ABSTRACT
Purpose
Guillain-Barré Syndrome (GBS) as a consequence of influenza vaccination is a relevant topic, yet to be clarified, which raises concern both amongst health care personnel and the general population. Every study and pharmacovigilance system point to need of further research and the importance of continuous monitoring of safety regarding influenza vaccines. The aim of the present study is to investigate the publication of new data since the realisation of our meta-analysis of GBS and influenza vaccines (published in 2015).
Methods
A systematic revision of PubMed, Embase, and Web of Knowledge (WOS) databases has been carried out. These report observational studies assessing GBS risk after the administration of influenza vaccines from May 2014 up to July 20th, 2017.
Results
The research yielded 107 articles. Only three studies met established inclusion criteria and referred to an estimation GBS risk after some influenza vaccine. Two studies investigated GBS risk by the pandemic A/H1N1 vaccine, while only one looked into season vaccines.
Conclusions
The present systematic review, conducted after the publication of our previous meta-analysis, seems to confirm its previous results. Therefore, GBS should be considered an infrequent adverse effect of influenza vaccination, which should not negatively influence its acceptance. Unfortunately, very few of the systematically surveyed studies meeting inclusion criteria. This fact sharply contrasts with the current consensus as to the need of continuously monitoring the safety of influenza vaccines.
Keywords: A/H1N1 vaccine, Guillain-Barré syndrome, influenza vaccines
RESUMEN
Introducción
El síndrome de Guillain-Barré (GBS) después de la administración de la vacuna frente a la gripe es un tema actual que sigue causando preocupación tanto en el personal sanitario como en la población y que permanece sin esclarecer. El objetivo del presente trabajo es investigar la publicación de nuevos datos desde la realización de nuestro metaanálisis sobre el GBS y las vacunas frente a la gripe (publicado en 2015).
Métodos
Se ha realizado una revisión sistemática en las bases de datos PubMed, Embase y Web of Science (WOS) de estudios observacionales que evaluarán el riesgo de GBS después de la administración de vacunas influenza, desde mayo de 2014 hasta el 20 de julio de 2017.
Resultados
El resultado de las búsquedas fue de 107 artículos. Finalmente, solo 3 estudios cumplían con los criterios de inclusión establecidos y referían una estimación del riesgo de GBS después de alguna de las vacunas antigripales. Dos estudios investigaron el riesgo de GBS con la vacuna pandémica A/ H1N1 y un estudio investigó las vacunas estacionales.
Conclusiones
Esta revisión sistemática parece confirmar los hallazgos obtenidos en nuestro metaanálisis. El SGB se podría considerar como un posible efecto adverso poco frecuente de las vacuna antigripales, lo cual no debería afectar negativamente en su aceptación. Desafortunadamente, en nuestra revisión sistemática, hemos encontrado muy pocos estudios que cumplieran los criterios de inclusión, este hecho resulta llamativo ya que el consenso actual señala la necesidad de una monitorización continua sobre la seguridad de las vacunas antigripales.
Palabras clave: vacuna A/H1N1, Síndrome de Guillain-Barré, gripe
INTRODUCTION
Guillain-Barré syndrome (GBS) consists of an acute demyelinating neuropathy involving the peripheral nervous system. It causes weakness, paralysis, and, in some cases, leads to death [1-3]. GBS is regarded to be a rare autoimmune disease, in which the body is attacked by its own immune system [3-5].
GBS incidence ranges from 0.8 to 1.9 per 100,000 persons/ year, being more frequent among males, and the incidence increase with age [1, 2, 6, 7]. So far, the precise causes that trigger the disease are not well known. It has been reported that GBS is preceded by an infection of the gastrointestinal or respiratory tract in 2/3 of cases. It has been also linked to some viral infections, and even to influenza vaccination [5, 8-11].
The association of GBS with influenza vaccination was first reported in 1976, when the seasonal vaccination campaign was stopped in the United States due to an excess of GBS cases (relative risk [RR]: 7-8) [12]. However, few studies addressing the potential relationship of GBS to influenza vaccination were published between 1976 and 2009. Since the pandemic outbreak of influenza A in 2009, the vaccine A/ H1N1/2009 were rapidly developed, manufactured and commercialised, and surveillance systems were reinforced, adapted or set up with the aim of identifying as early as possible any incidence excess of GBS, notably in the United States, wherein an increased risk of GBS associated to influenza vaccine was found [13, 14].
While isolated studies on vaccination campaigns and active and passive notification of GBS cases by surveillance systems have been conducted; so far, little research has been devoted to synthesising the results from epidemiological studies [14, 15]. In 2015, we carried out a meta-analysis with the aim of studying the possible relationship between GBS and influenza vaccination. Now, in this article, we present the results of a systematic review of the literature, whose objective was to analyze the new data that has been appearing since the publication of our meta-analysis [16].
METHODS
We reviewed the databases PubMed, EMBASE, and Web of Knowledge (WoS) covering the period 1-May-2014 / 20-July-2017. We used the same search terms and study selection criteria as in our prior meta-analysis [16]. The search was conducted by combining the terms Influenza vaccine* AND Guillain-Barré syndrome, with no restrictions. Criteria for the inclusion of studies were the following: (a) observational studies evaluating the risk of GBS associated with any of the influenza vaccines, and (b) studies reporting risk measures expressed as relative risk (RR), odds ratio (OR), relative incidence (RI), or incidence rate ratio (IRR); though, in the present review, we included a new risk measure as well, namely the hazard ratio (HR). In all cases, we considered the respective 95% confidence intervals (95% CI).
RESULTS
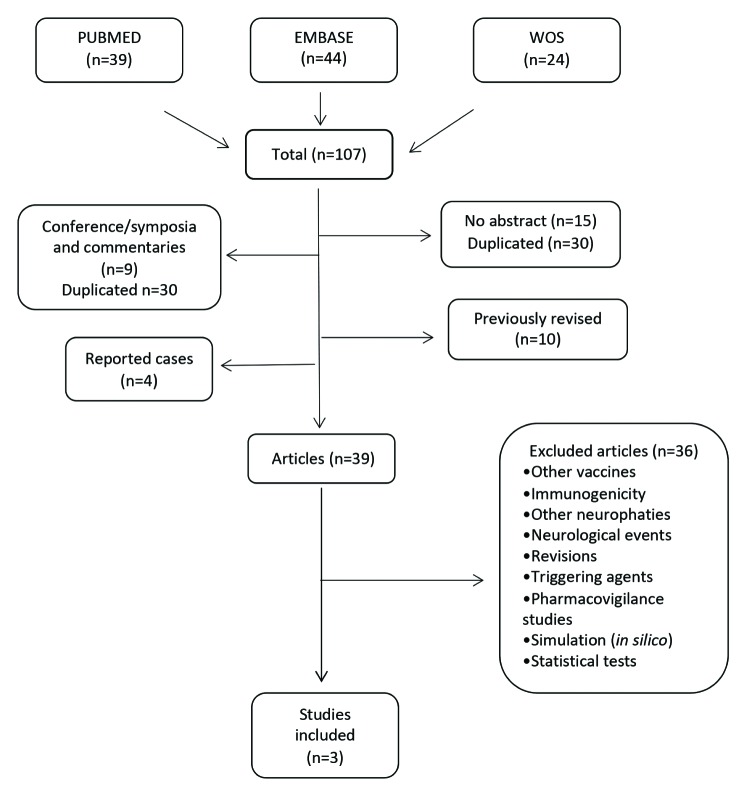
The bibliographic review enabled us to identify 107 studies (figure 1): PubMed (n=39), EMBASE (n=44), and WoS (n=24). First, we eliminated the articles lacking an abstract (n=15), those containing lectures and oral comments (n=7), those notifying isolated cases (n=3), duplicated articles (n=30), and articles previously reviewed or included in our earlier meta-analysis (n=10) [16]. Then, we read the article abstracts and/or the whole texts of the potentially eligible studies. Some of the exclusion criteria were the following: a) studies based on the results from surveillance system notifications of serious and non-serious adverse effects supposedly related to any of the influenza vaccines that did not report any estimates of GBS risk following influenza vaccination [17-22], b) pharmacovigilance studies on GBS that included any types of paediatric immunisation [23], c) studies addressing the potential triggering agents for GBS [24], c) studies based on risk simulation of GBS and other autoimmune diseases associated with either infections or influenza vaccination [25, 26], d) studies whose design or analysis of data on vaccine safety was considered not to be suitable [27, 28, 29], and e) studies focusing on other influenza vaccination adverse effects, such as polyneuropathies, neurological events, Zika virus or surgery
Figure 1.
Flow diagram showing identification of studies meeting inclusion criteria.
Only 3 studies met all the eligibility criteria (table 1), as follows: Kim et al.; 2015 [30], in which an increased risk of GBS was observed in South Korea following the administration of the adjuvanted and non-adjuvanted pandemic vaccine A/ H1N1, with the risk expressed as a rate ratio (RR=1.46, 95% CI: 1.26-1.68); Ghaderi et al.; 2015 [31], in which the authors found an increased risk of GBS in Norway within 42 days after the administration of the pandemic vaccine A/H1N1 (Pandemrix®), with the risk expressed as a hazard ratio (HR= 1.1, 95% CI: 0.51-2.43); and Sandhu et al.; 2017 [7], which focused on the outcomes for four influenza vaccination campaigns (from 2010/11 to 2013/14). In this latter study, the authors reported a statistically non-significant increased risk of GBS (RR=1.25, 95% CI: 0.96-1.63) for the season 2010/1011 among a Medicare population (USA); with the risk being much lower than that observed in the season 2009/10 (RR=1,98, 95% CI: 1.42- 2.76), while an increased risk was not found in the remaining three vaccination campaigns (i. e. from 2011/12 to 2013/14).
Table 1.
Characteristics of selected observational studies.
| Authors/Year | Study location | GBS cases | Design | Influenza vaccine | Risk |
|---|---|---|---|---|---|
| Ghaderi et al. 2016 | Norwey | 410 | Cohort | A(H1N1) 2009 | HR=1.11 (95% CI 0.51-2.43) |
| Kim et al. 2016 | Korea | 245 | Cohort | A(H1N1) 2009 | RR=1.46 (95% CI: 1.26-1.68) |
| Sandhu et al. 2017 | USA | SCRI | 2010/11 seasonal | RR=1.25 (95% CI: 0.96-1.63) |
SCRI: Self-controlled risk interval (SCRI) design; HR: Hazard ratio; RR=risk relative.
DISCUSSION
Our systematic review focused on the new data about influenza vaccination and its potential association with GBS published after the publication of our prior meta-analysis [16]. The review of the published articles enabled us to identify only three epidemiological studies that fulfilled the eligibility criteria [7, 30, 31]. It is striking the shortage of published studies estimating the magnitude of the GBS risk linked to or following the administration of the influenza vaccines when taking into account that the relevant health authorities have been emphasizing the necessity for continuously monitoring the potential adverse effects of influenza vaccination [21, 28, 29, 32] .Furthermore, it is well known the importance of having post-commercialisation studies on the influenza vaccines [17-20, 22, 33], as well as near real-time pharmacovigilance studies aimed at rapidly identifying any risk excess of GBS following influenza vaccination [7, 13]. Despite these studies have the disadvantage that it is difficult to quantify any causal associations; they present the advantage of enabling us to rapidly detect the signals for potential adverse events following immunization (AEFI). In addition, such studies may constitute the starting basis for further investigation on potential causal associations. Therefore, it should be emphasised the importance of conducting and reporting multicentre, collaborative, epidemiological studies with prolonged follow-up periods aimed to identifying and quantifying potential unexpected or rare adverse effects (e. g. GBS) following influenza vaccination [7, 15, 34, 35].
Concerning the risk reported in the observational studies selected for our systematic review (table 1) and our previously published meta-analysis (table 2), it should be pointed out that, in one of the studies selected for our review, Kim et al. [30] concluded that the pandemic vaccine (pH1N1) was associated with an increased risk of GBS expressed as a relative risk of 1.46; (95% CI, 1.26-1.68). This finding is in keeping with the results from two meta-analyses [15, 16], which reported a GBS risk estimate expressed as a relative incidence of 2.09 (95% CI: 1.28-3.42) and a relative risk of 1.84 (95% CI: 1.36- 2.50), respectively. The finding by Kim et al. is also in line with the results from other individual studies [36-40]. Another meta-analysis [14] reported a statistically non-significant increased risk of GBS for the vaccine H1N1 2009 expressed as an incidence rate ratio of 2.35 (95% CI: 1.42-4.01), which concurs with the results from several other individual studies [33, 41]. The second study identified by our systematic review was that by Ghaderi et al. [31], who reported that the pandemic vaccine (pH1N1) was not associated with an increased risk of GBS. This finding is in keeping with the results from some previous studies [34, 42]. Finally, the third study we selected, that is, that by Sandhu et al. [7] , focused on the outcomes of four influenza vaccination campaigns. The authors reported a statistically non-significant increased risk expressed as a relative risk (RR=1.25, 95% CI: 0.96-1.63) in the season 2010/11. However, they failed to observe any risk excess in the remaining three seasons. In this latter study, the authors found an increased risk of GBS (RR=1.98 95% CI: 1.42-2.76) for the season 2009/10. In contrast, an investigation based on both simulation models and previously published risk estimates [26] supported the hypothesis posed in an earlier study that influenza immunisation was protective against GBS, and, therefore, resulted in a decreased risk [43] . At any rate, it should be borne in mind that the differences in the risk magnitudes reported in each study are small and they only approximated to the value with statistical significance by either excess or defect. On the other hand, the coverage of influenza vaccination programmes is broader every year, and this is not positively correlated with the number of hospitalisations for GBS [44]. In addition, it is worth reminding that the financial burden associated with the complications derived from the infections caused by the influenza virus largely exceeds the financial costs of influenza vaccination [45].
Table 2.
Characteristics of previous published meta-analysis.
| Authors/Year | Study location | Design | Influenza vaccine | Risk |
|---|---|---|---|---|
| Salmon et al. 2013 | USA | Meta-analysis | A (H1N1) 2009 | IRR=2.35 (95% CI 1.2-4.01) |
| Dodd et al. 2013 | International * | Meta-analysis | A (H1N1) 2009 | RI= 2.09 (95%CI 1.28-3.42) |
| Martín Arias et al. 2015 | International | Meta-analysis | A(H1N1) 2009 Seasonal |
RR= 1.84 (95%CI 1.36-2.50) RR= 1.22 (95%CI 1.01-1.48) |
Australia, Canada, China, Denmark, Finland, The Netherlands, Singapore, Spain, The United Kingdom and The United States Databases.
IRR: Incidence rate ratio. RI: relative incidence. RR: relative risk.
Likewise, other suspected triggering agents of GBS, apart from influenza vaccination, should be taken into consideration. The current evidence indicates that previous infections are likely to play an important role in the development of GBS, notably the infections involving the upper respiratory tract or the gastrointestinal tract, those caused by the influenza virus and the so-called influenza-like infections (ILI) [5, 8-11, 46-47]. One of the studies selected for our systematic review supported the hypothesis that the influenza infection is a potential triggering agent among the Norwegian population. These authors found a post-influenza infection risk expressed as a hazard ratio (HR) of 4.89 (95% CI: 1.17-20.36), this risk magnitude being much higher than that observed after influenza vaccination (HR=1.11 95%CI: 0.51-2.43) [31]. Another study we selected for our review reported that in 82.5% of GBS cases there had been a previous respiratory or gastrointestinal infection [30]. This finding is in agreement with the results from a study reporting a strong association of GBS with either previous respiratory or gastrointestinal infections or previous unspecific viral infections (odds ratio=7.73, 95% CI: 3.60-16.61) [46]. The results from other studies also support the aforementioned hypothesis, since the authors noted an important increment in the risk of GBS following a previous infection. Thus, Tam et al. reported an odds ratio (OR) of 18.6, (95% CI: 7.5-46.4), whilst Stowe et al. spoke of a relative risk (RR) of 7.35, (95% CI: 4.36-12.38) within the first 60 and 90 days of an influenza-like illness (ILI), respectively [8, 10].
Another potential triggering factor for GBS reported in the studies we have reviewed is surgery. According to the results of a study conducted in Finland, the relative risk of developing GBS within the first 6 weeks after surgery is 13.1-fold higher (95% CI: 5.68-30.3, P ≤ 0.0001) [24]. This finding concurs with the results of other studies [48, 49]. Also, it has been reported an increase in the notified GBS cases related to the ZiKa virus infection [50].
With regard to the type of study, in our systematic review we found that some of the published studies were based on the notifications of supposed adverse reactions related to the different influenza vaccines, such as the notifications by the Vaccine Adverse Reporting System (VAERS). However, it should be kept in mind that, while these notifications are very useful for the quick detection of safety concerns, they are not of value for establishing potential cause-effect relationships.
Epidemiological studies with self-controlled design, like self-controlled case series, self-controlled risk-interval, case-centred and case-population studies, represent the most suitable approach in the field of observational studies, since, in this type of investigation, each case acts as its own control. In addition, these studies are adjusted for all confounding factors that may vary with time. In our earlier meta-analysis, 24 of the 39 weighted studies used the self-controlled design [16]. Nevertheless, few studies have reported the risk estimates adjusted for either seasonality or subjects’ previous infections [15, 34]. In the three studies selected for the present systematic review, the authors referred to seasonality as an important factor. In one of these studies, a larger number of cases was found in November, January and February [30], whereas another study showed a significant increased risk of GBS during the pandemic period compared to other time intervals [31].
The studies selected for our systematic review (i.e. 3/107) estimated the risk of GBS following influenza vaccination, showing a very small risk excess magnitude when taking into account the financial and health benefits obtained from immunisation. Indeed, the risk excess is very small; however, other potential explanatory factors should be considered, such as the low incidence rate of GBS, the temporal coincidence with the periods with the largest circulation of the influenza virus, the occurrence of respiratory and gastrointestinal infections, or the administration of the vaccine for either seasonal (October-November) or pandemic influenza. All the above factors may make it difficult to interpret the results from the epidemiological studies on the potential relationship between influenza vaccination and GBS.
In relation to the differences in the estimates of the risk as reported in the different studies, they can be combined or reconciled, because post-vaccination GBS is a rare condition [16]. According to two studies, the risk estimates obtained with the case-population approach (CPA) are consistent with the odds ratios in the case-control studies, and discrepancies were observed only with the vaccine A (H1N1) in Sweden and UK [27, 28]. In a review of the statistical methods used in vaccine surveillance studies, the authors indicated that up to 37 different methods can be used depending on the kind of analysis [29].
The studies selected for our systematic review reported that GBS was more prevalent among males and elderly people, which is in agreement with the results from earlier investigation [1-2, 14]. Thus, in 1983 a study was published, whose authors stated that GBS was more frequent in males, the incidence increased with age, and incidence rates were more heightened among white race individuals [51].
Another issue of concern for particularly sensitive populations is influenza vaccine safety for pregnant women and children. Further investigation is needed to provide information regarding influenza vaccination during pregnancy. This issue was addressed by only three of the studies we found in our initial search [22, 52, 53]. Thus, in a large cohort of pregnant women to whom the vaccine A/H1N1 was given, the authors did not find any cases of GBS within the first 42 days after vaccination [52]. Nevertheless, it was notified a case of GBS that occurred 24 days after the administration of a trivalent influenza vaccine (TIV) to a pregnant woman during the 2013/14 campaign [53]. The pharmacovigilance system deployed in Latin America and the Caribbean to monitor the potential adverse effects of the vaccine A/H1N1 reported increased adverse effect rates among pregnant women as compared to those among healthcare personnel and patients with chronic diseases [22], which might be explained by differences in the influenza virus strains that circulate in both hemispheres.
Few studies initially screened for our systematic review addressed the potential relationship between influenza vaccination and GBS in paediatric populations [47, 54-56]. A prospective epidemiological study conducted in the UK based on the UK pharmacovigilance system (British Paediatric Surveillance Unit System) [47] showed that GBS and “Miller Fisher syndrome” associated with the pandemic influenza vaccine were more prevalent among boys compared to girls. In addition, this study revealed that most of the affected children had suffered a previous infection, and that the cases associated with the pandemic vaccine were more frequent than those associated with the seasonal vaccine, though this difference did not reach statistical significance. This finding is in keeping with the results of another study [7], and coincides with those of our previous meta-analysis [16]. In a safety study in children aged 6-12 months conducted in Taiwan, the authors failed to find serious adverse events within the first 7 days after the administration of a killed TIV during the 2010/11 campaign [54]. In a USA pharmacovigilance study with the live quadrivalent influenza vaccine (LAIV4), it was found that the most frequently notified non-fatal serious adverse reactions were those involving the nervous and respiratory systems. Neurological adverse reactions were more frequent in children than among adults, and GBS was the second most frequently notified neurological event [56]. This children’s susceptibility may be related either to genetic heredity or to the type of vaccine, since live vaccines have not been sufficiently investigated in children aged under 2 years, and the established safety of a given influenza vaccine cannot be extrapolated to the remainder of vaccines [55].
The advent of inmunogenomics, proteomics, genetic engineering, biostatistics, and computational studies may help to find biomarkers that allow us to identify the differences in both individual and group pathophysiologic mechanisms. In turn, these differences might explain why certain individual or groups present a greater susceptibility to autoimmune diseases following vaccination. Some genetic polymorphisms (e. g. HLA-DQB1*) are known to render carriers more susceptible to develop GBS [57, 58], while computational simulation studies (in silico) have identified some genes involved in 4 types of autoimmune diseases, including GBS, a disease in which as many as 73 genes may be implicated [25]. Nonetheless, a meta-analysis based on Asian and Caucasian populations did not find any relationships between alleles and risk of GBS [59].
The present systematic review we conducted after the publication of our previous meta-analysis seems to confirm the results of such a meta-analysis. Therefore, GBS should be considered an infrequent adverse effect of influenza vaccination, which should not negatively influence the vaccination acceptance. However, unfortunately, very few of the studies meeting inclusion criteria that we found in our systematic review presented sufficient quality. This fact sharply contrasts with the current consensus as to the need of continuously monitoring the safety of influenza vaccines. Therefore, one would expect to find a larger number of competent studies that allow us to detect near real-time signals. However, today it is not easy to find such studies in the medical literature.
FUNDING
None to declare
CONFLICT OF INTEREST
The authors declare that they have not conflict of interest.
References
- 1.Sejvar JJ, Baughman AL, Wise M, Morgan OW. Population incidence of Guillain-Barre syndrome: a systematic review and meta-analysis. Neuroepidemiology. 2011;36(2):123-33. DOI: 10.1159/000324710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shui IM, Rett MD, Weintraub E, Marcy M, Amato AA, Sheikh SI, et al. . Guillain-Barre syndrome incidence in a large United States cohort (2000-2009). Neuroepidemiology. 2012;39(2):109-15. DOI: 10.1159/000339248 [DOI] [PubMed] [Google Scholar]
- 3.Yuki N, Hartung HP. Guillain-Barre syndrome. N Engl J Med. 2012;366(24):2294-304. DOI: 10.1056/NEJMra1114525 [DOI] [PubMed] [Google Scholar]
- 4.Hardy TA, Blum S, McCombe PA, Reddel SW. Guillain-barre syndrome: modern theories of etiology. Curr Allergy Asthma Rep. 2011;11(3):197-204. DOI: 10.1007/s11882-011-0190-y [DOI] [PubMed] [Google Scholar]
- 5.Vellozzi C, Iqbal S, Broder K. Guillain-Barre syndrome, influenza, and influenza vaccination: the epidemiologic evidence. Clin Infect Dis. 2014;58(8):1149-55. DOI: 10.1093/cid/ciu005 [DOI] [PubMed] [Google Scholar]
- 6.Benedetti MD, Pugliatti M, D’Alessandro R, Beghi E, Chio A, Logroscino G, et al. . A Multicentric Prospective Incidence Study of Guillain-Barre Syndrome in Italy. The ITANG Study. Neuroepidemiology. 2015;45(2):90-9. DOI: 10.1159/000438752 [DOI] [PubMed] [Google Scholar]
- 7.Sandhu SK, Hua W, MaCurdy TE, Franks RL, Avagyan A, Kelman J, et al. . Near real-time surveillance for Guillain-Barre syndrome after influenza vaccination among the Medicare population, 2010/11 to 2013/14. Vaccine. 2017;35(22):2986-92. DOI: 10.1016/j.vaccine.2017.03.087 [DOI] [PubMed] [Google Scholar]
- 8.Tam CC, O’Brien SJ, Rodrigues LC. Influenza, Campylobacter and Mycoplasma infections, and hospital admissions for Guillain-Barre syndrome, England. Emerg Infect Dis. 2006;12(12):1880-7. DOI: 10.3201/eid1212.051032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tam CC, O’Brien SJ, Petersen I, Islam A, Hayward A, Rodrigues LC. Guillain-Barre syndrome and preceding infection with campylobacter, influenza and Epstein-Barr virus in the general practice research database. PLoS One. 2007;2(4):e344 DOI: 10.1371/journal.pone.0000344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stowe J, Andrews N, Wise L, Miller E. Investigation of the temporal association of Guillain-Barre syndrome with influenza vaccine and influenzalike illness using the United Kingdom General Practice Research Database. Am J Epidemiol. 2009;169(3):382-8. DOI: 10.1093/aje/kwn310 [DOI] [PubMed] [Google Scholar]
- 11.Lehmann HC, Hartung HP, Kieseier BC, Hughes RA. Guillain-Barre syndrome after exposure to influenza virus. Lancet Infect Dis. 2010;10(9):643-51. DOI: 10.1016/S1473-3099(10)70140-7 [DOI] [PubMed] [Google Scholar]
- 12.Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, Keenlyside RA, Ziegler DW, Retailliau HF, et al. . Guillain-Barre syndrome following vaccination in the National Influenza Immunization Program, United States, 1976--1977. Am J Epidemiol. 1979;110(2):105-23. PMid: [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease C, Prevention. Preliminary results: surveillance for Guillain-Barre syndrome after receipt of influenza A (H1N1) 2009 monovalent vaccine-United States, 2009-2010. MMWR Morb Mortal Wkly Rep. 2010;59(21):657-61. PMid: [PubMed] [Google Scholar]
- 14.Salmon DA, Proschan M, Forshee R, Gargiullo P, Bleser W, Burwen DR, et al. . Association between Guillain-Barre syndrome and influenza A (H1N1) 2009 monovalent inactivated vaccines in the USA: a meta-analysis. Lancet. 2013;381(9876):1461-8. DOI: 10.1016/S0140-6736(12)62189-8 [DOI] [PubMed] [Google Scholar]
- 15.Dodd CN, Romio SA, Black S, Vellozzi C, Andrews N, Sturkenboom M, et al. . International collaboration to assess the risk of Guillain Barre Syndrome following Influenza A (H1N1) 2009 monovalent vaccines. Vaccine. 2013;31(40):4448-58. DOI: 10.1016/j.vaccine.2013.06.032 [DOI] [PubMed] [Google Scholar]
- 16.Martin Arias LH, Sanz R, Sainz M, Treceno C, Carvajal A. Guil-lain-Barre syndrome and influenza vaccines: A meta-analysis. Vaccine. 2015;33(31):3773-8. DOI: 10.1016/j.vaccine.2015.05.013 [DOI] [PubMed] [Google Scholar]
- 17.Haber P, Moro PL, McNeil MM, Lewis P, Woo EJ, Hughes H, et al. . Post-licensure surveillance of trivalent live attenuated influenza vaccine in adults, United States, Vaccine Adverse Event Reporting System (VAERS), July 2005-June 2013. Vaccine. 2014;32(48):6499-504. DOI: 10.1016/j.vaccine.2014.09.018 [DOI] [PubMed] [Google Scholar]
- 18.Moro PL, Winiecki S, Lewis P, Shimabukuro TT, Cano M. Surveillance of adverse events after the first trivalent inactivated influenza vaccine produced in mammalian cell culture (Flucelvax((R))) reported to the Vaccine Adverse Event Reporting System (VAERS), United States, 2013-2015. Vaccine. 2015;33(48):6684-8. DOI: 10.1016/j.vaccine.2015.10.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haber P, Moro PL, Cano M, Lewis P, Stewart B, Shimabukuro TT. Post-licensure surveillance of quadrivalent live attenuated influenza vaccine United States, Vaccine Adverse Event Reporting System (VAERS), July 2013-June 2014. Vaccine. 2015;33(16):1987-92. DOI: 10.1016/j.vaccine.2015.01.080 [DOI] [PubMed] [Google Scholar]
- 20.Haber P, Moro PL, Lewis P, Woo EJ, Jankosky C, Cano M. Post-licensure surveillance of quadrivalent inactivated influenza (IIV4) vaccine in the United States, Vaccine Adverse Event Reporting System (VAERS), July 1, 2013-May 31, 2015. Vaccine. 2016;34(22):2507-12. DOI: 10.1016/j.vaccine.2016.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayet A, Duron S, Meynard JB, Koeck JL, Deparis X, Migliani R. Surveillance of adverse events following vaccination in the French armed forces, 2011-2012. Public Health. 2015;129(6):763-8. DOI: 10.1016/j.puhe.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 22.Ropero-Alvarez AM, Whittembury A, Bravo-Alcantara P, Kurtis HJ, Danovaro-Holliday MC, Velandia-Gonzalez M. Events supposedly attributable to vaccination or immunization during pandemic influenza A (H1N1) vaccination campaigns in Latin America and the Caribbean. Vaccine. 2015;33(1):187-92. DOI: 10.1016/j.vaccine.2014.10.070 [DOI] [PubMed] [Google Scholar]
- 23.Top KA, Desai S, Moore D, Law BJ, Vaudry W, Halperin SA, et al. . Guillain-BarrE Syndrome After Immunization in Canadian Children (1996-2012). Pediatr Infect Dis J. 2015;34(12):1411-3. DOI: 10.1097/INF.0000000000000903 [DOI] [PubMed] [Google Scholar]
- 24.Sipila JO, Soilu-Hanninen M. The incidence and triggers of adult-onset Guillain-Barre syndrome in southwestern Finland 2004-2013. Eur J Neurol. 2015;22(2):292-8. DOI: 10.1111/ene.12565 [DOI] [PubMed] [Google Scholar]
- 25.McGarvey PB, Suzek BE, Baraniuk JN, Rao S, Conkright B, Lababidi S, et al. . In silico analysis of autoimmune diseases and genetic relationships to vaccination against infectious diseases. BMC Immunol. 2014;15:61 DOI: 10.1186/s12865-014-0061-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hawken S, Kwong JC, Deeks SL, Crowcroft NS, McGeer AJ, Ducharme R, et al. . Simulation study of the effect of influenza and influenza vaccination on risk of acquiring Guillain-Barre syndrome. Emerg Infect Dis. 2015;21(2):224-31. DOI: 10.3201/eid2102.131879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Théophile H, Moore N, Bégaud B, Pariente A. Application of the case-population approach to vaccine safety surveillance. Drug Saf. 2015;38(10):1014 DOI: 10.1007/s40264-015-0346-0 [DOI] [Google Scholar]
- 28.Théophile H, Pariente A, Moore N, Bégaud B. Is the case-population approach useful for vaccine safety surveillance? Pharmacoepidemiol and Drug Saf. 2015;24(S1):187 DOI: 10.1002/pds.3838 [DOI] [Google Scholar]
- 29.Leite A, Andrews NJ, Thomas SL. Near real-time vaccine safety surveillance using electronic health records-a systematic review of the application of statistical methods. Pharmacoepidemiol Drug Saf. 2016;25(3):225-37. DOI: 10.1002/pds.3966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim C, Rhie S, Suh M, Kang DR, Choi YJ, Bae GR, et al. . Pandemic influenza A vaccination and incidence of Guillain-Barre syndrome in Korea. Vaccine. 2015;33(15):1815-23. DOI: 10.1016/j.vaccine.2015.02.035 [DOI] [PubMed] [Google Scholar]
- 31.Ghaderi S, Gunnes N, Bakken IJ, Magnus P, Trogstad L, Haberg SE. Risk of Guillain-Barre syndrome after exposure to pandemic influenza A(H1N1)pdm09 vaccination or infection: a Norwegian population-based cohort study. Eur J Epidemiol. 2016;31(1):67-72. DOI: 10.1007/s10654-015-0047-0 [DOI] [PubMed] [Google Scholar]
- 32.Santuccio C, Trotta F, Felicetti P. Ongoing pharmacovigilance on vaccines. Pharmacol Res. 2015;92:2-5. DOI: 10.1016/j.phrs.2014.10.011 [DOI] [PubMed] [Google Scholar]
- 33.Yih WK, Lee GM, Lieu TA, Ball R, Kulldorff M, Rett M, et al. . Surveillance for adverse events following receipt of pandemic 2009 H1N1 vaccine in the Post-Licensure Rapid Immunization Safety Monitoring (PRISM) System, 2009-2010. Am J Epidemiol. 2012;175(11):1120-8. DOI: 10.1093/aje/kws197 [DOI] [PubMed] [Google Scholar]
- 34.Dieleman J, Romio S, Johansen K, Weibel D, Bonhoeffer J, Sturkenboom M, et al. . Guillain-Barre syndrome and adjuvanted pandemic influenza A (H1N1) 2009 vaccine: multinational case-control study in Europe. BMJ. 2011;343:d3908 DOI: 10.1136/bmj.d3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romio S, Weibel D, Dieleman JP, Olberg HK, de Vries CS, Sammon C, et al. . Guillain-Barre syndrome and adjuvanted pandemic influenza A (H1N1) 2009 vaccines: a multinational self-controlled case series in Europe. PLoS One. 2014;9(1):e82222 DOI: 10.1371/journal.pone.0082222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greene SK, Rett M, Weintraub ES, Li L, Yin R, Amato AA, et al. . Risk of confirmed Guillain-Barre syndrome following receipt of monovalent inactivated influenza A (H1N1) and seasonal influenza vaccines in the Vaccine Safety Datalink Project, 2009-2010. Am J Epidemiol. 2012;175(11):1100-9. DOI: 10.1093/aje/kws195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wise ME, Viray M, Sejvar JJ, Lewis P, Baughman AL, Connor W, et al. . Guillain-Barre syndrome during the 2009-2010 H1N1 influenza vaccination campaign: population-based surveillance among 45 million Americans. Am J Epidemiol. 2012;175(11):1110-9. DOI: 10.1093/aje/kws196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Wals P, Deceuninck G, Toth E, Boulianne N, Brunet D, Boucher RM, et al. . Risk of Guillain-Barre syndrome following H1N1 influenza vaccination in Quebec. JAMA. 2012;308(2):175-81. DOI: 10.1001/jama.2012.7342 [DOI] [PubMed] [Google Scholar]
- 39.Crawford NW, Cheng A, Andrews N, Charles PG, Clothier HJ, Day B, et al. . Guillain-Barre syndrome following pandemic (H1N1) 2009 influenza A immunisation in Victoria: a self-controlled case series. Med J Aust. 2012;197(10):574-8. PMid: [DOI] [PubMed] [Google Scholar]
- 40.Prestel J, Volkers P, Mentzer D, Lehmann HC, Hartung HP, Keller-Stanislawski B, et al. . Risk of Guillain-Barre syndrome following pandemic influenza A(H1N1) 2009 vaccination in Germany. Pharmacoepidemiol Drug Saf. 2014;23(11):1192-204. DOI: 10.1002/pds.3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang WT, Yang HW, Liao TL, Wu WJ, Yang SE, Chih YC, et al. . Safety of pandemic (H1N1) 2009 monovalent vaccines in taiwan: a self-controlled case series study. PLoS One. 2013;8(3):e58827 DOI: 10.1371/journal.pone.0058827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andrews N, Stowe J, Al-Shahi Salman R, Miller E. Guillain-Barre syndrome and H1N1 (2009) pandemic influenza vaccination using an AS03 adjuvanted vaccine in the United Kingdom: self-controlled case series. Vaccine. 2011;29(45):7878-82. DOI: 10.1016/j.vaccine.2011.08.069 [DOI] [PubMed] [Google Scholar]
- 43.Vellozzi C, Iqbal S, Stewart B, Tokars J, DeStefano F. Cumulative risk of Guillain-Barre syndrome among vaccinated and unvaccinated populations during the 2009 H1N1 influenza pandemic. Am J Public Health. 2014;104(4):696-701. DOI: 10.2105/AJPH.2013.301651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iqbal S, Li R, Gargiullo P, Vellozzi C. Relationship between Guillain-Barre syndrome, influenza-related hospitalizations, and influenza vaccine coverage. Vaccine. 2015;33(17):2045-9. DOI: 10.1016/j.vaccine.2015.02.064 [DOI] [PubMed] [Google Scholar]
- 45.Carias C, Reed C, Kim IK, Foppa IM, Biggerstaff M, Meltzer MI, et al. . Net Costs Due to Seasonal Influenza Vaccination--United States, 2005-2009. PLoS One. 2015;10(7):e0132922 DOI: 10.1371/journal.pone.0132922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greene SK, Rett MD, Vellozzi C, Li L, Kulldorff M, Marcy SM, et al. . Guillain-Barre Syndrome, Influenza Vaccination, and Antecedent Respiratory and Gastrointestinal Infections: A Case-Centered Analysis in the Vaccine Safety Datalink, 2009-2011. PLoS One. 2013;8(6):e67185 DOI: 10.1371/journal.pone.0067185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verity C, Stellitano L, Winstone AM, Stowe J, Andrews N, Miller E. Pandemic A/H1N1 2009 influenza vaccination, preceding infections and clinical findings in UK children with Guillain-Barre syndrome. Arch Dis Child. 2014;99(6):532-8. DOI: 10.1136/archdis- child-2013-304475 [DOI] [PubMed] [Google Scholar]
- 48.Gensicke H, Datta AN, Dill P, Schindler C, Fischer D. Increased incidence of Guillain-Barre syndrome after surgery. Eur J Neurol. 2012;19(9):1239-44. DOI: 10.1111/j.1468-1331.2012.03730.x [DOI] [PubMed] [Google Scholar]
- 49.Yang B, Lian Y, Liu Y, Wu BY, Duan RS. A retrospective analysis of possible triggers of Guillain-Barre syndrome. J Neuroimmunol. 2016;293:17-21. DOI: 10.1016/j.jneuroim.2016.02.003 [DOI] [PubMed] [Google Scholar]
- 50.Saiz JC, Vazquez-Calvo A, Blazquez AB, Merino-Ramos T, Escribano-Romero E, Martin-Acebes MA. Zika Virus: the Latest Newcomer. Front Microbiol. 2016;7:496 DOI: 10.3389/fmicb.2016.00496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hurwitz ES, Schonberger LB, Nelson DB, Holman RC. Guillain-Barre syndrome and the 1978-1979 influenza vaccine. N Engl J Med. 1981;304(26):1557-61. DOI: 10.1056/NEJM198106253042601 [DOI] [PubMed] [Google Scholar]
- 52.Nordin JD, Kharbanda EO, Vazquez-Benitez G, Lipkind H, Lee GM, Naleway AL. Monovalent H1N1 influenza vaccine safety in pregnant women, risks for acute adverse events. Vaccine. 2014;32(39):4985-92. DOI: 10.1016/j.vaccine.2014.07.017 [DOI] [PubMed] [Google Scholar]
- 53.Tomimatsu T, Sugihara M, Nagai T, Sunada Y, Kimura T, Shimoya K. Guillain-Barre syndrome after trivalent influenza vaccination during pregnancy. Eur J Obstet Gynecol Reprod Biol. 2016;201:225-6. DOI: 10.1016/j.ejogrb.2016.03.031 [DOI] [PubMed] [Google Scholar]
- 54.Hwang KP, Hsu YL, Hsieh TH, Lin HC, Yen TY, Wei HM, et al. . Immunogenicity and safety of a trivalent inactivated 2010-2011 influenza vaccine in Taiwan infants aged 6-12 months. Vaccine. 2014;32(21):2469-73. DOI: 10.1016/j.vaccine.2014.02.078 [DOI] [PubMed] [Google Scholar]
- 55.Halsey NA, Talaat KR, Greenbaum A, Mensah E, Dudley MZ, Proveaux T, et al. . The safety of influenza vaccines in children: An Institute for Vaccine Safety white paper. Vaccine. 2015;33 Suppl 5:F1-F67. DOI: 10.1016/j.vaccine.2015.10.080 [DOI] [PubMed] [Google Scholar]
- 56.Haber P, Moro PL, Cano M, Vellozzi C, Lewis P, Woo EJ, et al. . Post-Licensure Surveillance of Trivalent Live-Attenuated Influenza Vaccine in Children Aged 2-18 Years, Vaccine Adverse Event Reporting System, United States, July 2005-June 2012. J Pediatric Infect Dis Soc. 2015;4(3):205-13. DOI: 10.1093/jpids/piu034 [DOI] [PubMed] [Google Scholar]
- 57.Blum S, McCombe PA. Genetics of Guillain-Barre syndrome (GBS) and chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): current knowledge and future directions. J Peripher Nerv Syst. 2014;19(2):88-103. DOI: 10.1111/jns5.12074 [DOI] [PubMed] [Google Scholar]
- 58.Waddington CS, Walker WT, Oeser C, Reiner A, John T, Wilkins S, et al. . Safety and immunogenicity of AS03B adjuvanted split virion versus non-adjuvanted whole virion H1N1 influenza vaccine in UK children aged 6 months-12 years: open label, randomised, parallel group, multicentre study. BMJ. 2010;340:c2649 DOI: 10.1136/bmj.c2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jin PP, Sun LL, Ding BJ, Qin N, Zhou B, Xia F, et al. . Human Leukocyte Antigen DQB1 (HLA-DQB1) Polymorphisms and the Risk for Guillain-Barre Syndrome: A Systematic Review and Meta-Analysis. PLoS One. 2015;10(7):e0131374 DOI: 10.1371/journal.pone.0131374 [DOI] [PMC free article] [PubMed] [Google Scholar]