Abstract
Purpose of Review:
Pre-exposure prophylaxis (PrEP) is a potent HIV prevention strategy, but uptake of daily oral PrEP remains low. This review covers PrEP agents currently available and agents and modalities under investigation.
Recent Findings:
Injectable ARV preparations have high acceptability among users but are likely to require adherence to 8-week interval injections. Topical microbicide gels and vaginal rings have underperformed by intention-to-treat analyses in efficacy studies, at least in large part due to challenges with adherence and/or sustained use. However, daily oral TDF-FTC also underperformed in randomized, placebo-controlled trials compared to expectations and subsequent real-world pragmatic use.
Keywords: Pre-exposure prophylaxis (PrEP), HIV/AIDS, On-demand, Injectables, Microbicide gels, Vaginal rings
Summary:
On-demand (2–1-1 dosing strategy for MSM) and injectable PrEP appear to be acceptable among participants in clinical trials. These modalities are particularly compelling alternatives for individuals who either do not want to take a daily medication (both on-demand and injectable) and/or want to take PrEP without a long commitment (on-demand). Emerging modalities such as vaginal films, microneedles, and subdermal implants have numerous advantages but are still in early stages of development.
INTRODUCTION
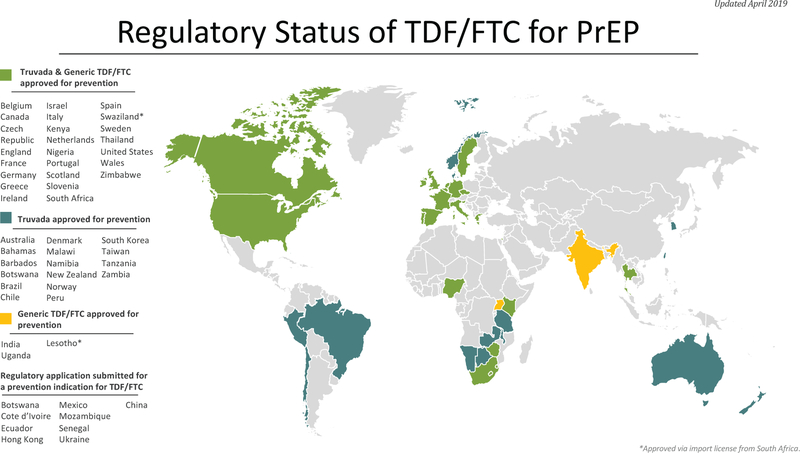
HIV pre-exposure prophylaxis (PrEP) is an HIV prevention strategy that has been shown to significantly reduce the sexual transmission of HIV. Models estimate that PrEP confers an HIV incidence reduction of 99% for men who have sex with men (MSM) and ≥90% for women, in the setting of consistent daily adherence [1, 2]. The first agent to receive regulatory approval for PrEP is a once-daily oral coformulation of tenofovir disoproxil fumarate with emtricitabine (TDF-FTC). In 2012, daily oral TDF-FTC was approved by the United States (U.S.) Food and Drug Administration (F.D.A.) for MSM, high-risk heterosexuals, and people who use injection drugs (PWID) [3]. In 2018, the indication was expanded to individuals weighing at least 35 kilograms (approximately 77 pounds) so that younger people could access PrEP [4]. Outside the U.S., TDF-FTC PrEP has regulatory approval in 43 other countries with additional regulatory applications pending (Figure 1) [5].
Fig. 1.
In the U.S., 1.2 million adults are estimated to have sufficient HIV acquisition risk to warrant PrEP use [6], but uptake has been sub-optimal with only approximately 70,000 unique individuals with an active PrEP prescription in the fourth quarter of 2017 [7]. Among individuals who decide to initiate PrEP, demonstration projects and clinical databases suggest that medication adherence is insufficient to provide high levels of HIV protection among select populations.
Medication adherence is just one potential barrier toward wider PrEP uptake. Other concerns about daily PrEP include resistance to TDF-FTC compromising first-line HIV treatment options globally. Concerns also exist over the potential for drug-drug interactions, especially for hormones and tuberculosis medications [8]. In addition, TDF-FTC is very safe and well-tolerated, but it does have signature toxicities including gastrointestinal intolerance, renal toxicity, and bone toxicity [9–11]. Particularly among women, gastrointestinal side effects seem to lead to more discontinuations [12]. Therefore, alternative PrEP agents with a similarly safe or better profile are needed. Lastly, the development of different family planning modalities shows that more choices increase the chances of finding a PrEP modality acceptable to a wider audience [13]. These options range from alternative dosing of oral products (both existing and novel); injectable preparations; topical products delivered as gels, rings, films, and long-acting/extended release (LA/ER) systems such as microneedles and implants. The purpose of this manuscript is to review alternative PrEP modalities as well as highlight potential strengths and drawbacks of each approach.
Currently Available Options
On-Demand/Nondaily PrEP
“On-demand” PrEP is a dosing strategy in which an individual takes oral PrEP only around the time of a sexual event, and is predicated on the ability to plan sexual activity (Table 1). A randomized, double-blind, placebo controlled trial in France and Canada among MSM, evaluated a dosing protocol of a double dose (2 tablets taken simultaneously) of oral TDF-FTC (or placebo) between two and 24 hours prior to sexual activity and then single tablets of oral TDF-FTC at 24 hours and 48 hours after the first dose, with ongoing daily dosing if exposures continue. Participants randomized to active TDF-FTC demonstrated 86% reduced incident HIV infections compared to those randomized to placebo [14]. Of note, the median number of doses was 15 tablets per month, approximating the 4 doses per week known to confer high levels of rectal protection during attempted daily dosing – and raising the question as to whether it was the on-demand strategy per se, or the high rates of dosing (regardless of proximity to intercourse) that most contributed to the overall findings. A post-hoc subgroup analysis suggests that on-demand PrEP remained effective in participants with less frequent sexual activity [15], and similar results were seen in the open-label extension of the double-blind randomized controlled trial [16]. Current guidelines for on-demand dosing are conflicting and pharmacokinetic (PK) data offer conflicting predictions of on-demand dosing efficacy at least in part due to lack of clarity as to the optimal PK correlate of HIV protection [17, 18]. However, increasing open-label data in follow-up to the initial randomized controlled trial supports the efficacy of the approach, leading to opportunities to individualize recommendations around TDF-FTC dosing regimens [19].
Table 1 -.
Compounds, Phase, and Frequency of Dosing for Investigational HIV Pre-Exposure Prophylaxis (PrEP) Modalities.
| Modality | Compounds Used | Phase | Frequency of Dosing | Next Phase |
|---|---|---|---|---|
| On-Demand/Nondaily PrEP | oral TDF-FTC | Post-3 | Pericoital | Regulatory approval in Europe, United Kingdom, and Canada. Pending approval in the United States |
| Oral tenofovir alafenamide with emtricitabine (TAF-FTC) | oral TAF-FTC | 3 | Daily | Pending results of Phase 3 efficacy studies |
| Injectable Agents | Rilpivirine | 2 | Every 8 Weeks | No Progression: Concerns about cold-chain requirements and a low barrier to resistance |
| Cabotegravir | 3 | Every 8 Weeks | Pending results of Phase 3 efficacy studies | |
| Topical Microbicide Gels | 1% Tenofovir Gel (Vaginal) | 3 | Daily | No Progression: Low observed efficacy |
| 1% Tenofovir Gel (Rectal) | 2 | Daily and Pericoital | Pending results of Phase 3 efficacy studies | |
| Vaginal Rings | Dapivirine | 3 | Once per Month | Open-label extensions are ongoing |
| Vaginal Films | Dapivirine | 1 | Pericoital | Additional Phase 1 studies |
| Tenofovir | 1 | Pericoital | Additional Phase 1 studies | |
| Microneedles/Microarray Patches | Rilpivirine | 0 | Undetermined | Trials are in the pre-clinical phase |
| Subdermal Implants | TAF | 0 | Undetermined | Trials are in the pre-clinical phase |
| Elvitegravir | 0 | Undetermined | Trials are in the pre-clinical phase | |
| Broadly Neutralizing HIV-1 Monoclonal Antibodies | VRC01 / VRC01LS | 2 | Undetermined | Pending results of Phase 2B efficacy studies |
Further complicating the debate, an open-label Phase 2 study among MSM, transgender women, and cisgender women compared the percent of sex acts “covered” by three dosing strategies: daily, time-driven (one dose of oral TDF-FTC twice weekly with a post-sex dose), and event-driven (one dose of oral TDF-FTC before and after sex). Among all populations, daily dosing provided higher rates of “coverage” of sex acts than did event- or time-driven dosing strategies [20, 21], suggesting that routinization of dosing is more likely to result in “coverage.” Importantly, no differences in safety were seen across these dosing strategies, disavowing conjecture that less than daily dosing may reduce side effects attributable to daily oral TDF-FTC use.
The advantages of on-demand PrEP are that it could be cost-saving when compared to daily oral TDF-FTC and may be helpful for people who have infrequent sex. Disadvantages include non-routinization, patient education on how to most effectively deploy and use on-demand PrEP, and the potential for unplanned sex compromising the precoital dose [22]. Most importantly, there is an absence of data for transwomen and ciswomen, limiting its potential use only for MSM.
Modalities Under Investigation
Daily oral TAF-FTC
The fixed dose combination of tenofovir alafenamide with emtricitabine (TAF-FTC) is currently under investigation as another form of daily oral PrEP. Macaque challenge models have demonstrated preventive efficacy for TAF-FTC against rectal and vaginal exposures [23, 24]. In contrast, PK studies have demonstrated poor rectal and vaginal tissue penetration [25].
TAF-FTC is currently under investigation for PrEP activity in a Phase 3 efficacy study comparing daily oral TDF-FTC to TAF-FTC in MSM and transgender women globally. Results were presented at CROI 2019, and while only 22 infections were observed, incidence rates in both the TAF/FTC (0.16 per 100 person-years) and TDF/FTC (0.34 per 100 person-years) were extremely low despite high rates of incident STIs (26). Regulatory submissions are under review.
Injectable Agents
Long-acting rilpivirine (RPV LA) and long-acting cabotegravir (CAB LA) are in advanced development for HIV prevention; both are crystalline, water insoluble nanoformulations for intramuscular injection. Because of their prolonged half-lives, injectable agent use is currently preceded by a four-week oral lead-in period of a short-acting tablet formulation of the product to assess safety and tolerability.
Rilpivirine
Rilpivirine, currently approved for HIV therapeutics (in combination with other antiviral agents), is a second-generation non-nucleoside reverse transcriptase inhibitor. RPV LA has demonstrated efficacy in preventing HIV transmission in humanized mouse models [27]. In humans, two Phase 1 trials have investigated the safety and PK of RPV LA, demonstrating good tolerability and rapid plasma accumulation after administration [28–30]. A Phase 2 trial among women administered 1200 mg of either RPV LA (administered as two 3 mL injection) or placebo every eight weeks followed participants up to a year after terminal injection. The trial found no significant difference in side effects or adverse events between RPV LA and placebo treated participants [31, 32]. RPV has not progressed to Phase 3 clinical trials, likely out of concerns for cold-chain requirements of the injectable product and a low barrier to resistance.
Cabotegravir
Cabotegravir is an investigational strand-transfer integrase inhibitor currently in development both for HIV treatment and HIV prevention. CAB LA has been shown in macaque models to provide protection against simian/human immunodeficiency virus (SHIV) challenge via vaginal, rectal, penile, and intravenous routes [33–36]. In Phase 2A trials, CAB LA was generally safe and well tolerated; although the majority of participants experienced local injection site reactions, these were overwhelmingly mild to moderate, and infrequently led to product discontinuation [37–39].
Initially studied as two simultaneous gluteal injections of CAB LA (2 mL each, 800 mg total) every 12 weeks [40], at that dose and dose-interval approximately 30% of participants had injection trough concentrations that were below the protein-adjusted IC90 (1 x PA-IC90), the protective threshold established in non-human primate rectal challenge models [34]. In response to these findings, a second Phase 2A trial in men and women globally was modified to evaluate an alternative dose of 600 mg (given as a single 3 mL injection) every 8 weeks after an initial 4-week injection interval; this dose/interval was also well tolerated, with similar rates and consequences of injection site reactions [41]. CAB LA is currently being evaluated in ongoing clinical Phase 3 studies to establish HIV prevention efficacy in comparison to daily oral TDF-FTC among MSM, transgender women, and cisgender women globally.
Potential advantages of injectable ARVs include less frequent dosing than oral TDF-FTC as well as the potential for reduced gastrointestinal toxicity [42]. Potential disadvantages of injectable ARVs include the current requirement of an oral lead-in period, injection site reactions, and a prolonged PK tail. The clinical consequences of seroconversion during the PK tail vis-à-vis selection for resistant HIV virus remain to be determined in the Phase 3 studies – but have the potential to compromise the activity of integrase-inhibitor based first line ARV therapy. The need for an oral lead-in period in clinical use will similarly be informed by the accumulating safety experience in Phase 2 and 3 clinical trials.
Topical Microbicide Gels
Topical microbicide gels contain an antiviral product that is applied to either the vagina or rectum in pericoital or daily dosing strategies. In a non-human primate model, both gel with 1% tenofovir (TFV) alone and with 5% emtricitabine (FTC) fully protected macaques from vaginal exposures to SHIV [43]. In a Phase 1 study investigating daily application of 1% TFV vaginal gel, the product was well tolerated over a two-week course with primarily mild adverse events [44].
The first Phase 3 study of an ARV-based topical microbicide gel randomly assigned HIV-uninfected women in South Africa to receive either 1% TFV gel or a placebo gel applied vaginally. TFV gel or placebo gel was applied in a pericoital dosing strategy consisting of a first application 12 hours before intercourse and the second dose of gel 12 hours after intercourse. The TFV gel arm demonstrated a use-dependent 39% reduction in HIV incidence when compared to the placebo gel arm [45]. However, subsequent studies of the 1% TFV gel when dosed daily have shown discrepant results based on adherence and patterns of use. Two Phase 3 trials among women using a daily dosing pattern reported no reduction in HIV incidence between treatment and placebo groups [46, 47]. Poor observed efficacy may be explained by low daily adherence.
In Phase 2 testing of tenofovir 1% rectal gels (TFV gel), there were no differences in safety profiles between daily TFV gel, TFV gel applied before and after receptive anal intercourse (RAI), and daily oral TDF-FTC [48]. Furthermore, 83% of participants had excellent (≥ 80%) adherence to daily gel and 93% had excellent (≥ 80%) adherence to the peri-coital (used before and after sex) TFV gel regimen [49].
Gels may be preferred by some consumers because they have the potential to be dosed intermittently, can act as a lubricant during sex, and may be both more discreet and have fewer side effects when compared to systemic agents. However, gels, similar to other topical agents, would only be expected to provide protection to the local compartment of application, absent being able to achieve systemic concentrations of the antiviral product. Three studies examining the safety, tolerability, and PK of dapivirine gel administered rectally are enrolling or in development.
Vaginal Rings
The vaginal rings, similar to products used for contraception, are currently in advanced development for HIV prevention. Rings provide a sustained, controlled release of antiviral agents over time. An elastomer ring containing dapivirine has demonstrated safety and tolerability in Phase 1 and 2 trials [50–54]. Notably, 96% of participants in a Phase 2 study of a dapivirine ring reported that the ring was comfortable, and 97% reported that they would be willing to use this modality in the future if proven effective [54].
Two Phase 3 trials investigating dapivirine rings have found similar results with an HIV incidence reduction of between 27% and 31% when comparing treatment and placebo groups [55, 56]. In both trials, there was no difference in the incidence of pregnancy between the dapivirine and placebo groups suggesting no clinically significant drug-drug interactions between dapivirine and hormonal contraception [55, 56].
Vaginal rings may be advantageous because they are more discreet than oral products. The disadvantages of dapivirine vaginal rings are relatively low efficacy in clinical trials, likely not achieving multicompartment protection, and a low barrier to resistance of dapivirine. Open-label extensions of these trials are ongoing, and suggest a 50% reduction in HIV incidence compared to a counterfactual background HIV incidence rate [57, 58]. The dapivirine ring is currently under review by regulatory agencies; novel ring designs with both dapivirine and alternative agent active products are in development, including multi-purpose rings that couple anti-HIV, anti-STD, and/or contraceptive agents into a single ring formulation [59, 60].
Vaginal Films
Vaginal films are dissolvable strips that are inserted into the vagina at least 15 minutes before intercourse and slowly release an antiviral substance. An in vitro study of a dapivirine film showed that more than half of dapivirine was released within the first ten minutes of application [61]. Animal studies have administered various intravaginal films to mice [62, 63] and pigs [62] and were found to be safe without apparent histological changes.
In a Phase 1 trial that assessed dapivirine film compared to dapivirine gel, placebo gel, or placebo film, both active agents were protective against HIV in ex vivo challenge assays [64]. Approximately 80% of the film users, compared with 68% of the gel users, reported that they would use the product in the future if it were found to be protective against HIV. In a two-arm, single-dose, crossover study of dapivirine gel and dapivirine film groups, both film and gel demonstrated reduced cervical tissue infectivity after ex vivo HIV challenge [65]. Similar results were seen for tenofovir film in both studies of daily dosing over a week as well as single dose administration [66, 67].
Advantages for these films include their discreet use and quick dissolvability. In comparison to gels, films have additional advantages in that they have a reduced tendency to leak and, by virtue of their smaller volume, would be anticipated to be less disruptive to endogenous immune function in the vaginal compartment [64]. The disadvantages for these films include more difficult insertion when compared to gels and the lack of systematic concentrations. An additional Phase 1 randomized trial is planned to evaluate the safety, acceptability, and length of time to dissolution of a placebo vaginal film among women.
Microneedles/Microarray Patches
Microneedles (also known as microarray patches) are synthetic material devices applied to the skin that deliver drug nanosuspensions trans dermally through numerous projections [68]. Microneedles have been used to successfully deliver vaccine products, demonstrating immunogenicity for the influenza vaccine comparable to when administered via IM injection [69]. Only one pre-clinical study has evaluated microneedles for ARV delivery [70].
The advantages to microneedles over injectable agents include the avoidance of needle-stick injuries, no requirement for skilled medical personnel to administer the dose, potential for at-home use, and no need for specialized disposal [68]. In addition, although the product has an extended release mechanism, it can still be removed quickly if there is an adverse event. Disadvantages include a large patch size as volume of medication increases and issues in scale-up of microneedle production. A microneedle patch with rilpivirine is under investigation [70].
Subdermal Implants
Subdermal implants, similar to currently available contraceptive implants, are porous polymer rods placed under the skin that provide a sustained release of an agent over a prolonged time interval. Three animal models evaluated either tenofovir alafenamide (TAF) or TAF and elvitegravir (EVG) and showed antiviral agents could be systemically delivered by this mechanism [71–73].
The advantage of implants is that they can be removed in the event of toxicity. Since the antiviral agent itself would be anticipated to have a short half-life, the liability of a prolonged PK tail would be avoided. The disadvantage is that implants have had varying degrees of acceptability, varying widely by geography and population. Human trials of HIV prevention agents delivered by implant are anticipated to begin in the near future.
Broadly neutralizing HIV-1 monoclonal antibodies (bNAbs)
Broadly neutralizing HIV-1 monoclonal antibodies (bNAbs) are human or humanized antibodies derived or modified from HIV-infected individuals who were observed to have unusually delayed or arrested HIV progression. bNAbs appear to bind conserved regions of the HIV virion. The portfolio of bNAbs is rapidly expanding and currently includes antibodies against four major sites on HIV: CD4, N332, V1V2, and gp41 MPER. The current generation of bNAbs entering clinical trials have been molecularly engineered to improve breadth, specificity, and pharmacokinetics.
bNabs have been shown to successfully protect macaques against repeated SHIV challenges [74–76] and mice against intravenous challenges [77]. Three Phase 1 studies reported that the VRC01 or VRC01LS administered either subcutaneously or intravenously were well tolerated [78–80]. Two Phase 2b studies are currently ongoing to examine the efficacy of VRC01 with the first study among men and transgender persons who have sex with men in North America, South America, and Europe and the second study among women in sub-Saharan Africa. In addition, a number of first-in-human studies of single and combinations of bNAbs as well as poly-functional bNAbs are in various stages of planning.
The advantages of bNAbs are their potential for long-acting HIV protection with infrequent dosing, their relative safety in early phase trials, and if successful, their preventive efficacy to inform potential vaccine design. The disadvantages of bNAbs include the need for parenteral administration, manufacturing complexities, costs, a small safety database to-date, and that anti-drug antibodies may be generated.
Other Formulations and Modalities
Additional delivery systems for PrEP agents are also being investigated. Studies are currently evaluating electrospun fibers [81–86], enemas [87, 88], mucoadhesive intravaginal tablets [89], thin-film polymer devices [90], vaginal foams, and vaginal sponges. Small early-phase studies of PK and tolerability are encouraging, but little additional data are available.
Conclusion
As the standard of care for HIV prevention improves, the pathway to regulatory approval of new HIV prevention products becomes fraught. It becomes increasingly complicated to assess the superiority or non-inferiority of new PrEP modalities when compared to control groups that have active comparators. For example, it is more difficult to discern the true efficacy of a new PrEP modality when the control arm allows for daily PrEP or on-demand PrEP. Novel trial design strategies, including counterfactual analyses that try to estimate hypothetical untreated/placebo incidence in the same or a similar population, will be paramount to the feasibility of studying new interventions. However, designs that are statistically sound, adaptive, and creative have yet to be accepted by regulatory agencies. It is important that there be widespread collaboration on such initiatives so as to ensure that promising prevention agents are evaluated quickly, and if appropriate, facilitated through regulatory and local approvals, implemented and scaled up as quickly as possible such that individuals who are at the most risk for HIV may benefit.
Research on new modalities of PrEP must integrate biomedical research and behavioral research to support conceptualization of processes required to move from awareness about the availability and efficacy of new PrEP formulations to uptake and persistence of these formulations. While long acting formulations of PrEP stand to address some of these issues, decision-making about whether to use these new PrEP modalities will depend on individual-level, interpersonal-level, community-level, and structural-level factors that must be considered in future research.
Acknowledgments
Funding
MRB was supported by the UCLA Postdoctoral Fellowship Training Program in Global HIV Prevention Research (Currier and Gorbach, PIs); T32MH080634.
IWH and RJL were supported by the Center for HIV Identification, Prevention, and Treatment (CHIPTS; NIMH grant MH58107) and the UCLA Center for AIDS Research (CFAR; NIAID grant AI028697)
IWH was supported by California HIV/AIDS Research Program (CHRP; grant RP15-LA-007) and the National Center for Advancing Translational Sciences through UCLA (CSTI; grant UL1TR000124). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
RJL has received research grants from, and served as a consultant to Gilead Sciences; and served as a consultant to Merck and Co, Inc.
Footnotes
Conflicts of Interest
MRB, IWH, and CP declare that they have no conflict of interest.
Human and Animal rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors
REFERENCES
- 1.Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV et al. Emtricitabine-Tenofovir Concentrations and Pre-Exposure Prophylaxis Efficacy in Men Who Have Sex with Men. Science Translational Medicine 2012;4(151). doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J et al. Antiretroviral Prophylaxis for HIV Prevention in Heterosexual Men and Women. N Engl J Med 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United States Public Health Service. Preexposure Prophylaxis for the Prevention of HIV Infection in the United States 2014. https://www.cdc.gov/hiv/pdf/prepguidelines2014.pdf Accessed January 26, 2017 2017.
- 4.Hosek SG, Landovitz RJ, Kapogiannis B, Siberry GK, Rudy B, Rutledge B et al. Safety and Feasibility of Antiretroviral Preexposure Prophylaxis for Adolescent Men Who Have Sex With Men Aged 15 to 17 Years in the United States. JAMA Pediatr 2017;171(11):1063–71. doi: 10.1001/jamapediatrics.2017.2007.• This study was responsible for expanding the TDF/FTC PrEP indication down to include youth (weight of 35 kg or more) by the U.S. Food and Drug Administration.
- 5.AIDS Vaccine Advocacy Coalition (AVAC). Regulatory status of Truvada for PrEP 2019. [Google Scholar]
- 6.Smith DK, Van Handel M, Huggins R. Estimated Coverage to Address Financial Barriers to HIV Preexposure Prophylaxis Among Persons With Indications for Its Use, United States, 2015. J Acquir Immune Defic Syndr 2017;76(5):465–72. doi: 10.1097/QAI.0000000000001532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegler A, Mouhanna F, Giler R Mera, Weiss KM, Pembleton E, Guest JL et al. The prevalence of PrEP use and the PrEP-to-need ratio in the fourth quarter of 2017, United States. Annals of Epidemiology 2018. doi: 10.1016/j.annepidem.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson PL, Reirden D, Castillo-Mancilla J. Pharmacologic Considerations for Preexposure Prophylaxis in Transgender Women. J Acquir Immune Defic Syndr 2016;72 Suppl 3:S230–4. DOI: 10.1097/QAI.0000000000001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gandhi M, Glidden DV, Mayer K, Schechter M, Buchbinder S, Grinsztejn B, et al. Association of age, baseline kidney function, and medication exposure with declines in creatinine clearance on pre-exposure prophylaxis: an observational cohort study. Lancet HIV 2016;3(11):e521–e8. DOI: 10.1016/S2352-3018(16)30153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solomon MM, Lama JR, Glidden DV, Mulligan K, McMahan V, Liu AY, et al. Changes in renal function associated with oral emtricitabine/tenofovir disoproxil fumarate use for HIV pre-exposure prophylaxis. AIDS 2014;28(6):851–9. DOI: 10.1097/QAD.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glidden DV, Mulligan K, McMahan V, Anderson PL, Guanira J, Chariyalertsak S, et al. Brief Report: Recovery of Bone Mineral Density After Discontinuation of Tenofovir-Based HIV Pre-exposure Prophylaxis. J Acquir Immune Defic Syndr 2017;76(2):177–82. DOI: 10.1097/QAI.0000000000001475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcus JL, Hurley LB, Hare CB, Nguyen DP, Phengrasamy T, Silverberg MJ, et al. Preexposure Prophylaxis for HIV Prevention in a Large Integrated Health Care System: Adherence, Renal Safety, and Discontinuation. Jaids-Journal of Acquired Immune Deficiency Syndromes 2016;73(5):540–6. DOI: 10.1097/QAI.0000000000001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Institute of Medicine (US) Committee on a Comprehensive Review of the HHS Office of Family Planning Title X Program; Stith Butler A, Wright Clayton E, editors. A Review of the HHS Family Planning Program: Mission, Management, and Measurement of Results Washington (DC): National Academies Press (US); 2009. 2, Overview of Family Planning in the United States; Available from: https://www.ncbi.nlm.nih.gov/books/NBK215219/ [PubMed] [Google Scholar]
- 14.Molina JM, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I et al. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. N Engl J Med 2015;373(23):2237–46. doi: 10.1056/NEJMoa1506273.• This was the first study to show that on-demand PrEP (2–1-1 dosing strategy for MSM) is efficacious for preventing HIV among MSM.
- 15.Antoni G, Tremblay C, Charreau I, Cua E, Rojas-Castro D, Hall N et al. On-demand PrEP with TDF/FTC remains highly effective among MSM with infrequent sexual intercourse: a sub-study of the ANRS IPERGAY trial International AIDS Society; 2017; Paris, France. [Google Scholar]
- 16.Molina JM, Charreau I, Spire B, Cotte L, Chas J, Capitant C et al. Efficacy, safety, and effect on sexual behaviour of on-demand pre-exposure prophylaxis for HIV in men who have sex with men: an observational cohort study. Lancet HIV 2017;4(9):e402–e10. doi: 10.1016/S2352-3018(17)30089-9. [DOI] [PubMed] [Google Scholar]
- 17.Seifert SM, Glidden DV, Meditz AL, Castillo-Mancilla JR, Gardner EM, Predhomme JA et al. Dose response for starting and stopping HIV preexposure prophylaxis for men who have sex with men. Clin Infect Dis 2015;60(5):804–10. doi: 10.1093/cid/ciu916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cottrell ML, Yang KH, Prince HM, Sykes C, White N, Malone S et al. A Translational Pharmacology Approach to Predicting Outcomes of Preexposure Prophylaxis Against HIV in Men and Women Using Tenofovir Disoproxil Fumarate With or Without Emtricitabine. J Infect Dis 2016;214(1):55–64. doi: 10.1093/infdis/jiw077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.United States Public Health Service. Preexposure Prophylaxis for the Prevention of HIV Infection in the United States - 2017 Update 2017. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf.
- 20.Grant RM, Mannheimer S, Hughes JP, Hirsch-Moverman Y, Loquere A, Chitwarakorn A et al. Daily and Nondaily Oral Preexposure Prophylaxis in Men and Transgender Women Who Have Sex With Men: The Human Immunodeficiency Virus Prevention Trials Network 067/ADAPT Study. Clin Infect Dis 2018;66(11):1712–21. doi: 10.1093/cid/cix1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bekker LG, Roux S, Sebastien E, Yola N, Amico KR, Hughes JP et al. Daily and non-daily pre-exposure prophylaxis in African women (HPTN 067/ADAPT Cape Town Trial): a randomised, open-label, phase 2 trial. Lancet HIV 2018;5(2):e68–e78. doi: 10.1016/S2352-3018(17)30156-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parsons JT, Rendina HJ, Grov C, Ventuneac A, Mustanski B. Accuracy of highly sexually active gay and bisexual men’s predictions of their daily likelihood of anal sex and its relevance for intermittent event-driven HIV pre-exposure prophylaxis. J Acquir Immune Defic Syndr 2015;68(4):449–55. doi: 10.1097/QAI.0000000000000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massud I, Mitchell J, Babusis D, Deyounks F, Ray AS, Rooney JF et al. Chemoprophylaxis With Oral Emtricitabine and Tenofovir Alafenamide Combination Protects Macaques From Rectal Simian/Human Immunodeficiency Virus Infection. J Infect Dis 2016;214(7):1058–62. doi: 10.1093/infdis/jiw312. [DOI] [PubMed] [Google Scholar]
- 24.Massud I, Cong ME, Ruone S, Mitchell J, Deyounks F, Holder A et al. Oral FTC/TAF Combination Prevents Vaginal SHIV Infection in Pigtail Macaques. Conference on Retroviruses and Opportunistic Infections; Boston, Massachusetts2018. [Google Scholar]
- 25.Cottrell ML, Garrett KL, Prince HMA, Sykes C, Schauer A, Emerson CW et al. Single-dose pharmacokinetics of tenofovir alafenamide and its active metabolite in the mucosal tissues. J Antimicrob Chemother 2017;72(6):1731–40. doi: 10.1093/jac/dkx064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hare CB, Coll J, Ruane P,Molina J, Mayer KH, Jessen H, et al. The Phase 3 Discover Study: Daily F/TAF Or F/TDF For HIV Preexposure Prophylaxis. Conference on Retroviruses and Opportunistic Infections; Seattle, Washington; 2019. [Google Scholar]
- 27.Denton PW, Garcia JV. Mucosal HIV-1 transmission and prevention strategies in BLT humanized mice. Trends Microbiol 2012;20(6):268–74. doi: 10.1016/j.tim.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGowan I, Dezzutti CS, Siegel A, Engstrom J, Nikiforov A, Duffill K et al. Long-acting rilpivirine as potential pre-exposure prophylaxis for HIV-1 prevention (the MWRI-01 study): an open-label, phase 1, compartmental, pharmacokinetic and pharmacodynamic assessment. Lancet HIV 2016;3(12):e569–e78. doi: 10.1016/S2352-3018(16)30113-8. [DOI] [PubMed] [Google Scholar]
- 29.Jackson AG, Else LJ, Mesquita PM, Egan D, Back DJ, Karolia Z et al. A compartmental pharmacokinetic evaluation of long-acting rilpivirine in HIV-negative volunteers for pre-exposure prophylaxis. Clin Pharmacol Ther 2014;96(3):314–23. doi: 10.1038/clpt.2014.118. [DOI] [PubMed] [Google Scholar]
- 30.Walensky RP, Jacobsen MM, Bekker LG, Parker RA, Wood R, Resch SC et al. Potential Clinical and Economic Value of Long-Acting Preexposure Prophylaxis for South African Women at High-Risk for HIV Infection. J Infect Dis 2016;213(10):1523–31. doi: 10.1093/infdis/jiv523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bekker LG, Li SS, Tolley B, Marzinke M, Mgodi NM, Justman J et al. HPTN 076: TMC278 LA Safe, Tolerable, and Acceptable for HIV Preexposure Prophylaxis. Conference on Retroviruses and Opportunistic Infections; Seattle, Washington2017. [Google Scholar]
- 32.Sista N, Li SS, Marzinke M, Mgodi NM, Justman J, Swaminathan S et al. HPTN 076: safety and pharmacokinetics of rilpivirine LA through week 76 in HIV-uninfected women International AIDS Society; Paris, France: 2017. [Google Scholar]
- 33.Radzio J, Spreen W, Yueh YL, Mitchell J, Jenkins L, Garcia-Lerma JG et al. The long-acting integrase inhibitor GSK744 protects macaques from repeated intravaginal SHIV challenge. Sci Transl Med 2015;7(270):270ra5. doi: 10.1126/scitranslmed.3010297. [DOI] [PubMed] [Google Scholar]
- 34.Andrews CD, Spreen WR, Mohri H, Moss L, Ford S, Gettie A et al. Long-acting integrase inhibitor protects macaques from intrarectal simian/human immunodeficiency virus. Science 2014;343(6175):1151–4. doi: 10.1126/science.1248707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dobard C, Makarova N, Nishiura K, Sterling M, Dinh C, Mitchell J et al. Long-Acting Cabotegravir Protects Macaques against Repeated Penile SHIV Exposures. Conference on Retroviruses and Opportunistic Infections; Boston, Massachusetts2018. [Google Scholar]
- 36.Andrews CD, Bernard LS, Poon AY, Mohri H, Gettie N, Spreen WR et al. Cabotegravir long acting injection protects macaques against intravenous challenge with SIVmac251. AIDS 2017;31(4):461–7. doi: 10.1097/QAD.0000000000001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spreen W, Ford SL, Chen S, Wilfret D, Margolis D, Gould E et al. GSK1265744 pharmacokinetics in plasma and tissue after single-dose long-acting injectable administration in healthy subjects. J Acquir Immune Defic Syndr 2014;67(5):481–6. doi: 10.1097/QAI.0000000000000301. [DOI] [PubMed] [Google Scholar]
- 38.Spreen W, Williams P, Margolis D, Ford SL, Crauwels H, Lou Y et al. Pharmacokinetics, safety, and tolerability with repeat doses of GSK1265744 and rilpivirine (TMC278) long-acting nanosuspensions in healthy adults. J Acquir Immune Defic Syndr 2014;67(5):487–92. doi: 10.1097/QAI.0000000000000365. [DOI] [PubMed] [Google Scholar]
- 39.Meyers K, Rodriguez K, Brill AL, Wu Y, La Mar M, Dunbar D et al. Lessons for Patient Education Around Long-Acting Injectable PrEP: Findings from a Mixed-Method Study of Phase II Trial Participants. AIDS Behav 2018;22(4):1209–16. doi: 10.1007/s10461-017-1871-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Markowitz M, Frank I, Grant RM, Mayer KH, Elion R, Goldstein D et al. Safety and tolerability of long-acting cabotegravir injections in HIV-uninfected men (ECLAIR): a multicentre, double-blind, randomised, placebo-controlled, phase 2a trial. Lancet HIV 2017;4(8):e331–e40. doi: 10.1016/S2352-3018(17)30068-1. [DOI] [PubMed] [Google Scholar]
- 41.Landovitz R, Li S, Grinsztejn B, Dawood H, Liu A, Magnus M et al. Safety, tolerability and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected women and men: HPTN 077 International AIDS Society; Paris, France: 2017.• This study showed that injectable cabotegravir as PrEP administered every eight weeks met pharmocokinetic targets and was well tolerated.
- 42.Landovitz RJ, Kofron R, McCauley M. The promise and pitfalls of long-acting injectable agents for HIV prevention. Current Opinion in Hiv and Aids 2016;11(1):122–8. doi: 10.1097/coh.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parikh UM, Dobard C, Sharma S, Cong ME, Jia H, Martin A et al. Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. J Virol 2009;83(20):10358–65. doi: 10.1128/JVI.01073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayer KH, Maslankowski LA, Gai F, El-Sadr WM, Justman J, Kwiecien A et al. Safety and tolerability of tenofovir vaginal gel in abstinent and sexually active HIV-infected and uninfected women. AIDS 2006;20(4):543–51. doi: 10.1097/01.aids.0000210608.70762.c3. [DOI] [PubMed] [Google Scholar]
- 45.Karim Q Abdool, Karim SS Abdool, Frohlich JA, Grobler AC, Baxter C, Mansoor LE et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010;329(5996):1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rees H D-MS, Lombard C. FACTS 001 Phase III Trial of Pericoital Tenofovir 1% Gel for HIV Prevention in Women. Conference on Retroviruses and Opportunistic Infections; February 23–26, 2015; Seattle, Washington2015. [Google Scholar]
- 47.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G et al. Tenofovir-Based Preexposure Prophylaxis for HIV Infection among African Women. N Engl J Med 2015;372(6):509–18. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cranston RD, Lama JR, Richardson BA, Carballo-Diéguez A, Na Ayudhya RP Kunjara, Liu K, et al. MTN-017: A Rectal Phase 2 Extended Safety and Acceptability Study of Tenofovir Reduced-Glycerin 1% Gel. Clin Infect Dis 2017;64(5):614–20. doi: 10.1093/cid/ciw832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carballo-Diéguez A, Balán IC, Brown W, Giguere R, Dolezal C, Leu CS, et al. High levels of adherence to a rectal microbicide gel and to oral Pre-Exposure Prophylaxis (PrEP) achieved in MTN-017 among men who have sex with men (MSM) and transgender women. PLoS One 2017;12(7):e0181607. doi: 10.1371/journal.pone.0181607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nel A, Haazen W, Nuttall J, Romano J, Rosenberg Z, van Niekerk N. A safety and pharmacokinetic trial assessing delivery of dapivirine from a vaginal ring in healthy women. AIDS 2014;28(10):1479–87. doi: 10.1097/QAD.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 51.Chen BA, Panther L, Marzinke MA, Hendrix CW, Hoesley CJ, van der Straten A et al. Phase 1 Safety, Pharmacokinetics, and Pharmacodynamics of Dapivirine and Maraviroc Vaginal Rings: A Double-Blind Randomized Trial. J Acquir Immune Defic Syndr 2015;70(3):242–9. doi: 10.1097/QAI.0000000000000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van der Straten A, Panther L, Laborde N, Hoesley CJ, Cheng H, Husnik MJ et al. Adherence and Acceptability of a Multidrug Vaginal Ring for HIV Prevention in a Phase I Study in the United States. AIDS Behav 2016;20(11):2644–53. doi: 10.1007/s10461-016-1299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Romano J, Variano B, Coplan P, Van Roey J, Douville K, Rosenberg Z et al. Safety and availability of dapivirine (TMC120) delivered from an intravaginal ring. AIDS Res Hum Retroviruses 2009;25(5):483–8. doi: 10.1089/aid.2008.0184. [DOI] [PubMed] [Google Scholar]
- 54.Nel A, Bekker LG, Bukusi E, Hellstrm E, Kotze P, Louw C et al. Safety, Acceptability and Adherence of Dapivirine Vaginal Ring in a Microbicide Clinical Trial Conducted in Multiple Countries in Sub-Saharan Africa. PLoS One 2016;11(3):e0147743. doi: 10.1371/journal.pone.0147743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V et al. Use of a Vaginal Ring Containing Dapivirine for HIV-1 Prevention in Women. N Engl J Med 2016;375(22):2121–32. doi: 10.1056/NEJMoa1506110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nel A, van Niekerk N, Kapiga S, Bekker LG, Gama C, Gill K et al. Safety and Efficacy of a Dapivirine Vaginal Ring for HIV Prevention in Women. N Engl J Med 2016;375(22):2133–43. doi: 10.1056/NEJMoa1602046. [DOI] [PubMed] [Google Scholar]
- 57.Baeten JM, Palanee T, Mgodi NM, Mayo A, Nel A, Rosenberg Z et al. High Uptake and Reduced HIV-1 Incidence in an Open-Label Trial of the Dapivirine Ring. Conference on Retroviruses and Opportunistic Infections; Boston, Massachusetts2018. [Google Scholar]
- 58.Nel A, Van Niekerk N, Van Baelen B, Rosenberg Z. HIV Incidence and Adherence in Dream: An Open-Label Trial of Dapivirine Vaginal Ring. Conference on Retroviruses and Opportunistic Infections; Boston, Massachusetts2018. [Google Scholar]
- 59.Derby N, Aravantinou M, Kenney J, Ugaonkar SR, Wesenberg A, Wilk J et al. An intravaginal ring that releases three antiviral agents and a contraceptive blocks SHIV-RT infection, reduces HSV-2 shedding, and suppresses hormonal cycling in rhesus macaques. Drug Deliv Transl Res 2017;7(6):840–58. doi: 10.1007/s13346-017-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith JM, Moss JA, Srinivasan P, Butkyavichene I, Gunawardana M, Fanter R et al. Novel multipurpose pod-intravaginal ring for the prevention of HIV, HSV, and unintended pregnancy: Pharmacokinetic evaluation in a macaque model. PLoS One 2017;12(10):e0185946. doi: 10.1371/journal.pone.0185946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akil A, Parniak MA, Dezzuitti CS, Moncla BJ, Cost MR, Li M et al. Development and Characterization of a Vaginal Film Containing Dapivirine, a Non- nucleoside Reverse Transcriptase Inhibitor (NNRTI), for prevention of HIV-1 sexual transmission. Drug Deliv Transl Res 2011;1(3):209–22. doi: 10.1007/s13346-011-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Machado A, Cunha-Reis C, Araujo F, Nunes R, Seabra V, Ferreira D et al. Development and in vivo safety assessment of tenofovir-loaded nanoparticles-in-film as a novel vaginal microbicide delivery system. Acta Biomater 2016;44:332–40. doi: 10.1016/j.actbio.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 63.Cunha-Reis C, Machado A, Barreiros L, Araujo F, Nunes R, Seabra V et al. Nanoparticles-in-film for the combined vaginal delivery of anti-HIV microbicide drugs. J Control Release 2016;243:43–53. doi: 10.1016/j.jconrel.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 64.Bunge KE, Dezzutti CS, Rohan LC, Hendrix CW, Marzinke MA, Richardson-Harman N et al. A Phase 1 Trial to Assess the Safety, Acceptability, Pharmacokinetics, and Pharmacodynamics of a Novel Dapivirine Vaginal Film. J Acquir Immune Defic Syndr 2016;71(5):498–505. doi: 10.1097/QAI.0000000000000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robinson JA, Marzinke MA, Bakshi RP, Fuchs EJ, Radebaugh CL, Aung W et al. Comparison of Dapivirine Vaginal Gel and Film Formulation Pharmacokinetics and Pharmacodynamics (FAME 02B). AIDS Res Hum Retroviruses 2017;33(4):339–46. doi: 10.1089/AID.2016.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bunge KE, Dezzutti CS, Hendrix C, Marzinke MA, Spiegel H, Moncla B et al. Phase I Trial to Assess Safety, PK, and PD of Film and Gel Formulations of Tenofovir. Conference on Retroviruses and Opportunistic Infections; Boston2016. [Google Scholar]
- 67.Robinson JA, Marzinke MA, Fuchs EJ, Bakshi RP, Spiegel HML, Coleman JS et al. Comparison of the Pharmacokinetics and Pharmacodynamics of Single-Dose Tenofovir Vaginal Film and Gel Formulation (FAME 05). J Acquir Immune Defic Syndr 2018;77(2):175–82. doi: 10.1097/QAI.0000000000001587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Donnelly RF, Larraneta E. Microarray patches: potentially useful delivery systems for long-acting nanosuspensions. Drug Discov Today 2018;23(5):1026–33. doi: 10.1016/j.drudis.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 69.Arnou R, Eavis P, Pardo JR, Ambrozaitis A, Kazek MP, Weber F. Immunogenicity, large scale safety and lot consistency of an intradermal influenza vaccine in adults aged 18–60 years: Randomized, controlled, phase III trial. Hum Vaccin 2010;6(4):346–54. [DOI] [PubMed] [Google Scholar]
- 70.MCCrudden M Two-step casting of dissolving MN arrays for use in the sustained release of rilpivirine for HIV pre-exposure prophylaxis. Proc. 43rd Annu. Meet. Controlled Release Soc2016. [Google Scholar]
- 71.Gunawardana M, Remedios-Chan M, Miller CS, Fanter R, Yang F, Marzinke MA et al. Pharmacokinetics of long-acting tenofovir alafenamide (GS-7340) subdermal implant for HIV prophylaxis. Antimicrob Agents Chemother 2015;59(7):3913–9. doi: 10.1128/AAC.00656-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prathipati PK, Mandal S, Pon G, Vivekanandan R, Destache CJ. Pharmacokinetic and Tissue Distribution Profile of Long Acting Tenofovir Alafenamide and Elvitegravir Loaded Nanoparticles in Humanized Mice Model. Pharm Res 2017;34(12):2749–55. doi: 10.1007/s11095-017-2255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gatto G, Girouard N, Brand RM, Johnson LM, Marzinke M, Sudie R et al. Pharmacokinetics of Tenofovir Alafenamide by Subcutaneous Implant for HIV PrEP. Conference on Retroviruses and Opportunistic Infections; Boston, Massachusetts2018. [Google Scholar]
- 74.Gautam R, Nishimura Y, Pegu A, Nason MC, Klein F, Gazumyan A et al. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature 2016;533(7601):105–9. doi: 10.1038/nature17677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Julg B, Tartaglia LJ, Keele BF, Wagh K, Pegu A, Sok D et al. Broadly neutralizing antibodies targeting the HIV-1 envelope V2 apex confer protection against a clade C SHIV challenge. Sci Transl Med 2017;9(406). doi: 10.1126/scitranslmed.aal1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rosenberg YJ, Montefiori DC, LaBranche CC, Lewis MG, Sack M, Lees JP et al. Protection against SHIV Challenge by Subcutaneous Administration of the Plant-Derived PGT121 Broadly Neutralizing Antibody in Macaques. PLoS One 2016;11(3):e0152760. doi: 10.1371/journal.pone.0152760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pardi N, Secreto AJ, Shan X, Debonera F, Glover J, Yi Y et al. Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge. Nat Commun 2017;8:14630. doi: 10.1038/ncomms14630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ledgerwood JE, Coates EE, Yamshchikov G, Saunders JG, Holman L, Enama ME et al. Safety, pharmacokinetics and neutralization of the broadly neutralizing HIV-1 human monoclonal antibody VRC01 in healthy adults. Clin Exp Immunol 2015;182(3):289–301. doi: 10.1111/cei.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mayer KH, Seaton KE, Huang Y, Grunenberg N, Isaacs A, Allen M et al. Safety, pharmacokinetics, and immunological activities of multiple intravenous or subcutaneous doses of an anti-HIV monoclonal antibody, VRC01, administered to HIV-uninfected adults: Results of a phase 1 randomized trial. PLoS Med 2017;14(11):e1002435. doi: 10.1371/journal.pmed.1002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gaudinski MR, Coates EE, Houser KV, Chen GL, Yamshchikov G, Saunders JG et al. Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: A Phase 1 open-label clinical trial in healthy adults. PLoS Med 2018;15(1):e1002493. doi: 10.1371/journal.pmed.1002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tyo KM, Vuong HR, Malik DA, Sims LB, Alatassi H, Duan J et al. Multipurpose tenofovir disoproxil fumarate electrospun fibers for the prevention of HIV-1 and HSV-2 infections in vitro. Int J Pharm 2017;531(1):118–33. doi: 10.1016/j.ijpharm.2017.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grooms TN, Vuong HR, Tyo KM, Malik DA, Sims LB, Whittington CP et al. Griffithsin-Modified Electrospun Fibers as a Delivery Scaffold To Prevent HIV Infection. Antimicrob Agents Chemother 2016;60(11):6518–31. doi: 10.1128/AAC.00956-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ball C, Chou SF, Jiang Y, Woodrow KA. Coaxially electrospun fiber-based microbicides facilitate broadly tunable release of maraviroc. Mater Sci Eng C Mater Biol Appl 2016;63:117–24. doi: 10.1016/j.msec.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ball C, Woodrow KA. Electrospun solid dispersions of Maraviroc for rapid intravaginal preexposure prophylaxis of HIV. Antimicrob Agents Chemother 2014;58(8):4855–65. doi: 10.1128/AAC.02564-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krogstad EA, Woodrow KA. Manufacturing scale-up of electrospun poly(vinyl alcohol) fibers containing tenofovir for vaginal drug delivery. Int J Pharm 2014;475(1–2):282–91. doi: 10.1016/j.ijpharm.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carson D, Jiang Y, Woodrow KA. Tunable Release of Multiclass Anti-HIV Drugs that are Water-Soluble and Loaded at High Drug Content in Polyester Blended Electrospun Fibers. Pharm Res 2016;33(1):125–36. doi: 10.1007/s11095-015-1769-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hoang T, Date AA, Ortiz J Ortiz, Young TW, Bensouda S, Xiao P et al. Development of rectal enema as microbicide (DREAM): Preclinical progressive selection of a tenofovir prodrug enema. Eur J Pharm Biopharm 2018. doi: 10.1016/j.ejpb.2018.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xiao P, Gumber S, Marzinke M, Date AA, Hoang T, Hanes J et al. Hypo-osmolar Rectal Enema TFV Formulation Prevents SHIV Acquisition. Conference on Retroviruses and Opportunistic Infections; Boston, USA2018. [Google Scholar]
- 89.Khan AB, Thakur RS. Design and evaluation of mucoadhesive vaginal tablets of tenofovir disoproxil fumarate for pre-exposure prophylaxis of HIV. Drug Dev Ind Pharm 2018;44(3):472–83. doi: 10.1080/03639045.2017.1399272. [DOI] [PubMed] [Google Scholar]
- 90.Schlesinger E, Johengen D, Luecke E, Rothrock G, McGowan I, van der Straten A et al. A Tunable, Biodegradable, Thin-Film Polymer Device as a Long-Acting Implant Delivering Tenofovir Alafenamide Fumarate for HIV Pre-exposure Prophylaxis. Pharm Res 2016;33(7):1649–56. doi: 10.1007/s11095-016-1904-6. [DOI] [PMC free article] [PubMed] [Google Scholar]