Abstract
Background:
Survival estimates for soft tissue sarcomas (STS) and malignant bone tumors (BT) diagnosed in pediatric, adolescent, and young adult patients are not easily available. We present survival estimates based on a patient having survived a defined period of time (conditional survival). Conditional survival estimates for the short-term were calculated for patients from diagnosis to the first five years after diagnosis and for patients surviving in the long-term (up to 20 years after diagnosis).
Methods:
We identified 703 patients who were diagnosed with a STS or BT at age ≤25 years from January 1, 1986 to December 31, 2012 at a large pediatric oncology center in Salt Lake City, Utah, United States. We obtained cancer type, age at diagnosis, primary site, and demographic data from medical records, and vital status through the National Death Index. Cancer stage was available for a subset of the cohort through the Utah Cancer Registry. Cox proportional hazards models, adjusted for age and sex, calculated survival estimates for all analyses.
Results:
Short-term survival improves over time for both sarcomas. Short-term survival for STS from diagnosis (Year 0) did not differ by sex, but short-term survival starting from 1-year post diagnosis was significantly worse for male patients (Survival probability 1-year post-diagnosis [SP1]:77% [95% CI:71-83]) than female patients (SP1:86% [81–92]). Survival for patients who were diagnosed at age ≤10 years (Survival probability at diagnosis [SP0]:85% [79–91]) compared to diagnosis at ages 16–25 years (SP0:67% [59–75]) was significantly better at all time-points from diagnosis to 5-years post-diagnosis. Survival for axial sites (SP0:69% [63–75]) compared to extremities (SP0:84% [79–90]) was significantly worse from diagnosis to 1-year post-diagnosis. Survival for axial BT (SP0: 64% [54–74] was significantly worse than BT in the extremities (SP0:73% [68–79]) from diagnosis to 3-years post diagnosis. Relapsed patients of both sarcoma types had significantly worse shortterm survival than non-relapsed patients. Long-term survival for STS in this cohort is 65% at diagnosis, and improves to 86% 5-years post-diagnosis. BT survival improves from 51% at diagnosis to 78% at 5-years postdiagnosis.
Conclusion:
Conditional survival for short- and long-term STS and BT improve as time from diagnosis increases. Short-term survival was significantly affected by patients' sex, age at diagnosis, cancer site, and relapse status.
Keywords: Cancer, Cancer survival, Epidemiology, Pediatrics
1. Introduction
Soft tissue sarcomas and malignant bone tumors account for 20% of solid tumors diagnosed among children and 13% among adolescent and young adults [1]. These sarcomas have relatively low survival compared to more common childhood cancers (e.g. leukemia), particularly if disease is metastatic at diagnosis [1,2]. Survival estimates derived at diagnosis are commonly used in prognostication, but may not be as accurate for patients who complete therapy or attain remission [3]. Patients and families ask providers for updated survival information based on their current status, yet there is a scarcity of analyzed data available to address these pertinent medical questions after therapy ceases. As such, understanding conditional survival (the risk of mortality given that a patient has survived a defined number of years) is critical for providing prognostic information to patients and their caregivers prior to and after cancer therapy, which includes systemic chemotherapy and local control with surgery and/or radiation. Since the chemotherapy and high dose radiation therapy used to treat soft tissue sarcomas and malignant bone tumors can cause toxic effects in the heart and other organs, as well as second cancers [4], obtaining patient-specific survival estimates can inform surveillance and monitoring of patients who are in early to late survivorship, and reduce patient and caregiver anxiety.
At this time, few studies have specifically evaluated the conditional survival of pediatric, adolescent, and young adult patients diagnosed with soft tissue sarcoma and malignant bone tumors [5]. Previous conditional survival analyses of pediatric sarcomas report improvements in survival through 30 years of follow-up [6,7]. We provide short-term and long-term conditional survival estimates for pediatric, adolescent and young adult cancer patients diagnosed with soft tissue or malignant bone tumors by clinical characteristics at diagnosis. Here, short-term conditional survival is defined as surviving an additional five years from a specific time point (e.g. one year after diagnosis), and are presented annually from diagnosis to five years post-diagnosis. Short-term survival statistics are stratified by year of diagnosis, cancer site, sex, age at diagnosis, and cancer stage. Long-term conditional survival estimates are calculated for twenty years of follow-up, starting at diagnosis, and one, three, and five years post-diagnosis. Both followup time frames are longer than the median time for recurrence as noted in previous studies [8-10].
This study determines annual improvements in the conditional survival of soft tissue sarcomas and malignant bone tumors, and examines how annual improvements in conditional survival differ by year of diagnosis, cancer site, sex, age at diagnosis, cancer stage, and relapse from diagnosis to five years post-diagnosis. We also examine long-term survival at diagnosis, and at one, three, and five years post-diagnosis over twenty years of follow-up.
2. Materials and methods
2.1. Data collection
For this retrospective cohort study, we obtained records of patients diagnosed with cancer at age 25 or younger, between January 1, 1986 and December 31, 2012, and received treatment at any Intermountain Healthcare (IH) facility. IH is a Utah-based not-for-profit health system that includes Primary Children’s Hospital (PCH), the only pediatric oncology center in a five-state catchment region [11]. IH maintains an enterprise data warehouse (EDW) with an internal cancer registry that reports cancer diagnoses to the Utah Cancer Registry (UCR). IH and UCR data were linked to records from the Utah Population Database (UPDB), a population registry for containing vital records and other government records for Utah residents. The IH and the University of Utah Institutional Review Boards approved this study.
Subjects were eligible for inclusion if their cancer diagnosis was coded as “IX. Soft Tissue and Other Extraosseous Sarcomas” or “VIII. Malignant Bone Tumors” by the International Classification for Childhood Cancer (ICCC) III categorization system. Although most PCH oncology patients reside in Utah, PCH also sees patients from other states in the Intermountain West (e.g. Montana, Idaho, and Nevada) and the West Coast.
Subjects were followed from first diagnosis date until their date of death or January 1, 2014. Since cancer stage was only available for a subset of the cohort, short-term survival estimates by therapy and stage were analyzed in a separate cohort. Subjects that were alive at the end of follow-up were considered censored. We also conducted short-term survival analysis stratified by relapse, in which relapsed subjects were followed from their first relapse date to the end of follow-up or death.
Patients who visited an IH clinic after January 1, 2014 were considered alive. National Death Index (NDI) identified death date and cause of death as of January 1, 2014 for other subjects using date of birth, first, middle, last name, sex, and state of birth. If subjects had hyphenated last names, NDI received the combined and individual names.
2.2. Demographic and clinical variables
We obtained birthdate and sex from the IH data warehouse. Hispanic ethnicity was determined using the ethnicity classification stored in UPDB and matching participant surnames to the 1990 Census Heavily Hispanic Surname list [12,13]. Participants with hyphenated names were matched based on either last name. If a participant was Hispanic according to either source, we classified the participant as Hispanic [12].
Clinical variables were obtained from IH. Year of diagnosis was categorized as 1986 to 2000 and 2001 to 2012 based on the data distribution. Cancer site at diagnosis was identified using medical records, surgical notes, and coded variables. Sarcomas located in the shoulders and lower or upper limbs were labeled extremity sites; sarcomas in the head, neck, spine, torso or pelvic region were axial sites. Date of first relapse was obtained from IH.
Staging data from the UCR were matched to IH records by a unique identifying number. As this information was only available for patients who resided in Utah at the time of diagnosis, these analyses reflect a subset of our full sample that was treated at PCH. We distinguished local/regional tumors from distant/metastatic disease stage.
2.3. Statistical analyses
Analyses for soft tissue sarcomas and malignant bone tumors were conducted separately. Adjusted Cox proportional hazards models produced predicted survival probabilities for short-term survival statistics for five-year increments starting at diagnosis (Year 0), and then continued from one year post-diagnosis (Year 1) to five years post-diagnosis (Year 5) [14]. Short-term analyses were stratified by sex, year of diagnosis, and cancer site (axial vs extremity) to produce the estimated survival probabilities. We also obtained p-values for comparisons of yearly survival times between each group using the same Cox models. To determine which variables would be included for adjustment, we examined individual associations between covariates and mortality, and then compared results to a stepwise model with entry criteria of 0.1 and exit criteria of 0.05. All models were adjusted for age at diagnosis and sex where appropriate. We indicated significant differences (p ≤ 0.05) between groups at each year.
Adjusted Cox models also produced survival probabilities for longterm survival statistics from diagnosis, and survival curves for one, three, and five years post-diagnosis for all patients diagnosed between January 1, 1986 and December 31, 2012 to the end of follow-up on January 1, 2014. Analyses were conducted in SAS version 9.4.
3. Results
Our cohort contains 703 soft tissue sarcoma (n = 377) and malignant bone tumor patients (n = 326). Ninety-two percent of the 221 deaths during the study period are attributed to cancer-related causes. Rhabdomyosarcoma is the most common soft tissue sarcoma (n = 135, 35.8%). The two most common malignant bone tumors are Ewing sarcoma (n = 104, 31.9%) and osteosarcoma (n = 194, 59.5%). The remainder are bone tumors that are not of osteosarcoma or Ewing sarcoma origin (n = 22, 6.7%). The largest percent of soft tissue sarcoma and malignant bone tumor patients are 16 to 25 years of age at diagnosis (34.0% and 38.0%, respectively) (Table 1). The distribution of soft tissue and malignant bone tumor diagnoses are similar between females and males, and patients of Latino and Non-Latino ethnicity. Soft tissue sarcomas are most frequently reported in axial locations (65.3%), while malignant bone tumors are more commonly found in the extremities (72.7%). The average time to relapse is 3.2 years for soft tissue sarcomas and 2.2 years for malignant bone tumors.
Table 1.
Demographic and clinical characteristics of cohort.
| Total 2 | Soft tissue sarcoma |
Malignant bone tumor |
||||
|---|---|---|---|---|---|---|
| N = 377 | N = 326 | |||||
| N = 703 | n | Column% | n | Column% | ||
| Year of diagnosis | 1986–2000 | 342 | 174 | 46.2 | 168 | 51.5 |
| 2001–2012 | 360 | 202 | 53.6 | 158 | 48.5 | |
| – | ||||||
| Cancer site | Axial | 335 | 246 | 65.3 | 89 | 27.3 |
| Extremity | 368 | 131 | 34.7 | 237 | 72.7 | |
| – | ||||||
| Sex | Female | 300 | 169 | 44.8 | 131 | 40.2 |
| Male | 403 | 208 | 55.2 | 195 | 59.8 | |
| – | ||||||
| Age at diagnosis | 0–5 | 124 | 105 | 27.9 | 19 | 5.8 |
| 6–10 | 119 | 56 | 14.9 | 63 | 19.3 | |
| 11–15 | 208 | 88 | 23.3 | 120 | 36.8 | |
| 16–25 | 252 | 128 | 34.0 | 124 | 38.0 | |
| – | ||||||
| Relapse | Yes | 113 | 53 | 14.1 | 60 | 18.4 |
| No | 590 | 324 | 85.9 | 266 | 81.6 | |
| – | ||||||
| Ethnicity | Not Hispanic | 628 | 338 | 89.7 | 290 | 89.0 |
| Hispanic | 75 | 39 | 10.3 | 36 | 11.0 | |
| – | ||||||
| Number of subjects, by year of follow-up1 | Years after dx | - | - | - | ||
| 1 | 644 | 342 | - | 302 | - | |
| 2 | 558 | 300 | - | 258 | - | |
| 3 | 508 | 275 | - | 233 | - | |
| 4 | 457 | 252 | - | 205 | - | |
| 5 | 415 | 233 | - | 182 | - | |
| 10 | 289 | 163 | - | 126 | - | |
| 20 | 92 | 49 | - | 43 | - | |
| – | ||||||
| Number of deaths | _ | 221 | 112 | _ | 108 | _ |
Risk sets for analysis.
Total N’s that do not sum to 703 have missing values.
Short-term survival from diagnosis (Year 0) to five years post-diagnosis (Year 5) improves as time from diagnosis increases (Table 2). Survival from diagnosis to five years post-diagnosis for soft tissue sarcomas is 75% (95% confidence interval [CI] = 71-80). At one year from diagnosis (Year 1), survival for soft tissue sarcomas overall is 81% (CI = 77-86), and then improves to 94% (CI = 90-97) at five years post-diagnosis. For malignant bone tumors, survival from diagnosis to five years past diagnosis is 71% (CI = 66-76), and survival at one year post-diagnosis improves from 74% (CI = 69-79) to 93% (CI = 90-97) at five years post-diagnosis.
Table 2.
Adjusted 5-year survival statistics for pediatric, adolescent, and young adult soft tissue sarcoma and malignant bone tumor patients.
| Year 01 |
Year 1 |
Year 2 |
Year 3 |
Year 4 |
Year 5 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| Soft tissue sarcoma | ||||||||||||
| Overall | 75 | 71–80 | 81 | 77–86 | 86 | 83–91 | 90 | 87–94 | 92 | 88–96 | 94 | 90–97 |
| Years of diagnosis | ||||||||||||
| 1986 – 2000 | 74 | 68–81 | 82 | 76–88 | 85 | 79–91 | 90 | 85–95 | 93 | 88–97 | 93 | 89–98 |
| 2001–2012 (ref) | 76 | 70–82 | 81 | 75–87 | 88 | 83–94 | 91 | 86–96 | 91 | 86–97 | 95 | 90–100 |
| Cancer site | ||||||||||||
| Axial | 69* | 63–75 | 77* | 71–83 | 85 | 80–91 | 91 | 86–96 | 90 | 84–95 | 94 | 89–99 |
| Extremity (ref) | 84 | 79–90 | 87 | 82–93 | 88 | 82–94 | 90 | 84–96 | 94 | 90–00 | 94 | 89–99 |
| Sex | ||||||||||||
| Female | 80 | 74–86 | 86* | 81–92 | 92* | 88–97 | 94* | 90–98 | 96* | 93–100 | 99* | 97–100 |
| Male (ref) | 71 | 65–78 | 77 | 71–83 | 82 | 76–88 | 87 | 81–93 | 88 | 83–94 | 89 | 83–95 |
| Age at diagnosis | ||||||||||||
| 0–10 years | 85* | 79–91 | 91* | 86–96 | 94* | 90–98 | 98* | 95–100 | 97* | 94–100 | 98* | 95–100 |
| 11–15 years | 70 | 61–80 | 73 | 64–84 | 80 | 72–90 | 83 | 74–93 | 92 | 85–100 | 95 | 89–100 |
| 16–25 years (ref) | 67 | 59–75 | 75 | 67–83 | 81 | 74–90 | 85 | 78–93 | 85 | 78–94 | 87 | 80–96 |
| Bone tumor | ||||||||||||
| Overall | 71 | 66–76 | 74 | 69–79 | 83 | 78–88 | 85 | 81–90 | 91 | 86–95 | 93 | 90–97 |
| Years of diagnosis | ||||||||||||
| 1986 – 2000 | 64* | 57–72 | 69 | 62–77 | 80 | 74–88 | 84 | 77–90 | 89 | 84–95 | 94 | 89–98 |
| 2001–2012 (ref) | 78 | 71–85 | 79 | 71–86 | 86 | 79–93 | 88 | 82–95 | 93 | 87–99 | 93 | 86–100 |
| Cancer site | ||||||||||||
| Axial | 64* | 54–74 | 66* | 56–77 | 70* | 60–83 | 76* | 65–88 | 85 | 75–96 | 90 | 81–100 |
| Extremity (ref) | 73 | 68–79 | 77 | 71–83 | 87 | 82–93 | 89 | 84–94 | 93 | 88–97 | 94 | 90–99 |
| Sex | ||||||||||||
| Female | 75 | 68–83 | 76 | 68–84 | 83 | 76–91 | 86 | 79–94 | 91 | 87–99 | 93 | 87–99 |
| Male (ref) | 67 | 61–74 | 73 | 66–80 | 82 | 76–89 | 85 | 79–92 | 91 | 85–96 | 94 | 89–99 |
| Age at diagnosis | ||||||||||||
| 0–10 years | 77* | 68–87 | 82* | 73–91 | 85 | 77–91 | 84 | 74–94 | 90 | 82–99 | 93 | 86–100 |
| 11–15 years | 75* | 68–84 | 80* | 72–88 | 87 | 80–94 | 88 | 81–95 | 91 | 84–98 | 91 | 85–98 |
| 16–25 years (ref) | 62 | 54–71 | 63 | 55–73 | 76 | 67–86 | 84 | 75–93 | 91 | 84–99 | 96 | 91–100 |
Significant at p≤0.05.
Year 0 starts at diagnosis.
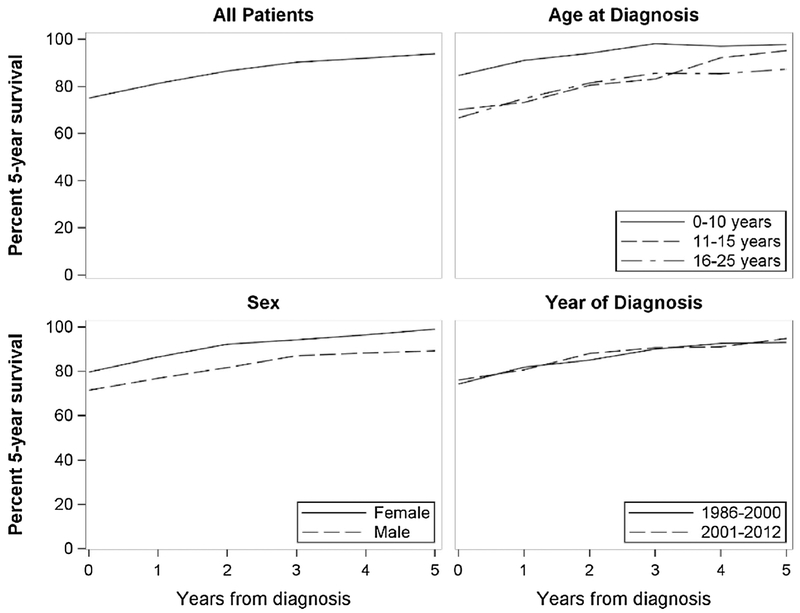
For soft tissue sarcoma, short-term estimates differ significantly by cancer site, sex, and age at diagnosis (Table 2 and Fig. 1). Survival for axial sarcomas is significantly worse from diagnosis and one year postdiagnosis than sarcomas found in the extremities, but those differences were not significant after two years. Survival among male patients is significantly worse than female patients at one year post-diagnosis, and did not improve with time. Patients diagnosed at 10 years or younger have significantly better survival from diagnosis through five years post-diagnosis than patients diagnosed between 16 and 25 years. Relapsed patients have significantly worse survival than non-relapsed patients until five years post-diagnosis, but still improves from 40% (CI = 29-55) at relapse to 78% (CI = 59-100) at five years post-relapse (non-relapses: Year 0 = 75%, CI = 70-80; Year 5 = 97%, CI = 94-99) (data not shown).
Fig. 1.
Conditional survival for an additional 5 years for each year from diagnosis of pediatric, adolescent, and young adult soft tissue sarcoma patients, by diagnosis year, age at diagnosis, and sex.
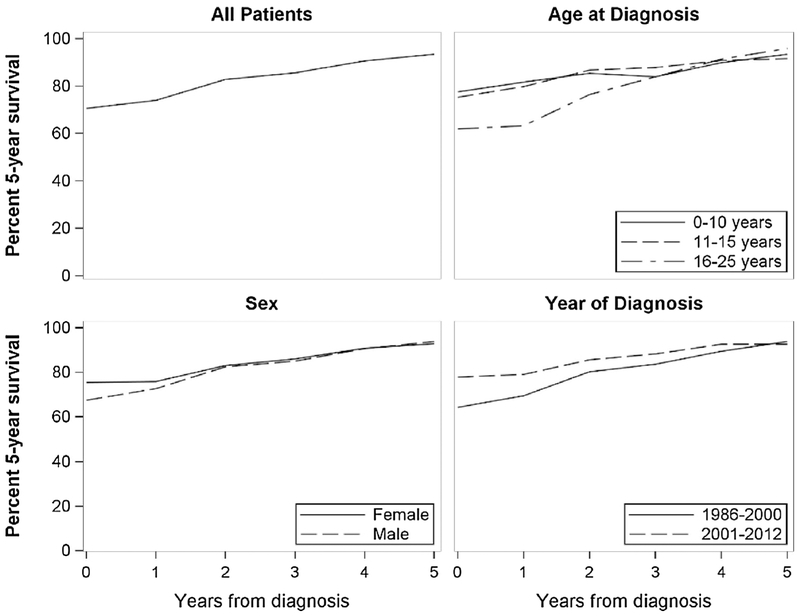
For localized malignant bone tumors, short-term conditional survival differs by year of diagnosis, cancer site, and age of diagnosis (Table 2 and Fig. 2). Differences in survival for patients diagnosed between 1986 and 2000 and patients diagnosed between 2001 and 2012 are significant at diagnosis, but not after one year post-diagnosis. Short-term survival at diagnosis to three years post-diagnosis for axial bone tumors is significantly worse than survival for bone tumors found in the extremities. Survival of malignant bone tumor patients diagnosed between 16 and 25 years is significantly lower at all time points than sarcomas diagnosed in patients aged 10 years or less. Survival for relapsed malignant bone tumor patients improves from 40% (CI = 29-55) at first relapse date to 78% (CI = 53-100) at five years post-relapse, but is significantly worse than non-relapsed patients at all time points.
Fig. 2.
Conditional survival for an additional 5 years for each year from diagnosis of pediatric, adolescent, and young adult malignant bone tumor patients, by diagnosis year, age of diagnosis, and sex.
In the subcohort of 428 patients with staging information from UCR (Table 3), 33.1% of soft tissue sarcomas are rhabdomyosarcomas, and 34.9% of malignant bone tumors are Ewing sarcomas and 63.5% are osteosarcomas. Improvements in local/regional soft tissue sarcomas (86%, CI = 81-91 to 94% at Year 0, CI = 90-99 at Year 5) and malignant bone tumors (79%, CI = 73-86 at Year 0 to 95%, CI = 90-100 at Year 5) were seen over the five year time frame (Table 4). Distant/metastatic stage sarcomas show a similar trend, but survival estimates for soft tissue sarcomas (33%, CI = 22-48) and distant/metastatic malignant bone tumors (38%, CI = 26-54) at diagnosis are significantly lower than estimates for local/regional stage sarcomas. These differences are not significant at five years post-diagnosis for soft tissue sarcomas, and four years for malignant bone tumors.
Table 3.
Classification by cancer stage and histology for pediatric, adolescent, and young adult soft tissue and malignant bone tumor patients.1
| All diagnoses | Soft Tissue Sarcoma |
Malignant bone tumor |
|||
|---|---|---|---|---|---|
| n = 236 |
n = 192 |
||||
| n | n | % | n | % | |
| Stage | |||||
| Distant | 98 | 51 | 21.6 | 47 | 24.5 |
| Local/Regional | 330 | 185 | 78.4 | 145 | 75.5 |
| Histology | |||||
| Rhabdomyosarcoma | 78 | 33.1 | - | - | |
| Other soft tissue sarcoma | 158 | 66.9 | - | - | |
| Ewing sarcoma | - | - | 67 | 34.9 | |
| Osteosarcoma | - | - | 122 | 63.5 | |
| Other bone tumors | - | - | 3 | 1.6 | |
Staging information was only available for patients with records in the Utah Cancer Registry; therefore, this table reflects a subset of the full cohort.
Table 4.
Five-year conditional survival of pediatric, adolescent, and young adult soft tissue and bone tumor patients by cancer stage.
| Year 01 |
Year 1 |
Year 2 |
Year 3 |
Year 4 |
Year 5 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | ||
| Soft Tissue Sarcoma | Stage | ||||||||||||
| Distant/Metastatic | 33* | 22–48 | 42* | 29–61 | 60* | 43–83 | 69* | 50–95 | 73* | 53–100 | 90 | 74–100 | |
| Local/Regional (ref) | 86 | 81–91 | 88 | 83–93 | 88 | 83–94 | 92 | 88–96 | 94 | 90–98 | 94 | 90–99 | |
| Malignant Bone Tumor | Stage | ||||||||||||
| Distant/Metastatic | 38* | 26–54 | 50* | 36–69 | 67* | 51–89 | 74* | 57–97 | 86 | 70–100 | 84 | 66–100 | |
| Local/Regional (ref) | 79 | 73–86 | 79 | 73–86 | 89 | 83–95 | 89 | 83–95 | 92 | 87–98 | 95 | 90–100 | |
p ≤ 0.05.
Year 0 starts at diagnosis.
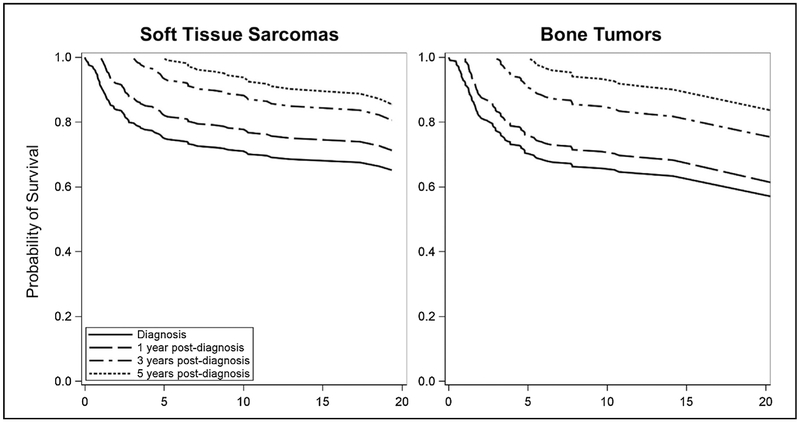
Long term survival estimates at diagnosis, and one, three and five years post-diagnosis for 20 years of follow-up show improvement as time from diagnosis increases (Table 5 and Fig. 3). Probability of survival at diagnosis for both localized and metastatic soft tissue sarcomas is 65% (CI = 59-71) Survival at 3-years post-diagnosis improves to 81% (CI = 74-87), and to 86% (CI = 79-92) at 5-years post-diagnosis. A similar pattern is found in malignant bone tumors with 51% (CI = 33-79) overall survival at diagnosis, 69% (CI = 49-97) at 3-years post-diagnosis and 78% (CI = 57-100) at 5-years post-diagnosis.
Table 5.
Twenty–year conditional survival estimates for pediatric, adolescent, and young adult soft tissue sarcoma and bone tumor, at starting at diagnosis and at one, three, and five years from diagnosis.
| Survival Estimates1 | 95% CI | ||
|---|---|---|---|
| Soft tissue sarcoma | At diagnosis | 65 | 59–71 |
| 1-year | 71 | 65–78 | |
| 3-years | 81 | 74–87 | |
| 5-years | 86 | 79–92 | |
| Bone tumor | At diagnosis | 51 | 33–79 |
| 1-year | 55 | 36–85 | |
| 3-years | 69 | 49–97 | |
| 5-years | 78 | 57–100 |
Adjusted for age at diagnosis and sex.
Fig. 3.
Conditional survival probabilities for 20-years after diagnosis in pediatric, adolescent, and young adult soft tissue and bone tumor survivors, at diagnosis and at one, three, and five years from diagnosis
4. Discussion
The 10,520 soft tissue sarcomas and 2650 malignant bone tumors diagnosed in the United States in 2010 are considered some of the more rare, aggressive, and deadly cancers due to delays in their diagnosis and potentially advanced stage at diagnosis [1]. Although survival at initial diagnosis for sarcomas is lower than the majority of childhood cancers, conditional survival for soft tissue sarcomas and malignant bone tumors five years after diagnosis is comparable to childhood cancers with survival rates of 90% or greater [15]. New data reports that if localized Ewing sarcoma and osteosarcoma patients diagnosed at less than 40 years of age survive for five years after initial diagnosis, the likelihood that they will survive another five years is greater than 90% [16]. We confirm these conditional survival estimates of 90% at five years postdiagnosis for soft tissue sarcomas and malignant bone tumors in our cancer population diagnosed at 25 years or younger. Further, we provide short-term survival statistics by specific clinical and demographic characteristics that allow providers to determine individualized prognostic information, which may aid in relieving patient and family stress [6,7,16].
The first five years after diagnosis is a high-risk period for mortality among cancer patients [6,7], so providing relevant survival estimates and identifying at risk patients by clinical characteristics are critical. We found improvements in overall short-term survival for both soft tissue sarcomas and malignant bone tumors.
Our analysis reports differences in short-term survival estimates between male and female, and differences in survival by cancer site. We found that survival for both bone and soft tissue sarcomas in axial sites is significantly worse than sarcomas found in the limbs. Poor survival of axial tumors could be attributed to difficulty with detecting tumors that form in the pelvis or torso before they reach advanced stages, damage caused to vital organs (i.e. spinal cord) with their growth, or the surgery necessary to remove them. Axial tumors are also associated with metastatic disease at presentation and may have different biologic presentation from tumors located in the extremities [5,17-20]. Ideally, both soft tissue and malignant bone tumors would be surgically removed with negative margins as part of the treatment protocol. This is most important for non-radiation sensitive tumors like osteosarcoma. However, most axial tumors cannot be primarily resected meaning that radiation therapy is more likely to be used for local control in these cases.
In our cohort, females were less frequently diagnosed with soft tissue sarcoma in childhood and adolescence, and have significantly better conditional survival than males starting one year post-diagnosis. Starting from diagnosis, we find that females have a higher probability of surviving an additional five years than males, although the difference is not significant. This survival statistic starting from diagnosis differs from the childhood cancer statistics reported by the National Cancer Institute's Cancer Surveillance, Epidemiology, and End-Result (SEER) Program, in which females are more likely to die from soft tissue sarcoma than males [21]. Although this may be a function of the differences between the populations and years included in this study, the difference in survival by sex does not change in the first five years postdiagnosis and should be assessed in other populations for confirmation.
Survival statistics for soft tissue and malignant bone tumor patients do not differ between patients diagnosed between 1986 and 2000, and 2000 and 2012. Unfortunately, there have not been major advances in therapy for these types of cancer over the past 30 years [22,23], so this pattern is expected. However, surgical techniques and supportive care have improved during this time frame with the goal of decreasing infections and excess morbidity due to surgical complications, and increasing quality of life in terms of limb salvage techniques. This may account for the significant difference in malignant bone tumor survival at diagnosis, which does not become significant at one year post-diagnosis when these changes in surgical procedures would be seen.
In our study, the largest age group for both soft tissue and malignant bone tumors are in the 16 to 25 year age range at diagnosis. These diseases remain rare, but are certainly more common in adolescent and young adult (AYA) patients. None of the available literature that we are aware of specifically documents conditional survival for AYA patients with sarcomas. As documented previously, AYA patients have survival deficits when compared to younger patients with similar disease characteristics [24]. Research into the reasons for this discrepancy is ongoing, and questions about differences in tumor biology remain [25,26].
Long-term survival also improves as time from diagnosis increases. Since 92% of deaths in this cohort were related to cancer, mortality from recurrence of the primary malignancy and other cancer-related causes is still a major concern in this population at 20 years from first diagnosis. As there is an inherent time lag between treatment for cancer and the long-term impacts of treatment on survivor health, these conditional survival estimates still do not fully capture the previously described premature mortality due to non-malignant causes in survivors of childhood cancer that occurs beyond 20 to 30 years after diagnosis [27,28].
Limitations of this data include the lack of minority populations and the limited data on treatment. African-Americans with Ewing sarcoma have a higher risk of death, and may have poorer conditional survival [5]. As our population is primarily white Caucasians, we are unable to comment on this association. We are unable to comment on how tumor size or grade affect survival statistics as our available data did not include those variables, but was recorded as local versus metastatic only, with small numbers of metastatic patients. Tumors larger than five centimeters at diagnosis, tumor grade, and higher stage of disease are linked to a high occurrence of recurrent tumors, secondary neoplasms, and increased risk of death in previous studies of soft tissue sarcoma [29]. These late effects occur among 9% of children with non-rhabdyomyosarcoma soft tissue sarcomas at 10 years from diagnosis [29], and should be included in future survival studies. We did not evaluate long-term survival by specific clinical and demographic characteristic. We also found that the ICCC chapter classification of “VIII. Malignant Bone Tumors” included soft tissue sarcomas that were treated as osteosarcomas (n = 8, 1.8%), and benign bone tumors (n = 2, 0.6%). Because our inclusion criteria was defined by the ICCC chapter classification, these tumors were considered as malignant bone tumors. As the cases represent 2% of the total sample, they are unlikely to influence our results but are important to capture per this classification system.
Our sample is limited to all sarcoma diagnoses seen at the only children’s hospital in the five-state catchment region. An earlier assessment found that 97% of children in Utah with cancer aged 0 to 9 years and 82% of aged 10 and 14 years attended PCH for their cancer treatment [17]. However, for adolescents ages 15 to 19 years, virtually all bone tumors were seen at the children’s hospital, whereas only 52% of soft tissue sarcomas were seen there for this age group [17]. This potentially explains the large number of malignant bone tumor cases relative to soft tissue sarcomas and is likely due to differences in referral. Although this may lead to selection bias as certain cancers in adolescents are shown to have better outcomes when treated on pediatric protocols, our results are based on cancer registry data from the only children’s hospital in a five-state region, and capture the majority of sarcoma cases for younger patients.
Although we see disparities in survival by sex, cancer site, and age at diagnosis, both short- and long-term conditional survival estimates for soft tissue and malignant bone tumors improve with each year of survival. For patients, families, and clinicians alike these estimates are vital to expressing an accurate prognosis for a particular individual who has survived to date, something that overall survival estimates do not provide. Since conditional survival estimates are greater than overall survival at diagnosis, with each year of survival, patients and families can be more reassured about survival as prognosis improves and previous risk factors are no longer important. Since many differences in short-term survival are not significant at five years post-diagnosis, five years of survival without a relapse may be a milestone in a patients’ treatment and possibly the appropriate time to transition to survivorship care. Although intuitive, it is helpful to use conditional statistics to reassure families and bring the definition of “cure” to them in an understandable and meaningful way.
Acknowledgements
We thank Kent Korgenski at Intermountain Health and Jessica Smith for their assistance.
Funding sources
This project was funded by Intermountain Foundation. Research reported in this publication utilized the Utah Population Database Shared Resource and was supported by the National Cancer Institute of the National Institutes of Health under Award Number P30CA042014. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations:
- EDW
Enterprise data warehouse
- IH
Intermountain Healthcare
- PCH
Primary Children’s Hospital
- UCR
Utah Cancer Registry
- UPDB
Utah Population Database
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
References
- [1].Burningham Z, Hashibe M, Spector L, Schiffman JD, The epidemiology of sarcoma, Clin. Sarcoma Res. 2 (1) (2012) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Herzog CE, Overview of sarcomas in the adolescent and young adult population, J. Pediatr. Hematol. Oncol. 27 (4) (2005) 215–218. [DOI] [PubMed] [Google Scholar]
- [3].Baade PD, Youlden DR, Chambers SK, When do I know I am cured? Using conditional estimates to provide better information about cancer survival prospects, Med. J. Aust. 194 (2) (2011) 73–77. [DOI] [PubMed] [Google Scholar]
- [4].Longhi A, Ferrari S, Tamburini A, Luksch R, Fagioli F, Bacci G, et al. , Late effects of chemotherapy and radiotherapy in osteosarcoma and Ewing sarcoma patients: the Italian Sarcoma Group Experience (1983–2006), Cancer 118 (20) (2012) 5050–5059. [DOI] [PubMed] [Google Scholar]
- [5].Davenport JR, Vo KT, Goldsby R, West DC, DuBois SG, Conditional survival and predictors of late death in patients with ewing sarcoma, Pediatr. Blood Cancer 63 (6) (2016) 1091–1095. [DOI] [PubMed] [Google Scholar]
- [6].Mertens AC, Yong J, Dietz AC, Kreiter E, Yasui Y, Bleyer A, et al. , Conditional survival in pediatric malignancies: analysis of data from the Childhood Cancer Survivor Study and the Surveillance, Epidemiology, and End Results Program, Cancer 121 (7) (2015) 1108–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kaul S, Kirchhoff AC, Boucher KM, Dietz AC, Conditional survival for pediatric and adolescent patients with cancer: implications for survivorship care, Cancer Epidemiol. 39 (6) (2015) 1071–1077. [DOI] [PubMed] [Google Scholar]
- [8].Leary SES, Wozniak AW, Billups CA, Wu J, McPherson V, Neel MD, et al. , Survival of pediatric patients after relapsed osteosarcoma: the St. Jude Children’s Research Hospital experience, Cancer 119 (14) (2013) 2645–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sawamura C, Springfield DS, Marcus KJ, Perez-Atayde AR, Gebhardt MC, Factors predicting local recurrence, metastasis, and survival in pediatric soft tissue sarcoma in extremities, Clin. Orthop. 468 (11) (2010) 3019–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Potter DA, Glenn J, Kinsella T, Glatstein E, Lack EE, Restrepo C, et al. , Patterns of recurrence in patients with high-grade soft-tissue sarcomas, J. Clin. Oncol. 3 (3) (1985) 353–366. [DOI] [PubMed] [Google Scholar]
- [11].Albritton KH, Wiggins CH, Nelson HE, Weeks JC, Site of oncologic specialty care for older adolescents in Utah, J. Clin. Oncol. 25 (29) (2007) 4616–4621. [DOI] [PubMed] [Google Scholar]
- [12].Clarke LC, Rull RP, Ayanian JZ, Boer R, Deapen D, West DW, et al. , Validity of race, ethnicity, and national origin in population-based cancer registries and rapid case ascertainment enhanced with a Spanish surname list, Med. Care 54 (1) (2016) e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Word DL, Perkins RC, Census USBot Building a Spanish Surname List for the 1990's?: A New Approach to an Old Problem: Population Division, US Bureau of the Census, Washington, DC, 1996. [Google Scholar]
- [14].Zhang X, Loberiza FR, Klein JP, Zhang M-J, A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model, Comput. Methods Programs Biomed. 88 (2) (2007) 95–101. [DOI] [PubMed] [Google Scholar]
- [15].Ward E, DeSantis C, Robbins A, Kohler B, Jemal A, Childhood and adolescent cancer statistics, CA. Cancer J. Clin. 64 (2) (2014) 83–103. [DOI] [PubMed] [Google Scholar]
- [16].Miller BJ, Lynch CF, Buckwalter JA, Conditional survival is greater than overall survival at diagnosis in patients with osteosarcoma and Ewing's sarcoma, Clin. Orthop. 471 (11) (2013) 3398–3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Weiss KR, Biau DJ, Bhumbra R, Griffin AM, Blackstein ME, Chung P, et al. , Axial skeletal location predicts poor outcome in ewing's sarcoma: a single institution experience, Sarcoma 2011 (2011) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Isakoff MS, Barkauskas DA, Ebb D, Morris C, Letson GD, Poor survival for osteosarcoma of the pelvis: a report from the Children's Oncology Group, Clin. Orthop. 470 (7) (2012) 2007–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Marina N, Granowetter L, Grier HE, Womer RB, Randall RL, Marcus KJ, et al. , Age, tumor characteristics, and treatment regimen as event predictors in ewing: a children's oncology group report, Sarcoma 2015 (2015) 927123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rodriguez-Galindo C, Liu T, Krasin MJ, Wu J, Billups CA, Daw NC, et al. , Analysis of prognostic factors in ewing sarcoma family of tumors: review of St: jude Children's Research Hospital studies, Cancer 110 (2) (2007) 375–384. [DOI] [PubMed] [Google Scholar]
- [21].National Cancer Institute Surveillance E, and End Results Programe, Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995, Cancer Statistics Branch, Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, Maryland, 1999. [Google Scholar]
- [22].Demetri GD, Antonia S, Benjamin RS, Bui MM, Casper ES, Conrad EU 3rd, et al. : soft tissue sarcoma, J. Nat. Compr. Cancer Network: JNCCN. 8 (6) (2010) 630–674. [DOI] [PubMed] [Google Scholar]
- [23].National Comprehensive Cancer Network, NCCN Clinical Practice Guidelines in Oncology: Bone Cancer Fort Washington, PA: National Comprehensive Cancer Network, (2013) ([Available from:), http://www.alabmed.com/uploadfile/2014/0423/20140423023613360.pdf. [Google Scholar]
- [24].Janeway KA, Barkauskas DA, Krailo MD, Meyers PA, Schwartz CL, Ebb DH, et al. , Outcome for adolescent and young adult patients with osteosarcoma: a report from the Children's Oncology Group, Cancer 118 (18) (2012) 4597–4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hayes-Jordan AA, Spunt SL, Poquette CA, Cain AM, Rao BN, Pappo AS, et al. , Nonrhabdomyosarcoma soft tissue sarcomas in children: is age at diagnosis an important variable? J. Pediatr. Surg. 35 (6) (2000) 948–953 (discussion 53-4). [DOI] [PubMed] [Google Scholar]
- [26].Tricoli JV, Blair DG, Anders CK, Bleyer WA, Boardman LA, Khan J, et al. , Biologic and clinical characteristics of adolescent and young adult cancers: acute lymphoblastic leukemia, colorectal cancer, breast cancer, melanoma, and sarcoma, Cancer 122 (7) (2016) 1017–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Armstrong GT, Chen Y, Yasui Y, Leisenring W, Gibson TM, Mertens AC, et al. , Reduction in late mortality among 5-Year survivors of childhood cancer, New Engl. J. Med. 374 (9) (2016) 833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Prasad PK, Signorello LB, Friedman DL, Boice JD, Pukkala E, Long-Term non-Cancer mortality in pediatric and young adult cancer survivors in Finland, Pediatr. Blood Cancer 58 (3) (2012) 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sung L, Anderson JR, Donaldson SS, Spunt SL, Crist WM, Pappo AS, Late events occurring five years or more after successful therapy for childhood rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group, Eur. J. Cancer (Oxford, England; 1990) 40 (12) (2004) 1878–1885. [DOI] [PubMed] [Google Scholar]