Abstract
African Americans bear a disproportionate burden of osteoarthritis (OA), but they have been underrepresented in trials of behavioral interventions for pain. This trial examined a culturally tailored pain coping skills training (CST) program, compared to a wait list control group, among 248 African Americans with knee or hip OA. The pain CST program involved 11 telephone-based sessions over 3 months. Outcomes were assessed at baseline, 3 months (primary), and 9 months, and included the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale (primary outcome), WOMAC total score and function subscale, PROMIS Pain Interference, Short-Form 12 Mental and Physical Composite Subscales, Coping Strategies Questionnaire—Total Coping Attempts, Pain Catastrophizing Scale, Patient Health Questionnaire-8, Arthritis Self-Efficacy Scale, and Patient Global Impression of Arthritis Symptom Change. Linear mixed models were fit for all outcomes. There were no significant between-group differences in WOMAC pain score at 3 months (20.63 [95% confidence interval 21.45, 0.18]; P = 0.128) or 9 months (20.84 [95% confidence interval 21.73, 0.06]; P = 0.068). Among secondary outcomes, at 3 months, there were significant differences, in favor of the CST group, for Coping Strategies Questionnaire Total Coping Attempts, Pain Catastrophizing Scale, Arthritis Self-Efficacy, and Patient Global Impression of Arthritis Symptom Change (P < 0.01). Coping Strategies Questionnaire Total Coping Attempts, Arthritis Self-Efficacy, and Patient Global Assessment Change were also significantly improved at 9 months in the CST group, compared with wait list (P < 0.01). The culturally tailored pain CST program did not significantly reduce pain severity but did improve key measures of pain coping and perceived ability to manage pain among African Americans with OA.
Keywords: Coping, Pain, Arthritis, Disparities, Clinical trial, African Americans
1. Introduction
African Americans bear a greater burden of osteoarthritis (OA) than non-Hispanic Whites, including higher prevalence and more severe pain and functional limitations.1,4,5,61,66 This is consistent with the greater levels of chronic pain observed generally among African Americans.40 Although these disparities are well documented, efforts toward developing interventions to improve pain-related outcomes among African Americans have been limited,7,23 as have culturally tailored behavioral interventions in general.10
Pain coping skills training (CST) may be a promising intervention for African Americans with OA. First, prior studies have shown that pain CST can improve outcomes among individuals with OA in general,19,22,29,32,35,38,62 although studies have included primarily non-Hispanic Whites. Second, African Americans report higher levels of pain catastrophizing,24,47,48,56 lower perceived ability to cope with and control pain,64 and more frequent use of maladaptive coping strategies3,5,31,47,64 compared with non-Hispanic Whites. Pain CST uses cognitive and behavioral approaches that can modify maladaptive coping behaviors and enhance use and perceived effectiveness of pain coping strategies.32,33,35,39,53 Third, research suggests pain coping and other psychological variables are key factors underlying racial differences in OA-related pain.4,5 Therefore, these factors are logical targets for interventions to improve pain-related outcomes among African Americans with OA.
Although pain CST programs have empirical support, there have been no studies specifically among African Americans with OA, and most participants in previous studies have been non-Hispanic Whites.12,13,16,28,29,34,38,54,62,67 Prior pain CST studies have not compared effectiveness by race, but the broader literature illustrates the importance of culturally tailoring interventions to improve potential for success when focusing on minority populations.41
However, there has been limited engagement of African Americans to obtain perspectives on cultural appropriateness of behavioral interventions for pain. It is critical that these interventions consider cultural values and pain experiences of African Americans, which may differ from other demographic groups.9,49 For example, African Americans often experience a greater number and different types of stressful events than non-Hispanic Whites, and these can contribute to the pain experience.52 Also, one cultural value of many African Americans is religion or spirituality, and African Americans tend to use more religious coping strategies than non-Hispanic Whites.20,52 Attention to these experiences and values is particularly important because pain CST programs weave coping skills into contexts, activities, and relationships.
The Pain Coping Skills Training for African American with OsteoaARThritis (STAART) study engaged African Americans with OA, their support partners, clinicians, and public health representatives in a process of enhancing a pain CST program for culturally appropriate content and delivery.59 We then examined the effectiveness of this pain CST program among African Americans with OA, relative to usual OA care. As a secondary aim, we explored whether intervention effects differed on the basis of baseline pain catastrophizing, comorbid illnesses, and duration of OA symptoms that were specified a priori.
2. Methods
This clinical trial was registered on clinicaltrials.gov (; Pain Coping Skills Training for African Americans with OA) before enrolling participants. This study was reviewed and approved by the institutional review boards of the University of North Carolina at Chapel Hill, Duke University Medical Center, Durham Veterans Affairs Medical Center (VAMC), and East Carolina University. Recruitment occurred from May 2016 to August 2017, and follow-up assessments were completed in May 2018. Detailed methods have been published previously.59
2.1. Study design and setting
The STAART study was a parallel-group design, randomized controlled trial with 248 participants assigned with equal allocation to a pain CST group and a usual care, wait list (WL) control group. Participants were enrolled from the Durham VA Health Care System (DVAHCS) and the University of North Carolina (UNC) Health Care System (n = 124 at each site). Randomization was stratified according to enrollment site and sex with a block size of 4; randomization schedules were computer-generated by the statistician. After completion of the follow-up assessments, participants in the WL control group were offered the pain CST program. All study participants continued with their usual medical care for OA during the study period.
2.2. Participants and recruitment
Study inclusion criteria were: (1) self-reported Black or African American race, (2) diagnosis of knee or hip OA (based on participant self-report), (3) and self-report of pain, aching, stiffness, or swelling in or around a hip or knee with OA on most days for the past month. Exclusion criteria are shown in Box 1. Most exclusion criteria were evaluated from both electronic medical record review and self-report at screening; lower-extremity paralysis and personality disorders were evaluated through medical record only, and participation in other OA or CST studies was assessed only through self-report at screening. Participants were recruited using 3 methods: (1) proactive recruitment of patients with evidence of knee or hip OA in DVAHCS and UNC medical records; these individuals were mailed an introductory letter, (2) advertisements at study sites and surrounding communities, and (3) referrals from health care providers at both study sites. All potentially eligible individuals were screened via telephone; those meeting eligibility criteria were invited to an in-person visit to complete consent and baseline assessments. We used a culturally targeted informed consent process that included materials developed by the National Medical Association as part of Project IMPACT—Increase Minority Participation and Awareness of Clinical Trials.51 Specifically, before study enrollment, potential participants were mailed the “You’ve Got the Power!” booklet, and at the baseline visit, they were shown the “What You Should Know About Clinical Trials” video, which includes basic information about clinical trials and perspectives from African Americans who have participated. After baseline assessments, participants were given their randomization assignment over telephone by the study co-ordinator or CST counselor because they were not blinded to treatment assignment.
Box 1. Exclusion criteria.
Diagnosis of gout (in knee or hip), rheumatoid arthritis, fibromyalgia, or other systemic rheumatic disease
Dementia or other memory loss condition
Active diagnosis of psychosis, serious personality disorder, or current uncontrolled substance abuse disorder
Total hip/knee replacement surgery, other hip/knee surgery, anterior cruciate ligament tear, or other significant knee/hip injury in the past 6 months
Scheduled for or on a waiting list for joint replacement surgery
Severely impaired hearing or speech (patients must be able to participate in telephone sessions)
Unable to speak English
Participating in another osteoarthritis intervention or coping skills training study
Unwilling to be randomized to either study arm
Lower-extremity paralysis
2.3. Pain coping skills training program
The pain CST program was based on prior clinical trials among patients with various chronic pain conditions,32,35,38,62 as well as African American men with prostate cancer.18 In the first phase of this project, we worked with a group of stakeholders (African Americans with OA, public health representatives, and health care providers) to enhance the program with attention to issues of cultural relevance; details of this process, as well as the enhancements made, have been described previously, and Appendix 1 provides a summary of stakeholder feedback and modifications (available at http://links.lww.com/PAIN/A759).59 The CST program involved 11 weekly sessions, approximately 30 to 45 minutes each, delivered through telephone by a trained counselor. The content for each session is shown in Table 1, and details of each topic have been previously published.59 During intervention phone calls, a counselor provided instruction in cognitive and behavioral pain coping skills and led participants in guided rehearsals of these skills. Participants were asked to engage in home-based practice of the skills to enhance their application in pain-related situations. During each phone call, the counselor reviewed participants’ home practice, including successes and barriers, encouraged problem solving, and worked to set goals for application of skills. Participants were given handouts to facilitate each session, along with an audio recording to guide progressive muscle relaxation.
Table 1.
Content of pain CST sessions.
| Session | Topic(s) and skills |
|---|---|
| 1 | CST program introduction, education in rationale for CST, progressive muscle relaxation |
| 2 | Mini-relaxation practices, communication with significant others about pain and coping |
| 3 | Managing unhelpful mood (congnitive restructuring)—part I |
| 4 | Managing unhelpful mood (congnitive restructuring)—part II |
| 5 | Activity pacing |
| 6 | Pleasant activities |
| 7 | Pleasant imagery and other distraction techniques |
| 8 | Physical activity and osteoarthritis |
| 9 | Weight management and osteoarthritis |
| 10 | Skills review and problem solving |
| 11 | Relapse prevention and maintenance |
CST, coping skills training.
Coping skills training counselors received training in the pain CST protocol, including role-play sessions, from experienced coinvestigators (T.S. and L.C.). Before delivering the CST protocol, study therapists were certified in the CST protocol by the experienced investigators. Therapist certification included delivering each of the 11 study sessions to “mock” participants. These training sessions were audio-recorded and rated by an experienced coinvestigator; to certify, therapists were required to receive at least a 4 out of 5 for both quality of intervention delivery and adherence to the study protocol. Counselor training also included issues related to cultural sensitivity (LC). In particular, counselors were encouraged to use active listening to identify opportunities to demonstrate that pain coping skills are compatible with cultural, spiritual, religious, or other values.59 For example, in the context of cognitive restructuring, if a participant described religious beliefs or values, the counselor would explore how those beliefs could be integrated into new, more adaptive thoughts about pain coping. Coping skills training sessions were audio-recorded, and study counselors had hour-long weekly sessions with the experienced investigators to review recorded sessions and receive ongoing feedback as part of a quality assurance process.
2.4. Measures
2.4.1. Overview of measures
Assessments were conducted at baseline, 3 months after baseline (coinciding with intervention completion for those randomized to CST), and 9 months after baseline by trained research assistants blinded to participants’ randomization assignments. Baseline and 3-month assessments were conducted in person, and 9-month assessments were conducted through telephone. When participants could not complete 3-month assessments in person, allowance was made for these to be conducted through telephone. Participants were paid $50 for completing baseline and 3-month assessments and $25 for completing 9-month assessments
2.4.2. Primary outcome
Western Ontario and McMasters Universities Osteoarthritis Index (WOMAC) Pain Subscale: The WOMAC pain subscale, a commonly used and well-validated measure among patients with lower-extremity OA,11 includes 5 items, all rated on a Likert scale of 0 (no symptoms) to 4 (extreme symptoms) with a range of 0 to 20. The subscale refers to the severity of pain during the past 2 weeks for different activities (walking, going up or down stairs, at night while in bed, sitting or lying, and standing).
2.4.3. Secondary outcomes
2.4.3.1. WOMAC total score and function subscale
In addition to the pain subscale, the WOMAC includes stiffness (2 items) and function (17 items) subscales. All items are listed rated on a Likert scale of 0 (no symptoms) to 4 (extreme symptoms), with ranges of 0 to 96 for the total score (pain, stiffness, and function subscales) and 0 to 68 for the function subscale.
2.4.3.2. PROMIS Pain Interference Instrument (Short-Form 6a)
This instrument measures the self-reported consequences of pain, over the past 7 days, across aspects of life including social, cognitive, emotional, physical, and recreational activities.6 This validated scale has 6 items with 5 response options with Likert scale of 1 to 5.
2.4.3.3. Short-Form 12 Health Survey (SF-12)
This 12-item measure covers domains of general health, physical health, work and activity limitations, and emotional health.69 We computed both Mental Health and Physical Health Composite Scores; each range from 0 to 100 with lower scores indicating poorer health.
2.4.3.4. Coping Strategies Questionnaire
This scale includes 48 items that have subscales that assess 6 different cognitive pain coping strategies (catastrophizing, diverting attention, ignoring sensations, coping self-statements, reinterpreting pain sensations, and praying-hoping) and 1 behavioral coping strategy (increasing behavioral activities).27,55 Each subscale includes 6 items, and participants rate the frequency of their use of specific coping strategies on a 7-point Likert scale from 0 (“Never do that”) to 6 (“Always do that”). We created a Total Coping Attempts score, which includes 5 cognitive subscales and 1 behavioral sub-scale but excludes the catastrophizing domain, similar to prior studies.13,35,36,37,50 The catastrophizing subscale from the Coping Strategies Questionnaire (CSQ) was not included in these analyses; catastrophizing was measured using the Pain Catastrophizing Scale (PCS), which includes 5 of the 6 pain catastrophizing items from the CSQ.
2.4.3.5. Pain Catastrophizing Scale
This 13-item instrument asks participants to describe their thoughts and feelings when in pain, assessing domains of rumination, magnification, and helplessness; all items are rated on a scale of 0 to 4, with higher scores indicating more pain catastrophizing.63
2.4.3.6. Patient Health Questionnaire-8 (PHQ-8)
This 8-item survey of depressive symptoms includes items corresponding to the depression criteria listed in the Diagnostic and Statistics Manual Fourth Edition (DSM-IV).42 All items are scored as 0 (not at all) to 3 (nearly every day), referring to symptoms during the past 2 weeks, with higher scores indicating more depressive symptoms.
2.4.3.7. Arthritis Self-Efficacy Scale
This scale includes 8 items asking respondents how certain they are that they can manage arthritis pain and keep it from interfering with specific activities.46,60 All items are scored on a scale of 1 (very uncertain) to 10 (very certain), with higher scores indicating greater self-efficacy for managing arthritis symptoms.
2.4.3.8. Yale Physical Activity Survey
This scale was developed to assess physical activity in older adults and includes common activities (such as housework, yardwork, leisure activity, moderate-intensity physical activity, caretaking, and recreational activity) performed during a typical week in the past month; higher scores are associated with greater activity level.21
2.4.3.9. Patient Global Impression of Arthritis Symptom Change
This single-item measure asks participants to describe their change in arthritis symptoms on a 7-point rating scale with the following options: 1 = “very much improved,” “much improved,” “minimally improved,” “no change,” “minimally worse,” “much worse,” and 7 = “very much worse;” lower scores indicate more improvement. At both follow-up time points, this item was asked relative to change since baseline.
2.4.4. Demographic and clinical characteristics
Self-reported participant characteristics include age, race and ethnicity (using US Census items), sex (male or female), marital status (married or living with a partner vs single, divorced, separated, or widowed), household financial state (with low income defined as self-report of “just meeting basic expenses” or “don’t even have enough to meet basic expenses” vs “meet your basic expenses with a little left over for extras” or “live comfortably”), education level (high school education or lower vs education beyond high school), work status (working full or part time vs unemployed, retired, disabled, or student), duration of OA symptoms, and comorbid illnesses (Self-Administered Comorbidity Questionnaire57).
2.5. Statistical methods
2.5.1. Sample size
The sample size estimate of n = 124 per arm was based on being able to detect a 1.3-point difference in mean WOMAC pain scores at 9 months between the CST group and the WL control group with 80% power and a type-I error rate of 0.05. We conservatively powered on the 9-month follow-up although 3 months was our primary time point. Sample size calculations used methods appropriate for analysis of covariance type analyses.15,25 We assumed a correlation of 0.6 between time points, an SD of 3.9%, and 20% attrition rate by 9 months.
2.5.2. Statistical analysis
Our primary hypothesis was that participants who received the pain CST intervention would have greater improvement in mean WOMAC pain score than participants in the WL group at 3-month follow-up. Analyses involved all randomly assigned participants, using all data collected for each participant.30 Observations were not deleted due to missing follow-up data.44 The estimation procedure for our analytic technique (linear mixed models) implicitly accommodates missingness when related to prior outcome, or to other baseline covariates included in the model (missing at random). To assess the model’s robustness to missing observations, we multiply imputed missing WOMAC pain follow-up scores using a Markov chain Monte Carlo algorithm incorporating additional variables that were related to missing outcomes (sex, body mass index, working status, education level, and baseline score for PHQ-8, arthritis self-efficacy, PCS, CSQ pain catastrophizing score and its rumination and magnification subscales, and CSQ reinterpreting pain sensation sub-scale) to strengthen the missing-at-random assumption.
For continuous outcomes, linear mixed models were fit using the SAS procedure PROC MIXED with unstructured covariance structure to account for the repeated measures over time. For the YALE Physical Activity Survey score, a square root transformation was used, and for the SF-12 Mental Health Component score, change from baseline to 3-month follow-up and baseline to 9-month follow-up were used as the outcomes, due to normality assumptions.
The predictors in all models, unless otherwise noted, included dummy-coded follow-up time effect(s) and indicator variables for the intervention interacting with the follow-up time effect(s).25 We assumed equal baseline means across study groups, as is generally appropriate for a randomized controlled trial l.25,45 Final models also included stratification variables site and sex. For the SFS-12 Mental Health Composite change score outcome (as described above), model fixed-effect terms included baseline YALE score along with indicators for intervention and 9-month follow-up and interaction of intervention indicator by 9-month follow-up. Because Patient Global Impression of Change was only assessed at 3- and 9-month follow-up, predictors included indicators for intervention and 3-month follow-up and interaction of intervention indicator by 9-month follow-up.
To explore whether intervention effects differed for our primary outcome, WOMAC pain, based on participants’ baseline PCS score, comorbid illnesses,57 and duration of OA symptoms (defined a priori), we added the baseline characteristic as a main effect, as well as 2- and 3-way interactions with time and treatment indicators to the primary analysis model described above.
As we had 35% of participants (43 of 124) completing less than 7 of the 11 possible intervention sessions in the CST arm, we conducted a post hoc sensitivity analysis to estimate the effect of receiving a more complete “dose” of the intervention, known as the complier average causal effect (CACE).43 Compliance was defined a priori as completing at least 7 intervention calls. We selected this number of sessions because all the “core” content of pain CST was covered during the first 7 sessions (Table 1; sessions 8 and 9 covered physical activity and weight management, which are not typically part of pain CST programs but were added here for more of a comprehensive behavioral approach. Sessions 10–11 focused on skills review and maintenance.) Because noncompliers were also more likely to drop out, we first multiply imputed missing 3- and 9-month outcomes. Twenty multiple imputations were generated using the MCMC option in PROC MI. The change between baseline and month 3 and baseline and month 9 was calculated for each outcome and imputed data set. The CACE was then calculated on these change scores following the formulas presented in the study by Liang et al.43 and averaged across the 20 imputed data sets. Confidence intervals (95% CIs) for the CACE estimates were computed using 1000 bootstrapped samples of the 20 multiply imputed data sets.58
2.6. Role of the funding source
The funding agency, Patient-Centered Outcomes Research Institute (PCORI), did not have a role in study design or the collection, management, analysis, or interpretation of data.
3. Results
3.1. Participants, retention, and intervention delivery
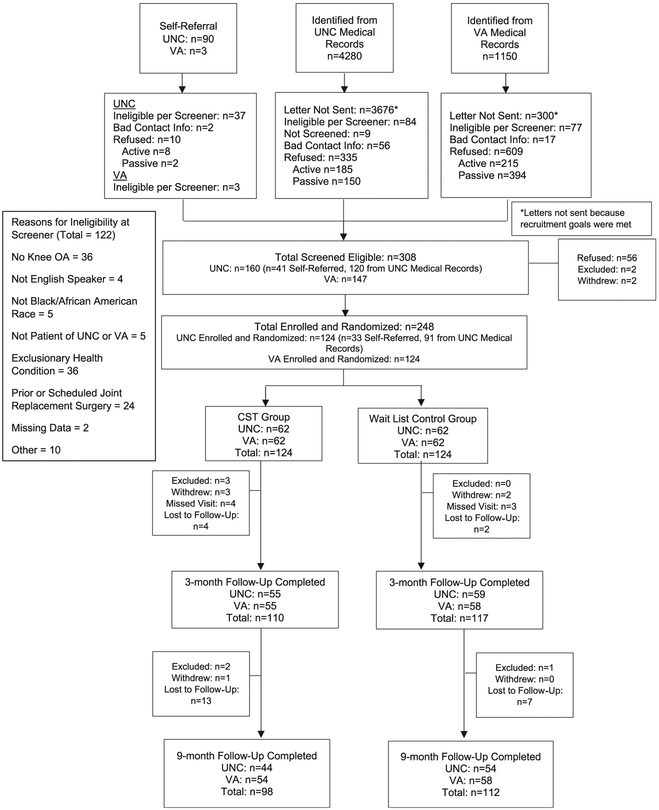
We identified 5430 potentially eligible patients from electronic medical records (4280 UNC, 1150 DVAHCS); Figure 1. Additional 93 participants self-referred to the study (90 UNC, 3 DVAHCS). Among 1547 patients who were mailed a letter or self-referred to the study, 248 were eligible, enrolled, and randomized. At 3-month follow-up, 92% of participants completed assessments (89% CST group, 94% WL group). At 9-month follow-up, 85% of participants completed assessments (79% CST group, 90% WL group). Participant characteristics are shown in Table 2. The mean number of pain CST sessions completed was 8.0 (SD = 4.3); 20% of participants completed 0 or 1 session, and 61% completed 9 to 11 sessions. There were no study-related events in either the CST or WL groups.
Figure 1.
CONSORT diagram. CST, coping skills training; OA, osteoarthritis.
Table 2.
Characteristics of STAART participants, overall and by arm.
| Characteristic | Total sample mean ± SD or N (%) | Pain CST mean ± SD or N (%) | WL control mean ± SD or N (%) |
|---|---|---|---|
| Demographic and clinical characteristics | |||
| Age | 59.0 ± 10.3 | 59.2 ± 9.8 | 58.9 ± 10.9 |
| Female | 122 (49.2%) | 61 (49.2%) | 61 (49.2%) |
| Hispanic | 7 (2.9%) | 3 (2.5%) | 4 (3.3%) |
| Some education above high school | 187 (75.4%) | 92 (74.2%) | 95 (76.6%) |
| Working | 86 (34.7%) | 43 (34.7%) | 43 (34.7%) |
| Married or living with partner | 103 (41.5%) | 51 (41.1%) | 52 (41.9%) |
| Low perceived income* | 83 (33.6%) | 48 (38.7%) | 35 (28.5%) |
| Body mass index (kg/ms) | 35.2 ± 8.2 | 35.6 ± 8.4 | 34.8 ± 7.9 |
| No. of self-reported comorbidities | 8.5 ± 3.9 | 8.2 ± 3.9 | 8.8 ± 4.0 |
| Duration of arthritis symptoms | 13.0 ± 10.0 | 12.4 ± 9.6 | 13.6 ± 10.3 |
| Primary and secondary outcomes | |||
| WOMAC pain | 11.0 ± 3.9 | 11.2 ± 4 | 10.8 ± 3.8 |
| WOMAC total | 53.0 ± 17.8 | 52.9 ± 16.4 | 53.0 ± 19.1 |
| WOMAC function | 37.0 ± 13.2 | 37.1 ± 12.2 | 36.9 ± 14.2 |
| PROMIS Pain Interference Score | 63.8 ± 6.9 | 64.0 ± 6.3 | 63.5 ± 7.5 |
| Short-Form-12—physical | 33.1 ± 9.1 | 33.1 ± 8.9 | 33.0 ± 9.2 |
| Short-Form-12—mental | 50.7 ± 11.1 | 51.0 ± 10.4 | 50.4 ± 11.8 |
| CSQ—total coping attempts | 93.9 ± 36.6 | 94.3 ± 37.5 | 93.5 ± 35.9 |
| Pain Catastrophizing Scale | 19.8 ± 12.3 | 19.9 ± 12.8 | 19.6 ± 11.8 |
| PHQ-8 | 6.2 ± 5.3 | 6.0 ± 5.1 | 6.4 ± 5.6 |
| Arthritis Self-Efficacy Scale | 5.9 ± 2.0 | 5.6 ± 1.9 | 5.8 ± 2.0 |
| Yale Physical Activity Scale | 4020.1 ± 3472.5 | 4318.0 ± 3905.5 | 3725.1 ± 2971.9 |
Self-report of “just meet basic expenses” or “don’t even have enough to meet basic expenses.”
CSQ, Coping Strategies Questionnaire; CST, coping skills training.; DVAHCS, Durham VA Health Care System; PHQ, Patient Health Questionnaire; UNC, University of North Carolina; WL, wait list; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
3.2. Primary outcome
At 3-month follow-up, WOMAC pain scores did not differ significantly between the pain CST group and the WL group (estimated treatment difference = −0.63, 95% CI = −1.45, 0.18; P = 0.128; Table 3). At 9-month follow-up, WOMAC pain scores in the pain CST group continued to decrease from 3 months but were not statistically different from the WL group (estimated treatment difference = −0.84, 95% CI = −1.73, 0.06; P = 0.068). Results were similar in multiple imputation analyses of WOMAC pain scores. At 3-month follow-up, the estimated treatment difference was −0.63 (95% CI = −1.46, 0.20; P = 0.137). At 9-month follow-up, the estimated treatment difference was −0.92 (95% CI = −1.81, −0.04; P = 0.042). Proportions of participants in the pain CST group who improved at least 14% (which represents a clinically relevant change8) were 49% and 51% at 3 and 9-month follow-up, respectively. CACE methods showed that receipt of at least 7 intervention sessions resulted in larger and clinically important improvements, although not statistically different from the control group at either 3 or 9 months (difference in CACE estimated mean change = −1.2, 95% CI = −2.4, 0.2 for 3 months and = −1.4, 95% CI = −2.8, 0.1 for 9 months). In our exploratory analysis, we found no evidence of differential intervention effects on WOMAC pain by baseline pain catastrophizing score, comorbid illnesses, or duration of OA symptoms (results not shown).
Table 3.
Estimated means and 95% confidence intervals from linear mixed models.
| Outcome | Time point | Pain CST (N = 124) mean (95% CI) |
WL control (N = 124) mean (95% CI) |
Treatment difference: pain CST-WL mean (95% CI) | P |
|---|---|---|---|---|---|
| WOMAC pain subscale | Baseline | 11.01 (10.52,11.49) | |||
| 3 mo | 9.42 (8.77, 10.06) | 10.05 (9.42, 10.68) | −0.63 (−1.45, 0.18) | 0.128 | |
| 9 mo | 8.77 (8.04, 9.50) | 9.61 (8.91,10.30) | −0.84 (−1.73, 0.06) | 0.068 | |
| WOMAC total subscale | Baseline | 52.99 (50.79,55.19) | |||
| 3 mo | 46.39 (43.49, 49.29) | 49.01 (46.16, 51.86) | −2.62 (−6.01, 0.77) | 0.129 | |
| 9 mo | 44.18 (40.96, 47.40) | 47.49 (44.39, 50.59) | −3.31 (−7.07, 0.44) | 0.084 | |
| WOMAC function | Baseline | 37.00 (35.37, 38.64) | |||
| 3 mo | 32.93 (30.76, 35.10) | 35.54 (32.41, 36.67) | −1.61 (−4.14, 0.91) | 0.209 | |
| 9 mo | 31.40 (29.00, 33.80) | 33.60 (31.29, 35.90) | −2.20 (5.03, 0.63) | 0.128 | |
| PROMIS Pain Interference | Baseline | 63.75 (62.89,64.62) | |||
| 3 mo | 61.12 (60.04, 62.21) | 61.96 (60.90, 63.01) | −0.83 (−2.18, 0.51) | 0.222 | |
| 9 mo | 61.00 (59.74, 62.27) | 62.14 (60.92, 63.36) | −1.14 (−2.60, 0.33) | 0.128 | |
| SF-12 Physical Component Health Score | Baseline | 33.08 (31.95,34.2) | |||
| 3 mo | 35.67 (33.99, 37.36) | 33.96 (32.31, 35.61) | 1.71 (−0.40, 3.82) | 0.111 | |
| 9 mo | 35.55 (33.82, 37.27) | 34.81 (33.17, 36.45) | 0.73 (−1.42, 2.88) | 0.502 | |
| SF-12 Mental Health Component Score* | 3 mo | −0.72 (−2.08, 0.64) | −1.48 (−2.82, −0.15) | 0.76 (−1.15, 2.66) | 0.433 |
| 9 mo | −0.57 (−2.13, 0.99) | −2.28 (−3.76, −0.79) | 1.71 (−0.45, 3.86) | 0.120 | |
| CSQ Total Coping Attempts | Baseline | 105.38 (100.62, 110.13) | |||
| 3 mo | 121.84 (116.48, 127.19) | 106.53 (101.28, 111.78) | 15.31 (9.09, 21.53) | 0.001 | |
| 9 mo | 114.65 (108.88, 120.41) | 102.97 (97.42, 108.52) | 11.67 (5.08, 18.27) | <0.001 | |
| Pain Catastrophizing Scale | Baseline | 19.73 (18.19,21.28) | |||
| 3 mo | 17.81 (15.81, 19.81) | 20.84 (18.87, 22.81) | −3.03 (−5.25, −0.8) | 0.008 | |
| 9 mo | 16.98 (14.86, 19.09) | 18.35 (16.33, 20.38) | −1.38 (−3.85, 1.09) | 0.273 | |
| PHQ-8 | Baseline | 6.19 (5.53, 6.85) | |||
| 3 mo | 5.87 (4.99, 6.75) | 6.37 (5.51,7.23) | −0.50 (−1.57, 0.57) | 0.356 | |
| 9 mo | 5.30 (4.36, 6.23) | 6.31 (5.43, 7.20) | −1.02 (−2.19, 0.15) | 0.087 | |
| Arthritis Self-Efficacy Scale | Baseline | 5.87 (5.62, 6.11) | |||
| 3 mo | 6.67 (6.35, 6.99) | 5.66 (5.35, 5.98) | 1.01 (0.61, 1.41) | <0.001 | |
| 9 mo | 6.33 (5.99, 6.67) | 5.66 (5.33, 5.99) | 0.67 (0.24, 1.09) | 0.002 | |
| YALE Physical Activity Scale† | Baseline | 58.55 (55.09, 62.01) | |||
| 3 mo | 60.72 (56.16, 65.28) | 55.72 (51.29, 60.15) | 5.00 (−0.93, 10.93) | 0.078 | |
| 9 mo | 56.66 (51.85, 61.46) | 53.80 (49.38, 58.22) | 2.85 (−3.05, 8.75) | 0.727 | |
| Patient Global Assessment of Pain Change‡ | |||||
| 3 mo | 2.91 (2.67, 3.14) | 4.18 (3.95, 4.40) | −1.27 (−1.60, −0.95) | <0.001 | |
| 9 mo | 3.25 (2.99, 3.52) | 4.13 (3.87, 4.38) | −0.87 (−1.24, −0.51) | <0.001 |
Analyzed as change score from baseline (follow-up 2 baseline).
Square root transformed variable.
Assessed at 3 and 9 months only and reported as change from baseline.
CI, confidence interval; CSQ, Coping Strategies Questionnaire; CST, coping skills training; WL, wait list.
3.3. Secondary outcomes
WOMAC total and function scores, PROMIS Pain Interference, and SF-12 Mental and Physical Health Component Scores did not differ significantly between pain CST and WL groups at either 3 or 9 months (Table 3). The CSQ Pain Coping Attempts score increased more in the pain CST group than in the WL control group at both 3 months (estimated treatment difference = 15.31, 95% CI = 9.09, 21.53; P < 0.001) and 9 months (estimated treatment difference = 11.67, 95% CI = 5.08, 18.27; P < 0.001). Pain Catastrophizing Scale scores decreased more in the pain CST group than in the WL group at 3 months (estimated treatment difference = −3.03, 95% CI = −5.25, −0.80; P = 0.008); at 9 months, PCS scores continued to decline in the pain CST group, but the difference compared with the WL control group was smaller and no longer statistically significant (P = 0.273). PHQ-8 scores did not differ between pain CST and WL control groups at either time point. Arthritis Self-Efficacy scores improved more in the pain CST group than in the WL control group at both 3 months (estimated treatment difference = 1.01, 95% CI = 0.61, 1.41; P < 0.001) and 9 months (estimated treatment difference = 0.67, 95% CI = 0.24, 1.09; P = 0.02). Yale Physical Activity Survey scores did not differ between groups at either 3 or 9 months. Patient Global Impression of Arthritis Symptom Change scores were lower (indicating more improvement since baseline) in the pain CST group than in the WL control group at both 3 months (estimated difference = −1.27, 95% CI = −1.60, −0.95; P < 0.001) and 9 months (estimated difference = −0.87, 95% CI = −1.24, −0.51; P < 0.001).
4. Discussion
4.1. Summary of findings
This trial examined the effects of a culturally tailored pain CST program among a sample of African Americans with OA who differed substantially from participants in prior trials with respect to sociodemographic variables, pain severity, pain coping, and number of comorbid illnesses.2 The pain CST program did not result in statistically different or clinically meaningful changes in WOMAC pain score (the primary outcome), compared with a WL control group. Although the between-group difference in WOMAC pain scores at 9-month follow-up was statistically significant in the multiple imputation analysis, this difference was still modest with most values in the 95% confidence interval of the difference below the threshold for a clinically meaningful between-group difference (12%).8 CACE analyses showed that intervention effects on WOMAC pain were more robust for participants who received >7 visits, although these between-group differences were also not statistically significant and were on the border of clinical relevance. For secondary outcomes including WOMAC total and function, PROMIS Pain Interference, SF-12, PHQ-8, and Yale Physical Activity Survey, observed patterns were similar to those of WOMAC pain scores, with somewhat more favorable changes in the pain CST group but no significant between-group differences at either time point. However, the pain CST program did improve key measures of pain coping and perceived ability to manage pain among African Americans with OA. In particular, CST participants decreased pain catastrophizing, and increased arthritis self-efficacy, relative to the WL group. These are important outcomes, given prior research showing that African Americans are more prone to engage in pain catastrophizing and report lower self-efficacy for managing arthritis, compared with non-Hispanic Whites.3,5,24,31,47,48,56,64 After treatment, participants in the CST group also perceived changes in their arthritis symptoms more favorably than participants in the WL group.
4.2. Comparison with other studies
With regard to the effects of pain CST for improving pain severity in patients with OA, prior studies have been mixed, with some reporting significant improvement34,62 but others reporting no difference relative to a usual care control group.16,28,38,68 These studies have primarily involved non-Hispanic White participants, with less than 1/3 of participants being African American.2 Meta-analyses of cognitive behavioral interventions (including pain CST) have concluded that there are small effect sizes for pain severity among patients with arthritis and other chronic pain conditions.22,70 Findings of this study among African Americans with OA therefore concur with prior research because there was some improvement in WOMAC pain scores after the CST intervention, but changes were relatively small and not significantly different from changes in the WL control group. There is increasing recognition that patients with chronic pain vary in their response to psychological and other treatments, and one recent study found that among participants with OA who completed a pain CST intervention, effects on a composite variable (including pain severity) varied based on several demographic and clinical variables.17,65 It is important to understand which patients may benefit most from pain CST interventions because this informs dissemination and implementation efforts. Although we observed no differential effects of the intervention on the primary study outcome based on a priori selected variables baseline pain catastrophizing, comorbid illnesses, and duration of OA symptoms, future exploratory analyses will assess whether intervention response varied by multidimensional subgroups.59
Findings of this study regarding secondary outcomes also align with prior research.16,28,34,38,62,68 Of particular importance were the significant improvements in pain catastrophizing and arthritis self-efficacy in the pain CST group. Prior studies of pain CST for OA have consistently shown increases in arthritis self-efficacy13,16,34,54,62; studies have been mixed with regard to pain catastrophizing, with some studies showing no effect.16,62 African Americans in this study varied considerably from participants in prior studies of CST for OA, having worse pain and function, greater pain catastrophizing, and more risk factors for negative pain-related outcomes.2 Therefore, findings of this study importantly illustrate that a culturally tailored pain CST can improve perceived ability to manage pain and maintain activities in a racial minority group that is at high risk for poor pain-related outcomes. The findings regarding changes in pain catastrophizing are particularly important because multiple studies have shown higher catastrophizing among African Americans.24,47,48,56 African Americans in this study had significant improvements in pain catastrophizing after the CST intervention, and although there was no significant difference at 9 months compared with the WL group, pain catastrophizing scores continued to decrease in the CST group between 3- and 9-month follow-up. This suggests that CST participants continued to improve in this key coping-related construct even after the intervention ended.
Another interesting finding was the significant difference in global assessment of arthritis symptom change, despite the lack of difference in WOMAC pain scores. It is possible that CST affected how participants’ viewed their symptoms more generally, resulting in a more positive perception, although the more specific domain of pain severity did not change substantially. A recent study of Internet-delivered pain CST and exercise for patients with hip OA also found improvements in global impression of change despite a lack of significant change in pain severity.14
4.3. Strengths and limitations of the study
This is, to the best of our knowledge, the first study of a pain CST intervention particularly among African Americans with a chronic musculoskeletal pain condition, which is important, given the disproportionate burden of pain and poorer pain-related out-comes in this racial group. Other strengths include the multisite design, inclusion of a larger proportion of men than in most OA studies, inclusion of Veterans (who also bear a disproportionate burden of OA), proactive recruitment of participants, and inclusion of a follow-up time point that allowed for assessment of maintenance. Limitations include: (1) lack of de novo radio-graphs to confirm OA status, (2) use of self-reported race as a proxy for the broader construct of culture (whereas life experiences and culture undoubtedly varied among study participants), (3) lack of direct assessment of how cultural adaptations may have impacted intervention effectiveness, (4) lack of fidelity ratings for all CST sessions, (5) no comparison to a more generic pain CST program (before cultural tailoring) or an attention control group, (6) inclusion of patients in one geographic region, and (7) inclusion of a relatively well-educated sample (75% had some education above high school) that may limit generalizability. In addition, although we had low rates of attrition, there were some limitations in the level of intervention adherence, particularly that about 20% of participants attended <2 CST sessions. Given that the mean number of sessions completed was 8 out of 11, a future intervention may need to be reconfigured to provide the most critical skills in as few sessions as possible. We believe the suboptimal intervention attendance illustrates the real-world challenges of identifying individuals who are most likely to engage with the intervention and the need to further evaluate intervention design to help participants to complete pain CST even in the face of many competing life responsibilities.
4.4. Conclusion and implications
This study demonstrated the feasibility of enrolling a large number of African American participants, who had high mean levels of comorbidity, into a trial of pain CST and engaging them in an 11-session telephone-based intervention. Although there was no significant effect of pain CST on the primary outcome of pain severity in this study, African Americans experienced improvements in other key outcomes related to the pain experience. These changes are particularly notable because participants in this sample had poorer pain coping patterns than participants in prior studies of pain CST for OA. We believe these findings support the value of efforts to disseminate pain CST among African Americans with OA, as a part of overall efforts to mitigate racial disparities in OA-related outcomes. There are challenges to more widespread dissemination, including access to pain CST; efforts are needed to explore models of delivering pain CST programs specifically among African Americans, potentially in partnership with community-based organizations. Future research efforts should also examine strategies for enhancing adherence, perhaps through briefer interventions, and identify other factors associated with treatment response; both of these areas will help to optimize effectiveness of pain CST programs. In addition, future efforts should consider cost-effectiveness of pain CST programs, including evaluation of participant subgroups and briefer interventions.
Results of this study also have implications for populations other than African Americans living in the United States because there are pain disparities based on socioeconomic status, education, urban/rural geography, and occupational status.26 This study shows a successful model of culturally tailoring a behavioral intervention to match the needs and backgrounds of a demographic group at risk for poor pain-related outcomes. This model can be applied in other contexts and populations to increase the use and impact of behavioral pain interventions.41
Supplementary Material
Acknowledgements
The authors acknowledge the contributions of study team members Kimberlea Grimm, Bernadette Benas, Ashley Gwyn, Alexander Gunn, Erin Haley, Caroline Nagle, Scott Ravyts, Leah Schrubbe, and Catherine Stanwyck. The authors expresses gratitude to the stakeholder panel for this project, without whom this work would not be possible: Ms Mae Karim, Ms Sandy Walker, LPN (Chapel Hill Children’s Clinic), Mr Ralph Brown, Dr Yashika Watkins, PhD, MPH (Movement is Life/University of Illinois at Chicago), Dr Teresa J. Brady, PhD (Centers for Disease Control and Prevention), Dr Elaine Hart-Brothers, MD, MPH (Community Health Coalition), and Ms Laura C. Marrow (Arthritis Foundation). The authors also thank all the participants taking part in this research.
Research reported in this manuscript was funded through a Patient-Centered Outcomes Research Institute (PCORI) Award (AD-1408–19519). The statements presented in this manuscript are solely the responsibility of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors, or Methodology Committee. Drs Allen, Coffman, and Oddone receive support from the Center of Innovation to Accelerate Discovery and Practice Transformation, Durham VA Health Care System (CIN 13–410). Dr Allen receives support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases Multidisciplinary Clinical Research Center (P60 AR062760).
Footnotes
Conflict of interest statement
The authors do not have any conflicts of interest to declare.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/A759.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.painjournalonline.com).
References
- [1].Allen KD. Racial and ethnic disparities in osteoarthritis phenotypes. Curr Opin Rheumatol 2010;22:528–32. [DOI] [PubMed] [Google Scholar]
- [2].Allen KD, Arbeeva L, Cené CW, Coffman CJ, Grimm KF, Haley E, Keefe FJ, Nagle CT, Oddone EZ, Somers TJ, Watkins Y, Campbell LC. Pain coping skills training for African Americans with osteoarthritis study: baseline participant characteristics and comparison to prior studies. BMC Musculoskelet Disord 2018;19:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Allen KD, Golightly YM, Olsen MK. Pilot study of pain and coping among patients with osteoarthritis: a daily diary analysis. J Clin Rheumatol 2006; 12:118–23. [DOI] [PubMed] [Google Scholar]
- [4].Allen KD, Helmick CG, Schwartz TA, DeVellis B, Renner JB, Jordan JM. Racial differences in self-reported pain and function among individuals with radiographic hip and knee osteoarthritis: the Johnston County Osteoarthritis Project. Osteoarthritis Cartilage 2009;17:1132–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Allen KD, Oddone EZ, Coffman CJ, Keefe FJ, Lindquist JH, Bosworth HB. Racial differences in osteoarthritis pain and function: potential explanatory factors. Osteoarthritis Cartilage 2010;18:160–7. [DOI] [PubMed] [Google Scholar]
- [6].Amtmann D, Cook KF, Jensen MP, Chen WH, Choi S, Revicki D, Cella D, Rothrock N, Keefe F, Callahan L, Lai JS. Development of a PROMIS item bank to measure pain interference. PAIN 2010;150:173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Anderson KO, Green CR, Payne R. Racial and ethnic disparities in pain: causes and consequences of unequal care. J Pain 2009;10:1187–204. [DOI] [PubMed] [Google Scholar]
- [8].Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum 2001;45:384–91. [DOI] [PubMed] [Google Scholar]
- [9].Baker TA. Chronic pain in older Black Americans: the influence of health and psychosocial factors. Ethn Dis 2005;15:179–86. [PubMed] [Google Scholar]
- [10].Barrera M Jr, Castro FG, Strycker LA, Toobert DJ. Cultural adaptations of behavioral health interventions: a progress report. J Consult Clin Psychol 2013;81:196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833–40. [PubMed] [Google Scholar]
- [12].Bennell KL, Ahamed Y, Jull G, Bryant C, Hunt MA, Forbes AB, Kasza J, Akram M, Metcalf B, Harris A, Egerton T, Kenardy JA, Nicholas MK, Keefe FJ. Physical therapist-delivered pain coping skills training and exercise for knee osteoarthritis: randomized controlled trial. Arthritis Care Res (Hoboken) 2016;68:590–602. [DOI] [PubMed] [Google Scholar]
- [13].Bennell KL, Nelligan R, Dobson F, Rini C, Keefe F, Kasza J, French S, Bryant C, Dalwood A, Abbott JH, Hinman RS. Effectiveness of an internet-delivered exercise and pain-coping skills training intervention for persons with chronic knee pain: a randomized trial. Ann Intern Med 2017; 166:453–62. [DOI] [PubMed] [Google Scholar]
- [14].Bennell KL, Nelligan RK, Rini C, Keefe FJ, Kasza J, French S, Forbes A, Dobson F, Abbott JH, Dalwood A, Harris A, Vicenzino B, Hodges PW, Hinman RS. Effects of internet-based pain coping skills training before home exercise for individuals with hip osteoarthritis (HOPE trial): a randomised controlled trial. PAIN 2018;159:1833–42. [DOI] [PubMed] [Google Scholar]
- [15].Borm GF, Fransen J, Lemmens WA. A simple sample size formula for analysis of covariance in randomized clinical trials. J Clin Epidemiol 2007; 60:1234–8. [DOI] [PubMed] [Google Scholar]
- [16].Broderick JE, Keefe FJ, Bruckenthal P, Junghaenel DU, Schneider S, Schwartz JE, Kaell AT, Caldwell DS, McKee D, Reed S, Gould E. Nurse practitioners can effectively deliver pain coping skills training to osteoarthritis patients with chronic pain: a randomized, controlled trial. PAIN 2014;155:1743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Broderick JE, Keefe FJ, Schneider S, Junghaenel DU, Bruckenthal P, Schwartz JE, Kaell AT, Caldwell DS, McKee D, Gould E. Cognitive behavioral therapy for chronic pain is effective, but for whom? PAIN 2016; 157:2115–23. [DOI] [PubMed] [Google Scholar]
- [18].Campbell LC, Keefe FJ, Scipio C, McKee DC, Edwards CL, Herman SH, Johnson LE, Colvin OM, McBride CM, Donatucci C. Facilitating research participation and improving quality of life for African American prostate cancer survivors and their intimate partners: a pilot study of telephone-based coping skills training. Cancer 2007;109(2 suppl):414–24. [DOI] [PubMed] [Google Scholar]
- [19].Carson JW, Keefe FJ, Affleck G, Rumble ME, Caldwell DS, Beaupre PM, Kashikar-Zuck S, Sandstrom M, Weisberg JN. A comparison of conventional pain coping skills training and pain coping skills training with a maintenance training component: a daily diary analysis of short- and long-term treatment effects. J Pain 2006;7:615–25. [DOI] [PubMed] [Google Scholar]
- [20].Cochran S, Mays V. Applying social psychological models to predicting HIV-related sexual risk behaviors among African Americans. J Black Psychol 1993;19:142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dipietro L, Caspersen CJ, Ostfeld AM, Nadel ER. A survey for assessing physical activity among older adults. Med Sci Sports Exerc 1993;25: 628–42. [PubMed] [Google Scholar]
- [22].Dixon KE, Keefe FJ, Scipio CD, Perri LM, Abernethy AP. Psychological interventions for arthritis pain mangement in adults: a meta-analysis. Health Psychol 2007;26:241–50. [DOI] [PubMed] [Google Scholar]
- [23].Dzau VJ, Pizzo PA. Relieving pain in America: insights from an Institute of Medicine committee. JAMA 2014;312:1507–8. [DOI] [PubMed] [Google Scholar]
- [24].Edwards RR, Moric M, Husfeldt B, Buvanendran A, Ivankovich O. Ethnic similarities and differences in the chronic pain experience: a comparison of African American, Hispanic, and White patients. Pain Med 2005;6: 88–98. [DOI] [PubMed] [Google Scholar]
- [25].Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. Hoboken: Wiley-Interscience, 2004. [Google Scholar]
- [26].Goldberg DS, McGee SJ. Pain as a global public health priority. BMC Public Health 2011;11:770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hastie BA, Riley JL, Fillingim RB. Ethnic differences in pain coping: factor structure of the coping strategies questionnaire and coping strategies questionnaire-revised. J Pain 2004;5:304–16. [DOI] [PubMed] [Google Scholar]
- [28].Helminen EE, Sinikallio SH, Valjakka AL, Vaisanen-Rouvali RH, Arokoski JP. Effectiveness of a cognitive-behavioural group intervention for knee osteoarthritis pain: a randomized controlled trial. Clin Rehabil 2015;29: 868–81. [DOI] [PubMed] [Google Scholar]
- [29].Hurley MV, Walsh NE, Mitchell HL, Pimm TJ, Patel A, Williamson E, Jones RH, Dieppe P, Reeves BC. Clinical effectiveness of a rehabilitation program integrating exercise, self-management, and active coping strategies for chronic knee pain: a cluster randomized trial. Arthritis Care Res 2007;57:1211–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].ICH E9 Expert Working Group. ICH harmonised tripartite guideline—statistical principles for clinical trials. Stat Med 1999;18: 1905–42. [PubMed] [Google Scholar]
- [31].Jones AC, Kwoh CK, Groeneveld PW, Mor M, Geng M, Ibrahim SA. Investigating racial differences in coping with chronic osteoarthritis pain. J Cross Cult Gerontol 2008;23:339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Keefe FJ, Caldwell D, Williams DA, Gil KM, Mitchell D, Robertson C, Martinez S, Nunley J, Beckham JC, Helms M. Pain coping skills training in the management of osteoarthritic knee pain-II: follow-Up results. Behav Ther 1990;21:435–47. [Google Scholar]
- [33].Keefe FJ, Caldwell DS. Cognitive behavioral control of arthritis pain. Med Clin North Am 1997;81:277–90. [DOI] [PubMed] [Google Scholar]
- [34].Keefe FJ, Caldwell DS, Baucom D, Salley A, Robinson E, Timmons K, Beaupre P, Weisberg J, Helms M. Spouse-assisted coping skills training in the management of osteoarthritic knee pain. Arthritis Care Res 1996;9: 279–91. [DOI] [PubMed] [Google Scholar]
- [35].Keefe FJ, Caldwell DS, Baucom D, Salley A, Robinson E, Timmons K, Beaupre P, Weisberg J, Helms M. Spouse-assisted coping skills training in the management of knee pain in osteoarthritis: long-term followup results. Arthritis Care Res 1999;12:101–11. [DOI] [PubMed] [Google Scholar]
- [36].Keefe FJ, Caldwell DS, Queen K, Gil KM, Martinez S, Crisson JE, Ogden W, Nunley J. Osteoarthritis knee pain: a behavioral analysis. PAIN 1987; 28:309–21. [DOI] [PubMed] [Google Scholar]
- [37].Keefe FJ, Caldwell DS, Queen KT, Gil KM, Martinez S, Crisson JE, Ogden W, Nunley J. Pain coping strategies in osteoarthritis patients. J Consulting Clin Psychol 1987;55:208–12. [DOI] [PubMed] [Google Scholar]
- [38].Keefe FJ, Caldwell DS, Williams DA, Gil KM, Mitchell D, Robertson C, Martinez S, Nunley J, Beckham JC, Crisson JE, Helms M. Pain coping skills training in the management of osteoarthritic knee pain: a comparative study. Behav Ther 1990;21:49–62. [Google Scholar]
- [39].Keefe FJ, Blumenthal J, Baucom D, Affleck G, Waugh R, Caldwell DS, Beaupre P, Kashikar-Zuck S, Wright K, Egert J, Lefebvre J. Effects of spouse-assisted coping skills training and exercise training in patients with osteoarthritic knee pain: a randomized controlled study. PAIN 2004; 110:539–49. [DOI] [PubMed] [Google Scholar]
- [40].Kim HJ, Yang GS, Greenspan JD, Downton KD, Griffith KA, Renn CL, Johantgen M, Dorsey SG. Racial and ethnic differences in experimental pain sensitivity: systematic review and meta-analysis. PAIN 2017;158: 194–211. [DOI] [PubMed] [Google Scholar]
- [41].Kreuter MW, Lukwago SN, Bucholtz RD, Clark EM, Sanders-Thompson V. Achieving cultural appropriateness in health promotion programs: targeted and tailored approaches. Health Educ Behav 2003;30:133–46. [DOI] [PubMed] [Google Scholar]
- [42].Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord 2009;114:163–73. [DOI] [PubMed] [Google Scholar]
- [43].Liang Y, Ehler BR, Hollenbeak CS, Turner BJ. Behavioral support intervention for uncontrolled hypertension: a complier average causal effect (CACE) analysis. Med Care 2015;53:e9–e15. [DOI] [PubMed] [Google Scholar]
- [44].Little RJA, Rubin DB. Statistical analysis with missing data. Hoboken: John Wiley & Sons, Inc, 2002. [Google Scholar]
- [45].Liu GF, Lu K, Mogg R, Mallick M, Mehrotra DV. Should baseline be a covariate or dependent variable in analyses of change from baseline in clinical trials? Stat Med 2009;28:2509–30. [DOI] [PubMed] [Google Scholar]
- [46].Lorig K, Chastain RL, Ung E, Shoor S, Holman HR. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum 1989;32:37–44. [DOI] [PubMed] [Google Scholar]
- [47].Meints SM, Miller MM, Hirsh AT. Differences in pain coping between Black and White Americans: a meta-analysis. J Pain 2016;17:642–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Meints SM, Stout M, Abplanalp S, Hirsh AT. Pain-related rumination, but not magnification or helplessness, mediates race and sex differences in experimental pain. J Pain 2017;18:332–9. [DOI] [PubMed] [Google Scholar]
- [49].Miranda J, Bernal G, Lau A, Kohn L, Hwang WC, LaFromboise T. State of the science on psychosocial interventions for ethnic minorities. Annu Rev Clin Psychol 2005;1:113–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Parker JC, Smarr KL, Buescher KL, Phillips LR, Frank RG, Beck NC, Anderson SK, Walker SE. Pain control and rational thinking. Implications for rheumatoid arthritis. Arthritis Rheum 1989;32:984–90. [DOI] [PubMed] [Google Scholar]
- [51].Project IMPACT. Increase minority participation and awareness of clinical trials. Available at: http://impact.nmanet.org/wordpress/. Accessed February 26, 2019.
- [52].Resnicow K, Braithwaite RL, Dilorio C, Glanz K. Applying theory to culturally diverse and unique populations In: Glanz K, Rimer BK, Viswanath K, editors. Health Behavior and Health Education: Theory, Research, and Practice. 3rd ed San Francisco: Jossey-Bass, 2002. [Google Scholar]
- [53].Riddle DL, Keefe FJ, Nay WT, McKee D, Attarian DE, Jensen MP. Pain coping skills training for patients with elevated pain catastrophizing who are scheduled for knee arthroplasty: a quasi-experimental study. Arch Phys Med Rehabil 2011;92:859–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Rini C, Porter LS, Somers TJ, McKee DC, DeVellis RF, Smith M, Winkel G, Ahern DK, Goldman R, Stiller JL, Mariani C, Patterson C, Jordan JM, Caldwell DS, Keefe FJ. Automated Internet-based pain coping skills training to manage osteoarthritis pain: a randomized controlled trial. PAIN 2015;156:837–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship of patient characteristics and current adjustment. PAIN 1983;17:33–44. [DOI] [PubMed] [Google Scholar]
- [56].Ruehlman LS, Karoly P, Newton C. Comparing the experiential and psychosocial dimensions of chronic pain in african americans and Caucasians: findings from a national community sample. Pain Med 2005; 6:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The self-administered comorbidity questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum 2003;49:156–63. [DOI] [PubMed] [Google Scholar]
- [58].Schomaker M, Heumann C. Bootstrap inference when using multiple imputation. Stat Med 2018;37:2252–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Schrubbe LA, Ravyts SG, Benas BC, Campbell LC, Cené CW, Coffman CJ, Gunn AH, Keefe FJ, Nagle CT, Oddone EZ, Somers TJ, Stanwyck CL, Taylor SS, Allen KD. Pain coping skills training for African Americans with osteoarthritis (STAART): study protocol of a randomized controlled trial. BMC Musculoskelet Disord 2016;17:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sharma L, Cahue S, Song J, Hayes K, Pai YC, Dunlop D. Physical functioning over three years in knee osteoarthritis: role of psychosocial, local mechanical, and neuromuscular factors. Arthritis Rheum 2003;48:3359–70. [DOI] [PubMed] [Google Scholar]
- [61].Smith DM, Parmelee PA. Within-day variability of fatigue and pain among African Americans and non-Hispanic whites with osteoarthritis of the knee. Arthritis Care Res (Hoboken) 2016;68:115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Somers TJ, Blumenthal JA, Guilak F, Kraus VB, Schmitt DO, Babyak MA, Craighead LW, Caldwell DS, Rice JR, McKee DC, Shelby RA, Campbell LC, Pells JJ, Sims EL, Queen R, Carson JW, Connelly M, Dixon KE, Lacaille LJ, Huebner JL, Rejeski WJ, Keefe FJ. Pain coping skills training and lifestyle behavioral weight management in patients with knee osteoarthritis: a randomized controlled study. PAIN 2012;153:1199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess 1995;7:524–32. [Google Scholar]
- [64].Tan G, Jensen MP, Thornby J, Anderson KO. Ethnicity, control appraisal, coping, and adjustment to chronic pain among black and white Americans. Pain Med 2005;6:18–28. [DOI] [PubMed] [Google Scholar]
- [65].Turk DC. The potential of treatment matching for subgroups of patients with chronic pain: lumping versus splitting. Clin J Pain 2005;21:44–55; discussion 69–72. [DOI] [PubMed] [Google Scholar]
- [66].Vina ER, Ran D, Ashbeck EL, Kwoh CK. Natural history of pain and disability among African-Americans and Whites with or at risk for knee osteoarthritis: a longitudinal study. Osteoarthr Cartil 2018;26:471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Vitiello MV, McCurry SM, Shortreed SM, Balderson BH, Baker LD, Keefe FJ, Rybarczyk BD, Von Korff M. Cognitive-behavioral treatment for comorbid insomnia and osteoarthritis pain in primary care: the lifestyles randomized controlled trial. J Am Geriatr Soc 2013;61:947–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Vitiello MV, Rybarczyk B, Von Korff M, Stepanski EJ. Cognitive behavioral therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. J Clin Sleep Med 2009;5: 355–62. [PMC free article] [PubMed] [Google Scholar]
- [69].Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220–33. [DOI] [PubMed] [Google Scholar]
- [70].Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev 2012;11:CD007407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.