Abstract
The directed differentiation of pluripotent stem cells to a desired lineage often involves complex and lengthy protocols. In order to study the requirements for differentiation in a systematic way, we present here methodology for an iterative approach using combinations of small molecules and biological factors. The factors are used in a cyclical process in which the best combination of factors and concentrations are selected in one round of testing, followed by a modification of the combination and subsequent rounds. While this may produce the desired differentiation in the cell population under study, it is also possible that other strategies may be needed to optimize the differentiation process. These strategies are described in this chapter.
Keywords: Pluripotent stem cells, nonhuman primates, differentiation, algorithms, gene expression
1. Introduction
There is an ongoing need to devise efficient protocols for the differentiation of pluripotent stem cells into defined lineages. This may be required for basic science studies, or for translational and therapeutic investigations. The methodology described in this chapter comprises a combinatorial approach, using factors hypothesized or demonstrated to promote differentiation into a desired lineage, such as neural, cardiac, immune system, and so on. The approach taken here is to rationally combine selected compounds in protocols that take advantage both of the results of prior studies and also of the increasing knowledge of the intracellular signaling pathways that have been identified as being involved. The methodology requires the identification of suitable molecular markers needed for differentiation; these may represent various developmental stages in the specific lineage being studied. A major concern is that the adaptation of protocols across species (for example, from humans to nonhuman primates, NHPs) requires many adjustments to the concentrations of the factors being used and in the timing of their use in the protocol.
This chapter outlines a general method for neural differentiation of pluripotent stem cells, with specific reference to an NHP stem cell line, marmoset iPS cells. Methods for the differentiation of pluripotent stem cells into defined lineages typically use small molecule inhibitors or agonists, combined with biological factors, in various combinations and concentrations, used for various periods of time and added in various orders. Such methods can become extremely complex and offer huge numbers of potential combinations (factors, concentrations, time, order of addition). A systematic approach to this challenge is needed, and this is the approach outlined in this chapter.
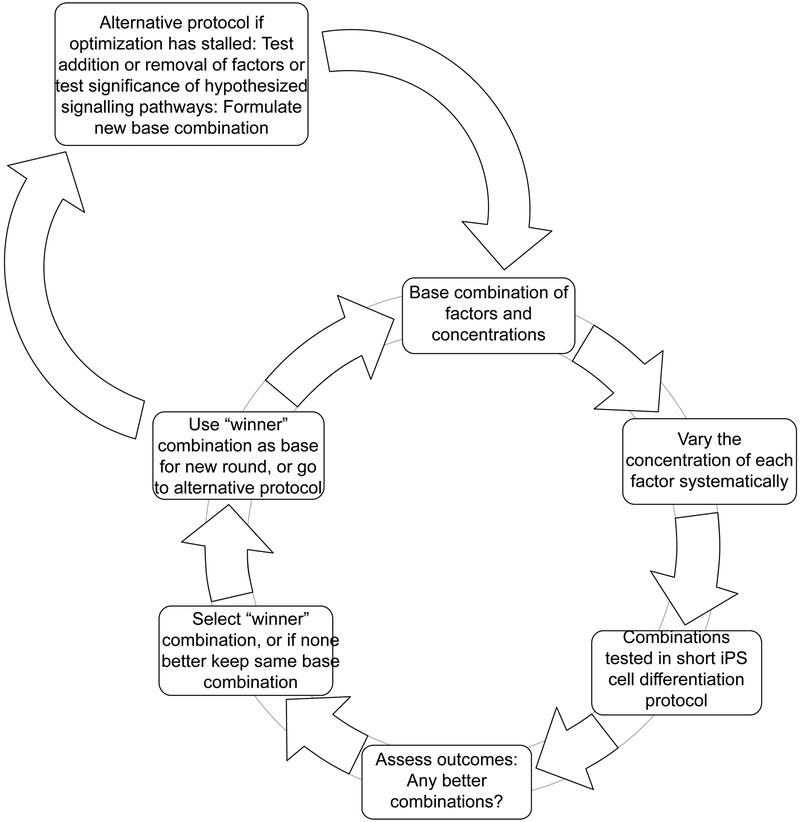
In previous publications we demonstrated that such a combinatorial approach, using small molecules and biological factors, can assist in the rapid optimization of a differentiation protocol (1). We use a cyclical approach in which systematic variations in the concentrations of several factors are used to select a “winning” combination in each cycle. This cyclical approach has been termed feedback system control (2). The protocol described here uses a “hill-climbing” algorithm in which systematic variations in the combinations and concentrations of factors used moves the cell population toward a maximum state of differentiation (1). We assume that the degree of differentiation of the cells following combination treatments is evaluable as a single value, so permitting a combination of factors at specific concentrations to be declared the “winner” at each round. See Note 1.
Here we present this approach, with further variations included as alternatives to the main “hill-climbing” cycle, as shown in Fig. 1.
Fig. 1.
Cyclical combinatorial scheme for differentiation of pluripotent cells, with variations added to permit analysis of specific issues. See Introduction for details. For an example of the outcome of a single round of testing, see Fig. 2; for examples of the outcome of the alternative approaches, other than the main cycle, see Figs. 3 and 4.
2. Materials
2.1. Pluripotent stem cells
An appropriate pluripotent stem cell line: here we used marmoset iPS cells B8 as previously characterized (3).
2.2. Cell culture medium, related materials
E8 medium (STEMCELL Technologies)
DMEM/F12 (Sigma)
Knockout Serum Replacement (KSR; Invitrogen)
Fetal bovine serum (GlobalStem)
Y-27632; inhibitor of Rho-associated, coiled-coil containing protein kinase (ROCK) (Fisher Scientific)
Accutase (BioExpress, Kaysville, UT)
2.3. Small molecule differentiation factors
Y-27632
Dorsomorphin (Fisher Scientific); a selective small molecule inhibitor of BMP signaling
SB431542 (Selleckchem); inhibitor of the TGF-β/Activin/NODAL pathway that inhibits ALK5, ALK4 and ALK7
PD325901 (Biotang); inhibitor of MEK 1 and 2
BIO (Enzo); GSK-3 inhibitor
IWR-1 (Sigma); Wnt inhibitor, promotes β-catenin destruction DAPT (Tocris); Notch inhibitor
all-trans retinoic acid (Sigma)
BMP4 ligand (Peprotech)
TGF-β1 (R&D Systems)
FGF2 (basic FGF) (Stemgent)
2.4. Materials for analysis by qPCR
ABI Prism 7900 HT Sequence Detection System (Applied Biosystems; ABI) and SDS analysis software.
RNA-Bee (Tel-test)
Superscript II reverse transcriptase kit (Invitrogen)
Random primers (Promega)
SYBR green (ABI)
Primers: designed using Primer Express 2.0 software (ABI) against predicted mRNA sequence for marmoset based upon a sequence comparison between the marmoset genome (UCSC Genome Browser) and human Refseq mRNA; β-actin (ACTB) primers as reference gene.
3. Methods
Select an appropriate pluripotent stem cell line for these studies. Here we illustrate the methodology using a marmoset iPS cell line, B8, which we have previously characterized (3). Propagate the cells under conditions that maintain the pluripotent characteristics of the cells (e.g. E8 medium plus 10% fetal bovine serum; ref. 4); this will be specific for the cell line chosen.
In the case of B8 marmoset iPS cells, begin by removing the cells from the culture surface with Accutase and place the cells in nonadhesive U-bottom 96-well plates to create cell aggregates (1). Use DME/F12 with 20% KSR (1). This was found to be optimal for this cell line, but other cell lines may require different protocols (see Note 2).
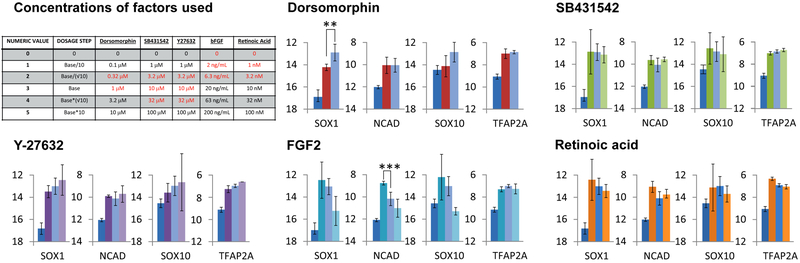
After 24 hours, change the medium on the cell aggregates to a basal differentiation medium (DME/F12 with 20% KSR) to which is added various combinations of factors to be tested. The table in Fig. 2 shows the combinations of factors used, based on prior results obtained with this cell line (1). Adjust the combinations of factors used based on what is already known for the cell line under study (see Note 3).
For each factor under investigation, begin with combinations of factors set at level “3” (3 is an arbitrary designation for the initially used level, allowing decreases to levels 2 or 1, or increases to levels 4 or 5; however greater changes might be needed). This nomenclature follows that in the literature (2). Select the value for level 3 based on values shown to be effective in the literature, whenever that information is available (see Note 4). Derive the other concentration levels (1, 2, 4, 5, etc.) from the concentration level “3” by decreasing or increasing the concentration by a factor of the square root of 10 (see Note 5). In the first round, use combinations that vary from level 3 by one step (2 or 4).
On days 2 and 4, add 75 μl per well of the appropriate medium plus the same factors as added on day 0.
On day 6, harvest the aggregates of cells and prepare total RNA by any applicable method, for example the RNA Bee protocol (1).
Select genes whose expression will be monitored to assess the degree of differentiation of the cells in the desired lineage (see Note 6). Design primers for these genes using appropriate software.
Use standard qPCR techniques to measure the mRNA levels for the selected set of genes (1). Based on an assessment of the levels of mRNA for these genes, choose a “winner” combination for this round of combinations of factors (see example in Fig. 2) (see Note 7).
Repeat the process in steps 2 through 8 above to select another “winner” combination. Repeat the process as long as substantial increases in the expression of the selected differentiation genes are observed.
The process outlined above may be continued as necessary, but additionally other strategies may be employed in order to potentially improve the efficiency of arriving at an optimal combination of factors (Fig. 1) (See Note 8). These alternative strategies may re-start the optimization process if no further improvement is noted using the combination of factors currently being tested (optimization is stalled). These are: (1) testing the appropriate concentrations of factors to be used by assessing their effect on specific intracellular signaling pathways; (2) testing new factors for their potential effects on differentiation, before including them in the combinations being tested; (3) assessing whether assessing changes in mRNAs at times other than the standard (6 days as used here) may be useful; (4) testing whether measuring mRNAs for other genes may be useful. For example, test genes that are in the lineage being targeted (neural) versus other lineages (e.g. mesodermal) or indicators of pluripotency. Based on the outcome of these studies, perform further rounds of testing as in steps 2 through 8. The following steps (11 through 16) outline these procedures.
For testing effectiveness of small molecule factors in specific intracellular signaling pathways, use monolayer culture, e.g. in 12-well plates. Change to E8 medium containing appropriate biological factors with the addition of the small molecules under investigation. Steps 12 through 14 provide three examples.
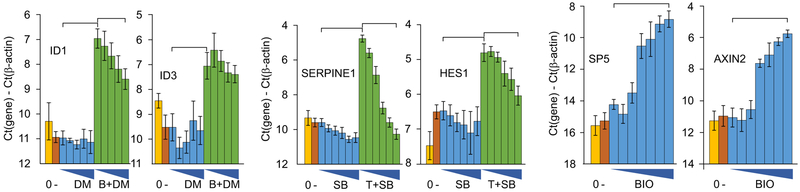
To assess the effectiveness of dorsomorphin on BMP signaling, add recombinant BMP4 together with various concentrations of dorsomorphin (5). Select potentially BMP- responsive target genes, such as SMAD target genes ID1 and ID3 (6, 7). The results of this test on marmoset B8 cells are illustrated in Fig. 3.
To assess the effectiveness of the TGF-β/activin/nodal inhibitor SB431542, remove TGFβ−1 from the medium for culture of the cells (typically the medium contains 2 ng/ml TGFβ-1), then add a higher level of TGF-β1 (10 ng/ml) together with the inhibitor. Use appropriate target genes, such as SERPINE1 and HES1; expression of these genes is inhibited by SB431542 (8 – 10) (Fig. 3).
The small molecule BIO activates the Wnt pathway by inhibiting GSK3. To assess the appropriate concentration of BIO, add various concentrations in E8 medium and assess the expression of Wnt-responsive genes such as SP5 and AXIN2 (11). (Fig. 3).
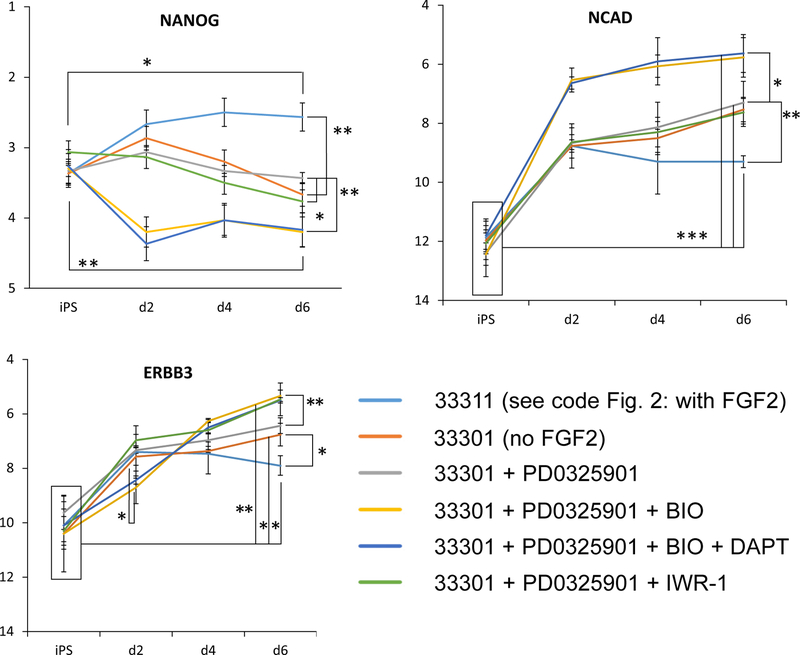
As indicated in Fig. 1, the cyclical “hill-climbing” process may require adjustment by adding other factors to the mix being tested. To test whether other factors may be required for optimal differentiation, perform the process in steps 2 through 8 above with the addition of these new factors. For example, test whether FGF2 may be replaced by other factors by omitting FGF2 and adding BIO, PD0325901, DAPT, or IWR-1 (Fig. 4).
As a further variation, harvest the aggregates at times other than the standard (here, we used 2 and 4 days instead of the standard 6 days) (Fig. 4).
Fig. 2.
Example of the results of one round of testing of combinations of various factors on differentiation of marmoset iPS cells. The concentrations shown in red in the table in the upper left were used in this round; other concentrations (initially level “3”) were used in prior rounds, or may be used in future rounds. Only two concentrations of dorsomorphin were used because the higher concentrations were toxic. Expression of the neuroectoderm marker genes SOX1 and NCAD, and the neural crest markers SOX10 and TFAP2A, was assessed. The y axis represents the mRNA level as Ct(gene)-Ct(β-actin); higher bars indicate higher levels of mRNA. In each case the first bar shows the value before the 6 days protocol and the other 2 or 3 bars show the values following the treatment with the factors as indicated in the table. Data are presented as means +/− SEM (n=3). * = p<0.05, ** = p<0.01, *** = p<0.001. See Note 9.
Fig. 3.
Analysis of the effects of three small-molecule factors on genes that respond to activation of three different intracellular pathways in marmoset iPS cells. In each case “0” = mRNA level before treatment and “-” = level following 24 hours in basal medium only. DM = dorsomorphin at a range of concentrations from 0 to 2 μM; B = 40 ng/ml BMP4. BMP4 treatment induced a statistically significant upregulation of ID1 and ID3 mRNA; co-treatment with dorsomorphin resulted in lowered ID1, but not a statistically significant lowering of ID3. SB = SB4321542 at a range of concentrations from 0 to 3 μM; T = 10 ng/ml TGF-β1. Treatment with TGF-β1 increased expression of SERPINE and HES1, while SB431542 coadministration resulted in dosage-dependent reduction in expression of both genes. B = BIO at a range of concentrations from 0 to 2 μM. BIO increased the expression of Wnt/β-catenin targets SP5 and AXIN2. See Note 10.
Fig. 4.
Analysis of the potential effects of including new factors (BIO, PD0325901, DAPT, IWR-1) on differentiation of marmoset iPS cells. In this case gene expression was tested after 2 and 4 days of treatment as well as 6 days; results for three representative genes, NANOG, NCAD and ERBB3, are shown. We compared two baseline conditions (with and without FGF2; coded as 33311 and 33301, see Fig. 2) with the addition of 1 μM PD0325901, 2 μM BIO, 10 μm DAPT, or 3 μM IWR-1. Statistically significant differences are indicated as ***(p<0.001), ** (p<0.01) and * (p<0.05). See Note 11.
4. Notes
The underlying assumptions employed in the “hill-climbing” approach have been discussed in a previous publication (1). Versions of the original feedback control system on which this is based (2) have been used in a variety of studies (12 – 17). These versions are often more complex than the approach described here because they attempt to avoid local maxima (a peak other than the global maximum). However, we make the simpler assumption that there is only a global maximum because examples in differentiation protocols have not yet shown a situation where this simple assumption has proved to be invalid. In these experiments we pick the “winning” combination of factors and concentrations by an overall assessment of the expression of a set of genes. In a future expansion of this work, this assessment could potentially be done mathematically; however, at the present stage of development of this approach we do not have sufficient information to formulate a purely mathematical method for “winner” selection.
The neural differentiation of pluripotent stem cells has been demonstrated under both monolayer conditions (18) and suspension culture (19). Additionally, reports of induction of neural crest cells from human pluripotent stem cells have been reported in both monolayer and suspension conditions. In our system, one aggregate (often referred to as an embryoid body) was generated per well of a U-bottom 96-well plate, similar to those utilized in other SFEBq protocols (19). Other cell lines may perform better under monolayer conditions, and therefore preliminary studies should establish which conditions are optimal for the specific cell line.
A significant advance in the field of neural differentiation from pluripotent cells was the introduction of the concept of FGF withdrawal coupled with dual SMAD inhibition (18). Initially this was accomplished by the combination of noggin and SB431542, while later versions substituted chemical inhibitors such as dorsomorphin for the more expensive noggin (20, 21).
In some cases, starting concentrations used may be based on an already completely defined, published protocol. In most cases this would have been defined for mouse or human pluripotent cells; while the optimal combinations and concentrations might be quite different for an NHP cell line, or other species, it is reasonable to begin with any combination shown to be effective in human or mouse pluripotent stem cells.
In practice the step change in concentration for most factors may be set at the square root of 10, but occasionally it may be necessary to change this; for example, concentrations may be stepped up or stepped down by a factor of 10.
It is important to select an appropriate set of genes for expression testing; this may be based on what is already established for differentiation in cell culture, or on what is known for embryological development in the desired lineage. Preliminary testing may be needed before an appropriate set of genes is defined.
Any regular qPCR protocol may be used; we used the SYBR green method, but any quantitative protocol may be employed.
As illustrated in Fig. 1, the normal cycle (“hill-climbing” protocol) may be interspersed with alternative strategies to improve the extent of differentiation. This may be especially necessary when using NHP cells, or generally any species other than human or mouse. It may be necessary to verify that an inhibitor commonly used in those species works in the NHP species under investigation, or if the effective concentration range may differ substantially. Because of the complexity of many differentiation protocols, and because the molecular targets of the drugs/factors used are not always known, it is not yet clear whether a simple “hill climbing” iterative approach to optimizing differentiation will always be appropriate. More complex algorithms might be necessary. This will require enough testing to ensure that the theoretical possibility that the search becomes stalled on a local maximum or plateau rather than a global maximum is unlikely. However, if practical experience shows that this happens with some frequency, algorithms should be adjusted to avoid this.
The results obtained here show only modest improvements over the starting combination for this round (e.g. dorsomorphin effect on SOX1) and show that other factors are already optimal (e.g. FGF2). Therefore these data indicate the need for alternative strategies to be tested (Figs. 3 and 4).
Under monolayer culture of marmoset iPS cells, we found that dorsomorphin partially suppressed BMP4-induced transcription of a known BMP target: ID1, at 2 μM, but failed to significantly suppress ID3. These results suggest that dorsomorphin produces an incomplete inhibitory effect on BMP signaling in these marmoset iPS cells, similar to that reported for human cells (22, 23). These data for SB431542 with marmoset iPS cells are in line with SB431542 potency reported for human cells, (0.5–1 μM; ref. 23). Our data suggest that SB431542 has Activin/Nodal/TGF-β inhibition potency in marmoset iPS cells similar to that reported for human cells. BIO stimulated target genes at concentrations similar to that reported in other systems.
The figure shows the effects of introducing four new small molecule factors on the neuroectodermal marker NCAD, the neural crest marker ERBB3, and the pluripotency marker NANOG. The starting combination was the that coded as 33311 or 33301, see Table in Fig. 2, depending if FGF2 was included or not. Data were also collected for the neuroectodermal markers SOX1, PAX6, and MSI1; for the floor plate marker FOXA2; for the neural crest markers SOX10 and TFAP2A; for the endodermal marker SOX17; for the mesodermal markers BRACHYURY and TBX6; for the mesoderm/neural crest markers SNAI1, SNAI2, and SOX9; and for the ectodermal marker GBX2. The combination of BIO and PD0325901, with or without DAPT, significantly improved differentiation over the baseline conditions (with or without FGF2). PD0325901 alone, or with IWR-1, did not increase the differentiation level, thus indicating that for future rounds of combinations of factors the role of BIO would be worth exploring.
Table 1.
Concentrations of factors used. For details see Figure 2.
| NUMERIC VALUE | DOSAGE STEP | Dorsomorphin | SB431542 | Y27632 | bFGF | Retinoic Acid |
|---|---|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | Base/10 | 0.1 μM | 1 μM | 1 μM | 2 ng/mL | 1 nM |
| 2 | Base/(√10) | 0.32 μM | 3.2 μM | 3.2 μM | 6.3 ng/mL | 3.2 nM |
| 3 | Base | 1 μM | 10 μM | 10 μM | 20 ng/mL | 10 nM |
| 4 | Base*(√10) | 3.2 μM | 32 μM | 32 μM | 63 ng/mL | 32 nM |
| 5 | Base*10 | 10 μM | 100 μM | 100 μM | 200 ng/mL | 100 nM |
5. References
- 1.Farnsworth SL, Qiu Z, Mishra A, Hornsby PJ (2013) Directed neural differentiation of induced pluripotent stem cells from non-human primates. Exp. Biol. Med 238:276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsutsui H, Valamehr B, Hindoyan A, Qiao R, Ding X, Guo S, Witte ON, Liu X, Ho CM, Wu H (2011) An optimized small molecule inhibitor cocktail supports long-term maintenance of human embryonic stem cells. Nat. Commun 2:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Y, Zhang Y, Mishra A, Tardif SD, Hornsby PJ (2010) Generation of induced pluripotent stem cells from newborn marmoset skin fibroblasts. Stem Cell Res. 4:180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mishra A, Qiu Z, Farnsworth SL, Hemmi JJ, Li M, Pickering AV, Hornsby PJ (2016) Induced pluripotent stem cells from nonhuman primates. Methods Mol. Biol 1357:183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong CC, Yu PB (2009) Applications of small molecule BMP inhibitors in physiology and disease. Cytokine Growth Factor Rev. 20:409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hollnagel A, Oehlmann V, Heymer J, Ruther U, Nordheim A (1999) Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J. Biol. Chem 274:19838–45. [DOI] [PubMed] [Google Scholar]
- 7.Ying QL, Nichols J, Chambers I, Smith A (2003) BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 115:281–92. [DOI] [PubMed] [Google Scholar]
- 8.Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS (2002) SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol. Pharmacol 62:65–74. [DOI] [PubMed] [Google Scholar]
- 9.Farberov S, Meidan R (2016) Thrombospondin-1 Affects Bovine Luteal Function via Transforming Growth Factor-Beta1-Dependent and Independent Actions. Biol. Reprod 94:25–. [DOI] [PubMed] [Google Scholar]
- 10.Gudey SK, Sundar R, Heldin CH, Bergh A, Landstrom M (2017) Pro-invasive properties of Snail1 are regulated by sumoylation in response to TGFbeta stimulation in cancer. Oncotarget 8:97703–97726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Jaime-Soguero A, Aulicino F, Ertaylan G, Griego A, Cerrato A, Tallam A, Del Sol A, Cosma MP, Lluis F (2017) Wnt/Tcf1 pathway restricts embryonic stem cell cycle through activation of the Ink4/Arf locus. PLoS Genet. 13:e1006682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang Y, Hodges A, Ong E, Roberts W, Piermarocchi C, Paternostro G. (2014) Identification of drug combinations containing imatinib for treatment of BCR-ABL+ leukemias. PLoS One. 9:e102221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding X, Matsuo K, Xu L, Yang J, Zheng L. (2015) Optimized combinations of bortezomib, camptothecin, and doxorubicin show increased efficacy and reduced toxicity in treating oral cancer. Anticancer Drugs. 26:547–54. [DOI] [PubMed] [Google Scholar]
- 14.Weiss A, Ding X, van Beijnum JR, Wong I, Wong TJ, Berndsen RH, Dormond O, Dallinga M, Shen L, Schlingemann RO, Pili R, Ho CM, Dyson PJ, van den Bergh H, Griffioen AW, Nowak-Sliwinska P. (2015) Rapid optimization of drug combinations for the optimal angiostatic treatment of cancer. Angiogenesis. 18:233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss A, Berndsen RH, Ding X, Ho CM, Dyson PJ, van den Bergh H, Griffioen AW, Nowak-Sliwinska P. (2015) A streamlined search technology for identification of synergistic drug combinations. Sci Rep. 5:14508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Q, Zhang C, Ding X, Deng H, Zhang D, Cui W, Xu H, Wang Y, Xu W, Lv L, Zhang H, He Y, Wu Q, Szyf M, Ho Cm, Zhu J. (2015) Preclinical optimization of a broad-spectrum anti–bladder cancer tri-drug regimen via the Feedback System Control (FSC) platform. Sci Rep. 5:11464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding X, Njus Z, Kong T, Su W, Ho CM, Pandey S. (2017) Effective drug combination for Caenorhabditis elegans nematodes discovered by output-driven feedback system control technique. Sci Adv. 3:eaao1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L (2009) Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotech 27:275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, Wataya T, Nishiyama A, Muguruma K, Sasai Y (2008) Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3:519–32. [DOI] [PubMed] [Google Scholar]
- 20.Zhou J, Su P, Li D, Tsang S, Duan E, Wang F (2010) High-efficiency induction of neural conversion in human ESCs and human induced pluripotent stem cells with a single chemical inhibitor of transforming growth factor beta superfamily receptors. Stem Cells 28:1741–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morizane A, Doi D, Kikuchi T, Nishimura K, Takahashi J (2011) Small-molecule inhibitors of bone morphogenic protein and activin/nodal signals promote highly efficient neural induction from human pluripotent stem cells. J. Neurosci. Res 89:117–26. [DOI] [PubMed] [Google Scholar]
- 22.Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT (2008) Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat. Chem. Biol 4:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogt J, Traynor R, Sapkota GP (2011) The specificities of small molecule inhibitors of the TGF and BMP pathways. Cell Signal. 23:1831–42. [DOI] [PubMed] [Google Scholar]