Abstract
Neuroendocrine tumors (NETs) of the gastrointestinal tract can be grossly divided into two general types: carcinoid and pancreatic endocrine tumors. The former develop in the luminal intestine whereas the latter occur within the pancreas. To ascertain whether pituitary adenylate cyclase-activating polypeptide (PACAP) has a biological effect on the regulation of secretion or growth, we studied the well-established NET cell line, BON. BON cells have been shown previously to contain chromogranin A, neurotensin, and serotonin. In response to mechanical stimulation, BON cells have been demonstrated to release serotonin. The current article demonstrates that the high-affinity PAC1 receptor is expressed on the NET cell line BON. These results indicate that PACAP may regulate the biological release of peptides and serotonin from BON cells and that, like in solid tumors, PACAP could potentially stimulate the growth of BON cells.
Keywords: BON, carcinoid tumor, PACAP
INTRODUCTION
Neuroendocrine tumors (NETs) include both carcinoid and pancreatic endocrine tumors. Originally these tumors were referred to as argentaffin tumors with intracytoplasmic granules owing to their staining characteristics against chromogranin A, neuron-specific enolase, or synaptophysin.1 It is presumed that the majority of these tumors originate in the neuroendocrine cells of the gastrointestinal tract. Patients experience clinical syndromes that are related to the release of biologically active peptides and/or amines. For example, in patients presenting with carcinoid tumors, flushing and diarrhea are the characteristic symptoms.1
A useful way to investigate the biology of NETs is to study the BON cell line. This cell line that was originally developed by C.M. Townsend Jr., (University of Texas, Galveston, TX) is derived from a metastatic lymph node in a patient with a pancreatic NET.2 BON cells have been demonstrated to release a variety of peptides including transforming growth factor (TGF), fibroblast growth factor (FGF), and epidermal growth factor receptor (EGFR) as well as peptides, such as pancreastatin, neurotensin, and the amine 5-HT.2 The release of secretory products, results in the clinical symptoms associated with these tumors.1
We have previously shown that receptors for pituitary adenylate cyclase-activating polypeptide (PACAP) can be identified on tumors originating from the lung, breast, and colon where stimulation by PACAP is linked to proliferative signaling pathways and tumor growth.3–5 We therefore proposed that, similar to solid tumors, receptors for PACAP are expressed on NETs and may mediate biological effects induced by PACAP, such as secretion and growth.
MATERIALS AND METHODS
Materials
BON tumor cells were obtained from Dr. Courtney Townsend. Cells were cultured using DMEM F:12 (Hyclone, Logan, UT) supplemented with 10% (v/v) fetal bovine serum (FBS), kanamycin (Gibco, Carlsbad, CA), and gentamicin (Sigma, St. Louis, MO) antibiotics. Cells were grown to confluency in a humidified atmosphere of 5% CO2 at 37°C.
Molecular Identification of PAC1
RT-PCR was used to demonstrate molecular expression of PAC1 R. These studies were performed on total RNA obtained from cells that were grown to confluency, using BD Biosciences’ Nucleospin RNA II Kit (Palo Alto, CA) according to the manufacturer’s instructions. PCR was performed in low salt Taq + DNA polymerase buffer and five units Taq + DNA polymerase (Stratagene, La Jolla, CA) in the presence of oligonucleotide primers under the following conditions: initial step (one cycle): 94°C for 2 min, 57°C for 1 min, and 72°C for 2 min; followed by 94°C for 1 min, 57°C for 1 min, and 72°C for 2 min (30 cycles); and a final extension step (one cycle) at 94°C for 1 min, 57°C for 1 min, and 72°C for 15 min. The sense primers used were: (SENSE 1) 5ʹCGAGTGGACAGTGGCAGGCGGTGA3ʹ (52–77); and (SENSE 2) 5ʹGCTCTCCCTGACTGCTCTCCTGCTG 3ʹ (145–170). The antisense primers used were: (ANTISENSE 1) 5ʹCAGTAGTGAGGGTGGCGAGGGAAGT3ʹ (611–636) and (ANTISENSE 2) 5ʹCAGTAGGTGTCCCCCAGCCGATGAT3ʹ (935–960).
Immunocytochemical Analysis of PAC1 R in BON Cells
In order to demonstrate the expression of PAC1 R on BON cells, we used immunofluorescent staining with antibodies specific to PAC1 R (CURE Antibodies Core), as described previously.5 Confocal microscopy was used to characterize the expression of PAC1 R. To perform these studies, BON cells were plated on polylysine-treated coverslips overnight and fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton-X, and then incubated at 4°C with polyclonal rabbit anti-PAC1 R (1:1000). To exclude nonspecific binding of the antibodies, BON cells were incubated with rabbit IgG (1µg/mL) as a negative control overnight at 4°C. The following day, the cells were washed with PBS, and then incubated with Alexa-488 (Molecular Probes, Eugene, Oregon)-conjugated secondary antibodies—goat anti-rabbit IgG antibodies. Cells were visualized using a Zeiss LSM 510 Laser Scanning Microscope (Carl Zeiss, Thornwood, NY).
RESULTS
Molecular Determination of PAC1 Expression
Specific primer pairs to detect expression of PAC1 R and VPAC1 R were chosen for these studies as described previously.6–8 RNA extracted from confluent BON cells only showed expression of PAC1 R and VPAC1-R. VPAC2 R was not detected in the test samples. These results demonstrate that high levels of PAC1 R and VPAC1 R mRNAs but not VPAC2 R mRNA are present in BON cells.
Immunocytochemistry
Immunocytochemistry and confocal microscopy were used to visualize PAC1 R in BON cells (Fig. 1). The cells were incubated with polyclonal rabbit anti-PAC1 antibodies or rabbit IgG as a negative control, followed by goat anti-rabbit FITC-conjugated antibodies. Expression of PAC1 R was detected at the surface of the BON cells. As a negative control, we used rabbit IgG followed by the secondary antibodies, to ascertain the specificity of the PAC1 R staining.
FIGURE 1.
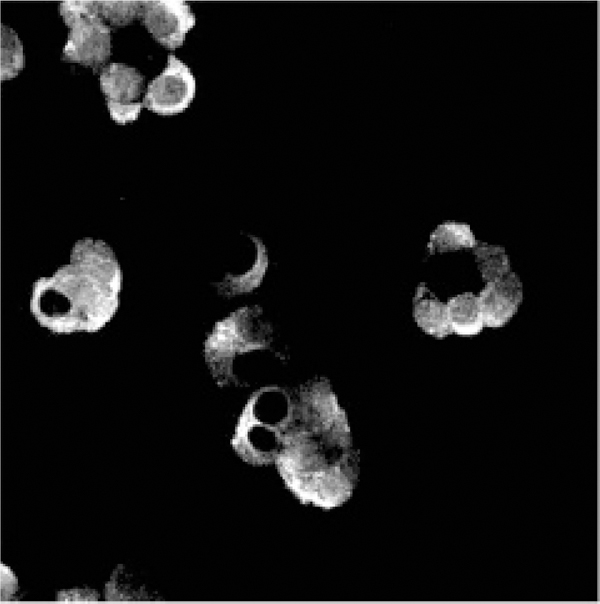
Immunocytochemistry demonstrating PAC1 R expression in BON cells. The cells were incubated with anti-PAC1 R rabbit polyclonal antibodies followed by Alexa-488-conjugated goat anti-rabbit IgG as a second antibody.
DISCUSSION
PACAP is a member of the vasoactive intestinal polypeptide (VIP) family of peptides.6 The cloning of the high-affinity receptor for PACAP (PAC1 R), specific for PACAP-27 and PACAP-38, has identified this receptor as a member of the seven transmembrane G protein–coupled receptors family.6,9,10 PACAP binds with a high affinity to PAC1 R and with a lower affinity to VPAC1 R and VPAC2 R.11 Knowledge of the receptor structure has permitted the development of specific antibodies to detect receptors in native cell systems.12 PAC1 has been shown to be expressed on a number of tumor cell lines derived from lung, breast, and colonic solid tumors.3–5 In addition, PAC1 R has been shown to be expressed on the enterochromaffin-like cells of the gastric mucosa. In all these organs, PACAP stimulation leads to the proliferation of the cancer cells.4,13,14 Expression of PAC1 R on neuroendocrine tumoral cells, such as BON, has not been shown previously.
In this study, we report the presence of PAC1 receptors in BON cells by means of RT-PCR and immunohistochemical staining. Immunohistochemical analysis using confocal laser scanning microscopy, performed in BON cells, using specific rabbit polyclonal anti-PAC1 R antibodies, has provided evidence of PAC1 R expression on the cell membranes. To determine whether activated PACAP receptors expressed on the surface of BON cells lead to the activation of intracellular signaling pathways, involved in cellular proliferation and secretion it will be necessary to confirm these results with functional pharmacological and biochemical assays. The precise signaling pathway involved in activating mitogen-activated protein kinases (MAPKs) would presumably originate from the activation of Gαq. It has been previously demonstrated in solid tumors that the Gαq pathway is involved in this process, because inhibition of phospholipasc (PLC) or protein kinase C (PKC) results in blocking PACAP-induced activation of MAPKs.13 These observations suggest that PAC1 R, expressed on BON cells, could result in the activation of PKC, and thereby activate MAPK leading to cell proliferation. This hypothesis needs to be tested in BON cells. The expression of PAC1 R on BON cells suggests that PACAP plays a role in stimulating the growth and survival of human NETs in vivo. These observations support the hypothesis that NETs cells can be stimulated by the neuropeptide PACAP.
In conclusion, the present data indicate that PAC1 R is expressed on neuroendocrine cells and suggests that PACAP may play a key role in the regulation of their growth. These data therefore may have important clinical implications by providing novel therapeutic targets to regulate the growth and development of NETs.
ACKNOWLEDGMENTS
This work was supported by the Department of Veterans Affairs Merit Review Grant (JRP) and National Institutes of Health DK37240 (HJC).
REFERENCES
- 1.PISEGNA JR & SAWICKI MP. 2001. Neuroendocrine pancreas. In Cancer Treatment HASKELL CM & BEREK JS Eds: 1065–1081. Saunders; Philadelphia, PA. [Google Scholar]
- 2.PAREKH D, ISHIZUKA J, TOWNSEND CM JR.., et al. 1994. Characterization of a human pancreatic carcinoid in vitro: morphology, amine and peptide storage, and secretion. Pancreas 9: 83–90. [DOI] [PubMed] [Google Scholar]
- 3.ZIA F, FAGARASAN M, BITAR K, et al. 1996. PACAP receptors regulate the growth of non-small cell lung cancer cells. Cancer Res 55: 4886–4891. [PMC free article] [PubMed] [Google Scholar]
- 4.LEYTON J, GOZES Y, PISEGNA JR, et al. 1999. PACAP (6–38) is a PACAP receptor antagonist for breast cancer cells. Breast Cancer Res. Treat 56: 177–186. [DOI] [PubMed] [Google Scholar]
- 5.LE SV, YAMAGUCHI DJ, MC ARDLE CA, et al. 2002. PAC1 and PACAP expression, signaling, and effect in the growth of HCT8, human colonic tumor cells. Regul. Pept 109: 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.PISEGNA JR & WANK SA. 1996. Cloning and characterization of the signal transduction of four splice variants of the human pituitary adenylate cylcase activating polypeptide receptor. Evidence for dual coupling to adenylate cyclase and phospholipase C. J. Biol. Chem 271: 17267–17274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ISHIHARA T, SHIGEMOTO R, MORI K, et al. 1992. Functional expression and tissue distribution of a novel receptor for vasoactive intestinal polypeptide. Neuron 8: 811–819. [DOI] [PubMed] [Google Scholar]
- 8.LUTZ EM, SHEWARD WJ, WEST KM, et al. 1993. The VIP2 receptor: molecular characterization of a cDNA encoding a novel receptor for vasoactive intestinal peptide. FEBS 334: 3–8. [DOI] [PubMed] [Google Scholar]
- 9.PISEGNA JR & WANK SA. 1993. Molecular cloning and functional expression of the pituitary adenylate cylcase-activating polypeptide type I receptor. Proc. Natl. Acad. Sci. USA 90: 6345–6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.SPENGLER D, WAEBER C, PANTALONI C, et al. 1993. Differential signal transduction by five splice variants of the PACAP receptor. Nature 365: 170–175. [DOI] [PubMed] [Google Scholar]
- 11.HARMER AJ, ARIMURA A, GOZES I, et al. 1998. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol. Rev 50: 265–270. [PMC free article] [PubMed] [Google Scholar]
- 12.MIAMPAMBA M, GERMANO PM, ARLI S, et al. 2002. Expression of pituitary adenylate cyclase-activating polypeptide and PACAP type I receptor in the rat gastric and coloine myenteric neurons. Regul. Pept 105: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.PISEGNA JR, LEYTON J, COELHO T, et al. 1997. Differential activation of immediate-early gene expression by four splice variants of the human pituitary activating polypeptide receptor: evident for activation by PACAP hybrid and the phospholipase C inhibitor U73122. Life Sci 61: 631–639.9250719 [Google Scholar]
- 14.ZENG N, ATHMANN C, KANG T, et al. 1999. PACAP type I receptor activation regulates ECL cells and gastric acid secretion. J. Clin. Invest 104: 1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]


