Abstract
Background:
Opioid agonist treatment (OAT) is an effective biomedical intervention to manage opioid use disorder among persons who inject drugs (PWID). Preliminary evidence suggests that OAT may also disrupt the social communicability of injection drug use (IDU) practices by established PWID. We therefore aim to investigate the association between OAT enrollment and initiating others into IDU among PWID in Vancouver, Canada.
Methods:
Preventing Injecting by Modifying Existing Responses (PRIMER; NIDA DP2-DA040256–01) is a prospective multi-cohort study seeking to identify structural interventions that reduce the risk that PWID initiate others into IDU. The present analysis was conducted using data from a participating cohort of PWID in Vancouver, Canada, between December 2014 and May 2017. Multivariable logistic regression models were built to assess the association between reporting active (i.e., within the past six months) OAT enrollment and assisting others in injection initiation. A final model was determined using a manual stepwise approach whereby covariates were excluded if their removal altered the coefficient of interest by <5%.
Results:
Participants (n=1740) were predominantly male (62.3%); 35.1% reported daily injecting (n=611); 860 (49.4%) reported active OAT enrollment, and 80 (4.6%) reported recently providing injection initiation assistance. In a multivariable model, participants who reported active OAT enrollment had significantly lower odds of recently providing injection initiation assistance (Adjusted Odds Ratio=0.52, 95% Confidence Interval: 0.31–0.87, P=0.01).
Conclusion:
Results suggest a protective association between OAT and the expansion of IDU practices among vulnerable populations, suggesting its potential use as ‘addiction treatment as prevention.’
Keywords: Opioid Agonist Treatment, Overdose Prevention, HIV Prevention, HCV Prevention, Persons Who Inject Drugs, Methadone, Suboxone, Treatment as Prevention
1. Introduction
For many individuals with opioid use disorder (OUD), worsening disease severity is associated with injection drug use (IDU), a risk factor for overdose and bloodborne disease transmission (Compton et al., 2016; Degenhardt et al., 2013; UNODC, 2018). Among the 12 million persons who inject drugs (PWID) worldwide, estimates suggest that between 17–30% are HIV-seropositive and 75–82% are HCV-seropositive (Grebely et al., 2014; Jacka et al., 2016; UHRI, 2013). In North America, increased heroin use and elevated levels of opioid prescribing have contributed to a continental epidemic of opioid-related overdose, which is now the leading cause of death for Americans under the age of 50 (Cicero et al., 2015; Compton et al., 2016; Mack et al., 2017). While promising reductions in HIV among PWID in North America have been achieved, increasing incidence of opioid overdose mortality has undermined many of the advances made in managing the harms of drug injecting and OUD through evidence-based approaches such as opioid agonist treatment (OAT) (Broers et al., 1998; Gowing et al., 2011; Karki et al., 2016). Public health experts have therefore renewed calls to move HIV and OUD prevention efforts upstream towards the prevention of IDU initiation (Bluthenthal and Kral, 2015; Werb et al., 2017).
PWID play a critical role in facilitating the entry of others into IDU. Specifically, evidence suggests that PWID may facilitate the exposure of others to IDU by socializing injecting behaviors and assisting injection-naïve persons who use drugs [PWUD] in their first injection event (Bluthenthal et al., 2015b; Roy et al., 2003). This phenomenon appears largely consistent across settings (Guise et al., 2017) with between 14% and 38% of sampled PWID reporting ever initiating others into IDU (Bluthenthal et al., 2015a; Bluthenthal et al., 2015b; Kral et al., 2015; Mittal et al., 2017; Rafful et al., 2017; Roy et al., 2003). Emerging research has also identified factors that heighten the risk that PWID will respond to IDU initiation requests. These include the use of non-injection opioids (which may increase interaction between networks of PWID and injection-naïve PWUD at risk of initiating IDU), daily IDU (which may increase the frequency of others’ exposure to IDU practices), and injecting in public settings (which may increase the number of individuals exposed to IDU practices) (Bluthenthal et al., 2015a; Bluthenthal et al., 2015b; Hamida et al., 2017; Melo et al., 2018; Rafful et al., 2017). It is posited that these factors, all of which may increase the visibility of injection practices to noninjectors, may also therefore increase the risk that PWID are asked to provide injection initiation assistance. Previous studies have also reported that opioid withdrawal symptoms place PWID in a particularly vulnerable position to accept requests for injection initiation assistance, a phenomenon that can be mitigated with effective OUD treatment (Guise et al., 2017).
Vancouver, Canada, like other settings in North America, is experiencing an acute increase in overdose mortality due primarily to the rising availability and use of fentanyl (Hayashi et al., 2018), a high-potency opioid analogue, among a large population of street-involved PWID (DeBeck et al., 2009). There is a scientific consensus that OAT effectively reduces the frequency of opioid injecting (Gowing et al., 2011; Karki et al., 2016; Volkow et al., 2014), and it has also been shown to reduce public injecting (Ickowicz et al., 2017). As such, we hypothesize that active OAT enrollment may also be associated with a decreased risk that PWID assist others in their first injection in Vancouver, Canada, a setting disproportionately impacted by public injecting and untreated OUD.
2. Methods
2.1. Study design
The Preventing Injecting by Modifying Existing Responses (PRIMER; NIDA DP2-DA040256–01) protocol seeks to assess the impact of socio-structural factors on the risk that PWID facilitate the entry of others into IDU (Werb et al., 2016). The PRIMER study protocol and rationale have been previously described in full (Werb et al., 2016). For the present analysis, data were drawn from three linked prospective cohort studies of PWUD in Vancouver, Canada: The At-Risk Youth Study (ARYS; including street-involved youth aged 14 to 26), the AIDS Care Cohort to Evaluate Access to Survival Services study (ACCESS; HIV-seropositive PWUD), and the Vancouver Injection Drug Users Study (VIDUS; HIV-seronegative PWID) during six-month follow-up visits from December 2014 to May 2017. To be eligible for ARYS, participants had to be 14–26 years old, street-involved (i.e., homeless or having used street youth services) and report illegal drug use other than cannabis in the past month (Cheng et al., 2018; Werb et al., 2016). To be eligible for ACCESS, participants had to be ≥18 years old, living with HIV, and report illegal drug use other than cannabis in the past month (Prangnell et al., 2017). To be eligible for VIDUS, participants had to be ≥18 years old, HIV-negative, and report injecting drugs at least once in the past month (Cheng et al., 2018; Werb et al., 2016). All cohort participants had to reside in the greater Vancouver area and provide written informed consent at baseline. For the current study, participants were included only if they reported ever injecting any type of drugs. We opted to include individuals who reported non-opioid injecting given a high level of polysubstance use among the cohort and previous research from this study site demonstrating that OAT enrollment may influence the use of non-opioid substances (Kerr et al., 2005), including stimulants. The PRIMER baseline is defined as the visit when specific survey items soliciting reports of assisting others in their first injection were introduced into the cohort questionnaires. Importantly, all three cohort studies use identical survey items in a combined questionnaire allowing for cross-cohort data analysis. Participants provided written informed consent. This study was approved by the University of California San Diego Human Research Protection Program and the University of British Columbia/Providence Health Care Research Ethics Board.
2.2. Measures
At the PRIMER baseline, participants completed an interviewer-administered questionnaire assessing sociodemographics, IDU practices, and enrollment in health services including OAT (i.e., methadone, Suboxone [buprenorphine/naloxone in combination]) in the previous six months. The primary outcome was defined as recently assisting others in their first injection (confirmed via endorsement of the following survey item: “In the past 6 months, have you helped someone inject who had never injected before?”). The independent variable of interest was active OAT enrollment, defined via endorsement of the statement: “In the past 6 months have you been in (methadone/methadose program or Suboxone) treatment?” Covariates were selected based on previous literature and included: cohort, age, gender, race/ethnicity, marital status, homelessness status, current neighborhood of residence, any non-injection drug use, recent IDU frequency (defined as daily vs. less than daily vs. none), recent public injection, recent cocaine IDU, recent methamphetamine IDU, recent heroin IDU, recent speedball (i.e., heroin and cocaine in combination) IDU, recent prescription opioid IDU, and type of OAT (Bluthenthal et al., 2015b; Fuller et al., 2005; Hamida et al., 2017; Ickowicz et al., 2017). The categories for current neighborhood of residence (i.e., Downtown Eastside [DTES], Downtown South [DTS], vs Other) were chosen based on data showing distinct high-risk drug practices in these two areas. For example, Vancouver’s open-air street drug market is in the DTES (Shannon et al., 2006), while initiation of IDU has been shown to be over twice as high in the DTS than in the DTES among a local sample of street-involved youth (Werb et al., 2010). Additionally, perceived OAT dose appropriateness (Strain et al., 1999), was measured by the following survey item: “Is the dose of methadone or Methadose you receive…?” and endorsement of any of the following options: “too low, about right, or too high.”
2.3. Statistical analyses
Cross-sectional univariate associations between potential risk factors and the provision of IDU initiation assistance were assessed using cross-tabulation, Fisher’s exact test for categorical variables, and Wilcoxon rank sum test for continuous variables. Multivariable logistic regression models were performed to study the independent association of reporting active (i.e., past six months) OAT use on recent assistance of others in their first injection. Potential confounders included: cohort, age, gender, race/ethnicity, homelessness, current neighborhood of residence, recent IDU frequency, recent public injection, recent cocaine IDU, recent methamphetamine IDU, recent heroin IDU, and recent speedball IDU. The confounding model-building approach used a manual stepwise protocol wherein a covariate was removed from the model if the coefficient of interest was altered by <5% upon its removal (Rothman et al., 2008). Therefore, the final parsimonious model consists of only those variables that meaningfully influence the association between the outcome and the independent variable of interest. Finally, in a sub-analysis restricted to participants actively enrolled in OAT, we assessed the proportion of participants’ recent reports of providing injection initiation assistance stratified by the perceived appropriateness of their OAT dose, defined as ‘too low’, ‘about right’, or ‘too high’. Analyses were performed using R software (version 3.3.2). P-values less than 0.05 were considered statistically significant.
3. Results
Participants (n=1740) were predominantly male (n=1082, 62.3%) and were 57.1% (n=992) White individuals and 35.5% (n=617) persons who self-identified as Indigenous. Median participant age was 43.4 years old (Interquartile Range: 33–53). At the PRIMER baseline, 35.1% (n=611) of the sample reported daily injecting, and 24.25% (n=422) had ever assisted others in their first injection. Overall, 80 participants (4.6%) reported recently assisting others in their first injection. Less than half of participants (n=860; 49.4%) reported active OAT enrollment, of which 821 (47.2%) reported enrollment in methadone only, 62 (3.6%) in Suboxone only, and 23 (1.3%) reported enrollment in both methadone and Suboxone. The largest proportion of participants reported residing in the Downtown Eastside (DTES) neighborhood (n=862, 49.6%). A quarter of participants (n=437, 25.1%) were homeless in the past 6 months. The largest proportion of recent IDU type among participants was heroin (n=772, 44.4%), and 556 (32.0%) reported recently injecting in public.
Table 1 presents univariate associations between covariates and the outcome, defined as assisting others in their first injection. As shown, participants who reported recently assisting others were significantly younger (i.e., mean age 33.0 years [Interquartile Range: 23.0–40.3; Fisher’s exact P<0.001]). When compared by age group, participants ≤ 30 years old (n=39) had a significantly higher proportion of recently assisting others compared with participants in older age groups (i.e., 31–40, 41–50, and ≥51 years old; Fisher’s exact P<0.001). Further, a significantly higher proportion of participants who reported daily IDU in the past six months also reported recently assisting others (9.2%) compared with those who reported injecting less than daily (3.9%) or not at all (0.5%; P<0.001). We also observed a significantly higher proportion of participants in the ARYS cohort reporting recently assisting others (10.2% vs 3.1% ACCESS vs 3.5% VIDUS, Fisher’s exact P<0.001). We did not observe a statistically significant difference between male and female participants with respect to reporting assisting others in their first injection (4.2% among female participants vs 4.1% among male participants, Fisher’s exact P=0.91). Finally, a significantly smaller proportion of participants actively enrolled in OAT reported recently assisting others in their first injection when compared with non-enrolled participants (3.1% vs 6.0%, Fisher’s exact P=0.004).
Table 1.
Baseline characteristics stratified by reports of assisting others in their first injection in the past 6 months in Vancouver, Canada, 2014–2017 (n=1740).
| Variable | Did not assist others in their first injection in the past 6 months (n=1660) | Assisted others in their first injection in the past 6 months (n=80) | P-valuea | Odds Ratio | 95% CI | ||
|---|---|---|---|---|---|---|---|
| Cohort | |||||||
| ACCESS | 572 | (97.0%) | 18 | (3.1%) | <0.001 | 1.00 | |
| ARYS | 291 | (89.8%) | 33 | (10.2%) | 3.60 | (2.00–6.51) | |
| VIDUS | 797 | (96.5%) | 29 | (3.5%) | 1.16 | (0.64–2.10) | |
| Age in years, mean (IQR) | 43.4 | (33–53) | 33.0 | (23.0–40.3) | <0.001 | 0.93 | (0.91–0.95) |
| Genderb | |||||||
| Female | 677 | (95.8%) | 30 | (4.2%) | 0.91 | 1.00 | |
| Male | 1162 | (95.9%) | 49 | (4.1%) | 0.99 | (0.62–1.57) | |
| Race/Ethnicityb | |||||||
| White | 951 | (95.9%) | 41 | (4.1%) | 0.29 | 1.00 | |
| Indigenous | 587 | (95.1%) | 30 | (4.9%) | 1.19 | (0.73–1.92) | |
| Other | 118 | (92.9%) | 9 | (7.1%) | 1.77 | (0.84–3.73) | |
| Marital Statusb | |||||||
| Married | 228 | (96.2%) | 9 | (3.8%) | 0.74 | 1.00 | |
| Other | 1431 | (95.3%) | 70 | (4.7%) | 1.24 | (0.61–2.52) | |
| Homeless† | |||||||
| Yes | 407 | (93.1%) | 30 | (6.9%) | 0.012 | 1.85 | (1.16–2.94) |
| No | 1253 | (96.2%) | 50 | (3.8%) | 1.00 | ||
| Neighborhood of residence† | |||||||
| DTES | 818 | (94.9%) | 44 | (5.1%) | 0.02 | 1.00 | |
| DTS | 285 | (93.4%) | 20 | (6.6%) | 1.31 | (0.78–2.25) | |
| Other | 556 | (97.2%) | 16 | (2.8%) | 0.54 | (0.30–0.96) | |
| Any non-injection drug use† | |||||||
| Yes | 965 | (94.6%) | 55 | (5.4%) | 0.06 | 1.58 | (0.98–2.57) |
| No | 695 | (96.5%) | 25 | (3.5%) | 1.00 | ||
| Cocaine IDU† | |||||||
| Yes | 313 | (92.9%) | 24 | (7.1%) | 0.02 | 1.84 | (1.13–3.02) |
| No | 1347 | (96.0%) | 56 | (4.0%) | 1.00 | ||
| Crack IDU† | |||||||
| Yes | 30 | (90.9%) | 3 | (9.1%) | 0.19 | 2.12 | (0.62–7.09) |
| No | 1630 | (95.5%) | 77 | (4.5%) | 1.00 | ||
| Heroin IDU† | |||||||
| Yes | 715 | (92.6%) | 57 | (7.4%) | <0.001 | 3.28 | (2.00–5.37) |
| No | 945 | (97.6%) | 23 | (2.4%) | 1.00 | ||
| Speedball IDU† | |||||||
| Yes | 106 | (89.8%) | 12 | (10.2%) | 0.009 | 2.59 | (1.36–4.93) |
| No | 1554 | (95.8%) | 68 | (4.2%) | 1.00 | ||
| Methamphetamine IDU† | |||||||
| Yes | 543 | (89.9%) | 61 | (10.1%) | <0.001 | 6.60 | (3.91–11.16) |
| No | 1117 | (98.3%) | 19 | (1.7%) | 1.00 | ||
| Prescription opioid IDU† | |||||||
| Yes | 276 | (89.9%) | 31 | (10.1%) | <0.001 | 3.17 | (1.99–5.07) |
| No | 1384 | (96.6%) | 49 | (3.4%) | 1.00 | ||
| IDU frequency† | |||||||
| None | 587 | (99.5%) | 3 | (0.5%) | <0.001 | 1.00 | |
| Less than daily | 518 | (96.1%) | 21 | (3.9%) | 7.93 | (2.35–26.75) | |
| Daily | 555 | (90.8%) | 56 | (9.2%) | 19.743 | (6.14–63.44) | |
| Public Injecting† | |||||||
| Yes | 499 | (89.8%) | 57 | (10.3%) | <0.001 | 5.74 | (3.50–9.42) |
| No | 1156 | (98.1%) | 23 | (2.0%) | 1.00 | ||
| OAT enrollment | |||||||
| Yes | 833 | (96.9%) | 27 | (3.1%) | 0.004 | 0.51 | (0.32–0.81) |
| No | 827 | (94.0%) | 53 | (6.0%) | 1.00 | ||
| Type of OAT† | |||||||
| Methadone only | 774 | (97.0%) | 24 | (3.0%) | 0.48 | (0.30–0.79) | |
| Buprenorphine/naloxone only | 38 | (97.4%) | 1 | (2.6%) | 0.01 | 0.41 | (0.06–3.05) |
| Both | 21 | (91.3%) | 2 | (8.7%) | 1.49 | (0.34–6.51) | |
| None | 827 | (94.0%) | 53 | (6.0%) | 1.00 | ||
Fisher’s exact test;
Change in sample size due to missing observations.
The variable refers to activities during the previous six months; Note: IQR: Interquartile Range; CI: Confidence Interval; DTES: Downtown Eastside, Vancouver’s drug use epicenter; DTS: Downtown South; IDU: Injection Drug Use; OAT: Opioid Agonist Treatment; ARYS: At-Risk Youth Study; ACCESS: The AIDS Care Cohort to Evaluate Access to Survival Services study; VIDUS: Vancouver Injection Drug Users Study
As shown in Table 2, the final multivariable confounding regression model includes participants who reported ever injecting drugs (n=1740) and included OAT enrollment, age, sex, cohort, homelessness status, recent injecting frequency, recent methamphetamine IDU, and speedball IDU. After adjusting for these covariates, participants who reported active OAT enrollment had significantly lower odds of reporting recently assisting others in their first injection (Adjusted Odds Ratio [AOR]: 0.52, 95% Confidence Interval [CI]: 0.31–0.87, P=0.01).
Table 2.
Multivariable logistic regression model assessing the association between assisting others in their first injection and OAT enrollment in Vancouver, Canada.
| Variable | AOR | (95% CI) | P-value |
|---|---|---|---|
| OAT enrollment† | 0.52 | (0.31–0.87) | 0.01 |
| Age | 0.94 | (0.91–0.97) | <0.001 |
| Male gender | 1.16 | (0.70–1.91) | 0.57 |
| Cohort membership*: | |||
| ARYS | 0.70 | (0.30–1.70) | 0.42 |
| VIDUS | 0.82 | (0.43–1.57) | 0.56 |
| Homeless† | 0.54 | (0.32–0.93) | 0.03 |
| Injection Frequency† | |||
| Less than daily | 4.43 | (1.21–16.20) | 0.02 |
| Daily | 8.37 | (2.34–29.7) | 0.001 |
| Methamphetamine IDU† | 2.20 | (1.20–4.04) | 0.01 |
| Speedball IDU† | 1.98 | (0.99–4.00) | 0.05 |
The variable refers to activities during the previous six months; AOR: Adjusted Odds Ratio; 95% CI: 95% Confidence Interval; IDU: Injection Drug Use; OAT: Opioid Agonist Treatment. ARYS: At-Risk Youth Study; VDUS: Vancouver Injection Drug Users Study.
Reference category: ACCESS (The AIDS Care Cohort to Evaluate Access to Survival Services study).
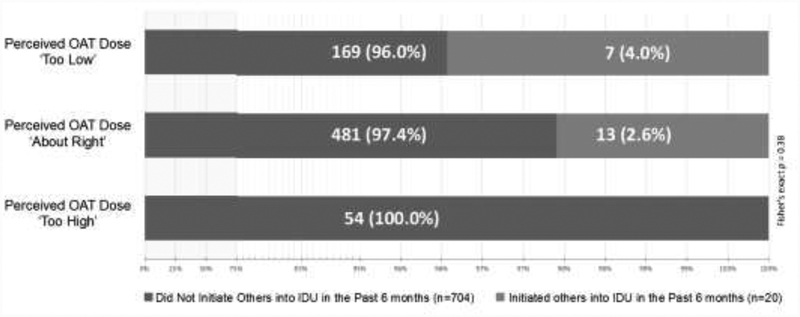
Figure 1 presents findings from a sub-analysis of participants currently enrolled in OAT (n=724) at the PRIMER baseline stratified by the perceived appropriateness of their OAT dose. The subsample reporting that their OAT dose was suboptimal (i.e., ‘too low’) also had the highest proportion of participants assisting others in their first injection (4.0%, n=7) compared with those who reported that their OAT dose was satisfactory (2.6%, n=13) or excessive (0%, n=0), though the small subsample size precluded testing statistical significance.
Figure 1.
Perceived OAT Dose and Recent Injection Initiation Assistance Provision among PWID Enrolled in OAT at the PRIMER baseline in Vancouver, Canada (n=724). OAT: Opioid Agonist Treatment; PWID: Persons Who Use Drugs; IDU: Injection Drug Use
4. Discussion
Among a sample of PWID in Vancouver, active OAT enrollment was associated with a 48% reduction in the odds of assisting others in their first injection event. These results are, to our knowledge, the first to identify an association between active enrollment in OAT and a reduced risk of facilitating others’ entry into drug injecting. This suggests that OAT may provide both an individual-level benefit to individuals seeking to manage OUD and a potential population-level protective effect on the expansion of the epidemics of IDU (Guise et al., 2017). This is a mechanism consistent with ‘HIV treatment as prevention,’ which is predicated on the fact that effective management of HIV infection also reduces the transmissibility of the virus to others (Granich et al., 2010). If further research confirms the preliminary findings reported herein, OAT may be considered in a similar role as HIV antiretroviral therapy within an ‘addiction treatment as prevention’ framework to prevent the expansion of epidemics of IDU and untreated OUD (Vashishtha et al., 2017).
Effective OUD treatment (i.e., OAT) reduces opioid withdrawal symptoms and injection frequency (Gowing et al., 2011; Karki et al., 2016; Volkow et al., 2014). Given that injection frequency was an independent covariate in our final model, we posit that OAT’s reduction of withdrawal symptoms among study participants rendered them less vulnerable to respond to requests for injection initiation assistance. Previous research has shown that PWID who had described IDU or injected in front of injection-naïve individuals were at higher odds of being asked to provide injection initiation assistance (Bluthenthal et al., 2015a). However, most studies have reported that accepting these requests is linked to opioid withdrawal symptoms (Guise et al., 2017). Therefore, by effectively managing withdrawal symptoms, active OAT enrollment could help reduce the economic and addiction-related vulnerability of PWID to initiate requests within drug-using networks. This may in turn also reduce the number of requests for injection initiation assistance.
While PWID enrolled in OAT had an almost 50% reduction in the odds of facilitating the entry of others into IDU, we nevertheless found that a higher proportion of participants who perceived their OAT dose as ‘too low’ reported assisting others compared with OAT-enrolled participants with ‘adequate’ or ‘high’ OAT doses. Because of the small sample size, we were unable to assess the significance of this difference. Additional research should therefore seek to confirm a potential protective effect of OAT enrollment in our study setting and elsewhere and to further determine whether it is enhanced among PWID receiving optimal OAT doses. This is particularly relevant in the U.S., as some OAT programs are significantly more likely than others to provide low OAT doses to African Americans, who continue to experience a high prevalence of OUD and related harms (D’Aunno et al., 2019). Currently, clinical options for OAT delivery are expanding, and this treatment modality will become available in a larger number of North American settings experiencing disproportionately high levels of IDU-related morbidity and mortality (Rapoport and Rowley, 2017). Researchers and key stakeholders should leverage these opportunities to help address effective OAT dose levels to reduce the harms associated with OUD and help mitigate the risk of injection initiation assistance.
The association between younger age and a higher risk of assisting others in their first injection we identified is particularly relevant given the increased risk of opioid misuse experienced during young adulthood (Zibbell et al., 2015). Youth are not only at higher risk of initiating IDU, but these results suggest younger PWID in Vancouver are also at higher risk of initiating others (Bluthenthal et al., 2015b). This suggests an enhanced role for pediatricians engaged in clinic-based OUD education, prevention, and treatment efforts to consider how the provision of clinical care may influence the process of IDU initiation (Levy et al., 2016).
We observed an association between homelessness and assisting others in their first injection. We found this negative association to be counterintuitive given that homelessness among daily opioid users in Vancouver has been associated with less likelihood of being linked to addiction care, OAT enrollment, and OAT retention in the past decade (Socías et al., 2018; Kerr et al., 2005). This finding may reflect the social isolation and community disconnection that individuals with no housing support face in Vancouver (Patterson et al., 2013), and they may therefore not be as likely to experience requests for injection initiation assistance from others. Additionally, homeless PWID may not have access to semi-private settings such as single-room occupancy hotels, which have been associated with higher-risk drug use behaviors in this setting (Shannon et al., 2006). Further study of the association between OAT enrollment among homeless PWID and injection initiation assistance is needed, particularly with current efforts in Vancouver which are seeking to improve OAT adherence among homeless adults with OUD (Parpouchi et al., 2017).
Finally, we found that measures of higher drug use activity—such as injection frequency and polydrug IDU—were significantly associated with an increased risk that study participants reported providing injection initiation assistance. This adds to a small but growing evidence base identifying these as risk factors for the provision of injection initiation assistance among PWID (Bluthenthal et al., 2015b; Hamida et al., 2017; Rafful et al., 2017). Given that methamphetamine IDU was a strong predictor of initiating others, future OAT-based intervention research should also examine polydrug use to address the role of opioid use in driving PWID’s increased risk for initiating others. In this context, OAT-based interventions to prevent injection initiation as well as behavioral approaches such as Change the Cycle and Break the Cycle, which also include safer IDU education, could be used in isolation or in combination with OAT (Vashishtha et al., 2017; Werb et al., 2017).
This study has limitations typical of observational studies of PWID. First, we cannot assume causality between OAT enrollment and the assistance of others into drug injecting. Second, similar to other prospective studies of PWID, data are not from random samples, and we therefore cannot assume their representativeness to the broader PWID population in Vancouver (Wood et al., 2006). Additionally, we rely on self-reported behavioral data which may result in underreporting of drug-related behaviors (Magura, 2010); we note that the assistance of others’ entry into drug injecting is likely to be particularly under-reported given that it has been shown to be highly stigmatized among PWID across a range of settings (Guise, 2018).
5. Conclusion
We observed a protective association between OAT enrollment and facilitating the entry of others into IDU in a setting marked by a high prevalence of untreated OUD and public injecting behaviors. These findings suggest that along with OAT’s effectiveness at managing OUD, this clinical intervention may also have a secondary protective effect on the expansion of IDU among vulnerable populations. Given the high levels of morbidity and mortality associated with untreated OUD, as well as the intensification of this condition resulting from IDU, further study is required to determine whether OAT may be utilized within an ‘addiction treatment as prevention’ framework to manage both individual- and population-level patterns of IDU.
Highlights.
People who inject drugs (PWID) who reported OAT enrollment had lower odds of recently initiating others into IDU
Younger age was associated with an increased risk of initiating others into injection drug use (IDU)
Findings suggest a potential role for opioid agonist treatment (OAT) to prevent incident cases of IDU initiation
Acknowledgements
The authors thank the study participants for their contribution to the research as well as current and past VIDUS/ARYS/ACCESS/PRIMER researchers and staff. Dr. Mittal is supported by the Fogarty International Center of the National Institutes of Health (NIH) Award Numbers D43TW008633 and R25TW009343, UC San Diego Center for AIDS Research (CFAR) NIAID P30AI36214, and National Institute on Drug Abuse (NIDA) grants T32DA023356 and 3R01DA040648–02S1. Dr. Milloy is supported by NIDA (U01-DA0251525), a New Investigator award from the Canadian Institutes of Health Research, and a Scholar award from the Michael Smith Foundation for Health Research. His institution has received an unstructured gift from NG Biomed Ltd., a private firm seeking a government license to produce cannabis, to support him. He is the Canopy Growth professor of cannabis science, a position created through arms’ length gifts to the University of British Columbia from the Government of British Columbia’s Ministry of Mental Health and Addictions and Canopy Growth, a licensed producer of cannabis. Dr. Hayashi is supported by a Canadian Institutes of Health Research New Investigator Award (MSH-141971), a Michael Smith Foundation for Health Research Scholar Award, and the St. Paul’s Foundation. Dr. Werb is supported by a US NIDA Avenir Award (DP2-DA040256–01), a New Investigator Award from the Canadian Institutes of Health Research, and an Early Researcher Award from the Ontario Ministry of Research, Innovation and Science. The VIDUS and ARYS Studies are supported by NIDA grant U01DA038886. The ACCESS Study is supported by NIDA grant U01DA021525.
Role of the Funding Source
Research reported in this publication was supported in part by the national institute on drug abuse (nida) award numbers dp2-da040256–01, u01da038886, u01da021525, t32da023356. The content is solely the responsibility of the authors and does not necessarily represent the official views of the national institutes of health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflicts of interests to declare.
References
- Bluthenthal R, Wenger L, Chu D, Lorvick J, Quinn B, Thing JP, Kral AH, 2015a. Factors associated with being asked to initiate someone into injection drug use. Drug Alcohol Depend 149, 252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthenthal R, Wenger L, Thing J, Chu D, Lorvick J, Quinn B, Kral A, 2015b. Factors associated with precursors to initiating other people into injection drug use. Drug Alcohol Depend 146, e224–e225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthenthal RN, Kral AH, 2015. Next steps in research on injection initiation incidence and prevention. Addiction 110, 1258–1259. [DOI] [PubMed] [Google Scholar]
- Broers B, Junet C, Bourquin M, Déglon J-J, Perrin L, Hirschel B, 1998. Prevalence and incidence rate of HIV, hepatitis B and C among drug users on methadone maintenance treatment in Geneva between 1988 and 1995. AIDS 12, 2059–2066. [DOI] [PubMed] [Google Scholar]
- Cheng T, Small W, Nosova E, Hogg B, Hayashi K, Kerr T, DeBeck K, 2018. Nonmedical prescription opioid use and illegal drug use: Initiation trajectory and related risks among people who use illegal drugs in Vancouver, Canada. BMC Res. Notes 11, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Harney J, 2015. Shifting patterns of prescription opioid and heroin abuse in the United States. N. Engl. J. Med 373, 1789–1790. [DOI] [PubMed] [Google Scholar]
- Compton WM, Jones CM, Baldwin GT, 2016. Relationship between nonmedical prescription-opioid use and heroin use. N. Engl. J. Med 374, 154–163. [DOI] [PubMed] [Google Scholar]
- D’Aunno T, Park SE, Pollack HA, 2019. Evidence-based treatment for opioid use disorders: A national study of methadone dose levels, 2011–2017. J. Subst. Abuse Treat 96, 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBeck K, Small W, Wood E, Li K, Montaner J, Kerr T, 2009. Public injecting among a cohort of injecting drug users in Vancouver, Canada. J. Epidemiol. Community Health 63, 81–86. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Whiteford HA, Ferrari AJ, Baxter AJ, Charlson FJ, Hall WD, Freedman G, Burstein R, Johns N, Engell RE, 2013. Global burden of disease attributable to illicit drug use and dependence: Findings from the Global Burden of Disease Study 2010. Lancet 382, 1564–1574. [DOI] [PubMed] [Google Scholar]
- Fuller CM, Borrell LN, Latkin CA, Galea S, Ompad DC, Strathdee SA, Vlahov D, 2005. Effects of race, neighborhood, and social network on age at initiation of injection drug use. Am. J. Public Health 95, 689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowing L, Farrell MF, Bornemann R, Sullivan LE, Ali R, 2011. Oral substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst. Rev CD004145. [DOI] [PubMed] [Google Scholar]
- Granich R, Crowley S, Vitoria M, Smyth C, Kahn JG, Bennett R, Lo YR, Souteyrand Y, Williams B, 2010. Highly active antiretroviral treatment as prevention of HIV transmission: Review of scientific evidence and update. Curr. Opin. HIV AIDS 5, 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebely J, Lima VD, Marshall BD, Milloy M, DeBeck K, Montaner J, Simo A, Krajden M, Dore GJ, Kerr T, 2014. Declining incidence of hepatitis C virus infection among people who inject drugs in a Canadian setting, 1996–2012. PloS One 9, e97726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guise A, Horyniak D, Melo J, McNeill R, Werb D, 2017. The experience of initiating injection drug use and its social context: A qualitative systematic review and thematic synthesis. Addiction 112, 2098–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guise AMJ, Mittal ML, Rafful C, Cuevas-Mota J, Davidson P, Garfein R, Werb D, 2018. A fragmented code: The moral and structural context for providing assistance with injection drug use initiation in San Diego, USA. Int. J. Drug Policy 55, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamida AB, Rafful C, Jain S, Sun S, Gonzalez-Zuniga P, Rangel G, Strathdee SA, Werb D, 2017. Non-injection drug use and injection initiation assistance among people who inject drugs in Tijuana, Mexico. J. Urban Health 95, 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Milloy M-J, Lysyshyn M, DeBeck K, Nosova E, Wood E, Kerr T, 2018. Substance use patterns associated with recent exposure to fentanyl among people who inject drugs in Vancouver, Canada: A cross-sectional urine toxicology screening study. Drug Alcohol Depend 183, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ickowicz S, Wood E, Dong H, Nguyen P, Small W, Kerr T, Montaner JSG, Milloy M-J, 2017. Association between public injecting and drug-related harm among HIV-positive people who use injection drugs in a Canadian setting: A longitudinal analysis. Drug Alcohol Depend 180, 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacka B, Applegate T, Poon AF, Raghwani J, Harrigan PR, DeBeck K, Milloy M-J, Krajden M, Olmstead A, Joy JB, 2016. Transmission of hepatitis C virus infection among younger and older people who inject drugs in Vancouver, Canada. J. Hepatol 64, 1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki P, Shrestha R, Huedo-Medina TB, Copenhaver M, 2016. The impact of methadone maintenance treatment on HIV risk behaviors among high-risk injection drug users: A systematic review. Evid. Based Med. Public Health 2, e1229. [PMC free article] [PubMed] [Google Scholar]
- Kerr T, Marsh D, Li K, Montaner J, Wood E, 2005. Factors associated with methadone maintenance therapy use among a cohort of polysubstance using injection drug users in Vancouver. Drug Alcohol Depend 80, 329–335. [DOI] [PubMed] [Google Scholar]
- Kral A, Wenger L, Chu D, Quinn B, Thing J, Lorvick J, Bluthenthal R, 2015. Initiating people into illicit drug injection. Drug Alcohol Depend 146, e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S, Ryan SA, Gonzalez PK, Patrick SW, Quigley J, Siqueira L, Walker LR, Faden VB, Tau G, Jarrett R, 2016. Medication-assisted treatment of adolescents with opioid use disorders. Pediatrics 138, e20161893. [DOI] [PubMed] [Google Scholar]
- Mack KA, Jones CM, Ballesteros MF, 2017. Illicit drug use, illicit drug use disorders, and drug overdose deaths in metropolitan and nonmetropolitan areas—United States. Am. J. Transplant 17, 3241–3252. [DOI] [PubMed] [Google Scholar]
- Magura S, 2010. Validating self-reports of illegal drug use to evaluate national drug control policy: A reanalysis and critique. Eval. Prog. Plann 33, 234–237. [DOI] [PubMed] [Google Scholar]
- Melo J, Garfein R, Hayashi K, Milloy M, DeBeck K, Sun S, Jain S, Strathdee S, Werb D, 2018. Do law enforcement interactions reduce the initiation of injection drug use? An investigation in three North American settings. Drug Alcohol Depend 182, 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal ML, Vashishtha D, Sun S, Jain S, Cuevas-Mota J, Garfein R, Strathdee SA, Werb D, 2017. History of medication-assisted treatment and its association with initiating others into injection drug use in San Diego, CA. Subst. Abuse Treat. Prev. Policy 12, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpouchi M, Moniruzzaman A, Rezansoff S, Russolillo A, Somers J, 2017. Methadone therapy adherence among homeless adults in a housing first trial in Vancouver, Canada. Eur. J. Public Health 27, ckx187.671. [Google Scholar]
- Patterson ML, Rezansoff S, Currie L, Somers JM, 2013. Trajectories of recovery among homeless adults with mental illness who participate in a randomised controlled trial of Housing First: A longitudinal, narrative analysis. BMJ Open 3, e003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prangnell A, Dong H, Daly P, Milloy MJ, Kerr T, Hayashi K, 2017. Declining rates of health problems associated with crack smoking during the expansion of crack pipe distribution in Vancouver, Canada. BMC Public Health 17, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafful C, Melo J, Medina-Mora ME, Rangel G, Sun X, Jain S, Werb D, 2017. Cross-border migration and initiation of others into drug injecting in Tijuana, Mexico. Drug Alcohol Rev 37, S277–S284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport AB, Rowley CF, 2017. Stretching the scope— Becoming frontline addiction-medicine providers. N. Engl. J. Med 377, 705–707. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Lash TL, Greenland S, 2008. Modern epidemiology, 3rd Edition Lippincott Williams and Wilkins, Philadelphia, PA. [Google Scholar]
- Roy É, Haley N, Leclerc P, Cédras L, Blais L, Boivin J-F, 2003. Drug injection among street youths in Montreal: Predictors of initiation. J. Urban Health 80, 92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon K, Ishida T, Lai C, Tyndall MW, 2006. The impact of unregulated single room occupancy hotels on the health status of illicit drug users in Vancouver. Int. J. Drug Policy 17, 107–114. [Google Scholar]
- Socías ME, Wood E, Kerr T, Nolan S, Hayashi K, Nosova E, Montaner J, Milloy MJ, 2018. Trends in engagement in the cascade of care for opioid use disorder, Vancouver, Canada, 2006–2016. Drug Alcohol Depend 189, 90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain EC, Bigelow GE, Liebson IA, Stitzer ML, 1999. Moderate-vs high-dose methadone in the treatment of opioid dependence: A randomized trial. JAMA 281, 1000–1005. [DOI] [PubMed] [Google Scholar]
- Urban Health Research Initiative (UHRI), 2013. Drug situation in Vancouver BC Centre for Excellence in HIV/AIDS, Urban Health Research Initiative, Vancouver: pp. 5. [Google Scholar]
- United Nations Office of Drugs and Crime (UNODC), 2018. World Drug Report 2018 United Nations publication, Vienna. [Google Scholar]
- Vashishtha D, Mittal ML, Werb D, 2017. The North American opioid epidemic: Current challenges and a call for treatment as prevention. Harm Reduct. J 14, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Frieden TR, Hyde PS, Cha SS, 2014. Medication-assisted therapies—Tackling the opioid-overdose epidemic. N. Engl. J. Med 370, 2063–2066. [DOI] [PubMed] [Google Scholar]
- Werb D, Bluthenthal R, Kolla G, Strike C, Kral A, Uusküla A, Des Jarlais D, 2017. Preventing injection drug use initiation: State of the evidence and opportunities for the future. J. Urban Health 95, 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb D, Garfein R, Kerr T, Davidson P, Roux P, Jauffret-Roustide M, Auriacombe M, Small W, Strathdee SA, 2016. A socio-structural approach to preventing injection drug use initiation: Rationale for the PRIMER study. Harm Reduct. J 13, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb D, Kerr T, Fast D, Qi J, Montaner JSG, Wood E, 2010. Drug-related risks among street youth in two neighborhoods in a Canadian setting. Health Place 16, 1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood E, Tyndall MW, Zhang R, Stoltz JA, Lai C, Montaner JS, Kerr T, 2006. Attendance at supervised injecting facilities and use of detoxification services. N. Engl. J. Med 354, 2512–2514. [DOI] [PubMed] [Google Scholar]
- Zibbell JE, Iqbal K, Patel RC, Suryaprasad A, Sanders KJ, Moore-Moravian L, Serrecchia J, Blankenship S, Ward JW, Holtzman D, 2015. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤ 30 years— Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morb. Mortal. Wkly. Rep 64, 453–458. [PMC free article] [PubMed] [Google Scholar]