Abstract
Uterine tumor resembling ovarian sex cord tumor (UTROSCT) is a rare and distinctive neoplasm of unclear histogenesis, and uncertain malignant potential. These neoplasms morphologically resemble sex-cord stromal tumors of the ovary, and possess a polyphenotypic immunophenotype. Their molecular pathogenesis has yet to be elucidated; notably, however, tumors lack alterations found in other uterine tumors bearing sex-cord-like differentiation, such as endometrial stromal sarcoma. Following identification of an index patient with an ESR1-NCOA3 fusion gene by RNA-Sequencing, we undertook a retrospective review for additional cases of UTROSCT. We identified a total of 4 patients, with an average age of 53 years (range, 38–68). RNA-Sequencing was performed in all cases, revealing an ESR1-NCOA3 fusion in 2 cases and one case each with related ESR1-NCOA2 and GREB1-NCOA2 fusions. Each of the tumors showed histologic and an immunophenotype features within the previously reported spectrum of UTROSCT; interestingly, one case contained prominent spindle cell fascicles and another was largely comprised of sheets of small round cells. Our results demonstrate UTROSCT are defined by recurrent fusions involving NCOA2 or NCOA3, a finding that is directly amenable to diagnostic evaluation. This study confirms UTROSCT is molecularly distinct from endometrial stromal sarcoma, but raises intriguing new questions into the pathogenesis of these neoplasms and possible relationship with other NCOA-fusion positive uterine tumors.
Keywords: Uterine tumor resembling ovarian sex cord tumor, uterus, sex cord, ESR1, GREB1, NCOA2, NCOA3
INTRODUCTION
Sex-cord-like differentiation has been reported to occur as a secondary phenomenon in uterine neoplasms such as endometrial stromal sarcoma,(1, 2) and Müllerian adenosarcoma.(3, 4) In contrast, sex-cord-like differentiation is considered an intrinsic attribute of so-called ‘uterine tumor resembling ovarian sex-cord tumor’ (UTROSCT),(5–7) a rare mesenchymal neoplasm of unclear histogenesis.(8) The World Health Organization currently classifies uterine tumor resembling ovarian sex-cord tumor under the rubric of endometrial stromal tumors.(9) These are uncommon neoplasms unique to the uterus, and rarely cervix.(10) Tumors predominate in middle-aged women.(7, 10, 11) Most patients present with symptoms of abnormal bleeding, pain and/or an enlarged uterus.(5–7) Hysterectomy is generally curative,(7, 12) and most cases were thought to follow a benign course.(9) A recent series, however, reported that 23.5% of patients developed metastases and 8.8% of patients died of their tumor; as a result, it has been suggested that these tumors are more appropriately considered of uncertain malignant potential.(8)
The molecular pathogenesis of UTROSCT has yet to be elucidated. In contrast to endometrial stromal sarcoma with sex-cord-like differentiation, UTROSCT lacks evidence of either JAZF1-SUZ12 fusion genes,(13, 14) or PHF1 rearrangement.(15) Furthermore, these tumors lack FOXL2 and DICER1 mutations typical of ovarian adult granulosa cell tumor and Sertoli-Leydig cell tumor, respectively.(16, 17) Following the incidental discovery of an ESR1-NCOA3 fusion gene by RNA-Sequencing (RNA-Seq) in a patient with UTROSCT, we investigated 3 additional cases to better understand the molecular landscape in UTROSCT.
MATERIALS & METHODS
Cases
A primary uterine tumor with a morphology and immunophenotype compatible with UTROSCT was reviewed by one of the authors (BCD), and tested by diagnostic RNA-Seq to exclude the presence of a gene fusion associated with endometrial stromal sarcoma. The result revealed an ESR1-NCOA3 fusion gene candidate. As a result, a retrospective archival review was performed for additional cases diagnosed as UTROSCT (Mount Sinai Hospital, 2010–2018). Each case was examined by RNA-Seq and the findings independently confirmed by fluorescence in situ hybridization (FISH). This study was performed following institutional Research Ethics Board approval.
Immunohistochemistry
Formalin-fixed paraffin-embedded tissue blocks were cut at 4 microns and stained for calretinin, inhibin, desmin, smooth muscle actin, H-caldesmon, S100, keratin (AE1/AE3), WT-1, HMB45, MART-1, estrogen receptor and androgen receptor, using standard techniques (Supplementary Table 1, Supplemental Digital Content 1, http://links.lww.com/PAS/A683 ). Positive on-slide controls were applied throughout.
RNA Sequencing
RNA was extracted from formalin-fixed paraffin-embedded tissue scrolls (10 micron sections, 3–4 per case) or tissue cut onto positively charged glass slides (4 micron section, 7–10 per case) using the ExpressArt FFPE Clear RNA Ready kit (Amsbio, Cambridge, MA). Total RNA was quantified using the Qubit RNA HS Assay Kit (ThermoFisher Scientific, Mississauga, ON). RNA-seq libraries were prepared following the manufacturer’s instructions using an input of 20–100 ng RNA and the TruSight RNA Fusion Panel (Illumina, San Diego, CA). The results were analysed using both STAR and BOWTIE2 aligners, and Manta and JAFFA fusion callers, respectively.(18, 19)
In order to evaluate for similarities in transcriptional signatures, the expression profile of the study group was compared to 13 cases of molecularly confirmed endometrial stromal sarcoma (JAZF1-SUZ12, N=7; ZC3H7B-BCOR, N=7; BRD8-PHF1, N=2; JAZF1-PHF1, N=2). In addition, the expression profile was compared to over 100 other sarcoma cases that have been previously tested on the same RNA-Seq platform; this did not include other gynecologic neoplasms (e.g., ovarian sex-cord stromal tumors, leiomyosarcoma, Müllerian adenosarcoma).
Fluorescence in situ hybridization
Fluorescence in situ hybridization for ESR1, NCOA2 and NCOA3 was performed as previously reported in detail.(20) The UCSC genome browser was used to design custom bacterial artificial chromosome (BAC) clone probes flanking the target genes (http://genome.ucsc.edu). These were ordered from the BACPAC Resources Center at the Children’s Hospital (Oakland, CA; https://bacpacresources.org) (Supplementary Table 2, Supplemental Digital Content 2, http://links.lww.com/PAS/A684 ).(21) Each DNA BAC probe was labelled with fluorochromes by nick translation. Staining was performed using standard techniques, briefly: 4 micron formalin-fixed paraffin-embedded tissue sections were deparaffinized, pretreated, and then hybridized with denatured probes. The slides were incubated overnight, then rinsed, stained with 4’,6-diamidino-2-phenylindole (DAPI), mounted, and examined under a Zeiss fluorescence microscope (Zeiss Axioplan, Oberkochen, Germany).
RESULTS
In the course of routine diagnostic RNA-Seq testing, which was performed to exclude the possibility of endometrial stromal sarcoma with sex-cord-like differentiation, an ESR1-NCOA3 fusion gene was identified in a patient with UTROSCT. A subsequent retrospective archival review identified three additional patients with UTROSCT (N=4). The average patient age was 53 years (range, 38–68 years). All tumors were uterine in location. The average size was 2.4 cm (range, 0.7–3.3 cm). Two tumors were surrounded by myometrium; one was centred in the myometrium and focally abutted endometrium; and, one was a radiologically polypoid and diagnosed on curettage, thus myometrial involvement could not be assessed. One of the tumors was circumscribed; two showed myometrial infiltration, one with tongue-like protrusions reminiscent of low-grade endometrial stromal sarcoma on low magnification.
There was considerable morphologic heterogeneity amongst, and within, the tumors. They contained retiform, trabecular/cord, tubular and/or nested patterns. The tumor from Patient 1 was predominantly composed of small round cells with a reticular pattern, along with a secondary population of larger polygonal cells (Figure 1). The predominant pattern in Patient 2’s tumor included broad anastomosing trabeculae/cords comprised of polygonal cells, along with more retiform areas (Figure 2). The tumor of Patient 3 was almost exclusively composed of sheets and nests of small round-ovoid cells, with focal tubular and cord-like patterns (Figure 3). Patient 4 had a biphasic tumor with spindle cell fascicles, and a minor component of interspersed tubules (Figure 4). Overall, the cells ranged from round-polygonal-spindle shaped. The cytoplasm ranged from scant to ample and brightly eosinophilic. The nuclei were round-ovoid; occasionally they contained prominent clefts and/or angulation, vesicular chromatin and prominent small nucleoli. Mitotic activity was not conspicuous (0–1 per 10 HPFs [FD=0.55 mm]). Small nests of cells with abundant foamy cytoplasm, a recognized secondary finding,(6, 22) were noted in two cases. A single case contained lymphovascular invasion. None of the tumors showed necrosis. Patient 1 was noted to have adenomyosis (not shown).
Figure 1.
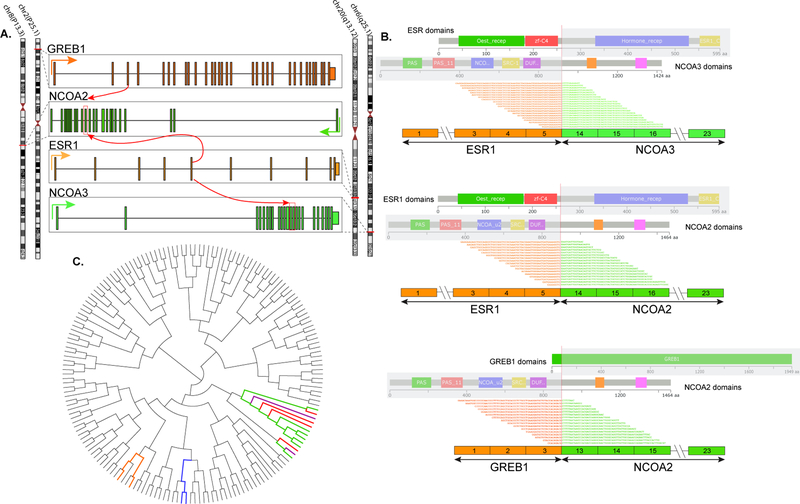
ESR1-NCOA3 gene fusion in a myometrial UTROSCT (Patient 1): (A) Tumor centred in myometrium with a retiform pattern of epithelioid cells, and area of interstitial hyalinization. (B) Tubules lined by epithelioid cells with scant cytoplasm. (C) Nests of plump epithelioid cells with abundant eosinophilic cytoplasm. (D) Aggregates of cells with foamy cytoplasm. Fluorescence in situ hybridization showing break-apart signals for (E) ESR1, and (F) NCOA3 (white arrows, red, centromeric; green telomeric).
Figure 2.
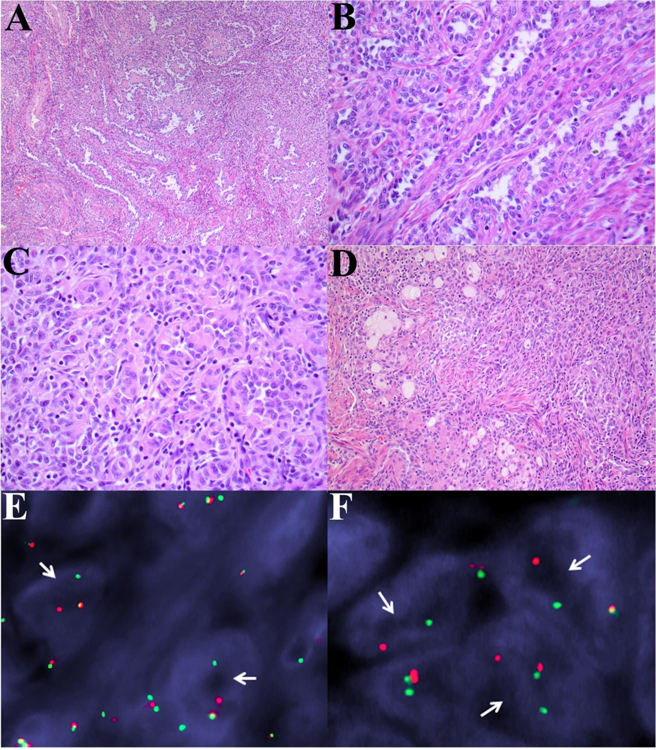
ESR1-NCOA3 gene fusion in a polypoid UTROSCT (Patient 2): (A-B) Broad anastomosing trabeculae comprised of epithelioid cells. Immunohistochemistry showing staining for (C) keratin (AE1/AE3), (D) desmin, (E) WT-1, and (F) Mart-1.
Figure 3.
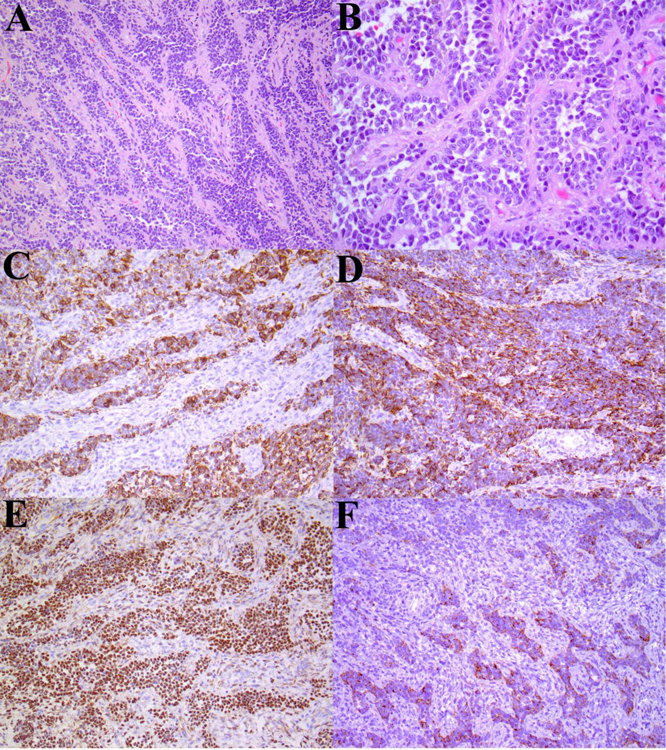
ESR1-NCOA2 gene fusion in a myometrial UTROSCT (Patient 3): (A) At low-magnification areas of the tumor contain tongue-like myometrial invasion reminiscent of endometrial stromal sarcoma. (B) Nests of plump epithelioid cells with abundant eosinophilic cytoplasm, and vesicular nuclei. (C) Sheets of small round-polygonal cells. (D) Occasional epithelioid cells with a tubular pattern. Immunohistochemistry showing staining for (E) calretinin, and (F) estrogen receptor.
Figure 4.
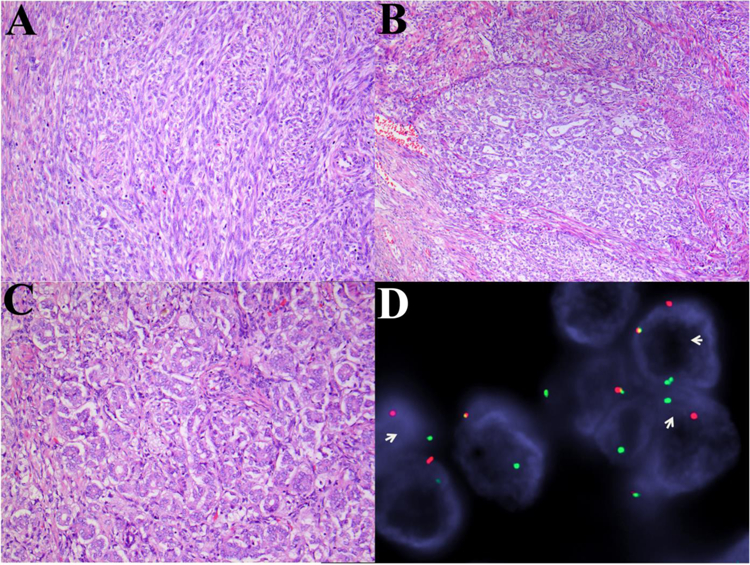
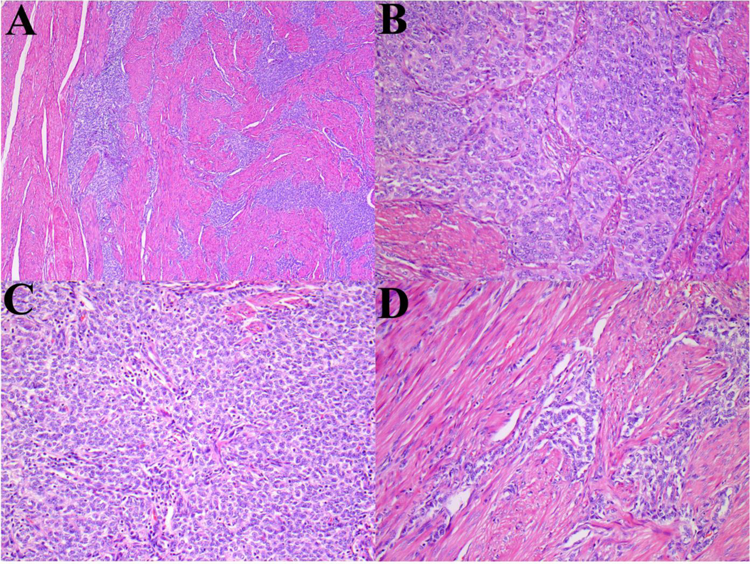
GREB1-NCOA2 gene fusion in a myometrial UTROSCT (Patient 4): (A) Areas of plump spindle cells with a prominent fascicular-herringbone pattern reminiscent of monophasic synovial sarcoma. (B) Spindle cell fascicles admixed with epithelioid cells with a tubular pattern. (C) Epithelioid cells with tubular pattern. (D) Fluorescence in situ hybridization showing break-apart signals for NCOA2 (white arrows, red, centromeric; green telomeric).
Each of the tumors tested showed diffuse immunoreactivity for calretinin, WT-1 (nuclear), and estrogen receptor. There was frequent expression of androgen receptor, keratin, muscle markers and Mart-1, though these tended to less extensive (Table 1). None of the tumors were found to express HMB45. Immunohistochemistry for S100, CD10 and cyclin D1 was performed on a single case and found to be negative (not shown).
TABLE 1:
Summary of immunohistochemical findings among sample cohort.
| Patient | Immunohistochemistry | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CRTN | IHBN | Desmin | SMA | H-C | AE1/3 | WT1 | HMB45 | Mart-1 | AR | ER | |
| 1 | 5+ | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 5+ |
| 2 | 5+ | 1+ | 3+ | 2+ | 0 | 4+ | 5+ | 0 | 2+ | 2+ | 5+ |
| 3 | 5+ | 1+ | 1+ | N/A | 0 | 1+ | 5+ | 0 | 0 | 4+ | 5+ |
| 4 | 5+ | 1+ | 1+ | N/A | 1+ | 5+ | 5+ | 0 | 1+ | 1+ | 5+ |
AE1/A3 (pancytokeratin, AE1/AE3); AR (androgen receptor); CRTN (calretinin); ER (estrogen receptor); H-C (H-caldesmon); IHBN; N/A (not assessed); and, SMA (smooth muscle actin). Scoring of tumors was based on the percentage of positive cells (0: no staining; 1+: <5%; 2+: 5% to 25%; 3+: 26% to 50%; 4+: 51% to 75%; and 5+: 76% to 100%). NB: WT1 (nuclear staining).
The tumors with NCOA3 rearrangement involved either exon 14 or 15 (NCBI Reference Sequence: NM_181659.2), which was fused to ESR1 exon 3 (NM_000125.3). Two cases with NCOA2 rearrangement involved exon 14 (NM_001321703.1), which was fused with either ESR1 exon 3 (NM_000125.3), or GREB1 exon 2 (NM_014668.3). Rearrangement of ESR1, NCOA2 and NCOA3 was independently confirmed by fluorescence in situ hybridization. All 4 cases showed significant up-regulation of ESR1 mRNA levels compared to >100 other sarcomas available on the same RNA-Seq platform (Supplementary Figure 1, Supplemental Digital Content 3, http://links.lww.com/PAS/A685 ). There was no significantly increased expression of either NCOA2 or NCOA3 RNA compared to other tumor types (not shown). GREB1 was not represented on the fusion panel, so mRNA expression could not be assessed. A gene signature was also obtained of these 4 cases compared to the other tumors on the array, showing significant up-regulation of WT1, AR, HOXA10, HOXA11 and PBX1 (Supplementary Figure 1, Supplemental Digital Content 3, http://links.lww.com/PAS/A685).
By unsupervised hierarchical clustering of the RNA-Seq data, the four study cases clustered together, separate from all other sarcoma types. In order to investigate the potential transcriptional signature overlap with endometrial stromal sarcomas, we then included an additional group of endometrial stromal sarcomas with various gene fusions tested on the same platform. The repeat unsupervised hierarchical clustering showed that the study cohort grouped closely to certain molecular subsets of endometrial stromal sarcoma; namely, low grade endometrial stromal sarcomas with JAZF1-SUZ12 and JAZF1-PHF1 fusions, but not the others (Figure 5).
Figure 5. Fusion structures and molecular correlates.
(A) Diagrammatic representation of the 3 different fusions, including ESR1-NCOA3, resulting from a t(6;20)(q25.1;q13.12); ESR1-NCOA2, resulting from a t(8;20)(p13.3;q13.12); and GREB1-NCOA2 from a t(2;8)(p25.1;p13.3). The gene loci are indicated with red lines on the vertical chromosomes on both sides. The exonic breakpoint location is indicated by red arrows and red boxes. Green, yellow, orange arrows indicate the direction of transcription of individual gene. (B) Detailed RNA sequencing fusion junction reads, exon composition of the fusion transcripts and protein domain structures of each protein. (C) Unsupervised clustering using RNA-Seq data showing the 4 study cases (red lines) cluster closely in a tight group separate from most other sarcoma types (grey lines) available on the same platform. Interestingly, the tumors appear to cluster with low-grade endometrial stromal sarcomas containing JAZF1-SUZ12 (green) and JAZF1-PHF1 (purple) fusions genes, but not BRD8-PHF1 (orange) or high-grade endometrial stromal sarcomas with ZC3H7B-BCOR (blue) fusion products.
DISCUSSION
Uterine tumor resembling ovarian sex-cord tumor is a rare neoplasm of unclear histogenesis, which is classified under the rubric of ‘endometrial stromal and related tumours’ in the World Health Organization classification scheme.(9) Prior attempts to molecularly characterize these neoplasms have failed to show a relationship to either endometrial stromal tumors,(13–15) or ovarian sex cord tumors.(16, 17) Following identification of an ESR1-NCOA3 fusion gene in an index patient, we investigated additional cases of UTROSCT in attempt to better characterize the morphologic, molecular and ontological nature of these tumors.
Clement and Scully are credited with the first detailed description of UTROSCT. (5, 23) In their series uterine tumors with sex-cord differentiation were divided into two groups: (Group I) tumors which are identical to endometrial stromal tumors but with focal epithelial-like differentiation resembling ovarian sex-cord tumors, and (Group II) tumors with a predominant or exclusive pattern resembling an ovarian sex-cord tumor.(5) The relationship of Group I tumors to endometrial stromal sarcoma has recently been confirmed molecularly, where six cases were found to harbor PHF1 rearrangement.(24) However, the nature of Group II tumors (UTROSCT) remains controversial. They have been proposed to originate from epithelial, stromal, or myoid elements; represent a distinct entity more closely related to ovarian sex-cord tumors;(6, 7, 25, 26) or, perhaps originate from an as yet unknown uncommitted cell with the capacity for multidirectional differentiation.(11)
Uterine tumor resembling ovarian sex-cord tumor is comprised of epithelioid cells that may assume a variety of architectural patterns (e.g., cords, nests, trabeculae, tubules, and sheets, as well as glandular and retiform patterns).(6, 10, 11, 13, 26, 27) A similar array of morphologies was encountered in our series; interestingly, one case was enriched with spindle cells in a fascicular pattern and a second case predominantly contained sheets of small round cells. These tumors have a polyphenotypic immunoprofile.(7, 11) They are generally positive for keratins (AE1/AE3, Cam5.2), calretinin, vimentin, WT-1 (nuclear/cytoplasmic), and hormone receptors (androgen, estrogen, and progesterone receptors); with more variable immunoreactivity for epithelial membrane antigen, inhibin, FOXL2, steroidogenic factor-1, desmin, smooth muscle actin, calponin, H-caldesmon, CD10, CD56, and Melan-A.(6, 10, 11, 17, 26, 28) To date, the molecular pathogenesis of UTROSCT remains to be elucidated.
Following the incidental discovery of an ESR1-NCOA3 fusion gene in a patient with UTROSCT in the course of routine diagnostic RNA-Seq testing, we examined three additional cases by RNA-Seq (N=4) to further assess their molecular pathogenesis. Two patients were found to have ESR1-NCOA3 fusions genes, one had an ESR1-NCOA2 fusion gene, and one had a GREB-NCOA2 fusion product. Notably, NCOA2/3 fusions have previously been identified in uterine neoplasms. A GREB1-NCOA2 fusion gene was recently reported in a uterine tumor designated ‘sarcoma, not otherwise classifiable.’(29) This tumor was centred in the uterine corpus and composed of spindle-polygonal cells, with immunoreactivity for keratin and hormone receptors. Interestingly, this case appears to show morphologic and immunohistochemical overlap with Patient 4 in our cohort, though our case additionally contained a tubular component facilitating classification as UTROSCT. In addition, in a series of 20 uterine adenosarcomas Piscuoglio et al. identified two cases with ESR1 rearrangement—one partnered with NCOA2 and the other NCOA3.(30) Interestingly, one of these cases was reported to contain sex-cord-like elements.(30) Additional studies, with larger cohorts, are necessary to investigate the relationship between UTROSCT and other uterine neoplasms with NCOA gene fusions. Such studies would also be anticipated to further illuminate the diversity of potential fusion genes possible amongst these neoplasms (i.e., range of NCOA genes, and potential partners).
ESR1 mRNA up-regulation was observed in all 4 of the cases in this series, including the GREB1-NCOA2 positive tumor. ESR1 encodes estrogen receptor 1, a ligand-dependent transcription factor.(31) The NCOA1–3 genes encode for nuclear receptor co-activators 1–3, and belong to the steroid receptor coactivator p160/SRC family (murine SRC1–3), exerting pleiotropic roles on a wide spectrum of physiologic systems, including a critical role for SRC2 in progesterone-dependent uterine function in the mouse.(32) Fusion genes involving NCOA2 have been reported in several soft tissue tumors, including: mesenchymal chondrosarcoma (HEY1-NCOA2 fusion),(33) soft tissue angiofibroma (NCOA2 fused to various partners e.g., AHRR, GTF2I),(34, 35) congenital spindle cell rhabdomyosarcoma (NCOA2 fused with SRF, TEAD1, VGLL2),(21, 36) and rare examples of alveolar rhabdomyosarcoma (PAX3-NCOA2)(37). The three members of the NCOA protein family have 50–55% sequence homology,(37) thus it is perhaps not surprising these genes may substitute for one another in various fusion gene pairs. There is a precedent for this in alveolar rhabdomyosarcoma, where both NCOA1 and NCOA2 have the potential to pair with PAX3;(37, 38) and, in our cohort ESR1 was found to partner with both NCOA3 and NCOA2. GREB1, growth regulation by estrogen in breast cancer, is an ESR1-upregulated protein that mediates estrogen activity. In humans GREB1 is expressed in all ESR1-expressing tissues within the reproductive tract.(39) Furthermore, GREB1 is overexpressed in a variety of epithelial cancers (e.g., breast, ovary, prostate),(40) and in ovarian cancer cell lines is associated with increased proliferation and induction of a mesenchymal morphology.(39)
In addition to ESR1, the gene signature of UTROSCT included upregulation of Müllerian-related genes, such as AR and WT1, as well as overexpression of transcription factors HOXA10, HOXA11 and PBX1 (Supplemental Figure 1, Supplemental Digital Content 3, http://links.lww.com/PAS/A685). HOXA10 and HOXA11 are expressed in the uterus of both mice and humans.(41) Pbx is an essential Hox cofactor and is required for Hox activity, they cooperatively promote cell proliferation and have been implicated as proto-oncogenes in human leukemia,(42) and associated with epigenetic regulation in Ewing sarcoma.(43) Although the 4 cases in our cohort clustered separately from other sarcoma types, they appeared to group closely to a subset of low grade endometrial stromal sarcomas, specifically those with JAZF1-SUZ12 and JAZF1-PHF1 fusions. Our sample size is admittedly limited; therefore, while intriguing, there is currently insufficient data to extrapolate a direct relationship between UTROSCT and low-grade endometrial stromal sarcoma.
The projected ESR1-NCOA2/3 fusion oncoproteins retain the estrogen receptor domain (ESD) and the Zinc finger domain (ZF) encoded from the first 5 exons of ESR1; and the nuclear receptor coactivator (NRC) from the last 10 exons of NCOA2/3. Similar breakpoints were reported in 2 cases of adenosarcoma, with the fusions retaining the first 3 exons of ESR1 and last 10 exons of NCOA2/3.(29) Furthermore, similar NCOA2 break points were also detected in other mesenchymal neoplasms with NCOA2 gene rearrangements, including mesenchymal chondrosarcoma, angiofibroma, etc. As uterine tissues typically show high ESR1 and GREB1 expression, a likely mechanism for the current translocations is hijacking of the active promoter region to dysregulate expression of the retained nuclear receptor coactivator domain of NCOA2/3.
In summary, we report the molecular characterization of four cases of uterine tumor resembling ovarian sex-cord tumor. The cases were found to contain fusion genes involving ESR1-NCOA3 (N=2), ESR1-NCOA2 (N=1) and GREB1-NCOA2 (N=1). Our findings support the hypothesis that UTROSCT represents a distinct entity, and these results can be directly applied as a means of diagnostic confirmation. Further studies are necessary to characterize the breadth of molecular events possible in UTROSCT, and establish the relationship, if any, to other uterine neoplasms with NCOA rearrangement.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Ms. Evangeline Agro and Ms. Sharon Crafter for facilitating molecular testing, and are grateful to Ms. Grace Murray (Illumina) for providing RNA fusion test kits.
Supported in part by: P50 CA140146-01 (CRA); P30-CA008748 (CRA); Kristen Ann Carr Foundation (CRA); Cycle for Survival (CRA)
Footnotes
Conflicts of Interest: None
References
- 1.McCluggage WG, Date A, Bharucha H, et al. Endometrial stromal sarcoma with sex cord-like areas and focal rhabdoid differentiation. Histopathology 1996;29:369–374. [DOI] [PubMed] [Google Scholar]
- 2.Oliva E, Clement PB, Young RH. Endometrial stromal tumors: an update on a group of tumors with a protean phenotype. Adv Anat Pathol 2000;7:257–281. [DOI] [PubMed] [Google Scholar]
- 3.Clement PB, Scully RE. Mullerian adenosarcomas of the uterus with sex cord-like elements. A clinicopathologic analysis of eight cases. Am J Clin Pathol 1989;91:664–672. [DOI] [PubMed] [Google Scholar]
- 4.Hirschfield L, Kahn LB, Chen S, et al. Mullerian adenosarcoma with ovarian sex cord-like differentiation. A light- and electron-microscopic study. Cancer 1986;57:1197–1200. [DOI] [PubMed] [Google Scholar]
- 5.Clement PB, Scully RE. Uterine tumors resembling ovarian sex-cord tumors. A clinicopathologic analysis of fourteen cases. Am J Clin Pathol 1976;66:512–525. [DOI] [PubMed] [Google Scholar]
- 6.Krishnamurthy S, Jungbluth AA, Busam KJ, et al. Uterine tumors resembling ovarian sex-cord tumors have an immunophenotype consistent with true sex-cord differentiation. Am J Surg Pathol 1998;22:1078–1082. [DOI] [PubMed] [Google Scholar]
- 7.Irving JA, Carinelli S, Prat J. Uterine tumors resembling ovarian sex cord tumors are polyphenotypic neoplasms with true sex cord differentiation. Mod Pathol 2006;19:17–24. [DOI] [PubMed] [Google Scholar]
- 8.Moore M, McCluggage WG. Uterine tumour resembling ovarian sex cord tumour: first report of a large series with follow-up. Histopathology 2017;71:751–759. [DOI] [PubMed] [Google Scholar]
- 9.Oliva E, Carcangiu ML, Carinelli SG, et al. Mesenchymal tumours. In: Kurman RJ, Carcangiu ML, Herrington CS, et al. , eds. WHO Classification of Tumours of Female Reproductive Organs Lyon: International Agency for Research on Cancer; 2014:142–144. [Google Scholar]
- 10.Kabbani W, Deavers MT, Malpica A, et al. Uterine tumor resembling ovarian sex-cord tumor: report of a case mimicking cervical adenocarcinoma. Int J Gynecol Pathol 2003;22:297–302. [DOI] [PubMed] [Google Scholar]
- 11.Hurrell DP, McCluggage WG. Uterine tumour resembling ovarian sex cord tumour is an immunohistochemically polyphenotypic neoplasm which exhibits coexpression of epithelial, myoid and sex cord markers. J Clin Pathol 2007;60:1148–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pradhan D, Mohanty SK. Uterine tumors resembling ovarian sex cord tumors. Arch Pathol Lab Med 2013;137:1832–1836. [DOI] [PubMed] [Google Scholar]
- 13.Staats PN, Garcia JJ, Dias-Santagata DC, et al. Uterine tumors resembling ovarian sex cord tumors (UTROSCT) lack the JAZF1-JJAZ1 translocation frequently seen in endometrial stromal tumors. Am J Surg Pathol 2009;33:1206–1212. [DOI] [PubMed] [Google Scholar]
- 14.Umeda S, Tateno M, Miyagi E, et al. Uterine tumors resembling ovarian sex cord tumors (UTROSCT) with metastasis: clinicopathological study of two cases. Int J Clin Exp Pathol 2014;7:1051–1059. [PMC free article] [PubMed] [Google Scholar]
- 15.Nucci MR, Schoolmeester JK, Sukov W, et al. Uterine tumors resembling ovarian sex cord tumor (UTROSCT) lack rearrangement of PHF1 by FISH. Modern Pathology 2014;27:298A. [Google Scholar]
- 16.Chiang S, Staats PN, Senz J, et al. FOXL2 mutation is absent in uterine tumors resembling ovarian sex cord tumors. Am J Surg Pathol 2015;39:618–623. [DOI] [PubMed] [Google Scholar]
- 17.Croce S, de Kock L, Boshari T, et al. Uterine Tumor Resembling Ovarian Sex Cord Tumor (UTROSCT) Commonly Exhibits Positivity With Sex Cord Markers FOXL2 and SF-1 but Lacks FOXL2 and DICER1 Mutations. Int J Gynecol Pathol 2016;35:301–308. [DOI] [PubMed] [Google Scholar]
- 18.Liu S, Tsai WH, Ding Y, et al. Comprehensive evaluation of fusion transcript detection algorithms and a meta-caller to combine top performing methods in paired-end RNA-seq data. Nucleic Acids Res 2016;44:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X, Schulz-Trieglaff O, Shaw R, et al. Manta: rapid detection of structural variants and indels for germline and cancer sequencing applications. Bioinformatics 2016;32:1220–1222. [DOI] [PubMed] [Google Scholar]
- 20.Kao YC, Sung YS, Zhang L, et al. EWSR1 Fusions With CREB Family Transcription Factors Define a Novel Myxoid Mesenchymal Tumor With Predilection for Intracranial Location. Am J Surg Pathol 2017;41:482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosquera JM, Sboner A, Zhang L, et al. Recurrent NCOA2 gene rearrangements in congenital/infantile spindle cell rhabdomyosarcoma. Genes Chromosomes Cancer 2013;52:538–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fekete PS, Vellios F, Patterson BD. Uterine tumor resembling an ovarian sex-cord tumor: report of a case of an endometrial stromal tumor with foam cells and ultrastructural evidence of epithelial differentiation. Int J Gynecol Pathol 1985;4:378–387. [PubMed] [Google Scholar]
- 23.Morehead RP, Bowman MC. Heterologous Mesodermal Tumors of the Uterus: Report of a Neoplasm Resembling a Granulosa Cell Tumor. Am J Pathol 1945;21:53–61. [PMC free article] [PubMed] [Google Scholar]
- 24.D’Angelo E, Ali RH, Espinosa I, et al. Endometrial stromal sarcomas with sex cord differentiation are associated with PHF1 rearrangement. Am J Surg Pathol 2013;37:514–521. [DOI] [PubMed] [Google Scholar]
- 25.McCluggage WG. Uterine tumours resembling ovarian sex cord tumours: immunohistochemical evidence for true sex cord differentiation. Histopathology 1999;34:375–376. [DOI] [PubMed] [Google Scholar]
- 26.de Leval L, Lim GS, Waltregny D, et al. Diverse phenotypic profile of uterine tumors resembling ovarian sex cord tumors: an immunohistochemical study of 12 cases. Am J Surg Pathol 2010;34:1749–1761. [DOI] [PubMed] [Google Scholar]
- 27.Nogales FF, Stolnicu S, Harilal KR, et al. Retiform uterine tumours resembling ovarian sex cord tumours. A comparative immunohistochemical study with retiform structures of the female genital tract. Histopathology 2009;54:471–477. [DOI] [PubMed] [Google Scholar]
- 28.Oliva E, Young RH, Amin MB, et al. An immunohistochemical analysis of endometrial stromal and smooth muscle tumors of the uterus: a study of 54 cases emphasizing the importance of using a panel because of overlap in immunoreactivity for individual antibodies. Am J Surg Pathol 2002;26:403–412. [DOI] [PubMed] [Google Scholar]
- 29.Brunetti M, Panagopoulos I, Gorunova L, et al. RNA-sequencing identifies novel GREB1-NCOA2 fusion gene in a uterine sarcoma with the chromosomal translocation t(2;8)(p25;q13). Genes Chromosomes Cancer 2018;57:176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piscuoglio S, Burke KA, Ng CK, et al. Uterine adenosarcomas are mesenchymal neoplasms. J Pathol 2016;238:381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeselsohn R, Buchwalter G, De Angelis C, et al. ESR1 mutations-a mechanism for acquired endocrine resistance in breast cancer. Nat Rev Clin Oncol 2015;12:573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukherjee A, Soyal SM, Fernandez-Valdivia R, et al. Steroid receptor coactivator 2 is critical for progesterone-dependent uterine function and mammary morphogenesis in the mouse. Mol Cell Biol 2006;26:6571–6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L, Motoi T, Khanin R, et al. Identification of a novel, recurrent HEY1-NCOA2 fusion in mesenchymal chondrosarcoma based on a genome-wide screen of exon-level expression data. Genes Chromosomes Cancer 2012;51:127–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin Y, Moller E, Nord KH, et al. Fusion of the AHRR and NCOA2 genes through a recurrent translocation t(5;8)(p15;q13) in soft tissue angiofibroma results in upregulation of aryl hydrocarbon receptor target genes. Genes Chromosomes Cancer 2012;51:510–520. [DOI] [PubMed] [Google Scholar]
- 35.Arbajian E, Magnusson L, Mertens F, et al. A novel GTF2I/NCOA2 fusion gene emphasizes the role of NCOA2 in soft tissue angiofibroma development. Genes Chromosomes Cancer 2013;52:330–331. [DOI] [PubMed] [Google Scholar]
- 36.Alaggio R, Zhang L, Sung YS, et al. A Molecular Study of Pediatric Spindle and Sclerosing Rhabdomyosarcoma: Identification of Novel and Recurrent VGLL2-related Fusions in Infantile Cases. Am J Surg Pathol 2016;40:224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sumegi J, Streblow R, Frayer RW, et al. Recurrent t(2;2) and t(2;8) translocations in rhabdomyosarcoma without the canonical PAX-FOXO1 fuse PAX3 to members of the nuclear receptor transcriptional coactivator family. Genes Chromosomes Cancer 2010;49:224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wachtel M, Dettling M, Koscielniak E, et al. Gene expression signatures identify rhabdomyosarcoma subtypes and detect a novel t(2;2)(q35;p23) translocation fusing PAX3 to NCOA1. Cancer Res 2004;64:5539–5545. [DOI] [PubMed] [Google Scholar]
- 39.Hodgkinson K, Forrest LA, Vuong N, et al. GREB1 is an estrogen receptor-regulated tumour promoter that is frequently expressed in ovarian cancer. Oncogene 2018. [DOI] [PMC free article] [PubMed]
- 40.Hodgkinson KM, Vanderhyden BC. Consideration of GREB1 as a potential therapeutic target for hormone-responsive or endocrine-resistant cancers. Expert Opin Ther Targets 2014;18:1065–1076. [DOI] [PubMed] [Google Scholar]
- 41.Taylor HS, Vanden Heuvel GB, Igarashi P. A conserved Hox axis in the mouse and human female reproductive system: late establishment and persistent adult expression of the Hoxa cluster genes. Biol Reprod 1997;57:1338–1345. [DOI] [PubMed] [Google Scholar]
- 42.Moens CB, Selleri L. Hox cofactors in vertebrate development. Dev Biol 2006;291:193–206. [DOI] [PubMed] [Google Scholar]
- 43.Svoboda LK, Harris A, Bailey NJ, et al. Overexpression of HOX genes is prevalent in Ewing sarcoma and is associated with altered epigenetic regulation of developmental transcription programs. Epigenetics 2014;9:1613–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.