Abstract
Persons with multiple sclerosis (MS) experience cognitive and physical decline despite more effective disease modifying therapies (DMTs), and symptomatic treatments currently have limited efficacy. The best treatment of MS disability may therefore be prevention of decline. Here we present a working model of reserve and brain maintenance, with a focus on modifiable risk and protective factors. At disease onset patients have varying degrees of reserve, broadly conceptualized as the dynamic availability of cerebral resources to support functional capacity. A clinical focus on prevention aims to minimize factors that deplete reserve (e.g., disease burden, comorbidities) and maximize factors that preserve reserve (e.g., DMTs, cardiovascular health). We review evidence for cardiovascular health, diet, and sleep as three potentially important modifiable factors that may modulate cerebral reserve generally, but also in disease-specific ways. We frame the brain as a limited capacity system in which inefficient usage of available cerebral capacity (reserve) leads to or exacerbates functional deficits, and we provide examples of factors that may lead to such inefficiency (e.g., poor mood, obesity, cognitive-motor dual-tasking). Finally, we discuss the challenges and responsibilities of MS neurologists and patients in pursuing comprehensive brain maintenance as a preventative approach.
Keywords: Multiple Sclerosis, Cognitive Reserve, Protective Factors, Risk Factors, Comorbidity
Introduction
Persons with multiple sclerosis (MS) strive to live full, happy, productive, and functionally independent lives free from cognitive decline, physical disability, and socioemotional distress. Toward this end, the field has dramatically improved the efficacy of disease-modifying therapies (DMTs), which have attenuated but not eliminated risk for disability.1 It is clear that no magic pill (or injection or infusion) exists to fully protect against disease-related functional decline, and symptomatic interventions to treat functional deficits have mixed efficacy.2 Taken together, the best treatment of disability is likely prevention of decline. Here we discuss the concepts of reserve and brain maintenance as components of a working model of protection against decline, including discussion of key modifiable factors that may modulate risk for decline. We emphasize that prevention of disability cannot be fully accomplished by any one person, but instead requires a coordinated effort by clinicians, patients, and support systems.
Reserve and Maintenance of Reserve
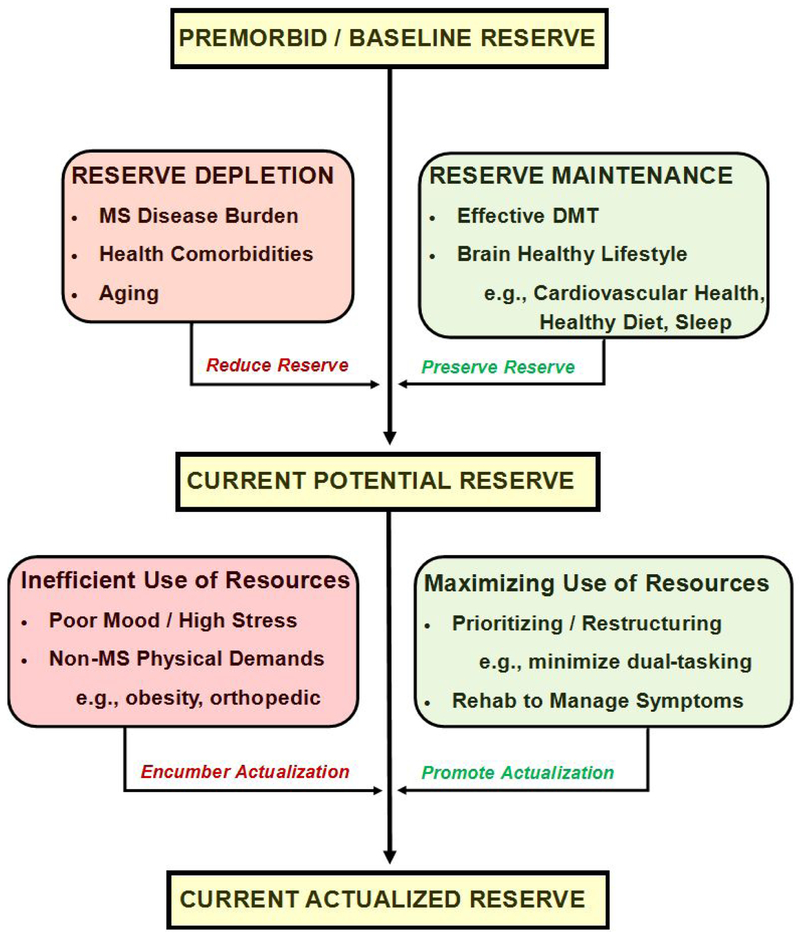
Persons with the same neurologic disease and similar disease burden (e.g., T2 lesion volume) often have very different functional outcomes. This observation was the impetus for the concept of reserve: the notion that some persons are better able to withstand MS disease without (or with less) functional decline.3,4 Here we present a working conceptual model for preventing decline (Figure), which begins with premorbid (baseline) reserve conceptualized broadly as cerebral resources underlying functional capacity. This varies across persons, and varies across functional domains within a person (e.g., memory versus balance). Baseline reserve is depleted by deleterious factors (e.g., aging, disease burden) and preserved by protective factors (e.g., effective treatment, healthy lifestyle), some of which are modifiable. It is unclear whether a person’s level of baseline reserve can be improved, so the best way to reduce risk for decline may be to preserve as much reserve as possible (see brain maintenance below).
FIGURE.
This working model of reserve and brain maintenance against disability proposes that persons have variable baseline reserve prior to MS onset, which is reduced by deleterious factors (red) or preserved by protective factors (green), leading to one’s current potential reserve. Actualization of this current potential reserve in the limited capacity CNS is reduced by inefficiency (waste) due to such factors as poor mood and obesity (red), or maximized by factors that promote efficiency, such as prioritizing a specific goal while eliminating distractions.
PREMORBID / BASELINE RESERVE
Research often interprets MS disease progression or DMTs as if they affect a universal brain and spinal cord: a central nervous system that is the same across all patients. Although we are more alike than different, there is notable interpersonal variability in structural and functional aspects of each person’s central nervous system, which is the product of unique genetic and environmental influences (e.g., early life nutrition, medical history, intellectual and physical activity). That is, each person brings a unique brain and spinal cord to the disease, and certain features of one’s CNS increase or decrease risk for disease progression and functional decline.
Research on brain reserve shows that larger brain growth during development (proxy of neuronal and synaptic count, estimated with intracranial volume) reduces risk for cognitive or physical decline in persons with MS.3,4 Theoretically, greater neuronal and synaptic counts may lead to more robust functional network development or provide more degrees of freedom to plastically adapt networks in the face of disease. Of course, proxies of premorbid brain reserve provide only rough estimates of gross interpersonal differences in premorbid brain structure. Estimates of premorbid cognitive reserve or capacity (estimated with premorbid intelligence) provide a window into the functional capacity of one’s brain. Persons with greater premorbid cognitive reserve are better able to maintain cognition despite disease progression.3 Although research on functional aspects of reserve has focused on cognition, premorbid physical capacity may represent a domain-specific form of reserve. For instance, a person with excellent premorbid balance and coordination may be less likely to experience gait impairment than a person with average premorbid balance. Although this needs further investigation, the concept of physical reserve may provide a rationale for balance training (e.g.,5) and motor rehabilitation6 generally to improve functional reserve prior to the onset of gait disturbance.
BRAIN MAINTENANCE
Reserve changes over time, with deleterious effects leading to depletion of reserve and protective factors leading to preservation of reserve. This modulation of reserve over time is reflected by the concept of brain maintenance,7,8 which we propose as a helpful framework from the aging literature to identify risk and protective factors for functional outcomes in MS, especially factors modifiable by treatment or lifestyle changes. Here we highlight key factors with evidence bases in the MS literature, but this working model should be further developed and adjusted over time.
Disease Progression & DMTs
Reserve is not a static construct. Proxies of premorbid reserve are fixed (e.g., maximal lifetime brain growth, premorbid intelligence), but actual reserve is dynamically depleted by age-related brain changes, neurologic disease progression, and health comorbidities, among other known and unknown factors. In the context of MS, disease progression is the most deleterious factor depleting a person’s reserve, therefore effective DMTs are a major influence in preserving reserve. Indeed, DMTs help preserve cerebral volume, leading to lower risk for disability.9
Brain Healthy Lifestyle
In addition to disease progression and disease modification, there are surely many other factors that dynamically deplete or preserve reserve. Here we focus on three potentially modifiable factors with research support directly relevant to MS. We emphasize that this is a working model of prevention, and many other factors (and interactions among factors) will need to be added as the model is further developed. We also acknowledge that most data on protective factors in MS is observational, so future randomized trials are necessary.
Cardiovascular Risk Factors:
Vascular diseases have deleterious effects on overall brain health outside MS (e.g. increased white matter signal abnormalities,10 reduced grey matter volume11). Retrospective analysis from the North American Research Committee on Multiple Sclerosis (NARCOMS) Registry demonstrated a link between vascular comorbidity with rapid disability progression in MS, such that patients reporting ≥1 vascular comorbidity at time of MS diagnosis had increased risk of ambulatory disability.12 MS patients with one or more cardiovascular risk factors (e.g. hypertension, heart disease, smoking, obesity, diabetes) were shown to have increased lesion burden and more advanced brain atrophy than patients without these risks.13 Smoking is a known risk factor for incident MS, but smoking is also linked to worsened quality of life and disability.14 Recently, higher body mass index was associated with worse brain atrophy over 5 years in MS patients.15 Although more work is needed to parse the underlying mechanisms, body mass index is a good proxy of cardiovascular health. To underscore the importance of such concomitant vascular disease, a nationwide Danish study found that the mortality rate due to cardiovascular disease was higher in MS patients than the matched general population.16
Given the link between cardiovascular disease and MS outcomes, interest has grown in the association of lipid profiles with MS disease burden and disability. Elevated total cholesterol has been associated with worsened disability and decreased brain parenchymal fraction while higher high-density lipoprotein cholesterol levels were associated with lower contrast-enhancing lesion volume.17 In patients with clinically isolated syndrome (CIS), higher low-density lipoprotein and total cholesterol levels were associated with increased cumulative number of new T2 lesions over a two-year period.18 Translation of this work to the clinical domain involved several trials of statin therapy in early MS and CIS, which largely failed to meet primary endpoints (e.g.19). A phase two study of high-dose simvastatin in secondary progressive MS did demonstrate benefit, with a 43% reduction in the annualized rate of whole-brain atrophy.20 It is important to note, however, that although statin trials are discussed here in the context of cardiovascular comorbidity in MS, they have more potential immunomodulatory and neuroprotective actions that are cholesterol-independent and it is unclear whether observed effects are in any way related to traditional lipid-lowering mechanisms. Taken together, however, it appears that better understanding and treatment of cardiovascular comorbidity (or promotion of cardiovascular health) may provide an opportunity for enhancing reserve and improving outcomes for patients.
A vast literature on physical exercise in persons with MS ranges from resistance training to aerobic exercise trials,21 with some observational work linking proxies of cardiorespiratory fitness to greater integrity of cerebral white matter and greater grey matter volume (e.g.,22), thereby drawing a link between fitness and preservation of reserve. Importantly, however, observational links between proxies of cardiovascular health (e.g., cardiorespiratory fitness, BMI) and structural integrity of the brain provide proofs of concept that cardiovascular health is important in preserving reserve in persons with MS, but more work is needed to elucidate precise mechanisms of action (especially for statins), ideal timing of interventions,23 and to assess causality with randomized trials.
Diet:
There is already evidence for a role for diet in preserving reserve within the aging literature. For example, adherence to a Mediterranean-style diet has been associated with total brain volume, gray matter volume, and white matter volume in older adults.24 While aging and MS represent distinct pathophysiologic entities, there are also shared features and these observations lend credibility to the overall concept that dietary factors have the ability to impact brain health, particularly with regard to reserve. Preclinical models in MS further support the biological plausibility of this idea. From a mechanistic standpoint, metabolites derived directly from the diet or produced by gut microbiota in response to dietary intake have the capability for important immunomodulatory, neuroprotective, and even remyelinating or regenerative effects.25 Illustrating one potential mechanistic link for impact inside the CNS relevant to MS, recent research has modeled the ability of tryptophan derived from the diet (cruciferous vegetables) and processed by gut microbiota to diffuse across the blood brain barrier and bind to the aryl hydrocarbon receptor (AhR) on astrocytes, with important downstream effects.26 Given the considerable supporting role of astrocytes and widespread effects of AhR activation, this mechanism is potentially particularly significant with regard to maintenance of reserve in MS.
Dietary factors with demonstrated efficacy in limiting severity of disease and promoting recovery in MS animal models include polyunsaturated fats (particularly omega-3 fatty acids), short chain fatty acids, high polyphenol content molecules and foods, and calorie-restricted diets whereas diets high in saturated fat and sodium seem to be detrimental (reviewed in25,27). However, there are multiple barriers to studying the role of diet in MS, and translating these animal model observations into clinical studies has proved challenging; further work is needed regarding the clinical impact of dietary components and patterns in MS patients. Current evidence in MS is based mostly on epidemiologic studies, registry data, and relatively limited observational studies and pilot-type clinical trials, the details of which are reviewed elsewhere.25,27 Until further work is completed, providing specific dietary advice for promoting the maintenance of reserve in MS patients is challenging. However, one aspect that can be translated into reasonable clinical recommendations comes from registry studies demonstrating an association between overall dietary quality and patient-reported measures of disability and quality of life.28,29 Ultimately, we need sizable longitudinal studies and interventional clinical trials rigorously studying outcomes. In the meantime observations from these registry studies and the aforementioned negative impact of cardiovascular comorbidities lend enough support for clinicians to recommend that patients follow general dietary guidelines for good health such as limiting the intake of processed foods and emphasizing the intake of fresh fruits and vegetables. While we continue to pursue specific evidence regarding dietary contributions to reserve in MS, we can feel confident that these types of recommendations are likely to have an overall positive impact on patient health.
Sleep:
Persons with MS often report sleep difficulty, but little is known about relationships between sleep and MS disease processes. Outside of MS, less sleep has been linked to lower integrity of normal appearing white matter in otherwise healthy adults (e.g.30). It is important to remember that persons with MS are not immune from the negative effects of aging on brain health, so that factors associated with depletion of reserve due to aging should also lead to an overall reduction in reserve in MS patients, including cardiovascular risk factors, poor diet, and poor sleep. There is, however, reason to also expect a disease specific contribution of sleep disturbance to reduced reserve in MS patients. Experimental work in rodents has linked sleep deprivation to increased blood-brain barrier permeability,31 which may increase risk for lesion formation and diffuse cerebral white matter damage in patients. Also, work in rodents has linked sleep to oligodendrocyte precursor cell proliferation and myelin repair,32 which is of clear importance for persons with MS. Moreover, chronic sleep deprivation leads to worse clinical disability in experimental autoimmune encephalomyelitis, an animal model of MS.33
Interactions across Risk Factors.
There is increasing evidence linking lower socioeconomic status to worse disability in persons with MS.34 Although precise mechanisms of this relationship are not fully understood, cardiovascular risk, poor diet, and risk for any comorbidity likely partially mediate this relationship. It is clearly unrealistic to target socioeconomic status in an effort to improve outcomes; however, attention to modifiable mediating factors may help to reduce health disparities. As an example of how risk factors may interact, data suggest that as the number of comorbid conditions increases, likelihood of starting an MS DMT decreases,35 which may be due to lack of drug safety information in patients with certain comorbid illnesses. Even in the age of highly effective DMTs, there is still need for improved long-term patient outcomes (particularly in progressive MS) and aggressive targeting of comorbid illness may provide benefit.
ACTUALIZATION of POTENTIAL RESERVE
In the proposed working model of prevention (Figure), a person’s current potential reserve against functional decline is the result of premorbid (baseline) reserve minus depletion by deleterious factors, which is mitigated by preservation through protective factors. This current potential reserve is instantiated in the structural and functional integrity of neural networks. We posit that other factors modulate actualization of this potential reserve to achieve goals.
The brain is a limited capacity system and available resources must be used efficiently to yield maximal performance. The example of a cerebral economy may be helpful. Everyone begins with a certain amount of cerebral resources (reserve), which varies across people. These resources are depleted or preserved as discussed above. A person must then use remaining resources efficiently to achieve outcomes; however, resources can be depleted by inefficiency (waste) in the system. Research on “walking and talking” in MS provides a nice proof of concept of the limited capacity model. Patients with even mild imbalance have more difficulty than healthy persons when asked to perform cognitive tasks while walking,36,37 likely because patients must divert limited capacity attentional resources away from cognition to attend to balance. In this situation, patients with imbalance would better actualize their cognitive potential if they performed cognitive tasks while seated. This improves efficiency within the cerebral economy.
Anxiety, depression, and stress likely also exhaust limited capacity attentional resources, thereby limiting patients’ actualization of potential reserve to achieve a cognitive (or physical) goal by depleting resources. Indeed, anxiety and depression are associated with worse cognition in MS.38 Interventions aiming to improve mood or reduce stress may reduce inefficacy (waste) in the system. Within the physical domain, factors beyond MS (e.g., obesity, orthopedic injuries) may encumber actualization of reserve by placing greater demands on balance and stability, therefore exhausting limited capacity resources.
This component of the model needs elaboration, which will require theoretical models of domain general and domain specific resource allocation and capacity, and how these systems are impacted by variables such as mood, obesity, and other known and as-yet-unknown factors. Of course, links between mood and cognition are likely more complicated than depicted here, and there may be one aspect of mood that affects cognition by depleting attentional resources (reducing actualization of reserve), and a separate aspect that affects cognition through effects of stress on mesial temporal structure and function (poor brain maintenance). The same is true for obesity and other factors (e.g., sleep). A future goal is to better understand the different ways modifiable factors impact brain maintenance, actualization of reserve, or both.
Addressing factors related to brain maintenance is a long-term endeavor aiming to prevent functional decline by preserving reserve (brain structure and function). This should be initiated at diagnosis and sustained thereafter. Actualization of remaining reserve is promoted by changes leading to more efficient use of limited resources. Interventions to promote actualization of reserve (to improve outcomes) can be initiated before or after functional decline occurs.
PROMOTING BRAIN MAINTENANCE IN CLINICAL PRACTICE
The roles and responsibilities of an MS neurologist are ever expanding. Quality MS care now involves intimate knowledge and comfort with powerful immunomodulatory drugs with associated safety monitoring, familiarity with advanced neuroimaging, complex and aggressive symptom management, and consideration for the overall health and wellness of our patients. To provide excellent care, MS providers must collaborate with colleagues from a variety of fields (e.g. psychiatry, physiatry, and urology). All MS providers ought to acknowledge the importance of good primary care to our patients as we learn more about the impact that comorbidities have on MS disability. As such, MS providers should also focus on lifestyle factors and health care maintenance (e.g. diet, exercise, smoking cessation, and psychological health), and recognize the importance of educating patients in these modifiable aspects of health. Incorporating routine questions about diet, smoking, exercise and psychological wellbeing into visits will signal to patients that positive changes in these domains are as vital as their DMT and radiologic surveillance towards attaining good functional outcomes. Creating a network of supporting physicians and providers to help ensure comprehensive care for MS patients is key while also engaging with patients’ support systems (i.e., friends and family).
Patients must take a proactive role in preserving their reserve to reduce risk for future disability. Adherence to DMTs is an important and necessary responsibility, but patients must also make cardiovascular health, diet, and sleep a priority. Mental health and stress reduction are also key, which may include mindfulness, relaxing and pleasurable leisure, and socialization, as well as seeking professional help when necessary. These suggestions are challenging to follow when patients are juggling myriad work, social, household, and familial responsibilities. MS is almost always diagnosed between ages 20 and 40, a period when demand for productivity is highest and self-care may seem like a luxury. It is therefore necessary to reframe self-care as a necessary part of treatment by educating patients about brain maintenance, and by signaling the importance of healthy lifestyles by routinely monitoring patients’ progress toward health goals.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health [R01 HD082176 to JFS].
Footnotes
Disclosures:
Rachel Brandstadter and Ilana Katz Sand have nothing to disclose.
James F. Sumowski has received compensation from Biogen and Genzyme for educational programs.
REFERENCES
- 1.University of California SFMSET, Cree BAC, Hollenbach JA, et al. Silent Progression in Disease Activity-Free Relapsing Multiple Sclerosis. Annals of neurology. March 9 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amatya B, Khan F, Galea M. Rehabilitation for people with multiple sclerosis: an overview of Cochrane Reviews. The Cochrane database of systematic reviews. January 14 2019;1:CD012732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sumowski JF, Rocca MA, Leavitt VM, et al. Brain reserve and cognitive reserve protect against cognitive decline over 4.5 years in MS. Neurology. May 20 2014;82(20):1776–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sumowski JF, Rocca MA, Leavitt VM, et al. Brain reserve against physical disability progression over 5 years in multiple sclerosis. Neurology. May 24 2016;86(21):2006–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prosperini L, Fortuna D, Gianni C, Leonardi L, Marchetti MR, Pozzilli C. Home-based balance training using the Wii balance board: a randomized, crossover pilot study in multiple sclerosis. Neurorehabilitation and neural repair. Jul-Aug 2013;27(6):516–525. [DOI] [PubMed] [Google Scholar]
- 6.Prosperini L, Piattella MC, Gianni C, Pantano P. Functional and Structural Brain Plasticity Enhanced by Motor and Cognitive Rehabilitation in Multiple Sclerosis. Neural plasticity. 2015;2015:481574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stern Y, Arenaza-Urquijo EM, Bartres-Faz D, et al. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. September 14 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabeza R, Albert M, Belleville S, et al. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nature reviews. Neuroscience. November 2018;19(11):701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sormani MP, Arnold DL, De Stefano N. Treatment effect on brain atrophy correlates with treatment effect on disability in multiple sclerosis. Annals of neurology. January 2014;75(1):43–49. [DOI] [PubMed] [Google Scholar]
- 10.Fukuda H, Kitani M. Differences between treated and untreated hypertensive subjects in the extent of periventricular hyperintensities observed on brain MRI. Stroke. September 1995;26(9):1593–1597. [DOI] [PubMed] [Google Scholar]
- 11.Enzinger C, Fazekas F, Matthews PM, et al. Risk factors for progression of brain atrophy in aging: six-year follow-up of normal subjects. Neurology. May 24 2005;64(10):1704–1711. [DOI] [PubMed] [Google Scholar]
- 12.Marrie RA, Rudick R, Horwitz R, et al. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology. March 30 2010;74(13):1041–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kappus N, Weinstock-Guttman B, Hagemeier J, et al. Cardiovascular risk factors are associated with increased lesion burden and brain atrophy in multiple sclerosis. Journal of neurology, neurosurgery, and psychiatry. February 2016;87(2):181–187. [DOI] [PubMed] [Google Scholar]
- 14.Briggs FB, Gunzler DD, Ontaneda D, Marrie RA. Smokers with MS have greater decrements in quality of life and disability than non-smokers. Multiple sclerosis (Houndmills, Basingstoke, England). November 2017;23(13):1772–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mowry EM, Azevedo CJ, McCulloch CE, et al. Body mass index, but not vitamin D status, is associated with brain volume change in MS. Neurology. 2018;91(24):e2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bronnum-Hansen H, Koch-Henriksen N, Stenager E. Trends in survival and cause of death in Danish patients with multiple sclerosis. Brain : a journal of neurology. April 2004;127(Pt 4):844–850. [DOI] [PubMed] [Google Scholar]
- 17.Weinstock-Guttman B, Zivadinov R, Mahfooz N, et al. Serum lipid profiles are associated with disability and MRI outcomes in multiple sclerosis. Journal of neuroinflammation. October 4 2011;8:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinstock-Guttman B, Zivadinov R, Horakova D, et al. Lipid profiles are associated with lesion formation over 24 months in interferon-beta treated patients following the first demyelinating event. Journal of neurology, neurosurgery, and psychiatry. November 2013;84(11):1186–1191. [DOI] [PubMed] [Google Scholar]
- 19.Sorensen PS, Lycke J, Eralinna JP, et al. Simvastatin as add-on therapy to interferon beta-1a for relapsing-remitting multiple sclerosis (SIMCOMBIN study): a placebo-controlled randomised phase 4 trial. The Lancet. Neurology. August 2011;10(8):691–701. [DOI] [PubMed] [Google Scholar]
- 20.Chataway J, Schuerer N, Alsanousi A, et al. Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS-STAT): a randomised, placebo-controlled, phase 2 trial. Lancet (London, England). June 28 2014;383(9936):2213–2221. [DOI] [PubMed] [Google Scholar]
- 21.Motl RW, Sandroff BM, Kwakkel G, et al. Exercise in patients with multiple sclerosis. The Lancet Neurology. 2017/October/01/ 2017;16(10):848–856. [DOI] [PubMed] [Google Scholar]
- 22.Prakash RS, Snook EM, Motl RW, Kramer AF. Aerobic fitness is associated with gray matter volume and white matter integrity in multiple sclerosis. Brain research. June 23 2010;1341:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riemenschneider M, Hvid LG, Stenager E, Dalgas U. Is there an overlooked "window of opportunity" in MS exercise therapy? Perspectives for early MS rehabilitation. Multiple sclerosis (Houndmills, Basingstoke, England). June 2018;24(7):886–894. [DOI] [PubMed] [Google Scholar]
- 24.Gu Y, Brickman AM, Stern Y, et al. Mediterranean diet and brain structure in a multiethnic elderly cohort. Neurology. November 17 2015;85(20):1744–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katz Sand I The Role of Diet in Multiple Sclerosis: Mechanistic Connections and Current Evidence. Current nutrition reports. September 2018;7(3):150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothhammer V, Mascanfroni ID, Bunse L, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nature medicine. June 2016;22(6):586–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mische LJ, Mowry EM. The Evidence for Dietary Interventions and Nutritional Supplements as Treatment Options in Multiple Sclerosis: a Review. Current treatment options in neurology. March 17 2018;20(4):8. [DOI] [PubMed] [Google Scholar]
- 28.Hadgkiss EJ, Jelinek GA, Weiland TJ, Pereira NG, Marck CH, van der Meer DM. The association of diet with quality of life, disability, and relapse rate in an international sample of people with multiple sclerosis. Nutritional neuroscience. April 2015;18(3):125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitzgerald KC, Tyry T, Salter A, et al. Diet quality is associated with disability and symptom severity in multiple sclerosis. Neurology. January 2 2018;90(1):e1–e11. [DOI] [PubMed] [Google Scholar]
- 30.Yaffe K, Nasrallah I, Hoang TD, et al. Sleep Duration and White Matter Quality in Middle-Aged Adults. Sleep. September 1 2016;39(9):1743–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He J, Hsuchou H, He Y, Kastin AJ, Wang Y, Pan W. Sleep restriction impairs blood-brain barrier function. The Journal of neuroscience : the official journal of the Society for Neuroscience. October 29 2014;34(44):14697–14706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellesi M, Pfister-Genskow M, Maret S, Keles S, Tononi G, Cirelli C. Effects of sleep and wake on oligodendrocytes and their precursors. The Journal of neuroscience : the official journal of the Society for Neuroscience. September 4 2013;33(36):14288–14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He J, Wang Y, Kastin AJ, Pan W. Increased sleep fragmentation in experimental autoimmune encephalomyelitis. Brain, behavior, and immunity. May 2014;38:53–58. [DOI] [PubMed] [Google Scholar]
- 34.Harding KE, Wardle M, Carruthers R, et al. Socioeconomic status and disability progression in multiple sclerosis: A multinational study. Neurology. March 26 2019;92(13):e1497–e1506. [DOI] [PubMed] [Google Scholar]
- 35.Zhang T, Tremlett H, Leung S, et al. Examining the effects of comorbidities on disease-modifying therapy use in multiple sclerosis. Neurology. April 5 2016;86(14):1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamilton F, Rochester L, Paul L, Rafferty D, O'Leary CP, Evans JJ. Walking and talking: an investigation of cognitive-motor dual tasking in multiple sclerosis. Multiple sclerosis (Houndmills, Basingstoke, England). October 2009;15(10):1215–1227. [DOI] [PubMed] [Google Scholar]
- 37.Leone C, Patti F, Feys P. Measuring the cost of cognitive-motor dual tasking during walking in multiple sclerosis. Multiple sclerosis. February 2015;21(2):123–131. [DOI] [PubMed] [Google Scholar]
- 38.Whitehouse CE, Fisk JD, Bernstein CN, et al. Comorbid anxiety, depression, and cognition in MS and other immune-mediated disorders. Neurology. 2019;92(5):e406. [DOI] [PMC free article] [PubMed] [Google Scholar]