Abstract
We have identified pituitary adenylate cyclase activating peptide (PACAP) receptors on small cell lung cancer cell line NCI-N417 in a previous study. In this study, the role of PACAP in the growth and signal transduction of non-small cell lung cancer cells was investigated. Northern blot analysis with a full-length human PACAP receptor cDNA probe revealed a major 7.5-kb hybridizing transcript when total RNA extracted from NCI-H838 cells was used. PACAP bound with high affinity (Kd = 1 nM) to a single class of sites (Bmax = 14,000/cell) when NCI-H838 cells were used. Specific 125I-labeled PACAP binding was inhibited with high affinity by PACAP-27 and PACAP-38, with moderate affinity by PACAP(6–38), and with low affinity by vasoactive intestinal polypeptide, PACAP(28–38), and PACAP(16–38). PACAP-27 elevated cAMP in a dose-dependent manner, and the increase in cAMP caused by PACAP was reversed by PACAP(6–38). PACAP-27, but not vasoactive intestinal polypeptide, elevated cytosolic Ca2+ in individual NCI-H838 cells. PACAP-27 stimulated arachidonic acid release, and the increase caused by PACAP was reversed by PACAP(6–38). PACAP-27 stimulated colony formation in NCI-H838 cells, whereas the PACAP antagonist PACAP(6–38) reduced colony formation in the absence or presence of exogenous PACAP-27. In nude mice bearing NCI-H838 xenografts, PACAP(6–38) slowed tumor growth significantly. These data suggest that biologically active type 1 PACAP receptors are present on human non-small cell lung cancer cells, which exhibit dual signal transduction pathways and regulate cell proliferation.
INTRODUCTION
PACAP4 and VIP are 27- and 28-amino acid peptides that have 65% sequence homology and are related to the larger family of peptide hormones, which includes secretin, glucagon, calcitonin, parathyroid hormone, and glucagon-like peptide 1 (1, 2). PACAP occurs as two biologically active peptides, PACAP-27 and PACAP-38, that share the same 27 NH2-terminal amino acids. Early radioligand binding studies have suggested the existence of distinct high-affinity receptors for PACAP, VIP, and helodermin (3–6). VIP1, VIP2, and type 1 PACAP receptors that have 459, 430, and 495 amino acids, respectively, have been cloned previously (7–10). Each of the receptors has seven transmembrane domains and binds PACAP with high affinity; however, only the VIP1 and VIP2 receptors, and not PACAP type 1 receptor, bind VIP with high affinity.
VIP is derived from a 170-amino acid precursor protein (11, 12). The presence of VIP and VIP receptor on specific NSCLC cell lines has been shown by VIP immunoreactivity, as well as by radioligand binding studies using 125I-labeled VIP (13). In addition, Northern blot analysis has confirmed the specific expression of VIP, as well as that of VIP1 receptor, mRNA in these cells. Using NCI-H727 cells, VIP agonist stimulation elevates cAMP. The stimulated increase in adenylate cyclase activity and colony formation can be antagonized by VIP hybrid (14). VIP hybrid slows NSCLC xenograft formation in nude mice in vivo (14).
The type 1 PACAP receptor has recently been cloned in rats and humans, and the deduced nucleotide sequence encodes a 495-amino acid protein that shares 45% homology with the VIP1 receptor (15). Unlike the VIP receptors, however, PACAP receptors have been shown in several cell lines to have a dual cascade of intracellular responses that include activation of adenylate cyclase and phospholipase C. Previously, we found that PACAP bound with high affinity to SCLC cell line NCI-N417 (16). Specific 125I-PACAP-27 binding to NCI-N417 was inhibited with high affinity by PACAP-27 and the COOH-terminally extended peptide PACAP-38 and with lower affinity by the truncated versions of the peptide [PACAP(6–38) and PACAP(16–38)]. PACAP-27 induced stimulation of adenylate cyclase, as well as PI turnover, an effect that was antagonized by PACAP(6–38). PACAP-27 and PACAP-38 stimulated colony formation in vitro, an effect that also was antagonized by PACAP(6–38). Because of its effects in SCLC cells, in this study, we investigated the effects of PACAP on NSCLC cells. We report that PACAP-induced receptor activation is coupled to an increase in intracellular Ca2+, cAMP. and proliferation in NSCLC cells.
MATERIALS AND METHODS
Cell Culture.
NSCLC cells were cultured in RPMI 1640 containing 10% heat-inactivated fetal bovine serum. When a monolayer had formed, the adherent cells were washed with PBS and treated with trypsin/EDTA. The cells were pelleted and resuspendcd in serum-supplemented medium and incubated at 37°C in 5% CO2/95% air. The cells were studied during their exponential growth phase and were mycoplasma free (17).
Receptor Binding.
The binding assays were conducted using 125I-PACAP-27 (2200 Ci/mmol). NSCLC cells (cell line, NCI-H838; 5 × 104) were placed in 24-well plates coated with human fibronectin (20 µg).When a monolayer of cells had formed, the cells were washed four times with SIT medium and then incubated in receptor-binding medium (RPMI 1640 containing SIT medium plus 1% BSA and 1 mg/ml bacitracin). The cells were incubated with 125I-PACAP-27 for 30 min at 37°C. Next, they were washed four times in receptor-binding medium at 4°C. The cells that contained bound peptide were dissolved in 0.2 N NaOH and counted in a γ-counter.
Northern Blot Anaylsis.
For Northern blot analysis, NSCLC cells were cultured with SIT medium containing 0.5% fetal bovine serum. Cells were washed in PBS and frozen in liquid nitrogen. Total RNA was isolated using the guanidinium isothiocyanate method (18). Ten µg of total RNA were separated in a 0.66-M formaldehyde 1% agarose gel (19). The RNA was blotted onto a Nytran membrane overnight, and the membrane was hybridized with full-length cDNA probes labeled with 32P[dCTP] using a Bethesda Research Laboratories random priming kit (BRL). The membrane was exposed and analyzed using a Molecular Dynamics Phosphorimager.
cAMP.
Cyclic AMP was assayed by RIA (20). The cell line NCI-H838 was harvested and resuspended in SIT medium containing 1% BSA. 1 mg/ml bacitracin, and 100 µM isobutyl-methyl-xanthine. After 5 min, the reaction was quenched by the addition of an equal volume (0.5 ml) of ethanol. The samples were mixed and frozen at −80°C until assay.
Cytosolic Ca2+.
Cytosolic Ca2+ was assayed in individual NCI-H838 cells. NCI-H838 cells (104) were cultured on fibronectin-treated Lab-Tek coverslip chambers. The cells were fed 1 day before the experiment, and, on the day of the experiment, the cells were rinsed with 1 ml of buffer (150 mM NaCl, 1 mM MgCU2, 5 mM KC1, 10 mM glucose, 1 mM CaCl2, and 20 mM HEPES/NaOH (pH 7.4) containing 1% BSA). After 5 min at 37°C, the buffer was removed, and new buffer containing 5 µM Indo-1AM was added. The cells were incubated for 30 min at 37°C, the old buffer was removed, and the cells were treated with new CaCl2-free buffer. After 5 min at 37°C, the cells were treated with new buffer and assayed for Ca2+ using an ACAS 570 Interactive Laser Cytometer (Meridian Instruments). The excitation wavelength was 320 nm, and the fluorescence emission was monitored at 485 nm (Ca2+-free emission) and 405 nm (Ca2+-bound emission). The emission ratio was determined every 30 s before and after the addition of peptide.
Arachidonic Acid Release.
NSCLC cells (5 × 104) were placed in 24-well plates coated with human fibronectin (20 µg). After a monolayer of cells had formed (5 days), ([3H]5, 6, 8, 9, 11, 12, 14, 15)arachidonic acid (2.5 × 105 cpm) was added (21). After 16 h, the cells were washed twice in 1 ml of SIT medium containing 0.2% fatty acid-free BSA. New medium that contained PACAP-like peptides was added. After 40 min, 100 µl of medium was removed from each well, placed in a scintillation vial, scintillation fluid added and sample counted in a β-counter (21).
Proliferation Assays.
Growth studies were performed in vitro using NCI-H838 cells and the agarose cloning system described previously (22). The base layer consisted of 3 ml of 0.5% agarose in SIT medium containing 5% fetal bovine serum in 6-well plates. The top layer consisted of 3 ml of SIT medium in 0.3% agarose, PACAP-like peptides, and 5 × 104 single viable cells. For each cell line and peptide concentration, triplicate wells were plated. After 2 weeks, 1 ml of 0.1% p-iodonitrotetrazolium violet was added, and, after 16 h at 37°C, the plates were screened for colony formation; the number of colonies larger than 50 µm in diameter were counted using an Omnicon image analysis system.
Growth was assessed in vivo using nude mice bearing NSCLC xenografts. Female athymic BALB/c nude mice, 4–5 weeks old, were housed in a pathogen-free, temperature-controlled isolation room and fed autoclaved rodent food and autoclaved water ad libitum. NCI-H838 cells (1 × 107) were injected into the right flank of each mouse by s.c. injection. Palpable tumors were observed in approximately 90% of the mice after 2 weeks, and PBS (100 µl) or PACAP(6–38) (10 µg/day s.c.) was injected during weeks 2–6. The tumor volume (height × width × depth) was determined weekly by using calipers and was recorded. When the tumor became necrotic, the growth studies were terminated.
RESULTS
Receptor binding.
To evaluate NSCLC cell lines for the presence of PACAP type 1 receptor-specific binding sites, several cell lines were studied, as shown in Table 1. 125I-PACAP-27 bound with high affinity in all cell lines tested, suggesting that all of the cell lines possessed PACAP receptors. The specific:nonspecific binding ratio ranged from 2:1 to 4:1, depending on the cell line used. Because cell line NCI-H838 bound 125I-PACAP-27 best, it was used in subsequent studies.
Table 1.
Radiolabeled PACAP bindinga
| Cell line | cpm bound |
|---|---|
| NCI-H157 | 5598 |
| NCI-H727 | 9100 |
| NCI-H838 | 10860 |
| NCI-H1299 | 8758 |
The amount of specifically bound l25I-PACAP-27 was determined using radiolabeled PACAP-27 (0.2 nM) and 106 cells. The mean of four determinations is indicated, and the standard error was approximately 10%.
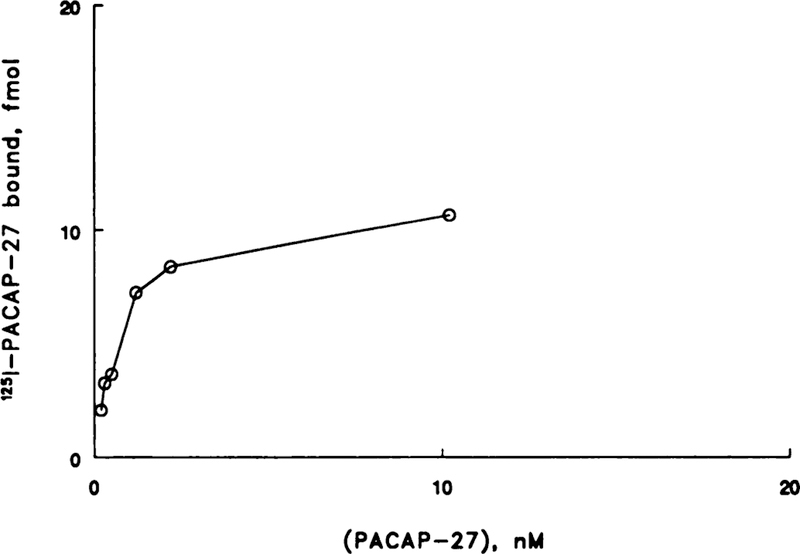
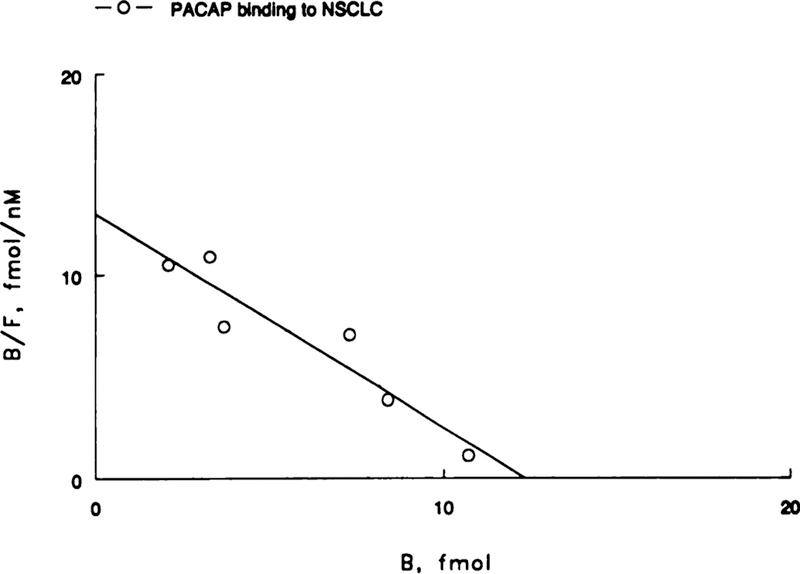
125I-PACAP-27 bound with high affinity to NSCLC cell line NCI-H838 in a dose-dependent manner (Fig. 1). Binding was a linear function of PACAP concentration at low doses (<1 nM), whereas binding appeared to saturate at high doses (10 nM). A Scatchard plot of the specific binding data was linear (Fig. 2). PACAP bound with high affinity (Kd = 1 nM) to a single class of sites (Bmax = 14,000/cell).
Fig. 1.
The amount of specifically bound 125I-PACAP-27 is indicated as a function of PACAP-27 concentration. The mean of four deteminations is indicated. The line is point to point.
Fig. 2.
A Scatchard plot of the specific binding data is shown. The line represents the best fit.
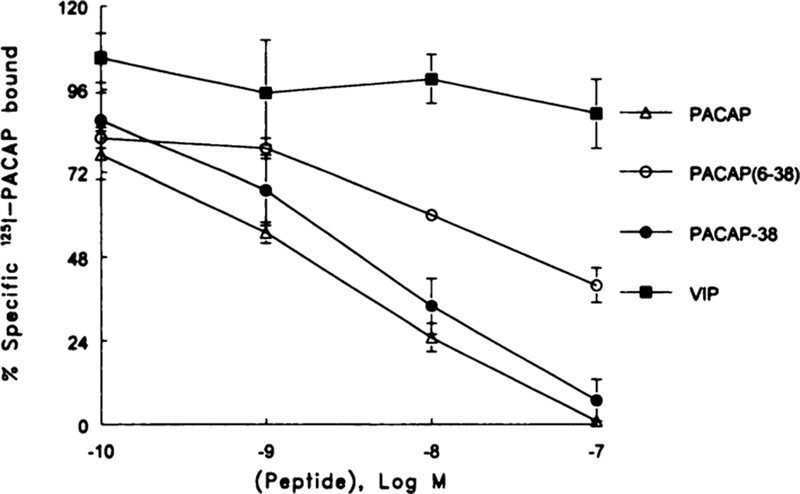
The specificity of binding was investigated. Fig. 3 shows that specific 125I-PACAP binding was inhibited slightly by 0.1 nM PACAP-27 and strongly by 1000 nM PACAP-38. The concentration of PACAP-27 required to inhibit 50% of the specific 125I-PACAP-27 binding (IC50) was 1 nM. Because the dose-response curve was shallow, it is possible that PACAP-27 may bind with low affinity to an additional class of sites. PACAP-38 and PACAP(6–38) were less potent; they had IC50 values of 3 and 20 nM, respectively. VIP, PACAP(28–38), and PACAP(16–38) did not significantly inhibit 125I-PACAP binding at a 1000-nM dose. Similar binding data were obtained using cell line NCI-H1299 or H727 (data not shown).
Fig. 3.
The percent of specific 25I-PACAP bound was determined as a function of PACAP-27 (Δ), PACAP-38 (●), PACAP(6–38) (○), and VIP (■) concentration. The mean of three experiments each repeated in quadruplicate is indicated. Bars, SE.
Northern Blot Analysis.
To further confirm the presence of PACAP type 1 receptor mRNA in NSCLC cells, high-stringency Northern blot analysis of the total RNA was performed. A full-length, 32P-labeled, human, type 1 PACAP receptor cDNA probe hybridized to a single 7.5-kb transcript in the NCI-H838 cells (Fig. 4). In addition, SCLC NCI-N417 cells, AR42-J, and human brain RNA showed similar hybridizing transcripts (8).
Fig. 4.
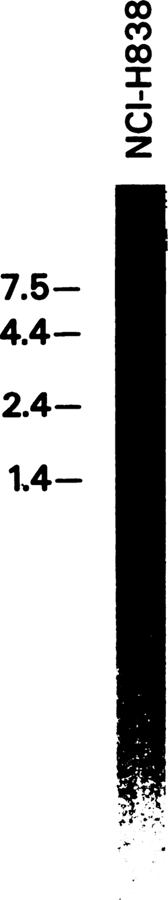
Northern blot analysis. A main band of radioactivity at 7.5 kb was detected using guanidine isothiocyanate extracts of NCl-H838 cells.
cAMP.
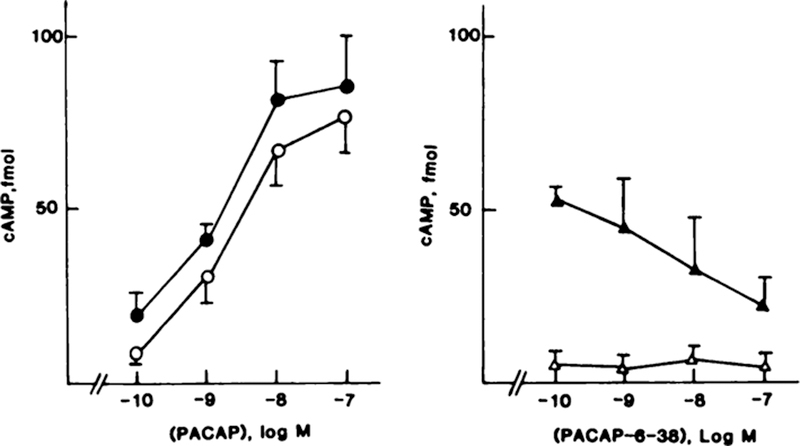
To demonstrate that the receptors are coupled to adenylate cyclase, PACAP-27 and PACAP-38 dose-response stimulation of cAMP was determined in NCI-H838 cells. Fig. 5 (left) shows that 0.1 nM PACAP-27 elevated cAMP 2-fold, whereas 10 nM PACAP-27 elevated cAMP 12-fold. The half-maximal effective concentration (EC50) was 3 nM. Likewise, PACAP-38 elevated cAMP, and the EC50 was 2 nM. In contrast, PACAP(6–38) and PACAP(16–38) had little effect on basal cAMP, but PACAP(6–38) inhibited the increase in cAMP caused by 10 nM PACAP-27 (Fig. 5, right). PACAP(6–38) (100 nM) half maximally inhibited the increase in cAMP caused by 10 nM PACAP. PACAP(16–38) and PACAP(28–38) had no effect on cAMP at a 1-μM dose.
Fig. 5.
Left, cAMP was determined in NCI-H838 cells as a function of PACAP-27 (○) and PACAP-38 (●) concentration. Right, cAMP was determined as a function of PACAP(6–38) concentration in the absence (Δ) and presence (▲) of 10 nM PACAP. The mean of four determinations is indicated. Bars, SE.
Cytosolic Ca2+.
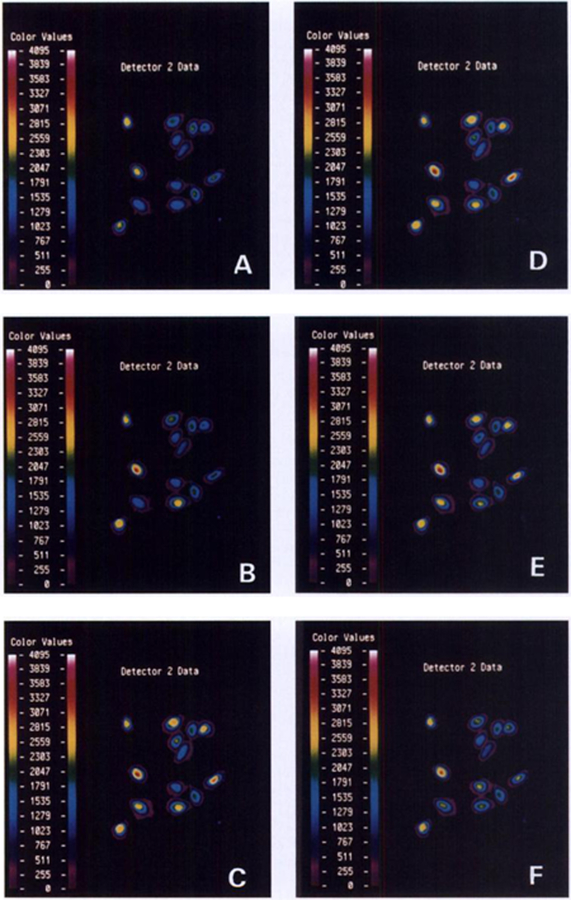
PACAP elevated cytosolic Ca2+ in Indo-1 AM-loaded NCI-H838 cells. Fig. 6A shows that the basal Ca2+ was determined in a field of 13 cells. Thirty s after the addition of 100 nM PACAP-27, Ca2+ started to increase in some cells, and the increase was maximal after 60 s (Fig. 6C). Cytosolic Ca2+ increased in 9 of 13 cells and started to decline after 90 s (Fig. 6D). Cytosolic Ca2+ continued to decline (Fig. 6E) and returned to baseline after approximately 150 s (Fig. 6F). The effects of PACAP were dose dependent; 10 and 100 nM, but not 1 nM, PACAP-27 elevated cytosolic Ca2+. Likewise, 100 nM PACAP-38, but not PACAP(16–38), PACAP(28–38), or VIP, increased the cytosolic Ca2+. PACAP(6–38) (1 uM) inhibited the increase in cytosolic Ca2+ caused by 100 nM PACAP-27 (data not shown).
Fig. 6.
NCI-H838 cells were loaded with Indo-1AM, and the basal cytosolic Ca2+ was determined in a field of 13 cells. PACAP-27 (100 nM) was added, and cytosolic Ca2+ determined after 0 min (A), 0.5 min (B), 1 min (C), 1.5 min (D), 2 min (E), and 2.5 min (F). This experiment is representative of two others.
Arachidonic Acid Release.
PACAP stimulated arachidonic add release. NCI-H838 readily incorporated AA into endogenous phospholipids after overnight incubation. When the cells were washed twice to remove free AA, only 106 cpm were released into the medium during a 45-min incubation (Table 2). When PACAP-27 (100 nM) was added, the rate of AA release increased 6-fold. The addition of PACAP(6–38) had no effect on basal AA release but inhibited, in a concentration-dependent manner, PACAP-27-induced arachidonic acid release.
Table 2.
Effects of PACAP on arachidonic acid releasea
| Addition | cpm |
|---|---|
| None | 106 ± 7b |
| PACAP-27, 100 nM | 607 ± 104 |
| PACAP-27 + PACAP (6–38), 1 nM | 507 ± 43 |
| PACAP-27 + PACAP (6–38), 10 nM | 489 ± 60 |
| PACAP-27 + PACAP (6–38), 100 nM | 430 ± 81c |
| PACAP-27 + PACAP (6–38), 1000 nM | 286 ± 57b |
The mean ± SD of four determinations is indicated.
P < 0.05; relative to 100 nM PACAP using Student’s t-test.
P < 0.01.
Proliferation.
PACAP stimulated colony formation in NCI-H838 cells. PACAP(6–38) (100 nM) significantly inhibited NCI-H727 colony number (Table 3). PACAP-27 (10 nM) significantly stimulated NCI-11727 colony number (3-fold), and the increase caused by PACAP was inhibited by 100 nM PACAP(6–38). In addition, 100 nM PACAP(6–38) reduced basal colony formation 7-fold. PACAP(16–38) or PACAP(28–38) had no effect on NCI-H838 growth (data not shown).
Table 3.
Effect of PACAP analogues on growtha
| Additions | NCI-H727 colonies |
|---|---|
| None | 27 ± 7 |
| PACAP-27, 10 nM | 85 ± 3b |
| PACAP (6–38), 100 nM | 4 ± 3c |
| PACAP-27 + PACAP (6–38) | 33 ± 7 |
The mean ± SE of three determinations is indicated.
P < 0.05; relative to control.
P < 0.01.
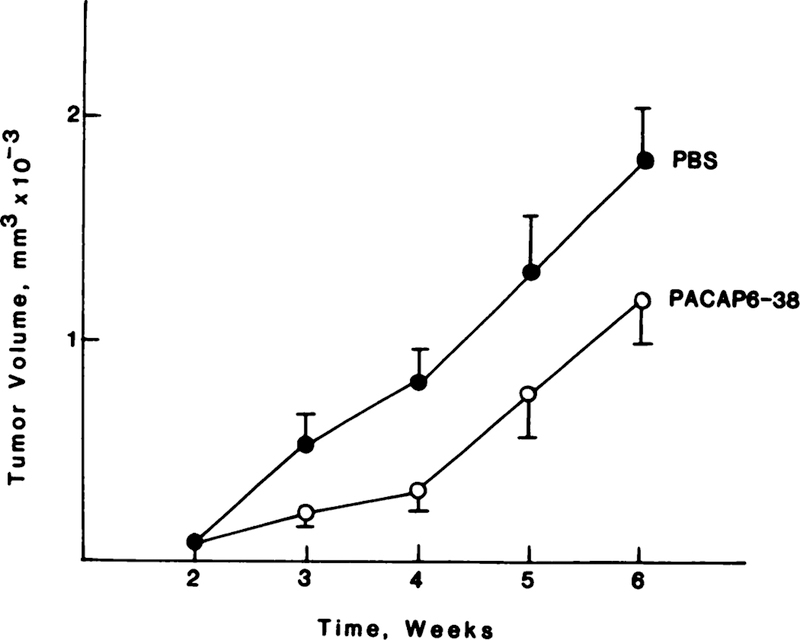
PACAP(6–38) inhibited NSCLC growth in vivo. In nude mice that received injections of NCI-H838 cells, a palpable mass formed at week 2. Fig. 7 shows that, in control mice, the tumors grew rapidly, and, at week 6, the tumor volume was 1909 mm3. In mice that received PACAP(6–38) injections of 10 µg/day s.c., tumor proliferation was slowed significantly at weeks 3–6, and, at week 6, the mean tumor volume was 1112 mm3. Similar data were obtained using NCI-H727 xenografts.
Fig. 7.
Nude mice received injections of NCI-H838 cells, and, after 2 weeks, a xenograft formed. PBS and PACAP(6–38) (10 µg/day s.c.) were injected daily during weeks 2–6, and the xenografts were measured weekly.
DISCUSSION
PACAP is the most recently discovered peptide in the VIP/secretin/glucagon family of peptides (2). Since its identification, numerous-studies have identified specific PACAP binding sites on normal and tumoral tissues (3, 16). With the recent cloning of the rat and human PACAP receptors (8, 9, 23), specific molecular probes are now available to distinguish the type 1 PACAP receptor from the VIP1, and VIP2 receptors. We have shown previously the presence of VIP receptors on NSCLC cell lines (24, 25), and, herein, we report the effects of PACAP on NSCLC cells.
125I-PACAP-27 shows high-affinity binding to NSCLC cells. In NCI-H838 cells, 125I-VIP binds with high affinity to VIP receptors only, whereas 125I-PACAP-27 binds with high affinity to both PACAP and VIP receptors. These observations are similar to those observed in native cells, as well as in cells transfected with VIP or PACAP receptor cDNAs (15). VIP receptors chacterized previously on NSCLC cells demonstrated that 125I-VIP binding was inhibited by both VIP and PACAP. In this study, 125I-PACAP-27 bound to NCI-H838 cells with high affinity (Kd = 1 nM) to a single class of receptor sites (Bmax = 1.4 × 104/cells). The order of peptide potency is PACAP-38 = PACAP-27 ≫ PACAP(6–38) ≫ VIP = PACAP(16–38) > PACAP(28–38). These data suggest that 125I-PACAP-27 bind to type 1 PACAP receptors.
Unlike the VIP receptor, which couples to NSCLC adenylate cyclase, PACAP receptors have been shown in both native and transfected cells to couple to both adenylate cyclase and phospholipase C. PACAP stimulation of NCI-H838 cells, therefore, was evaluated to couple to both signal transduction pathways. PACAP-27 and PACAP-38 increased cAMP similarly; they had EC50 of 3 and 2 nM, respectively. The ability of both peptides to stimulate cAMP with similar potency has been observed in native cells and in cells transfected with the type 1 PACAP receptor (15, 16). In astrocytes, low doses of VIP are reported to elevate adenylate cyclase and translocate protein kinase C (26, 27).
To evaluate the ability of PACAP receptors to couple to phospholipase C, we evaluated the downstream response of intracellular Ca2+ signaling in Indo-1AM- loaded NCI-H838 cells. In contrast with the adenylate cyclase response, PACAP-27 was half maximally potent at 100 nM at stimulating an increase in cytosolic Ca2+. These results suggest that PACAP-27 is 50-fold less potent at stimulating phospholipase C in NCI-H838 cells than is adenylate cyclase. These results are consistent with the PI turnover observed for PACAP-stimulated NIH 3T3 cells stably transfected with the human type 1 PACAP receptor (15). Because PACAP induces an elevation in cytosolic Ca2+ in the presence or absence of EGTA, Ca2+ may be released from the endoplasmic reticulum. PACAP-induced stimulation of phospholipase C also may result in protein kinase C activation. This signal transduction response suggests that PACAP receptors at low ligand occupancy are capable of maximal adenylate cyclase stimulation as a result of receptor spareness. Receptor spareness is not observed for Ca2+ stimulation, as suggested by the nearly superimposable dose response for Ca2+ stimulation and ligand-binding affinity curves. These data suggest that the PACAP receptor is more tightly coupled to a stimulatory guanine nucleotide binding protein (G protein), such as Gαs, because it is coupled principally to adenylate cyclase rather than phospholipase C. However, as suggested for the D2 dopaminer gic receptor, alternatively spliced receptor splice variants may couple to additional G proteins, leading to different effector responses.
Five splice variants of the rat PACAP type 1 receptor, which differ between the fifth and sixth transmembrane domains (third intracellular loop), have been cloned. The basic PACAP receptor has 467 amino acids and a 14-amino acid third intracellular domain (9). The hip PACAP receptor (495 amino acids) has the basic 14 amino acids plus a 28-amino acid insert at the third intracellular domain. The hop1 PACAP receptor (495 amino acids) has the basic 14 amino acids plus a different 28-amino acid insert at the third intracellular domain, whereas the hop2 PACAP receptor is similar to the hop1, variant, but has a deletion of 1 amino acid from the hop1 insert. The hip/hop PACAP receptor (523 amino acids) has the basic 14 amino acids plus both 28-amino acid inserts at the third cytosolic domain.
Differential expression of the rat PACAP receptor splice variants results in differential coupling to the intracellular effectors, cAMP, and total PI. These structural and functional differences in the splice variants are important because they may explain the different intracellular signal transduction responses that occur with PACAP-stimulation in specific cell types. The rat PACAP-R basic and hop1 splice variants showed an ability to couple to both intracellular pathways, whereas the hip splice variant couples to adenylate cyclase only (9). Although the purpose of our study was not aimed specifically at identifying splice variant specific expression for PACAP receptors on human NSCLC cells, our signal transduction results suggest that human NSCLC cells may express the basic PACAP-R and/or hop1 splice variants. At present, we are investigating the specificity of this splice expression in NSCLC cells.
The existence of PACAP receptors was further confirmed by high-stringency Northern blot analysis of NCI-H838 cells showing a single hybridizing transcript of 7.5 kb, which is consistent in size with the rat and human PACAP receptors published previously (9, 10). VIP receptor mRNA shows transcript sizes of 5, 2.5, and 1.5 kb when hybridized with a VIP1 receptor cDNA probe.5
Previously, epidermal growth factor receptors were detected in high density in NSCLC cells (28). Recently, we found that EGF caused prostaglandin E2 production in NSCLC cells, which resulted in proliferation (29). Herein, PACAP caused arachidonic acid release from NSCLC cells. PACAP may stimulate phospholipase A2 activity as a result of increased phosphorylation of phospholipase A2 by protein kinase C.
The proliferation of SCLC cells in vitro is maximally stimulated by 10 nM bombesin or 10 nM VIP, which cause PI turnover and elevate cAMP, respectively (25, 30). One hypothesis is that PACAP may be more potent than VIP and may maximally stimulate NCI-H838 colony formation at 1 nM, because it stimulates PI turnover and elevates cAMP at the same time. Recently, we found that PACAP significantly increases c-fos gene expression at a 1 nM (31). The c-fos gene in NSCLC cells may have both a phorbol ester- and cAMP-responsive element.
PACAP(6–38) (10 µg/day s.c.) slowed NCI-H838 xenograft formation by approximately 45%. Previously, we found that VIP hybrid (10 µg/day s.c.) slowed NSCLC xenograft formation in nude mice by 80% (14). These data indicate that NSCLC tumor growth may be slowed by either PACAP or VIP receptor antagonist. VIP hybrid may be more potent than PACAP(6–38) in vivo because of the slower rate of degradation by blood proteases (32).
In summary, PACAP binds with high affinity, elevates cAMP, causes PI turnover, releases arachidonic acid, and stimulates growth of NSCLC cells. The actions of PACAP are antagonized by PACAP(6–38). Therefore, PACAP receptors are present on NSCLC cells, where they may function as potential regulators of growth and differentiators of these tumor cells. The development of more potent PACAP receptor antagonists will permit a potential antitumor strategy using selective PACAP receptor antagonists to reduce proliferation of NSCLC cells.
ACKNOWLEDGMENTS
The authors thank Drs. R. Jensen and S. Jakowlew for helpful discussions.
This research was supported in part by National Institutes of Health Grant DK-36107.
Footnotes
The abbreviations used are: PACAP, pituitary adenylate cyclase activating peptide; VIP, vasoactive intestinal peptide; NSCLC, non-small cell lung cancer; PI, phosphatidylinosilol; SCLC, small cell lung cancer; SIT, 3×10−8 m Na2SeO3, 5 µg/ml insulin, and 10 µg/ml transferrin; AA, [3H]arachidonic acid.
T. Moody, unpublished observation.
Contributor Information
Farah Zia, Departments of Microbiology, Biochemistry, and Molecular Biology, George Washington University Medical Center, Washington, District of Columbia 20037.
Mirela Fagarasan, Departments of Microbiology, Biochemistry, and Molecular Biology, George Washington University Medical Center, Washington, District of Columbia 20037.
Kamil Bitar, Department of Medicine, Peptide Research Laboratory, Tulane University School of Medicine, New Orleans, Louisiana 70112.
David H. Coy, Department of Medicine, Peptide Research Laboratory, Tulane University School of Medicine, New Orleans, Louisiana 70112
Joe R. Pisegna, Digestive Diseases Branch, National Institute of Diabetes, Digestive and Kidney Disease, National Institutes of Health, Bethesda, Maryland 20892
Steve A. Wank, Digestive Diseases Branch, National Institute of Diabetes, Digestive and Kidney Disease, National Institutes of Health, Bethesda, Maryland 20892
Terry W. Moody, Biomarkers and Prevention Research Branch, National Cancer Institute, Rockville, Maryland 20850.
REFERENCES
- 1.Said SI, and Mutt V Polypeptide with broad biological activity: isolation from the small intestine. Science (Washington DC), 69: 1217–1218, 1970. [DOI] [PubMed] [Google Scholar]
- 2.Miyata A, Jiang L, Dahl RR, Kitada C, Kubo K, Fujino M, Minamino N, and Arimura A Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP-38). Biochem. Biophys. Res. Commun, 170: 643–648, 1990. [DOI] [PubMed] [Google Scholar]
- 3.Gottschall PE, Tatsuno I, Miyata A, and Arimura A Characterization and distribution of binding sites for the hypothalamic peptide, pituitary adenyate cyclase activating polypeptide (PACAP): characterization and molecular identification. Endocrinology, 127: 272–277, 1990. [DOI] [PubMed] [Google Scholar]
- 4.Luis J, and Said S Characterization of VIP and helodermin-preferring receptors on human small cell lung carcinoma ceil lines. Peptides, 11: 1239–1244, 1990. [DOI] [PubMed] [Google Scholar]
- 5.LaBurthe M, Boissard C, Chevalier G, Zweibaum A and Rosselin G Peptide receptors in human lung tumor cells in culture: vasoactive intestinal peptide (VIP) and secretin interaction with the Calu-1 and SW-900 cell lines. Regul. Peptides, 2: 219–230, 1981. [DOI] [PubMed] [Google Scholar]
- 6.Cauvin A, Buscail L, Gourlet P, DeNeef P, Gossen D, Arimura A, Miyata A, Coy DH, Robberecht P, and Christophe J The novel VIP-like hypothalamic polypeptide PACAP interacts with high affinity receptors in the human neuroblastoma cell line NB-OK. Peptides, 11: 773–777, 1990. [DOI] [PubMed] [Google Scholar]
- 7.Ishihara T, Shigemoto R, Mori K, Takahashi K, and Nagata S, Functional expression and tissue distribution of a novel receptor for vasoactive intestinal polypeptide. Neuron, 8: 811–819, 1992. [DOI] [PubMed] [Google Scholar]
- 8.Pisegna JR, and Wank SA Molecular cloning and functional expression of the pituitary adenylate cyclase-activating polypeptide type 1 receptor. Proc. Natl. Acad. Sci. USA, 90: 6345–6349, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spengler D, Waebcr C, Pantaloni C, Holsboer F, Bockaert J, Seebcrg PH, and Joumot L Differential signal transduction by five splice variants of the PACAP receptor. Nature (Lond.), 365: 170–175, 1993. [DOI] [PubMed] [Google Scholar]
- 10.Lutz EM, Sheward WJ, West KM, Morrow JA, and Harmar AJ The VIP2 receptor: molecular characterization of a cDNA encoding a novel receptor for vasoactive intestinal peptide. FEBS Lett, 334: 3–8, 1993. [DOI] [PubMed] [Google Scholar]
- 11.Bloom SR, Christofides ND, Delamarter J, Buell G, Kawashima E, and Polak JM Diarrhoea in VIPoma patients associated with cosecretion of a second active peptide (peptide histidine isoleucine) explained by a single coding gene. Lancet, 2: 1163–1165, 1983. [DOI] [PubMed] [Google Scholar]
- 12.Bodner M, Fridkin M, and Gozes I VIP and PHM-27 sequences are located on two adjacent exons in the human genome. Proc. Natl. Acad. Sci. USA, 82: 3548–3551, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gozes I, Davidson M, Draoui M, and Moody TW The VIP gene is expressed in non-small cell lung cancer cell lines. Biomed. Res, 13 (Suppl. 2): 37–40, 1993. [Google Scholar]
- 14.Moody TW, Zia F, Brenneman D, Fridkin M, Davidson A, and Gozes I A VIP antagonist inhibits the growth of non-small cell lung cancer. Proc. Natl. Acad. Sci. USA, 90: 4345–4349, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pisegna JR and Wank SA Molecular cloning and characterization of the signal transduction pathways for the four splice variants of the human PACAP receptor (hPACAP-R), J. Biol. Chem, in press, 1995. [DOI] [PMC free article] [PubMed]
- 16.Moody TW, Zia F, and Makheja A PACAP elevates cytosolic calcium in small cell lung cancer cell lines. Peptides, 14: 241–246, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Carney DN, Gazdar AF, Bepler G, Guccion JG, Marangos PJ, Moody TW, Zweig MH, and Minna JD Establishment and identification of small cell lung cancer cell lines having classic and variant features. Cancer Res, 45: 2913–2923, 1985. [PubMed] [Google Scholar]
- 18.Chirgwin JM, Przybyla AE, MacDonald RJ, and Rutter WJ Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry, 18: 5294–5299, 1979. [DOI] [PubMed] [Google Scholar]
- 19.Davis L, Dibner M, and Battey JF Preparation and analysis of RNA from eukaryotic cells. Basic Methods in Molecular Biology, pp. 319–396. New York: Elsevier, 1994. [Google Scholar]
- 20.Korman LY, Carney DN, Citron ML, and Moody TW Secretin/VIP stimulated secretion of bombesin-like peptides from human small cell lung cancer. Cancer Res, 46: 1214–1218, 1986. [PubMed] [Google Scholar]
- 21.Moody TW, Faragasan M, Zia F, Cesnjaj M, and Goldstein AL Thymosin α−1 downregulates the growth of human non-small cell lung cancer. Cancer Res, 53: 5214–5218, 1993. [PubMed] [Google Scholar]
- 22.Mahmoud S, Staley J, Taylor J, Bogden A, Moreau JP, Coy D, Avis I, Cuttitta F, Mulshine JL, and Moody TW (Psi-13, 14) Bombesin analogues inhibit growth of small cell lung cancer in vitro and in vivo. Cancer Res, 51: 1798–1802, 1991. [PubMed] [Google Scholar]
- 23.Svoboda M, Tastenoy M, Ciccarelli E, Stievenart M, Christophe J, Cloning of a splice variant of the pituitary adenylate cyclase-activating polypeptide (PACAP) type 1 receptor. Biochem. Biophys. Res. Commun 195: 881–888, 1993. [DOI] [PubMed] [Google Scholar]
- 24.Lee M, Jensen RT, Huang SC, Bepler G, Korman L, and Moody TW Vasoactive intestinal polypeptide binds with high affinity to non-small cell lung cancer cells and elevates cyclic AMP levels. Peptides, 11: 1205–1209, 1990. [DOI] [PubMed] [Google Scholar]
- 25.Shaffer MM, Carney DN, Korman LY, Lebovic GS, and Moody TW High affinity binding of VIP to human lung cancer cell lines. Peptides, 8: 1101–1106, 1987. [DOI] [PubMed] [Google Scholar]
- 26.Fatatis A, Holtzclaw LW, Avidor R, Brenneman DE, and Russel J Vasoactive intestinal peptide increases intracellular calcium in astroglia: Synergism with α-adrenergic receptors. Proc. Natl. Acad. Sci. USA, 91: 2036–2040, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olah Z, Lehel C, Anderson WB, Brenneman DE, and Agoston DV Subnanomolar concentrations of VIP induces the nuclear translocation of protein kinase C in neonatal rat cortical astrocytes. J. Neurosci. Res, 355–363, 1994. [DOI] [PubMed]
- 28.Moody TW, Lee M, Kris RM, Bellot F, Bepler G, Oie H, and Gazdar A Lung carcinoid cell lines have bombesin-like peptides and EGF receptors. J. Cell. Biochem, 43: 139–147, 1990. [DOI] [PubMed] [Google Scholar]
- 29.Moody TW, Hida T, Mulshine J, Makheja A, Zia H, and Hla T Cyclooxygenase inhibitors reduce prostaglandin E2 and the growth of non-small cell lung cancer cells. Cancer Res. Proc 36: 601, 1995. [Google Scholar]
- 30.Carney DN, Cuttitta F, Moody TW, and Minna JD Selective stimulation of small cell lung cancer clonal growth by bombesin and gastrin releasing peptide. Cancer Res, 47: 821–825, 1987. [PubMed] [Google Scholar]
- 31.Zia F, Park M, Jakowlew S, Birrer M, and Moody TW Pituitary adenylate cyclase activating peptide stimulates c-fos gene expression in small cell lung cancer cells. Cancer Res. Proc 36: 564, 1995. [Google Scholar]
- 32.Moody TW, Zia F, Bitar K, and Coy DH PACAP (6–38) is a PACAP type 1 receptor antagonist on small lung cancer cells. Rosselin G (ed.), Vasoactive Intestinal Peptide, Pituitary Adenylate Cyclase Activating Polypeptide and Related Regulatory Peptides: From Molecular Biology to Clinical Applications, pp. 537–544. Singapore: World Scientific, 1994. [Google Scholar]