Abstract
Objectives:
To assess the safety of carvedilol among heart failure (HF) patients with a cocaine-use disorder (CUD).
Background:
Although carvedilol is recommended among certain patients with heart failure (HF), the safety and efficacy of carvedilol among HF patients with a CUD is unknown.
Methods:
This was a single center study of patients with HF hospitalization. Cocaine use was self-reported or defined as having a positive urine toxicology. Patients were stratified by carvedilol prescription. Subgroup analyses were performed by strata of ejection fraction (EF) (≤40%, 41-49%, ≥50%). Major adverse cardiovascular events (MACE) was defined as CV mortality and 30-day HF readmission.
Results:
From a cohort of 2,578 patients hospitalized with HF in 2011, 503 patients with a CUD were identified, among whom 404 (80%) were prescribed carvedilol and 99 (20%) were not. Both groups had similar characteristics; however, those prescribed carvedilol had a lower LVEF, heart rate, admission and discharge NT-proBNP and more coronary artery disease. Over a median follow-up of 19 months, there were 169 MACE events. MACE was similar between the carvedilol and the non-carvedilol groups (32 vs. 38%, p=0.16), among those with a preserved EF (30 vs. 33%, p=0.48) and was lower among those with a reduced EF on carvedilol (34 vs. 58%, p=0.02). In a multivariate model, carvedilol use was associated with lower MACE among HF patients with a CUD (HR=0.67, CI=0.481–0.863).
Conclusions:
Our findings suggest that carvedilol is safe among HF patients with a CUD and may be effective among those with a reduced EF.
Keywords: Beta-blockers, Cocaine-use disorder, Heart Failure
Introduction
Data from the National Survey on Drug Use and Health have shown that that over 5 million people in the United States have a Cocaine-use disorder (CUD), making it one of the most commonly abused substances in the United States. (1) Cocaine, via effects on norepinephrine and dopamine blockade, may have a myriad of adverse cardiovascular effects, principally from augmented sympathetic stimulation. (2) In the late 1970’s, propranolol, a selective beta-blocker, was suggested as a treatment for cocaine toxicity. (3–5) However, animal models of cocaine exposure suggested reduced survival and this approach was subsequently abandoned. This reduced survival with beta-blockers (BB) was thought to be related to the unopposed alpha-adrenergic activity seen with administration of a selective beta-antagonist. (6–8) Use of non-cardioselective beta-blockers that also offer alpha-adrenergic blocking activity, such as labetalol and carvedilol were previously contraindicated in the management of myocardial infarction and NSTE-ACS (9); currently, they may be used but remain a class IIB indication among individuals with a history of cocaine use presenting with non-ST elevation acute coronary syndrome (NSTE-ACS) and unstable angina. (10) The use of beta-blockers is strongly recommended among patients with heart failure (HF) with a reduced ejection fraction. (11) However, current HF guidelines, citing a lack of data, note that the safety and efficacy of beta-blockers among individuals with recent or active cocaine use is unclear. (11, 12) However, there are pharmacological differences between beta-blockers approved for heart failure and non-cardioselective beta-blockers may be safe among patients with a CUD. Therefore, the aim of this study was to address this knowledge gap on the use of non-cardioselective beta-blockers, specifically carvedilol, among individuals with HF with a CUD. We leveraged a large single center HF registry from a tertiary care center with a high background prevalence of a CUD to test our hypothesis.
Methods
After obtaining Institutional Board Review (IRB) approval, we created a prospective observational registry of all patients admitted to a US tertiary care hospital (Bronx-Lebanon Hospital Center of Icahn School of Medicine at Mount Sinai, Bronx, New York) in 2011 with HF. Full details of the entire cohort have previously been reported. (13–15) The use of cocaine, diagnoses of HF, as well as other clinically relevant variables, were ascertained through manual review of each of the individual electronic health records (EHR).
Covariates
Data on traditional HF risk factors (including hypertension, dyslipidemia, diabetes mellitus, coronary artery disease (CAD), body mass index (BMI), prior or active cigarette smoking, and prior or active cocaine use, mode of cocaine use) as well as education, employment history, left ventricular ejection fraction (LVEF), electrocardiogram (ECG) variables, history of sleep apnea and medication use were collected from the index HF hospitalization by EHR review. LVEF was used to categorize HF as HF with reduced ejection fraction (HFrEF, LVEF ≤ 40%), HF with mid-range ejection fraction (HFmrEF, LVEF 41-49%), and HF with preserved ejection fraction (HFpEF, LVEF ≥ 50%) in keeping with international guidelines (11, 12). In addition, data were collected on laboratory parameters such as serum creatinine and NT-proBNP on admission and at discharge, in addition to clinical parameters including systolic blood pressure (SBP), diastolic blood pressure (DBP) and non-invasive derivation of pulmonary artery systolic pressure (PASP) from echocardiography. Based on standardized criteria, cocaine use was self-reported or defined as having a positive urine toxicology. (16–19) Based on self-report, the frequency of cocaine use was stratified into active users (those using it at least once a week), monthly users (not exceeding once a month) and occasional users (those reporting use infrequently/once a year). (20) Monthly or occasional users were classified as prior users. The mode of administration was classified as intranasal, smoking or intravenous. (21) The types of cocaine were described as either crack, pure cocaine or a combination of cocaine mixed with a non-cocaine drug. (1, 22). Based on local hospital practices, carvedilol was the only beta-blocker administered to individuals with HF with CUD.
Outcomes
The follow-up period began on the date of discharge from the first HF hospitalization in 2011. Our primary outcome was the occurrence of major adverse cardiovascular events (MACE), which was a composite of CV mortality and 30-day heart failure readmission. Death was determined through Social Security death index (SSDI) and cause of death was confirmed by physician-adjudicated individual EHR review.
Statistical Analysis
Continuous variables are presented as mean and SD or median (IQR), as appropriate based on normality, and categorical variables are presented as percentages. Continuous data were compared with the use of unpaired Student t tests or Wilcoxon rank-sum tests, as appropriate. Categorical data were compared using the chi-square or the Fisher exact test. Survival curves were plotted using Kaplan-Meier curves. Univariate and adjusted multivariate regression analyses using Cox proportional hazard regression were performed to determine the association between covariates and the occurrence of MACE. Statistical significance was defined using a two-tailed p-value <0.05. Statistical analyses were performed using SPSS software version 24.
Results
Demographics and Baseline Characteristics:
There were 2,578 patients hospitalized with acute decompensated HF over a single academic year. From these, 503 (20.0%) individuals were defined as either active (n=348, 69%) or with a prior CUD (n=155, 31%) (monthly or occasionally). Among those with a CUD, 404 (80%) were prescribed carvedilol while 99 (20%) were not on any beta-blocker. Baseline demographics are shown in Table 1. Compared to individuals not on carvedilol, those who were on carvedilol had a lower EF (40±12 vs. 44±12%, p = 0.003), heart rate (75±21 vs. 83±21 bpm, p < 0.001) and NT-proBNP on admission (median 4121 pg/mL, IQR 2421-7857 vs. median 4489, IQR 2674-8208, p = 0.041) as well as NT-proBNP at discharge (median 2364, IQR 1107-5052 vs. median 2711, IQR 1227-5476, p=0.033). CAD was more prevalent among those on carvedilol compared to those not on carvedilol (39 vs. 26%, p = 0.046); otherwise, there were no significant differences between groups with respect to age, race, cardiovascular risk factors, pulmonary artery systolic pressure (PASP), blood pressure, ECG parameters, renal function, body mass index (BMI), prevalence of sleep apnea, New York Heart Association (NYHA) heart failure class, education, unemployment or other medications. The study cohort was also stratified based on frequency of cocaine use (weekly, monthly or occasionally), mode of administration (intranasal, smoking or intravenous) as well as type of cocaine (crack, cocaine alone or a combination of cocaine with a non-cocaine drug) (Table 2). There were no significant differences noted between carvedilol cohorts.
Table 1:
Baseline characteristics comparing patients on carvedilol vs. not on carvedilol
| Total Cohort | Carvedilol | No carvedilol | p-value | |
|---|---|---|---|---|
| (n=503) | (n=404) | (n=99) | ||
| Females | 263 (52%) | 214 (53%) | 49 (49%) | 0.535 |
| Age (yrs, mean±SD) | 60±9.3 | 60±9.5 | 61±9.3 | |
| Race | ||||
| Hispanic | 197 (39%) | 157 (39%) | 40 (40%) | |
| African American | 203 (40%) | 165 (41%) | 38 (38%) | 0.199 |
| Others | 103 (20%) | 82 (20%) | 21 (21%) | |
| Socioeconomic parameters | ||||
| High School /GED completion | 315 (63%) | 251 (62%) | 64 (64%) | 0.643 |
| Unemployment | 62 (12%) | 52 (13%) | 10 (10%) | 0.452 |
| Cardiovascular risk factors | ||||
| Diabetes | 184 (37%) | 149 (37%) | 35 (35%) | 0.777 |
| Hypertension | 334 (66%) | 271 (67%) | 63 (63%) | 0.516 |
| Hyperlipidemia | 188 (37%) | 149 (37%) | 39 (39%) | 0.643 |
| Smoking | 233 (46%) | 189 (47%) | 44 (44%) | 0.676 |
| Sleep apnea | 99 (20%) | 81 (20%) | 18 (18%) | 0.675 |
| CAD | 175 (35%) | 149 (39%) | 26 (26%) | 0.046 |
| LVEF (%, mean±SD) | 42±12.0 | 40±12.0 | 44±12.2 | 0.003 |
| LVEF ≤ 40% | 230 (46%) | 211 (52%) | 19 (19%) | |
| LVEF 41-49 % | 94 (19%) | 72 (18%) | 22 (22%) | |
| LVEF ≥ 50% | 179 (36%) | 121 (30%) | 58 (59%) | |
| PASP (mmHg, mean±SD) | 42±9.0 | 43±9.2 | 42±9.0 | 0.331 |
| SBP (mmHg) | 137±27.2 | 135±27.5 | 139±27.3 | 0.195 |
| DBP (mmHg) | 78±18 | 78±18.2 | 79±17.8 | 0.623 |
| HR (bpm) | 80±21.4 | 75±21.3 | 83±21.7 | <0.001 |
| BMI (kg/m2, mean±SD) | 29±6.3 | 30±6.4 | 29±6.3 | 0.163 |
| QRS duration (ms) | 114±24.3 | 115±24.3 | 113±24.6 | 0.464 |
| QTc duration (ms) | 423±28.4 | 425±28.4 | 422±28.7 | 0.348 |
| Serum creatinine (mg/dL) | 1.28±1.0 | 1.29±1.0 | 1.27±1.2 | 0.864 |
| NT-proBNP at admission (pg/mL) | 4222 (2504-8118) | 4121 (2421-7857) | 4489 (2674-8208) | 0.041 |
| NT-proBNP on discharge (pg/mL) * | 2497 (1184-5212) | 2364 (1107-5052) | 2711 (1227-5476) | 0.033 |
| HF NYHA class | 0.308 | |||
| NYHA class 1-2 | 221 (44%) | 173 (43%) | 48 (48%) | |
| NYHA class 3-4 | 282 (56%) | 231 (57%) | 51 (51%) | |
| Medications | ||||
| ACE/ARB | 430 (85%) | 347 (86%) | 83 (83%) | 0.603 |
| Spironolactone | 71 (14%) | 61 (15%) | 10 (10%) | 0.201 |
| Furosemide | 382 (76%) | 307 (78%) | 75 (75%) | 0.961 |
BMI= body mass index, LVEF = left ventricular ejection fraction, PASP= pulmonary artery systolic pressure, CAD= coronary artery disease, ACE I= angiotensin converting enzyme inhibitor, ARB= angiotensin receptor blocker,
NT-proBNP on discharge available in 141 (28%) patients with a CUD.
Table 2:
Cocaine parameters: Comparing Frequency, Mode and Type of cocaine use among patients on carvedilol vs. those not on carvedilol
| Total cohort | Carvedilol | No carvedilol | p-value | |
|---|---|---|---|---|
| (n=503) | (n=404) | (n=99) | ||
| Self-reported frequency of cocaine use | ||||
| Active user (≥once a week) | 348 (69%) | 274 (68%) | 74 (74%) | |
| Once a month | 93 (19%) | 78 (19%) | 15 (15%) | 0.409 |
| Once a year/occasionally | 62 (12%) | 52 (13%) | 10 (10%) | |
| Mode of cocaine administration | ||||
| Intranasal | 148 (29%) | 117 (29%) | 31 (31%) | |
| Smoking | 220 (44%) | 178 (44%) | 42 (42%) | 0.214 |
| Intravenous | 135 (27%) | 109 (27%) | 26 (26%) | |
| Cocaine type | ||||
| Crack | 220 (44%) | 178 (44%) | 42 (42%) | |
| Cocaine alone | 185 (37%) | 149 (37%) | 36 (36%) | 0.244 |
| Combination (Cocaine + non-cocaine drug) | 98 (19%) | 77 (19%) | 21 (21%) | |
Stratification based on category of HF
Among the 503 individuals with HF with a CUD, 230 (46%) were categorized as HFrEF, 94 (19%) as HFmrEF, and 179 (36%) as HFpEF. Carvedilol was prescribed for 211 (92%) of HFrEF cohort, 72 (77%) of HFmrEF cohort, and 121 (68%) of HFpEF cohort.
Outcomes
Over a median follow-up of 19 months, there were 169 MACE events. Factors associated with MACE on univariate analysis included a history of CAD, lower EF, increased PASP, increased NT-proBNP, lower prescription of carvedilol and ACE I/ARB, in addition to socioeconomic parameters such as low education level and unemployment (Table 4). In a multivariable model, the following parameters remained independent predictors of MACE among cocaine users with HF: history of CAD, lower EF, elevated PASP, higher NT-proBNP, lower education level, unemployment and lower use of ACE I/ARB and beta blockers (Table 5).
Table 4:
Univariate analysis testing the covariates associated with MACE among those with a CUD with HF
| Hazard ratio | 95% CI | p-value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Gender | 1.123 | 0.821 | 1.723 | 0.381 |
| Age | 1.079 | 0.923 | 1.286 | 0.283 |
| BMI (kg/m2) | 0.959 | 0.755 | 1.117 | 0.241 |
| Diabetes | 1.351 | 0.837 | 2.119 | 0.477 |
| Hypertension | 1.068 | 0.682 | 1.844 | 0.724 |
| H/o CAD | 1.371 | 1.187 | 1.511 | <0.001* |
| LVEF (%) | 0.775 | 0.629 | 0.973 | 0.008* |
| PASP (mmHg) | 1.224 | 1.046 | 1.464 | 0.003* |
| NT-proBNP (pg/mL) | 1.432 | 1.044 | 3.028 | 0.004* |
| SBP (mmHg) | 1.132 | 0.722 | 1.778 | 0.632 |
| DBP (mmHg) | 1.088 | 0.668 | 1.622 | 0.602 |
| HR (bpm) | 1.111 | 0.833 | 1.571 | 0.433 |
| QRS duration (ms) | 1.154 | 1.036 | 1.942 | 0.021 |
| QTc duration (ms) | 1.083 | 1.007 | 1.841 | 0.013 |
| SA | 1.122 | 0.614 | 1.354 | 0.806 |
| Education (GED completion) | 0.722 | 0.443 | 0.927 | 0.006* |
| Unemployment | 1.178 | 1.010 | 1.898 | 0.009* |
| Carvedilol | 0.722 | 0.552 | 0.924 | 0.006* |
| ACE-I/ARB | 0.865 | 0.441 | 0.824 | 0.008* |
| Spironolactone | 1.109 | 0.823 | 1.563 | 0.716 |
| Furosemide | 1.271 | 0.755 | 2.223 | 0.543 |
p<0.01,
CAD= coronary artery disease, SA= sleep apnea, CAD= coronary artery disease, ACE I= angiotensin converting enzyme inhibitor, ARB= angiotensin receptor blocker, LVEF= left ventricular ejection fraction, PASP= pulmonary artery systolic pressure, BMI= body mass index.
NT-proBNP= log transformed
Table 5:
Multivariate analysis (Outcome-MACE)
| Hazard ratio | 95% CI | p-value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| H/o CAD | 1.312 | 1.104 | 1.868 | <0.001 |
| LVEF | 0.703 | 0.617 | 0.901 | 0.006 |
| PASP | 1.243 | 1.075 | 1.911 | 0.009 |
| NT-proBNP | 1.372 | 1.072 | 2.557 | 0.010 |
| Education | 0.655 | 0.341 | 0.867 | 0.019 |
| Unemployment | 1.179 | 1.006 | 1.676 | 0.027 |
| ACE/ARB | 0.544 | 0.441 | 0.917 | 0.024 |
| Carvedilol | 0.665 | 0.481 | 0.863 | 0.010 |
Cox proportional hazard regression for multivariate analysis for primary outcome (MACE).
This model included all the covariates with p<0.01 on univariate analysis (Table 4).
MACE= 30-day HF readmission or CV mortality. NT-proBNP=log transformed.
Entire Cohort:
The MACE event rate among all with a CUD with HF on carvedilol did not differ significantly when compared to those not on carvedilol (32 vs. 38%, p = 0.16; Figure 1A).
Figure 1:
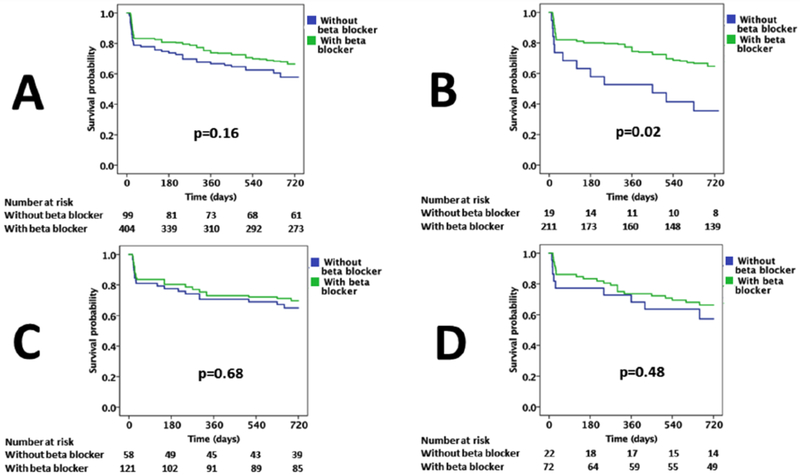
Kaplan Meier survival curves comparing MACE (including 30-day readmission and CV mortality) with and without the use of carvedilol in cocaine users among (A) all patients with heart failure (B) patients with HFrEF (C) patients with HFpEF and (D) patients with HFmrEF. Log rank p-values are recorded.
Reduced EF:
Among 230 individuals with reduced EF, there were significant differences in the rate of MACE among those on carvedilol compared to those not on carvedilol (34 vs. 58%, p=0.02) (Table 3, Figure 1B, Central Illustration).
Table 3:
Outcomes (MACE including CV mortality and 30-day readmission)
| Pts on Carvedilol | Pts not on Carvedilol | p-value | |
|---|---|---|---|
| HF (total) | n=404 | n=99 | |
| 131 (32%) | 38 (38%) | 0.26 | |
| HFrEF | n=211 | n=19 | |
| 72 (34%) | 11 (58%) | 0.04 | |
| HFpEF | n=121 | n=58 | |
| 36 (30%) | 19 (33%) | 0.67 | |
| HFmrEF | n=72 | n=22 | |
| 23 (32%) | 8 (36%) | 0.70 |
HF = heart failure, HFrEF = heart failure with reduced ejection fraction, HFpEF = heart failure with preserved ejection fraction, HFmrEF = heart failure with mid-range ejection fraction
Central Illustration: Cardiovascular Outcomes Associated with Carvedilol in HF patients with Cocaine Use Disorder (CUD).
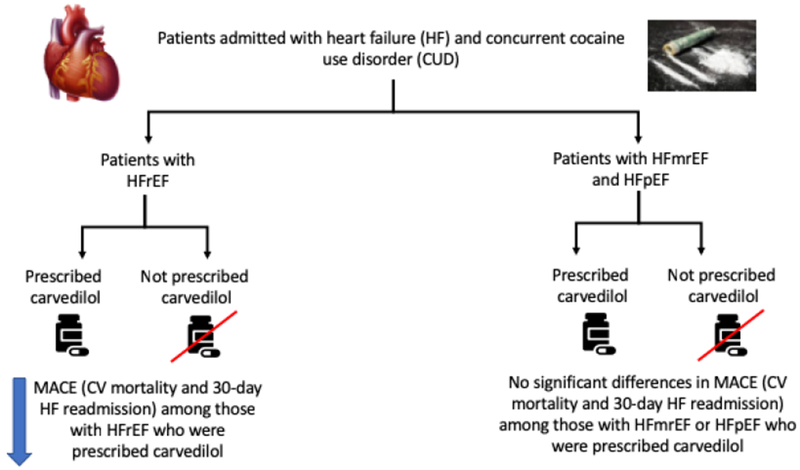
In this study, HFrEF patients with CUD who were prescribed carvedilol had lower incidence of MACE (CV mortality and 30-day HF readmission), compared to those not prescribed carvedilol. No significant differences were seen in the HFmrEF and HFpEF cohorts.
Preserved EF:
Of the 179 individuals with preserved EF, 121 were on carvedilol and 58 were not. There was no significant difference in outcomes between these two groups (30 vs. 33%, p=0.68) (Table 3, Figure 1C, Central Illustration).
Mid-Range EF:
Out of 94 individuals with mid-range EF, 72 were on carvedilol while 22 were not. There were no significant differences in outcomes between these two groups (32% vs. 36%, p=0.48) (Table 3, Figure 1D, Central Illustration).
Discussion
In this study, we leveraged a large single-center HF registry, in a population with a relatively high prevalence of a CUD (20% of all patients hospitalized with HF), to present data on the safety and efficacy of carvedilol use among HF patients with a CUD. We found that CV mortality and 30-day HF readmission were similar between CUD patients with all types of HF prescribed and not prescribed carvedilol. However, when stratified by category of HF, carvedilol use was associated with a lower rate of CV mortality and 30-day HF readmission among patients with HFrEF. Additionally, carvedilol use in this cohort was also associated with a lower NT-proBNP level on both admission and discharge. To our knowledge, this is the first large cohort registry study evaluating the safety of carvedilol among HF patients with a CUD.
Several prior randomized controlled trials have demonstrated the utility of beta-blockers, carvedilol, metoprolol succinate or bisoprolol, to improve symptoms, reduce hospitalization and enhance survival among patients with HF with a reduced EF. (23–25) Current HF guidelines recommend administration of one of these beta-blockers in individuals with current or prior HF and a left ventricular EF ≤ 40%. (11) Citing a lack of data, current guidelines do not provide suggestions on the safety and efficacy of beta-blockers in chronic HF among patients with a CUD. (11) There are limited prior data on the use of carvedilol among patients with HF with a CUD and no prior data evaluating the effect of carvedilol on clinical outcomes among those with a CUD with HF. In a single case series of 4 patients with HF and ongoing cocaine use, carvedilol use, compared to pre-carvedilol, was associated with an improvement in NYHA functional class and LVEF over a 2-year follow up. (26) Similarly, Lopez et al, demonstrated an improved exercise tolerance and LVEF with carvedilol in 72 patients with HFrEF and active cocaine use. (27) Our study extends these prior findings and tested the effect of carvedilol on clinical events among patients with HF with a CUD. We found that the use of carvedilol was not associated with an increase in clinical events in either HF with preserved EF or HF with a reduced EF. Further, clinical events were reduced among cocaine users with HFrEF who were prescribed carvedilol. There are also data from other models of acute cardiac events suggesting a safer profile for non-selective beta-blockers like carvedilol in the presence of cocaine. Specifically, Boehrer et al (28) compared the effect of labetalol (a non-selective beta blocker with effects on both α- and β-adrenergic receptors similar to carvedilol) vs. saline after cocaine administration and noted no pathophysiological changes in the coronary artery in those subjects who received labetalol, findings also noted in both canine and porcine models showing a neutral effect with labetalol (29–31). The alpha-adrenergic receptor blocking activity of carvedilol may allow cocaine users with HFrEF to safely derive similar benefits of beta-blockade as non-cocaine users, without the potential deleterious effects of unopposed alpha-adrenergic activity seen with co-administration of a selective beta-antagonist and cocaine.
There is reasonable scientific plausibility to support why carvedilol may be helpful among patients with HF and a CUD. Principally, there is significant overlap between several of the adverse pathophysiological changes seen with cocaine use and the pathophysiological changes that drive HF in broad groups. (32) Mechanistically, the adverse CV effects of cocaine are due to an increase in catecholamines leading to impaired handling of intracellular calcium, elevated reactive oxygen species and myocyte apoptosis with sequelae including elevated LV wall stress, LV dilatation, myocardial fibrosis and enhanced arrhythmogenesis. (33) Beta-blockers such as carvedilol block several of these adverse pathophysiological changes among broad groups of patients with HF and, likely, among patients with HF and a CUD. Carvedilol is a lipophilic, non-cardioselective ß- and α1-adrenergic receptor blocker and the use of carvedilol leads to a reduction in catecholamines, increased intracellular calcium, reduced reactive oxygen species, a decrease in myocyte apoptosis and arrhythmogenesis. (34) Our study demonstrated a lower heart rate among those on carvedilol compared to those not on it and this favorable reduction in heart rate is likely related to down-regulation of catecholamine release with a resultant decrease in myocardial oxygen demand. (35, 36) In addition, carvedilol has also been demonstrated to inhibit pathological left ventricular remodeling and fibrosis and reduce LV wall stress (37) – findings observed after exposure to cocaine. (38) Natriuretic peptides including atrial natriuretic peptide (ANP) and NT-proBNP are elevated with increasing wall stress (40) and are elevated in animal models of cocaine toxicity. (40) Our study also demonstrated lower levels of NT-proBNP both on admission as well as at discharge among those individuals on carvedilol suggesting that part of the protective effect of carvedilol in patients with HF and a CUD may be mediated in part via a reduction in wall stress. While not the focus of the study, there is also reasonable data on a dose-dependent efficacy for carvedilol during cocaine-withdrawal. For example, in a study by Sofuoglu et al, compared to 50 mg daily of carvedilol, 25 mg daily of carvedilol was associated with lower rate of positive urine toxicology during their 17-week trial. (39) This dose-dependent lower rate of a positive urine toxicology for cocaine while on carvedilol, was postulated to be due to lower doses of carvedilol preferentially blocking β-receptors and at higher doses blocking both α-1 and β-receptors. Their study suggested not using more than 25 mg carvedilol to avoid increases in cocaine and opioid use.
Our study has limitations which merit discussion. In our study, all patients on beta blockers were prescribed carvedilol and this prescription was based on institutional practice on the use of beta-blockers in the presence of documented cocaine use. Therefore, this study did not evaluate other beta-blockers. (41, 42) The accuracy of self-reporting data may be suboptimal. However, published reports have shown a strong association between self-reported and corroborative positive lab assays (43). In this study, individuals were stratified based on prescription of carvedilol and not confirmed use of carvedilol. Adherence to medications may be less than optimal among patients with active substance abuse; however, the lower heart rate among the cohort prescribed carvedilol, somewhat supports adherence to the medication. (44) Prescription of medication was based on chart review of the electronic medical record and cannot accurately assess medication adherence. Sicker patients with HFrEF may be intolerant of beta-blockade (e.g. due to hypotension or low cardiac output); thus, the worse outcomes observed among HFrEF patients not prescribed carvedilol may reflect a selection bias, whereby sicker patients with HFrEF were intolerant of carvedilol. However, the LVEF was higher in the non-carvedilol group and the heart rate and blood pressure were broadly similar.
Conclusions
While management should include counselling as to the risks of cocaine use among all patients and those with HF, our data suggest that prescribing carvedilol to individuals with HF with a CUD did not result in worse outcomes compared to those not on carvedilol and may be associated with a lower rate of adverse cardiovascular events among those with a reduced EF. Further research in this field is necessary to ascertain these benefits and to replicate such results prospectively.
Perspectives
Competency in Medical Knowledge
Both cardioselective and non-cardioselective beta blockers have been demonstrated to improve cardiac function, morbidity and survival in certain groups of people with HF. However, the use of beta-blockers among HF patients with CUD remains controversial. Our study suggests that carvedilol is safe and may even be effective among those with reduced ejection fraction heart failure and CUD.
Translational Outlook
Further research is necessary to corroborate these findings.
Supplementary Material
Acknowledgments
Funding/Support:
Dr. Banerji and Dr. Alvi were supported by NIH/NHLBI 5T32HL076136.
Dr. T. Neilan was supported in part through a gift from A. Curt Greer and Pamela Kohlberg, the National Institutes of Health/National Heart, Lung, and Blood Institute (1R01HL130539-01A1; 1R01HL137562-01A1; K24HL113128-06) and National Institutes of Health/Harvard Center for AIDS Research (P30 AI060354).
Abbreviations:
- HF
heart failure
- LVEF
left ventricular ejection fraction
- HFrEF
reduced ejection fraction heart failure
- HFmrEF
mid-range ejection fraction heart failure
- HFpEF
preserved ejection fraction heart failure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
TGN reports acting as a consultant for Parexel, Bristol Myers-Squibb, Aprea Therapeutics and Intrinsic Imaging unrelated to the current research.
References
- 1.NIDA. Cocaine. National Institute on Drug Abuse website. https://www.drugabuse.gov/publications/research-reports/cocaine May 6, 2016. Accessed December 19, 2018 [Internet]. 2016.
- 2.Finkel JB, Marhefka GD. Rethinking cocaine-associated chest pain and acute coronary syndromes. Mayo Clin Proc. 2011;86(12):1198–207. doi: 10.4065/mcp.2011.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rappolt RT Sr., Gray GR, Inaba DS. Letter: Propranolol in the treatment of cardiopressor effect of cocaine. N Engl J Med. 1976;295(8):448. doi: 10.1056/NEJM197608192950816. [DOI] [PubMed] [Google Scholar]
- 4.Rappolt RT, Gay GR, Inaba DS. Propranolol: a specific antagonist to cocaine. Clin Toxicol. 1977;10(3):265–71. doi: 10.3109/15563657708992422. [DOI] [PubMed] [Google Scholar]
- 5.Rappolt RT, Gay G, Inaba DS, Rappolt NR. Propranolol in cocaine toxicity. Lancet. 1976;2(7986):640–1. [DOI] [PubMed] [Google Scholar]
- 6.Catravas JD, Waters IW. Acute cocaine intoxication in the conscious dog: studies on the mechanism of lethality. J Pharmacol Exp Ther. 1981;217(2):350–6. [PubMed] [Google Scholar]
- 7.Damodaran S. Cocaine and beta-blockers: the paradigm. Eur J Intern Med. 2010;21(2):84–6. doi: 10.1016/j.ejim.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Smith M, Garner D, Niemann JT. Pharmacologic interventions after an LD50 cocaine insult in a chronically instrumented rat model: are beta-blockers contraindicated? Ann Emerg Med. 1991;20(7):768–71. [DOI] [PubMed] [Google Scholar]
- 9.McCord J, Jneid H, Hollander JE, de Lemos JA, Cercek B, Hsue P, et al. Management of cocaine-associated chest pain and myocardial infarction: a scientific statement from the American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology. Circulation. 2008;117(14):1897–907. doi: 10.1161/CIRCULATIONAHA.107.188950. [DOI] [PubMed] [Google Scholar]
- 10.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr., et al. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61(23):e179–347. doi: 10.1016/j.jacc.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 12.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 13.Alvi RM, Neilan AM, Tariq N, Awadalla M, Afshar M, Banerji D, et al. Protease Inhibitors and Cardiovascular Outcomes in Patients With HIV and Heart Failure. J Am Coll Cardiol. 2018;72(5):518–30. doi: 10.1016/j.jacc.2018.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvi RM, Neilan AM, Tariq N, Awadalla M, Rokicki A, Hassan M, et al. Incidence, Predictors, and Outcomes of Implantable Cardioverter-Defibrillator Discharge Among People Living With HIV. J Am Heart Assoc. 2018;7(18):e009857 Epub 2018/10/30. doi: 10.1161/JAHA.118.009857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvi RM, Tariq N, Malhotra A, Awadalla M, Triant VA, Zanni MV, et al. Sleep Apnea and Heart Failure With a Reduced Ejection Fraction Among Persons Living With Human Immunodeficiency Virus. Clin Infect Dis. 2018;67(3):447–55. Epub 2018/03/14. doi: 10.1093/cid/ciy216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samet S, Waxman R, Hatzenbuehler M, Hasin DS. Assessing addiction: concepts and instruments. Addict Sci Clin Pract. 2007;4(1):19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hjorthoj CR, Hjorthoj AR, Nordentoft M. Validity of Timeline Follow-Back for self-reported use of cannabis and other illicit substances--systematic review and meta-analysis. Addict Behav. 2012;37(3):225–33. doi: 10.1016/j.addbeh.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 18.Newton AS, Gokiert R, Mabood N, Ata N, Dong K, Ali S, et al. Instruments to detect alcohol and other drug misuse in the emergency department: a systematic review. Pediatrics. 2011;128(1):e180–92. doi: 10.1542/peds.2010-3727. [DOI] [PubMed] [Google Scholar]
- 19.Preston KL, Epstein DH, Cone EJ, Wtsadik AT, Huestis MA, Moolchan ET. Urinary elimination of cocaine metabolites in chronic cocaine users during cessation. J Anal Toxicol. 2002;26(7):393–400. [DOI] [PubMed] [Google Scholar]
- 20.Substance Abuse and Mental Health Services Administration, Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2014 . 2014. [Google Scholar]
- 21.Jeffcoat AR, Perez-Reyes M, Hill JM, Sadler BM, Cook CE. Cocaine disposition in humans after intravenous injection, nasal insufflation (snorting), or smoking. Drug Metab Dispos. 1989;17(2):153–9. [PubMed] [Google Scholar]
- 22.Solimini R, Rotolo MC, Pellegrini M, Minutillo A, Pacifici R, Busardo FP, et al. Adulteration Practices of Psychoactive Illicit Drugs: An Updated Review. Curr Pharm Biotechnol. 2017;18(7):524–30. doi: 10.2174/1389201018666170710184531. [DOI] [PubMed] [Google Scholar]
- 23.Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362(9377):7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 24.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353(9169):2001–7. [PubMed] [Google Scholar]
- 25.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353(9146):9–13. [PubMed] [Google Scholar]
- 26.Littmann L, Narveson SY, Fesel NM, Marconi SL. Beta blocker treatment of heart failure patients with ongoing cocaine use. Int J Cardiol. 2013;168(3):2919–20. doi: 10.1016/j.ijcard.2013.03.187. [DOI] [PubMed] [Google Scholar]
- 27.Lopez PD, Akinlonu A, Mene-Afejuku TO, Dumancas C, Saeed M, Cativo EH, et al. Clinical outcomes of Beta-blocker therapy in cocaine-associated heart failure. Int J Cardiol. 2018. doi: 10.1016/j.ijcard.2018.08.058. [DOI] [PubMed] [Google Scholar]
- 28.Boehrer JD, Moliterno DJ, Willard JE, Hillis LD, Lange RA. Influence of labetalol on cocaine-induced coronary vasoconstriction in humans. Am J Med. 1993;94(6):608–10. [DOI] [PubMed] [Google Scholar]
- 29.Shannon RP, Stambler BS, Komamura K, Ihara T, Vatner SF. Cholinergic modulation of the coronary vasoconstriction induced by cocaine in conscious dogs. Circulation. 1993;87(3):939–49. [DOI] [PubMed] [Google Scholar]
- 30.Kenny D, Pagel PS, Warltier DC. Attenuation of the systemic and coronary hemodynamic effects of cocaine in conscious dogs: propranolol versus labetalol. Basic Res Cardiol. 1992;87(5):465–77. [DOI] [PubMed] [Google Scholar]
- 31.Vargas R, Gillis RA, Ramwell PW. Propranolol promotes cocaine-induced spasm of porcine coronary artery. J Pharmacol Exp Ther. 1991;257(2):644–6. [PubMed] [Google Scholar]
- 32.Schwartz BG, Rezkalla S, Kloner RA. Cardiovascular effects of cocaine. Circulation. 2010;122(24):2558–69. doi: 10.1161/CIRCULATIONAHA.110.940569. [DOI] [PubMed] [Google Scholar]
- 33.Havakuk O, Rezkalla SH, Kloner RA. The Cardiovascular Effects of Cocaine. J Am Coll Cardiol. 2017;70(1):101–13. doi: 10.1016/j.jacc.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura K, Murakami M, Miura D, Yunoki K, Enko K, Tanaka M, et al. Beta-Blockers and Oxidative Stress in Patients with Heart Failure. Pharmaceuticals (Basel). 2011;4(8):1088–100. doi: 10.3390/ph4081088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isner JM, Estes NA 3rd, Thompson PD, Costanzo-Nordin MR, Subramanian R, Miller G, et al. Acute cardiac events temporally related to cocaine abuse. N Engl J Med. 1986;315(23):1438–43. doi: 10.1056/NEJM198612043152302. [DOI] [PubMed] [Google Scholar]
- 36.Lange RA, Hillis LD. Cardiovascular complications of cocaine use. N Engl J Med. 2001;345(5):351–8. doi: 10.1056/NEJM200108023450507. [DOI] [PubMed] [Google Scholar]
- 37.Palazzuoli A, Bruni F, Puccetti L, Pastorelli M, Angori P, Pasqui AL, et al. Effects of carvedilol on left ventricular remodeling and systolic function in elderly patients with heart failure. Eur J Heart Fail. 2002;4(6):765–70. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Huang C, Zhang B, Huang Q, Chen J, Xu L. The effects of carvedilol on cardiac structural remodeling: the role of endogenous nitric oxide in the activity of carvedilol. Mol Med Rep. 2013;7(4):1155–8. doi: 10.3892/mmr.2013.1329. [DOI] [PubMed] [Google Scholar]
- 39.Sofuoglu M, Poling J, Babuscio T, Gonsai K, Severino K, Nich C, et al. Carvedilol does not reduce cocaine use in methadone-maintained cocaine users. J Subst Abuse Treat. 2017;73:63–9. doi: 10.1016/j.jsat.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Casartelli A, Dacome L, Tessari M, Pascali J, Bortolotti F, Trevisan MT, et al. Cocaine-associated increase of atrial natriuretic peptides: an early predictor of cardiac complications in cocaine users? Heart Asia. 2014;6(1):100–7. doi: 10.1136/heartasia-2013-010482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fanari Z, Kennedy KK, Lim MJ, Laddu AA, Stolker JM. Comparison of in-hospital outcomes for beta-blocker use versus non-beta blocker use in patients presenting with cocaine-associated chest pain. Am J Cardiol. 2014;113(11):1802–6. doi: 10.1016/j.amjcard.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 42.Ibrahim M, Maselli DJ, Hasan R, Hamilton A. Safety of beta-blockers in the acute management of cocaine-associated chest pain. Am J Emerg Med. 2013;31(3):613–6. doi: 10.1016/j.ajem.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 43.Large MM, Smith G, Sara G, Paton MB, Kedzior KK, Nielssen OB. Meta-analysis of self-reported substance use compared with laboratory substance assay in general adult mental health settings. Int J Methods Psychiatr Res. 2012;21(2):134–48. doi: 10.1002/mpr.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gonzalez A, Mimiaga MJ, Israel J, Andres Bedoya C, Safren SA. Substance use predictors of poor medication adherence: the role of substance use coping among HIV-infected patients in opioid dependence treatment. AIDS Behav. 2013;17(1):168–73. doi: 10.1007/s10461-012-0319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


