Abstract
An ASTM International subcommittee on Respiratory Protection, F23.65 is currently developing a consensus standard for assessing respirator fit capability (RFC) criteria of half-facepiece air-purifying particulate respirators. The objective of this study was to evaluate if the test methods being developed for half-facepiece respirators can reasonably be applied to non-powered full-facepiece–air-purifying respirators (FF–APR).
Benchmark RFC test data were collected for three families of FF–APRs (a one-size-only family, a two-size family, and a three-size family). All respirators were equipped with P100 class particulate filters. Respirators were outfitted with a sampling probe to collect an in-mask particle concentration sample in the breathing zone of the wearer. Each of the six respirator facepieces was tested on the National Institute for Occupational Safety and Health 25-subject Bivariate Panel. The RFC test assessed face seal leakage using a PortaCount fit test. Subjects followed the corresponding Occupational Safety and Health Administration-accepted fit test protocol.
Two donnings per subject/respirator model combination were performed. The panel passing rate (PPR) (number or percentage of subjects in the panel achieving acceptable fit on at least one of two donnings) was determined for each respirator family at specified fit factor passing levels of 500, 1,000, and 2,000. As a reasonable expectation based on a previous analysis of alpha and beta fit test errors for various panel sizes, the selected PPR benchmark for our study was >75%.
At the fit factor passing level of 500 obtained on at least one of two donnings, the PPRs for three-, two-, and one-size families were 100, 79, and 88%, respectively. As the fit factor passing criterion increased from 500 to 1,000 or 2,000, PPRs followed a decreasing trend. Each of the three tested families of FF–APRs are capable of fitting ≥ 75% of the intended user population at the 500 fit factor passing level obtained on at least one of two donnings. The methods presented here can be used as a reference for standards development organizations considering developing RFC test requirements.
Keywords: Fit test, NIOSH-approved respirator, respirator fit methods
Introduction
Respirators, when properly selected and used, reduce exposures to inhalation hazards. In the United States, National Institute for Occupational Safety and Health (NIOSH)-approved respirators are required in workplaces with respiratory protection programs operating under Occupational Safety and Health Administration (OSHA) requirements.[1] Individual workplace respirator fit testing is performed to determine that respirators are capable of providing their expected level of protection.
The development of a respirator fit capability (RFC) test for air-purifying half-facepiece respirators (both filtering facepiece respirators [FFR] and elastomeric half-facepiece respirators [EHR]) using a 25-subject test panel has recently been studied by NIOSH.[2] In that paper, the RFC concept was developed as a way to assess a respirator model’s (or group of multi-sized models [referred to as “family” in that study]) ability to fit a specified user population of diverse facial sizes. In that study, 101 respirator models (57 N95 FFRs, 43 EHRs, and one-quarter-mask elastomeric respirator) were evaluated for their ability to pass five different criteria for determining acceptable respirator fit.
The study concluded that by grouping multi-sized respirators into families and evaluating fit test results over two successive donnings per test subject, a balance can be made between meeting users’ expectations of respirators being able to fit a specified user population while at the same time creating a reasonable performance criterion for manufacturers designing respirators.[2] This work was important in developing a method to assess the fit of filtering facepiece air-purifying respirators (FFR) because NIOSH does not currently include this assessment during the respirator approval process. Numerous researchers and external organizations such as the National Academy of Medicine (formerly the Institute of Medicine) have urged NIOSH to develop a process to incorporate a certification requirement for FFRs to ensure that they are capable of fitting a specified percentage of their intended user population.[3–5] The RFC concept for half-facepiece respirators is continuing to be evaluated and has not been adopted by NIOSH as a certification requirement.
Tight-fitting full-facepiece air-purifying respirators (FF-APR) are designed to form a seal around the entire perimeter of the face using a flexible, elastomeric sealing material; this design incorporates a transparent lens for the user’s vision. FF-APRs are equipped with an appropriate particulate filter, gas/vapor cartridge, or a combination cartridge for filtering both particulates and gasses and vapors. OSHA designates an assigned protection factor (APF) (the expected workplace level of protection under a complete respiratory protection program) of 50 for FF–APRs.[6] The APF of 50 means that if the respirator is properly selected, maintained, and used in accordance with all elements of an OSHA respiratory protection program (as detailed in 29 CFR 1910.134),[6] the wearer of a fit tested FF–APR can expect that the ambient contaminant concentration will be reduced by a minimum of 50 times inside of the respirator facepiece.
As an exploratory study, this paper evaluates RFC criteria for FF-APRs utilizing an updated subject test panel, the NIOSH Bivariate Panel (Bivariate Panel). NIOSH approval of FF–APRs (excluding those which are submitted for particulate protection only) undergo a fit assessment which is currently performed under an established NIOSH standard test procedure which differs from the RFC methods presented here. The established NIOSH test uses different pass/fail criteria, test exercises, a differently-sized anthropometric subject panel developed by the Los Alamos National Laboratory (LANL) (the Los Alamos Scientific Laboratory Male-and-Female 25-Member Panel for Testing of Full-Face Masks), and uses a qualitative fit test assessment (isoamyl acetate vapor) with respirators fitted with organic vapor cartridges.[7] At the time of this writing, NIOSH is phasing out the use of the LANL panel for this test and as of February 1, 2019, NIOSH implemented use of the Bivariate Panel during evaluation of all new approvals of air-purifying respirators (half-facepiece and full-facepiece, excluding non-powered, particulate only air-purifying respirators).
The boundaries of the Bivariate Panel are set so that >95% of the U.S. working population fit within its boundaries. Furthermore, the previously developed LANL full-facepiece panel (developed using data from subjects in 1967–1968 military surveys) excluded15.3% of the NIOSH survey subjects used to develop the Bivariate Panel (data collected in 2003).[8] Subjects measured in the 2003 NIOSH facial anthropometry survey had larger key face dimensions (face length and face width) than the subjects in the 1967–1968 military survey. Thus, it was concluded that the LANL respirator fit test panels did not well represent the current U.S. civilian work force. Additionally, the 2003 NIOSH survey is more representative of the age and racial/ethnic distributions of the current civilian population.
Our current study evaluates the RFC of FF–APRs using the Bivariate Panel and a quantitative PortaCount fit test method to establish an initial benchmark for standards development organizations (SDO) to consider if they choose to develop RFC requirements for this class of respirator.
Methods
Subjects
To evaluate our proposed RFC test criteria, 25 test subjects (14 men and 11 women) participated. Subjects made two separate visits to the laboratory to perform fit testing. This study was approved by the NIOSH Institutional Review Board (protocol no. 14-NPPTL-02). Subjects were recruited from a pool of subjects who periodically participate in NIOSH respirator fit testing research; thus, subjects had experience wearing respirators. Subjects were medically cleared using the OSHA Respirator Medical Evaluation Questionnaire found in Appendix C of the OSHA Respiratory Protection Standard (29 CFR 1910.134).[1] Inclusionary criteria were ages 18–65, subjects being in good physical, cardiac and pulmonary health, body mass index (BMI) of 16–35 kg/m2, and facial measurements bounded by bizygomatic breadth (face width) of 125.5 mm to 158.5 mm and a menton-sellion length (face length) of 98.5 mm to 138.5 mm. Exclusionary criteria were a history of uncontrolled chronic asthma, pneumonia, and high blood pressure. Subjects provided written informed consent. Subjects were monetarily reimbursed for their participation on a per visit basis.
Subject facial measurements were used to determine subject panel cell placement according to the Bivariate Panel (used for the RFC analysis) and also the NIOSH Principal Component Analysis (PCA) panel for study of mean fit factor performance (not part of the RFC analysis). Thirteen traditional anthropometric measurements between craniofacial landmarks (bony and soft tissue landmarks) were collected with spreading calipers, sliding calipers, and steel measuring tape. The dimensions measured were: head breadth, minimal frontal breadth, nasal root breadth, interpupillary breadth, face width (bizygomatic breadth), nose breadth, bigonial breadth, lip length, nose length, nose protrusion, face length (menton-sellion length), menton subnasale length, and head circumference. Face length and face width measurements were used to determine a subject’s panel cell in the Bivariate Panel. All measurements previously listed with the exception of lip length, nose length, and head circumference were used to determine a subject’s panel cell in the PCA panel. The selection of the 10 dimensions for the PCA panel was also based on literature review, expert opinions, and correlation analyses between all dimensions.[8]
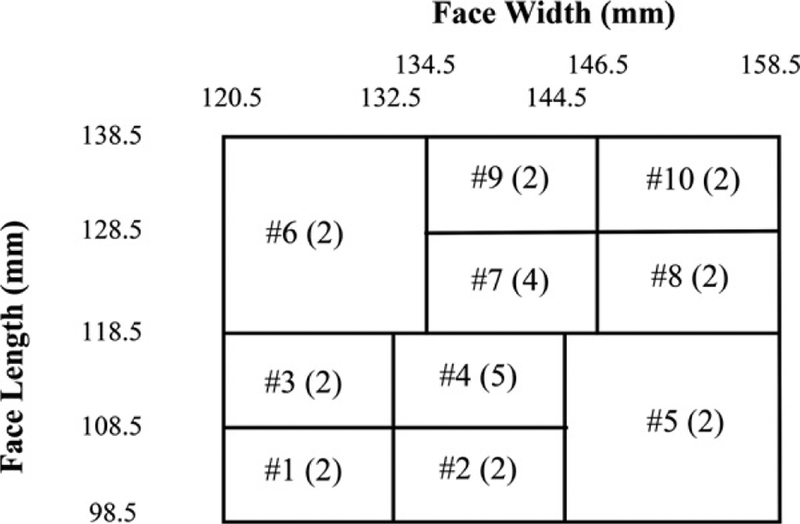
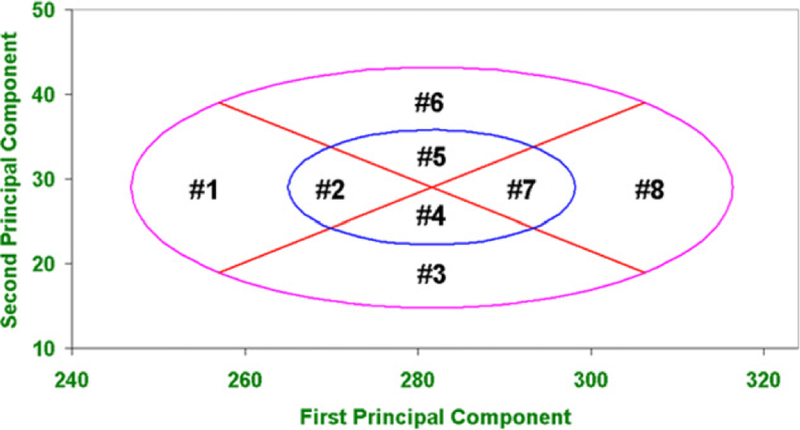
The RFC analysis uses the Bivariate Panel containing 25 test subjects (Figure 1). Zhuang et al.[8] developed this panel from a large scale anthropometric survey of U.S. workers. The Bivariate Panel contains 10 cells which are each defined by the dimensions of face length and face width. Two principal components represent the axes on the PCA panel. The first principal component (PC1) on the x-axis represents the overall size of the face (small, medium, or large) and the second principal component (PC2) on the y-axis determines the shape of a face, (long/narrow or short/wide). The PCA panel is comprised of eight cells representing overall face size and shape (Figure 2).
Figure 1.
National Institute for Occupational Safety and Health (NIOSH) bivariate panel, based on face length and face width. The cells are numbered 1–10 and the numbers in parentheses indicate the number of subjects sampled from each cell. When the subject’s face length or face width fall on the boundaries, the subject is classified into the higher number cell with greater face dimensions.
Figure 2.
NIOSH principal component analysis (PCA) panel. A subject’s cell number is determined by their PC1 and PC2 coordinates on the PCA panel. Cell 1 is considered small face size, Cells 2, 4, 5, and 7 are considered medium face size, cell 8 is considered large face size, Cell 3 is considered short/wide, and Cell 6 is considered long/narrow.
Respirators
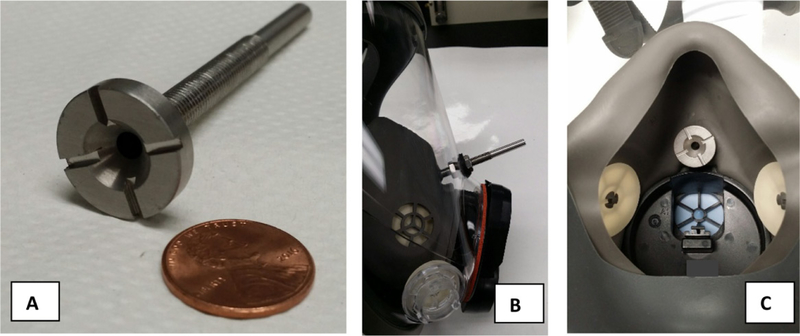
Three families (a family refers to set of different sized models from the same manufacturer) of FF–APRs were evaluated: a three-size model family, a two-size model family, and a one-size-only model family. These families were randomly selected from the list of NIOSH-approved industrial class families at the time of the study. A sampling probe was inserted into the facepiece by first drilling a hole through the face shield and the inner nose cup. A probe (hollow metal tube, length = 7 cm, inner diameter ~3 mm) having metal threads on its exterior surface was then secured to the facepiece by placing rubber gaskets and metal nuts on each side of the face shield (Figure 3). The probe inlet had a 2 cm diameter flange; the flange was placed flush to the inner wall of the nose cup. This probe location sampled air inside the nose cup to approximate the actual particle concentration that the wearer would breathe. The inlet flange had four notched depressions (each ~2 mm deep) on its outer perimeter, each equidistant apart; these notches are intended to transport the aerosol into the probe in the event the main center inlet becomes accidentally blocked.
Figure 3.
Fit test probe, typically used by National Institute for Occupational Safety and Health (NIOSH) to complete laboratory respirator protection level testing. A) probe inlet with affixed sampling tube (shown with U.S. penny for scale), B) side view of respirator showing probe inserted through face shield, and C) interior view of nose cup showing probe inlet.
Fit test methodology
Six respirator models were tested. The study design had the goal of each test subject performing one fit test (i.e., one donning) of each of the six different models (i.e., all sizes of all families). Each donning of each model was to be performed on two separate laboratory visits; thus, the study design set out to obtain a total of 300 data points (25 subjects × 6 respirator models × 1 donning/model/visit × 2 visits/subject = 300 donnings (i.e., 300 data points). In total, only 286 data points were collected because four of the subjects did not perform one or both of the donnings for some models, as follows: one subject did not test three of the models because of reporting discomfort (reporting the models were too small for the subject’s face), resulting in the loss of six data points; one subject did not return for the second visit, thus did not test the second trial for all six models (resulting in the loss of six data points); and finally, two subjects did not perform the second donning on a respirator model (resulting in the loss of two data points).
Fit testing was performed inside a walk-in chamber (length × width × height: 10’ × 8’ × 9’). An aerosol generator (4100/250F single collision atomizer; SFP Services, Dorset, UK) was used to generate sodium chloride (NaCl) aerosol by atomizing a 2 w/v% NaCl solution in deionized water. The particle concentration in the test chamber was maintained at 15–25 × 103 particles/cm3. A light-scattering laser photometer (DustTrak II 8530, TSI, Inc., Shoreview, MN) was used to monitor the chamber aerosol concentration. Because fit testing was performed with a particulate aerosol (NaCl), particulate filters were required. Respirators were equipped with P100 particulate filters as part of the approved respirator assembly for each model. The chamber particle size distribution was periodically measured with a scanning mobility particle sizer spectrometer (SMPS) (model 3936, TSI, Inc., Shoreview, MN) system consisting of a classifier controller (model 3080, TSI, Inc.), a differential mobility analyzer (DMA) (model 3081, TSI, Inc.), a condensation particle counter (CPC) (model 3772, TSI, Inc.), and an aerosol neutralizer (model 3077A, TSI, Inc.). The particle size distribution had a count median diameter in the range of 80–90 nm and geometric standard deviation of approximately 1.8.
Subjects were given training on each respirator model’s donning, adjustment procedures, and user seal check procedures using the user instructions provided by the manufacturer. Subjects were then asked to don the respirator and perform a user seal check. Test technicians observed the subject donning the respirator. If the donning was improper then the subject was instructed to correct the positioning of the respirator. Test technicians did not physically assist test subjects.
If the subject failed the user seal check, subjects were asked to readjust the respirator on their face and to readjust strap tension as necessary. Once these adjustments were made, they were asked to perform another user seal check. This process was repeated until the subject passed the user seal check. The intention of repeating this process was to have the wearer achieve a good fit based on their perception of the user seal check. All subjects were eventually able to pass a user seal check through this process. Subjects then wore the respirator for five minutes to acclimate; following the acclimation, they proceeded into the chamber for the fit test.
Quantitative fit testing was performed with a PortaCount Pro + Respirator Fit Tester (model 8038; TSI, Inc.). The PortaCount uses condensation nuclei counting (CNC) technology to determine a quantitative estimate of respirator fit. The standard blue and white Twin-Tube sampling hose assembly provided with the PortaCount was used to alternately sample between the mask sample and the chamber sample. Prior to the subjects donning each mask, a short length of tubing (~2 cm) was connected to the mask probe sampling outlet. After the respirator was donned, the mask sample hose was attached via a hose barb to the short length of tubing attached to the sample probe. FitPro + Fit Test software (V3.2.0, TSI, Inc.) installed on a desktop computer was used to collect fit test data and calculate fit factors.
The OSHA ambient aerosol condensation nuclei counter (CNC) quantitative fit test protocol (PotaCount protocol) found in Appendix A of 29 CFR 1910.134) was used.[1] Subjects conducted eight fit test exercises: normal breathing, deep breathing, turning head side to side, moving head up and down, talking (reciting the “rainbow passage”), grimacing, bending over (bending at the waist as if to touch the toes), and normal breathing. For each individual test exercise (with the exception of “grimace”), an individual exercise fit factor (FFe) was calculated as the ratio of the ambient particle concentration to the in-mask particle concentration. The overall fit factor (FFo) (the harmonic average of the individual FFe results (with the exclusion of “grimace”) was calculated by the FitPro + Fit Test software at the end of the full set of exercises. For FF–APRs, the OSHA specified passing criterion is FFo ≥ 500 using this method. Our RFC study assessed the passing criteria of FFo ≥ 500, FFo ≥ 1000, and FFo ≥ 2000.
Data analysis
The panel passing rate (PPR) is defined as the percentage (or number) of subjects in a 25-subject panel that a respirator model or family of respirator models is capable of achieving acceptable fit as defined by specific passing criteria selected. As a reasonable expectation based on a previous analysis of alpha and beta fit test errors for various panel sizes, the selected PPR benchmark for our study was >75%.[9]
For the current study, three different criteria for determining acceptable respirator fit were assessed:
Criterion 1: Achieve FFo ≥ the designated FFo (500, 1,000, or 2,000) on at least one of the two donnings.
Criterion 2: Achieve FFo ≥ the designated FFo (500, 1,000, or 2,000) on the first donning only; the second donning was not evaluated for this analysis.
Criterion 3: Achieve a FFo ≥ the designated FFo (500, 1,000, or 2,000) on both of two donnings.
For the PPR data analysis, data were used corresponding to the Bivariate Panel size of the subject and the respirator size they could test for a particular family. For Family A (the three-size model family), data from the small-size facepiece were used for subjects in panel cell sizes 1–3 (6 subjects needed), data from the medium-size facepiece were used for subjects in panel cells 4–7 (13 subjects needed), and data from the large-size facepiece were used for subjects in panel cell sizes 8–10 (6 subjects needed). If the subject did not test the facepiece size corresponding to these cell sizes (i.e., these were missing data points), or if the subject achieved a FFo ≤ the test criterion on both donnings, then data were used from another facepiece size accordingly: data from a medium-size facepiece were used for subjects in Cells 1–3, data from a small-size facepiece were used for subjects in Cells 4–5, data from a large-size facepiece were used for Cells 6–7, and data from a medium-size facepiece were used for Cells 8–10.
For data analysis of the two-size family, data from the regular-size facepiece were used for subjects in panel cell sizes 1–5 (where 13 subjects are needed for panel cell requirements) and data for the large-size facepiece were used for subjects in panel cells 5–10 (12 subjects needed). If the subject did not test the facepiece size corresponding to these cell sizes or if the subject achieved a FFo below the test criterion on both donnings, then data from the alternative size facepiece were used. For the one-size only family, all subjects (regardless of panel cell size) tested this single size model.
A special note must be made for the analyses of Criterion 1 and 3. Both of these criteria specifically evaluate that the subject complete two donnings on the same respirator size. Because Subject #257 did not perform a second donning on all six models, this subject could not be used for the two donning analysis; therefore, only 24 subjects could be used to evaluate Criterion 1 and 3.
Chi-square tests were performed to test the hypotheses that the PPRs among the three respirator families were not significantly different using the three acceptable fit criteria (Criterion 1, Criterion 2, and Criterion 3). The chi-square tests were performed at the three different FFo passing levels (500, 1,000, and 2,000). SAS (version 9.3, SAS Institute, Inc., Cary, NC) was used for all calculations and analyses.
To further analyze the data (outside of the RFC test requirements), geometric mean (GM) FFo and geometric standard deviation (GSD) by model were calculated by panel size for both the Bivariate and PCA Panels. These analyses on GM FFo are of interest to better understand mean respirator fit values using the two new panels. All FFo values were log-transformed and then averaged because FFo are highly variable and are usually log-normally or near log-normally distributed.[10] A general linear model procedure followed by a Duncan’s Multiple Range test for post hoc analysis were used to analyze differences in GM FFo by panel size for each model (a significance level of 0.05 was selected to test the null hypothesis that means were not different between panel sizes within a respirator model).
Results
Panel passing rates for the different respirator families and corresponding test criteria are summarized in Table 1. The PPRs within a family follow the general trend of Criterion 1 PPR > Criterion 2 PPR > Criterion 3 PPR. This trend is not surprising when considering the increasing difficulty to meet the requirements of each successive Criterion. For Criterion 1 (passing at least one out of two fit tests) at the specified fit factor level of 500 (i.e., the minimum FFo to pass the individual workplace OSHA-accepted fit test), the PPRs for three-, two-, and one-size families were 100, 79, and 88%, respectively. At the specified fit factor level of 1,000 for Criterion 1, when ≥ 75% of panel subjects was the chosen PPR, all three families can pass with PPRs of 96, 75, and 79% for the three-, two-, and one-size families, respectively. At the 2,000 required fit factor level for Criterion 1, the PPRs for the three-, two-, and onesize families were 96, 63, and 76%, respectively.
Table 1.
Panel passing rate (PPR)* for different respirator families and evaluation criteria.
| Respirator Family | Test Criterion†,‡,§ | Subjects (n) | FFo ≥ 500 (PPR %) | FFo ≥ 1,000 (PPR %) | FFo ≥ 2,000 (PPR %) |
|---|---|---|---|---|---|
| Three-Size Family | Criterion 1 | 24 | 100 | 96 | 96 |
| Criterion 2 | 25 | 96 | 96 | 92 | |
| Criterion 3 | 24 | 96 | 92 | 92 | |
| Two-size family | Criterion 1 | 24 | 79 | 75 | 63 |
| Criterion 2 | 25 | 76 | 72 | 60 | |
| Criterion 3 | 24 | 63 | 38 | 29 | |
| One-size family | Criterion 1 | 24 | 88 | 79 | 76 |
| Criterion 2 | 25 | 64 | 52 | 52 | |
| Criterion 3 | 24 | 54 | 38 | 33 |
PPR is the panel passing rate for the number of subjects tested.
Criterion 1: Achieve FFo ≥ 500 on at least one of the two donnings.
Criterion 2: Achieve FFo ≥ 500 on the first donning only; the second donning was not evaluated for this analysis.
Criterion 3: Achieve a FFo ≥ 500 for both of the two donnings.
For Criterions 2 and 3, the three-size family achieved PPRs ≥ 75% for all three required fit factor levels of 500, 1,000, and 2,000. The two-size family had its highest PPR of 76% for Criterion 2 at the required fit factor level of 500; all other Criterion 2 and 3 PPRs were <75%. For the one-size only family, all of the PPRs for Criterion 2 and 3 were <75% for all three required fit factor levels.
Geometric mean FFo results for the Bivariate Panel are summarized in Table 2 by respirator family, model size, and subject Bivariate Panel size. Within each family and model size grouping, the Duncan’s test on means indicates that mean FFo results are not significantly different between the subjects’ panel size (i.e., they all have the same alphabetical grouping “A”). This is an interesting result because for the three-size family, subjects tested all model sizes regardless of their panel size (e.g., small size panel subjects tested large size masks, and large size panel subjects tested small size masks); however, our experimental results here do not infer anything about actual respirator assignment in the workplace.
Table 2.
Geometric mean overall fit factors (GM FFo) by respirator family, mask size, and bivariate panel size.
| Respirator Family | Model Size | Bivariate Panel Size* | Number of Donnings | GM FFo | GSD FFo | Min FFo | Max FFo | Duncan’s Grouping† |
|---|---|---|---|---|---|---|---|---|
| Three-size family | Large | Large | 11 | 11,677 | 3.0 | 1,905 | 46,527 | A |
| Medium | 26 | 8,454 | 5.9 | 14 | 40,496 | A | ||
| Small | 12 | 7,246 | 9.7 | 17 | 53,298 | A | ||
| Medium | Large | 9 | 4,118 | 14.3 | 15 | 53,538 | A | |
| Medium | 25 | 6,228 | 8.2 | 43 | 43,255 | A | ||
| Small | 12 | 2,519 | 14.6 | 14 | 46,293 | A | ||
| Small | Large | 9 | 1,407 | 14.7 | 20 | 20,364 | A | |
| Medium | 26 | 4,508 | 5.5 | 72 | 49,284 | A | ||
| Small | 12 | 6,374 | 10.9 | 10 | 39,227 | A | ||
| Two-Size Family | Large | Large | 11 | 216 | 22.0 | 2 | 20,576 | A |
| Medium | 26 | 1,516 | 11.7 | 13 | 34,375 | A | ||
| Small | 12 | 213 | 31.8 | 2 | 42,380 | A | ||
| Regular | Large | 9 | 36 | 10.6 | 2 | 1,231 | A | |
| Medium | 26 | 259 | 13.0 | 4 | 15,641 | A | ||
| Small | 11 | 214 | 11.4 | 3 | 11,609 | A | ||
| One-size family | One-Size Only | Large | 11 | 1,251 | 6.5 | 106 | 12,143 | A |
| Medium | 26 | 2,163 | 7.0 | 10 | 20,950 | A | ||
| Small | 12 | 935 | 19.1 | 13 | 24,246 | A |
1–3 constitute “small”, Cells 4–7 constitute “medium,” Cells 8–10 constitute “large.”
Groups that have statistically different GM FFo will have different letters.
Geometric mean FFo results for the PCA panel are summarized in Table 3 by respirator family, model size, and PCA panel size. Within each family and model size grouping, the Duncan’s test on means indicates that the only significantly different means are for the two-size family within the “regular” mask size; the means for the subjects falling outside the panel (indicated by PCA size “–“) and for those of the “small” PCA size (both sizes with Duncan’s grouping “A”), are significantly different than those of all other PCA sizes (having Duncan’s grouping of “B”). The differences in GM’s for the “A” grouping as compared to the “B” grouping may be attributed to the small number of subjects in the panel sizes of the “A” grouping (a reduced number of subjects could have resulted in a higher standard error of the mean)—only two subjects were outside the panel range (“–” size) and only three subjects tested in the small panel size; in contrast, the other panel size in the “B” grouping had between 8 and 23 subjects.
Table 3.
Geometric mean overall fit factors (GM FFo) by respirator family, mask size, and principal component analysis (PCA) panel size.
| Respirator Family | Model Size | PCA Panel Size*,†,‡ | Number of Donnings | GM FFo | GSD FFo | Min FFo | Max FFo | Duncan’s Grouping§ |
|---|---|---|---|---|---|---|---|---|
| Three-size family | Large | - | 2 | 11,999 | 4.1 | 4,441 | 32,419 | A |
| L/N | 10 | 6,217 | 10.5 | 14 | 36,869 | A | ||
| Large | 10 | 14,378 | 2.9 | 1,628 | 46,527 | A | ||
| Medium | 23 | 8,892 | 6.0 | 17 | 53,298 | A | ||
| Small | 4 | 4,645 | 7.7 | 616 | 31,875 | A | ||
| Medium | - | 2 | 3,771 | 1.3 | 3,093 | 4,597 | A | |
| L/N | 7 | 3,181 | 10.8 | 263 | 43,255 | A | ||
| Large | 10 | 6,600 | 5.7 | 209 | 53,538 | A | ||
| Medium | 23 | 4,402 | 17.1 | 14 | 46,293 | A | ||
| Small | 4 | 4,306 | 4.7 | 663 | 26,000 | A | ||
| Small | - | 2 | 449 | 1.0 | 435 | 463 | A | |
| L/N | 8 | 6,906 | 4.5 | 381 | 26,019 | A | ||
| Large | 10 | 3,038 | 12.1 | 68 | 20,379 | A | ||
| Medium | 23 | 4,017 | 9.7 | 10 | 49,284 | A | ||
| Small | 4 | 6,527 | 4.9 | 1,229 | 25,484 | A | ||
| Two-size family | Large | - | 2 | 870 | 181.3 | 22 | 34,375 | A |
| L/N | 10 | 2,182 | 9.2 | 9 | 30,185 | A | ||
| Large | 10 | 362 | 12.2 | 9 | 17,817 | A | ||
| Medium | 23 | 399 | 23.7 | 2 | 42,380 | A | ||
| Small | 4 | 816 | 66.1 | 3 | 22,014 | A | ||
| Regular | - | 2 | 5,424 | 2.9 | 2,569 | 11,452 | A | |
| L/N | 8 | 70 | 8.3 | 4 | 1,711 | B | ||
| Large | 10 | 78 | 16.4 | 2 | 4,234 | B | ||
| Medium | 23 | 152 | 10.2 | 3 | 11,609 | B | ||
| Small | 3 | 5,125 | 2.7 | 2,559 | 15,641 | A | ||
| One-size family | One-size only | - | 2 | 1,473 | 2.7 | 727 | 2,986 | A |
| L/N | 10 | 1,424 | 9.4 | 22 | 20,950 | A | ||
| Large | 10 | 3,134 | 4.4 | 191 | 14,464 | A | ||
| Medium | 23 | 1,239 | 13.3 | 10 | 24,246 | A | ||
| Small | 4 | 1,297 | 7.4 | 170 | 20,194 | A |
“–” indicates subjects with anthropometric measurements that placed them outside of the limits of the panel.
“L/N” means “Long/Narrow” panel size.
None of the subjects were measured to be in the “Short/Wide” panel size.
Groups that have statistically different GM FFo will have different letters.
The chi-square tests ran to test the hypotheses that the PPRs of the three respirator families were not significantly different using the three acceptable fit criteria (Criterion 1, Criterion 2, and Criterion 3) are as follows: Using FFo ≥ 500 and Criterion 1, the chi-square test did not indicate any significant difference in PPR among the three families of respirators (P value > 0.05); using FFo ≥ 500 for both Criterion 2 and 3, the chi-square test showed there is significant difference in PPR among the three families of respirators (P value < 0.05). The same trend was found using FFo ≥ 1,000 (only Criterion 2 and 3 were significant). For FFo ≥ 2,000, the chi-square test showed there is significant difference in PPR for all three families for all the test criteria (Criterion 1, Criterion 2, and Criterion 3; see the Supplemental Data Tables).
Discussion
NIOSH approval of FF-APRs used in this study was performed under a NIOSH standard test procedure which uses different pass/fail criteria, test exercises, and uses a qualitative fit test assessment using isoamyl acetate.[7] The RFC methodology described in this paper was performed as a research effort which incorporates quantitative fit testing using the PortaCount, the updated Bivariate Panel, and considers different criteria for assessing acceptable respirator fit. Any of three RFC test criteria (Criterion 1, Criterion 2, or Criterion 3) can be considered by an SDO interested in developing an RFC test for FF–APRs.
The ASTM International subcommittee on Respiratory Protection, F23.65 is continuing their work to develop a consensus standard for assessing respirator fit capability (RFC) criteria of half-facepiece air-purifying particulate respirators. As there is no consensus standard yet for RFC test requirements, we chose to evaluate FFAPRs using the test criteria of the previously published Zhuang et al. study which evaluated PPR for families of air-purifying half-piece respirators.[2]
The SDO would need to determine what test methods and criteria are appropriate. Among the factors an SDO can consider in establishing an RFC test for FF–APRs are: (1) what PPR to establish, (2) the fit factor passing level (e.g., 500, 1,000, or 2,000), (3) the type of criteria (e.g., number of passing donnings required), and (4) if any of these choices would vary based on the type of respirator family (i.e., how many models make up a particular family). For selecting an appropriate PPR, SDOs can consider the analysis by Landsittel et al.[9] where a binomial model was used to quantify alpha and beta fit test errors for various subject panel sizes.
Our approach to testing subjects with different sizes of a respirator family mirrors a recommended approach for employers when implementing a respiratory protection program.[11] In the workplace practice, workers are initially tested with one size of a respirator family, and if there is a failure, they can be tested on an alternate size model of the same family to determine if a different size fits adequately.
Our results were limited in the respect that we did not obtain a complete data set for some subjects who did not perform all donnings on some models. The overall PPR results may have been slightly different if a complete data set was obtained.
Conclusions
The test method developed for half-facepiece particulate only respirators can be reasonably applied for the RFC test of FF–APRs. Using the analyses presented in this paper, it is reasonable to test respirators as a family and to select a PPR ≥ 75% for performing two fit tests where the acceptable fit criterion is achieving an overall fit factor ≥ 500 for at least one out of two tests (Criterion 1). Increasing the required fit factor level to 1,000 or 2,000 and/or increasing the rigor of the test requirement (e.g., using Criterion 2 or Criterion 3) have the effect of decreasing the PPR. The methods presented here can be considered by SDOs when developing RFC test requirements.
Supplementary Material
Acknowledgments
The authors would like to thank our NIOSH NPPTL colleagues that reviewed draft versions of this manuscript: Colleen Miller, Jeff Palcic, Dr. Patrick Yorio, Dr. Lewis Radonovich, and Judi Coyne. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention. Mention of commercial product or trade name does not constitute endorsement by the National Institute for Occupational Safety and Health.
Funding
This study was funded by NIOSH.
Footnotes
Supplemental data for this article can be accessed at tandfonline.com/uoeh. AIHA and ACGIH members may also access supplementary material at http://oeh.tandfonline.com/.
References
- [1].“Respiratory Protection: Final Rule,” Code of Federal Regulations Title 29, Part 1910 1998. pp. 1152–1300. [Google Scholar]
- [2].Zhuang Z, Bergman M, Lei Z, Niezgoda G, and Shaffer R: Recommended test methods and pass/fail criteria for a respirator fit capability test of half-mask air-purifying respirators. J. Occup. Environ. Hyg 14:473–481 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Institute of Medicine: Preventing Transmission of Pandemic Influenza and Other Viral Respiratory Diseases: Personal Protective Equipment for Healthcare Workers: Update 2010. Larson EL and Liverman CT, Editors. Washington, DC: The National Academies Press, 2011. [PubMed] [Google Scholar]
- [4].Lofgren D: A must for NIOSH: Certify fit performance of the half mask particulate respirator. J. Occup. Environ. Hyg 9:D191–195 (2012). [DOI] [PubMed] [Google Scholar]
- [5].Institute of Medicine: Certifying personal protective technologies: Improving worker safety. Washington, DC: The National Academies Press, 2011. [PubMed] [Google Scholar]
- [6].“Assigned Protection Factors: Final Rule,” Code of Federal Regulations Title 29, Part 1910 2006. pp. 50122–50192. [Google Scholar]
- [7].U.S. National Institute for Occupational Safety and Health: Determination of Qualitative Isoamyl Acetate (IAA) Facepiece Fit, Air-Purifying Respirators Standard Testing Procedure (STP). Procedure No. TEB-APR-STP-0005-05A-06, 2008. [Google Scholar]
- [8].Zhuang Z, Bradtmiller B, and Shaffer RE: New respirator fit test panels representing the current US civilian work force. J. Occup. Environ. Hyg 4:647–659 (2007). [DOI] [PubMed] [Google Scholar]
- [9].Landsittel D, Zhuang Z, Newcomb W, and Berry Ann R: Determining sample size and a passing criterion for respirator fit-test panels. J. Occup. Environ. Hyg 11:77–84 (2014). [DOI] [PubMed] [Google Scholar]
- [10].Nicas M, and Neuhaus J: Variability in respiratory protection and the assigned protection factor. J. Occup. Environ. Hyg 1:99–109 (2004). [DOI] [PubMed] [Google Scholar]
- [11].U.S Occupational Safety and Health Administration: Hospital Respiratory Protection Program Toolkit Resources for Respirator Program Administrators. U.S. Department of Labor, 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.