Abstract
Under normal physiological conditions, gastric acid production is controlled by a negative feedback mechanism. Proton pump inhibitors, such as pantoprazole, inhibit gastric acid secretion by irreversibly binding and inactivating luminally active hydrogen potassium ATPase. Recovery of acid production after treatment with a proton pump inhibitor is driven by new pump synthesis, activation of existing cytoplasmic pumps, or reversal of proton pump inhibition. The authors measured the time course of the inhibition and recovery of acid secretion in healthy volunteers following intravenous administration of pantoprazole to determine the rate of proton pump activation under maximally stimulated conditions. Gastric acid production was measured in 27 Helicobacter pylori negative healthy volunteers (mean age = 31 ± 7 years; 17 men, 10 women) who received single doses of intravenous pantoprazole (20, 40, 80, or 120 mg) in the presence of a continuous intravenous infusion of 1 ug/kg/h of pentagastrin. From the time profile of acid secretion, the authors described the rate of change of acid output using an irreversible pharmacodynamic response model represented by the equation and correlated the parameter values with demographic factors and gastric acid measurements. Mean stimulated acid output secretion was 21.6 ± 18.4 mEq/h (range: 1.6–90.5) prior to the administration of pantoprazole and remained steady for 25 hours after placebo administration. Intravenous pantoprazole inhibited acid output in a dose-response fashion, with maximal inhibition (99.9%) occurring after an 80 mg dose. Mean proton pump recovery time was 37.1 ± 21.0 hours (range: 6.7–75), and recovery was independent of the dose of pantoprazole. There was no association noted between proton pump recovery time and gender, age, race, body weight, or pantoprazole dose. However, there was an inverse correlation between acid output during baseline stimulation and recovery of acid secretion. Mean proton pump recovery time in stimulated normal human volunteers was 37.1 ± 21.0 hours, with a range of 6.7 to 75 hours. The authors hypothesize that there may be a normal homeostatic mechanism that maintains acid secretory capability within a normal range by altering the rate of proton pump activation dependent on the individual’s parietal cell mass. Abnormalities of this process may be responsible for the development of acid peptic disease in susceptible individuals.
Gastric acid secretion from parietal cells occurs in response to stimulation from neurocrine, paracrine, and hormonal stimuli following binding by the major known secretagogues (including gastrin, acetylcholine, PACAP, and histamine) with their respective receptors on the basolateral surface of the cell.1,2 Following the generation of intracellular second messengers that activate protein kinases, acid secretion is stimulated by activation of parietal cell hydrogen-potassium ATPase enzymes (proton pumps) that fuse with the secretory canalicular surface of the parietal cell leading to the generation of acid.1–3 Proton pumps exchange intracellular hydrogen ions for luminal potassium ions in a ratio of 1 to 1 to maintain intracellular electrical neutrality.4 Acid production by stimulated canalicular proton pumps is thus the final common pathway in the generation of gastric acid.1,3,5
The hydrogen potassium ATPase enzyme consists of two subunits: an alpha and a beta subunit.3,6 Animal studies suggest that messenger RNA for both subunits is synthesized in the endoplasmic reticulum, and processing of the actual pumps themselves is then accomplished in the Golgi apparatus.1,6,7 Preformed pumps are transported through the cytoplasm in secretory tubules before being inserted into the secretory canaliculus where they are activated.1,6,7 Animal studies suggest that the half-life for insertion under stimulated conditions is approximately 5 minutes.1,7–9 A retrieval mechanism whereby activated proton pumps are returned to the cytoplasm and recirculated has also been described.1,8,9 Retrieved pumps are either recirculated and reinserted into the secretory canaliculus or degraded by lysosomal action.1,8,9 Animal studies suggest that the half-life for retrieval is approximately 60 minutes under stimulated conditions.1,7–9 It is important to note that intracellular proton pumps are not functional until they reach the secretory canaliculus.1,3,6,7 In addition to causing acid secretion from activated proton pumps, secretagogues that generate intracellular cyclic AMP (such as histamine or PACAP) are also felt to be important for the synthesis of new proton pumps and for activation of preformed pumps.1,2,10
Gastric acid production under normal physiological conditions is controlled by a negative feedback mechanism.1,3,7,11 Acid causes a drop in pH within the gastric lumen. In turn, this physiologic change feeds back on somatostatin-releasing D cells in the gastric antrum and body to ultimately inhibit gastric acid secretion and restore homeostasis. The major action of gastric somatostatin, a paracrine octapeptide, is to inhibit release of the hormone, gastrin, from G cells in the gastric antrum (although it probably also has a direct effect on parietal and ECL cells in the gastric body as well).1,7,12 Gastrin stimulates acid secretion by two mechanisms: the aforementioned direct effect on parietal cells (its minor action) and a second indirect effect on gastric ECL cells, which release histamine in response to stimulation by gastrin (its major action).1,7,10,11 Thus, acid production by stimulated parietal cells indirectly inhibits the stimulus for further acid production. On the other hand, inhibition of gastric acid secretion by proton pump inhibitors (or other antisecretory agents) causes a rise in gastric pH, which in turn results in decreased somatostatin production, uncontrolled gastrin release, excessive ECL cell stimulation, histamine release, and ongoing stimulation of parietal cells.1,5,9,11,13–15
Studies in rats have shown a direct link between exposure to the proton pump inhibitor, omeprazole, and both inhibition of antral D-cell somatostatin and induction of H+/K(+)-ATPase parietal cell gene expression.15 The duration of this effect lasted for about 36 hours.15 35S-methionine degradation studies in rats suggested that the gastric H+/ K+,ATPase alpha subunit half-life is about 54 hours.9
The life cycle of the gastric acid proton pump in humans has not been well studied because proton pump turnover is affected by a number of variables that are difficult to control for. Since proton pump inhibitors inhibit gastric acid secretion by irreversibly binding and inactivating luminally active hydrogen potassium ATPase,5,9,14–16 recovery of acid production after treatment with a proton pump inhibitor will be driven by new pump synthesis or activation of existing cytoplasmic pumps. It has been estimated that covalent inhibition of human parietal cell proton pumps has an effective half-life of about 48 hours.17 In the current study, we measured the time course of inhibition and recovery of acid secretion in healthy volunteers following intravenous administration of a proton pump inhibitor, pantoprazole (Wyeth Ayerst Research, Radnor, PA), to determine the rate of proton pump recovery under maximally stimulated conditions.
METHODS
The database for the current evaluation was established by analyzing data previously collected for a study assessing gastric acid inhibition of stimulated acid output in normal Helicobacter pylori negative individuals.18 In that study,18 27 of the 35 study subjects received increasing doses of intravenous pantoprazole (20, 40, 80, or 120 mg) by a single 15-minute infusion to assess inhibition of stimulated gastric acid output in the presence of a continuous intravenous infusion of 1 ug/kg/h of pentagastrin (a maximally effective dose).19 Four additional subjects were tested on famotidine, and 8 received placebo, but the latter two arms of that study were not included in the current analysis since they did not receive intravenous PPI therapy. Acid output was collected continuously for 25 hours starting from 1 hour prior to dosing of pantoprazole or placebo. Gastric analysis was performed as previously reported.18,20 Briefly, after an overnight fast, a nasogastric tube was passed into the dependent portion of the stomach and its position was verified by the water recovery method to ensure adequate recovery of gastric juice. After removal of residual gastric volume over 15 minutes, gastric juice was collected in 15-minute intervals from 1 hour before study drug administration until 2 hours after study drug administration and then in 30-minute intervals for the next 24 hours using low, continuous suction. The acid content of each sample was measured automatically by titration to pH 7.0 with sodium hydroxide (0.05 N NaOH) using 1 ml of aspirate diluted in 39 ml of distilled water, and acid output was expressed in mEq/h.18,20 All studies were performed in the NIH-sponsored clinical research center at UCLA’s Center for Ulcer Research (CURE) following institutional review board (IRB) approval.
From the time profile of acid secretion, it was possible to describe the rate of change of acid output using an irreversible pharmacodynamic response model published previously.21 The data for this analysis were derived from the 27 individuals receiving intravenous pantoprazole and the 8 individuals receiving placebo in the aforementioned study.18 This model (previously published; see Ferron et al21) is described by the following equation:
| (1) |
where R is the rate of acid output, Ro is the rate of acid output before pantoprazole administration (i.e., the maximum rate of acid production in the presence of pentagastrin), −k is the apparent reaction rate constant of pantoprazole with the proton pumps, kdeg is the degradation rate constant of acid production, and Cpanto is the concentration of pantoprazole in plasma at the time of acid output measurement. When pantoprazole is present in the body, gastric acid production is inhibited. However, as the concentration of pantoprazole declines to negligible levels, gastric acid production recovers to approach the rate of production that was present before the pantoprazole was administered (i.e., Ro). This change occurs at a first-order rate that is proportional to kdeg and the difference between R and Ro. The time in which the difference between R and Ro decreases by half is called the apparent proton pump recovery (or turnover) time (PPR) and is equal to Ln2/kdeg.21 Thus, by substituting Ln2/PPR for kdeg, equation (1) can be rearranged as follows:
| (2) |
Values for Cpanto were taken from our previous pharmacokinetic study,21 which also included a different cohort of 12 healthy H. pylori negative white men who received intravenous pantoprazole (10, 20, 40, or 80 mg) by short 15-minute infusion in a randomized crossover fashion, followed by repeated plasma pantoprazole measurements for 24 hours. In that study,21 the pharmacokinetics of pantoprazole was described using a two-compartment model:
| (3) |
and
| (4) |
where Inf is the pantoprazole infusion rate, Cpanto concentration of pantoprazole in plasma, Cpanto, tissue is the concentration of pantoprazole in tissue, CL is the elimination clearance of pantoprazole, CLd is the distribution clearance of pantoprazole, Vc is the central volume of distribution, and Vtissue is the volume of distribution of pantoprazole in tissue. Blood samples were analyzed using a validated high-performance liquid chromatographic assay with ultraviolet detection after a fully automated precolumn sample cleanup.22 The limit of quantitation for Cpanto was 0.025 mg/L, and the assay was linear up to 5.0 mg/L.22
The pharmacodynamic analysis was performed using unweighted nonlinear regression and WinNonlin software (version 1.1, Pharsight Corporation, Palo Alto, CA). Since the pharmacokinetics of pantoprazole is linear, the mean pharmacokinetic parameter values were used to drive the concentrations up to a dose of 120 mg when computing the individual pharmacodynamic parameters. Analysis of the possible relationship between the pharmacodynamic parameters and demographic variables, pantoprazole doses, and baseline stimulated acid production was done with analysis of variance and regression analysis using SAS software (version 6.12, Cary, NC). The level of significance was set at an alpha value of 0.05.
RESULTS
Twenty-seven of the subjects described in our initial report18 received intravenous pantoprazole and form the core of the current analysis. Table I shows the clinical characteristics of these 27 normal volunteers. There were 17 men and 10 women. The mean age (and standard deviation) was 31 ± 7 years with a mean weight of 76 ± 17 kilograms. Eighteen subjects were white, 7 were African American, 1 was Hispanic, and 1 was Asian. All study subjects were H. pylori negative, and none had a history of acid-peptic disease. Mean stimulated acid output secretion in the presence of 1 ug/kg/h of pentagastrin prior to the administration of study drug was 21.6 ± 18.4 mEq/h, with a range of 1.6 to 90.5 mEq/h.
Table I.
Clinical Characteristics of 27 Subjects Receiving Intravenous Pantoprazole
| Mean age (± SD) (years) | 31 | (7) |
| Female (number [%]) | 10 | (37) |
| Mean weight (± SD) (kg) | 76 | (17) |
| Race (number [%]) | ||
| White | 18 | (67) |
| African American | 7 | (26) |
| Hispanic | 1 | (4) |
| Asian | 1 | (4) |
| H. pylori infection | 0 | (0) |
| Pentagastrin-stimulated acid output (mEq/h) | 21.6 | (18.4) |
Under conditions of continuous pentagastrin infusion, there was significant inhibition of acid output after a single administration of intravenous pantoprazole with a clear dose response from 20 to 80 mg. Maximal inhibition of stimulated acid output (99.9% within 1.5 h of pantoprazole administration) occurred with an 80 mg dose of pantoprazole. The highest dose administered (120 mg) did not inhibit acid secretion any further (Figure 1). In comparison, acid secretion remained steady for 25 hours, averaging 35 mEq/h (Figure 1) in the 8 subjects receiving continuous pentagastrin infusion and placebo. These patients were not studied further, but their secretory responses demonstrate the absence of tachyphylaxis to pentagastrin infusion in our model.
Figure 1.
Upper panel: Mean (SE) pantoprazole concentration-time profiles of pantoprazole after administration as 15-minute IV infusions of 10, 20, 40, and 80 mg doses in healthy subjects (see Ferron et al21). Curves were obtained by simultaneously fitting equations (3) and (4). Dose-linear pharmacokinetics was assumed to estimate the concentrations after administration of a 120 mg dose. Lower panel: Acid output measurements in pentagastrin-stimulated normal individuals after 15-minute infusions of placebo (closed circles) or intravenous pantoprazole at 10, 20, 40, 80, or 120 mg (open circles, triangles, squares, inverted triangles, or diamonds, respectively). These data have previously been published.18,21 The curves show estimated “best fits” for these data within each dosage group as calculated by our pharmacodynamic model (see Methods section). The 27 subjects in the lower panel who received pantoprazole form the core of the current analysis. The 8 subjects who received placebo are presented for comparison to show that there is no tachyphylaxis to continuous pentagastrin infusion.
The pharmacodynamic parameter values were calculated for each subject receiving intravenous pantoprazole. The mean reaction rate constant was 0.75 ± 1.01 L/mg/h (range: 0.16–4.95), and the mean proton pump recovery time was 37.1 ± 21.0 hours (range: 6.7–75.0 h). After maximum inhibition occurs, acid production recovers at the same rate regardless of the dose of pantoprazole administered. Indeed, the rate of recovery of acid secretion is linked to the proton pump recovery time since pantoprazole (with its very short serum half-life) is no longer present in the system at effective concentrations (Figure 1).
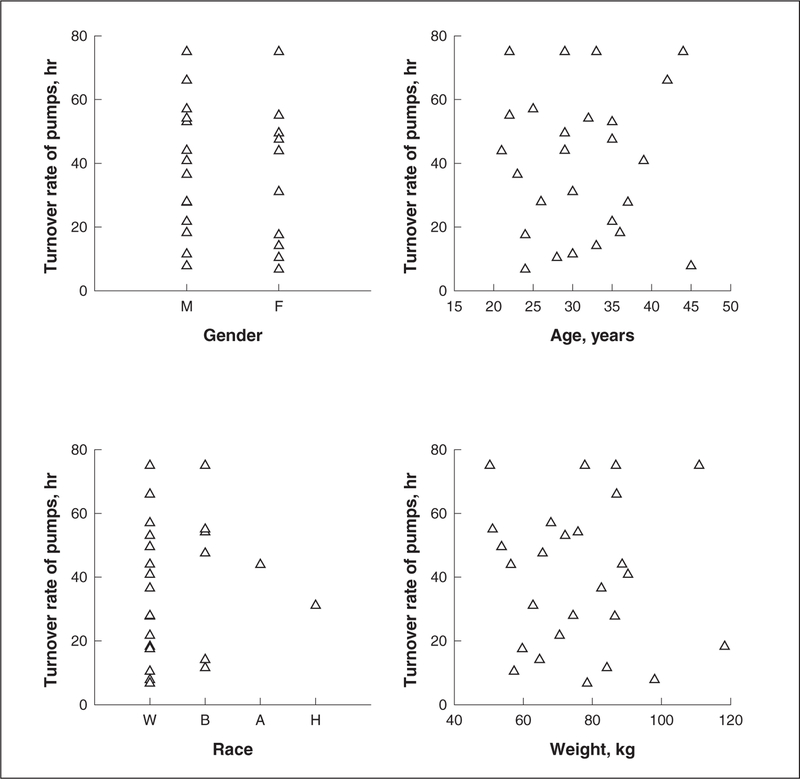
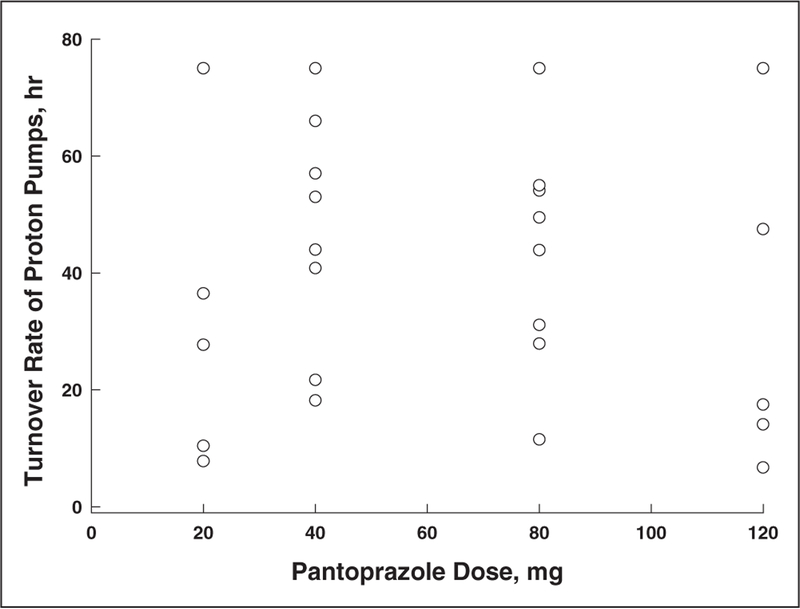
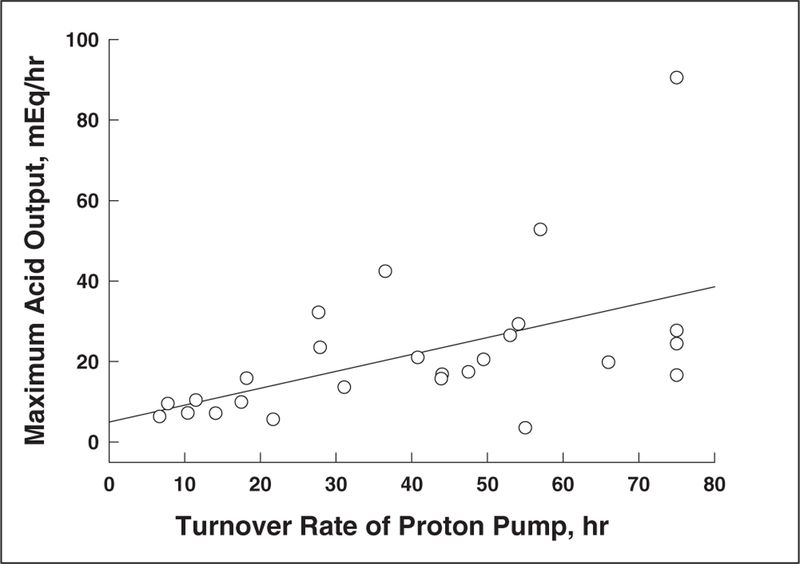
Figure 2 shows the lack of correlation between proton pump recovery time and various demographic variables. There was no association noted between proton pump recovery time and gender, age, race, or body weight. Furthermore, there was also no association noted between proton pump recovery time and the dose of intravenous pantoprazole administered (Figure 3). Figure 4 shows the relationship between pentagastrin-stimulated acid output prior to drug administration and proton pump recovery time in the 27 study volunteers receiving intravenous pantoprazole. There was an inverse correlation between stimulated acid output and recovery of acid secretion. The slope of this curve was 0.42 mEq/h, and the y-intercept was 4.94 mEq/h.
Figure 2.
Correlation of proton pump recovery time with various demographic variables. Upper left panel: Correlation with gender. Upper right panel: Correlation with age. Lower left panel: Correlation with race. Lower right panel: Correlation with body weight.
Figure 3.
Correlation of proton pump recovery time with intravenous pantoprazole dose.
Figure 4.
Correlation of proton pump recovery time with pentagastrin-stimulated acid output in 27 Helicobacter pylori negative normal volunteers. There is an inverse relationship with a slope of 0.42 mEq/h and a y-intercept of 4.94 mEq/h.
DISCUSSION
Little is known about the life cycle of the proton pump in humans. Studies in animal models have suggested a continuous turnover process whereby synthesized alpha and beta subunits of the proton pump are packaged in the Golgi apparatus and transported through the cytoplasm for insertion into the secretory canaliculus where they actively secrete acid.1,5–10 This process is felt to be gastrin driven and histamine dependent.1,9,10 Active pumps are later retrieved and recycled into the cytoplasm.1,8,9 The latter process is also likely dependent on parietal cell stimulation by secretagogues such as histamine and acetylcholine.1 It has been estimated that covalent inhibition of human parietal cell proton pumps has an effective half-life of about 48 hours.17 This figure is not much higher than the values we obtained and is well within the range of values that we calculated (6.7–75.0 h).
In the current study, we attempted to determine the rate of insertion or activation of proton pumps in humans by studying subjects under maximally stimulated conditions following inhibition of acid secretion with various doses of the intravenous proton pump inhibitor, pantoprazole. Our data suggest a variable pump activation rate that is inversely related to the underlying acid secretory capacity of the individual. Prior studies have demonstrated that maximal acid output correlates closely with parietal cell mass.1,23,24 Therefore, these data suggest that there may be a normal homeostatic mechanism that maintains acid secretory capability within a normal range by altering the rate of proton pump activation dependent on the individual’s parietal cell mass. Thus, persons with large acid secretory capabilities do not need to activate their pumps rapidly to maintain acid secretion within this normal range, whereas persons with lower acid secretory capabilities need to respond more rapidly and activate their existing pumps more quickly.
The conclusions from our study are based on a number of important caveats. First, this is a relatively small study in which only 27 healthy normal volunteers were studied. Second, we derived our irreversible pharmacodynamic response model based on pharmacokinetic data obtained from another normal volunteer cohort also in a small number of subjects.21 However, the data obtained from the initial pharmacokinetic trial reveal a small intersubject variability in pantoprazole pharmacokinetics, and the values obtained in our study (including the short half-life of less than 1 h) have been reproduced in other oral and intravenous pharmacokinetic studies as well.16,25 Furthermore, studies with other proton pump inhibitors also reveal similar short half-lives, suggesting that the pharmacokinetic parameters for proton pump inhibitors, as a class, are similar.5,26,27 Third, the pharmacodynamic equation that we have derived may not be a fully accurate representation of what actually goes on at the parietal cell proton pump level. For example, we have assumed that the pantoprazole effect is irreversible. It is conceivable that covalently bound drug may dissociate from the proton pump, permitting recirculation to occur despite the presence of proton pump inhibition. In fact, prior studies in rats9 reveal a discordant measurement for pump gene transcription (half-life of 54 h) as compared with the recovery of K(+)-stimulated ATPase activity (half-life of 15 h), suggesting that recirculation may occur to some extent. We cannot exclude the possibility that a component of the turnover we calculated may be due to recirculation of pumps that disassociate from pantoprazole. Nevertheless, we feel that the hypothesis underlying our model is sound. It should be noted that the slopes of the acid output recovery curve (another indirect measure of proton pump turnover) mirrored the turnover rate that was calculated using the model.
Despite the above limitations to our study, we believe it to be of some importance because it shows for the first time in humans that recovery of acid secretion after pharmacological inhibition occurs in a predictable manner. This recovery is inversely related to maximal acid secretory capability and is likely a normal homeostatic mechanism to maintain secretory capability within a normal range. The lack of correlation in our study between acid secretory recovery and demographic variables is curious, especially since at least three of the factors we analyzed (i.e., gender, age, and body weight but not race) are believed to be predictive of parietal cell mass.24,28–30 Women tend to make lesser amounts of acid than men,28 the amount of acid produced in normal individuals is related to lean body mass,28 and some but not other29 authorities have claimed that acid secretion decreases with age (although the latter point is quite controversial and may be a consequence of H. pylori infection and not intrinsic to the normal human stomach).30 The current study was not large enough for us to allow a regression analysis, but it is feasible that after correcting for parietal cell mass, some or all of these factors may in fact play a role.
As was mentioned above, the current hypothesis of proton pump activation is based on a two-stage process—that is, initial synthesis and packaging of alpha and beta proton pump subunits in the nucleus and Golgi apparatus, followed thereafter by insertion of preformed cytoplasmic (or retrieved) pumps into the secretory canaliculus.1,7–9 Both steps appear to be gastrin dependent and histamine driven,1,2,10 but the former is likely to take some time (through activation of ECL cells and, to a lesser extent, directly at the parietal cell), whereas the latter is likely to occur more rapidly (as a consequence of basal ECL cell stimulation, which in turn is dependent on gastrin and PACAP release). Since the current study evaluated proton pump recovery soon after inhibition with intravenous pantoprazole, it is likely that we were only measuring recovery of acid secretion as a consequence of activation of preformed cytoplasmic pumps (recirculation of pumps from the secretory canaliculus to the cytoplasm is likely to be minimal in such a setting because secretory canalicular pumps are irreversibly inhibited by covalent binding with pantoprazole).5,16 It may be possible to measure the rate of nuclear synthesis and packaging of proton pumps by performing a similar analysis in patients treated with multiple doses of intravenous pantoprazole over a few days before holding therapy and measuring acid recovery, as was done in this study.
The current study only gives an idea of acid secretory capabilities in normal individuals given proton pump inhibitors. It will be of specific interest to analyze whether a similar inverse relationship between acid secretory recovery and parietal cell secretory capability holds true in disease states such as H. pylori gastritis, Zollinger-Ellison syndrome (and other hypersecretory conditions), gastroesophageal reflux disease, or idiopathic peptic ulceration. It is possible that these disease states may result from a loss of the normal homeostatic mechanism that we have identified in normal individuals.
Acknowledgments
This manuscript is dedicated to the memory of Dr. John H. Walsh. This study was funded in part by Wyeth Ayerst Research, St. Davids, Pennsylvania.
Contributor Information
David C. Metz, University of Pennsylvania Health System, Philadelphia.
Geraldine M. Ferron, Wyeth-Ayerst Research, Radnor, Pennsylvania.
Jeffrey Paul, Wyeth-Ayerst Research, Radnor, Pennsylvania.
Mary Beth Turner, Wyeth-Ayerst Research, Radnor, Pennsylvania.
Elaine Soffer, Wyeth-Ayerst Research, Radnor, Pennsylvania.
Joseph R. Pisegna, CURE/West LA VAMC and UCLA, Los Angeles.
Wieslaw J. Bochenek, Wyeth-Ayerst Research, Radnor, Pennsylvania.
REFERENCES
- 1.Modlin IM, Sachs G: The regulation of acid secretion, in: Modlin IM, Sachs G (eds.), Acid Related Diseases: Biology and Treatment Konstanz, Germany: Schnetztor-Verlag, 1998;37–112. [Google Scholar]
- 2.Zeng N, Kang T, Wong H, et al. : PACAP type I receptor activation regulates ECL cells and gastric acid secretion. J Clin Investigation 1999;104:1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Modlin IM, Sachs G: The production of acid in the stomach, in: Modlin IM, Sachs G (eds.), Acid Related Diseases: Biology and Treatment Konstanz, Germany: Schnetztor-Verlag, 1998;1–36. [Google Scholar]
- 4.Stewart B, Wallmark B, Sachs G: The interactions of H+ and K+ with the parietal reactions of gastric H,K-ATPase. J Biol Chem 1981; 256:2682–2690. [PubMed] [Google Scholar]
- 5.Fellenius E, Berglindh T, Sachs G, et al. : Substituted benzimidazoles inhibit gastric acid secretion by blocking (H+-K+)ATPase. Nature 1981;290:159–161. [DOI] [PubMed] [Google Scholar]
- 6.Rabon EC, Reuben MA: The mechanism and structure of the gastric H,K-ATPase. Ann Rev Physiol 1990;52:321–344. [DOI] [PubMed] [Google Scholar]
- 7.Reuben MA, Lasater LS, Sachs G: Characterization of a beta subunit of the gastric H+/K+-transporting ATPase. Proc Natl Acad Sci USA 1990;87:6767–6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forte TM, Machen TE, Forte JG: Ultrastructural changes in oxyntic cells associated with secretory function: a membrane recycling hypothesis. Gastroenterology 1977;73:941. [PubMed] [Google Scholar]
- 9.Gedda K, Scott D, Besancon M, et al. : The turnover of the gastric H,K ATPase (subunit and its relationship to inhibition of gastric acid secretion). Gastroenterology 1995;109:1134–1141. [DOI] [PubMed] [Google Scholar]
- 10.Tari A, Yamamoto G, Sumii K, et al. : Role of histamine 2 receptor in increased expression of rat gastric H+-K+-ATPase alpha-subunit induced by omeprazole. Am J Physiol 1993;265:G752–G758. [DOI] [PubMed] [Google Scholar]
- 11.Prinz C, Kajimura M, Scott DR, et al. : Histamine secretion from rat enterochromaffin-like cells. Gastroenterology 1993;105:449. [DOI] [PubMed] [Google Scholar]
- 12.Lewin MJM: The somatostatin receptor in the GI tract. Ann Rev Physiol 1992;54:455. [DOI] [PubMed] [Google Scholar]
- 13.Lamberts R, Creutzfeldt W, Strueber HG, et al. : Long-term omeprazole therapy in peptic ulcer disease: gastrin, endocrine cell growth and gastritis. Gastroenterology 1993;104:1356. [DOI] [PubMed] [Google Scholar]
- 14.Modlin IM, Sachs G. Pharmacology of acid secretion, in: Modlin IM, Sachs G (eds.), Acid Related Diseases: Biology and Treatment Konstanz, Germany: Schnetztor-Verlag, 1998;113–146. [Google Scholar]
- 15.Tari A, Wu V, Sumii M, et al. : Regulation of rat gastric H+/K(+)-ATPase alpha-subunit by omeprazole. Biochim Biophys Acta 1991;1129:49–56. [DOI] [PubMed] [Google Scholar]
- 16.Fitton A, Wiseman L: Pantoprazole, a review of its pharmacology and therapeutic use in acid-related disorders. Drugs 1996;51: 460–482. [DOI] [PubMed] [Google Scholar]
- 17.Sachs G, Prinz C, Loo D, et al. : Gastric acid secretion: activation and inhibition. Yale J Biol Med 1994;67:81–95. [PMC free article] [PubMed] [Google Scholar]
- 18.Pisegna JR, Martin P, McKeand W, et al. : Inhibition of pentagastrin-induced gastric acid secretion by intravenous pantoprazole: a dose-response study. Am J Gastroenterol 1999;94: 2874–2880. [DOI] [PubMed] [Google Scholar]
- 19.Mason MC, Giles GR, Clark CG: Continuous intravenous pentagastrin as a stimulant of maximal acid secretion. Gut 1969;10: 34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lew EA, Pisegna JR, Starr JA, et al. : Intravenous pantoprazole rapidly controls gastric acid hypersecretion in patients with Zollinger-Ellison syndrome. Gastroenterology 2000;118:696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferron GM, McKeand W, Mayer PR: Pharmacodynamic modeling of pantoprazole’s irreversible effect on gastric acid secretion in humans and rats. J Clin Pharmacol 2001;41:149–156. [DOI] [PubMed] [Google Scholar]
- 22.Huber R, Muller W: High-performance liquid chromatographic determination of the H+/K+ ATPase inhibitor (by 1023/SK&F 96 0220) and its sulfone metabolite in serum or plasma by direct injection and fully automated pre-column sample cleanup. J Chromatogr 1990;529:389–401. [DOI] [PubMed] [Google Scholar]
- 23.Card W, Marks I: The relationship between the acid output of the stomach following “maximal” histamine stimulation and the parietal cell mass. Clin Sci 1960;19:147–163. [PubMed] [Google Scholar]
- 24.Makhlouf GM, McManus JPA, Card WI: Action of the pentapeptide (ICI 50123) on gastric secretion in man. Gastroenterology 1966;51: 455–465. [PubMed] [Google Scholar]
- 25.Pue MA, Laroche J, Meineke I, et al. : Pharmacokinetics of pantoprazole following single intravenous and oral administration to healthy male subjects. Eur J Clin Pharmacol 1993;44:575–578. [DOI] [PubMed] [Google Scholar]
- 26.Maton PN: Omeprazole. N Engl J Med 1991;324:965. [DOI] [PubMed] [Google Scholar]
- 27.Gerloff J, Mignot A, Barth H, et al. : Pharmacokinetics and absolute bioavailability of lansoprazole. Eur J Clin Pharmacol 1996;50: 293–297. [DOI] [PubMed] [Google Scholar]
- 28.Metz DC, Walsh JH: Gastroduodenal ulcer disease and gastritis, in: Humes HD (ed.), Kelley’s Textbook of Internal Medicine Philadelphia: Lippincott, Williams & Wilkins, in press. [Google Scholar]
- 29.Collen MJ, Abdulian JD, Chen YK: Age does not affect basal gastric secretion in normal subjects or in patients with acid-peptic disease. Am J Gastroenterol 1994;89:712–716. [PubMed] [Google Scholar]
- 30.Haruma K, Kamada T, Kawaguchi H, et al. : Effect of age and Helicobacter pylori infection on gastric acid secretion. J Gastroenterol Hepatol 2000;15:277–283. [DOI] [PubMed] [Google Scholar]