo de Promoción de la Salud a la Comisión de Salud Pública del Consejo Interterritorial del Sistema Nacional de Salud. Octubre 2003. [Internet]. 2003.
- 217.Glosario de Promoción de la Salud (OMS) 1998. [Internet]. 1998.
- 218.Marco de competencias del CIE para la enfermera generalista [Internet]. 2003.
- 219.Carta de Ottawa para el Fomento de la Salud Primera Conferencia Internacional sobre Fomento de la Salud, Ottawa. [Internet]. 1986.
- 220.Organización Mundial de la Salud Promoción de la Salud. 9ª Conferencia Mundial de Promoción de la Salud, Shanghai (2016) Available at: http://www.whoint/healthpromotion/conferences/9gchp/es/ 2016. [Google Scholar]
- 221.Nikula A, Puukka P, Leino-Kilpi H. Vaccination competence of grad-uating public health nurse students and nurses. Nurse Educ Today. 2012;32(8):850-6. DOI: 10.1016/j.nedt.2011.10.008 [DOI] [PubMed] [Google Scholar]
- 222.Nikula A, Nohynek H, Puukka P, Leino-Kilpi H. Vaccination com-petence of graduating public health nurse students. Nurse Educ Today. 2011;31(4):361-7. DOI: 10.1016/j.nedt.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 223.Bar-Tal Y, Barnoy S. Factors influencing the decision to comply with nurse recommendations to take or avoid influenza vaccination. Nurs Inq. 2016;23(4):338-45. DOI: 10.1111/nin.12145 [DOI] [PubMed] [Google Scholar]
- 224.Hutchison BG. Effect of computer-generated nurse/physician re-minders on influenza immunization among seniors. Fam Med. 1989;21(6):433-7. [PubMed] [Google Scholar]
- 225.Chan SS, Leung DY, Leung AY, Lam C, Hung I, Chu D, et al. . A nurse-delivered brief health education intervention to improve pneumococcal vaccination rate among older patients with chron-ic diseases: a cluster randomized controlled trial. Int J Nurs Stud. 2015;52(1):317-24. DOI: 10.1016/j.ijnurstu.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Smith JG, Metzger NL. Evaluation of pneumococcal vaccination rates after vaccine protocol changes and nurse education in a ter-tiary care teaching hospital. J Manag Care Pharm. 2011;17(9):701-8. DOI: 10.18553/jmcp.2011.17.9.701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Whelan NW, Steenbeek A, Martin-Misener R, Scott J, Smith B, D’Angelo-Scott H. Engaging parents and schools improves uptake of the human papillomavirus (HPV) vaccine: examining the role of the public health nurse. Vaccine. 2014;32(36):4665-71. DOI: 10.1016/j.vaccine.2014.06.026 [DOI] [PubMed] [Google Scholar]
- 228.Nyamathi A, Liu Y, Marfisee M, Shoptaw S, Gregerson P, Saab S, et al. . Effects of a nurse-managed program on hepatitis A and B vac-cine completion among homeless adults. Nurs Res. 2009;58(1):13-22. DOI: 10.1097/NNR.0b013e3181902b93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Nowalk MP, Tabbarah M, Hart JA, Fox DE, Raymund M, Wilson SA, et al. . Office manager and nurse perspectives on facilitators of adult immunization. Am J Manag Care. 2009;15(10):755-60. [PubMed] [Google Scholar]
- 230.Nowalk MP, Zimmerman RK, Feghali J. Missed opportunities for adult immunization in diverse primary care office settings. Vaccine. 2004;22(25-26):3457-63. DOI: 10.1016/j.vaccine.2004.02.022 [DOI] [PubMed] [Google Scholar]
- 231.Nowalk MP, Bardella IJ, Zimmerman RK, Shen S. The physician’s of-fice: can it influence adult immunization rates ? Am J Manag Care. 2004;10(1):13-9. [PubMed] [Google Scholar]
- 232.Patel AR, Breck AB, Law MR. The impact of pharmacy-based immunization services on the likelihood of immunization in the United States. J Am Pharm Assoc (2003). 2018. DOI: 10.1016/j.japh.2018.05.011 [DOI] [PubMed] [Google Scholar]
- 233.Westrick SC, Patterson BJ, Kader MS, Rashid S, Buck PO, Rothholz MC. National survey of pharmacy-based immunization services. Vaccine. 2018. DOI: 10.1016/j.vaccine.2018.07.027 [DOI] [PubMed] [Google Scholar]
- 234.Lin JL, Bacci JL, Reynolds MJ, Li Y, Firebaugh RG, Odegard PS. Com-parison of two training methods in community pharmacy: Project VACCINATE. J Am Pharm Assoc (2003). 2018;58(4s):S94-S100.e3. DOI: 10.1016/j.japh.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 235.Alsabbagh MW, Church D, Wenger L, Papastergiou J, Raman-Wilms L, Schneider E, et al. . Pharmacy patron perspectives of community pharmacist administered influenza vaccinations. Res Social Adm Pharm. 2018. DOI: 10.1016/j.sapharm.2018.04.015 [DOI] [PubMed] [Google Scholar]
- 236.Hohmeier KC, Randolph DD, Smith CT, Hagemann TM. A mul-timodal approach to improving human papillomavirus vac-cination in a community pharmacy setting. SAGE Open Med. 2016;4:2050312116682128 DOI: 10.1177/2050312116682128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Calo WA, Gilkey MB, Shah P, Marciniak MW, Brewer NT. Parents’ willingness to get human papillomavirus vaccination for their ad-olescent children at a pharmacy. Prev Med. 2017;99:251-6. DOI: 10.1016/j.ypmed.2017.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Pickren E, Crane B. Impact on CDC Guideline Compliance After Incor-porating Pharmacy in a Pneumococcal Vaccination Screening Process. Hosp Pharm. 2016;51(11):894-900. DOI: 10.1310/hpj5111-894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Long AG, Roberts CM, Hayney MS. Pharmacy student involve-ment with increasing human papillomavirus (HPV) vaccination among international college students. J Am Pharm Assoc (2003). 2017;57(1):127-8. DOI: 10.1016/j.japh.2016.11.009 [DOI] [PubMed] [Google Scholar]
- 240.Rhodes LA, Branham AR, Dalton EE, Moose JS, Marciniak MW. Im-plementation of a vaccine screening program at an independent community pharmacy. J Am Pharm Assoc (2003). 2017;57(2):222-8. DOI: 10.1016/j.japh.2016.10.009 [DOI] [PubMed] [Google Scholar]
- 241.Kirkdale CL, Nebout G, Megerlin F, Thornley T. Benefits of phar-macist-led flu vaccination services in community pharmacy. Ann Pharm Fr. 2017;75(1):3-8. DOI: 10.1016/j.pharma.2016.08.005 [DOI] [PubMed] [Google Scholar]
- 242.Chun GJ, Sautter JM, Patterson BJ, McGhan WF. Diffusion of Pharmacy-Based Influenza Vaccination Over Time in the United States. Am J Public Health. 2016;106(6):1099-100. DOI: 10.2105/ajph.2016.303142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Rawson SJ, Conway JH, Hayney MS. Addressing vaccine hesitan-cy in the pharmacy. J Am Pharm Assoc (2003). 2016;56(2):209-10. DOI: 10.1016/j.japh.2016.02.008 [DOI] [PubMed] [Google Scholar]
- 244.Hill JD, Anderegg SV, Couldry RJ. Development of a Pharmacy Technician-Driven Program to Improve Vaccination Rates at an Academic Medical Center. Hosp Pharm. 2017;52(9):617-22. DOI: 10.1177/0018578717722788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Voidazan S, Morariu SH, Tarcea M, Moldovan H, Curticapian I, Do-breanu M. Human Papillomavirus (HPV) Infection and HPV Vacci-nation: Assessing the Level of Knowledge among Students of the University of Medicine and Pharmacy of Tirgu Mures, Romania. Acta Dermatovenerol Croat. 2016;24(3):193-202. [PubMed] [Google Scholar]
- 246.Anderson C, Thornley T. Who uses pharmacy for flu vaccinations ? Population profiling through a UK pharmacy chain. Int J Clin Pharm. 2016;38(2):218-22. DOI: 10.1007/s11096-016-0255-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Maharajan MK, Rajiah K, Sze Fang KN, Lui LY. Cervical Cancer Prevention in Malaysia: Knowledge and Attitude of Undergrad-uate Pharmacy Students Towards Human Papillomavirus In-fection, Screening and Vaccination in Malaysia. J Cancer Educ. 2017;32(1):166-74. DOI: 10.1007/s13187-015-0957-2 [DOI] [PubMed] [Google Scholar]
- 248.Poulose S, Cheriyan E, Cheriyan R, Weeratunga D, Adham M. Phar-macist-administered influenza vaccine in a community pharmacy: A patient experience survey. Can Pharm J (Ott). 2015;148(2):64-7. DOI: 10.1177/1715163515569344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Comboroure JC, Mueller JE. [Perception of vaccination and role of the pharmacist: A survey among final year pharmacy students in France]. Ann Pharm Fr. 2014;72(2):122-31. DOI: 10.1016/j.phar-ma.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 250.Hook S, Windle J. Community pharmacy influenza immunisation increases vaccine uptake and gains public approval. Aust N Z J Pub-lic Health. 2013;37(5):489-90. DOI: 10.1111/1753-6405.12109 [DOI] [PubMed] [Google Scholar]
- 251.Banh HL. Alberta pharmacy students administer vaccinations in the University Annual Influenza Campaign. Can Pharm J (Ott). 2012;145(3):112-5. DOI: 10.3821/145.3.cpj112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Al-lela OQ, Bahari MB, Elkalmi RM, Jawad Awadh AI. Incorporating an immunization course in the pharmacy curriculum: Malaysian experience. Am J Pharm Educ. 2012;76(10):206 DOI: 10.5688/ajpe7610206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Olier E. Una estrategia vacunal para España. Notas estratégicas del Instituto Choiseul ISSN 2444-4006. 2017.
- 254.Choiseul I. El impacto económico de las vacunas. Madrid: Instituto Choiseul ISSN 2444-4006; 2017. [Google Scholar]
- 255.Stack ML, Ozawa S, Bishai DM, Mirelman A, Tam Y, Niessen L, et al. . Estimated Economic Benefits During The ‘Decade Of Vaccines’ Include Treatment Savings, Gains In Labor Productivity. Health Aff (Millwood). 2011;30(6):1021–8. [DOI] [PubMed] [Google Scholar]
- 256.Davies PD. Vaccine given bad press. Bmj. 1993;307(6902):506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Freed GL, Katz SL, Clark SJ. Safety of vaccinations. Miss America, the media, and public health. Jama. 1996;276(23):1869-72. [DOI] [PubMed] [Google Scholar]
- 258.Cohn V. Vaccines and risks. The responsibility of the media, scien-tists, and clinicians. Jama. 1996;276(23):1917-8. [DOI] [PubMed] [Google Scholar]
- 259.Eavey RD. Vaccine safety, media reporting, and Miss America. Jama. 1997;278(4):290-1. [PubMed] [Google Scholar]
- 260.Anderson P. Another media scare about MMR vaccine hits Britain. Bmj. 1999;318(7198):1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Danovaro-Holliday MC, Wood AL, LeBaron CW. Rotavirus vaccine and the news media, 1987-2001. Jama. 2002;287(11):1455-62. [DOI] [PubMed] [Google Scholar]
- 262.Dobson R. Media misled the public over the MMR vaccine, study says. Bmj. 2003;326(7399):1107 DOI: 10.1136/bmj.326.7399.1107-a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Ma KK, Schaffner W, Colmenares C, Howser J, Jones J, Poehling KA. Influenza vaccinations of young children increased with media coverage in 2003. Pediatrics. 2006;117(2):e157-63. DOI: 10.1542/peds.2005-1079 [DOI] [PubMed] [Google Scholar]
- 264.Smith MJ, Ellenberg SS, Bell LM, Rubin DM. Media coverage of the measles-mumps-rubella vaccine and autism controversy and its relationship to MMR immunization rates in the United States. Pediatrics. 2008;121(4):e836-43. DOI: 10.1542/peds.2007-1760 [DOI] [PubMed] [Google Scholar]
- 265.Roehr B. Media induced anti-vaccination sentiment can even affect health workers, vaccine researcher says. Bmj. 2012;344:e1563 DOI: 10.1136/bmj.e1563 [DOI] [PubMed] [Google Scholar]
- 266.Glanz JM, Wagner NM, Narwaney KJ, Kraus CR, Shoup JA, Xu S, et al. . Web-based Social Media Intervention to Increase Vaccine Ac-ceptance: A Randomized Controlled Trial. Pediatrics. 2017;140(6). DOI: 10.1542/peds.2017-1117 [DOI] [PMC free article] [PubMed] [Google Scholar]